Submitted:
11 July 2025
Posted:
14 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
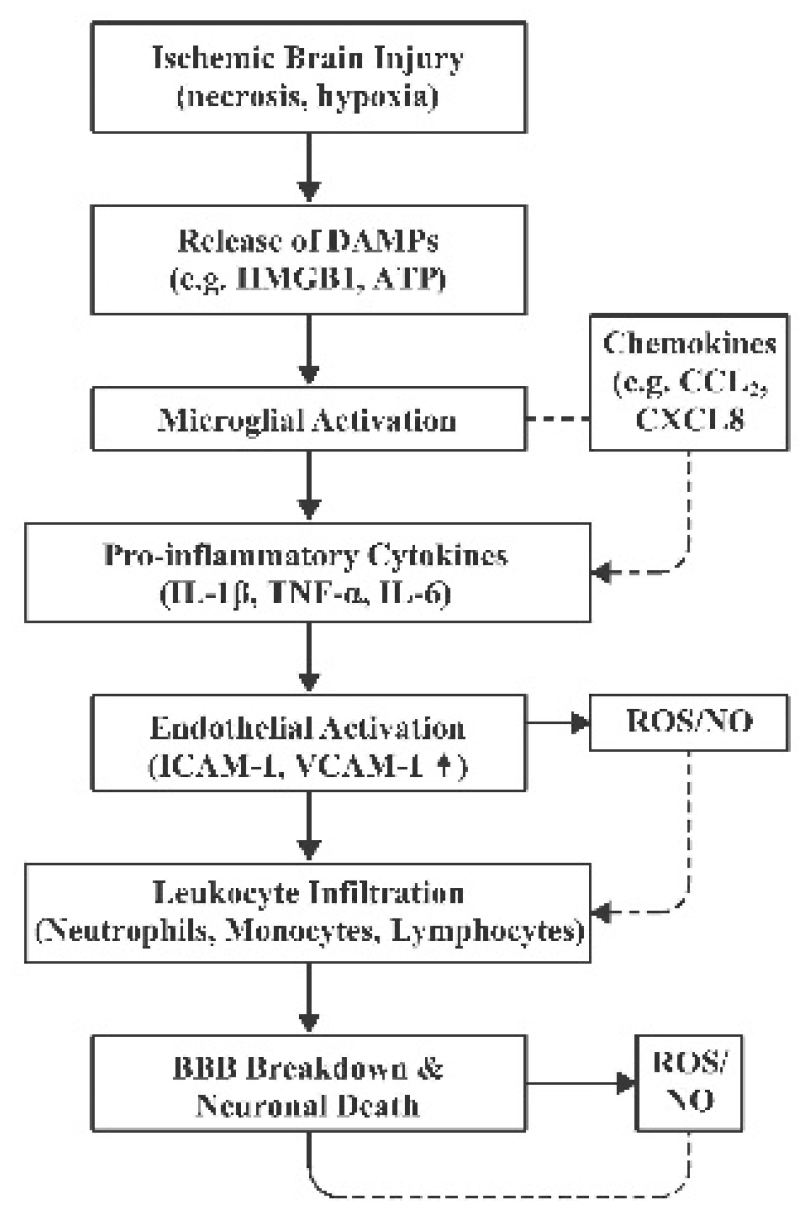
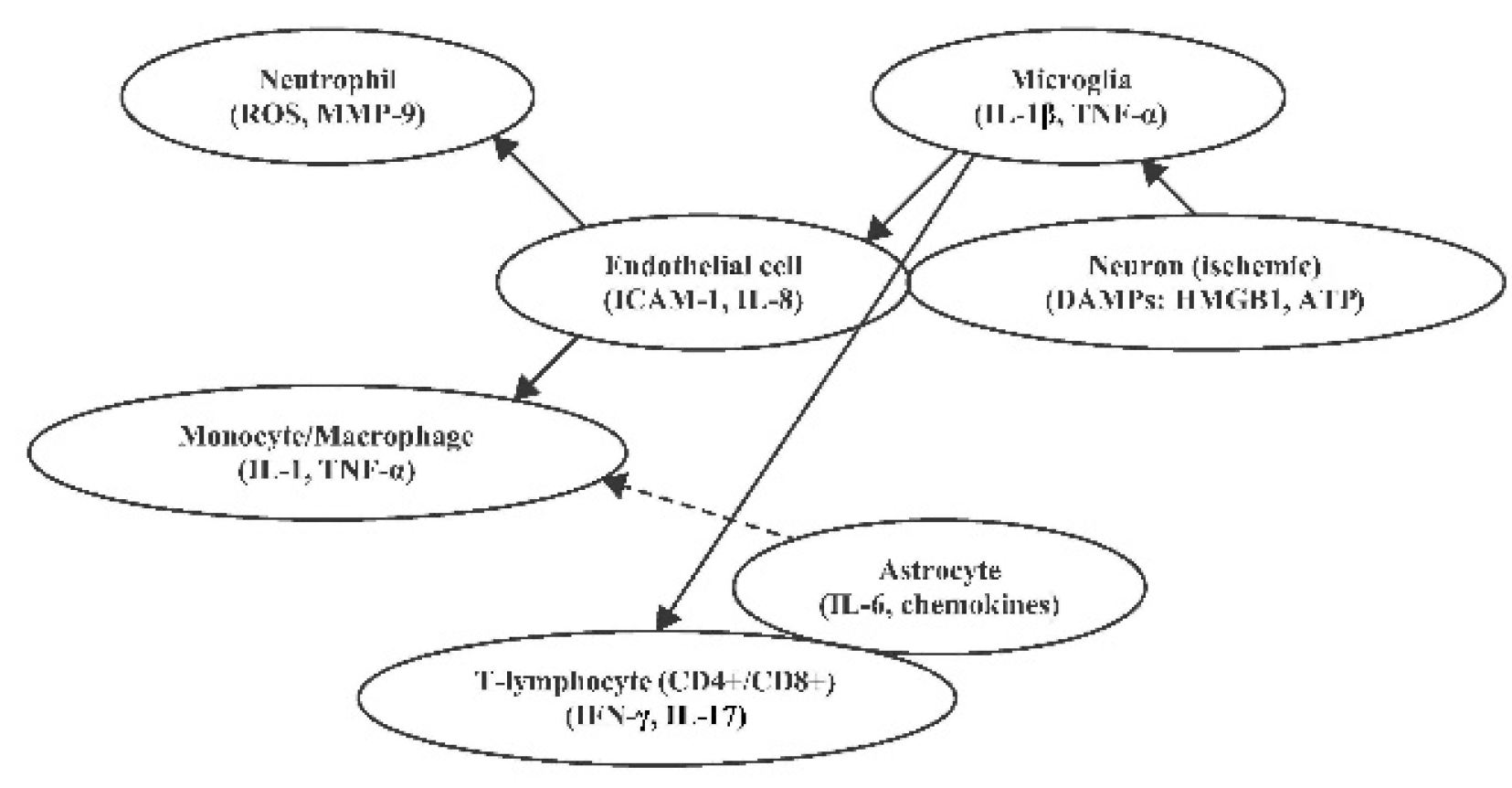
2. Inflammation and Immune Response in Ischemic Stroke
2.1. Key Inflammatory Biomarkers in Ischemic Stroke
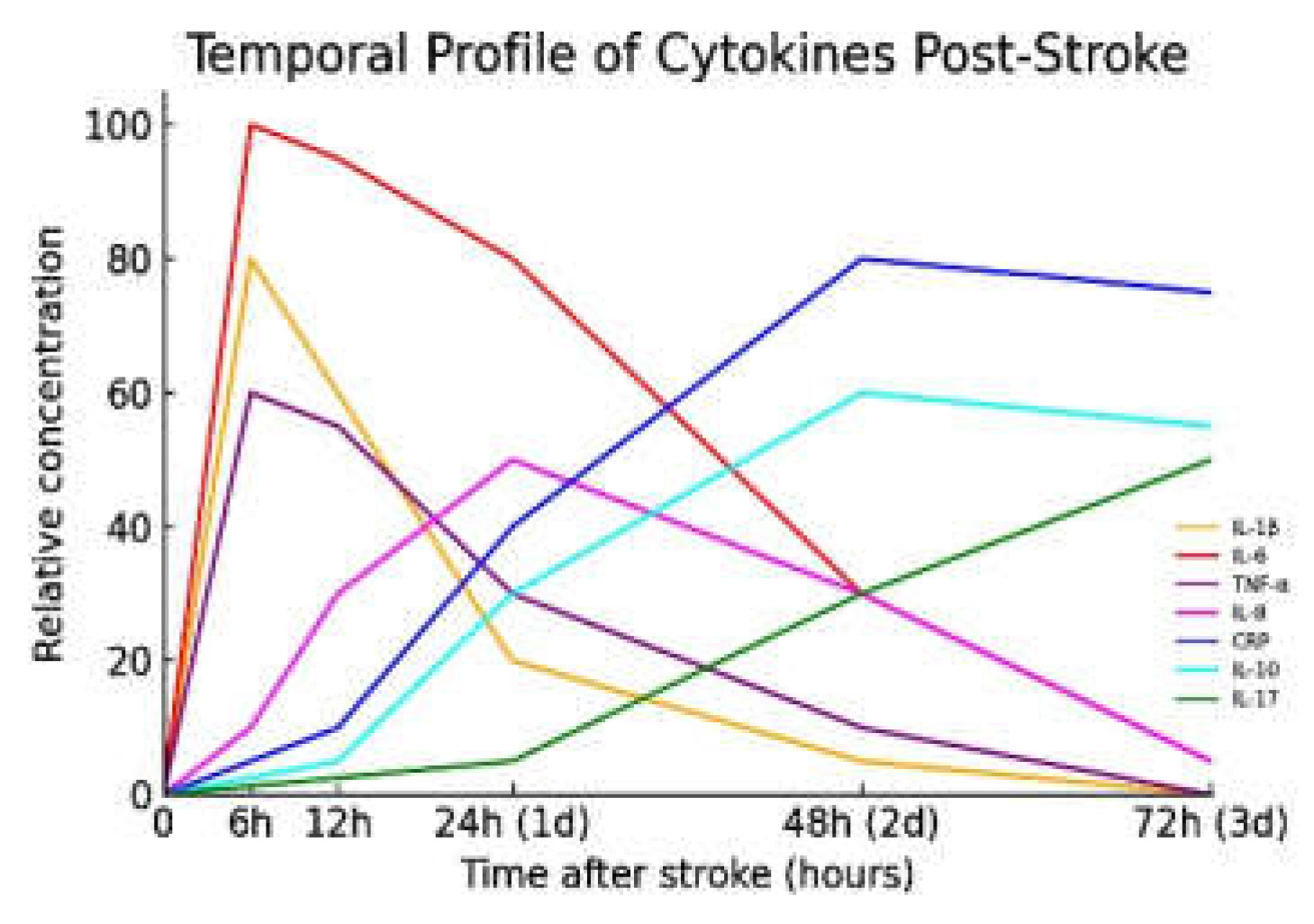
2.1.1. Interleukin-1β (IL-1β)
2.1.2. Tumour Necrosis Factor-α (TNF-α)
2.1.3. Interleukin-6 (IL-6)
2.1.4. C-Reactive Protein (CRP)
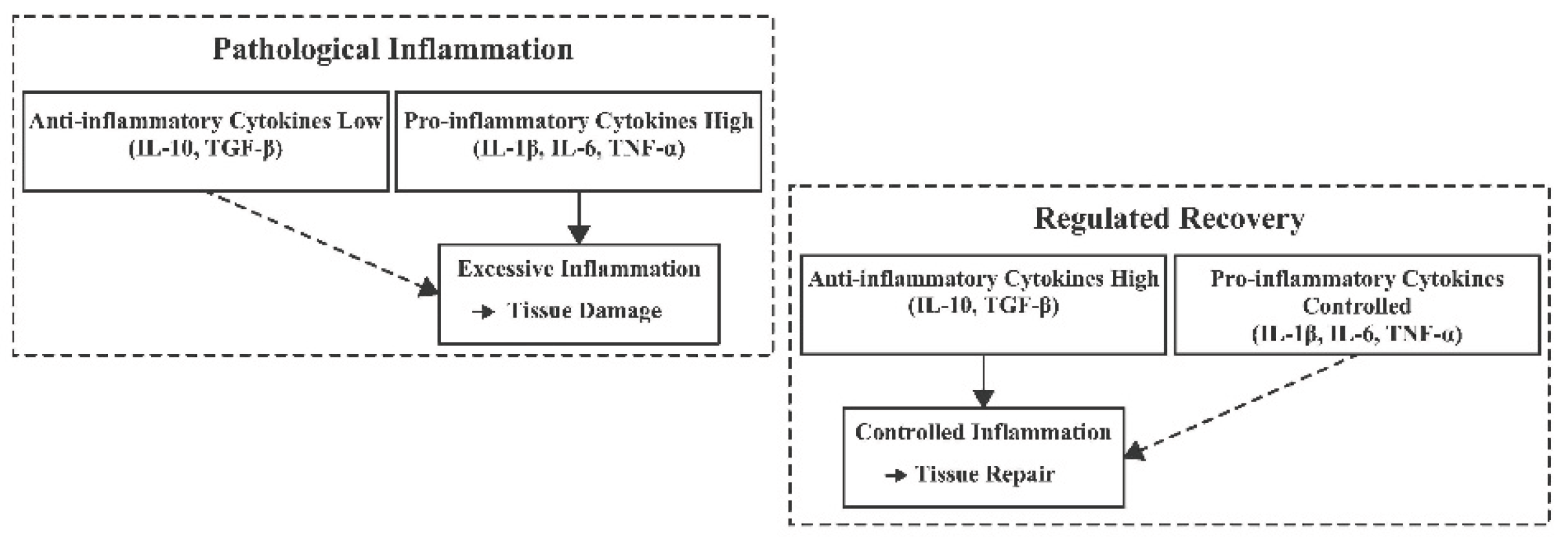
2.1.5. Interleukin-10 (IL-10)
2.2. Other Important Inflammatory Biomarkers
2.2.1. Interleukin-8 (CXCL8)
2.2.2. Interleukin-17 (IL-17). IL-17, predominantly produced by Th17 cells and γδ T cells, rises slightly later, typically 24–72 hours post-stroke, as part of the adaptive immune response. IL-17 amplifies inflammation by stimulating the release of IL-1β, TNF-α, and chemokines that recruit neutrophils [20,42,61]. It also directly impairs endothelial function and contributes to BBB disruption. Elevated IL-17 in plasma or CSF has been observed in patients with more severe strokes, and preclinical studies demonstrate that blocking IL-17 or IL-23 reduces infarct size and improves outcomes [6,30].2.2.3. Other relevant biomarkers:
3. Prognostic Value of Immune Biomarkers in Stroke
4. Therapeutic Modulation of Inflammation: Implications for Treatment and Prevention
5. Discussion and Future Directions
5.1. Personalized Inflammatory Profiling
5.2. Real-Time and Point-of-Care Inflammation Monitoring
5.3. Biomarker-Guided Immunotherapy Trials
5.4. Synergy with Neurorestorative Interventions
5.5. Inflammation as a Target for Primary Prevention
5.6. Translational and Clinical Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Adenosine Triphosphate |
| BBB | Blood–Brain Barrier |
| BDNF | Brain-Derived Neurotrophic Factor |
| CD | Cluster of Differentiation |
| CRP | C-Reactive Protein |
| CXCL8 | C-X-C Motif Chemokine Ligand 8 (also known as IL-8) |
| DAMPs | Damage-Associated Molecular Patterns |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| HMGB1 | High-Mobility Group Box 1 |
| hs-CRP | High-Sensitivity C-Reactive Protein |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IFN-γ | Interferon-Gamma |
| IL | Interleukin |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 (CXCL8) |
| IL-10 | Interleukin-10 |
| IL-17 | Interleukin-17 |
| IL-18 | Interleukin-18 |
| IL-21 | Interleukin-21 |
| IL-23 | Interleukin-23 |
| LFA-1 | Lymphocyte Function-Associated Antigen-1 |
| Mac-1 | Macrophage-1 Antigen |
| MMP-9 | Matrix Metalloproteinase-9 |
| mRS | Modified Rankin Scale |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| NLRP3 | NOD-, LRR- and Pyrin Domain-Containing Protein 3 |
| NO | Nitric Oxide |
| PLR | Platelet-to-Lymphocyte Ratio |
| ROS | Reactive Oxygen Species |
| SII | Systemic Immune-Inflammation Index |
| SIRI | Systemic Inflammation Response Index |
| SIS | Stroke-induced Immunosuppression |
| TGF-β | Transforming Growth Factor-Beta |
| Th1 | T Helper Type 1 |
| Th2 | T Helper Type 2 |
| Th17 | T Helper Type 17 |
| TIA | Transient Ischemic Attack |
| TNF-α | Tumour Necrosis Factor-Alpha |
