1. Introduction
The close contribution of bacterial flora to the aggravation and amelioration of atopic dermatitis (AD) has been reported previously [
1,
2]. Therefore, oral treatment with probiotics has long been investigated as an effective alternative for preventing and ameliorating AD symptoms in humans and companion animals [3-5].
Lactobacillus paracasei (
L. paracasei) is a representative probiotic that is effective against several types of allergic diseases, including AD, atopic eczema, asthma, and allergic rhinitis [6-9]. Expected impact of
L. paracasei is also as an immune modulator, and previous studies have investigated that the oral treatment of
L. paracasei exhibited the immunomodulatory effects
in vivo and
in vitro [10-12]. Dendritic cells (DCs) are one of the major targets of
L. paracasei and are key players in acquiring and presenting antigen information on allergic symptoms [
13]. Mileti et al. [
14] demonstrated that
L. paracasei minimally induced the release of cytokines, while it also inhibited the potential of DCs both to produce inflammatory cytokines such as interleukin (IL)-12 and tumor necrosis factor (TNF) α and to drive Th1 T cells in response to Salmonella. As shown in previous reports, the immunomodulatory effects of
L. paracasei alone are not strong; therefore, herein, we focused on a mixed microbial culture of
L. paracasei, Pichia membranifaciens (P. membranifaciens) and
Saccharomyces cerevisiae (
S. cerevisiae) (LS) as a new probiotic to ameliorate allergic diseases. In addition, most previous studies have highlighted oral exposure to these probiotics, whereas allergic inflammation occurs in local tissues such as the skin and respiratory system. Therefore, the objective of this study was to examine the therapeutic and preventive effects of dermal LS treatment in a mouse model of atopic dermatitis (AD). Immunomodulatory and anti-inflammatory effects of LS were confirmed using dendritic cells and keratinocytes.
2. Materials and Methods
