Introduction
The presence of type 1 diabetes mellitus (T1DM) during pregnancy significantly increases the risk of both maternal and perinatal complications. Maternal risks include miscarriage, preterm birth, caesarean delivery, preeclampsia, and hypertension, while perinatal risks involve macrosomia, congenital anomalies, neonatal hypoglycaemia, hyperbilirubinemia, and neonatal death [
1,
2]. Maternal hyperglycaemia, particularly during the second and third trimesters, has been associated with a higher incidence of preeclampsia, preterm delivery, large-for-gestational-age (LGA) and small-for-gestational-age (SGA) neonates, and neonatal hypoglycaemia [
2]. Optimal glycaemic control plays a critical role throughout pregnancy. Achieving tight metabolic control in the first trimester reduces the risk of congenital malformations and perinatal mortality, while maintaining normoglycaemia in the second and third trimesters lowers the risk of hypertensive disorders, LGA neonates, premature delivery, and the need for neonatal intensive care unit (NICU) [
3]. However, stringent glycaemic targets are accompanied by an increased risk of maternal hypoglycaemia, particularly in early gestation [
1].
Murphy et al. (2021) conducted a comprehensive 5-year national population-based cohort study in the UK, which included 4,089 pregnancies in women with T1DM and 1,768 with type 2 diabetes mellitus (T2DM). The study revealed significant differences in baseline characteristics and pregnancy outcomes between the two groups. Women with T2DM were generally older, more frequently obese (66.9% vs. 27.7%), and had a higher prevalence of chronic hypertension (23.1% vs. 12.3%) than women with T1DM. Nevertheless, perinatal outcomes were consistently worse in the T1DM group. The incidence of congenital anomalies (4.3% vs. 2.1%), preterm birth before 37 weeks (37.3% vs. 21.7%), macrosomia (27.9% vs. 21.8%), and NICU admission (27.2% vs. 17.6%) was significantly higher among women with T1DM. The rate of stillbirth was also slightly higher in the T1DM group (0.8% vs. 0.5%). The study further highlighted the underutilization of preconception counselling, with only 41.3% of women with T1DM and 14.3% with T2DM receiving structured advice before conception. Moreover, fewer than 20% of women with T1DM achieved the recommended pre-pregnancy HbA1c target of <6.5%, compared to 38.3% of women with T2DM. Glycaemic control remained suboptimal throughout pregnancy, especially among women with T1DM [
4].
To improve pregnancy outcomes, current guidelines recommend a target HbA1c of <6.5% and a pregnancy-specific time in range (TIR) of ≥70%, defined as glucose values between 3.5 and 7.8 mmol/L [
1]. Achieving these targets requires intensive insulin therapy, either via multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII), combined with frequent glucose monitoring using self-monitoring of blood glucose (SMBG) or continuous glucose monitoring (CGM) [
2].
Sensor-augmented pump (SAP) therapy, which integrates real-time CGM with insulin pumps, offers enhanced glycaemic control by facilitating more precise insulin adjustments. More recently, advanced hybrid closed loop (AHCL) systems have emerged as a promising innovation. These systems automate basal insulin delivery based on CGM data, while still requiring user-initiated bolus administration for meals. In non-pregnant individuals with T1DM, AHCL systems have been shown to improve TIR, reduce glycaemic variability, and decrease the risk of hypoglycaemia. Despite these advantages, maintaining optimal glycaemic control during pregnancy remains complex. Hormonal fluctuations lead to progressive changes in insulin sensitivity, particularly insulin resistance in late gestation, and insulin absorption becomes increasingly variable. These factors complicate insulin dose titration and can impair glycaemic stability [
3]. While AHCL technology offers the potential to support optimal glycaemic control before and during pregnancy, regulatory approvals are still limited, and not all AHCL systems are currently approved for use in pregnancy [
1].