| TNFR1/2 | Tumour Necrosis Factor Receptor 1/2 |
| Treg | Regulatory T Cell |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| VEGF | Vascular Endothelial Growth Factor |
| VLA-4 | Very Late Antigen-4 |
| Cytokine / Interleukin | Classification | Main Functions | 1. Clinical / Prognostic Implications |
2. Timing of Elevation Post-Stroke |
|---|---|---|---|---|
| Interleukin-1β (IL-1β) | Pro-inflammatory | Initiates inflammatory cascade; activates microglia and endothelial cells | Elevated levels associated with larger infarct size and worse neurological outcomes | Peaks within 6–24 hours |
| Interleukin-6 (IL-6) | Pro-inflammatory | Modulates acute phase response; promotes leukocyte recruitment | High levels correlate with increased stroke severity and poor functional outcomes | Rises within hours; remains elevated for days |
| Tumour Necrosis Factor-α (TNF-α) | Pro-inflammatory | Promotes apoptosis; disrupts blood-brain barrier | Elevated levels linked to increased infarct size and neurological deterioration | Peaks within 6–24 hours |
| Interleukin-10 (IL-10) | Anti-inflammatory | Suppresses pro-inflammatory cytokine production; limits immune response | Higher levels associated with reduced infarct size and better outcomes; excessive levels may increase infection risk | Increases within 24–72 hours |
| Transforming Growth Factor-β (TGF-β) | 3. Anti-inflammatory/ Regulatory |
Regulates immune response; promotes tissue repair | Contributes to neuroprotection and recovery; exact prognostic value in stroke is under investigation | Elevation timing varies; involved in later stages |
| Interleukin-17 (IL-17) | Pro-inflammatory | Recruits neutrophils; amplifies inflammatory response | Elevated levels may exacerbate neuroinflammation and tissue damage | Peaks within 24–72 hours |
| Interleukin-8 (IL-8) | Pro-inflammatory | Attracts neutrophils; promotes inflammation | Higher levels associated with increased infarct volume and poorer outcomes | Rises early; peaks within 24 hours |
| Interleukin-1α (IL-1α) | Pro-inflammatory | Acts as an alarmin; initiates sterile inflammation | Early release may contribute to initial inflammatory response; specific prognostic implications in stroke require further study | Released immediately upon cell injury |
References
- Endres, M.; Moro, M.A.; Nolte, C.H.; Dames, C.; Buckwalter, M.S.; Meisel, A. Immune Pathways in Etiology, Acute Phase, and Chronic Sequelae of Ischemic Stroke. Circ. Res. 2022, 130, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and Foe for Ischemic Stroke. J. Neuroinflammation 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, S.; He, Q.; Zhang, D.; Chang, J. The Role of Immune Cells in Post-Stroke Angiogenesis and Neuronal Remodeling: The Known and the Unknown. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, S.; Li, Y.; Sun, Y.; Xiong, X.; Hu, X.; Chen, J.; Qiu, S. Interleukins and Ischemic Stroke. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, H.; Woźniak, A.; Tafelska-Kaczmarek, A.; Kosinska, A.; Pawluk, M.; Sergot, K.; Grochowalska, R.; Kołodziejska, R. The Role of IL-6 in Ischemic Stroke. Biomolecules 2025, 15, 470. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, A.C.S.; Timóteo, R.P.; Bazan, R.; Silva, M.V.; Da Silveira Filho, L.G.; Ratkevicius, C.M.A.; De Assunção, T.S.F.; De Oliveira, A.P.S.; Luvizutto, G.J. Association of Proinflammatory Cytokine Levels with Stroke Severity, Infarct Size, and Muscle Strength in the Acute Phase of Stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106187. [Google Scholar] [CrossRef] [PubMed]