The LS culture was provided by Litanial Bio Science, Co. Ltd. (Hyogo, Japan) [15]. This culture was prepared by co-cultivation of L. paracasei, P. membranifaciens and S. cerevisiae in a rice grain broth supplemented with 5% dextrose at 30℃ for 24 h. Sterility was assessed by cultivating the microbial mixture on heart infusion (HI) agar (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan). The immunomodulatory and anti-inflammatory effects of LS were examined in vitro using murine dendritic cell lines (DC2.4) and a human epidermal keratinocyte cell line (HaCaT). DC2.4 was obtained from the American Type Culture Collection (Manassas, VA, USA) and was cultured in RPMI 1640 medium (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) supplemented with 10% fetal calf serum (FCS; Sigma-Aldrich Co. LLC., Tokyo, Japan) and penicillin-streptomycin (FUJIFILM Wako Pure Chemical Corporation). HaCaT cells were obtained from CLS (Cell Lines Service) GmbH (Eppelheim, Germany) and cultured in Dulbecco’s modified Eagle’s medium (FUJIFILM Wako Pure Chemical Corporation) supplemented with 10 % FCS and penicillin-streptomycin. DC2.4 cells (1 × 104 cells/100 μL) at 70% confluency were seeded in a 96-well culture plate and exposed to several concentrations of LS (0, 0.125, 0.25, 0.5 and 1 mg/mL). The concentrations of IL-10 and TNFα in the supernatants were quantified by enzyme-linked immunosorbent assay (ELISA) (DuoSet ELISA kit, R&D Systems, Minneapolis, MN, USA). HaCaT cells (1 × 104 cells) were seeded in 100 μL of the medium at 70 % confluence in 96-well culture plates and stimulated with recombinant human TNFα and interferon (IFN) γ (PeproTech, Inc., Cranbury, NJ) for 1 h; next, 100 μL of several concentrations of LS (0, 0.125, 0.25, 0.5 and 1 mg/mL) in culture medium was added followed by incubation for 24 h. The concentrations of IL-8 and thymus activation-regulated chemokine (TARC) in the supernatants were quantified by ELISA. The therapeutic and preventive properties of topical LS treatment were examined in a mouse model of AD. The AD mouse model was generated by topical sensitization with Biostir AD (Dermatophagoides farinae extract) in addition to the topical application of 4% sodium dodecyl sulfate solution (FUJIFILM Wako Pure Chemical Corporation) in NC/Nga mice according to previous report [16]. The treatment regimen involved daily topical application (0.1 mL/mouse) of 10% of LS solution (n = 8) or DW vehicle (n = 7). Treatment was initiated on day 19 after the development of AD, when the mean AD score was 2.07. Trans epidermal water loss (TEWL), back skin thicknesses, and clinical scores were monitored once weekly during the experimental period. TEWL was measured using a VAPO SCAN (AS-VT100RS, ASCH JAPAN Co., LTD, Tokyo, Japan), and a clinical score of 0–4 was assigned as follows: no symptoms, 0; mild, 1; moderate, 2; severe, 3; and extreme, 4 for the ear and back, as previously described [17]. Auricular lymph node (LN) samples were collected from each mouse 1 d after the final sensitization. Single-cell suspensions isolated from the LN were prepared as described previously [18,19], and the total number of cells was counted using a CellDrop™ Cell Counting System (DeNovix Inc., DE, USA). The cells were analyzed using a BD FACSAria™ III cell sorter (BD Biosciences, Tokyo, Japan) with monoclonal antibodies (PE/Cyanine7-conjugated anti-mouse CD3, PE-conjugated anti-mouse CD4, PE-conjugated anti-mouse CD11b, APC-conjugated anti-mouse CD11c, PerCP/Cyanine5.5-conjugated anti-mouse CD19, APC-conjugated anti-mouse CD44, APC/Cyanine7-conjugated anti-mouse CD62L, FITC-conjugated anti-mouse IgE, FITC-conjugated anti-mouse MHC class II, and DAPI) (BioLegend Inc., CA, USA; Miltenyi Biotec K.K. and Sony Biotechnology Inc., Tokyo, Japan). Single-cell suspensions of LNs were used to evaluate cytokine release by T cells. Single-cell suspensions of LNs (5 × 105 cells/well) were incubated with mouse T-activator CD3/CD28 dynabeads (Thermo Fisher Scientific Inc., Kanagawa, Japan) for 24 h. The levels of interferon (IFN) γ, IL-4, IL-13, and IL-17 in the supernatant were evaluated using ELISA (DuoSet ELISA kit, R&D Systems). Semi-quantitative histopathological evaluation of a portion of the skin sample was performed in a blinded fashion using the following grading system: 0, within normal limits; 1, mild; 