Submitted:
27 June 2025
Posted:
30 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Effects of PGRs and CW on Asymbiotic Seed Germination
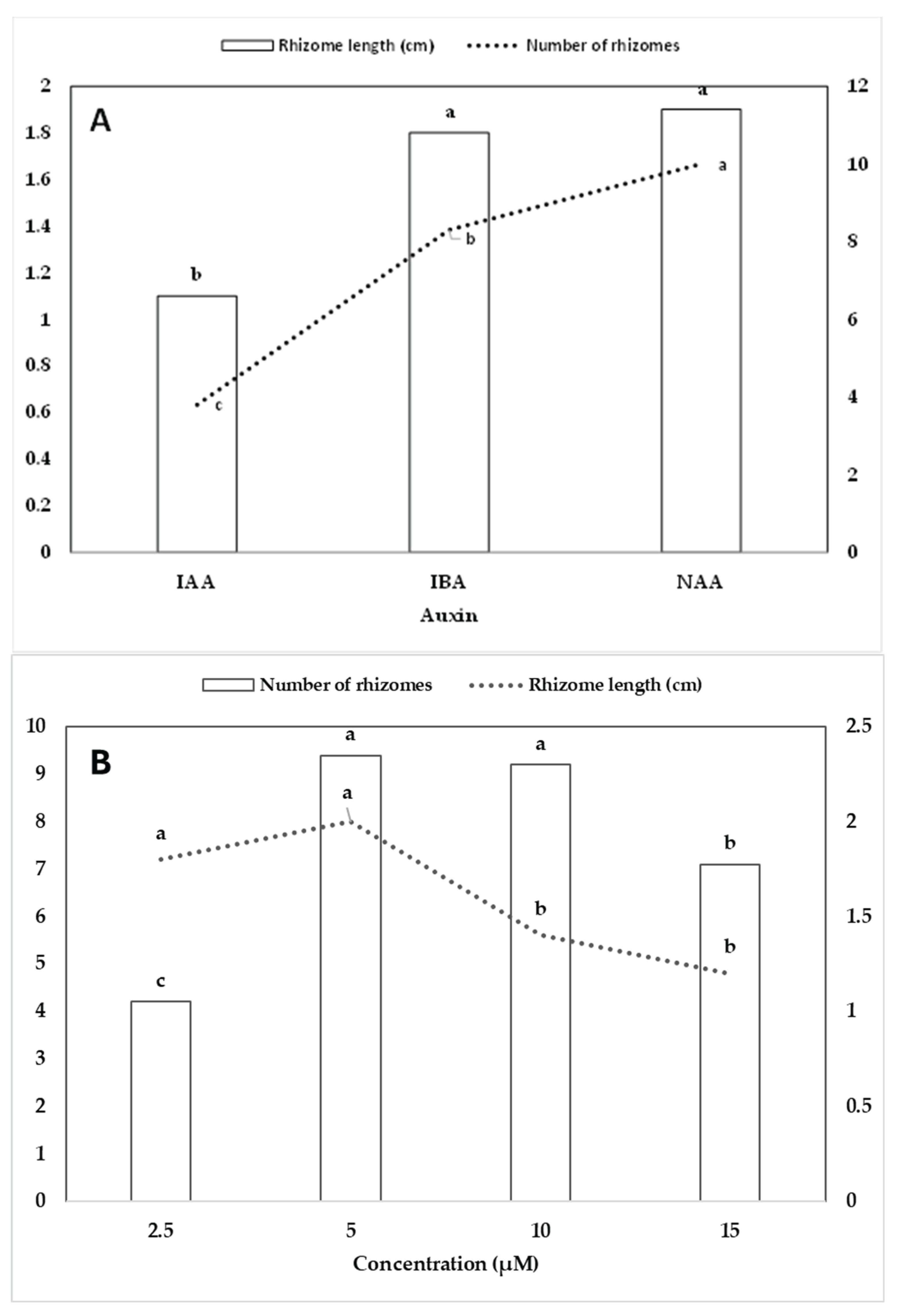
2.2. Effects of Auxins on Rhizome Proliferation
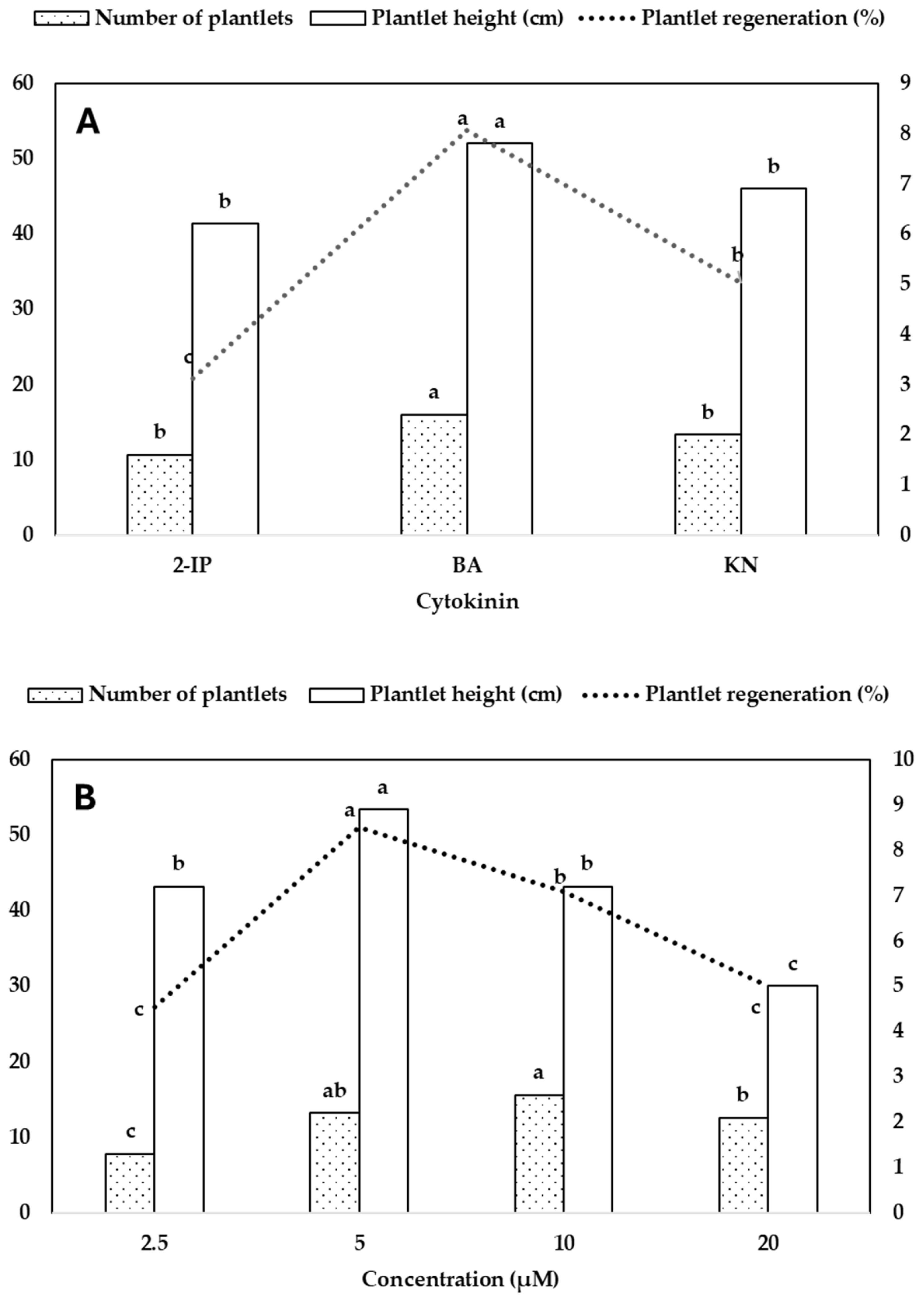
2.3. Effects of Cytokinins on Plantlet Regeneration
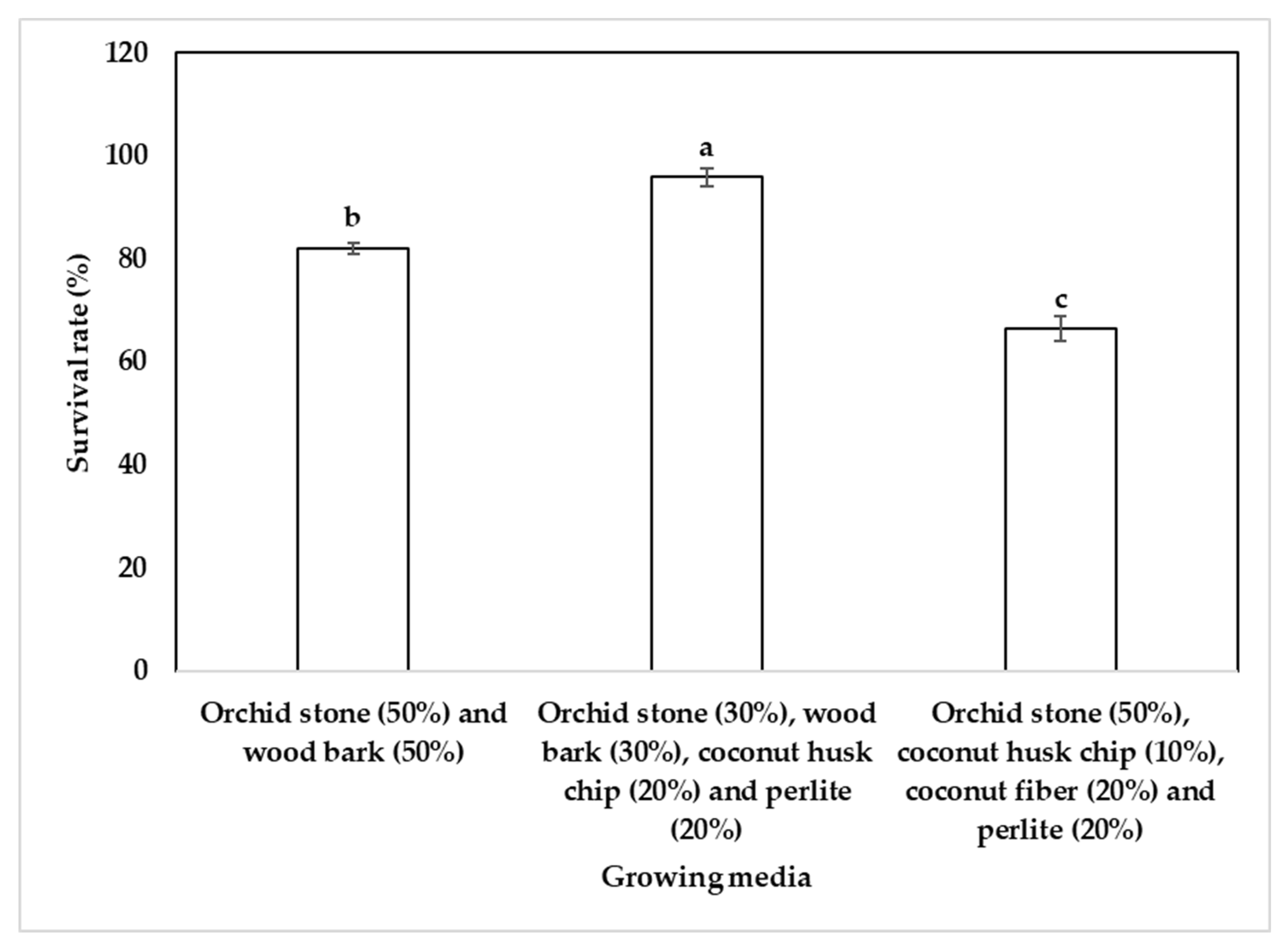
2.4. Acclimatization of Plantlets
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Surface Disinfection
4.2. Asymbiotic Seed Germination
4.3. Rhizome Proliferation
4.4. Plantlet Regeneration
4.5. Acclimatization
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Activated charcoal |
| CW | Coconut water |
| GA3 | Gibberellic acid |
| IAA | Indole-3-acetic acid |
| IBA | Indole-3-butyric acid |
| KN | Kinetin |
| MS | Murashige and Skoog |
| 2-IP | N6-(2-isopentenyl)adenine |
| BA | N6-benzyladenine |
| PPFD | Photosynthetic photon flux density |
| PGRs | Plant growth regulators |
| WLEDs | White light-emitting diodes |
| NAA | α-naphthalene acetic acid |
References
- Teoh, E.S. Cymbidium Sw. In Orchid Species from Himalaya and Southeast Asia, Teoh, E.S., Ed.; Springer Nature: Switzerland AG, Cham, Sw. In Orchid Species from Himalaya and Southeast Asia, Teoh, E.S., Ed.; Springer Nature: Switzerland AG, Cham, Switzerland, 2021; Volume 1, pp. 265–286. [Google Scholar]
