1. Global Carbon Dioxide Emissions: Trends, Challenges, and the Path to Neutrality
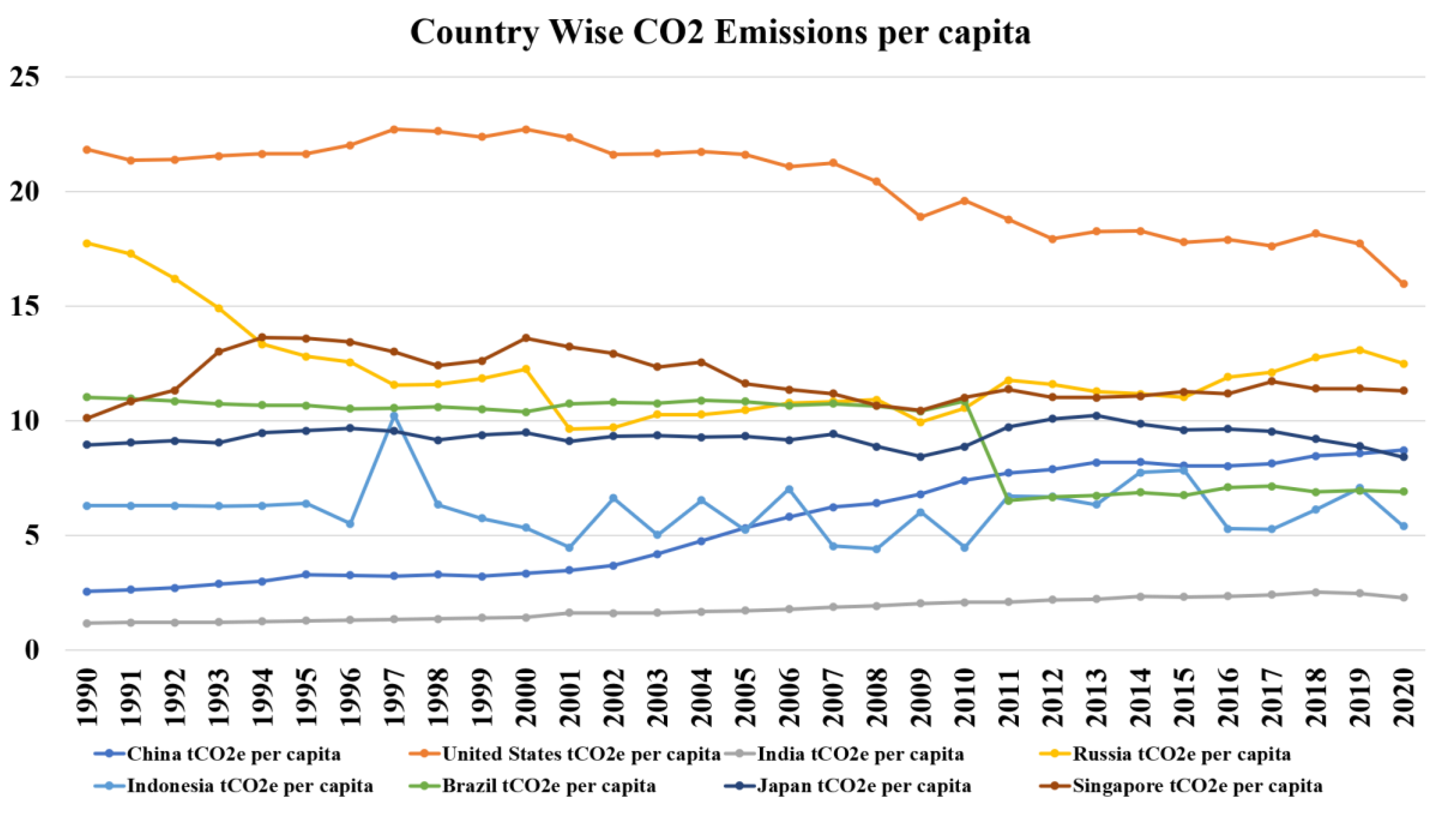
Carbon dioxide emissions were systematically measured beginning in 1894, and the records show startling levels of increase over the years. Analysis further shows that CO2 accounts for about 70-75% of total greenhouse gas emissions annually, putting it as the largest menace to environmental sustainability (Adams & Nsiah, 2019a; Aldy, 2006; Ezcurra, 2007; Pettersson et al., 2014; Schmalensee et al., 1998). Sectorial analysis concludes that energy production is the greatest contributor to emissions, followed by agriculture, and geographical distribution shows the highest per capita emissions in the United States, Russia, and Singapore, with the lowest per capita emissions in India even though it has an increasing total output. The figure shows an instructive trend: in the face of the Paris Climate Accord and its ideological commitment to reduce emissions the annual changes between 2000-2022 in CO2 emissions totaled an aggregate increase of 12.99 GtCO2. This is to consider the striking disconnect between recommendation and action, with the resultant levels of emissions far from mitigation levels of 1.5°C required to realize climate objectives. The thermodynamic instability of CO2, being a linear molecule non-polar and having a high Gibbs free energy of formation of −394.3 kJ/mol, is a massive scientific challenge to carbon capture technologies themselves as well, rendering mitigation increasingly hard (Alsaleh et al., 2023; Mascarenhas et al., 2019).
