1. Introduction
Graphene is a two-dimensional material consisting of a single layer of carbon atoms arranged in a hexagonal lattice pattern [
1], as shown in
Figure 1. Each carbon atom in graphene binds to its three neighbors through sp² hybridization, forming a sigma bond (
σ) in the field, and the pi bond (
π) delocalized above and below the field [
2]. Since it was first successfully isolated by Andre Geim and Konstantin Novoselov in 2004, graphene has garnered widespread attention among scientists due to its remarkable physical properties and potential for diverse applications in various technological fields. This discovery even received international recognition through the award of the Nobel Prize in Physics in 2010 [
3].
The advantages of graphene lie in its unique combination of properties, including extremely high electrical and thermal conductivity, outstanding mechanical strength, optical transparency, flexibility, and a large specific surface area [
5]. These properties are derived from two-dimensional atomic structures and sp² bonding systems, which are highly stable and enable the free and efficient movement of electrons in the field. These characteristics make them very promising for application in electronic devices, energy storage, sensors, composite materials, as well as biomedical and environmental technologies [
6].
The high prospects of graphene in diverse applications demand efficient and stable synthetic ways to produce quality graphene materials. For its application, graphene has been required to fulfill necessary properties including well-defined layer number, uniform lateral dimension, low defects, and high purity. These characteristics strongly influence graphene's performance in its final application since its electrical, mechanical, and chemical properties are very much dependent on its structure and composition [
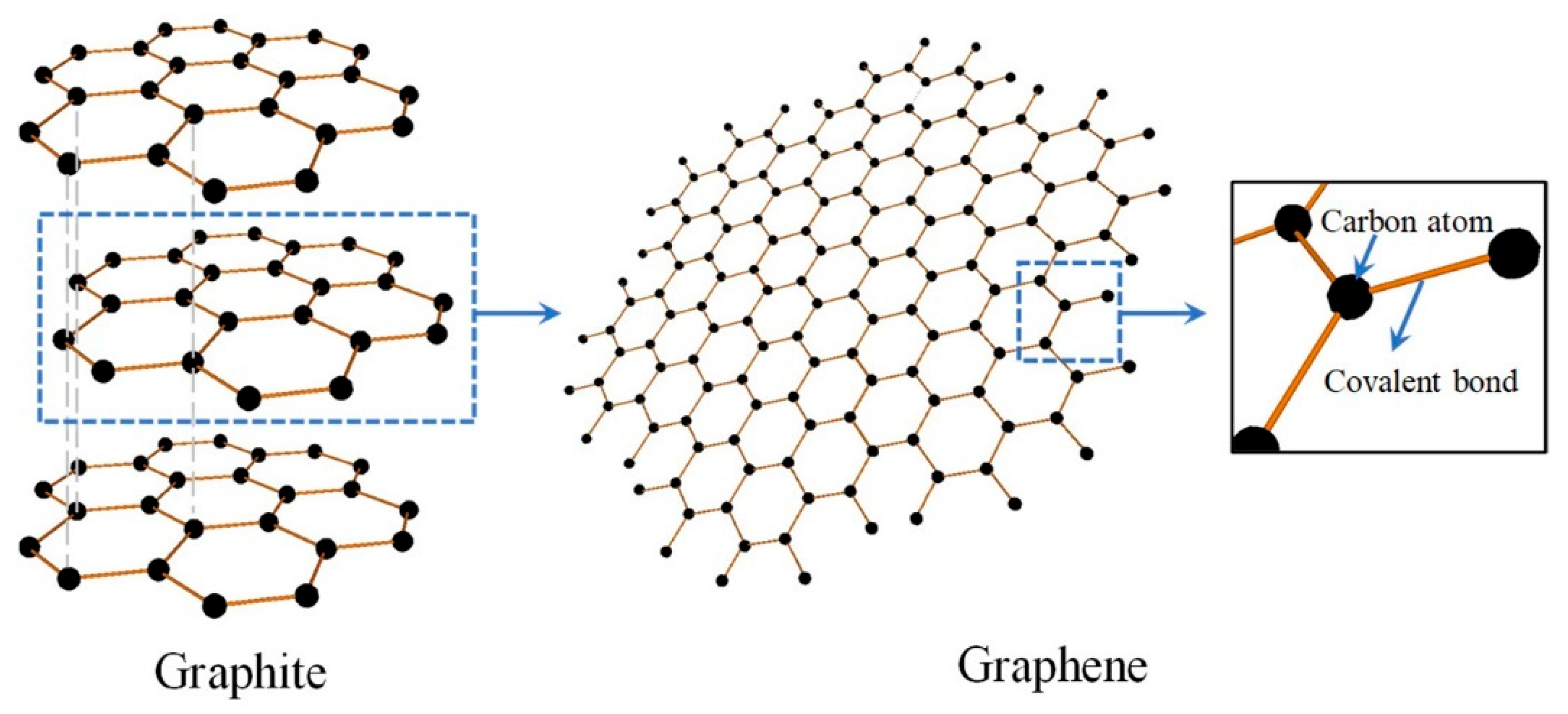
7]. The choice of raw materials is an important consideration to meet the application requirements of graphene specifications. Graphite is the most popular C precursor due to the fact that it can easily be exfoliated into graphene sheets due to its layered structure [
8]. The structural transition from graphite to graphene is depicted in
Figure 2. Meanwhile, graphite is known as an abundant material, has a relatively cheap price, and good chemical stability, and thus it can be an excellent precursor source for other graphene synthesis methods [
9].
In addition to the selection of raw materials, the diversity of graphene synthesis approaches that have been developed adds complexity in determining the most effective production strategy. Each of these methods can be used to give a different reaction product, based on reaction conditions, processing stage and added advanced material. Thus, a comprehensive understanding of these methods is necessary for selecting the one that meets most of the application requirements and research questions [
11]. However, despite the availability of various methods, the process of graphene synthesis, in general, still faces major challenges that cannot be considered simple. Regardless of the type of raw material and method used, this process requires highly controlled conditions for a layer of graphene to form without damaging its atomic structure [
12]. Errors in process control can result in greater structural defects, changes of particle size, or contamination, which reduces the quality and performance of the resultant graphene [
13].
As the synthesis of graphene is increasingly researched and developed, it is necessary to prepare a review that would capture all the approaches that have been undertaken as well as highlight the problems encountered and possible frameworks for further development. The purpose of this article is to provide a comprehensive review of different graphite-based graphene synthesis methods, looking into the main characteristics, concentrating on factors determining the quality of the end-product, prospective applications or innovations post-processing, and how these could further advance modern science. In this way, this review will enhance scientific understanding and development of graphene in various technological domains.
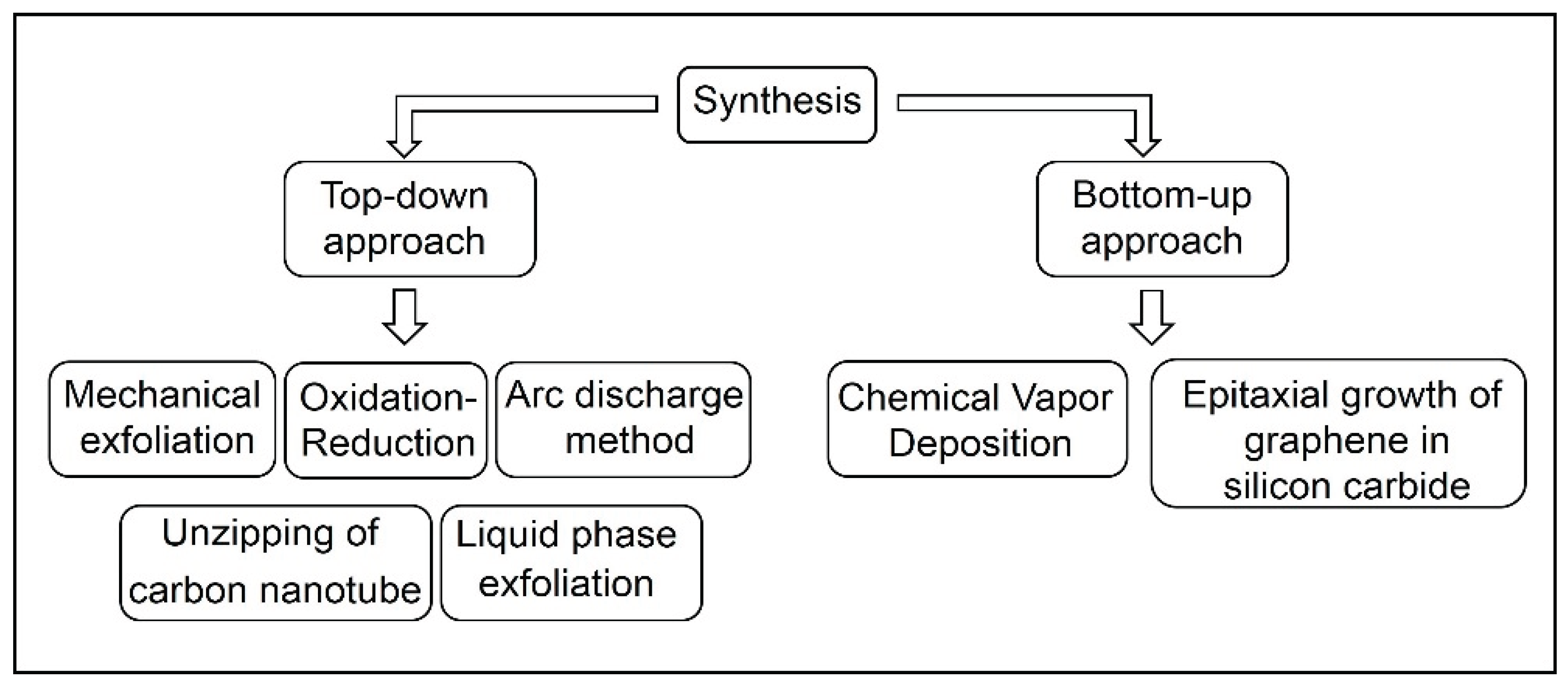
2. Graphene Synthesis
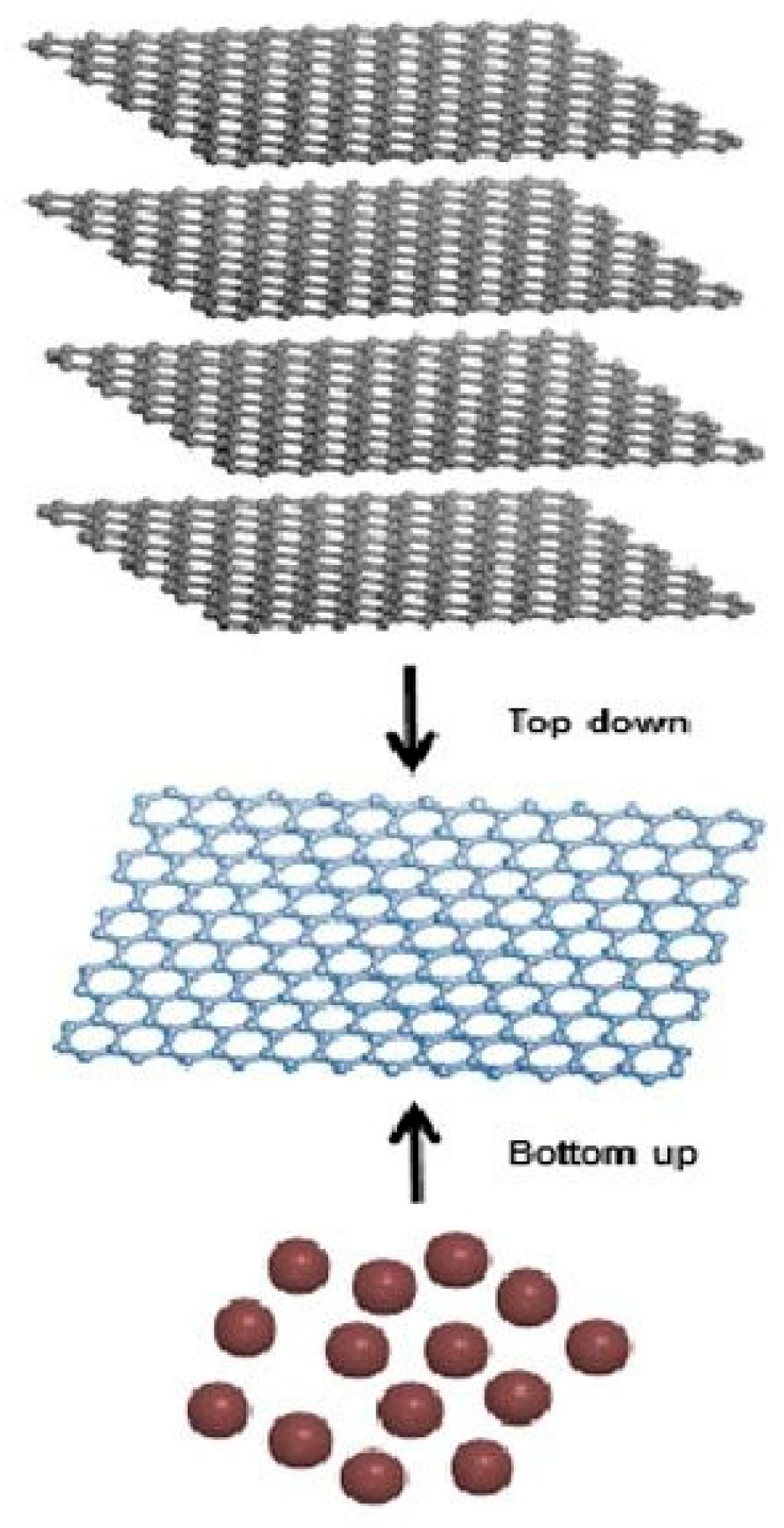
The synthesis of graphene is a fundamental stage in the widespread use of this material. Various methods have been developed to obtain graphene from carbon sources, especially graphite, which has a layered structure resembling graphene itself. In the scientific context, these synthesis techniques can generally be classified into two main approaches, namely top-down and bottom-up methods. These two contrasting approaches are illustrated in
Figure 3. This classification not only reflects the direction of the synthesis approach but also determines the characteristics of the resulting graphene products [
14].
The top-down approach involves separating the graphene layer from the bulk material, such as graphite, through an exfoliation process or chemical reaction, resulting in thin sheets of graphene [
16]. Meanwhile, the bottom-up approach forms graphene from simple carbon units through an atomic assembly process [
8]. Top-down methods are generally better suited for large-scale production at lower costs, although control over the number of layers and structural defects is often a challenge [
17]. In contrast, the bottom-up method offers better control over the structure of graphene but with greater complexity and process costs [
18]. The following is the schematic of the graphene synthesis method:
Figure 4.
Top Down and Bottom Up Graphene Synthesis Flowchart [
19].
Figure 4.
Top Down and Bottom Up Graphene Synthesis Flowchart [
19].