- Băcilă, C.-I.; Vlădoiu, M.-G.; Văleanu, M.; Moga, D.-F.-C.; Pumnea, P.-M. The Role of IL-6 and TNF-Alpha Biomarkers in Predicting Disability Outcomes in Acute Ischemic Stroke Patients. Life 2025, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, M.; Wang, J.; Hu, F. Association between Interleukin-6 Levels and Stroke: A Systematic Review and Meta-Analysis. J. Int. Med. Res. 2024, 52. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, H.; Woźniak, A.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. <p>The Role of Selected Pro-Inflammatory Cytokines in Pathogenesis of Ischemic Stroke</P>. Clin. Interv. Aging 2020, Volume 15, 469–484. [Google Scholar] [CrossRef]
- Pawluk, H.; Kołodziejska, R.; Grześk, G.; Kozakiewicz, M.; Woźniak, A.; Pawluk, M.; Kosinska, A.; Grześk, M.; Wojtasik, J.; Kozera, G. Selected Mediators of Inflammation in Patients with Acute Ischemic Stroke. Int. J. Mol. Sci. 2022, 23, 10614. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Gu, L.; Chen, L.; Hu, W.; Feng, X.; Qiu, F.; Fan, Z.; Chen, Q.; Qiu, J.; Shao, B. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Potential Predictors of Prognosis in Acute Ischemic Stroke. Front. Neurol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Liao, J.; Luo, X.; Chen, X. Prognostic Value of the Neutrophil-to-Lymphocyte Ratio in Older Patients with Acute Ischemic Stroke. J. Nippon Med. Sch. 2023, 90, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, I.; Vidya, T.A.; Ramachandran, K.; Hussain, A.; Aarthi, J.; Poovitha, M.; Madhavan, K.; Kumar, J.S. Platelet Indices as Prognostic Markers of Ischemic Stroke and Their Correlation with Lipid Profile. Clin. Neurol. Neurosurg. 2024, 237, 108119. [Google Scholar] [CrossRef] [PubMed]
- Brough, D.; Denes, A. Interleukin-1α and Brain Inflammation. IUBMB Life 2015, 67, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ren, W.; Wu, Q.; Liu, T.; Wei, Y.; Ding, J.; Zhou, C.; Xu, H.; Yang, S. NLRP3 Inflammasome Activation: A Therapeutic Target for Cerebral Ischemia–Reperfusion Injury. Front. Mol. Neurosci. 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Zietz, A.; Gorey, S.; Kelly, P.J.; Katan, M.; McCabe, J.J. Targeting Inflammation to Reduce Recurrent Stroke. Int. J. Stroke 2024, 19, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, H.; Hou, S.; Zhang, W.; Liu, H.; Zhu, L.; Meng, H. Inflammatory Cytokines and Stroke and Its Subtypes: A Genetic Correlation and Two-Sample Mendelian Randomization Study. Front. Mol. Neurosci. 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Tirandi, A.; Sgura, C.; Carbone, F.; Montecucco, F.; Liberale, L. Inflammatory Biomarkers of Ischemic Stroke. Intern. Emerg. Med. 2023, 18, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Q.; Wang, C.; Zhao, Y.; Jin, C.; Sun, M.; Ge, S. The Interplay between Cytokines and Stroke: A Bi-Directional Mendelian Randomization Study. Sci. Rep. 