Figure 1.
Country-wise CO2 emissions per capita.
Figure 1.
Country-wise CO2 emissions per capita.
2. Sectoral Distribution of CO2 Emissions and Mitigation Policies
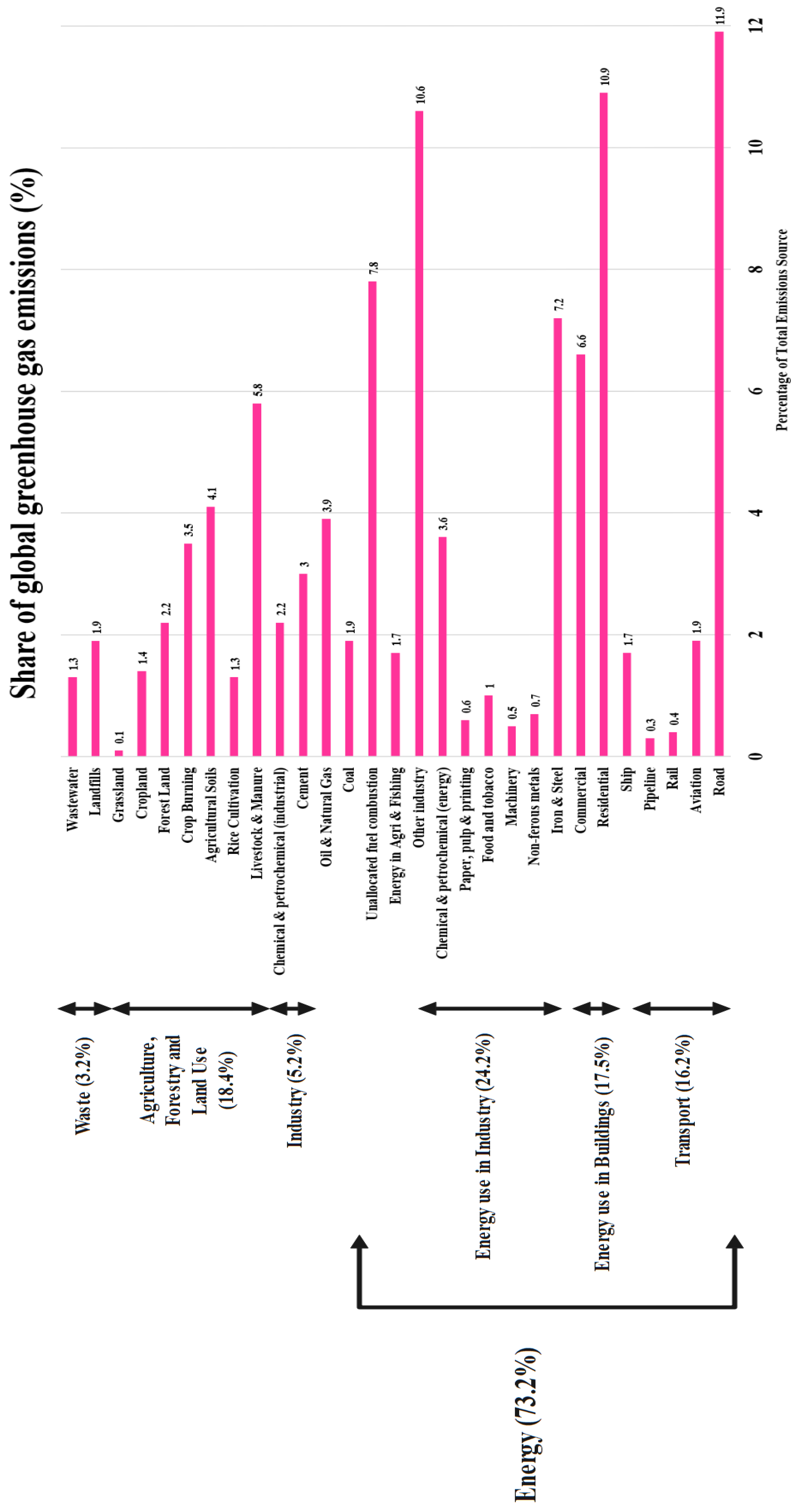
Carbon dioxide emissions are produced in four large sectors: Energy (73.2%), Direct Industrial Processes (5.2%), Waste (3.2%), and Agriculture, Forestry and Land Use (18.4%). The biggest sector is energy, which includes industrial energy consumption (24.2%), transport (11.9% alone for road transport), building energy consumption (17.9%), unallocated fuel combustion (7.8%), fugitive emissions (5.8%), and agricultural/fishing energy consumption (1.7%). Transportation emissions are also split between passenger and freight services within many modes such as road, air, shipping, and rail. Industrial processes create CO2 emissions directly from chemical conversions, more so in the production of cement (3%) and chemical processes (2.2%). Emissions from waste management occur in wastewater systems (1.3%) and landfills (1.9%), with agriculture and land use emissions taking place from diverse sources such as cropland management (1.4%), forestry change (2.2%), burning crop (3.5%), and livestock (5.8%). The multi-component nature of these emissions poses major challenges to carbon management, such as the thermodynamic infeasibility of CO2 as a reactant, dirty waste streams with changing compositions, and issues of large-scale flow management (Praveenkumar et al., 2024; Saraswat et al., 2025; Yadav, Mittal, et al., 2025b, 2025c). These challenges must be addressed by a complete three-pronged approach: carbon capture, concentration/purification, and utilization or storage each necessitating tailored implementation depending on individual emission sources and natures (Adams & Nsiah, 2019b; Magadum, Garg, et al., 2025; Murgod et al., 2025b).