Insulin Pumps with Automated Insulin Delivery
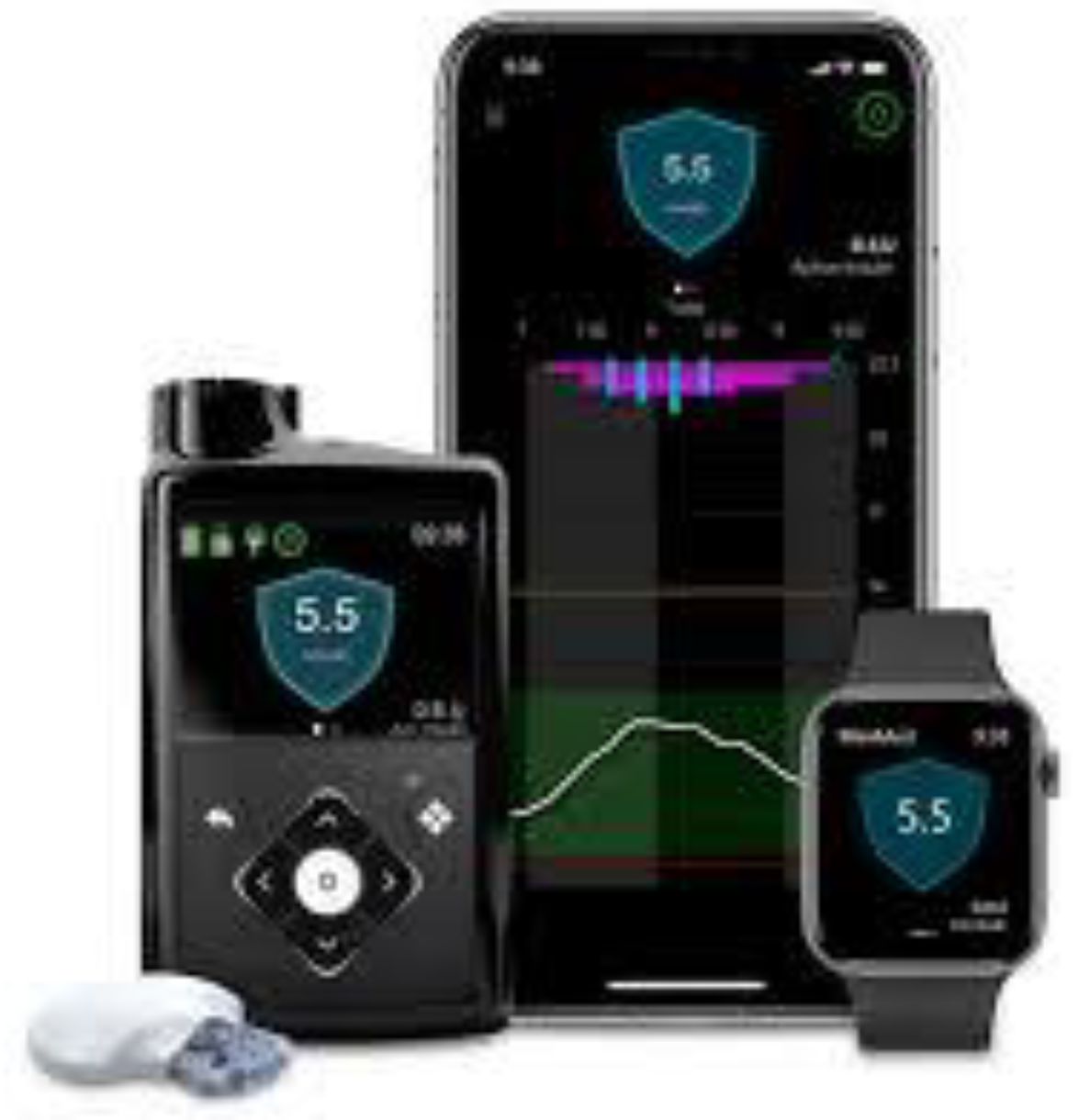
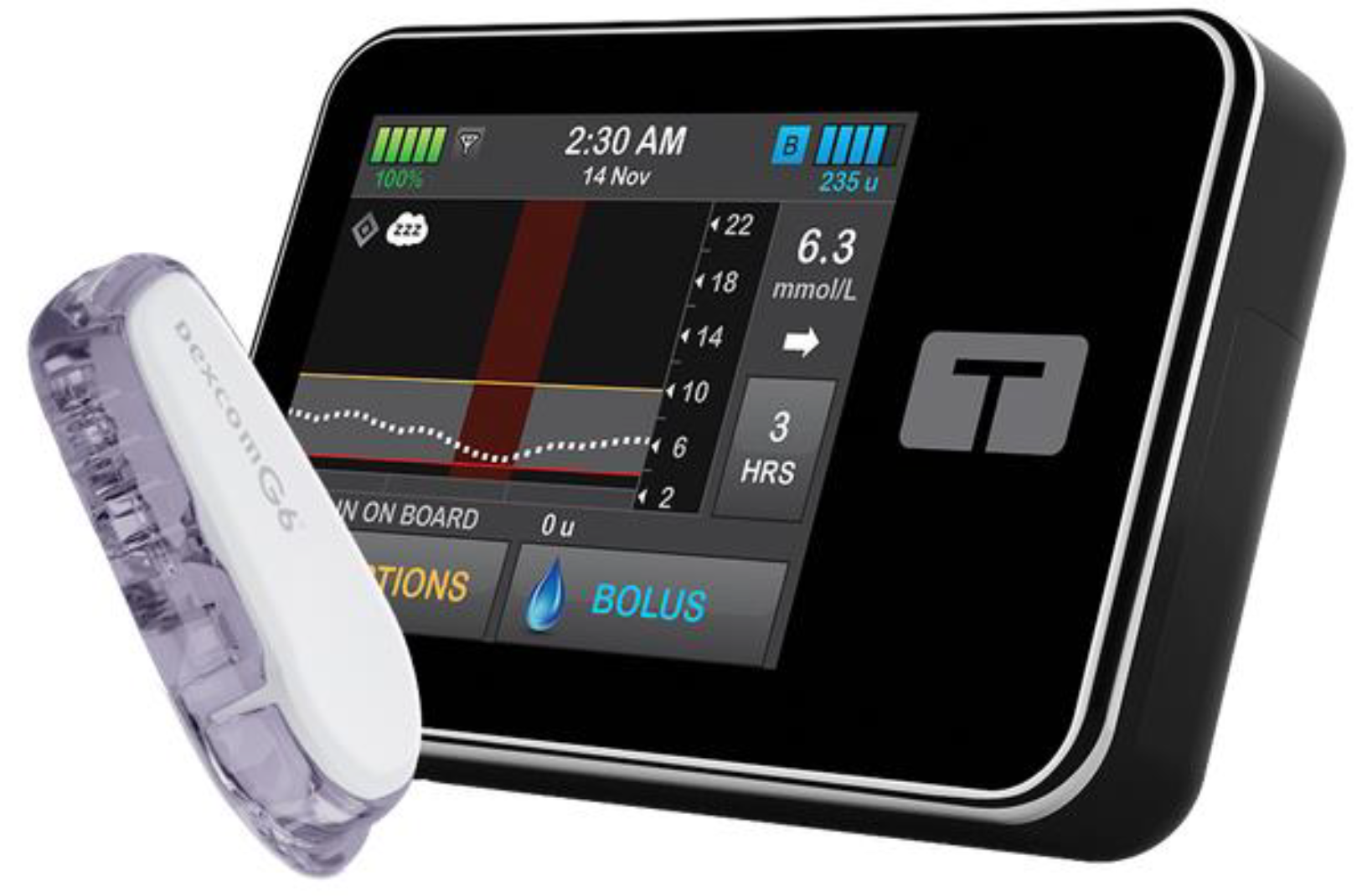
Automated insulin delivery (AID) systems combine CGM, an insulin pump, and a predictive algorithm to mimic the physiological function of the pancreas. Their use is increasingly integrated into the routine management of T1DM in both adults and children [
5]. AID systems operate as closed-loop platforms and consist of three main components: [
1] a CGM device that continuously measures interstitial glucose levels, [
2] an insulin pump for subcutaneous insulin delivery, and [
3] a computerised control algorithm that adjusts basal insulin delivery in real time based on glucose trends [
6]. AHCL systems represent the most sophisticated form of AID technology currently available. In AHCL systems, basal insulin delivery is automated based on sensor glucose data, while users are still required to manually administer prandial boluses using an integrated bolus calculator. These systems have demonstrated the ability to safely improve glycaemic control, increase TIR, and reduce glycaemic variability in non-pregnant individuals with T1DM, including paediatric populations [
1,
6]. Examples of widely used AHCL systems are illustrated in
Figure 1 and
Figure 2 [
1,
6].
