Submitted:
16 July 2025
Posted:
17 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Molecular Size of HA Dictates Distinct Biological Functions
3. HA in the Hepatic Sinusoidal Niche
4. HA Metabolism and Clearance in the Liver
4.1. HA Synthesis: Isoform-Specific Roles
4.2. HA Degradation and Clearance
5. HA as an Immune Modulator in Liver Fibrosis
5.1. Innate Immune Response
5.2. Adaptive Immune Response
6. HA in Chronic Liver Pathology: from MASLD to HCC
HA-Mediated Immune Reprogramming in HCC Progression
7. Therapeutic Targeting of HA Signaling and Metabolism in Liver Disease
7.1. Inhibition of HA Synthesis
7.2. Enzymatic Degradation of HA
7.3. Blockade of HA Receptors (CD44, RHAMM)
7.4. Integration with Immunotherapy and Combination Strategies
8. Concluding Remarks and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Cowman, M.K.; Turley, E.A. Functional Organization of Extracellular Hyaluronan, CD44, and RHAMM. Proteoglycan Research 2023, 1, e4. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol Rev 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Bollyky, P.L.; Falk, B.A.; Wu, R.P.; Buckner, J.H.; Wight, T.N.; Nepom, G.T. Intact Extracellular Matrix and the Maintenance of Immune Tolerance: High Molecular Weight Hyaluronan Promotes Persistence of Induced CD4+CD25+ Regulatory T Cells. J Leukoc Biol 2009, 86, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seki, E. Hyaluronan in Liver Fibrosis: Basic Mechanisms, Clinical Implications, and Therapeutic Targets. Hepatol Commun 2023, 7. [Google Scholar] [CrossRef] [PubMed]
- Gudowska, M.; Gruszewska, E.; Panasiuk, A.; Cylwik, B.; Flisiak, R.; Świderska, M.; Szmitkowski, M.; Chrostek, L. Hyaluronic Acid Concentration in Liver Diseases. Clin Exp Med 2016, 16, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Adams, L.A. Advances in Non-Invasive Assessment of Hepatic Fibrosis. Gut 2020, 69, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Park, S.J.; Noh, I.; Kim, C.H. The Effects of the Molecular Weights of Hyaluronic Acid on the Immune Responses. Biomater Res 2021, 25. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K.; Lee, H.G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front Immunol 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its Nature, Distribution, Functions and Turnover. J Intern Med 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Bonam, S.R.; Rasala, S.; Wankar, J.; Bohara, R.A.; Bayry, J.; Rochev, Y.; Pandit, A. Recent Advances and Prospects of Hyaluronan as a Multifunctional Therapeutic System. Journal of Controlled Release 2021, 336, 598–620. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P. Hyaluronan: From Extracellular Glue to Pericellular Cue. Nat Rev Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M.; Soares da Costa, D.; Paulo, P.M.R.; Reis, R.L.; Pashkuleva, I. Co-Localization and Crosstalk between CD44 and RHAMM Depend on Hyaluronan Presentation. Acta Biomater 2021, 119, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Hinneh, J.A.; Gillis, J.L.; Moore, N.L.; Butler, L.M.; Centenera, M.M. The Role of RHAMM in Cancer: Exposing Novel Therapeutic Vulnerabilities. Front Oncol 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Brenner, D.A. Toll-like Receptors and Adaptor Molecules in Liver Disease: Update. Hepatology 2008, 48, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Fan, J.; Yu, S.; Chen, S.; Luo, Y.; Prestwich, G.D.; Mascarenhas, M.M.; Garg, H.G.; Quinn, D.A.; et al. Regulation of Lung Injury and Repair by Toll-like Receptors and Hyaluronan. Nat Med 2005, 11, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, S.M.; Hawn, T.R.; Arrigoni, A.; Wight, T.N.; Bollyky, P.L. Tissue Integrity Signals Communicated by Highmolecular Weight Hyaluronan and the Resolution of Inflammation. Immunol Res 2014, 58, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Romo, M.; López-Vicario, C.; Pérez-Romero, N.; Casulleras, M.; Martínez-Puchol, A.I.; Sánchez, B.; Flores-Costa, R.; Alcaraz-Quiles, J.; Duran-Güell, M.; Ibarzábal, A.; et al. Small Fragments of Hyaluronan Are Increased in Individuals with Obesity and Contribute to Low-Grade Inflammation through TLR-Mediated Activation of Innate Immune Cells. Int J Obes 2022, 46, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Puré, E.; Cuff, C.A. A Crucial Role for CD44 in Inflammation. Trends Mol Med 2001, 7, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Arteel, G.E. Extracellular Matrix and Hepatic Wound Healing before Fibrosis. Semin Liver Dis 2024, 44, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Sanz-García, C.; Fernández-Iglesias, A.; Gracia-Sancho, J.; Arráez-Aybar, L.A.; Nevzorova, Y.A.; Cubero, F.J. The Space of Disse: The Liver Hub in Health and Disease. Livers 2021, 1, 2. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a Crucial Regulator of Inflammation. Front Immunol 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Patouraux, S.; Rousseau, D.; Bonnafous, S.; Lebeaupin, C.; Luci, C.; Canivet, C.M.; Schneck, A.S.; Bertola, A.; Saint-Paul, M.C.; Iannelli, A.; et al. CD44 Is a Key Player in Non-Alcoholic Steatohepatitis. J Hepatol 2017, 67, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Liver Sinusoidal Endothelial Cells — Gatekeepers of Hepatic Immunity. Nat Rev Gastroenterol Hepatol 2018, 15, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers (Basel) 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.E.; Laurent, T.C.; Engström-Laurent, A.; Laurent, U.G.B. ELIMINATION OF HYALURONIC ACID FROM THE BLOOD STREAM IN THE HUMAN. Clin Exp Pharmacol Physiol 1984, 11, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Larsen, A.K.; McCourt, P.; Smedsrød, B.; Sørensen, K.K. The Scavenger Function of Liver Sinusoidal Endothelial Cells in Health and Disease. Front Physiol 2021, 12, 757469. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and Signaling. Biochim Biophys Acta Gen Subj 2014, 1840, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Joy, R.A.; Vikkath, N.; Ariyannur, P.S. Metabolism and Mechanisms of Action of Hyaluronan in Human Biology. Drug Metab Pers Ther 2018, 33, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Noureddin, M.; Liu, C.; Ohashi, K.; Kim, S.Y.; Ramnath, D.; Powell, E.E.; Sweet, M.J.; Roh, Y.S.; Hsin, I.F.; et al. Hyaluronan Synthase 2-Mediated Hyaluronan Production Mediates Notch1 Activation and Liver Fibrosis. Sci Transl Med 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tian, X.; Lu, J.Y.; Boit, K.; Ablaeva, J.; Zakusilo, F.T.; Emmrich, S.; Firsanov, D.; Rydkina, E.; Biashad, S.A.; et al. Increased Hyaluronan by Naked Mole-Rat HAS2 Improves Healthspan in Mice. Nature 2023, 621, 196. [Google Scholar] [CrossRef] [PubMed]
- Rilla, K.; Oikari, S.; Jokela, T.A.; Hyttinen, J.M.T.; Kärnä, R.; Tammi, R.H.; Tammi, M.I. Hyaluronan Synthase 1 (HAS1) Requires Higher Cellular Udp-Glcnac Concentration than HAS2 and HAS3. Journal of Biological Chemistry 2013, 288, 5973–5983. [Google Scholar] [CrossRef] [PubMed]