Figure 2.
Share of sectoral Greenhouse Gas emissions in percentage.
Figure 2.
Share of sectoral Greenhouse Gas emissions in percentage.
3. Carbon Dioxide and Post-Combustion Capture Emission Reduction Technologies
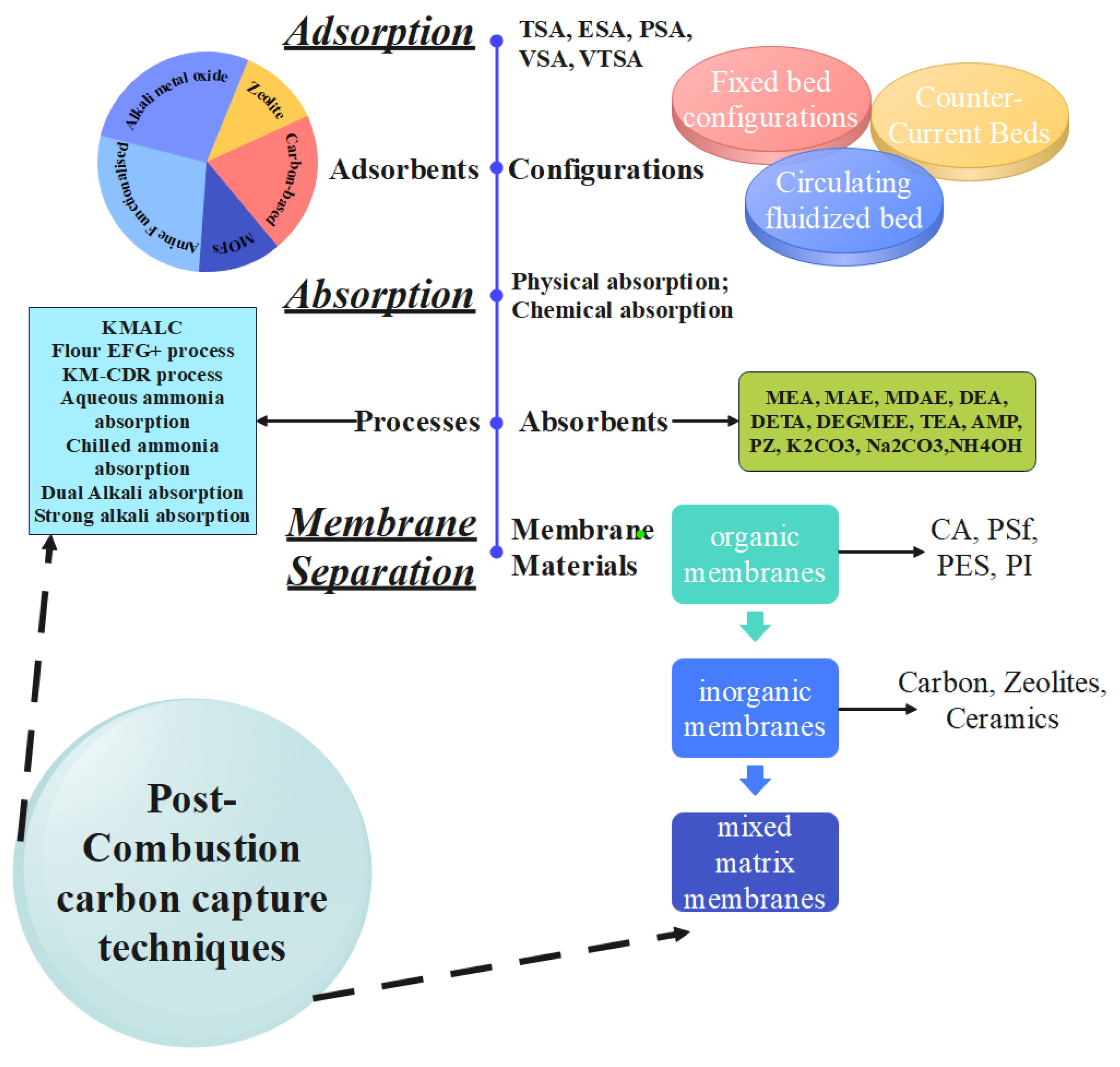
The mitigation of carbon dioxide emissions calls for a multi-faceted approach consisting of preventive and capture-based strategies. Eight principal strategies have emerged: low carbon footprint fuel switching, renewable energy geoengineering approaches, promotion of energy conservation, carbon capture and storage (CCS), energy efficiency enhancement, clean fuel utilization, and nuclear power setup. Of these, Carbon Capture technologies are the dominant path with three processes: post-combustion, oxy-combustion, and pre-combustion. Post-combustion capture, while it does incur an energy penalty of around 8% for coal-fired power generation and 6% for gas-fired power generation, does have the benefit of being simple to integrate with existing infrastructure. Among the post-combustion processes, adsorption processes have emerged into prominence due to their low cost and lack of operating flexibility (D’Alessandro et al., 2010; Lin et al., 2022; Mondal et al., 2012; Pérez-Fortes et al., 2016). These are Temperature Swing Adsorption, Pressure Swing Adsorption, Vacuum Swing Adsorption, and Electric Swing Adsorption with varying advantages in purity level, energy requirements, and efficiencies of separation. The effectiveness of such adsorptive technologies is highly dependent on geometries as well as careful selection of adsorbents with best-in-class chemical and structural properties, underscoring the technicality and tailoring involved in attaining effective application of carbon capture (Glenna et al., 2023; Gür, 2022a; Peng et al., 2018).
4. New Carbon Capture Technologies: Absorption, Membrane Separation, and Pre-Combustion Processes
Carbon dioxide capture systems have become more complex, from basic adsorption to more advanced absorption processes, membrane separation processes, and pre-combustion systems, each of which has a role to play in emissions reduction. Chemical absorption with amines in the form of the traditional industry 30% monoethanol amine liquid solution is the most developed system for CO2 capture but susceptible to thermal degradation and corrosion. New research