In addition to improving metabolic outcomes in the general T1DM population, AHCL systems have shown potential to optimise glycaemic control in women planning pregnancy. However, despite these promising results, the safety and efficacy of AHCL technology during pregnancy remain under investigation. Given the dynamic physiological changes of gestation and the stringent glycaemic targets required to minimise perinatal complications, further evidence is needed to confirm whether AHCL systems can be reliably and safely implemented throughout pregnancy [
1].
Recommendations for the Management of T1DM During Pregnancy
Glycaemic targets during pregnancy are more stringent compared to the non-pregnant state. Current clinical guidelines issued by the American Diabetes Association (ADA), the National Institute for Health and Care Excellence (NICE), and other international expert panels recommend achieving a preconception HbA1c below 6.5% (48 mmol/mol), with the goal of reaching values as close as possible to 6.0% (42 mmol/mol), provided this can be achieved without increasing the risk of hypoglycaemia. During pregnancy, recommended capillary blood glucose targets include fasting glucose levels between 3.9–5.3 mmol/L, 1-hour postprandial levels below 7.8 mmol/L, and 2-hour postprandial levels below 6.7 mmol/L. The 2019 consensus statement by the Advanced Technologies & Treatments for Diabetes (ATTD) recommends an HbA1c target <6.5% for pregnant women with T1DM. For those using CGM, a target TIR (defined as sensor glucose levels between 3.5–7.8 mmol/L) of >70% is advised. Additionally, time above range (TAR; >7.8 mmol/L) should remain <25%, time below range (TBR; <3.5 mmol/L) should be <4%, and TBR <3.0 mmol/L should not exceed 1% (
Figure 3) [
9].
Real-world data suggest that pregnant women with T1DM typically achieve TIRs of approximately 50%, 55%, and 60% during the first, second, and third trimesters, respectively. The recommended targets of TIR >70% and TAR <25% are commonly reached only in the final 3–4 weeks of gestation. Importantly, each 5% reduction in TIR and corresponding 5% increase in TAR during the second and third trimesters has been associated with a higher incidence of adverse neonatal outcomes, including macrosomia, neonatal hypoglycaemia, and admission to NICU [
10]. Despite increased adoption of CGM, CSII, and the use of rapid-acting insulin analogues, achieving and sustaining optimal glycaemic control in pregnant women with T1DM remains a significant clinical challenge [
6].
Review of Studies on Insulin Pump Use During Pregnancy
Stewart et al. (2016) conducted a randomized crossover trial to evaluate the efficacy and safety of an AHCL system in pregnant women with T1DM. Sixteen participants completed two 4-week intervention phases: one with conventional CSII combined with CGM, and the other with an AHCL system that autonomously adjusted basal insulin delivery based on sensor glucose levels. Use of the closed-loop system significantly increased time in the pregnancy-specific glycaemic target range (3.5–7.8 mmol/L), particularly during the night (75% vs. 59%;
p<0.001), without increasing the incidence of hypoglycaemia. Overall TIR improved from 61% with standard CSII to 69% with AHCL (
p=0.002), accompanied by a reduction in time spent in hyperglycaemia. No episodes of severe hypoglycaemia or diabetic ketoacidosis were reported. Participants subjectively reported improved sleep quality and reduced anxiety during the closed-loop treatment phase. The study concluded that AHCL insulin delivery is safe and enhances glycaemic control during pregnancy, particularly in the overnight period, underscoring its potential role in the management of T1DM in pregnant women [
11].
The CONCEPTT trial (Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy), conducted by Feig et al. (2017), was a pivotal multicentre, international, randomized controlled trial investigating the impact of real-time CGM on glycaemic control and pregnancy outcomes in women with T1DM. The study enrolled 325 women, including 215 who were already pregnant at the time of randomization. Pregnant participants were assigned to either real-time CGM or conventional SMBG, while continuing intensive insulin therapy via MDI or insulin pumps. The primary endpoint was the change in HbA1c from baseline to 34 weeks’ gestation. Although the CGM group demonstrated only a modest reduction in HbA1c compared to the SMBG group (mean difference −0.2%, p=0.02), CGM use resulted in a significantly greater percentage of TIR spent within the pregnancy-specific target glycaemic range (3.5–7.8 mmol/L), reduced time in hyperglycaemia, and no increase in hypoglycaemia.