Numerous top-down and bottom-up methods for graphene synthesis have been developed. Depending on the advanced treatment and reaction conditions, each method has a different set of working principles, stages, and outcomes. A thorough analysis of these methods is provided in the section that follows, beginning with a top-down strategy and moving on to a bottom-up strategy.
2.1. Top Down
One of the main methods for creating graphene is the top-down approach, which uses physical and chemical procedures to separate the graphene layer from the bulk material, like graphite. Large amounts of graphene are known to be produced using this method, but maintaining product quality is frequently difficult. The following are the top-down graphene synthesis techniques:
2.1.1. Mechanical Exfoliation
Mechanical exfoliation is a top-down method of producing graphene, where layers of graphite are separated using mechanical forces, such as normal or shear forces, to overcome the van der Waals tensile forces between layers. This method avoids major chemical reactions and is comparatively easy and economical by using mechanical energy to exfoliate graphite into single-layer or multilayer graphene [
20]. There are several physical methods for performing mechanical exfoliation, and each has unique properties. These are a few methods that are frequently employed in this approach.
Micromechanical Cleavage
Micromechanical cleavage is an exfoliation technique that utilizes the adhesion force of an adhesive (e.g., tape) to physically exfoliate the graphene layer [
21]. Geim and Novoselov first introduced this technique, and it was later refined using polymers such as PMMA and PDMS [
22]. The use of PMMA is more effective in producing monolayer graphene, while PDMS tends to produce multilayers. This suggests that PMMA is better suited for producing high-quality graphene with a thickness of one [
21].
In addition to the exfoliation method using adhesives, a new technique has been developed based on a single diamond tip that is very sharp. This method utilizes a single, very sharp diamond tip for precise exfoliation down to the atomic scale. This approach is capable of producing high-quality monolayer graphene with a thickness of tens of nanometers and a lateral size of about 900 × 300 μm. The use of ultrasonic vibrations (33.1 kHz, 2.1 V) was shown to be able to lower the I
D/I
G ratio from ~0.90 to ~0.73 and the crystallite size from ~24 nm to ~17 nm, indicating an improvement in the quality and stability of the graphene layer. TEM analysis showed a more organized structure as well as the emergence of nanohorns, which have the potential for gas storage applications [
23].
Sonication
Sonication is a graphene synthesis method that utilizes high-frequency ultrasonic waves to generate mechanical energy through the phenomenon of cavitation. Ultrasonic waves create microscopic bubbles that collapse rapidly, resulting in intense shear forces that can overcome the Van der Waals forces between graphite layers. As a result, graphite flakes efficiently into thin sheets of graphene without the need for aggressive chemical reagents. [
24].
The effectiveness of sonication techniques in graphene synthesis is highly dependent on the duration, sonication power, solvent type, and initial treatment of graphite materials. Sonication not only facilitates mechanical exfoliation but can also cause structural defects in graphene sheets, depending on process conditions. Therefore, characterization analysis such as FTIR, SEM, Raman, and XRD is important to evaluate sonication results, ranging from the presence of functional groups and morphology to the degree of defects and crystallinity of graphene [
25]. The following is a summary of the characterization data, as presented in
Table 1.
Sonication effectively exfoliates graphite into graphene, as evidenced by an increase in the ID/IG ratio (Raman), a flaky sheet morphology (SEM), and an angular shift of 2θ (XRD), indicating an increase in interlayer spacing. Changes in the intensity of functional groups in FTIR also indicate surface chemical modifications. Overall, sonication is a simple and effective method, although it requires optimization to produce high-quality graphene with minimal defects.
Ball Milling
The ball mill technique is a top-down method for graphene synthesis that involves grinding graphite using crusher balls in a rotating container. The impact that occurs during this process causes the graphite layer to peel off into graphene sheets. This method is known for its simplicity and low cost and is suitable for large-scale production of graphene [
31,
32].
There are two methods for the ball mill technique: wet methods and dry methods. The dry method is a simpler procedure because it doesn't require solvents. In contrast, the wet method uses solvents like ethanol or water to help create graphene suspensions, lower frictional heat, and speed up the graphite peeling process. However, it requires additional steps for the separation and drying of the final product [
33]. The following are the differences in the characteristics of graphene from dry and wet ball milling, as shown in
Table 2.
The wet and dry ball-mill methods both produce thinly coated graphene/GO with increased defects and decreased crystallinity. The wet method results in a more homogeneous structure and higher quality, suitable for electronics, sensors, and energy [
34]. The dry method produces graphene with higher oxygen functionalities, suitable for adsorption, catalysts, and composites, and simpler, cheaper, and more environmentally friendly for large-scale production [
42].
Fluid Dynamics
Fluid dynamics is a graphene synthesis technique that utilizes the movement of fluids and their interactions with solid surfaces. In graphene synthesis, this principle is used to exfoliate graphite into a thin layer of graphene without the use of harmful chemicals. This process relies on a high-speed flow of fluid that generates mechanical forces, such as friction, turbulence, cavitation, and differential pressure, to overcome the van der Waals forces between graphite layers. This technique is considered efficient, environmentally friendly, and has the potential to be developed on an industrial scale [
43].
The three primary fluid dynamics-based methods employed for graphene synthesis are the Vortex Fluidic Device (VFD), Pressure-driven Fluid Dynamics (PFD), and Mixer-driven Fluid Dynamics (MFD). VFD utilizes a fast-rotating tube to produce a thin layer of fluid and applies a gentle shear force to exfoliate graphite. PFD involves the flow of graphite suspension through a narrow channel under high pressure, triggering exfoliation through a combination of shear forces, turbulence, and cavitation. Meanwhile, MFDs utilize mixing tools such as rotor-stator mixers or blenders to produce high shear forces evenly, suitable for efficient large-scale graphene production [
44]. The following table summarizes the graphene characterization data generated by the three techniques, as shown in
Table 3.
Third, fluid dynamics techniques have great potential to produce quality graphene in a more environmentally friendly manner than chemical methods. VFDs are suitable for products with minimal defects, but production is limited. PFDs are efficient and capable of controlling size, but they require high pressure and a complex design. MFDs offer the most practical and economical solutions for large-scale production. With technological advancements, this approach presents a significant opportunity to support the commercially sustainable production of graphene.
Supercritical Fluids
A supercritical fluid is a substance that exists at temperatures and pressures above its critical point, where the boundary between the liquid and gaseous phases is lost. Under these conditions, the fluid has liquid-like properties with high density while also having high diffusivity, like gases. This combination of properties allows supercritical fluids to penetrate the interlayer gaps of graphite and facilitate exfoliation without the need for harsh chemicals. In addition, when the pressure is lowered, the rapid expansion of the fluid results in an effective separating force between graphite layers [
45].
In graphene synthesis, supercritical fluids can be divided into two main types: inert and reactive. Inert fluids such as CO₂ do not react chemically with graphene, making them suitable for producing pure graphene. In contrast, reactive fluids such as supercritical ethanol not only aid in exfoliation but can also modify the surface of graphene through chemical reactions. The selection of this type of fluid is adjusted to the needs of the desired graphene end application [
46]. The following table lists the differences in graphene characteristics generated through the two supercritical fluid approaches, as shown in
Table 4.
Exfoliation of graphene with pure SC-CO₂ tends to result in less homogeneous structures and a higher number of defects. In contrast, the use of SC-ethanol is more effective in producing graphene with a more stable thickness distribution, a more regular structure, and a lower defect rate. Thus, SC-ethanol is superior for the synthesis of high-quality graphene.
Detonation Technique
The detonation technique is a graphene synthesis method that utilizes the explosive reaction of carbon compounds, such as acetylene, in the presence of oxygen or oxidizing agents to produce graphene nanosheets [
51]. The high temperatures and pressures created during the detonation process break down carbon molecules and facilitate the formation of graphene structures [
52]. This method is very fast, does not require a catalyst, and is capable of producing high-quality graphene efficiently, making it suitable for large-scale production [
53]. The following table summarizes the graphene characterization data generated by this method, as shown in
Table 5.
The detonation technique is capable of producing high-quality graphene with a good crystalline structure, minimal defects (low ID/IG), and a varying number of layers. This process is fast, catalyst-free, and suitable for large-scale production, making it an efficient and promising method for graphene synthesis.
2.2. Oxidation-Reduction
The oxidation-reduction method is a graphene synthesis technique that involves oxidizing graphite to produce graphene oxide (GO), followed by exfoliation and chemical reduction to form reduced graphene oxide (rGO) [
55]. This process begins with the oxidation of graphite using strong oxidizers, such as sulfuric acid, nitric acid, or potassium permanganate, which introduces oxygen functional groups, including hydroxyl, epoxy, and carboxyl, into the graphite layer [
56]. This function group increases the distance between layers, allowing exfoliation into GO sheets through sonication in solvents such as water [
57]. The resulting GO is hydrophilic and easily dispersed; however, it lacks the conductive properties of graphene due to the disruption of the sp² structure caused by oxidation [
58].
The reduction stage is performed to remove the oxygen group and restore the sp² carbon structure, using reducing agents such as hydrazine, sodium borohydride, or thermal methods to produce reduced graphene oxide (rGO) [
59]. Although this method enables the production of large quantities of graphene at a low cost, rGO often exhibits structural defects and oxygen group residues that degrade its electronic properties compared to pure graphene [
60]. The quality of graphene can be improved by optimizing the reduction conditions, such as using environmentally friendly reducing agents or electrochemical reduction methods [
61]. The oxidation-reduction method is very popular due to its scalability and ease of process; however, the main challenge is minimizing defects for applications that require high-quality graphene [
62].
The different processes for oxidizing and reducing graphene will be covered in this section, along with how they affect the final product's quality and characteristics. The primary focus is on how these techniques affect graphene's conductivity, defects, and structure for the best possible applications.
2.2.1. Oxidation Method
The properties and applications of graphene can be greatly impacted by the classification of oxidation methods used in graphene synthesis, such as chemical, thermal, and electrochemical oxidation. It is essential to comprehend this method in order to optimize graphene for a variety of applications.
2.2.2. Chemical Oxidation
Chemical Oxidation is the initial stage in the synthesis of rGO, where graphite is converted to graphene oxide (GO) using a strong oxidizing agent in an acidic solution. The most common method is the Hummers method or its modification, which uses a combination of potassium permanganate (KMnO₄) and sulfuric acid (H₂SO₄), often with the addition of sodium nitrate (NaNO₃) [
63]. In some variations of these methods, oxidizing agents such as nitric acid (HNO₃), peroxide (H₂O₂), or a mixture of phosphoric acid (H₃PO₄) and potassium permanganate (KMnO₄) are also used [
64]. This reaction introduces various oxygen groups such as hydroxyl, epoxy, and carboxyl into the graphite structure, making GO water-soluble and ready for the reduction process to rGO [
65].
Thermal Oxidation
In order to introduce oxygen groups at high temperatures, thermal oxidation entails heating graphite with oxygen or other oxidizing gases. To increase the effectiveness of functional group recognition, this technique is frequently used in conjunction with chemical oxidation [
66]. Compared to chemical oxidation, thermal oxidation produces graphene with fewer defects because it allows for controlled oxidation at high temperatures. In addition, this method is often used in conjunction with chemical reduction to produce rGO that has better conductivity [
67].
Electrochemical Oxidation
This method utilizes an electric current to oxidize graphite in an electrolyte solution, which can be a neutral salt solution, such as sodium sulfate (Na₂SO₄), or an environmentally friendly electrolyte solution that is less harsh than a strong chemical agent [
68]. This process avoids the use of harmful chemical oxidizers such as KMnO₄ and strong acids, making it safer and more environmentally friendly [
69].
Reduction Method
The following is the reduction of graphene oxide (GO): The structure and electrochemistry of rGO are impacted by techniques like chemical, thermal, and electrochemical treatments, which in turn affect the properties and uses of materials.
Chemical Reduction
The cost-effectiveness and scalability of chemical reduction make it a popular choice. In order to eliminate the oxygen group from graphene oxide (GO) and partially restore the graphitic structure, reducing agents such as sodium borohydride, hydrazine, metals, and L-ascorbic acid are used in this process [
70]. Reduction capacity, toxicity, and environmental impact can all be impacted by changing the choice of reducing agents [
60].
Thermal Reduction
Thermal Reduction is achieved by heating graphene oxide (GO) in an inert atmosphere, such as argon or nitrogen, to remove oxygen groups as gases (e.g., CO and CO₂) [
71]. This method does not require any additional chemicals and can produce rGO with high conductivity. However, the process requires good temperature control to prevent damage to the carbon structure [
72].
Electrochemical Reduction
Electrochemical Reduction involves the use of an electric current to reduce graphene oxide (GO) inside an electrochemical cell [
73]. GO is used as an electrode, and when it is given a negative voltage, the oxygen groups on its surface are removed through electron transfer. This method is fast, clean, and allows for good control over the reduction results, making it widely considered for sustainable applications [
74]. The following table summarises the graphene characterization data obtained via the oxidation-reduction method, as shown in
Table 6.
The synthesis of rGO begins with the oxidation of graphite using thermal, chemical (Hummers), or electrochemical methods to introduce oxygen groups that facilitate exfoliation. Furthermore, reductions are carried out chemically (using NaBH₄, hydrazine, or ascorbic acid), thermally, or electrochemically to remove these oxygen groups, improve the sp² structure, and decrease the defects, which is reflected in the decrease in the Raman ID/IG ratio and changes in the XRD pattern.
2.2.3. Arc Discharge Method
The arc discharge method is a graphene synthesis technique that uses the discharge of electricity between graphite electrodes in a gaseous environment to form a carbon plasma, which condenses into graphene, usually few-layer graphene (FLG) [
82]. High-quality graphene with a good crystalline structure is produced by this process, which evaporates graphite at high temperatures. To guarantee homogeneity and purity, however, exact control of variables like gas pressure and current is necessary [
83]. Large-scale production is possible with this straightforward process, but minimizing contamination and flaws is a challenge [
84]. According to the research that has been done, the following factors influence the quality of graphene using the arc discharge method:
Effect of Buffer Gas Type
Table 7 summarizes how the type of buffer gas greatly affects the properties and quality of graphene produced by arc discharge.
By using various gases, the arc release method creates graphene with unique properties: argon for high-quality multilayers, hydrogen for pure graphene with little contamination, nitrogen-hydrogen mixtures for scalability and quality balance, and helium for monolayers. The particular requirements of the application are taken into consideration when choosing the gases.
Effect of Current Type
The structure, purity, and general quality of the synthesized graphene are greatly influenced by the type of electric current used in the arc discharge, whether it be direct current (DC) or alternating current (AC). The relative impact of the two existing types on different facets of graphene production is compiled in
Table 8.
Although the DC and AC arc release methods have their advantages, their selection depends on specific needs in graphene synthesis, such as the desired quality, structure, and production scale. The DC method is generally preferred to produce high-quality graphene with double or more layers and minimal defects, while the AC method is superior in large-scale production as it offers more flexible control over the structural characteristics of graphene.