proves that a combination of MEA with solvents such as DEGMEE or NMF can considerably promote absorption kinetics as well as diminish regeneration energy demand. Membrane separation has emerged as a new, chemical-free method involving various materials such as polymeric membranes, which exhibit high thermo-mechanical stability and carbon dioxide permeability although they need to be subjected to high initial pressure (20% or more CO2 concentration) for maximum efficiency. Pre-combustion capture, common in industry, involves partial oxidation of the fuels to produce CO2/CO and H2 mixture, and then subsequent separation of hydrogen as a clean fuel (Garg et al., 2025; Magadum, Murgod, Garg, et al., 2025; Yadav, S, et al., 2025). Physical and chemical absorbents are used, while physical absorbents involve less energy input to regenerate by pressure swing or low-temperature heating. While pre-combustion technology can be very effective at reducing carbon emissions (as much as 90.9% using Selexol), it is accompanied by large energy penalties and involves major auxiliary systems, again pointing to the challenge of maintaining capture efficiency against the cost (Ben-Mansour et al., 2016; Heydari-Gorji et al., 2011; Murgod et al., 2025a; Yadav et al., 2025).
Figure 3.
Post-combustion carbon capture techniques.
Figure 3.
Post-combustion carbon capture techniques.
5. Oxy-Fuel Combustion and Carbon Dioxide Usage Pathways
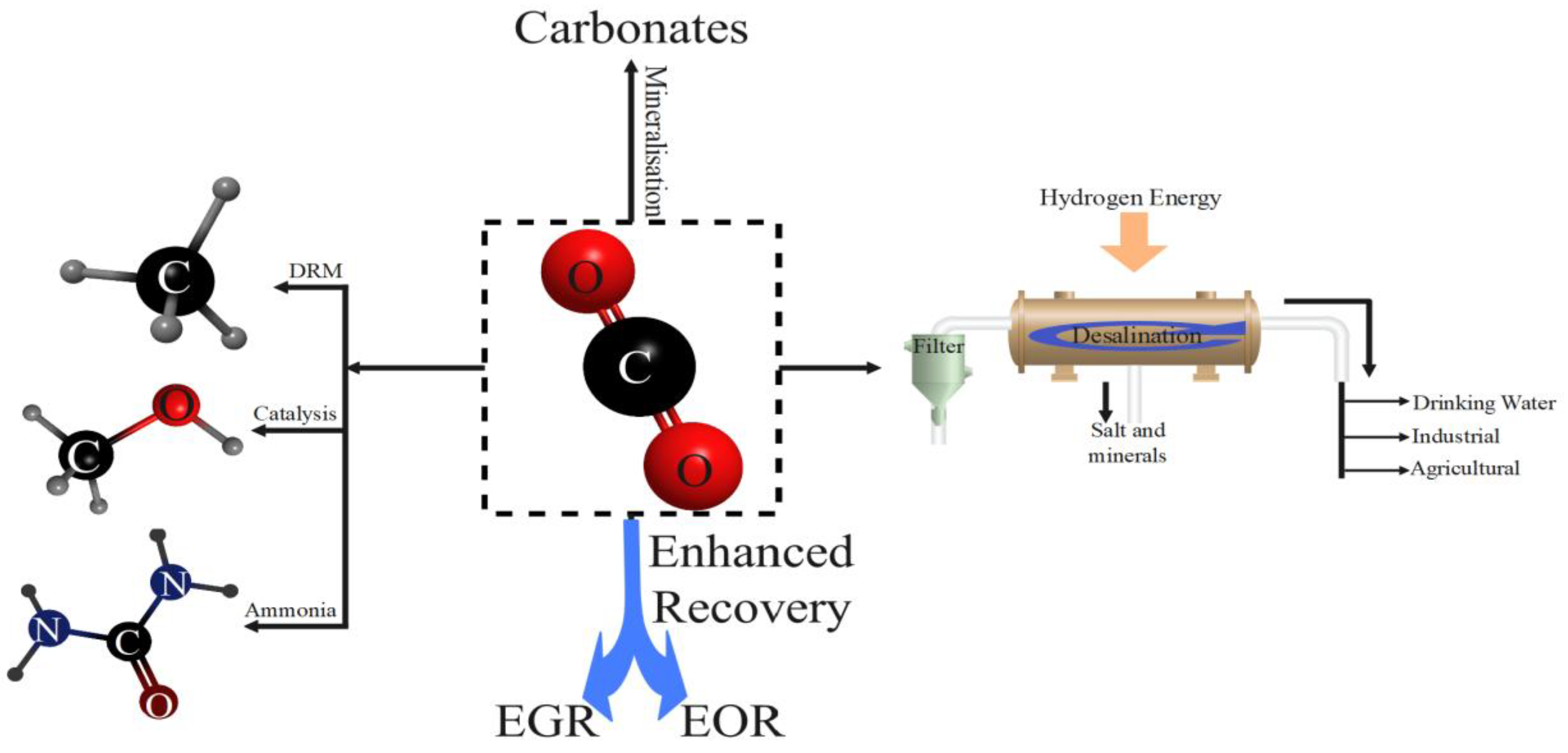
Oxy-fuel combustion is a carbon capture technology advancement where pure oxygen is utilized rather than air when burning, leaving a flue gas with elevated CO2 and H2O content but without nitrogen contamination. This process avoids the need for advanced carbon capture processes, as water vapor can be readily removed by cooling and compression, leaving virtually pure CO2 for storage or utilization. Although it has the efficiency benefits of increased flue gas temperatures that allow for heat recovery through partial recycling of gases, the primary cost driver of oxy-fuel combustion is the air separation unit used to provide oxygen. Compared to other carbon capture technologies, oxy-fuel combustion shows comparable efficiency in both coal-based (around 30%) and natural gas-based systems, especially with the Allam cycle breakthrough (Lasek et al., 2013; Mittal, Sehrawat, et al., 2025; Mittal & Kushwaha, 2024, 2025; Toftegaard et al., 2010). Apart from capture, the use of carbon dioxide presents various avenues for utilizing emissions in valuable products, with some current uses being refrigerants, extractants, carbonated drinks, fire suppressors, and enhanced oil recovery. Although current applications account for only 2-3% of all CO2 emissions, technological innovation is expanding utilization potential through four primary pathways: chemical conversion, mineralization, desalination, and improved recovery. Although the expanding list of CO2 utilization technologies is evidence that technology is gaining ground, economic viability remains a primary challenge, with fewer than 1% of atmospheric CO2 emissions being reused as raw material, indicating continued innovation is necessary to turn carbon dioxide from waste stream to valuable resource (Cuéllar-Franca & Azapagic, 2015; Hong, 2022; Rout et al., 2025; Yadav, Mittal, et al., 2025a).
Figure 4.
Pathways of Carbon Dioxide utilisation.
Figure 4.
Pathways of Carbon Dioxide utilisation.
6. Carbon Dioxide Conversion Technologies: Chemical and Biological Conversion Technologies
Carbon dioxide utilization technologies offer excellent prospects for reducing greenhouse gas emissions by chemical and biological conversion technologies. Chemical conversion of CO2 can lead to useful chemicals like organic carbonates cyclic carbonates, polycarbonates, formic acid, urea, and other fine chemicals (Centi et al., 2013; Chatzifragkou et al., 2011; Ibrahim et al., 2023; Maeda et al., 2014; Thomare et al., 2025). However, these processes are challenged by key issues like high operating conditions, complex reaction pathways, catalyst stability issues, and low levels of conversion. Biological conversion methods, both photosynthetic and non-photosynthetic, offer other routes with mixed benefits. Microalgae systems have special promise based on efficient photosynthesis and extensive use for wastewater treatment, feedstock, and biofuels, even though they are not economical. While limitations now prevail, the continuing improvement of catalysts, process engineering, and genetic modification of microorganisms show great potential for carbon-free chemical production systems compatible with sustainable development (Costentin et al., 2013; Mittal, Yadav, et al., 2025; Rashidi et al., 2013).