- Park, H.Y.; Kang, K.W.; Kim, D.H.; Sivanesan, I. In vitro propagation of Cymbidium goeringii Reichenbach fil. through direct adventitious shoot regeneration. Physiol. Mol. Biol. Plants 2018, 24, 307–313. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, H.J.; Kim, K.S. Night interruption promotes vegetative growth and flowering of Cymbidium. Sci. Hortic. 2011, 130, 887–893. [Google Scholar] [CrossRef]
- De, L.C.; Singh, R. Organic Production of Cymbidium Orchids. Acta Sci. Agric. 2018, 2, 30–35. [Google Scholar]
- Lee, J.S.; Choe, B.H. Distributions and red data of wild orchids in the Korean peninsula. Korean J. Plant Taxon. 2006, 36, 335–360. [Google Scholar] [CrossRef]
- Balilashaki, K.; Martinez-Montero, M.E.; Vahedi, M.; Cardoso, J.C.; Silva Agurto, C.L.; Leiva-Mora, M.; Feizi, F.; Musharof Hossain, M. Medicinal Use, Flower Trade, Preservation and Mass Propagation Techniques of Cymbidium Orchids—An Overview. Horticulturae 2023, 9, 690. [Google Scholar] [CrossRef]
- Hussien, M.; Kryuchkova, V.; Raeva-Bogoslovskaya, E.; Molkanova, O. Clonal Micropropagation of Cymbidium erythrostylum Rolfe. Int. J. Plant Biol. 2023, 14, 28–38. [Google Scholar] [CrossRef]
- Yao, M.; Wu, C.; Huang, W.; Fang, Z. Plant Growth Regulator-based Tissue Culture System Optimization for Cymbidium faberi Rolfe. HortScience 2024, 59, 1358–1368. [Google Scholar] [CrossRef]