Effects of Pressure
Because it directly affects the final graphene's morphology, purity, and structural quality, pressure is a crucial parameter in arc discharge techniques. The effects of various pressure ranges on graphene properties are compiled in
Table 9.
The trade-off between pressure regulation and the desired graphene properties must be taken into account, even though the arc release method works well for graphene synthesis. While medium and high pressures are more beneficial for creating graphene with fewer layers and higher purity, low pressures are less suitable for creating high-purity graphene. The pressure choice needs to be customized to meet the demands of the final graphene's particular application.
Effect of Reaction Temperature
Reaction temperature plays a crucial role in determining the number of layers, growth rate, and purity of graphene synthesized via arc discharge. The kinetic energy available at different temperatures influences the atomic mobility, plasma behavior, and overall structural integrity of the resulting material.
Table 10 summarizes the effects of various temperature ranges on graphene quality.
The arc release method is effective in producing graphene, but the temperature must be carefully controlled to optimize the number of layers and purity. High temperatures accelerate growth but can also contribute to defects, whereas low temperatures yield single-layer graphene with fewer defects. Medium temperatures provide balance, resulting in multi-layer graphene with high purity, which is crucial for tailoring it to specific applications.
Effect of Reaction Time
The number of layers, growth rate, and purity of graphene produced by arc discharge are all significantly influenced by the reaction temperature. Atomic mobility, plasma behavior, and the final material's overall structural integrity are all influenced by the kinetic energy accessible at various temperatures.
Table 11 summarizes the relationship between reaction time and graphene quality.
The arc removal method has the ability to control the number of layers and the purity of graphene, which is very important for adapting its properties to a specific application. Rapid synthesis is particularly suitable for applications that require high purity and fewer layers, whereas longer synthesis can produce graphene with more layers, making it suitable for applications that require thicker structures. However, the balance between the number of layers and purity must be carefully managed to optimize material performance according to the application's specific needs.
Effect of Chamber Type
The configuration of the reaction chamber influences the quality, purity, and layer number of graphene.
Table 12 summarizes the effects of different chamber types on graphene produced via arc discharge.
Although enclosed spaces typically produce graphene with high purity and fewer layers, this type may be less suitable for applications that demand large yields. In contrast, open and semi-open spaces, while at risk of producing more defects, are more suitable for mass production. Therefore, the selection of the type of space should be tailored to the needs of the application, taking into account the balance between the number of layers, purity, and yield.
2.2.4. Unzipping of Carbon Nanotube
The unzipping of Carbon Nanotubes (CNT) is a
top-down method for the synthesis of graphene, specifically graphene nanoribbons (GNR), by splitting the cylindrical structure of the CNT into flat graphene tapes. This process utilizes a CNT wall composed of rolled graphene sheets, resulting in GNR with width and length that depends on the dimensions of the CNT [
97,
98]. While effective for producing graphene with controlled edges, this method can cause structural damage [
99]. Various methods as follows, can unzip carbon nanotubes (CNT) into graphene nanoribbon (GNR):
Oxidative Zipper Retraction
This method utilizes strong oxidizers to break the carbon bonds in carbon nanotubes (CNTs), resulting in graphene nanoribbons (GNRs) [
100]. This process is general and flexible, allowing the regulation of GNR properties through oxidation rates [
101]. Nonetheless, harsh chemical conditions often damage the structure of CNT crystals and degrade their conductivity [
102].
Catalytic Zipper Opening
This technique uses microwaves and metal nanoparticles, like palladium, to efficiently accelerate the unzipping of carbon nanotubes. Although layered GNR can be produced using this process, the primary obstacles are the possibility of metal contamination and the requirement for stringent reaction control [
103].
Electrochemical Zipper Opening
The CNT zipper can be opened with a high degree of precision and little damage thanks to this method, which uses an electric field in the electrolyte medium [
104]. Although it necessitates specialized equipment and rigorous electrochemical condition control, this method works well for nitrogen-doped CNTs [
101].
Sonochemical Zipper Opening
This process utilizes ultrasonic waves to open the CNT mechanically. This method is relatively inexpensive and simple, and can produce GNR with smooth edges. However, the quality of the final product may vary due to limited control over the process [
105].
A comparison of the characteristics of graphene nanoribbon (GNR) generated through various carbon nanotube (CNT) unzipping methods is presented in the following table to highlight the advantages and limitations of each approach, as shown in
Table 13.
Each CNT unzipping method produces GNR with its advantages and limitations. The oxidative method is easy to do but poses many defects. Catalytic and electrochemical methods produce purer GNR with a more intact structure, although the process is more complex. Sonochemistry is relatively simple, yielding smooth-edged results, but it is not easy to control. The selection of methods depends on the target application and practical considerations such as purity, efficiency, and environmental impact.
2.2.5. Liquid Phase Exfoliation
Liquid-phase exfoliation (LPE) is a top-down method of graphene synthesis that utilizes solvents or chemicals to separate the graphene layers from the graphite. Unlike mechanical exfoliation, which is physical in nature, this method utilizes chemical interactions or reactions to weaken the forces between layers [
110]. Some of the techniques included in chemical exfoliation include:
Graphite Intercalation Compounds (GIC)
GIC is a compound that results from the insertion of chemicals (such as metals or acid molecules) into a graphite layer to widen the distance between layers to facilitate the process of exfoliating graphene through heating or ultrasonication [
111]. Intercalation with alkali metals, such as potassium, can increase the distance between graphite layers from 0.34 nm to approximately 0.53 nm. This increase weakens the inter-layer van der Waals force, making it easier to exfoliate into graphene [
112].
Intercalation with acid molecules, such as HClO₄, can produce graphene with high exfoliation efficiency. This intercalation widens the distance between graphite layers, as evidenced by the XRD peak at 2θ = 23.1°. After sonication, the separate layers reform into graphene. The Raman spectrum showed an increase in the peaks of D and G, signalling an increase in disorder and successful exfoliation [
110].
Chemical Exfoliation with Organic Solvents
Chemical exfoliation with organic solvents is a method of separating the graphene layer from graphite by utilizing a solvent with a surface tension close to that of graphene (30–40 mJ/m²) [
112]. Solvents such as N-methyl-2-pyrrolidone (NMP), dimethylformamide (DMF), and dimethyl sulfoxide (DMSO) are commonly used because they are able to stabilize graphene in suspension and improve exfoliation efficiency [
113].
Method Chemical Exfoliation with pure organic solvents produces low-concentration graphene (<0.1 mg/mL). The addition of organic salts such as sodium citrate, sodium tartrate, potassium sodium tartrate, and EDTA disodium has been shown to significantly improve exfoliation efficiency, resulting in high-quality graphene (1–3 layers, oxide-free and defective) with concentrations of up to ~1 mg/mL in 2 hours of sonication [
114].
Chemical Exfoliation with Ionic Liquid Exfoliation
Liquid phase exfoliation with ionic liquid is an environmentally friendly top-down method to produce graphene from graphite. The ionic liquid acts as a stable solvent with a corresponding surface tension, thus allowing the separation of the graphene layer without damaging its structure [
115], as shown in
Table 14.
[Ntf₂] based ionic liquids, specifically [C₄C₁im][Ntf₂] and [Pyrr₄,₁][Ntf₂], produce graphene with high concentrations, ≤5 layer counts, and the best purity. In contrast, ionic liquids with other anions, such as [C(CN)₃] and [Otf], show low efficiency and high oxidation rates, signalling the crucial role of ionic structures in exfoliation quality [
116]. Ionic liquids have been shown to produce high-concentration graphene dispersions without chemical modifications, but their use is still limited due to their high price and high viscosity, which affect the efficiency of exfoliation [
115].
Chemical Exfoliation with Surfactants
Liquid exfoliation with surfactants is a method to separate graphite into graphene sheets in solution using ultrasonication, where surfactants function to stabilize graphene sheets so that they do not clump together. This method is effective, simple, and suitable for large-scale graphene production [
117], as shown in
Table 15.
From
Table 15, the six types of surfaces studied, exfoliation with Triton X-100 resulted in the highest concentration of graphene (~0.29 mg/mL) in (~1 mg/mL) surfactant. TEM and AFM show thin sheets with a thickness of 1–3 nm and <5 layers. The average flake size of 46.83 μm² obtained from SDOC samples indicates a complete and quality graphene morphology. Raman and XPS in the Triton X-100 sample showed low defects and dominance of sp² structures. The best dispersion stability is also demonstrated by Triton X-100 and Tween 80, with more than 80% of the graphene remaining dispersed after 700 hours [
118].
Exfoliate with Low Boiling Point Solvent
Exfoliation with a low-boiling-point solvent is a method of separating a layer of graphite into graphene using a volatile solvent. High-boiling point solvents were previously widely used because they were effective, but they were difficult to vaporize and could cause clumping during drying. The use of low-boiling-point solvents is a solution to overcome this problem [
119], as shown in
Table 16.
Exfoliation with volatile solvents yields good quality graphene, with a thickness of <5 layers and a low defect rate based on TEM and Raman analyses. Solvents such as isopropanol and chloroform are not only easy to evaporate but are also able to maintain the stability of graphene dispersion. In contrast, high-boiling point solvents produce higher concentrations, but they tend to cause aggregation during drying, which can degrade the quality of graphene [
120].
Exfoliation with Electrochemistry
Electrochemical exfoliation is a fast and environmentally friendly method of producing graphene oxide from graphite using an electric current in an electrolyte solution [
121]. This process does not require strong oxidizers, is suitable for large-scale production, and is capable of producing <10-layer nanosheets with a lateral size of >1 μm [
122].
The shape of graphite has a significant effect on the results of electrochemical exfoliation. Compressed graphite powder produces graphene with a lateral size of >30 μm and a yield of 65% [
123]. Natural powder produces GO with an oxygen content of 25.3 at.%, an I
D/I
G ratio of 0.85, and ±9 layers [
124]. Graphite foil produces ~1.0 nm thick GO, consisting of 1–3 layers, with a yield of up to 96%, while rods break easily and flake exfoliate quickly but with a lower yield (<40%) [
121].
In addition, the type of electrolyte also determines the quality of the graphene formed. H₂SO₄ (0.5 M) electrolytes produce I
D/I
G of 0.35 with rapid blistering, Li₂SO₄ (0.5 M) produce I
D/I
G of 0.25 with moderate exfoliation, and NaClO₄ (1 M) produce I
D/I
G of 0.17 with non-destructive intercalation [
125]. Electrolytes such as HClO₄ and HNO₃ form epoxy and alkoxy groups, (NH₄)₂SO₄ allow doping of N and S, while ozone produces 1–3 layers of GO with 16.37 at.% oxygen and 1.21 I
D/I
G [
121]. This data confirms that the combination of graphite shape and the right type of electrolyte greatly determines the quality and efficiency of GO production.
2.3. Bottom up
The bottom-up approach is a graphene synthesis strategy that is carried out by gradually forming the structure of graphene from small molecular units such as carbon atoms, usually through chemical processes or deposition. This method generally produces high-quality graphene with a good level of structural control, although scalability and production costs are major challenges. The following techniques are used in the synthesis of graphene by the bottom-up method:
2.3.1. Chemical Vapor Deposition (CVD)
Chemical Vapor Deposition (CVD) is a vacuum deposition method to produce high-quality graphene through the chemical reaction of a gas precursor on a hot substrate, such as copper or nickel [
126]. This process produces the carbon atoms that make up graphene by breaking down gases like methane [
127]. For electronics and sensor applications, CVD makes it possible to create graphene with a large surface area and a consistent crystal structure [
128]. To reduce flaws in the graphene layer, this procedure necessitates rigorous regulation of temperature, pressure, and gas flow [
129].
Temperature
The temperature of synthesis has a major impact on the quality of graphene generated using the CVD process. The number of layers, purity, degree of defects, and interaction with the substrate are all influenced by temperature [
130]. High-quality graphene is characterized by uniform coatings, slight defects, high purity, and good substrate interactions, all depending on temperature settings during the process [
131]. The following sections discuss the impact of various low, high, and ultra-high temperature ranges on these aspects, as shown in
Table 17.
By increasing layer uniformity, decreasing defects, and enhancing substrate interactions, high temperatures enhance graphene quality; however, they can also increase energy consumption and cause substrate damage. Although they use less energy, low temperatures can cause quality degradation. For some applications, extremely high temperatures are advantageous, but they must be carefully managed to avoid aggravating flaws or damaging the substrate. These factors must be balanced in order to optimize the CVD process and produce graphene of the appropriate quality.
Pressure
The quality of graphene produced by CVD is greatly influenced by process pressures, whether atmospheric, low, or ultra-vacuum. This pressure affects the number of layers, defects, purity, and interaction with the substrate, with each condition offering its own advantages and challenges [
137], as shown in
Table 18.
Each pressure condition has its own advantages depending on the needs of the application. Ultra-vacuum is suitable for high-precision applications because it produces the purest graphene, while APCVD is more efficient and suitable for large-scale production despite higher defects.
Wall/Substrate
The quality of graphene synthesized with CVD is affected by the configuration of the walls/substrates, both cold walls and hot walls [
141]. Here are the differences in graphene results obtained from cold wall and hot wall configurations in the CVD method, as shown in
Table 19.
The hot-wall method is still useful for applications that value cost and scalability, but the cold-wall CVD method produces graphene with superior quality. By optimizing both approaches, research and technological advancements can lessen the disparity in quality between them.
Deposition Time
The quality of graphene produced by CVD is greatly influenced by deposition times, including continuous, disconnected, and pulsed methods. Each approach has a different impact on the number of layers, defects, and purity, so it is important to choose the right timing strategy to optimize the structural and functional properties of graphene [
135], as shown in
Table 20.
The continuous, intermittent, and pulsed CVD methods have their own advantages depending on the application. Continuous CVD produces the most uniform graphene and minimal defects; intermittent CVD is suitable for property modification, while pulsed CVD offers high control for customization.
Gas Flow State
The quality of graphene produced through CVD is affected by the gas flow method, which is an open and closed CVD system. Open systems use a continuous flow of gases, while closed systems maintain a static gas environment. These two methods affect the number of layers, the defect density, and the purity of graphene in different ways [
149], as shown in
Table 21.
The choice between open and closed CVD systems depends on the specific quality needs of the graphene application. Open systems are better suited for large-scale production with fewer uniformity and defects, while closed systems offer better control over the purity and thickness of the coating, ideal for applications that require high quality.
Activated Manner
The synthesis of graphene by various CVD methods, such as thermal, plasma, and laser, has a major effect on the number of layers, defect density, and purity. Each method has advantages and disadvantages that affect the final quality of graphene [
151], as shown in
Table 22.
The selection of the CVD method depends on the application requirements and the desired properties of graphene. PECVD is suitable for pure graphene with low defects; lasers are ideal for fast patterns, and superior thermal methods for large areas, albeit slower. Efficiency and quality are largely determined by the process parameters and the end goal of using graphene.