- Siiskonen, H.; Oikari, S.; Pasonen-Seppänen, S.; Rilla, K. Hyaluronan Synthase 1: A Mysterious Enzyme with Unexpected Functions. Front Immunol 2015, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three Isoforms of Mammalian Hyaluronan Synthases Have Distinct Enzymatic Properties. Journal of Biological Chemistry 1999, 274, 25085–25092. [Google Scholar] [CrossRef] [PubMed]
- Homann, S.; Grandoch, M.; Kiene, L.S.; Podsvyadek, Y.; Feldmann, K.; Rabausch, B.; Nagy, N.; Lehr, S.; Kretschmer, I.; Oberhuber, A.; et al. Hyaluronan Synthase 3 Promotes Plaque Inflammation and Atheroprogression. Matrix Biology 2018, 66, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Song, G.Y.; Shim, A.; Lee, J.H.; Eom, C. Bin; Liu, C.; Yang, Y.M.; Seki, E. Hyaluronan Synthase 2, a Target of MiR-200c, Promotes Carbon Tetrachloride-Induced Acute and Chronic Liver Inflammation via Regulation of CCL3 and CCL4. Exp Mol Med 2022, 54, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their Genomics, Structures, and Mechanisms of Action. Chem Rev 2006, 106, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.N.; Weigel, J.A.; Weigel, P.H. Endocytic Function, Glycosaminoglycan Specificity, and Antibody Sensitivity of the Recombinant Human 190-KDa Hyaluronan Receptor for Endocytosis (HARE). Journal of Biological Chemistry 2004, 279, 36201–36209. [Google Scholar] [CrossRef] [PubMed]
- Pandey, E.; Nour, A.S.; Harris, E.N. Prominent Receptors of Liver Sinusoidal Endothelial Cells in Liver Homeostasis and Disease. Front Physiol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Casalino-Matsuda, S.M.; Monzon, M.E.; Conner, G.E.; Salathe, M.; Forteza, R.M. Role of Hyaluronan and Reactive Oxygen Species in Tissue Kallikrein-Mediated Epidermal Growth Factor Receptor Activation in Human Airways. Journal of Biological Chemistry 2004, 279, 21606–21616. [Google Scholar] [CrossRef] [PubMed]
- Šoltés, L.; Mendichi, R.; Kogan, G.; Schiller, J.; Stankovská, M.; Arnhold, J. Degradative Action of Reactive Oxygen Species on Hyaluronan. Biomacromolecules 2006, 7, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.R.; Trowbridge, J.M.; Rudisill, J.A.; Termeer, C.C.; Simon, J.C.; Gallo, R.L. Hyaluronan Fragments Stimulate Endothelial Recognition of Injury through TLR4. Journal of Biological Chemistry 2004, 279, 17079–17084. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, S.S.; Karalis, T.; Heldin, P. Intracellular Hyaluronan: Importance for Cellular Functions. Semin Cancer Biol 2020, 62, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K.; Bhushan, B. Liver Regeneration: Biological and Pathological Mechanisms and Implications. Nat Rev Gastroenterol Hepatol 2021, 18, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Yang, M.; Yang, J.; Lin, S.; Wei, X.; Xu, X. Cellular Crosstalk during Liver Regeneration: Unity in Diversity. Cell Communication and Signaling 2022, 20. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; McAvoy, E.F.; Lam, F.; Gill, V.; De La Motte, C.; Savani, R.C.; Kubes, P. Interaction of CD44 and Hyaluronan Is the Dominant Mechanism for Neutrophil Sequestration in Inflamed Liver Sinusoids. Journal of Experimental Medicine 2008, 205, 915–927. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Jenne, C.N.; Zhuo, L.; Kimata, K.; Kubes, P. Kupffer Cells and Activation of Endothelial TLR4 Coordinate Neutrophil Adhesion within Liver Sinusoids during Endotoxemia. Am J Physiol Gastrointest Liver Physiol 2013, 305. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Wang, J. Therapeutic Targeting of Neutrophil Granulocytes in Inflammatory Liver Disease. Front Immunol 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Niemietz, I.; Moraes, A.T.; Sundqvist, M.; Brown, K.L. Hyaluronan Primes the Oxidative Burst in Human Neutrophils. J Leukoc Biol 2020, 108, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Niemietz, I.; Brown, K.L. Hyaluronan Promotes Intracellular ROS Production and Apoptosis in TNFα-Stimulated Neutrophils. Front Immunol 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Egan, C.E.; Daugherity, E.K.; Rogers, A.B.; Abi Abdallah, D.S.; Denkers, E.Y.; Maurer, K.J. CCR2 and CD44 Promote Inflammatory Cell Recruitment during Fatty Liver Formation in a Lithogenic Diet Fed Mouse Model. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Höchst, B.; Schildberg, F.A.; Sauerborn, P.; Gäbel, Y.A.; Gevensleben, H.; Goltz, D.; Heukamp, L.C.; Türler, A.; Ballmaier, M.; Gieseke, F.; et al. Activated Human Hepatic Stellate Cells Induce Myeloid Derived Suppressor Cells from Peripheral Blood Monocytes in a CD44-Dependent Fashion. J Hepatol 2013, 59, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Hagenstein, J.; Burkhardt, S.; Sprezyna, P.; Tasika, E.; Tiegs, G.; Diehl, L. CD44 Expression on Murine Hepatic Stellate Cells Promotes the Induction of Monocytic and Polymorphonuclear Myeloid-Derived Suppressor Cells. J Leukoc Biol 2024, 116, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Du, Y.; He, Y.; Liu, Y.; Zhang, G.; Yang, C.; Gao, F. INT-HA Induces M2-like Macrophage Differentiation of Human Monocytes via TLR4-MiR-935 Pathway. Cancer Immunology, Immunotherapy 2019, 68, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, A.S.; Pilling, D.; Gomer, R.H. High and Low Molecular Weight Hyaluronic Acid Differentially Regulate Human Fibrocyte Differentiation. PLoS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- DeGrendele, H.C.; Estess, P.; Siegelman, M.H. Requirement for CD44 in Activated T Cell Extravasation into an Inflammatory Site. Science (1979) 1997, 278, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Yago, T.; Shao, B.; Miner, J.J.; Yao, L.; Klopocki, A.G.; Maeda, K.; Coggeshall, K.M.; McEver, R.P. E-Selectin Engages PSGL-1 and CD44 through a Common Signaling Pathway to Induce Integrin ALβ2-Mediated Slow Leukocyte Rolling. Blood 2010, 116, 485–494. [Google Scholar] [CrossRef] [PubMed]
- van Steen, A.C.I.; Grönloh, M.L.B.; Joosten, S.; van Alphen, F.; van den Biggelaar, M.; Nolte, M.A.; Spaargaren, M.; van Buul, J.D.; Schoppmeyer, R. Endothelial ICAM-1 Adhesome Recruits CD44 for Optimal Transcellular Migration of Human CTLs. The Journal of Immunology 2023, 211, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.J.; Abuelela, A.F.; Merzaban, J.S. An Analysis of Trafficking Receptors Shows That CD44 and P-Selectin Glycoprotein Ligand-1 Collectively Control the Migration of Activated Human T-Cells. Front Immunol 2017, 8, 246772. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.L.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M. CD44 Mobilization in Allogeneic Dendritic Cell–T Cell Immunological Synapse Plays a Key Role in T Cell Activation. J Leukoc Biol 2008, 84, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Gomez, T.S.; Billadeau, D.D. T Cell Activation and the Cytoskeleton: You Can’t Have One Without the Other. Adv Immunol 2008, 97, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J.C. Oligosaccharides of Hyaluronan Activate Dendritic Cells via Toll-like Receptor 4. Journal of Experimental Medicine 2002, 195, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Scheibner, K.A.; Lutz, M.A.; Boodoo, S.; Fenton, M.J.; Powell, J.D.; Horton, M.R. Hyaluronan Fragments Act as an Endogenous Danger Signal by Engaging TLR2. The Journal of Immunology 2006, 177, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Bollyky, P.L.; Lord, J.D.; Masewicz, S.A.; Evanko, S.P.; Buckner, J.H.; Wight, T.N.; Nepom, G.T. Cutting Edge: High Molecular Weight Hyaluronan Promotes the Suppressive Effects of CD4+CD25+ Regulatory T Cells. The Journal of Immunology 2007, 179, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yokohama, S.; Yoneda, M.; Okamoto, S.; Tamaki, Y.; Ito, T.; Okada, M.; Aso, K.; Makino, I. High, but Not Low, Molecular Weight Hyaluronan Prevents T-Cell-Mediated Liver Injury by Reducing Proinflammatory Cytokines in Mice. J Gastroenterol 2004, 39, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Zelber-Sagi, S.; Lazarus, J.V.; Wong, V.W.-S.; Yilmaz, Y.; Duseja, A.; Eguchi, Y.; Castera, L.; Pessoa, M.G.; Oliveira, C.P.; et al. Global Consensus Recommendations for Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis. Gastroenterology 2025. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A Systemic Metabolic Disorder with Cardiovascular and Malignant Complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Dudek, M.; Knolle, P. Non-Alcoholic Fatty Liver Disease: The Interplay between Metabolism, Microbes and Immunity. Nat Metab 2021, 3, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Rauhala, L.; Jokela, T.; Kärnä, R.; Bart, G.; Takabe, P.; Oikari, S.; Tammi, M.I.; Pasonen-Seppänen, S.; Tammi, R.H. Extracellular ATP Activates Hyaluronan Synthase 2 (HAS2) in Epidermal Keratinocytes via P2Y2, Ca2+ Signaling, and MAPK Pathways. Biochemical Journal 2018, 475, 1755–1772. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, H.; Ge, X.; Nieto, N. High-Mobility Group Box-1 and Liver Disease. Hepatol Commun 2018, 2, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.M.; Swann, D.; Lee, P.F.; Lam, K.W. Inhibition of Oxidative Degradation of Hyaluronic Acid by Uric Acid. Curr Eye Res 1984, 3, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Jain, A.; Diehl, A.M.; Guy, C.D.; Portenier, D.; Sudan, R.; Singh, S.; Faulkner, C.; Richards, L.; Hester, K.D.; et al. Validation of Serum Test for Advanced Liver Fibrosis in Patients With Nonalcoholic Steatohepatitis. Clinical Gastroenterology and Hepatology 2019, 17, 1867–1876.e3. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, A.M.; Salvén, A.; Kärjä, V.; Rilla, K.; Matilainen, J.; Nieminen, P. Hyaluronan Histochemistry - A Potential New Tool to Assess the Progress of Liver Disease from Simple Steatosis to Hepatocellular Carcinoma. Glycobiology 2019, 29, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Lantier, L.; Kennedy, A.; Bonner, J.S.; Mayes, W.H.; Bracy, D.P.; Bookbinder, L.H.; Hasty, A.H.; Thompson, C.B.; Wasserman, D.H. Hyaluronan Accumulates with High-Fat Feeding and Contributes to Insulin Resistance. Diabetes 2013, 62, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat Rev Gastroenterol Hepatol 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, M.; Abraldes, J.G.; Liguori, E.; Kok, B.; Mura, V. La Intrahepatic Vascular Changes in Non-Alcoholic Fatty Liver Disease: Potential Role of Insulin-Resistance and Endothelial Dysfunction. World J Gastroenterol 2017, 23, 6777–6787. [Google Scholar] [CrossRef] [PubMed]