7. Carbon Dioxide Utilization and Storage: Enhanced Recovery, Mineralization, and Geological Sequestration
Carbon dioxide storage and utilization technologies provide promising opportunities to reduce atmospheric CO2 concentrations and create economic value. EOR/EGR utilizes injection of CO2 to pressure up reservoirs and mobilize and produce trapped hydrocarbons using processes like continuous gas injection and water alternating gas, but there are still issues with rock formation heterogeneity, viscous fingering, and corrosion (Álvarez-Murillo et al., 2016; Azdarpour et al., 2015; Magadum, Murgod, Mittal, et al., 2025; Sehrawat et al., 2025; Tsutsumi et al., 2010; Yadav et al., 2025). Mineralization processes irreversibly trap CO2 by precipitating stable carbonates by reacting with metal oxides, such as calcium and magnesium silicates, with both ex-situ and in-situ processes being promising despite kinetic constraints. CO2-based desalination presents other water treatment options, such as ammonia-carbon dioxide forward osmosis and hydrate-forming processes, although economic feasibility is difficult. Sedimentary formation storage in depths of more than 1,000 meters offers vast sequestration potential through several different trapping mechanisms structural, residual, solubility, and mineral trapping via depleted oil/gas fields, unmineable coal seams, and deep saline aquifers as main storage reservoirs. While currently with limited application, each of these technologies as a group constitutes crucial parts of effective carbon management policies that are needed for achieving climate targets (Gür, 2022b; Naims, 2016).
8. Conclusion
The wide-ranging review of carbon dioxide control technologies discloses optimistic innovations as well as overwhelming application hurdles. Although technological improvements have been achieved with capture technologies and storage a disappointing gap continues to exist between emission reduction commitments and real emission decreases. To meaningful climate influence, synergistic methodologies merging capture, utilization, and storage must be engineered specifically for the nature of particular emissions and site-specific factors, reinforced through policy guidelines and economic incentives augmenting cost favorability and moving towards acceleration in scale-up to industrial magnitude. Subsequent Research and development research endeavors need to revolve around streamlining energy input needs, refining conversion ratios, and delivering affordability options bridging the bridge from existing emission trajectories toward carbon zero in sustainable business growth models.
References
- Adams, S., & Nsiah, C. (2019a). Reducing carbon dioxide emissions; Does renewable energy matter? Science of The Total Environment, 693, 133288. [CrossRef]
- Adams, S., & Nsiah, C. (2019b). Reducing carbon dioxide emissions; Does renewable energy matter? Science of The Total Environment, 693, 133288. [CrossRef]
- Aldy, J. E. (2006). Per Capita Carbon Dioxide Emissions: Convergence or Divergence? Environmental & Resource Economics, 33(4), 533–555. [CrossRef]
- Alsaleh, M., Yang, Z., Chen, T., Wang, X., Abdul-Rahim, A. S., & Mahmood, H. (2023). Moving toward environmental sustainability: Assessing the influence of geothermal power on carbon dioxide emissions. Renewable Energy, 202, 880–893. [CrossRef]
- Álvarez-Murillo, A., Sabio, E., Ledesma, B., Román, S., & González-García, C. M. (2016). Generation of biofuel from hydrothermal carbonization of cellulose. Kinetics modelling. Energy, 94, 600–608. [CrossRef]
- Azdarpour, A., Guo, W., Asadullah, M., Asadullah, M., Mohammadian, E., Hamidi, H., Junin, R., & Karaei, M. A. (2015). A review on carbon dioxide mineral carbonation through pH-swing process. Chemical Engineering Journal. [CrossRef]
- Ben-Mansour, R., Habib, M. A., Habib, M. A., Bamidele, O. E., Bamidele, O. E., Basha, M., Basha, M., Qasem, N. A. A., Peedikakkal, A. M. P., Laoui, T., Ali, M., Ali, M., Ali, M., Ali, M., Ali, M., & Ali, Md. H. (2016). Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations - A review. Applied Energy. [CrossRef]
- Centi, G., Quadrelli, E. A., & Perathoner, S. (2013). Catalysis for CO2 conversion: a key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy and Environmental Science. [CrossRef]