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Matys, P.; Mirończuk, A.; Starosz, A.; Grubczak, K.; Kochanowicz, J.; Kułakowska, A.; Kapica-Topczewska, K. Expanding Role of Interleukin-1 Family Cytokines in Acute Ischemic Stroke. Int. J. Mol. Sci. 2024, 25, 10515. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.-Q.; Fang, Z.; Chen, X.-L.; Yang, S.; Zhou, Y.-F.; Mao, L.; Xia, Y.-P.; Jin, H.-J.; Li, Y.-N.; You, M.-F.; et al. Microglia-Derived TNF-α Mediates Endothelial Necroptosis Aggravating Blood Brain–Barrier Disruption after Ischemic Stroke. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Petrovic-Djergovic, D.; Goonewardena, S.N.; Pinsky, D.J. Inflammatory Disequilibrium in Stroke. Circ. Res. 2016, 119, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.A.; Jickling, G.C.; Winship, I.R. Neutrophil Dynamics and Inflammaging in Acute Ischemic Stroke: A Transcriptomic Review. Front. Aging Neurosci. 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- The Importance of Selected Markers of Inflammation and Blood-Brain Barrier Damage for Short-Term Ischemic Stroke Prognosis. J. Physiol. Pharmacol. 2019. [CrossRef]
- Xu, Q.; Liu, Y.; Tian, X.; Xia, X.; Zhang, Y.; Zhang, X.; Wang, Y.; Sun, P.; Meng, X.; Wang, A. Monocyte Chemoattractant Protein-1, Inflammatory Biomarkers, and Prognosis of Patients With Ischemic Stroke or Transient Ischemic Attack: Fndings From a Nationwide Registry Study. J. Am. Heart Assoc. 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Juli, C.; Heryaman, H.; Nazir, A.; Ang, E.-T.; Defi, I.R.; Gamayani, U.; Atik, N. The Lymphocyte Depletion in Patients with Acute Ischemic Stroke Associated with Poor Neurologic Outcome. Int. J. Gen. Med. 2021, Volume 14, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Manoj, H.; Gomes, S.M.; Thimmappa, P.Y.; Nagareddy, P.R.; Jamora, C.; Joshi, M.B. Cytokine Signalling in Formation of Neutrophil Extracellular Traps: Implications for Health and Diseases. Cytokine Growth Factor Rev. 2025, 81, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, M.; Ikram, A.; Suriya, S.; Saleem, S.; Quadri, S.A.; Robinson, M.; Ortega-Gutierrez, S.; Qeadan, F.; Leira, E.; Paul, S.; et al. Cytokine Registry In Stroke Patients (CRISP): Protocol of a Prospective Observational Study. Medicine (Baltimore) 2020, 99, e20921. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pradhan, R. Prognostic Relevance of C-Reactive Protein in Short Term Adverse Outcome in Patients with Acute Ischaemic Stroke. J. Clin. Diagn. Res. 2021. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Yuan, Y.; Wang, H.; Wang, Z.; Zhang, X. Th17 Cells and IL-17A in Ischemic Stroke. Mol. Neurobiol. 2024, 61, 2411–2429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ma, L.; Chu, Z.; Xu, H.; Wu, W.; Liu, F. Regulation of Microglial Activation in Stroke. Acta Pharmacol. Sin. 2017, 38, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Cruceru, M.L.; Enciu, A.-M.; Popa, A.C.; Albulescu, R.; Neagu, M.; Constantinescu, S.N. Signal Transduction Molecule Patterns Indicating Potential Glioblastoma Therapy Approaches. OncoTargets Ther. 2013, 1737. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Guo, H.; Zhou, Y.; Fang, R.; Zhang, W.; Mei, Z. Neutrophil Extracellular Traps in Cerebral Ischemia/Reperfusion Injury:Friend and Foe. Curr. Neuropharmacol. 2023, 21, 2079–2096. [Google Scholar] [CrossRef] [PubMed]