- Lupu, F.; Kinasewitz, G.; Dormer, K. The Role of Endothelial Shear Stress on Haemodynamics, Inflammation, Coagulation and Glycocalyx during Sepsis. J Cell Mol Med 2020, 24, 12258–12271. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Vink, H.; Hiramatsu, O.; Kajita, T.; Shigeto, F.; Spaan, J.A.E.; Kajiya, F. Role of Hyaluronic Acid Glycosaminoglycans in Shear-Induced Endothelium-Derived Nitric Oxide Release. Am J Physiol Heart Circ Physiol 2003, 285, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Evora, P.R.B.; Pearson, P.J.; Chua, Y.L.; Discigil, B.; Schaff, H.V. Exogenous Hyaluronidase Induces Release of Nitric Oxide from the Coronary Endothelium. Journal of Thoracic and Cardiovascular Surgery 2000, 120, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Luk, J.M.; Chu, A.C.; Ikeda, K.; Man, K.; Kaneda, K.; Fan, S.T. TNP-470 Blockage of VEGF Synthesis Is Dependent on MAPK/COX-2 Signaling Pathway in PDGF-BB-Activated Hepatic Stellate Cells. Biochem Biophys Res Commun 2006, 341, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Kim, Y.; Kim, H.; Kim, K.; Lee, Y.S.; Choe, J.; Hahn, J.H.; Lee, H.; Jeon, J.; Choi, C.; et al. Hyaluronic Acid Promotes Angiogenesis by Inducing RHAMM-TGFβ Receptor Interaction via CD44-PKCδ. Mol Cells 2012, 33, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Spee, B.; Carpino, G.; Schotanus, B.A.; Katoonizadeh, A.; Vander Borght, S.; Gaudio, E.; Roskams, T. Characterisation of the Liver Progenitor Cell Niche in Liver Diseases: Potential Involvement of Wnt and Notch Signalling. Gut 2010, 59, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P. Hyaluronan-CD44 Interactions in Cancer: Paradoxes and Possibilities. Clinical Cancer Research 2009, 15, 7462–7468. [Google Scholar] [CrossRef] [PubMed]
- Zöller, M. CD44: Can a Cancer-Initiating Cell Profit from an Abundantly Expressed Molecule? Nat Rev Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sheng, Y.; Shi, X.; Liu, Y.; He, Y.; Du, Y.; Zhang, G.; Gao, F. CD44/HA Signaling Mediates Acquired Resistance to a PI3Kα Inhibitor. Cell Death Dis 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Gagneja, S.; Capalash, N.; Sharma, P. Hyaluronic Acid as a Tumor Progression Agent and a Potential Chemotherapeutic Biomolecule against Cancer: A Review on Its Dual Role. Int J Biol Macromol 2024, 275. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liao, W.; Li, Y.; Wang, Y.; Chen, Q.; Jin, J.; He, S. Upregulation of Hyaluronan-Mediated Motility Receptor in Hepatocellular Carcinoma Predicts Poor Survival. Oncol Lett 2015, 10, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Tarullo, S.E.; He, Y.; Daughters, C.; Knutson, T.P.; Henzler, C.M.; Price, M.A.; Shanley, R.; Witschen, P.; Tolg, C.; Kaspar, R.E.; et al. Receptor for Hyaluronan-Mediated Motility (RHAMM) Defines an Invasive Niche Associated with Tumor Progression and Predicts Poor Outcomes in Breast Cancer Patients. Journal of Pathology 2023, 260, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, G.; Gu, J.; Chen, Z.; Wu, J. Role of Tumor-Associated Macrophages in Hepatocellular Carcinoma: Impact, Mechanism, and Therapy. Front Immunol 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Sezginer, O.; Unver, N. Dissection of Pro-Tumoral Macrophage Subtypes and Immunosuppressive Cells Participating in M2 Polarization. Inflammation Research 2024, 73, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Miyoshi, S.; Mikami, T.; Koyama, H.; Kitazawa, M.; Takeoka, M.; Sano, K.; Amano, J.; Isogai, Z.; Niida, S.; et al. Hyaluronan Deficiency in Tumor Stroma Impairs Macrophage Trafficking and Tumor Neovascularization. Cancer Res 2010, 70, 7073–7083. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wang, K.; Li, J.; Hu, H.; Yang, H.; Cai, M.; Liu, R.; Li, H.; Wang, N.; Shi, Y.; et al. Suppression of the Hyaluronic Acid Pathway Induces M1 Macrophages Polarization via STAT1 in Glioblastoma. Cell Death Discov 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Kim, J.; Wang, Z.; Kim, J.; Kim, S.Y.; Cho, G.J.; Lee, J.H.; Kim, S.M.; Tsuchiya, T.; Matsuda, M.; et al. Metastatic Tumor Growth in Steatotic Liver Is Promoted by HAS2-Mediated Fibrotic Tumor Microenvironment. J Clin Invest 2025, 135. [Google Scholar] [CrossRef] [PubMed]