Importantly, CGM was associated with improved neonatal outcomes. The incidence of LGA neonates was significantly lower in the CGM group compared to SMBG (53% vs. 69%,
p=0.02), as were the rates of neonatal hypoglycaemia and neonatal NICU admissions exceeding 24 hours (27% vs. 43%,
p=0.01). The authors concluded that CGM use in pregnant women with T1DM improves neonatal outcomes and confers modest benefits in maternal glycaemic control, supporting its integration into routine antenatal diabetes management [
3].
A retrospective observational study assessing the impact of combining CSII with CGM on glycaemic control and pregnancy outcomes in women with pregestational T1DM was conducted by
Lason et al. (2021). The analysis included 81 singleton pregnancies from 109 women treated between 2016 and 2017, stratified into three groups: CSII+CGM, CSII alone, and MDI. Women using CSII with CGM achieved significantly better glycaemic control throughout pregnancy and postpartum compared to the other groups. In this group, mean HbA1c remained consistently low: 5.3% in the first and second trimesters, 5.2% in the third, and 5.5% postpartum. Despite superior metabolic control, no significant differences were found between groups in obstetric outcomes, including gestational age at delivery, rates of preterm birth, or neonatal birth weight. Macrosomia remained prevalent (~20%) even among women with optimal glycaemic control. The study concluded that CGM combined with CSII, particularly with advanced safety algorithms (AID), significantly improves maternal glycaemic outcomes. However, the persistent incidence of macrosomia suggests that factors beyond maternal glycaemia may contribute to foetal overgrowth in T1DM pregnancies [
12].
A retrospective observational study evaluating the effectiveness of SAP therapy in pregnant women with T1DM was conducted by
Imafuku et al. (2023). The study compared maternal metabolic control and neonatal outcomes between women treated with SAP and those using MDI in combination with CGM. A total of 41 pregnant women with T1DM were included: 21 in the SAP group and 20 in the MDI+CGM group. Assessed outcomes included mean glucose levels, TIR (3.5–7.8 mmol/L), incidence of LGA neonates, neonatal birth weight, and frequency of maternal hypoglycaemia. The SAP group demonstrated superior glycaemic control, with significantly lower mean glucose levels and a higher proportion of TIR during the second and third trimesters. Moreover, SAP therapy was associated with a significantly lower incidence of LGA infants and fewer hypoglycaemic episodes compared to the MDI+CGM group. These findings support the clinical benefit of SAP therapy in optimizing metabolic control and reducing the risk of adverse neonatal outcomes in pregnant women with T1DM [
2].
The
AiDAPT study (Lee TTM et al.,
2023) was a multicentre, randomized controlled trial designed to assess the efficacy and safety of AID systems in pregnant women with T1DM. A total of 124 women were enrolled early in pregnancy, at a mean gestational age of 11 weeks, and randomly assigned to receive either an AID system (CamAPS FX closed-loop technology) or conventional SAP therapy without automation. The primary outcome was the percentage of time spent within the pregnancy-specific glycaemic target range (3.5–7.8 mmol/L) from 16 to 36 weeks of gestation. Women using the AID system achieved significantly better glycaemic control, spending on average 14 percentage points more TIR compared to the SAP group. The AID group also exhibited lower mean glucose concentrations and reduced time in hyperglycaemia, with no significant increase in time spent in hypoglycaemia. Although the trial was not powered to detect differences in maternal or neonatal clinical outcomes, there were no significant differences between the groups in the incidence of serious adverse events. The findings demonstrate that AID systems are both effective and safe for use in pregnancy, offering substantial improvements in glycaemic control. The authors concluded that AID should be considered a preferred therapeutic option for pregnant women with T1DM to optimize maternal glucose levels and potentially improve pregnancy outcomes [
6,
13].