2, moderate; 3, severe. The total lesion score was used for the statistical evaluation. Data are expressed as mean ± 1 standard error of the mean (SEM). Analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test were used to evaluate the results of the in vitro studies. In vivo experiments, 2-way ANOVA followed by Šídák's multiple comparisons test or Student's t-test was used to test the significance of differences between the two groups. Statistical significance was estimated at 5 % levels of probability, and data were analyzed using GraphPad Prism 10 (GraphPad Software, San Diego, CA, USA).
3. Results
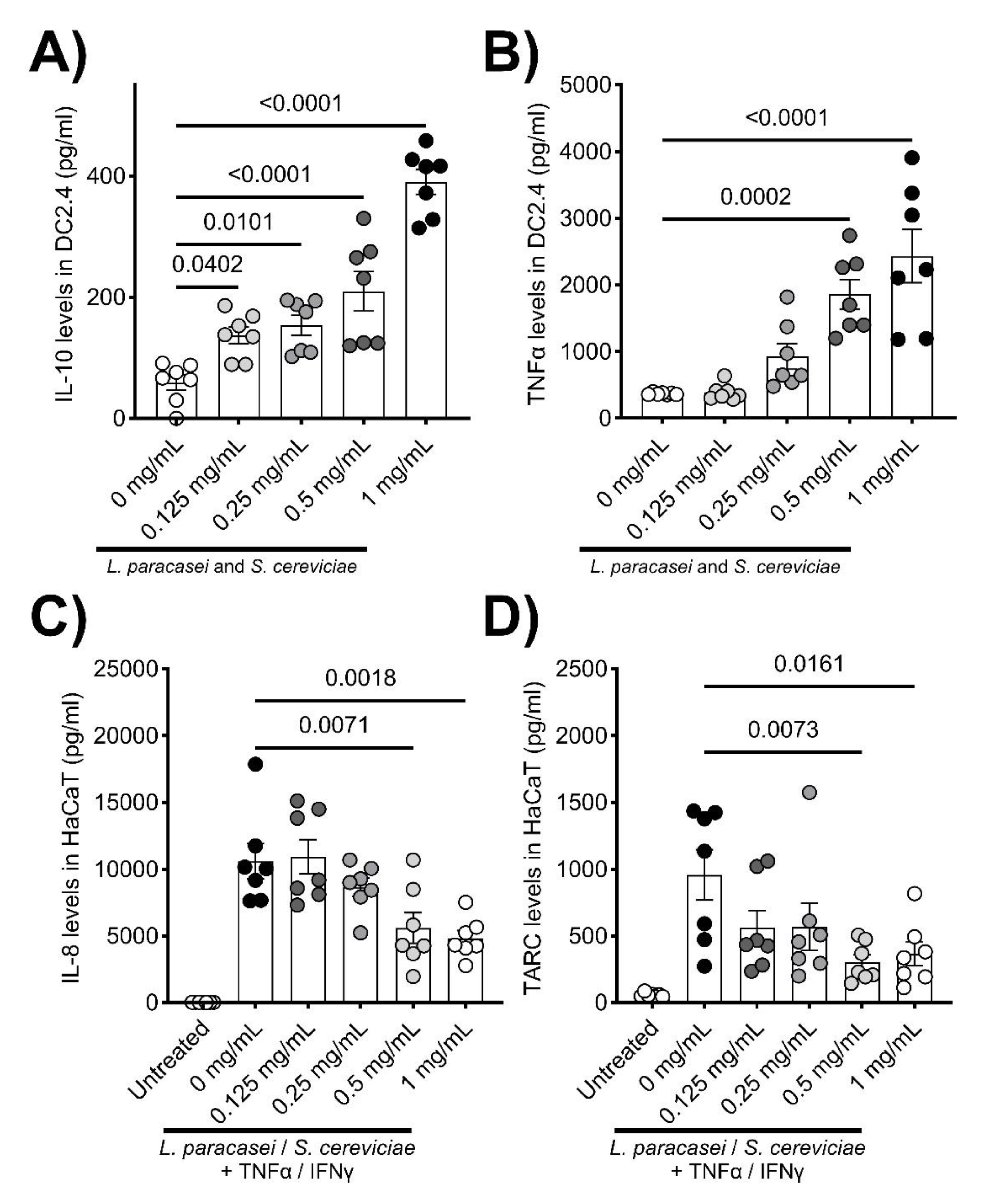
LS treatment significantly enhanced the secretions of IL-10 and TNFα by DC2.4 cells (
Figure 1A and B). In contrast, IL-8 and TARC production by stimulated HaCaT cells was significantly decreased by co-culturing with LS (
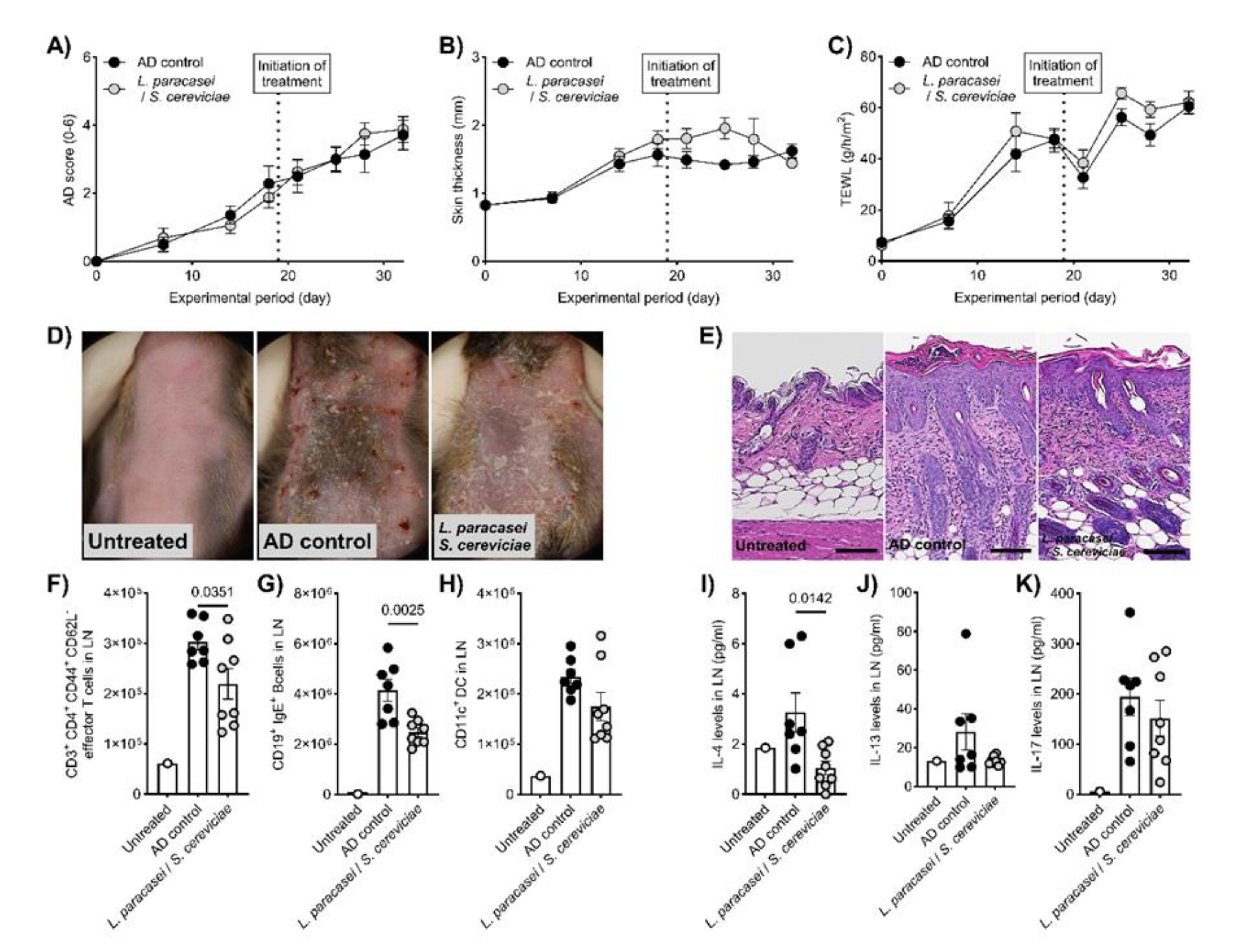
Figure 1C and D). Although there were no significant changes in clinical symptoms, skin thickness, and TEWL in the therapeutic setting of the AD mouse model (
Figure 2A-D), histological evaluations, including hyperplasia in the keratinized layer and crust in the epidermis, were significantly ameliorated by LS treatment (
Table 1,
Figure 2E). Allergy-related immune reactions, including the number of IgE-positive B cells and IL-4 levels in the local lymph nodes, also significantly decreased in the LS treatment group (
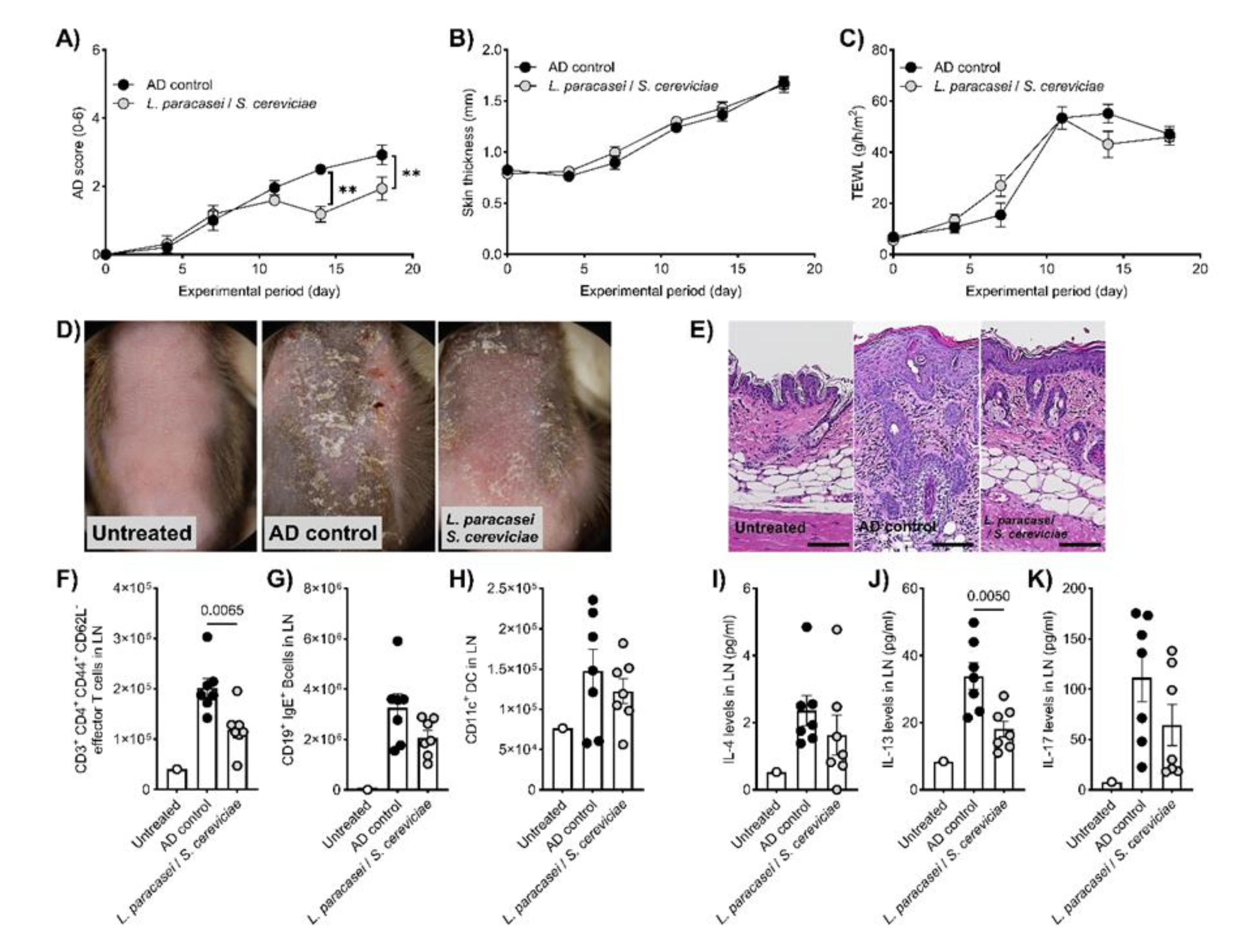
Figure 2F-K). The effects of LS were highlighted more in preventive treatment. LS treatment significantly decreased AD symptoms (
Figure 3A and D) and histological findings (
Table 1,
Figure 3E), whereas skin thickness and TEWL were unaffected (
Figure 3B and C). Effector T cells and IL-13 levels in the LN in the LS treatment group were significantly reduced compared to those in AD control mice (
Figure 3F-K).
4. Discussion