2.3.2. Epitaxial Growth of Graphene Silicon Carbide
Epitaxial growth of graphene on silicon carbide (SiC) substrates is a synthesis method in which a layer of graphene is formed directly on the surface of the SiC crystal through a process of thermal decomposition at high temperatures, generally above 1200 °C [
158]. At this temperature, the silicon atoms from the surface of SiC evaporate, leaving behind carbon atoms that then compose themselves into a graphene structure [
159]. This process is often carried out in the atmosphere of inert gases such as argon to control the sublimation rate and improve the uniformity of the graphene layer [
160]. The uniqueness of this method is that it does not require the process of transferring graphene to other substrates, thus reducing the risk of contamination and mechanical damage [
161].
In the process of epitaxial growth of graphene in silicon carbide, there are several important factors that significantly affect the quality and characteristics of the graphene layer produced. Factors that affect the quality of graphene with this method include as following:
Surface
SiC substrates have two surface sides, namely the silicon side (Si-face) and the carbon side (C-face), which significantly affect the growth outcome of graphene. In Si-face, growth usually results in a uniform monolayer graphene layer and bonds to the substrate via a buffer layer, which can modify the electronic properties of graphene [
162]. In contrast, the growth on the C-face produces a multilayer of graphene with a random orientation, resulting in electronic properties that resemble substrate-free graphene [
163]. Due to its advantages in uniformity, stability, and ability to produce graphene layers over large areas, this epitaxial method holds great promise for high-speed electronics applications and quantum metrology standards [
159]. The following are the results of graphene analysis with the difference between Si-face and C-face, as shown in
Table 23.
Graphene from Si-face has a regular epitactic structure, smooth surface, and controllable monolayer thickness, making it suitable for precision electronics applications such as field effect transistors (FETs) and sensors. In contrast, graphene from the C-face tends to grow in multilayer forms with random orientations and corrugated surfaces, yet offers higher electron mobility and weak interactions with substrates, making it more suitable for applications such as capacitors, batteries, or transparent conductors [
164].
Temperature
Growth temperature plays an important role in the quality of graphene synthesized through the epitaxial growth method on silicon carbide (SiC) because it directly affects the evaporation rate of silicon (Si) from the SiC surface and the formation of graphene layers [
165]. At high temperatures, Si atoms evaporate from the surface, leaving behind carbon atoms that are then composed into graphene structures [
166]. Temperatures that are too low can lead to incomplete or irregular graphene formation [
167], while too high a temperature can lead to too rapid evaporation of Si, resulting in a thick, non-uniform, or deformed layer of graphene [
168]. The following are the results of graphene analysis with temperature differences, as shown in
Table 24.
Optimal growth temperatures produce graphene monolayers with the highest electron mobility, while temperatures that are too low or too high produce low-quality graphene due to inadequate thickness or structural defects [
169].
Pressure
Pressure greatly affects the evaporation rate of silicon during the epitaxial growth of graphene from SiC. At low pressures, silicon evaporates faster, which can lead to uncontrolled growth of graphene and rough surfaces [
170]. In contrast, the use of an inert atmosphere, such as argon at high pressure, can slow the evaporation of silicon, allowing the carbon to be composed more stably into a finer and more uniform layer of graphene [
171]. The following are the results of graphene analysis with pressure differences, as shown in
Table 25.
The pressure during the epitaxial growth of graphene greatly affects its quality. At low pressures, silicon evaporates too quickly, so graphene is heavily deformed and morphologically rough. Whereas at inert atmospheric pressure, the sublimation rate is inhibited, resulting in a smoother, uniform, and high-quality layer of graphene [
167].
Catalyst
By aiding in the breakdown of silicon from the SiC surface, catalysts in the epitaxial growth of graphene in silicon carbide contribute to the acceleration and direction of the graphene layer formation process. Graphene can grow more regularly and with fewer defects if a catalyst is present to control the silicon sublimation rate. Because they make it easier for carbon atoms to come together to form graphene structures, metal-based catalysts like nickel and copper are frequently employed.
However, the use of metal catalysts must also be considered so as not to cause contamination that can degrade the quality of graphene, especially in its electronic properties. Additionally, catalysts can influence the morphology and size of graphene domains, enabling growth with larger crystals and more uniform layers [
172]. The following are the results of graphene analysis without a catalyst and with a catalyst, as shown in
Table 26.
The use of Ni–Cu catalysts on graphene epitaxial growth in 3C–SiC/Si significantly improved the quality of graphene, resulting in a monolayer structure with a low I
D/I
G ratio (~0.24), smooth surface, and uniform morphology. Without catalysts, the graphene produced is multilayer, high defects, and has a rough surface[
172].
3. Conclusions
Graphene is a two-dimensional material that is stronger, more conductive, and more heat-resistant than other materials. Scientists have come up with several techniques to produce graphene, employing both top-down and bottom-up methods. Top-down processes like mechanical exfoliation, oxidation-reduction, arc discharge, unzipping carbon nanotubes, and liquid phase exfoliation are all examples of procedures that are usually easy to use and may be employed to produce things on a large scale. But this method usually creates graphene that has structural problems and sizes that aren't always the same.
On the other hand, bottom-up technologies like Chemical Vapour Deposition (CVD) and Epitaxial Growth on Carbide may generate high-quality graphene with a nice crystal structure and a number of layers that can be controlled. However, this method does need intricate, costly, and inefficient equipment for making things in large quantities. Because of this, it is better for high-tech uses that need very high purity and accuracy.
There is still no one-size-fits-all approach to manufacturing graphene that works for all purposes. So, the way that graphene is made should alter depending on the final goal, which could be to generate the best graphene possible or to speed up the process. We need to work on building graphene synthesis procedures that are better for the environment and use green chemistry to help make production more sustainable in the future.
Author Contributions
Conceptualization, J.A.A.H., A.I.Y.T; methodology, Y.G.O.M., S.T.C.L.N., R.S.; software, Y.G.O.M., S.T.C.L.N., R.S.; validation, S.T.C.L.N., R.S.; investigation, Y.G.O.M.; resources, J.A.A.H., Y.G.O.M., S.T.C.L.N., R.S., R.G.; writing—original draft preparation, J.A.A.H., Y.G.O.M., S.T.C.L.N., R.S.; writing—review and editing, J.A.A.H., J.H.; supervision, J.A.A.H.; project administration, J.A.A.H.; funding acquisition, J.A.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PMMA |
Polymethyl methacrylate |
| PDMS |
Polydimethylsiloxane |
| FTIR |
Fourier Transform Infrared |
| SEM |
Scanning Electron Microscopy |
| XRD |
X-ray Diffraction |
| AFM |
Atomic Force Microscopy |
| TEM |
Transmission Electron Microscopy |
| DMF |
Dimethylformamide |
| NMP |
N-Methyl-2-pyrrolidone |
| SC |
Supercritical |
| RDX |
Research Department Explosive |
| ER-GO |
Explosively Reduced Graphene Oxide |
| SEG |
Solvent-Exfoliated Graphene |
| SDOC |
Sodium Deoxycholate |
| SDBS |
Sodium Dodecylbenzenesulfonate |
| SDS |
Sodium Dodecyl Sulfate |
| HTAB |
Hexadecyltrimethylammonium Bromide |
| CG |
Concentration of Graphene |
| Csur |
Concentration of Surfactant |
| CMC |
Critical Micelle Concentration |
| APCVD |
Atmospheric Pressure Chemical Vapor Deposition |
| LPCVD |
Low Pressure Chemical Vapor Deposition |
| PECVD |
Plasma Enhanced Chemical Vapor Deposition |
| TCVD |
Thermal Chemical Vapor Deposition |
References
- Ares, P.; Novoselov, K.S. Recent Advances in Graphene and Other 2D Materials. Nano Mater. Sci. 2022, 4, 3–9. [Google Scholar] [CrossRef]
- Sur, U.K. Graphene: A Rising Star on the Horizon of Materials Science. Int. J. Electrochem. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Hancock, Y. The 2010 Nobel Prize in Physics - Ground-Breaking Experiments on Graphene. J. Phys. D. Appl. Phys. 2011, 44. [Google Scholar] [CrossRef]
- Radadiya, T. An Properties of Graphene. Int. J. Mech. Eng. Inf. Technol. 2015, 3, 983–992. [Google Scholar] [CrossRef]
- Ali Tahir, A.; Ullah, H.; Sudhagar, P.; Asri Mat Teridi, M.; Devadoss, A.; Sundaram, S. The Application of Graphene and Its Derivatives to Energy Conversion, Storage, and Environmental and Biosensing Devices. Chem. Rec. 2016, 16, 1591–1634. [Google Scholar] [CrossRef] [PubMed]
- Luan, D. Applications of Graphene in Different Fields. MATEC Web Conf. 2023, 386, 03015. [Google Scholar] [CrossRef]
- Li, S. Analysis of Large-Scale High-Quality Graphene Production and Applications. Appl. Comput. Eng. 2024, 63, 84–89. [Google Scholar] [CrossRef]
- Yan, Y.; Nashath, F.Z.; Chen, S.; Manickam, S.; Lim, S.S.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of Graphene: Potential Carbon Precursors and Approaches. Nanotechnol. Rev. 2020, 9, 1284–1314. [Google Scholar] [CrossRef]
- Grayfer, E.D.; Makotchenko, V.G.; Nazarov, A.S.; Kim, S.J.; Fedorov, V.E. Graphene: Chemical Approaches to the Synthesis and Modification. Russ. Chem. Rev. 2011, 80, 751–770. [Google Scholar] [CrossRef]
- Li, H.; Zhao, G.; Zhang, H. Recent Progress of Cement-Based Materials Modified by Graphene and Its Derivatives. Materials (Basel). 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.S.; Coleman, K.S. Graphene Synthesis: Relationship to Applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Buzaglo, M.; Bar, I.P.; Varenik, M.; Shunak, L.; Pevzner, S.; Regev, O. Graphite-to-Graphene: Total Conversion. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.D.; Kim, H.; Kim, G. Various Defects in Graphene: A Review. RSC Adv. 2022, 12, 21520–21547. [Google Scholar] [CrossRef] [PubMed]
- Biliak, R. Methods of Obtaining Graphene. Comput. Probl. Electr. Eng. 2023, 13, 1–8. [Google Scholar] [CrossRef]
- Shams, S.S.; Zhang, R.; Zhu, J. Graphene Synthesis: A Review. Mater. Sci. Pol. 2015, 33, 566–578. [Google Scholar] [CrossRef]
- Patel, R. V.; Patel, R.H.; Chaki, S.H. Synthesis and Characterization of 2D Graphene Sheets from Graphite Powder. AIP Conf. Proc. 2018, 1961. [Google Scholar] [CrossRef]
- Tour, J.M. Top-down versus Bottom-up Fabrication of Graphene-Based Electronics. Chem. Mater. 2014, 26, 163–171. [Google Scholar] [CrossRef]
- Moreno, C.; Vilas-Varela, M.; Kretz, B.; Garcia-Lekue, A.; Costache, M. V.; Paradinas, M.; Panighel, M.; Ceballos, G.; Valenzuela, S.O.; Peña, D.; et al. Bottom-up Synthesis of Multifunctional Nanoporous Graphene. Science (80-. ). 2018, 360, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Santhiran, A.; Iyngaran, P.; Abiman, P.; Kuganathan, N. Graphene Synthesis and Its Recent Advances in Applications—A Review. C 2021, 7, 76. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A Review on Mechanical Exfoliation for the Scalable Production of Graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; khan, A. ullah Graphene Synthesis, Characterization and Its Applications: A Review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Sinclair, R.C.; Suter, J.L.; Coveney, P. V. Micromechanical Exfoliation of Graphene on the Atomistic Scale. Phys. Chem. Chem. Phys. 2019, 21, 5716–5722. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, B.; Subbiah, S. A Novel Mechanical Cleavage Method for Synthesizing Few-Layer Graphenes. Nanoscale Res. Lett. 2011, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.H.; Mai, P.T.; Nguyen, N.P.T.; Tran, H. Van; Nguyen, H.T.M.; Nguyen, A.T. Van; Nguyen, D.V.; Doan, P.D.; Phan, M.N.; Bui, T.H. Fabrication of Graphene from Graphite Using High-Powered Ultrasonic Vibrators. Mater. Res. Express 2024, 11, 1–16. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.P.; Valverde, J.L.; Sanchez-Silva, L.; Romero, A. Solvent-Based Exfoliation via Sonication of Graphitic Materials for Graphene Manufacture. Ind. Eng. Chem. Res. 2016, 55, 845–855. [Google Scholar] [CrossRef]
- Liyanage, C.D.; Kumar, H.; Perera, I.; Abeykoon, P.G.; Chen, F.; Joya, J.S.; Suib, S.L.; Adamson, D.H. Synthesis of Graphene Oxide: Effect of Sonicating during Oxidation. Carbon N. Y. 2024, 223. [Google Scholar] [CrossRef]