- Monica, H.; Kumaria, S. Exogenous application of chitosan, a potent biotic elicitor enhances micropropagation efficiency of Cymbidium aloifolium (L.) Sw., an orchid of medicinal and horticultural importance. Vegetos 2025, 38, 58–69. [Google Scholar] [CrossRef]
- Li, F.; Bao, J.; Sun, Y.; Liu, C.; Ma, H.; Zhang, T.; Chen, X. Embryo development and corresponding factors affecting in vitro germination of Cymbidium faberi × C. sinense hybrid seeds. Arch. Biol. Sci. 2016, 68, 541–550. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sharma, M.; da Silva, J.A.T.; Pathak, P. Seed germination and tissue culture of Cymbidium giganteum Wall. ex Lindl. Sci. Hortic. 2010, 123, 479–487. [Google Scholar] [CrossRef]
- Pradha, S.; Pant, B. In vitro seed germination in Cymbidium elegans Lindl. and Dendrobium densiflorum Lindl. ex Wall. (Orchidaceae). Botanica Orientalis. J. Plant Sci. 2010, 6, 100–102. [Google Scholar] [CrossRef]
- Pant, B.; Swar, S. Micropropagation of Cymbidium iridioides. Nepal J. Sci. Technol. 2011, 12, 91–96. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, S.; Rattan, S.; Warghat, A.R.; Kumar, D.; Bhargava, B. In vitro propagation and phyto-chemical assessment of Cymbidium aloifolium (L.) Sw.: An orchid of pharma-horticultural importance. S. Afr. J. Bot. 2022, 144, 261–269. [Google Scholar] [CrossRef]