- Lundin, J.I.; Peters, U.; Hu, Y.; Ammous, F.; Benjamin, E.J.; Bis, J.C.; Brody, J.A.; Cushman, M.; Fuller, H.; Gignoux, C.; et al. Epigenetic Mechanisms Underlying Variation of IL-6, a Well-Established Inflammation Biomarker and Risk Factor for Cardiovascular Disease. Atherosclerosis 2025, 407, 120219. [Google Scholar] [CrossRef] [PubMed]
- Amruta, N.; Rahman, A.A.; Pinteaux, E.; Bix, G. Neuroinflammation and Fibrosis in Stroke: The Good, the Bad and the Ugly. J. Neuroimmunol. 2020, 346, 577318. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.; Cabantan, D.; Monsour, M.; Borlongan, C.V. Neuroinflammation, Stem Cells, and Stroke. Stroke 2022, 53, 1460–1472. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Zhuang, H.; Huang, W.; Sun, J. Neuroinflammation and Energy Metabolism: A Dual Perspective on Ischemic Stroke. J. Transl. Med. 2025, 23. [Google Scholar] [CrossRef] [PubMed]
- Couch, C.; Mallah, K.; Borucki, D.M.; Bonilha, H.S.; Tomlinson, S. State of the Science in Inflammation and Stroke Recovery: A Systematic Review. Ann. Phys. Rehabil. Med. 2022, 65, 101546. [Google Scholar] [CrossRef] [PubMed]
- Monsour, M.; Croci, D.M.; Agazzi, S.; Borlongan, C.V. Contemplating IL-6, a Double-edged Sword Cytokine: Which Side to Use for Stroke Pathology? CNS Neurosci. Ther. 2023, 29, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, T.; Zhai, X.; Ma, Y.; Xie, L.; Lu, B.; Zhang, Y.; Li, Y.; Chen, Z.; Yin, J.; et al. Targeting Neutrophils as a Novel Therapeutic Strategy after Stroke. J. Cereb. Blood Flow Metab. 2021, 41, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Prapiadou, S.; Živković, L.; Thorand, B.; George, M.J.; Van Der Laan, S.W.; Malik, R.; Herder, C.; Koenig, W.; Ueland, T.; Kleveland, O.; et al. Proteogenomic Data Integration Reveals CXCL10 as a Potentially Downstream Causal Mediator for IL-6 Signaling on Atherosclerosis. Circulation 2024, 149, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.F.; Wang, D.L.; Zhou, Y.; Xiong, H. Association between the Interleukin-6-174 G/C Polymorphism and Risk of Ischemic Stroke: A Meta-Analysis. Genet. Mol. Res. 2015, 14, 13076–13083. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, L.; Wallén, H.; Aspberg, S.; De Faire, U.; Gigante, B. IL6 Trans-Signaling Associates with Ischemic Stroke but Not with Atrial Fibrillation. BMC Neurol. 