- Nguyen, Y.H.; Mai, P.T.; Nguyen, N.P.T.; Van Tran, H.; Nguyen, H.T.M.; Van Nguyen, A.T.; Nguyen, D.V.; Doan, P.D.; Phan, M.N.; Bui, T.H. Fabrication of Graphene from Graphite Using High-Powered Ultrasonic Vibrators. Mater. Res. Express 2024, 11. [Google Scholar] [CrossRef]
- Sargın, F.; Ak Azem, F.; Kanbur, K.; Birlik, I.; Türk, A. Evaluating the Impact of Sonication Process on Graphene Oxide Structural Properties. Ömer Halisdemir Üniversitesi Mühendislik Bilim. Derg. 2024, 13, 1139–1149. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Mariatti, M.; Chow, W.S.; Suda, Y.; Thant, A.A. Effect of Sonication Time on the Production of Graphene by Electrochemical Exfoliation Method. J. Phys. Conf. Ser. 2018, 1082. [Google Scholar] [CrossRef]
- Azimi, Z.; Alimohammadian, M.; Sohrabi, B. Graphene Quantum Dots Based on Mechanical Exfoliation Methods: A Simple and Eco-Friendly Technique. ACS Omega 2024, 9, 31427–31437. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.; Paul, G. Synthesis and Characterization of Graphene Oxide Nanosheets by Mechanical Exfoliation Using Ball Milling. J. Phys. Conf. Ser. 2024, 2818. [Google Scholar] [CrossRef]
- Awan, Z.; Naqvi, A.A.; Shahid, Z.; Butt, F.A.; Raza, F. Synthesis and Characterization of Graphene Sheets from Graphite Powder by Using Ball Milling. Rev. UIS Ing. 2022, 21, 71–76. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, Y.J.; Hwang, S.J.; Chung, H.S. High Energy Ball Milling of Catalytically Synthesized Carbon Nanotubes. Mater. Sci. Forum 2007, 534–536, 193–196. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, M.; Wu, F.; Wu, H.; Wang, L.; Chen, G. Preparation of Graphene by Exfoliation of Graphite Using Wet Ball Milling. J. Mater. Chem. 2010, 20, 5817–5819. [Google Scholar] [CrossRef]
- Hu, K.; Brambilla, L.; Sartori, P.; Moscheni, C.; Perrotta, C.; Zema, L.; Bertarelli, C.; Castiglioni, C. Development of Tailored Graphene Nanoparticles: Preparation, Sorting and Structure Assessment by Complementary Techniques. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Yan, L.; Huang, K.; Li, Y.; Ai, F.; Zhang, H.; Jiang, Z. Effect of Different Rotational Speeds on Graphene-Wrapped Sic Core-Shell Nanoparticles in Wet Milling Medium. Materials (Basel). 2021, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Myekhlai, M.; Munkhbayar, B.; Lee, T.; Tanshen, M.R.; Chung, H.; Jeong, H. Experimental Investigation of the Mechanical Grinding Effect on Graphene Structure. RSC Adv. 2014, 4, 2495–2500. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Stolle, A.; Stelter, M. Sustainable Synthesis of High-Surface-Area Graphite Oxide via Dry Ball Milling. ACS Sustain. Chem. Eng. 2018, 6, 6358–6369. [Google Scholar] [CrossRef]
- Brandão, A.T.S.C.; Costa, R.; Silva, A.F.; Pereira, C.M. Sustainable Preparation of Nanoporous Carbons via Dry Ball Milling: Electrochemical Studies Using Nanocarbon Composite Electrodes and a Deep Eutectic Solvent as Electrolyte. Nanomaterials 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cao, Y.; Zhang, J.; Zhang, W.; Xu, Y.; Guo, J.; Yang, W.; Liu, J. One-Step Preparation of Graphene Nanosheets via Ball Milling of Graphite and the Application in Lithium-Ion Batteries. J. Mater. Sci. 2016, 51, 3675–3683. [Google Scholar] [CrossRef]
- Dash, P.; Dash, T.; Rout, T.K.; Sahu, A.K.; Biswal, S.K.; Mishra, B.K. Preparation of Graphene Oxide by Dry Planetary Ball Milling Process from Natural Graphite. RSC Adv. 2016, 6, 12657–12668. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Choi, H.J.; Jung, S.M.; Seo, J.M.; Kim, M.J.; Dai, L.; Baek, J.B. Large-Scale Production of Edge-Selectively Functionalized Graphene Nanoplatelets via Ball Milling and Their Use as Metal-Free Electrocatalysts for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2013, 135, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Shen, Z.; Zhu, J. A Fluid Dynamics Route for Producing Graphene and Its Analogues. Chinese Sci. Bull. 2014, 59, 1794–1799. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. Fluid Dynamics: An Emerging Route for the Scalable Production of Graphene in the Last Five Years. RSC Adv. 2016, 6, 72525–72536. [Google Scholar] [CrossRef]
- Pang, Y.X.; Yew, M.; Yan, Y.; Khine, P.; Filbert, A.; Manickam, S.; Foo, D.C.Y.; Sharmin, N.; Lester, E.; Wu, T.; et al. Application of Supercritical Fluid in the Synthesis of Graphene Materials: A Review. J. Nanoparticle Res. 2021, 23. [Google Scholar] [CrossRef]
- Morales Ibarra, R.; Goto, M.; García-Serna, J.; García Montes, S.M. Graphene Exfoliation with Supercritical Fluids. Carbon Lett. 2021, 31, 99–105. [Google Scholar] [CrossRef]
- Gao, H.; Hu, G. Graphene Production via Supercritical Fluids. RSC Adv. 2016, 6, 10132–10143. [Google Scholar] [CrossRef]
- Shang, T.; Feng, G.; Li, Q.; Zheng, Y. Production of Graphene Nanosheets by Supercritical CO2 Process Coupled with Micro-Jet Exfoliation. Fullerenes Nanotub. Carbon Nanostructures 2017, 25, 691–698. [Google Scholar] [CrossRef]
- Rangappa, D.; Sone, K.; Wang, M.; Gautam, U.K.; Golberg, D.; Itoh, H.; Ichihara, M.; Honma, I. Rapid and Direct Conversion of Graphite Crystals into High-Yielding, Good-Quality Graphene by Supercritical Fluid Exfoliation. Chem. - A Eur. J. 2010, 16, 6488–6494. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Karimi-Sabet, J.; Moosavian, S.M.A.; Ghorbanian, S. Optimization of Graphene Production by Exfoliation of Graphite in Supercritical Ethanol: A Response Surface Methodology Approach. J. Supercrit. Fluids 2016, 107, 92–105. [Google Scholar] [CrossRef]
- Ye, B.Y.; Wang, J.Y.; Geng, X.H.; An, C.W.; Ding, P.H. One-Step Synthesis of Graphene Nanosheets through Explosive Process. Inorg. Nano-Metal Chem. 2017, 47, 1216–1219. [Google Scholar] [CrossRef]
- Wright, J.P.; Sigdel, S.; Corkill, S.; Covarrubias, J.; LeBan, L.; Nepal, A.; Li, J.; Divigalpitiya, R.; Bossmann, S.H.; Sorensen, C.M. Synthesis of Turbostratic Nanoscale Graphene via Chamber Detonation of Oxygen/Acetylene Mixtures. Nano Sel. 2022, 3, 1054–1068. [Google Scholar] [CrossRef]
- Nepal, A.; Singh, G.P.; Flanders, B.N.; Sorensen, C.M. One-Step Synthesis of Graphene via Catalyst-Free Gas-Phase Hydrocarbon Detonation. Nanotechnology 2013, 24. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Zhuo, D.X.; Wu, L.X.; Ma, L.; Weng, Z.X.; Wang, R. A Facile and Efficient Method to Prepare Exfoliated and Reduced Graphene Nanosheets by Detonation. Adv. Mater. Res. 2014, 937, 260–266. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon N. Y. 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically Derived Graphene Oxide: Towards Large-Area Thin-Film Electronics and Optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.M. The Reduction of Graphene Oxide. Carbon N. Y. 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical Reduction of Graphene Oxide: A Synthetic Chemistry Viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and Thermal Reduction of Graphene Oxide: Reaction Mechanisms, Product Structures, and Reaction Design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (RGO)*. Graphene 2017, 06, 1–18. [Google Scholar] [CrossRef]
- Paton-Carrero, A.; Valverde, J.L.; Garcia-Alvarez, E.; Lavin-Lopez, M.P.; Romero, A. Influence of the Oxidizing Agent in the Synthesis of Graphite Oxide. J. Mater. Sci. 2020, 55, 2333–2342. [Google Scholar] [CrossRef]
- Das, P.; Ibrahim, S.; Chakraborty, K.; Ghosh, S.; Pal, T. Stepwise Reduction of Graphene Oxide and Studies on Defect-Controlled Physical Properties. Sci. Rep. 2024, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yao, X.; Li, H.; Liu, Z.; Ma, W.; Liang, X. Thermal Stability of Oxygen-Containing Functional Groups on Activated Carbon Surfaces in a Thermal Oxidative Environment. J. Chem. Eng. Japan 2014, 47, 21–27. [Google Scholar] [CrossRef]
- Lee, B.J.; Jeong, G.H. Thermal Oxidation of Synthesized Graphenes and Their Optical Property Characterization. J. Nanosci. Nanotechnol. 2011, 11, 6084–6088. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.S.; Liu, W.W. A Review of the Effect of Different Electrolytes on the Synthesis of Graphene Sheets by Electrochemical Exfoliation. Int. J. Nanoelectron. Mater. 2024, 17, 279–283. [Google Scholar] [CrossRef]
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.M.; Ren, W. Green Synthesis of Graphene Oxide by Seconds Timescale Water Electrolytic Oxidation. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lesiak, B.; Trykowski, G.; Tóth, J.; Biniak, S.; Kövér, L.; Rangam, N.; Stobinski, L.; Malolepszy, A. Chemical and Structural Properties of Reduced Graphene Oxide—Dependence on the Reducing Agent. J. Mater. Sci. 2021, 56, 3738–3754. [Google Scholar] [CrossRef]
- Cataldo, F.; Putz, M. V.; Ursini, O.; Angelini, G.; Garcia-Hernandez, D.A.; Manchado, A. A New Route to Graphene Starting from Heavily Ozonized Fullerenes: Part 1 - Thermal Reduction under Inert Atmosphere. Fullerenes Nanotub. Carbon Nanostructures 2016, 24, 52–61. [Google Scholar] [CrossRef]
- Sengunthar, P.; Patel, S.; Thankachen, N.; Joshi, U.S.; Pandya, R.J. Controlled Synthesis of Reduced Graphene Oxide Sheets on Large Scale Using Thermal Exfoliation. ECS Trans. 2022, 107, 19943–19948. [Google Scholar] [CrossRef]
- Feng, X.; Chen, W.; Yan, L. Electrochemical Reduction of Bulk Graphene Oxide Materials. RSC Adv. 2016, 6, 80106–80113. [Google Scholar] [CrossRef]
- Kholib, N.S.; Liu, W.W. Graphene Synthesis by Electrochemical Reduction of Graphene Oxide and Its Characterizations. Int. J. Nanoelectron. Mater. 2023, 16, 717–724. [Google Scholar] [CrossRef]
- Nair, S.S.; Saha, T.; Dey, P.; Bhadra, S. Thermal Oxidation of Graphite as the First Step for Graphene Preparation: Effect of Heating Temperature and Time. J. Mater. Sci. 2021, 56, 3675–3691. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, L. ping; Lin, F. yun; Liu, H. xia Electrochemistry and Electrocatalysis of Polyoxometalate-Ordered Mesoporous Carbon Modified Electrode. Anal. Chim. Acta 2007, 587, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, L.; Jiao, X.; Qiu, Y.; Xu, W. The Electrochemical Performance of Reduced Graphene Oxide Prepared from Different Types of Natural Graphites. RSC Adv. 2021, 11, 4042–4052. [Google Scholar] [CrossRef] [PubMed]
- Mawatha, B.; Lanka, S. Spectroscopic Analysis of Mass-Scale Prepared GO and RGO from Vein Graphite through Compositional Improvement. Sri Lankan J. Phys. 2024, 25, 13–34. [Google Scholar] [CrossRef]
- Mhlongo, J.T.; Tlhaole, B.; Linganiso, L.Z.; Motaung, T.E.; Linganiso-Dziike, E.C. Microwave-Assisted Reduction of Graphene Oxide to Reduced Graphene Oxide. Processes 2025, 13, 1–15. [Google Scholar] [CrossRef]
- Hidayat, R.; Wahyuningsih, S.; Ramelan, A.H. Simple Synthesis of RGO (Reduced Graphene Oxide) by Thermal Reduction of GO (Graphene Oxide). IOP Conf. Ser. Mater. Sci. Eng. 2020, 858. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, S.; Pathania, P.; Pathak, D.; Rangra, V.S. SYNTHESIS of RGO-ZnO COMPOSITES for THERMAL, ELECTRICAL and ANTIBACTERIAL STUDIES. Surf. Rev. Lett. 2017, 24, 1–8. [Google Scholar] [CrossRef]
- Awoji, M.O.; Onoja, A.D.; Echi, M.I. Synthesis of Graphene Via Arc Discharge and Its Characterization: A Comparative Approach. East Eur. J. Phys. 2023, 2023, 252–257. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.; Ma, Y.; Huang, Y.; Li, N.; Zhang, F.; Chen, Y. Efficient and Large-Scale Synthesis of Few-Layered Graphene Using an Arc-Discharge Method and Conductivity Studies of the Resulting Films. Nano Res. 2010, 3, 661–669. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhao, K.; Shi, Z.; Gu, Z.; Xu, S. Large Scale Synthesis of N-Doped Multi-Layered Graphene Sheets by Simple Arc-Discharge Method. Carbon N. Y. 2010, 48, 255–259. [Google Scholar] [CrossRef]
- Wu, C.; Dong, G.; Guan, L. Production of Graphene Sheets by a Simple Helium Arc-Discharge. Phys. E Low-Dimensional Syst. Nanostructures 2010, 42, 1267–1271. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Dubey, P.K.; Kumar, P.; Tiwari, R.S.; Oh, I.K. Pressure-Dependent Synthesis of High-Quality Few-Layer Graphene by Plasma-Enhanced Arc Discharge and Their Thermal Stability. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Simple Method of Preparing Graphene Flakes by an Arc-Discharge Method. J. Phys. Chem. C 2009, 113, 4257–4259. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Yang, H.; Shi, Z. Large-Scale Synthesis of High-Quality Graphene Sheets by an Improved Alternating Current Arc-Discharge Method. RSC Adv. 2016, 6, 93119–93124. [Google Scholar] [CrossRef]
- Antisari, M.V.; Gattia, D.M.; Brandão, L.; Marazzi, R.; Montone, A. Carbon Nanostructures Produced by an AC Arc Discharge. Mater. Sci. Forum 2010, 638–642, 1766–1771. [Google Scholar] [CrossRef]
- Gattia, D.M.; Vittori Antisari, M.; Marazzi, R. AC Arc Discharge Synthesis of Single-Walled Nanohorns and Highly Convoluted Graphene Sheets. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Kane, A.; Hinkov, I.; Brinza, O.; Hosni, M.; Barry, A.H.; Cherif, S.M.; Farhat, S. One-Step Synthesis of Graphene, Copper and Zinc Oxide Graphene Hybrids via Arc Discharge: Experiments and Modeling. Coatings 2020, 10, 1–24. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Shi, Z.; Gu, Z. Low-Cost and Large-Scale Synthesis of Graphene Nanosheets by Arc Discharge in Air. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Levchenko, I.; Cvelbar, U.; Keidar, M. Graphene Flakes in Arc Plasma: Conditions for the Fast Single-Layer Growth. Graphene 2016, 05, 81–89. [Google Scholar] [CrossRef]