- Pradhan, S.; Regmi, T.; Parmar, G.; Pant, B. Effect of different media on in vitro seed germination and seedling development of Cymbidium aloifolium (L.) Sw. Nepal J. Sci. Technol. 2013, 14, 51–56. [Google Scholar] [CrossRef]
- Tawara, S.; Suraninpong, P.; Chanprame, S. Germination and Regeneration of Cymbidium findlaysonianum Lindl. on a medium supplemented with some organic sources. Walailak J. Sci. Technol. 2008, 5, 125–135. [Google Scholar]
- Fang, Z.; Huang, W.; Zeng, S.; Wu, K. In vitro propagation of Cymbidium nanutum YS Wu et SC Chen. Propag. Ornam. Plants 2011, 11, 149–155. [Google Scholar]
- Kim, J.Y.; Lee, J.S. Effect of growth regulators on rhizome growth and differentiation of Cymbidium lancifolium, a native Korean bamboo orchid. Korean Soc. Hortic. Sci. Acad. Pres. Summ. 1991, 9(1), 174–175. [Google Scholar]
- Kim, J.Y.; Lee, J.S. Effect of cultural conditions on rhizome growth and organogenesis of Cymbidium lancifolium native to Korea in vitro. J. Korean Soc. Hortic. Sci. 1992, 33, 471–476. [Google Scholar]
- Yeung, E.C. A perspective on orchid seed and protocorm development. Bot. Stud. 2017, 58, 33. [Google Scholar] [CrossRef]