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vorst, E.P.C.; Döring, Y.; Weber, C. Chemokines and Their Receptors in Atherosclerosis. J. Mol. Med. 2015, 93, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Gill, D.; Rannikmäe, K.; Traylor, M.; Anderson, C.D.; MEGASTROKE consortium of the International Stroke Genetics Consortium (ISGC); Lee, J. -M.; Kamatani, Y.; Hopewell, J.C.; Worrall, B.B.; et al. Genetically Determined Levels of Circulating Cytokines and Risk of Stroke: Role of Monocyte Chemoattractant Protein-1. Circulation 2019, 139, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Lemmens, R.; Tsivgoulis, G. Inflammation and Stroke Risk: A New Target for Prevention. Stroke 2021, 52, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Yang, K.; Li, J.; Lin, J.; Jing, J.; Xiong, Y.; Zhao, X.; Wang, Y.; Liu, L.; Meng, X.; et al. Mediation Effect of Stroke Recurrence in the Association between Post-stroke Interleukin-6 and Functional Disability. CNS Neurosci. Ther. 2023, 29, 3579–3587. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, A.; Palaiopanos, K.; Björkbacka, H.; Peters, A.; De Lemos, J.A.; Seshadri, S.; Dichgans, M.; Georgakis, M.K. Circulating Interleukin-6 Levels and Incident Ischemic Stroke: A Systematic Review and Meta-Analysis of Prospective Studies. Neurology 2022, 98. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-H.; Luo, M.-Y.; Liang, N.-; Gong, S.-X.; Chen, W.; Huang, W.-Q.; Tian, Y.; Wang, A.-P. Interleukin-6: A Novel Target for Cardio-Cerebrovascular Diseases. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.N.; deGoma, E.; Shapiro, M.D. IL-6 and Cardiovascular Risk: A Narrative Review. Curr. Atheroscler. Rep. 2024, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, J.; Pan, Y.; Wang, M.; Meng, X.; Li, H.; Wang, Y.; Zhao, X.; Qin, H.; Liu, L.; et al. Interleukin-6 and YKL-40 Predicted Recurrent Stroke after Ischemic Stroke or TIA: Analysis of 6 Inflammation Biomarkers in a Prospective Cohort Study. J. Neuroinflammation 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Buckley, L.; Sun, C.; Al Rifai, M.; Yu, B.; Nambi, V.; Virani, S.S.; Selvin, E.; Matsushita, K.; Hoogeveen, R.C.; et al. Association of Interleukin-6 and Interleukin-18 with Cardiovascular Disease in Older Adults: Atherosclerosis Risk in Communities Study. Eur. J. Prev. Cardiol. 2023, 30, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-I.; Kim, J.-S.; Bae, H.J.; Kim, W.Y. C-Reactive Protein for Stroke Detection in the Emergency Department in Patients With Dizziness Without Neurological Deficits. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Di Raimondo, D.; Pecoraro, R.; Maida, C.; Arnao, V.; Corte, V.D.; Simonetta, I.; Corpora, F.; Di Bona, D.; Maugeri, R.; et al. Early High-Dosage Atorvastatin Treatment Improved Serum Immune-Inflammatory Markers and Functional Outcome in Acute Ischemic Strokes Classified as Large Artery Atherosclerotic Stroke: A Randomized Trial. Medicine (Baltimore) 2016, 95, e3186. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Enciu, A.M.; Codrici, E.; Popescu, I.D.; Dudau, M.; Dobri, A.M.; Pop, S.; Mihai, S.; Gheorghișan-Gălățeanu, A.-A.; Hinescu, M.E. Fatty Acids, CD36, Thrombospondin-1, and CD47 in Glioblastoma: Together and/or Separately? Int. J. Mol. Sci. 2022, 23, 604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, H.; Wang, J.; Xi, X.; Cefalo, P.; Wood, L.J.; Luo, X.; Wang, Q.M. Multiplex Array Analysis of Serum Cytokines Offers Minimal Predictive Value for Cognitive Function in the Subacute Phase after Stroke. Front. Neurol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Freuer, D.; Schmitz, T.; Ertl, M.; Zickler, P.; Naumann, M.; Linseisen, J. Inflammation Biomarkers in Acute Ischemic Stroke According to Different Etiologies. Eur. J. Neurol. 2024, 31. [Google Scholar] [CrossRef] [PubMed]