- Volotskova, O.; Levchenko, I.; Shashurin, A.; Raitses, Y.; Ostrikov, K.; Keidar, M. Single-Step Synthesis and Magnetic Separation of Graphene and Carbon Nanotubes in Arc Discharge Plasmas. Nanoscale 2010, 2, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Kulkarni, N. V.; Nawale, A.B.; Lalla, N.P.; Mishra, R.; Sathe, V.G.; Bhoraskar, S. V.; Das, A.K. A Novel Approach towards Selective Bulk Synthesis of Few-Layer Graphenes in an Electric Arc. J. Phys. D. Appl. Phys. 2009, 42. [Google Scholar] [CrossRef]
- Pacheco, M.; Mendoza, D.; Valdivia-Barrientos, R.; Santana-Diaz, A.; Pacheco, J.; Alarcon, L.E.; Gutierrez, P.G.V.; Tu, X. Multilayer Graphene Growth Assisted by Sulfur Using the Arc Discharge Method at Ambient Conditions. IEEE Trans. Plasma Sci. 2018, 46, 2407–2412. [Google Scholar] [CrossRef]
- Kosynkin, D. V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal Unzipping of Carbon Nanotubes to Form Graphene Nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, L.; Wang, X.; Diankov, G.; Dai, H. Narrow Graphene Nanoribbons from Carbon Nanotubes. Nature 2009, 458, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Cano-marquez, A.G.; Rodríguez-macias, F.J.; Campos-delgado, J.; Espinosa-gonzalez, C.G.; Tristan-lopez, F.; Ramírez-gonzalez, D.; Cullen, D.A.; Smith, D.J.; Terrones, M.; Vega-cantu, Y.I. Ex-MWNTs: Graphene Sheets and Ribbons Produced by Lithium Intercalation and Exfoliation of Carbon Nanotubes. Nano Lett. 2009, 9, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Dimiev, A.M.; Khannanov, A.; Vakhitov, I.; Kiiamov, A.; Shukhina, K.; Tour, J.M. Revisiting the Mechanism of Oxidative Unzipping of Multiwall Carbon Nanotubes to Graphene Nanoribbons. ACS Nano 2018, 12, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lim, J.; Cho, S.Y.; Kim, H.; Lee, C.; Lee, G.Y.; Sasikala, S.P.; Yun, T.; Choi, D.S.; Jeong, M.S.; et al. Intact Crystalline Semiconducting Graphene Nanoribbons from Unzipping Nitrogen-Doped Carbon Nanotubes. ACS Appl. Mater. Interfaces 2019, 11, 38006–38015. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, B.H.; Farid, S.B.H.; Chyad, F.A. Modified Unzipping Technique to Prepare Graphene Nano-Sheets. J. Phys. Conf. Ser. 2018, 1003. [Google Scholar] [CrossRef]
- Janowska, I.; Ersen, O.; Jacob, T.; Vennégues, P.; Begin, D.; Ledoux, M.J.; Pham-Huu, C. Catalytic Unzipping of Carbon Nanotubes to Few-Layer Graphene Sheets under Microwaves Irradiation. Appl. Catal. A Gen. 2009, 371, 22–30. [Google Scholar] [CrossRef]
- Zheng, Q.F.; Guo, Y.; Liang, Y.; Shen, Q. Graphene Nanoribbons from Electrostatic-Force-Controlled Electric Unzipping of Single- And Multi-Walled Carbon Nanotubes. ACS Appl. Nano Mater. 2020, 3, 4708–4716. [Google Scholar] [CrossRef]
- Hirsch, A. Unzipping Carbon Nanotubes: A Peeling Method for the Formation of Graphene Nanoribbons. Angew. Chemie - Int. Ed. 2009, 48, 6594–6596. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hu, Y.; Huang, J.; Zhou, N.; Liu, Y.; Wei, L.; Chen, X.; Zhuang, N. One-Step Oxidation Preparation of Unfolded and Good Soluble Graphene Nanoribbons by Longitudinal Unzipping of Carbon Nanotubes. Nanotechnology 2018, 29, 0–14. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, S.; Da Ross, T.; Ostric, A.; Tošić, D.; Prekodravac, J.; Marković, Z.; Todorović Marković, B. Raman Spectroscopy of Graphene Nanoribbons Synthesized by Longitudinal Unzipping of Multiwall Carbon Nanotubes. Phys. Scr. Top. Issues 2014, T162. [Google Scholar] [CrossRef]
- Ko, D.; Choi, J.; Yan, B.; Hwang, T.; Jin, X.; Kim, J.M.; Piao, Y. A Facile and Scalable Approach to Develop Electrochemical Unzipping of Multi-Walled Carbon Nanotubes to Graphene Nanoribbons. J. Mater. Chem. A 2020, 8, 22045–22053. [Google Scholar] [CrossRef]
- Xie, L.; Wang, H.; Jin, C.; Wang, X.; Jiao, L.; Suenaga, K.; Dai, H. Graphene Nanoribbons from Unzipped Carbon Nanotubes: Atomic Structures, Raman Spectroscopy, and Electrical Properties. J. Am. Chem. Soc. 2011, 133, 10394–10397. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, M.; Gu, Y.; Guo, B.; Ma, H.X.; Wang, P.; Wang, X.; Zhang, R. Fast Chemical Exfoliation of Graphite to Few-Layer Graphene with High Quality and Large Size via a Two-Step Microwave-Assisted Process. Chem. Eng. J. 2020, 381. [Google Scholar] [CrossRef]
- Chacón-Torres, J.C.; Wirtz, L.; Pichler, T. Raman Spectroscopy of Graphite Intercalation Compounds: Charge Transfer, Strain, and Electron-Phonon Coupling in Graphene Layers. Phys. Status Solidi Basic Res. 2014, 251, 2337–2355. [Google Scholar] [CrossRef]
- MOOSA, A.A.; ABED, M.S. Graphene Preparation and Graphite Exfoliation. Turkish J. Chem. 2021, 45, 493–519. [Google Scholar] [CrossRef] [PubMed]
- Qamar, S.; Ramzan, N.; Aleem, W. Graphene Dispersion, Functionalization Techniques and Applications: A Review. Synth. Met. 2024, 307, 117697. [Google Scholar] [CrossRef]
- Du, W.; Lu, J.; Sun, P.; Zhu, Y.; Jiang, X. Organic Salt-Assisted Liquid-Phase Exfoliation of Graphite to Produce High-Quality Graphene. Chem. Phys. Lett. 2013, 568–569, 198–201. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Bordes, E.; Morcos, B.; Bourgogne, D.; Andanson, J.M.; Bussière, P.O.; Santini, C.C.; Benayad, A.; Gomes, M.C.; Pádua, A.A.H. Dispersion and Stabilization of Exfoliated Graphene in Ionic Liquids. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.; Nisi, K.; Pepper, J.; Harvey, A.; Szydłowska, B.M.; Coleman, J.N.; Backes, C. Effect of Surfactant Choice and Concentration on the Dimensions and Yield of Liquid-Phase-Exfoliated Nanosheets. Chem. Mater. 2020, 32, 2852–2862. [Google Scholar] [CrossRef]
- Wang, S.; Yi, M.; Shen, Z. The Effect of Surfactants and Their Concentration on the Liquid Exfoliation of Graphene. RSC Adv. 2016, 6, 56705–56710. [Google Scholar] [CrossRef]
- Choi, E.Y.; Choi, W.S.; Lee, Y.B.; Noh, Y.Y. Production of Graphene by Exfoliation of Graphite in a Volatile Organic Solvent. Nanotechnology 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Neill, A.O.; Khan, U.; Nirmalraj, P.N.; Boland, J.; Coleman, J.N.; Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; et al. Graphene Dispersion and Exfoliation in Low Boiling Point Solvents Graphene Dispersion and Exfoliation in Low Boiling Point Solvents. J. Phys. Chem. 2011, 115, 5422–5428. [Google Scholar] [CrossRef]
- Liu, W.W.; Aziz, A. Review on the Effects of Electrochemical Exfoliation Parameters on the Yield of Graphene Oxide. ACS Omega 2022, 7, 33719–33731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Casiraghi, C.; Parvez, K. Electrochemical Exfoliation of 2D Materials beyond Graphene. Chem. Soc. Rev. 2024, 53, 3036–3064. [Google Scholar] [CrossRef] [PubMed]
- Achee, T.C.; Sun, W.; Hope, J.T.; Quitzau, S.G.; Sweeney, C.B.; Shah, S.A.; Habib, T.; Green, M.J. High-Yield Scalable Graphene Nanosheet Production from Compressed Graphite Using Electrochemical Exfoliation. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Salverda, M.; Thiruppathi, A.R.; Pakravan, F.; Wood, P.C.; Chen, A. Electrochemical Exfoliation of Graphite to Graphene-Based Nanomaterials. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Bellani, V.; Sun, J.; Palermo, V. Electrochemical Exfoliation of Graphite in H2SO4, Li2SO4and NaClO4solutions Monitored: In Situ by Raman Microscopy and Spectroscopy. Faraday Discuss. 2021, 227, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Am. Chem. Soc. 2008, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science (80-. ). 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Bhaviripudi, S.; Jia, X.; Dresselhaus, M.S.; Kong, J. Role of Kinetic Factors in Chemical Vapor Deposition Synthesis of Uniform Large Area Graphene Using Copper Catalyst. Nano Lett. 2010, 10, 4128–4133. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.J.; Güneş, F.; Kim, K.K.; Kim, E.S.; Han, G.H.; Kim, S.M.; Shin, H.; Yoon, S.M.; Choi, J.Y.; Park, M.H.; et al. Synthesis of Large-Area Graphene Layers on Poly-Nickel Substrate by Chemical Vapor Deposition: Wrinkle Formation. Adv. Mater. 2009, 21, 2328–2333. [Google Scholar] [CrossRef]
- Kostogrud, I.A.; Trusov, K. V.; Smovzh, D. V. Influence of Gas Mixture and Temperature on AP-CVD Synthesis of Graphene on Copper Foil. Adv. Mater. Interfaces 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Memon, N.K.; Tse, S.D.; Chhowalla, M.; Kear, B.H. Role of Substrate, Temperature, and Hydrogen on the Flame Synthesis of Graphene Films. Proc. Combust. Inst. 2013, 34, 2163–2170. [Google Scholar] [CrossRef]
- Zhang, C.; Man, B.Y.; Jiang, S.Z.; Yang, C.; Liu, M.; Chen, C.S.; Xu, S.C.; Feng, D.J.; Bi, D.; Liu, F.Y.; et al. Facile Synthesis of Graphene on Single Mode Fiber via Chemical Vapor Deposition. Appl. Surf. Sci. 2014, 307, 327–332. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical Vapour Deposition of Graphene—Synthesis, Characterisation, and Applications: A Review. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mehta, B.R. A Parametric Study on the Influence of Synthesis and Transfer Conditions on the Quality of Graphene. J. Nanosci. Nanotechnol. 2017, 17, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical Vapour Deposition. Primer 2021, 1. [Google Scholar] [CrossRef]
- Thodkar, K.; Plodinec, M.; Gramm, F.; Kunze, K. ISCOPEM2D V1.0: An In Situ Method to Characterize and Compare Chemical Vapor Deposition Graphene Films Using Quality Matrix Approaches. Phys. Status Solidi - Rapid Res. Lett. 2024, 18. [Google Scholar] [CrossRef]
- Donglah, N.A.B.H.; Adenan, N.B.M.; Sabet, M. Effects of Pressure Variations in the Quality of Graphene Production through Chemical Vapor Deposition by Regression. AIP Conf. Proc. 2023, 2643, 10–14. [Google Scholar] [CrossRef]
- Tursunkulov, O.; Allabergenov, B.; Abidov, A.; Kim, S.-Y.; Jeon, H.-W.; Jeong, S.-W.; Kim, S. Comparison Characteristic of Large Area Graphene Films Grown by Chemical Vapor Deposition with Nano-Graphite Structures. Int. J. Mater. Mech. Manuf. 2013, 324–327. [Google Scholar] [CrossRef]
- Lee, B.; Chu, W.; Li, W. Effects of Process Parameters on Graphene Growth via Low-Pressure Chemical Vapor Deposition. J. Micro Nano-Manufacturing 2020, 8, 1–7. [Google Scholar] [CrossRef]
- KAHYAOĞLU, A.; ÜNLÜ, Ö. Graphene Growth in Different Thickness by Chemical Vapor Deposition Method. Düzce Üniversitesi Bilim ve Teknol. Derg. 2023, 11, 787–798. [Google Scholar] [CrossRef]
- Arjmandi-Tash, H.; Lebedev, N.; van Deursen, P.M.G.; Aarts, J.; Schneider, G.F. Hybrid Cold and Hot-Wall Reaction Chamber for the Rapid Synthesis of Uniform Graphene. Carbon N. Y. 2017, 118, 438–442. [Google Scholar] [CrossRef]
- Jia, K.; Ci, H.; Zhang, J.; Sun, Z.; Ma, Z.; Zhu, Y.; Liu, S.; Liu, J.; Sun, L.; Liu, X.; et al. Superclean Growth of Graphene Using a Cold-Wall Chemical Vapor Deposition Approach. Angew. Chemie - Int. Ed. 2020, 59, 17214–17218. [Google Scholar] [CrossRef] [PubMed]
- Bosc, A.; Ladron-de-Guevara, A.; Pedros, J.; Martinez, J.; Fandan, R.; Calle, F. Parameter Space for Graphene Chemical Vapour Deposition in Cold-Wall Reactors under High Precursor Flux. Cryst. Growth Des. 2023, 23. [Google Scholar] [CrossRef]
- Das, S.; Drucker, J. Nucleation and Growth of Single Layer Graphene on Electrodeposited Cu by Cold Wall Chemical Vapor Deposition. Nanotechnology 2017, 28. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.H.; Huang, L.; Ji, L.C.; Wang, T.; Ling, B.; Yang, H.F. Few-Layer Graphene Direct Deposition on Ni and Cu Foil by Cold-Wall Chemical Vapor Deposition. Proc. - 2010 8th Int. Vac. Electron Sources Conf. Nanocarbon, IVESC 2010 NANOcarbon 2010 2010, 467–468. [CrossRef]
- Deng, B.; Liu, Z.; Peng, H. Toward Mass Production of CVD Graphene Films. Adv. Mater. 2019, 31, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shen, C.-M.; Tian, Y.; Wang, G.-Q.; Lin, S.-X.; Zhang, Y.; Gu, C.-Z.; Li, J.-J.; Gao, H.-J. Influence of Reaction Parameters on Synthesis of High-Quality Single-Layer Graphene on Cu Using Chemical Vapor Deposition. Chinese Phys. B 2014, 23, 096803. [Google Scholar] [CrossRef]
- Anisur, M.R.; Raman, R.K.S.; Banerjee, P.C.; Al-Saadi, S.; Arya, A.K. Review of the Role of CVD Growth Parameters on Graphene Coating Characteristics and the Resulting Corrosion Resistance. Surf. Coatings Technol. 2024, 487, 130934. [Google Scholar] [CrossRef]
- Fauzi, F.B.; Ismail, E.; Ani, M.H.; Syed Abu Bakar, S.N.; Mohamed, M.A.; Majlis, B.Y.; Md Din, M.F.; Azam Mohd Abid, M.A. A Critical Review of the Effects of Fluid Dynamics on Graphene Growth in Atmospheric Pressure Chemical Vapor Deposition. J. Mater. Res. 2018, 33, 1088–1108. [Google Scholar] [CrossRef]
- Shinde, D.B.; Chaturvedi, P.; Vlassiouk, I. V.; Smirnov, S.N. Unique Role of Dimeric Carbon Precursors in Graphene Growth by Chemical Vapor Deposition. Carbon Trends 2021, 5, 100093. [Google Scholar] [CrossRef]
- Wang, J. Bin; Ren, Z.; Hou, Y.; Yan, X.L.; Liu, P.Z.; Zhang, H.; Zhang, H.X.; Guo, J.J. A Review of Graphene Synthesis at Low Temperatures by CVD Methods. Xinxing Tan Cailiao/New Carbon Mater. 2020, 35, 193–208. [Google Scholar] [CrossRef]
- Zafar, M.A.; Jacob, M. V. Plasma-Based Synthesis of Graphene and Applications: A Focused Review; Springer Nature Singapore, 2022; Vol. 6; ISBN 0123456789.