- Chatzifragkou, A., Chatzifragkou, A., Makri, A., Makri, A., Belka, A., Belka, A., Bellou, S., Bellou, S., Mavrou, M., Mavrou, M., Mastoridou, M., Mastoridou, M., Mystrioti, P., Mystrioti, P., Onjaro, G., Onjaro, G., Aggelis, G., Aggelis, G., Papanikolaou, S., & Papanikolaou, S. (2011). Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy. [CrossRef]
- Costentin, C., Robert, M., Robert, M., & Savéant, J.-M. (2013). Catalysis of the electrochemical reduction of carbon dioxide. Chemical Society Reviews. [CrossRef]
- Cuéllar-Franca, R., & Azapagic, A. (2015). Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. Journal of CO 2 Utilization. [CrossRef]
- D’Alessandro, D. M., Smit, B., & Long, J. R. (2010). Carbon Dioxide Capture: Prospects for New Materials. Angewandte Chemie. [CrossRef]
- Ezcurra, R. (2007). Is there cross-country convergence in carbon dioxide emissions? Energy Policy, 35(2), 1363–1372. [CrossRef]
- Garg, K., Mittal, H., Yadav, V., Sehrawat, A., Shah, V., & Kushwaha, O. (2025). Municipal Solid Waste (MSW) Management Prediction Through Machine Learning Models: An Ensemble Tree Regressor Analysis. [CrossRef]
- Glenna, D. M., Jana, A., Xu, Q., Wang, Y., Meng, Y., Yang, Y., Neupane, M., Wang, L., Zhao, H., Qian, J., & Snyder, S. W. (2023). Carbon Capture: Theoretical Guidelines for Activated Carbon-Based CO2 Adsorption Material Evaluation. Journal of Physical Chemistry Letters, 10693–10699. [CrossRef]
- Gür, T. M. (2022a). Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Progress in Energy and Combustion Science, 89, 100965. [CrossRef]
- Gür, T. M. (2022b). Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Progress in Energy and Combustion Science, 89, 100965. [CrossRef]
- Heydari-Gorji, A., Belmabkhout, Y., & Sayari, A. (2011). Polyethylenimine-impregnated mesoporous silica: effect of amine loading and surface alkyl chains on CO2 adsorption. Langmuir. [CrossRef]
- Hong, W. Y. (2022). A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future. Carbon Capture Science & Technology, 3, 100044. [CrossRef]
- Ibrahim, I., Salehmin, M. N. I., Balachandran, K., Me, M. F. H., Loh, K. S., Bakar, M. H. A., Jong, B. C., & Lim, S. S. (2023). Role of microbial electrosynthesis system in CO2 capture and conversion: a recent advancement toward cathode development. In Frontiers in Microbiology (Vol. 14). Frontiers Media SA. [CrossRef]
- Lasek, J., Janusz, M., Zuwała, J., Głód, K., & Iluk, A. (2013). Oxy-fuel combustion of selected solid fuels under atmospheric and elevated pressures. Energy. [CrossRef]
- Lin, Q., Zhang, X., Wang, T., Zheng, C., & Gao, X. (2022). Technical Perspective of Carbon Capture, Utilization, and Storage. Engineering, 14, 27–32. [CrossRef]
- Maeda, C., Miyazaki, Y., & Ema, T. (2014). Recent progress in catalytic conversions of carbon dioxide. Catalysis Science & Technology. [CrossRef]
- Magadum, T., Garg, K., Murgod, S., Yadav, V., Mittal, H., & Kushwaha, O. (2025). Geospatial Analysis in Machine Learning for CO2 Emissions Prediction Analysis in 2100: A Continent-Wise Analysis. [CrossRef]
- Magadum, T., Murgod, S., Garg, K., Yadav, V., Mittal, H. N., & Kushwaha, O. (2025). Africa Renewable Energy Development to 2050: Forecast Analysis through a Machine Learning Perspective. [CrossRef]
- Magadum, T., Murgod, S., Mittal, H., Anshu, D., & Kushwaha, O. (2025). <span style="mso-fareast-language: EN-IN;">Global Wind Energy Generation Trends and Projections: A Comprehensive Analysis to 2050. [CrossRef]
- Mascarenhas, J. dos S., Chowdhury, H., Thirugnanasambandam, M., Chowdhury, T., & Saidur, R. (2019). Energy, exergy, sustainability, and emission analysis of industrial air compressors. Journal of Cleaner Production, 231, 183–195. [CrossRef]
- Mittal, H., & Kushwaha, O. S. (2024). Emerging Nanofiber Technology for the Purification of Wastewater Treatment Facilities for Elimination of Ions. In Innovative and Hybrid Technologies for Wastewater Treatment and Recycling (pp. 306–325). CRC Press. [CrossRef]
- Mittal, H., & Kushwaha, O. S. (2025). Chapter 3 Dynamic blowers: classification, characteristics, applications, and industrial scalability. In Compressors and Blowers (pp. 55–70). De Gruyter. [CrossRef]
- Mittal, H., Sehrawat, A., & Kushwaha, O. (2025). Energy, Environment and Biomedical Applications of Boron Nitride and Boron Nitride Carbon Nanotubes: A Sustainable Industrial Scale-Up Exegesis. [CrossRef]