- Moran-Salvador, E.; Garcia-Macia, M.; Sivaharan, A.; Sabater, L.; Zaki, M.Y.W.; Oakley, F.; Knox, A.; Page, A.; Luli, S.; Mann, J.; et al. Fibrogenic Activity of MECP2 Is Regulated by Phosphorylation in Hepatic Stellate Cells. Gastroenterology 2019, 157, 1398–1412.e9. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Kuipers, H.F.; Marshall, P.L.; Wang, E.; Kaber, G.; Bollyky, P.L. Hyaluronan in Immune Dysregulation and Autoimmune Diseases. Matrix Biology 2019, 78–79, 292–313. [Google Scholar] [CrossRef] [PubMed]
- Halimani, N.; Nesterchuk, M.; Tsitrina, A.A.; Sabirov, M.; Andreichenko, I.N.; Dashenkova, N.O.; Petrova, E.; Kulikov, A.M.; Zatsepin, T.S.; Romanov, R.A.; et al. Knockdown of Hyaluronan Synthase 2 Suppresses Liver Fibrosis in Mice via Induction of Transcriptomic Changes Similar to 4MU Treatment. Sci Rep 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.M.; Onorato, A.; Cantero, M.J.; Domínguez, L.; Bayo, J.; Fiore, E.; García, M.; Atorrasagasti, C.; Canbay, A.; Malvicini, M.; et al. 4-Methylumbelliferone-Mediated Polarization of M1 Macrophages Correlate with Decreased Hepatocellular Carcinoma Aggressiveness in Mice. Sci Rep 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Weiz, G.; Molejon, M.I.; Malvicini, M.; Sukowati, C.H.C.; Tiribelli, C.; Mazzolini, G.; Breccia, J.D. Glycosylated 4-Methylumbelliferone as a Targeted Therapy for Hepatocellular Carcinoma. Liver International 2022, 42, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Tempero, M.A.; Sigal, D.; Oh, D.Y.; Fazio, N.; MacArulla, T.; Hitre, E.; Hammel, P.; Hendifar, A.E.; Bates, S.E.; et al. Randomized Phase III Trial of Pegvorhyaluronidase Alfa with Nab-Paclitaxel plus Gemcitabine for Patients with Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. Journal of Clinical Oncology 2020, 38, 3185–3194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dui, G.S.W.; Ma, S.L.B.; Yang, C.; Xu, W.; Xu, J. HMMR Inhibition by 4-Methylumbelliferone Is Effective in Preclinical Hepatocellular Carcinoma Models. Histol Histopathol 2025, 18937. [Google Scholar] [CrossRef]
- Saikia, P.; Bellos, D.; McMullen, M.R.; Pollard, K.A.; de la Motte, C.; Nagy, L.E. MicroRNA 181b-3p and Its Target Importin A5 Regulate Toll-like Receptor 4 Signaling in Kupffer Cells and Liver Injury in Mice in Response to Ethanol. Hepatology 2017, 66, 602–615. [Google Scholar] [CrossRef] [PubMed]
- You, N.; Chu, S.; Cai, B.; Gao, Y.; Hui, M.; Zhu, J.; Wang, M. Bioactive Hyaluronic Acid Fragments Inhibit Lipopolysaccharide- Induced Inflammatory Responses via the Toll-like Receptor 4 Signaling Pathway. Front Med 2021, 15, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbα Is a Mediator and Potential Interventional Target for NASH and Subsequent Liver Cancer. Nat Med 2019, 25, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Author Correction: Platelet GPIbα Is a Mediator and Potential Interventional Target for NASH and Subsequent Liver Cancer (Nature Medicine, (2019), 25, 4, (641-655), 10.1038/S41591-019-0379-5). Nat Med 2022, 28, 600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, X.; Jia, A.; Wang, C.; Jiang, H. Hyaluronic Acid-Based Prodrug Nanomedicines for Enhanced Tumor Targeting and Therapy: A Review. Int J Biol Macromol 2023, 249. [Google Scholar] [CrossRef] [PubMed]
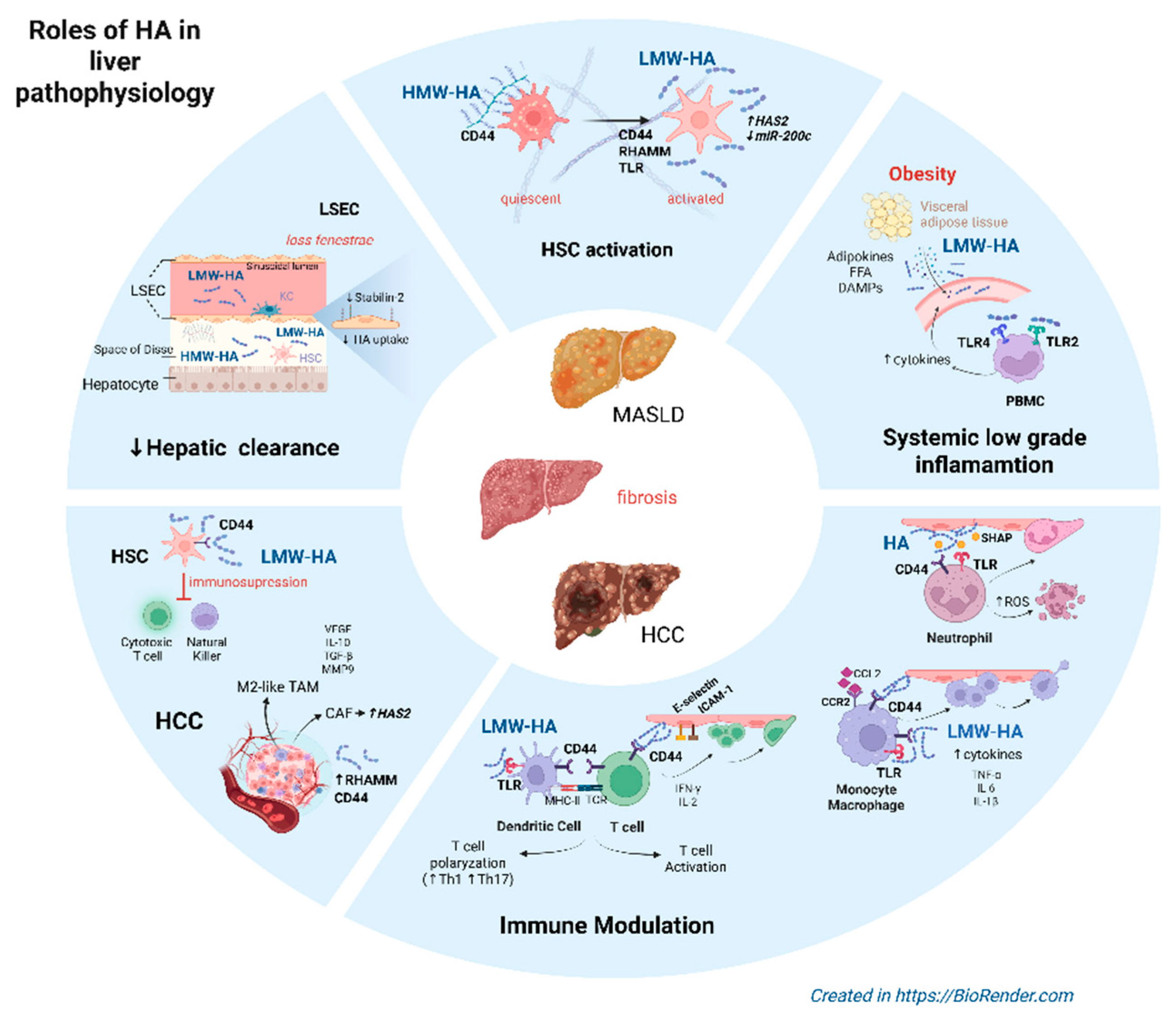