- Tikendra, L.; Singh, A.R.; Vendrame, W.A.; Nongdam, P. In Vitro Propagation of Endangered Vanda coerulea Griff. ex Lindl.: Asymbiotic Seed Germination, Genetic Homogeneity Assessment, and Micro-Morpho-Anatomical Analysis for Effective Conservation. Agronomy 2025, 15, 1195. [Google Scholar] [CrossRef]
- Paek, K.Y.; Murthy, H.N. Temperate oriental Cymbidium species. In Orchid biology reviews and perspectives, Kull, T., Arditti, J., Eds.; Kluwer Academic Publishers: Dordrecht, Netherlands, 2002; Volume 8. [Google Scholar]
- Li, Y.; Chen, H.; Kong, X.; Yin, Y.; Li, J.; Wu, K.; Zeng, S.; Fang, L. Excessive accumulation of auxin inhibits protocorm development during germination of Paphiopedilum spicerianum. Plant Cell Rep. 2025, 44, 23. [Google Scholar] [CrossRef] [PubMed]
- Deb, C.R.; Pongener, A. Asymbiotic seed germination and in vitro seedling development of Cymbidium aloifolium (L.) Sw: A multipurpose orchid. J. Plant Biochem. Biotechnol. 2011, 20, 90–95. [Google Scholar] [CrossRef]
- Utami, E.S.W.; Hariyanto, S. Organic compounds: Contents and their role in improving seed germination and protocorm development in orchids. Int. J. Agron. 2020, 2020, 2795108. [Google Scholar] [CrossRef]
- Anghelescu, N.E.; Vafaee, Y.; Ahmadzadeh, K.; Chen, J.T. Asymbiotic Seed Germination in Terrestrial Orchids: Problems, Progress, and Prospects. In: Advances in Orchid Biology Biotechnology and Omics, Tiwari, P., Chen, J.T., Eds.; Springer, Singapore, 2023, pp 221–260.