- Woehrl, N.; Ochedowski, O.; Gottlieb, S.; Shibasaki, K.; Schulz, S. Plasma-Enhanced Chemical Vapor Deposition of Graphene on Copper Substrates. AIP Adv. 2014, 4, 0–9. [Google Scholar] [CrossRef]
- Bekdüz, B.; Beckmann, Y.; Mischke, J.; Twellmann, J.; Mertin, W.; Bacher, G. Graphene Growth through a Recrystallization Process in Plasma Enhanced Chemical Vapor Deposition. Nanotechnology 2018, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Pei, Y.H.; Wang, X.; Wang, H.; Meng, Q.N.; Tian, H.W.; Zheng, X.L.; Zheng, W.T.; Liu, Y.C. Synthesis of Graphene on a Polycrystalline Co Film by Radio-Frequency Plasma-Enhanced Chemical Vapour Deposition. J. Phys. D. Appl. Phys. 2010, 43. [Google Scholar] [CrossRef]
- Lee, S.; Park, W.K.; Yoon, Y.; Baek, B.; Yoo, J.S.; Kwon, S. Bin; Kim, D.H.; Hong, Y.J.; Kang, B.K.; Yoon, D.H.; et al. Quality Improvement of Fast-Synthesized Graphene Films by Rapid Thermal Chemical Vapor Deposition for Mass Production. Mater. Sci. Eng. B 2019, 242, 63–68. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Lin, K.; Huang, Y. Laser-Assisted Chemical Vapor Deposition Setup for Fast Synthesis of Graphene Patterns. Rev. Sci. Instrum. 2017, 88. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.; Coletti, C.; Starke, U. Structural and Electronic Properties of Epitaxial Graphene on SiC(0001): A Review of Growth, Characterization, Transfer Doping and Hydrogen Intercalation. J. Phys. D. Appl. Phys. 2010, 43. [Google Scholar] [CrossRef]
- Emtsev, K. V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Röhrl, J.; et al. Towards Wafer-Size Graphene Layers by Atmospheric Pressure Graphitization of Silicon Carbide. Nat. Mater. 2009, 8, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Ouerghi, A.; Silly, M.G.; Marangolo, M.; Mathieu, C.; Eddrief, M.; Picher, M.; Sirotti, F.; El Moussaoui, S.; Belkhou, R. Large-Area and High-Quality Epitaxial Graphene on off-Axis Sic Wafers. ACS Nano 2012, 6, 6075–6082. [Google Scholar] [CrossRef] [PubMed]
- De Heer, W.A.; Berger, C.; Ruan, M.; Sprinkle, M.; Li, X.; Hu, Y.; Zhang, B.; Hankinson, J.; Conrad, E. Large Area and Structured Epitaxial Graphene Produced by Confinement Controlled Sublimation of Silicon Carbide. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16900–16905. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.; Coletti, C.; Iwasaki, T.; Zakharov, A.A.; Starke, U. Quasi-Free-Standing Epitaxial Graphene on SiC Obtained by Hydrogen Intercalation. Phys. Rev. Lett. 2009, 103, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tzalenchuk, A.; Lara-Avila, S.; Kalaboukhov, A.; Paolillo, S.; Syväjärvi, M.; Yakimova, R.; Kazakova, O.; Janssen, T.J.B.M.; Fal’Ko, V.; Kubatkin, S. Towards a Quantum Resistance Standard Based on Epitaxial Graphene. Nat. Nanotechnol. 2010, 5, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hwang, J.; Shields, V.B.; Tiwari, S.; Spencer, M.G.; Lee, J.W. SiC Surface Orientation and Si Loss Rate Effects on Epitaxial Graphene. Nanoscale Res. Lett. 2012, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Zhang, Y.; Lei, T.; Guo, H.; Wang, Y.; Tang, X.; Wang, H. Raman Analysis of Epitaxial Graphene on 6H-SiC (0001) Substrates under Low Pressure Environment. J. Semicond. 2011, 32. [Google Scholar] [CrossRef]
- Al-Temimy, A.; Riedl, C.; Starke, U. Growth and Characterization of Epitaxial Graphene on SiC Induced by Carbon Evaporation. Mater. Sci. Forum 2010, 645–648, 593–596. [Google Scholar] [CrossRef]
- Lei, T.M.; Deng, P.F.; Zhang, Y.M.; Guo, H. Epitaxial Graphene Growth on 6H-SiC (0001) Substrate by Confinement Controlled Sublimation of Silicon Carbide. Adv. Mater. Res. 2013, 709, 62–65. [Google Scholar] [CrossRef]
- Robinson, Z.R.; Jernigan, G.G.; Bussmann, K.M.; Nyakiti, L.O.; Garces, N.Y.; Nath, A.; Wheeler, V.D.; Myers-Ward, R.L.; Gaskill, D.K.; Eddy, C.R. Graphene Growth on SiC(000-1): Optimization of Surface Preparation and Growth Conditions. Carbon Nanotub. Graphene, Emerg. 2D Mater. Electron. Photonic Devices VIII 2015, 9552, 95520Y. [Google Scholar] [CrossRef]
- Vesapuisto, E.; Kim, W.; Novikov, S.; Lipsanen, H.; Kuivalainen, P. Growth Temperature Dependence of the Electrical and Structural Properties of Epitaxial Graphene on SiC(0001). Phys. Status Solidi Basic Res. 2011, 248, 1908–1914. [Google Scholar] [CrossRef]
- Palmer, J.; Kunc, J.; Hu, Y.; Hankinson, J.; Guo, Z.; Berger, C.; De Heer, W.A. Controlled Epitaxial Graphene Growth within Removable Amorphous Carbon Corrals. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Göckeritz, R.; Schmidt, D.; Beleites, M.; Seifert, G.; Krischok, S.; Himmerlich, M.; Pezoldt, J. High Temperature Graphene Formation on Capped and Uncapped SiC. Mater. Sci. Forum 2011, 679–680, 785–788. [Google Scholar] [CrossRef]
- Mishra, N.; Boeckl, J.J.; Tadich, A.; Jones, R.T.; Pigram, P.J.; Edmonds, M.; Fuhrer, M.S.; Nichols, B.M.; Iacopi, F. Solid Source Growth of Graphene with Ni-Cu Catalysts: Towards High Quality in Situ Graphene on Silicon. J. Phys. D. Appl. Phys. 2017, 50. [Google Scholar] [CrossRef]
Figure 1.
Graphene Structure Pictures [
4].
Figure 1.
Graphene Structure Pictures [
4].
Figure 2.
Graphite to Graphene Schematic Diagram [
10].
Figure 2.
Graphite to Graphene Schematic Diagram [
10].
Figure 3.
Top Down and Bottom Up Method Schema Diagram [
15].
Figure 3.
Top Down and Bottom Up Method Schema Diagram [
15].
Table 1.
Summary of characterization data produced by sonication methods.
Table 1.
Summary of characterization data produced by sonication methods.
| Methods |
FTIR |
SEM |
Raman |
XRD |
Ref. |
| Sonication during oxidation (Hummers method) |
Decrease in O–H and C=O functional groups due to strong sonication |
Flexible sheet structure with lower oxidation |
Increased (many defects) |
Intense (002) peak at 2θ = 26.5°, indicating dominant graphite phase |
[26] |
| Sonication for 1–5 hours (with Tween 80) |
– |
Lateral size decreased from ~5 μm to 317 nm |
Ratio increased gradually with longer sonication time |
– |
[27] |
| Sonication for 10/20 minutes (30/50 W) |
O–H and COOH peak intensities decreased (sample S1 to S4) |
Morphology becomes increasingly deformed (S1 to S4) |
Increased from 0.84 to 0.95, indicating increased structural disorder |
(002) peak shifted from 2θ = 11.34° to 11.14° |
[28] |
| Sonication for 15–45 minutes (electrochemical method) |
Appearance of C–O–H vibration, indicating the presence of hydroxyl group |
– |
– |
(002) peak intensity weakened; crystallite size decreased |
[29] |
| Sonication for 8.5 hours (ethanol: water = 20:80) |
Peaks observed O–H, C=C, and C–H |
Spherical morphology with particle size ~23–41 nm |
ID/IG ratio approximately 0.65, suggesting a moderate level of defects |
Broad (002) peak at 2θ ~25°; crystallite size ~20 nm |
[30] |
Table 2.
Summary of characterization data produced by dry and wet ball milling methods.
Table 2.
Summary of characterization data produced by dry and wet ball milling methods.
| Wet Ball-Mill Method |
| Sample |
FTIR |
Morphology (SEM/TEM) |
Raman (ID/IG) |
XRD |
Ref. |
| Graphite + DMF |
C=O stretching (~1700 cm⁻¹) |
Thin sheets with folded edges; few-layer structure (0.8–1.8 nm) |
Increased, indicating higher disorder due to milling |
(002) peak broadened |
[34] |
| Graphite + Water + KClO₄ |
C–O stretching (~1060 cm⁻¹) |
Small layered nanosheets, graphene oxide (GO) formed |
Increased with longer milling time |
(002) peak broadened |
[31] |
| Graphite + Water |
C=O functional group observed |
Large aggregates (BOTTOM60), finer sheets (TOP60); few-layer structure |
High value (TOP60), suggesting a small sheet size and more defects |
- |
[35] |
| Graphite + Ethanol: Water (7:3) |
- |
Graphene-encapsulated SiC; few-layer structure |
Decreased with increasing speed, indicating improved quality |
Graphite peak intensity decreased |
[36] |
| Graphite + Water |
- |
More uniform particles; presence of individual sheets |
Lowest value at 500 rpm (0.221), indicating high-quality graphene |
(002) peak became sharper |
[37] |
| Dry Ball-Mill Method |
| Sample |
FTIR |
Morphology (SEM/TEM) |
Raman (ID/IG) |
XRD |
Ref. |
| Graphite |
- |
Nanoparticles with irregular shapes |
Increased, suggesting greater defect formation |
Crystallinity decreased (weakened graphite peak) |
[38] |
| Graphite |
C=O, C–O functional groups |
Shaft-like structure with reduced particle size |
Increased, reflecting higher structural disorder |
Graphite peak intensity decreased |
[39] |
| Graphite |
C=O, OH, COOH groups |
Thin sheets with open structure; <10 layers |
Increased, indicating defect generation during milling |
Graphite peaks broadened |
[40] |
| Graphite |
OH, C=O groups |
Rough surface morphology; reduced particle size |
Increased (from 0.21 to 0.97), supporting oxidation process |
2θ peaks shifted and broadened |
[41] |
| Graphite |
- |
Thin and layered flake morphology |
- |
2θ peaks shifted and broadened |
[32] |
Table 3.
Summary of characterization data produced by fluid dynamics methods.
Table 3.
Summary of characterization data produced by fluid dynamics methods.
| Parameters |
VFD |
PFD |
MFD |
| Thickness |
Ranges from < 1 nm to > 20 nm |
Up to 79% ≤ 1.5 nm (after 8 hours at 15 MPa) |
Average ~1.5 nm; up to 92% ≤ 1.5 nm (after 3 hours) |
| Number of Layers |
1 to > 20 layers |
≤ 5 layers: 29% (0.5 h), 63% (4 h), 79% (8 h) |
Average < 5 layers; stable across various exfoliation durations |
| Lateral Size / Area |
< 1 μm |
Over 85% of flakes < 0.1 μm² (after 8 hours) |
Average ~320 nm (AFM); ~0.5 μm (Raman in protein medium) |
| Thickness Distribution |
Uneven; limited data available |
Becomes thinner and more uniform over time |
Remains stable around 1.5 nm; shifts toward thinner layers |
| Defect (Raman/XPS) |
Minimal defects |
Low defect levels, mainly at the edges |
Very low defect levels; basal planes largely defect-free |
Table 4.
Summary of characterization data produced by supercritical fluid methods.
Table 4.
Summary of characterization data produced by supercritical fluid methods.
| Sample |
AFM |
Raman |
XRD |
Ref. |
| Graphite + SC-CO₂ |
>10 layers |
Weak 2D peak; high ID/IG ratio indicating limited exfoliation |
Intense (002) peak with slight broadening, indicating minor delamination |
[47] |
| Graphite + SC-CO₂ |
Majority <3 layers (yield ~28%) |
88% <3 layers; sharp 2D peak confirms few-layer graphene |
Clear graphene structure; no signs of oxidation |
[48] |
| Graphite + SC ethanol |
~1.0–1.2 nm thickness; 6–10% monolayer content; stable |
Low ID/IG ratio (~0.17); symmetric 2D peak at 2684 cm⁻¹ |
(002) peak intensity decreased; increased interlayer spacing |
[49] |
| Graphite + SC ethanol |
Few layers (maximum yield ~18.5%) |
Slight increase in ID/IG ratio; minor defects introduced |
Decrease in (002) peak intensity; successful exfoliation |
[50] |
Table 5.
Summary of characterization data produced by detonation methods.
Table 5.
Summary of characterization data produced by detonation methods.
| Methods |
TEM |
Raman (ID/IG) |
XRD (2θ) |
Ref. |
| C₂H₂ + O₂ gas detonation (ratio 0.4–0.8) |
2–3 layers; monolayers observed; lateral size increases with higher O₂ ratio |
Decreases from ~1.33 to ~0.28, indicating reduced defects and improved crystallinity |
(002) peak at 26.05°, close to graphite (26.6°), indicating preserved graphite structure |
[53] |
| O₂/C₂H₂ detonation (O/C ratio 0.25–0.75) |
8–30 layers; turbostratic structure; lateral size 20–200 nm |
Decreases when O/C > 0.5, suggesting fewer defects and a more ordered structure |
(002) peak shifts from 25.33° to 25.74°, lower than graphite, indicating increased interlayer spacing |
[52] |
| Solid explosive: CaCO₃ + Mg + RDX |
<10 layers; transparent and crumpled sheets |
~0.26, indicating very few defects and high-quality graphene |
(002) peak at 26.04°, close to graphite, suggesting good crystallinity |
[51] |
| GO to ER-GO (thermal reduction at 100°C) |
Thin, transparent sheets |
No numerical value reported; D and G bands are present, indicating moderate defect density |
GO: 7.9° (d ≈ 1.09 nm); ER-GO: approximately 26.3°, indicating partial restoration of graphite-like structure |
[54] |
Table 6.
Summary of characterization data produced by oxidation-reduction methods.
Table 6.