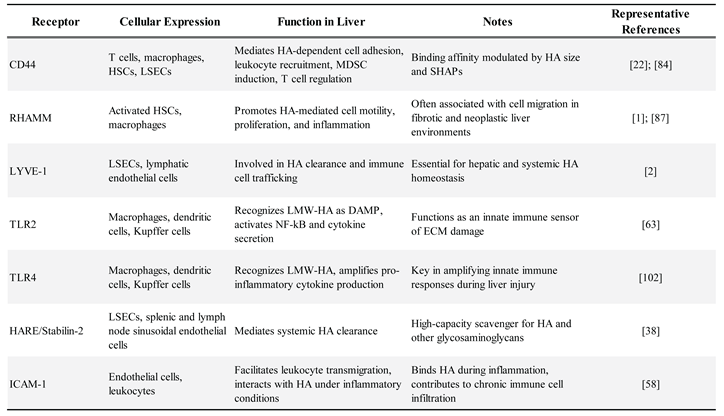 |
| HA Molecules | Function | References |
|---|---|---|
| LMW-HA | LMW-HA→TLR2,TLR4→MyD88→NF-κB→ ↑inflammatory cytokines (TNF-α, IL-6), immune cell infiltration → ↑ hepatic inflammation | [14]; [17] |
| LMW-HA | LMW-HA→TLR4→TRIF→type I interferon→ ↑ hepatic inflammation | [14] |
| HMW-HA | HMW-HA→CD44,RHAMM→Immune quiescence | [15]; [16] |
| LMW-HA | LMW-HA→CD44, RHAMM→inflammation and remodeling→↑iNOS, T cell activation and proliferation | [18] |
| HA | HA→activation and migration of HSC HA→CD44→Kupffer cell adhesion, polarization, immune responses of liver-resident and infiltrating macrophagues HA→CD44→trafficking, activation of T lymphocytes, DCs→immune surveillance |
[22]; [23] |
| HA | During endotoxin-derived liver inflammation: ↑HA in LSECs↔CD44 in neutrophils→neutrophil adhesion ↑TLR4→SHAP→HA↔CD44→↑neutrophil adhesion |
[46]; [47] |
| LMW-HA/HMW-HA | LMW-HA/HMW-HA→p38 MAPK→↑ROS in neutrophils→↑apoptosis→↑tissue damage HMW-HA+TNFα→↑↑ROS in neutrophils→↑↑apoptosis→↑↑tissue damage |
[49]; [50] |
| HA | In in vivo experimental models: ↓CD44→↓macrophague response to LPS, saturated fatty acids and DAMPs, ↑anti-inflammatory M2 phenotype→↓liver inflammation, ↓fibrosis | [23] |
| HA | HA→CD44↔CCL2-CCR2 axis→integrin activation, monocyte adhesion to LSECs in inflamed hepatic tissue | [51] |
| HA | HA→CD44 on activated HSC→ induction of MDSCs→immunosupression | [52]; [53] |
| LMW-HA | LMW-HA→TLR2, TLR4 on macrophagues→↑cytokine release (TNF-α, IL-6, IL-1β) →↑inflammation | [22]; [42] |
| HMW-HA | HMW-HA→↓TLR activation→ M2-like macrophague phenotype | [54] |
| HMW-HA/LMW-HA | HMW-HA→↓diferentiation of human fibrocytes, pro-fibrotic and inflammatory monocyte-derived cells LMW-HA→maturation of fibrocytes, pro-fibrotic and inflammatory monocyte-derived cells |
[55] |
| HA | HA expressed on vascular endothelium→CD44 in activated T cells→ adhesion and transendothelial migration | [56] |
| HA | HA→CD44→infiltration and retention of effector T cells in inflamed tissues HA→CD44↔E-selectin, ICAM-1→↑infiltration and retention of effector T cells in inflamed tissues |
[57]; [58]; [59] |
| HA | HA→CD44→CD44 co-localizes with lipid rafts and TCR-CD3 complex in T cells→stabilization of interactions between DCs and T cells Deficient CD44 DCs→↓T cell proliferation, cytokine production (IL-2, IFN-γ) CD44↔ezrin-radixin-moesin→synapse formation, T cell polarization |
[60]; [61] |
| LMW-HA | LMW-HA→TLR4 on DCs→MHC II, CD80, CD86→Th1, Th17 polarization | [62]; [63] |
| HMW-HA | In in vitro studies: HMW-HA→↓T cell proliferation, cytokine production | [3]; [64] |
| HMW-HA | In a T-cell mediated liver injury model: HMW-HA→ ↓pro-inflammatory cytokines (TNF-α, IFN-γ) | [65] |
| LMW-HA | LMW-HA→TLR2, TLR4 on monocytes, macrophagues and peripheral blood mononuclear cells→ hepatic inflammation | [22]; [17] |
| HA | In activated HSC→↑HAS2→HA→CD44→NOTCH1↔ ↓miR-200c→liver fibrosis | [30]; [36] |
| HA | Accumulation of HA in the space of Disse→ ↑leukocyte adhesion, transmigration | [76]; [46] |
| HA | In human and murine models of HCC: HA→CD44 on activated HSCs→ induction and expansion of MDSCs→ ↓CTL, ↓NK cell responses | [52]; [53] |
| LMW-HA | In HCC: LMW-HA→M2-like TAMs→↑VEGF, ↑IL-10, ↑MMP9, ↑TGF-β→ angiogenesis, matrix degradation, immunosupression | [22]; [54] |
| HA | Blockade of HA→↓CD44→ M1-like TAMs | [92] |
| HA | In in vivo studies: ↓HAS2 in activated HSCs→↓HA stromal content→↓immune infiltration, ↓fibrosis, ↓tumor burden | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