- Tan, S.N.; Yong, J.W.H.; Ge, L. Analyses of Phytohormones in Coconut (Cocos nucifera L.) Water Using Capillary Electrophoresis-Tandem Mass Spectrometry. Chromatography 2014, 1, 211–226. [Google Scholar] [CrossRef]
- Huh, Y.S.; Lee, J.K.; Nam, S.Y.; Paek, K.Y.; Suh, G.U. Improvement of asymbiotic seed germination and seedling development of Cypripedium macranthos Sw. with organic additives. J. Plant Biotechnol. 2016, 43, 138–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Kan, J.; Liu, X.; Song, F.; Zhu, K.; Li, N.; Zhang, Y. Chemical Components, Nutritional Value, Volatile Organic Compounds and Biological Activities In Vitro of Coconut (Cocos nucifera L.) Water with Different Maturities. Foods 2024, 13, 863. [Google Scholar] [CrossRef]
- Rohmah, K.N.; Taratima, W. Effect of chitosan, coconut water and potato extract on protocorm growth and plantlet regeneration of Cymbidium aloifolium (L.) Sw. Curr. Appl. Sci. Technol. 2022, 22, 1–10. [Google Scholar]
- Parmar, G. In vitro seed germination and seedling development of Cymbidium devonianum Paxton (Orchidaceae). Bull. Dept. Plant Res. 2014, 36, 61–64. [Google Scholar]
- Kim, D.H.; Kang, K.W.; Sivanesan, I. In vitro propagation of Cymbidium hybrid. Propag. Ornam. Plants 2017, 17, 48–54. [Google Scholar]
- Paul, M.; Islam, T.; Sarker, R.H.; Hoque, M.I. In vitro mass propagation of Cymbidium aloifolium (L.) Sw. Plant Tissue Cult. Biotechnol. 2019, 29, 73–79. [Google Scholar] [CrossRef]
- Tao, J.; Yu, L.; Kong, F.; Zhao, D. Effects of plant growth regulators on in vitro propagation of Cymbidium faberi Rolfe. Afr. J. Biotechnol. 2011, 10, 15639–15646. [Google Scholar] [CrossRef]
- Gogoi, K.; Kumaria, S.; Tandon, P. Ex situ conservation of Cymbidium eburneum Lindl.: A threatened and vulnerable orchid, by asymbiotic seed germination. 3 Biotech. 2012, 2, 337–343. [Google Scholar] [CrossRef]
- Nyorak, J.; Sonar, C.B. In vitro mass multiplication of Cymbidium iridioides D. Don–a medicinal orchid from Arunachal Pradesh, India. Pleione 2020, 14, 49–55. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Huang, L.; Su, J. In Vitro Mass Scale Propagation of Wild Cymbidium lowianum with a Rare and Endangered Plant. Am. J. Plant Sci. 2013, 4, 34755. [Google Scholar] [CrossRef]
- Kang, T.J.; Yang, D.C. Days to germination and effect of growth regulator on rhizome growth in Cymbidium goeringii Hybrid. Korean J. Plant Res. 2003, 6, 144–148. [Google Scholar]
- Teixeira da Silva, J.A.; Tsavkelova, E.A.; Ng, T.B.; Parthibhan, S.; Dobránszki, J.; Cardoso, J.C.; Rao, M.V.; Zeng, S. Asymbiotic in vitro seed propagation of Dendrobium. Plant Cell Rep. 2015, 34, 1685–1706. [Google Scholar] [CrossRef]