- Mittal, H., Yadav, V., Shah, V., & Kushwaha, O. (2025). Finite Element Analysis of Highly Stable Boron Nitride Nanotubes- Based Storage Tanks. [CrossRef]
- Mondal, M. K., Balsora, H. K., Varshney, P., & Varshney, P. (2012). Progress and trends in CO2 capture/separation technologies: A review. Energy. [CrossRef]
- Murgod, S., Garg, K., Magadum, T., Yadav, V., Mittal, H., & Kushwaha, O. (2025a). AI Powered Renewable Energy Balancing, Forecasting and Global Trend Analysis using ANN-LSTM Integration. [CrossRef]
- Murgod, S., Garg, K., Magadum, T., Yadav, V., Mittal, H., & Kushwaha, O. S. (2025b). CO₂ Emissions Projections for 2100: A Comparative Machine Learning Study of U.S. and Multimodal Approach of Global Trends. [CrossRef]
- Naims, H. (2016). Economics of carbon dioxide capture and utilization-a supply and demand perspective. Environmental Science and Pollution Research. [CrossRef]
- Peng, J., Iruretagoyena, D., & Chadwick, D. (2018). Hydrotalcite/SBA15 composites for pre-combustion CO 2 capture: CO 2 adsorption characteristics. Journal of CO 2 Utilization. [CrossRef]
- Pérez-Fortes, M., Pérez-Fortes, M., Schöneberger, J. C., Boulamanti, A., & Tzimas, E. (2016). Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Applied Energy. [CrossRef]
- Pettersson, F., Maddison, D., Acar, S., & Soderholm, P. (2014). Convergence of Carbon Dioxide Emissions: A Review of the Literature. International Review of Environmental and Resource Economics, 7(2), 141–178. [CrossRef]
- Praveenkumar, V., Mittal, H. N., & Kushwaha, O. S. (2024). Analysing Industrial Scale-Up of Carbon Dioxide Capture in Aqueous Amino Acids for Sustainable Technologies (pp. 39–55). [CrossRef]
- Rashidi, N. A., Yusup, S., & Hameed, B. H. (2013). Kinetic studies on carbon dioxide capture using lignocellulosic based activated carbon. Energy. [CrossRef]
- Rout, D., Shyamsukha, N., Mittal, H., & Kushwaha, O. S. (2025). Solar energy generation and power prediction through computer vision and machine intelligence. In Computer Vision and Machine Intelligence for Renewable Energy Systems (pp. 103–123). Elsevier. [CrossRef]
- Saraswat, V., Magadum, T., Mittal, H., & Kushwaha, O. (2025). AI-Driven Modeling of Microbial Carbon Capture Systems for ESG-Linked Carbon Accounting and Disclosures. [CrossRef]
- Schmalensee, R., Stoker, T. M., & Judson, R. A. (1998). World Carbon Dioxide Emissions: 1950–2050. Review of Economics and Statistics, 80(1), 15–27. [CrossRef]
- Sehrawat, A., Bhatnagar, R. M., Magadum, T., Mittal, H., & Kushwaha, O. (2025). Comparative Analysis of Bio-Based and Traditional Plastics: Life Cycle Assessment, Cost-Benefit Analysis, and Health Impact Evaluation. [CrossRef]
- Thomare, C., Magadum, T., & Mittal, H. (2025). Conversion of Cow Dung to Electricity: Process Analysis and Energy Yield Assessment. [CrossRef]
- Toftegaard, M. B., Brix, J., Jensen, P. A., Glarborg, P., & Jensen, A. D. (2010). Oxy-fuel combustion of solid fuels. Progress in Energy and Combustion Science. [CrossRef]
- Tsutsumi, Y., Yamakawa, K., Yoshida, M., Ema, T., & Sakai, T. (2010). Bifunctional Organocatalyst for Activation of Carbon Dioxide and Epoxide To Produce Cyclic Carbonate: Betaine as a New Catalytic Motif. Organic Letters. [CrossRef]
- Yadav, V., Deepanshu, Mittal, H., Shah, V., & Kushwaha, O. S. (2025). Fuel Cell Degradation Prediction Using Machine Learning Models: A Study on Proton Exchange Membrane (PEM) Fuel Cell Dataset. [CrossRef]
- Yadav, V., Mittal, H., Shah, V., & Kushwaha, O. (2025a). Green Synthesis and Characterization of Cellulose Acetate Phthalate/Carboxylated MWCNT Composites for Circular Economy Applications. [CrossRef]
- Yadav, V., Mittal, H., Shah, V., & Kushwaha, O. S. (2025b). Environmental Conversation and Safety Analysis of Ammonia Storage Tanks: An Indian Perspective from Lab to Industrial Scale. [CrossRef]
- Yadav, V., Mittal, H., Shah, V., & Kushwaha, O. S. (2025c). Sustainability Analysis of Polymers, Fibres and Nanomaterials for Ballistic Applications. [CrossRef]
- Yadav, V., S, K., Sehrawat, A., Magadum, T., Mittal, H., Shah, V., & Kushwaha, O. (2025). Sustainable Development and Advanced Technologies: Properties, Perspectives, and Applications of Synthetic Aerogels. [CrossRef]
- Yadav, V., Sehrawat, A., Magadum, T., Mittal, H., Shah, V., & Kushwaha, O. (2025). Green Hydrogen Generation Through Novel Electrolysers Towards Low Carbon Economy: An Opiniated Perspective. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).