P. membranifaciens and S. cerevisiae have been increasingly recognized for their immunomodulatory potential, such as IL-6 production by dendritic cells (Yee et al., 2024). In contrast, only a modest increase in IL-10, apivotal player in immunomodulation (Asgari et al., 2025), was observed after S. cerevisiae treatment. A previous study reported that oral administration of LS significantly increased protection against atypical Aeromonas salmonicida infection in the common carp, indicating that P. membranifaciens and S. cerevisiae may play a role as a prebiotic for L. paracasei (Kodama et al., 2011). In fact, our findings indicating significant upregulation of IL-10 and TNFα by LS treatment demonstrate the potential of LS as an immune modulator. Significant immunomodulatory effects were also observed in the therapeutic setting of the AD mouse model, whereas no influence was observed on the clinical signs and cutaneous inflammation. However, the preventive use of LS topical treatments significantly ameliorated the AD score compared to that in the vehicle control group, in addition to the immune modulation seen in effector T cell infiltration and IL-13 levels in local LN. Our findings indicated that the immunomodulatory and anti-inflammatory effects of LS can prevent AD development in humans and companion animals.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, M.K., C.O., and T.F.; methodology, M.K., C.O., T.M., A.H., Y.I., and T.F.; software, M.K., C.O., and T.F.; validation, M.K., C.O., and T.F.; formal analysis, M.K., C.O., and T.F.; investigation, M.K., C.O., and T.F.; resources, H.T..; data curation, M.K., C.O., and T.F.; writing—original draft preparation, M.K., C.O., and T.F. project administration, .T.F.; funding acquisition, T.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Litanial Bioscience Laboratory, 2-26 Shinobe-Kitamachi, Befu-cho, Kakogawa, Hyogo 675-0121, Japan
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of Azabu University (protocol code 240620-1 and June, 20, 2024).” for studies involving animals.
Data Availability Statement
The original contributions of this study are included in this article. Further inquiries can be di-rected to the corresponding author.
Acknowledgments
We would like to thank Editage (
www.editage.jp) for the English language editing.