- Rezaeitalab, F.; Esmaeili, M.; Saberi, A.; Vahidi, Z.; Emadzadeh, M.; Rahimi, H.R.; Ramezani, N.; Mirshabani-Toloti, S.Z. Predictive Value of Inflammatory Markers for Functional Outcomes in Patients with Ischemic Stroke. Curr. J. Neurol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Amantea, D.; Micieli, G.; Tassorelli, C.; Cuartero, M.I.; Ballesteros, I.; Certo, M.; Moro, M.A.; Lizasoain, I.; Bagetta, G. Rational Modulation of the Innate Immune System for Neuroprotection in Ischemic Stroke. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, C.; Wang, H.; Nan, S. Serum Interleukin-37 Increases in Patients after Ischemic Stroke and Is Associated with Stroke Recurrence. Oxid. Med. Cell. Longev. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhang, X.-G.; Jiang, H.-Y.; Cui, X.-K.; Zhang, D.; Yao, Z.-W.; Yue, Y.-H. An Increase in Neutrophil-to-Lymphocyte Ratio Predicts Poor Functional Outcomes in Older Patients with Acute Ischemic Stroke: A Retrospective Study. J. Integr. Neurosci. 2021, 20. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cai, L.; Yi, T.; Yi, X.; Hu, Y. Neutrophil-to-lymphocyte Ratio Is Associated with Stroke Progression and Functional Outcome in Patients with Ischemic Stroke. Brain Behav. 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.; Lv, C.; Li, F. The Prognosis of Neutrophil-to-Lymphocyte Ratio and Lymphocyte-to-Monocyte Ratio in Elderly with Acute Ischemic Stroke. Clin. Interv. Aging 2024, Volume 19, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Quan, K.; Wang, A.; Zhang, X.; Meng, X.; Chen, P.; Li, H.; Wang, Y. Neutrophil to Lymphocyte Ratio and Adverse Clinical Outcomes in Patients with Ischemic Stroke. Ann. Transl. Med. 2021, 9, 1047–1047. [Google Scholar] [CrossRef] [PubMed]
- Department of Neurology, Kirikkale University, Faculty of Medicine, Kirikkale, Turkey; Alpua, M.; Say, B.; Yardimci, I.; Ergün, U.; Kisa, U.; Ceylan, O.D. First Admission Neutrophil–Lymphocyte Ratio May Indicate Acute Prognosis of Ischemic Stroke. Rambam Maimonides Med. J. 2021, 12, e0021. [Google Scholar] [CrossRef] [PubMed]
- Memiş, Z.; Gürkaş, E.; Özdemir, A.Ö.; Acar, B.A.; Ögün, M.N.; Aytaç, E.; Akpınar, Ç.K.; Akıl, E.; Çabalar, M.; Özkul, A.; et al. Impact of Neutrophil-to-Lymphocyte Ratio on Stroke Severity and Clinical Outcome in Anterior Circulation Large Vessel Occlusion Stroke. Diagnostics 2024, 14, 2880. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, G.; Liu, F.; Zheng, J.; Jiang, Z.; Hu, S.; Shi, X.; Wang, W.; Xu, L.; Wang, Z. Association of Neutrophil-to-Lymphocyte Ratio with Stroke Morbidity and Mortality: Evidence from the NHANES 1999–2020. Front. Med. 2025, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, S.; Dai, Q.; Li, J. Neutrophil Lymphocyte Ratio Predicts Early Neurological Deterioration in Patients with Anterior Circulation Stroke. Int. J. Gen. Med. 2024, Volume 17, 5325–5331. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Heo, M.Y.; Joo, H.J.; Shim, G.Y.; Chon, J.; Chung, S.J.; Soh, Y.; Yoo, M.C. Neutrophil-to-Lymphocyte Ratio as a Predictor of Short-Term Functional Outcomes in Acute Ischemic Stroke Patients. Int. J. Environ. Res. Public. Health 2023, 20, 898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhu, T.; Li, H.; He, Y.; Zhang, Y.; Huang, N.; Zhang, G.; Li, Y.; Chang, D.; Li, X. <p>Plasma Interleukin-37 Is Elevated in Acute Ischemic Stroke Patients and Probably Associated With 3-Month Functional Prognosis </P>. Clin. Interv. Aging 2020, Volume 15, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Iordache, M.P. Reframing Functional Neurological Disorders in Modern Medicine. Cureus 2025. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Feng, X.; Shao, M.; Liu, W.; Ma, Q.; Wang, E.; Chen, J.; Shao, B. Serum Interleukin-33 as a Novel Marker for Long-term Prognosis and Recurrence in Acute Ischemic Stroke Patients. Brain Behav. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
| Cytokine / Interleukin | Classification | Main Functions | Clinical / Prognostic Implications |
Timing of Elevation Post-Stroke |
|---|---|---|---|---|
| Interleukin-1β (IL-1β) | Pro-inflammatory | Initiates inflammatory cascade; activates microglia and endothelial cells | Elevated levels associated with larger infarct size and worse neurological outcomes | Peaks within 6–24 hours |
| Interleukin-6 (IL-6) | Pro-inflammatory | Modulates acute phase response; promotes leukocyte recruitment | High levels correlate with increased stroke severity and poor functional outcomes | Rises within hours; remains elevated for days |
| Tumour Necrosis Factor-α (TNF-α) | Pro-inflammatory | Promotes apoptosis; disrupts blood-brain barrier | Elevated levels linked to increased infarct size and neurological deterioration | Peaks within 6–24 hours |
| Interleukin-10 (IL-10) | Anti-inflammatory | Suppresses pro-inflammatory cytokine production; limits immune response | Higher levels associated with reduced infarct size and better outcomes; excessive levels may increase infection risk | Increases within 24–72 hours |
| Transforming Growth Factor-β (TGF-β) | Anti-inflammatory/ Regulatory |
Regulates immune response; promotes tissue repair | Contributes to neuroprotection and recovery; exact prognostic value in stroke is under investigation | Elevation timing varies; involved in later stages |
| Interleukin-17 (IL-17) | Pro-inflammatory | Recruits neutrophils; amplifies inflammatory response | Elevated levels may exacerbate neuroinflammation and tissue damage | Peaks within 24–72 hours |
| Interleukin-8 (IL-8) | Pro-inflammatory | Attracts neutrophils; promotes inflammation | Higher levels associated with increased infarct volume and poorer outcomes | Rises early; peaks within 24 hours |
| Interleukin-1α (IL-1α) | Pro-inflammatory | Acts as an alarmin; initiates sterile inflammation | Early release may contribute to initial inflammatory response; specific prognostic implications in stroke require further study | Released immediately upon cell injury |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