- Thummavongsa, T.; Musimun, C.; Watthana, S.; Gale, S.; Choeyklin, R.; Wiriyathanawudhiwong, N.; Muangsan, N. Enhancing Germination of Habenaria janellehayneana (Orchidaceae): Insight from Asymbiotic and Symbiotic Methods. J. Ornam. Plants 2024, 14, 11–23. [Google Scholar]
- Manrique, J.P.; Fernandex-Lizarazo, C.; Suarez-Silva, A. Evaluation of the effect of three growth regulators in the germination of Comparetia falcate seeds under in vitro conditions. In Vitro Cell. Dev. Biol. Plant 2005, 41, 838–843. [Google Scholar] [CrossRef]
- Kang, H.; Kang, K.W.; Kim, D.H.; Sivanesan, I. In Vitro Propagation of Gastrochilus matsuran (Makino) Schltr., an Endangered Epiphytic Orchid. Plants 2020, 9, 524. [Google Scholar] [CrossRef]
- Paek, K.Y.; Kozai, T. Micropropagation of temperate Cymbidium via rhizome culture. HortTechnology 1998, 8, 283–288. [Google Scholar] [CrossRef]
- Nayak, N.R.; Rath, S.P.; Patnaik, S. In vitro propagation of three epiphytic orchids, Cymbidium aloifolium (L.) Sw., Dendrobium aphyllum (Roxb.), Fisch. and Dendrobium moschatum (Buch-Ham) Sw. through thidiazuron-induced high frequency shoot proliferation. Sci. Hortic. 1997, 71, 243–250. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Liu, Y. In vitro plant regeneration from the immature seeds of Cymbidium faberi. Plant Cell Tissue Organ Cult. 2005, 81, 247–251. [Google Scholar] [CrossRef]
- Paek, K.Y.; Yeung, E.C. The effects of 1-naphthaleneacetic acid and N6-benzyladenine on the growth of Cymbidium forrestii rhizomes in vitro. Plant. Cell Tissue Organ. Cult. 1991, 24, 65–71. [Google Scholar] [CrossRef]
- Huang, C. L.; Okubo, H. In vitro morphogenesis from rhizomes of Cymbidium sinense. J. Fac. Agr., Kyushu Univ. 2005, 50, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, K.; Uemoto, S. Micropropagation of a terrestrial Cymbidium species using rhizomes developed from seeds and pseudobulbs. Plant Cell Tissue Organ Cult. 1990, 22, 237–244. [Google Scholar] [CrossRef]
- Nayak, N.R.; Chand, P.K.; Rath, S.P.; Patnaik, S. Influence of some plant growth regulators on the growth and organogenesis of Cymbidium aloifolium (L.) Sw. seed derived rhizomes in vitro. In Vitro Cell. Dev. Biol. Plant 1998, 34, 185–188. [Google Scholar] [CrossRef]

| PGRs (µM) | Coconut water (mL/L) | Seed germination (%) | |||
| IAA | NAA | KN | GA3 | ||
| 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 ± 0.1 l |
| 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 ± 0.3 l |
| 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.2 ± 0.1 kl |
| 2.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.4 ± 0.5 jk |
| 4.0 | 0.0 | 0.0 | 0.0 | 0.0 | 8.3 ± 1.2 ij |
| 8.0 | 0.0 | 0.0 | 0.0 | 0.0 | 10.2 ± 1.1 hi |
| 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 3.0 ± 0.4 kl |
| 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 6.2 ± 0.8 ijk |
| 0.0 | 2.0 | 0.0 | 0.0 | 0.0 | 8.9 ± 1.1 hij |
| 0.0 | 4.0 | 0.0 | 0.0 | 0.0 | 16.8 ± 1.5 f |