Summary of characterization data produced by oxidation-reduction methods.
| Oxidation |
Reduction |
FTIR |
Raman (ID/IG) |
XRD |
Ref. |
| Thermal |
Thermal |
High intensity of –OH, C=O, and C–O functional groups |
Decreases with increasing temperature and time |
(002) peak at 26.5°, interlayer spacing d ≈ 3.36 Å |
[75] |
| Chemical (Hummers) |
Chemical (NaBH₄) |
High –OH and C=O intensity; epoxy group is reduced |
0.98 |
Interlayer spacing d = 0.388 nm; average number of layers ≈ 1.4; crystallite size ≈ 22 nm |
[70] |
| Electrochemical |
Chemical (hydrazine) |
Presence of –OH, C–O–C, C–C, and C=O groups |
0.849 |
2θ = 26.52°; grain size ≈ 23 nm |
[76] |
| Chemical (Hummers) |
Electrochemical |
Decrease in –OH, C=O, and C–O functional groups |
1.24 |
Interlayer spacing d = 0.3554 nm |
[77] |
| Chemical (Hummers) |
Chemical (Ascorbic acid) |
Decrease in C=O, C–OH, and C–O–C; partial restoration of sp² structure |
Decreases from 0.805 to 0.788 with increasing temperature |
GO peak at ~11.9°; rGO peaks between 24.8° and 25.2°; d-spacing ≈ 3.55 Å |
[78] |
| Chemical (Hummers) |
Chemical (hydrazine) |
C=O and C–O functional groups are reduced |
2.23 |
GO: ~10.9° (001); rGO: ~26.4° (002); interlayer spacing decreases |
[79] |
| Chemical (Hummers) |
Thermal |
C=O and C–OH groups are reduced |
- |
GO: ~10.5°; rGO: 24.7° to 26.2°; d-spacing for GO ≈ 0.84 nm; for rGO ≈ 0.34–0.36 nm |
[80] |
| Chemical (Hummers) |
Chemical (Zn metal) |
Decrease in C–O–C, C–OH, and C=O groups; oxygen-containing groups are weakened |
1.01 |
GO (002) peak at ~26°; additional ZnO peak with wurtzite structure observed |
[81] |
Table 7.
Summary of the effect of gas type on the quality of graphene via arc discharge methods.
Table 7.
Summary of the effect of gas type on the quality of graphene via arc discharge methods.
| Types of Gas |
Result |
Ref. |
| Ar |
~12% graphene sheets comprising 1–10 layers; interlayer spacing of 0.34–0.39 nm |
[85] |
| Ar |
High-quality, very pure 4-layer graphene |
[86] |
| H2
|
2–4-layer graphene, free of nanotube contaminants |
[87] |
| H2-N2
|
Up to 5 layers of graphene, low defect density, suitable for mass production |
[88] |
Table 8.
Summary of the effect current type on the quality of graphene via arc discharge methods.
Table 8.
Summary of the effect current type on the quality of graphene via arc discharge methods.
| Aspects |
Air conditioning |
DC |
Ref. |
| Structure |
Nanohorns, carbon onions, 1–5 layers of graphene |
Carbon nanotubes, 2–4 layers of graphene |
[89],[88],[86],[87] |
| Purity |
Very pure; minimal carbon contamination |
Less pure; mixed with non-graphitic carbon |
[89], [86] |
| Process & Control |
Flexible, frequency-controlled process |
Less flexible; dependent on gas, metal catalyst & pressure |
[89],[90],[91]
|
| Graphene Quality |
Low defects, optimal with N₂/H₂ mixture |
Minimal defects, optimal with argon gas |
[88],[86] |
| Scalability |
Large-scale, suitable for industry |
Suitable for small–to medium-scale and specialized applications |
[88],[91] |
| Efficiency & Results |
High efficiency when optimizing gas composition & frequency |
Generally lower efficiency than the air-conditioning method |
[88],[90] |
Table 9.
Summarizes the impact of pressure using arch discharge techniques on graphene quality.
Table 9.
Summarizes the impact of pressure using arch discharge techniques on graphene quality.
| Pressure |
Number of Layers |
Purity |
Ref. |
| Low |
Formation of nanohorns and nanospheres, no coated graphene layers |
Low purity, a hybrid of various carbon nanostructures |
[92] |
| Moderate |
Approximately 4-layer graphene, thermally and structurally stable |
High purity, well-ordered structure without toxic intercalates |
[86] |
| High |
Graphene with 2–10 layers, pronounced condensation observed |
High purity, uniform, defect-free graphene structure |
[92] |
Table 10.
Summary of reaction temperature against quality graphene via arch discharge methods.
Table 10.
Summary of reaction temperature against quality graphene via arch discharge methods.
| Temperature |
Number of Layers |
Purity |
Ref. |
| Low |
Single-layer graphene grown at low energy |
High purity, low-carbon atomic mobility, minimal defects |
[93] |
| Moderate |
2–4 layer graphene with balanced growth energy |
High purity, stable plasma, minimal fouling |
[86], [87] |
| High |
> 4-layer graphene; rapid growth due to high energy |
Lower purity, increased mobility lead to more defects and contamination |
[93], [94] |
Table 11.
Summary of reaction time against quality graphene via arc discharge methods.
Table 11.
Summary of reaction time against quality graphene via arc discharge methods.
| Duration |
Number of Layers |
Purity |
Ref. |
| Short |
2–4 layer graphene |
High purity; slightly distorted structure |
[87], [95] |
| Moderate |
4–6 layer graphene |
High purity; some layer non-uniformity and minor structural flaws |
[82], [94] |
| Long |
Up to ~20 layers graphene |
Decreased purity; more defects, mitigable with buffer gas |
[83] |
Table 12.
Summary of reaction chamber type against quality graphene via arch discharge methods.
Table 12.
Summary of reaction chamber type against quality graphene via arch discharge methods.
| Chamber Type |
Number of Layers |
Purity |
Ref. |
| Closed chamber |
Approximately 4 layers of graphene |
High purity, minimal defects |
[82],[86] |
| Semi-open chamber |
Moderate, depending on parameters |
Moderate purity, possible contamination |
[82] |
| Open chamber |
Multilayer graphene (many layers) |
Low purity, high defect density |
[96] |
Table 13.
Summary of characteristics of GNR against various methods for unzipping CNT.
Table 13.
Summary of characteristics of GNR against various methods for unzipping CNT.
| Method |
Number of Layers |
Purity |
Ref. |
| Oxidative |
Single to multi-layer graphene |
Multiple defects; presence of oxygen-containing functional groups |
[100],[106],[107] |
| Catalytic |
4 – 8 layers graphene |
Slight defects, traces of residual metal catalyst |
[103] |
| Electrochemical |
Single to multi-layer graphene |
High purity, minimal defects |
[108] |
| Sonochemistry |
Predominantly bilayer graphene, with some monolayer |
Smooth edges, low interference |
[109] |
Table 14.
Summary of ionic liquids type against characteristic graphene via LPE.
Table 14.
Summary of ionic liquids type against characteristic graphene via LPE.
| Ionic Liquids |
SEG (mg/mL) |
Number of Layers |
| [C₄C₁im][Ntf₂] |
~1.8 |
≤5 |
| [Pyrr₄,₁][Ntf₂] |
~1.8 |
≤5 |
| [N₄,₁,₁,₁][Ntf₂] |
~1.8 |
≤5 |
| [C₁₀C₁im][Ntf₂] |
<1.8 |
≤5 |
| [BnzmC₁im][Ntf₂] |
<1.8 |
≤5 |
| [C₄C₁im][C(CN)₃] |
<0.5 |
>30 |
| [C₂C₁im][N(CN)₂] |
<0.5 |
≤5 |
| [C₄C₁im][C₁SO₄] |
<0.5 |
≤5 |
| [C₂C₁im][OTF] |
<0.5 |
<5 |
Table 15.
Summary of Surfactants on Characteristics of Graphene via LPE.
Table 15.
Summary of Surfactants on Characteristics of Graphene via LPE.
| Surfactant |
CG Max (mg/mL) |
Optimal Csur (mg/mL) |
CMC (mg/mL) |
| SDOC |
~0.10 |
~1.0 |
5.0 |
| SDBS |
~0.11 |
~0.7 |
0.7 |
| SDS |
~0.09 |
~2.0 |
2.3 |
| HTAB |
~0.12 |
~0.3 |
0.33 |
| Tween 80 |
~0.08 |
~0.015 |
0.0157 |
| Triton X-100 |
~0.29 |
~1.0 |
0.343 |
Table 16.
Summary of Solvent type against effectiveness via LPE.
Table 16.
Summary of Solvent type against effectiveness via LPE.
| Solvent |
Boiling Point (°C) |
Concentration (mg/mL) |
Result |
| Acetone |
56 |
~0.08 |
Low concentration, suitable for low-boiling dispersion. |
| Chloroform |
61 |
~0.5 |
Stable (≥75 % remains suspended after 100 h), produces medium-sized flakes. |
| Isopropanol |
82 |
~0.5 |
Highly stable (>90% fixed suspended >200 hours); produces good quality flakes |
| Cyclohexa-none |
156 |
~1.0 |
Effectively exfoliates graphene; high boiling point makes solvent removal challenging. |
| NMP |
204 |
~1.0 |
Effectively exfoliates graphene; high boiling point makes solvent removal challenging. |
| DMF |
153 |
~1.0 |
Effectively exfoliates graphene; high boiling point makes solvent removal challenging. |
Table 17.
Summary of temperature reactor conditions against graphene characterisation via CVD.
Table 17.
Summary of temperature reactor conditions against graphene characterisation via CVD.
| CVD Temperature |
Layers |
Defects |
Substrate Interactions |
Ref. |
| Low |
Slow film growth, non-uniform coverage |
High defects, insufficient energy |
Weak interactions, delamination potential. |
[132] |
| High |
Fast carbon diffusion, uniform and continuous graphene film |
Reduced defects, more perfect structure |
Strong interaction, adhesion and stability are better. |
[133], [134] |
| Ultra-High |
Rapid growth, increased film thickness |
Further reduced defects, though new defects may emerge |
Potential substrate etching and adverse interactions |
[135],[136] |
Table 18.
Summary of Pressure reactor conditions against graphene characterization via CVD.
Table 18.
Summary of Pressure reactor conditions against graphene characterization via CVD.
| CVD Pressure |
Coating & Uniformity |
Defects |
Substrate Interactions |
Ref. |
| APCVD |
Large area, fairly uniform, limited control |
High defect, decreased purity |
Strong substrate interaction, difficult to transfer |
[138],[133] |
| LPCVD |
Uniform, high-quality monolayer |
Low defects, high purity |
Weak interaction, easy to transfer |
[139] |
| Ultra-Vacuum |
Highly uniform, high precision |
Very low defects, optimal purity |
Minimal interaction, ideal for transfer |
[140] |
Table 19.
Summary of cold wall and hot wall configurations against graphene characterization via CVD.
Table 19.
Summary of cold wall and hot wall configurations against graphene characterization via CVD.
| Aspects |
Cold Wall CVD |
Hot Wall CVD |
Ref. |
| Number of Layers |
Uniform and thin layer formation |
Thicker layers, less uniform |
[142],[143] |
| Defects |
Low defects, high purity |
Higher defect density, reduced purity |
[142],[144] |
| Substrate Influence |
Better process control, high-quality graphene |
Greater substrate influence; increased defect formation |
[145],[133] |
Table 20.
Summary of deposition times against graphene characterization via CVD.
Table 20.
Summary of deposition times against graphene characterization via CVD.
| CVD Method |
Coating & Uniformity |
Defects |
Ref. |
| Continuous |
Uniform, suitable for large areas |
Low defect density, high material purity |
[146],[147] |
| Diconected |
Less uniform |
Higher defect density, lower purity |
[148] |
| Pulsed |
Controlled and tunable deposition |
Low defect density, high material purity |
[135],[136] |
Table 21.
Summary of gas flow configurations against graphene characterization via CVD.
Table 21.
Summary of gas flow configurations against graphene characterization via CVD.
| CVD System |
Gas Flow & Dynamics |
Defects & Purity |
Scalability |
Ref. |
| Open |
Continuous flow (methane, H₂), stable reaction |
Slight defects, high purity |
Suitable for large-scale, even gas distribution |
[138] |
| Closed |
Static gas, layer growth control |
High crystallinity & purity, sensitive to reaction conditions |
Less suitable for large-scale use without process optimization |
[150] |
Table 22.
Summary of CVD techniques against the quality and efficiency of graphene synthesis.
Table 22.
Summary of CVD techniques against the quality and efficiency of graphene synthesis.
| Method |
Defects & Purity |
Layer |
Efficiency |
Ref. |
| PECVD |
Low defects, high purity |
SLG–FLG (dependent on parameters) |
Fast, low temperature, suitable for large-scale |
[152],[153],
[154],[155] |
| TCVD |
Higher defect density; RT-CVD offers improvement |
Generally monolayer |
Slow, high temperature, RT-CVD is more efficient |
[156] |
| Laser-CVD |
Low defects with precise control |
Multi-layer (dependent on laser parameters) |
Fast, ideal for specific patterns |
[157] |
Table 23.
Summary of the effect of substrates on graphene characteristics via SiC methods.
Table 23.
Summary of the effect of substrates on graphene characteristics via SiC methods.
| Parameters |
Si-face |
C-Face |
| Number of layers |
1–2 layers (monolayer) |
Up to ~30 layers (multilayer) |
| Raman Spectrum |
ID/IG < 0.02 (sharp G and 2D peaks) |
ID/IG < 0.05, broader peaks with creases |
| Electron mobility |
Relatively low |
Relatively high |
Table 24.
Summary of Temperature growth against graphene characteristics via SiC methods.
Table 24.
Summary of Temperature growth against graphene characteristics via SiC methods.
| Temperature |
Number of Layers |
Electron Mobility |
Description |
| Low |
0 – 0.6 layers |
Very low (up to 81 cm²/Vs) |
Graphene is not fully formed; significant exposure of SiC surface remains |
| Optimal |
~1.2 – 1.4 layers |
Highest (~370 cm²/Vs) |
Nearly monolayer graphene, maximum mobility, uniform surface morphology |
| High |
>1.6 layers |
Decreasing (up to 77 cm²/Vs) |
Multilayer graphene, presence of grain boundaries and "giraffe stripe" patterns |
Table 25.
Summary of pressure effects against graphene characteristics via SiC methods.
Table 25.
Summary of pressure effects against graphene characteristics via SiC methods.
| Pressure |
Raman |
Surface Morphology |
Graphene Quality |
| Low Pressure (∼10⁻⁷ mbar) |
Weak G and 2D bands, dominant D band |
Rough surface with many defects |
Low-quality, non-uniform graphene |
| Inert Atmosphere (∼10⁻³ mbar) |
Strong G and 2D bands, D band nearly absent |
Smooth surface with uniform morphology |
High-quality, thin, and uniform graphene layers |
Table 26.
Summary of Catalyst effects against graphene characteristics via SiC methods.
Table 26.
Summary of Catalyst effects against graphene characteristics via SiC methods.
| Method |
Number of Layers |
Raman |
Surface Morphology |
Graphene Quality |
| No Catalyst |
6–7 layers |
G and 2D wideband, ID/IG > 0.4 |
Rough surface, many defects, non-uniform |
Low-quality, multilayer graphene with small crystallite |
| With Ni–Cu Catalyst |
Monolayer |
G and 2D sharp band, ID/IG ~0.24, 2D > G |
Smooth surface, uniform morphology |
High quality, uniform monolayer graphene with large crystallites (35–60 nm) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).