Conflicts of Interest
Hideo Togase directed the Litanial Bioscience Laboratory. The remaining authors declare no potential conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AD |
Atopic dermatitis |
| ANOVA |
Analysis of variance |
| DCs |
Dendritic cells |
| FCS |
Fetal calf serum |
| HaCaT |
Human epidermal keratinocytes |
| IFN |
Interferon |
| Ig |
Immunoglobulin |
| IL |
Interleukin |
| LN |
Lymph nodes |
| LS |
Mixed microbial culture of Lactobacillus paracasei, Pichia membranifaciens and Saccharomyces cerevisiae
|
| SEM |
Standard error of the mean |
| TARC |
Thymus and activation-regulated chemokine |
| TEWL |
Transepidermal water loss |
| TNF |
Tumor necrosis factor |
References
- Li, W.; Li, A. Exploring the causal relationship between gut microbiota and atopic dermatitis: A Mendelian randomization study. Medicine (Baltimore) 2024, 103, e40193. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Z.; Qu, L. The changes of intestinal flora and metabolites in atopic dermatitis mice. Front Microbiol 2024, 15, 1462491. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Kunstner, A.; Wohlers, I.; Olbrich, M.; Lenfers, T.; Osumi, T.; Shimazaki, Y.; Nishifuji, K.; Ibrahim, S.M.; Watson, A.; et al. A comprehensive analysis of gut and skin microbiota in canine atopic dermatitis in Shiba Inu dogs. Microbiome 2023, 11, 232. [Google Scholar] [CrossRef]
- Wang, L.; Xu, L. The impact of prebiotics, probiotics and synbiotics on the prevention and treatment of atopic dermatitis in children: an umbrella meta-analysis. Front Pediatr 2025, 13, 1498965. [Google Scholar] [CrossRef]
- Xi, Z.; Fenglin, X.; Yun, Z.; Chunrong, L. Efficacy of probiotics in the treatment of allergic diseases: a meta-analysis. Front Nutr 2025, 12, 1502390. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Steinhoff, M. Meta-analysis on preventive and therapeutic effects of probiotic supplementation in infant atopic dermatitis. J Dtsch Dermatol Ges 2023, 21, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Puisto, R.; Gomez-Gallego, C.; Collado, M.C.; Turta, O.; Isolauri, E.; Rautava, S. The Role of Infant Gut Microbiota Modulation by Perinatal Maternal Probiotic Intervention in Atopic Eczema Risk Reduction. Neonatology 2025, 122, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Park, H.J.; Kim, Y.K.; Choi, Y.; Park, H.S. Lactobacillus paracasei-derived extracellular vesicles alleviate neutrophilic asthma by inhibiting the JNK pathway in airway epithelium. Allergol Int 2024, 73, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Song, X.; Shu, T.; Zhang, S.; Zhang, Z.; Hu, C.; Pan, J.; Dai, X.; Hao, H.; Xiao, G.; et al. Prevention and alleviation of allergic rhinitis by oral administration of Lacticaseibacillus paracasei GOLDGUT-Lpc969. Front Immunol 2024, 15, 1444778. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Aoki, T.; Iwabuchi, S.; Arai, S.; Iwabuchi, N.; Motobayashi, H.; Tanaka, M.; Hashimoto, S. Immunomodulatory activity of heat-killed Lacticaseibacillus paracaseiMCC1849 based on the activation of plasmacytoid dendritic cells in the peripheral blood of healthy adults. Food Sci Nutr 2024, 12, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Arai, S.; Sato, S.; Iwabuchi, N.; Takara, T.; Tanaka, M. Effects of Heat-Killed Lacticaseibacillus paracasei MCC1849 on Immune Parameters in Healthy Adults-A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Morikawa, M.; Yamamoto-Fujimura, M.; Iwata, A.; Maki, A.; Kato-Nagaoka, N.; Oana, K.; Kiyoshima-Shibata, J.; Matsuura, Y.; Kaji, R.; et al. Diverse impact of a probiotic strain, Lacticaseibacillus paracasei Shirota, on peripheral mononuclear phagocytic cells in healthy Japanese office workers: a randomized, double-blind, controlled trial. Biosci Microbiota Food Health 2023, 42, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wiese-Szadkowska, M.; Helmin-Basa, A.; Eljaszewicz, A.; Gackowska, L.; Januszewska, M.; Motyl, I.; Andryszczyk, M.; Wieczynska, J.; Michalkiewicz, J. Selected commensal bacteria change profiles of Helicobacter pylori-induced T cells via dendritic cell modulation. Helicobacter 2019, 24, e12614. [Google Scholar] [CrossRef] [PubMed]
- Mileti, E.; Matteoli, G.; Iliev, I.D.; Rescigno, M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One 2009, 4, e7056. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).