| 0.0 | 8.0 | 0.0 | 0.0 | 0.0 | 12.6 ± 1.3 gh |
| 0.0 | 4.0 | 0.0 | 0.0 | 25 | 18.1 ± 1.1 ef |
| 0.0 | 4.0 | 0.0 | 0.0 | 50 | 26.2 ± 2.0 d |
| 0.0 | 4.0 | 0.0 | 0.0 | 75 | 20.9 ± 1.6 e |
| 0.0 | 4.0 | 0.0 | 0.0 | 100 | 14.3 ± 1.3 fg |
| 0.0 | 4.0 | 2.3 | 0.0 | 50 | 32.2 ± 1.7 c |
| 0.0 | 4.0 | 4.7 | 0.0 | 50 | 27.8 ± 1.5 d |
| 0.0 | 4.0 | 9.4 | 0.0 | 50 | 28.3 ± 1.7 d |
| 0.0 | 4.0 | 2.3 | 0.3 | 50 | 33.3 ± 2.0 c |
| 0.0 | 4.0 | 2.3 | 1.4 | 50 | 40.6 ± 1.8 b |
| 0.0 | 4.0 | 2.3 | 2.9 | 50 | 46.8 ± 1.8 a |
| Auxin (µM) | Number of rhizomes | Rhizome length (cm) | ||
| IAA | IBA | NAA | ||
| 0.0 | 0.0 | 0.0 | 1.3 ± 0.2 h | 0.5 ± 0.1 f |
| 2.5 | 0.0 | 0.0 | 1.9 ± 0.4 gh | 1.0 ± 0.1 ef |
| 5.0 | 0.0 | 0.0 | 2.8 ± 0.4 gh | 1.4 ± 0.2 cde |
| 10.0 | 0.0 | 0.0 | 4.1 ± 0.6 fg | 1.0 ± 0.1 ef |
| 15.0 | 0.0 | 0.0 | 6.2 ± 1.0 ef | 1.1 ± 0.2 de |
| 0.0 | 2.5 | 0.0 | 3.0 ± 0.6 gh | 2.0 ± 0.2 ab |
| 0.0 | 5.0 | 0.0 | 8.0 ± 1.0 de | 2.5 ± 0.1 a |
| 0.0 | 10.0 | 0.0 | 11.0 ± 1.0 bc | 1.7 ± 0.2 bc |
| 0.0 | 15.0 | 0.0 | 5.8 ± 0.7 ef | 1.2 ± 0.2 cde |
| 0.0 | 0.0 | 2.5 | 7.8 ± 0.8 de | 2.4 ± 0.2 a |
| 0.0 | 0.0 | 5.0 | 17.4 ± 0.9 a | 2.1 ± 0.2 ab |
| 0.0 | 0.0 | 10.0 | 12.4 ± 1.0 b | 1.6 ± 0.2 bcd |
| 0.0 | 0.0 | 15.0 | 9.4 ± 0.9 cd | 1.3 ± 0.2 cde |
| ANOVA | R-Square | 0.748 | 0.491 | |
| Coefficient of variation | 34.86 | 32.94 | ||
| Auxin | p<0.0001 | p<0.0001 | ||
| Concentration | p<0.0001 | p<0.0001 | ||
| Auxin*Concentration | p<0.0001 | p<0.0208 | ||
| Cytokinin (µM) | Plantlet regeneration (%) | Number of plantlets per rhizome culture |
Plantlet height (cm) |
||
| 2-IP | BA | KN | |||
| 0.0 | 0.0 | 0.0 | 0.0 ± 0.0 j | 0.0 ± 0.0 g | 0.0 ± 0.0 1 |
| 2.5 | 0.0 | 0.0 | 21.7 ± 2.0 fg | 1.1 ± 0.1 f | 5.9 ± 0.6 e-h |
| 5.0 | 0.0 | 0.0 | 38.9 ± 1.6 e | 2.1 ± 0.3 b-e | 7.0 ± 0.4 c-e |
| 10.0 | 0.0 | 0.0 | 13.9 ± 1.6 hi | 1.7 ± 0.2 c-f | 6.3 ± 0.6 e-g |
| 20.0 | 0.0 | 0.0 | 8.9 ± 1.4 i | 1.6 ± 0.2 c-f | 5.5 ± 0.5 f-h |
| 0.0 | 2.5 | 0.0 | 33.9 ± 3.2 e | 1.3 ± 0.2 ef | 7.6 ± 0.5 cde |
| 0.0 | 5.0 | 0.0 | 67.8 ± 3.0 b | 2.3 ± 0.2 bc | 10.2 ± 0.9 a |
| 0.0 | 10.0 | 0.0 | 79.4 ± 1.8 a | 4.4 ± 0.5 a | 8.5 ± 0.5 a-c |
| 0.0 | 20.0 | 0.0 | 61.7 ± 2.0 c | 2.7 ± 0.3 b | 4.9 ± 0.5 gh |
| 0.0 | 0.0 | 2.5 | 26.1 ± 2.3 f | 1.4 ± 0.2 def | 7.9 ± 0.7 bcd |
| 0.0 | 0.0 | 5.0 | 46.7 ± 2.4 d | 2.2 ± 0.4 bcd | 9.5 ± 0.8 ab |
| 0.0 | 0.0 | 10.0 | 34.4 ± 1.5 e | 1.8 ± 0.3 c-f | 6.7 ± 0.5 d-g |
| 0.0 | 0.0 | 20.0 | 19.4 ± 2.1 gh | 2.0 ± 0.3 b-e | 4.4 ± 0.3 h |
| ANOVA | R-Square | 0.919 | 0.537 | 0.503 | |
| Coefficient of variation | 17.62 | 40.01 | 25.37 | ||
| Cytokinin | p<0.0001 | p<0.0001 | p<0.0003 | ||
| Concentration | p<0.0001 | p<0.0001 | p<0.0001 | ||
| Cytokinin*Concentration | p<0.0001 | p<0.0001 | p<0.0171 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