Rankin et al. (2023) examined healthcare professionals’ perspectives on implementing AHCL systems in pregnant women with T1DM. As AID technologies become more widely available, understanding the practical and systemic barriers to their integration into antenatal care is essential. The study involved semi-structured interviews with 29 clinicians—including diabetologists, obstetricians, diabetes specialist nurses, and midwives—across multiple NHS centres in the UK. Thematic analysis revealed overall support for AHCL use in pregnancy, citing benefits such as improved glycaemic control, reduced hypoglycaemia risk, and decreased psychological burden. However, several concerns were raised, including the complexity of the technology, increased workload for clinical staff, the need for specialised training, and disparities in access between centres. Participants highlighted the importance of multidisciplinary education, clear care pathways, and robust patient support systems. System-level factors, including funding, device availability, and workflow integration, were identified as critical to successful implementation. The authors concluded that while there is strong clinical enthusiasm for AHCL use in pregnancy, effective rollout requires coordinated planning, resource allocation, and efforts to ensure equitable access across healthcare settings [
14].
As part of a qualitative sub-study embedded in the AiDAPT randomized controlled trial,
Lawton et al. (2023) explored the experiences of pregnant women with T1DM using AHCL systems. Semi-structured interviews were conducted with 26 women who used the CamAPS FX system during pregnancy. Participants reported a marked reduction in the mental and emotional burden of diabetes management. Automated insulin adjustments provided a sense of safety—particularly overnight—and contributed to improved sleep, greater confidence, and a feeling of normalcy. Women also noted enhanced glycaemic control and increased day-to-day flexibility. Despite these benefits, some challenges were identified, including device wearability issues, occasional technical difficulties, and the continued need for user input for meal bolusing. The study concluded that AHCL systems offer significant psychological and practical benefits for pregnant women with T1DM. The authors emphasized the importance of incorporating patient experiences into clinical care models and service planning to support equitable and patient-centred implementation of this technology in routine antenatal diabetes management [
15].
According to
NICE (2023), HCL therapy should be offered to adults with T1DM who have an HbA1c level of 7.5% (58 mmol/mol or higher) despite optimized use of CSII or CGM, or to those experiencing disabling hypoglycaemia. In contrast, children, adolescents, and women who are pregnant or planning pregnancy are eligible for HCL therapy regardless of their current HbA1c level. These recommendations are supported by clinical trial data and real-world evidence demonstrating improvements in glycaemic control, including reductions in HbA1c and TAR, as well as increased time in the target glucose range (3.5–7.8 mmol/L). Additionally, patients report reduced psychological distress and fear of hypoglycaemia when using these systems. HCL systems are classified by NICE as a technology class rather than by brand; however, commercially available systems such as the Medtronic MiniMed 780G, Tandem t: slim X2 with Control-IQ, and CamAPS FX fall within the scope of the recommendation [
16].
The CRISTAL study (Benhalima et al.,
2024) was a multicentre, open-label, randomized controlled trial evaluating the efficacy and safety of AHCL insulin therapy in pregnant women with T1DM. A total of 124 women (≥18 years, ≤13+6 weeks’ gestation, HbA1c ≤9%) were randomized 1:1 to receive either AHCL therapy with the MiniMed 780G system or standard insulin therapy (MDI or CSII, with or without CGM) across nine Belgian centres. Participants were followed until delivery. The primary endpoint—TIR (3.5–7.8 mmol/L) from 14 to 34 weeks’ gestation—was significantly higher in the AHCL group (68.4%) compared to standard care (55.6%), with an adjusted mean difference of 12.8 percentage points (95% CI: 7.8–17.8;
p<0.0001). AHCL users also had lower mean glucose levels, reduced time in hyperglycaemia, and significantly lower HbA1c at 34 weeks (6.2% vs 6.6%), with no increase in hypoglycaemia. Neonatal outcomes favoured the AHCL group, with lower rates of LGA infants (31% vs 41%) and neonatal hypoglycaemia (33% vs 50%), though differences were not statistically significant. No cases of severe hypoglycaemia or diabetic ketoacidosis were reported. The study concluded that AHCL therapy during pregnancy significantly improves glycaemic control without increasing adverse events, supporting its use as a preferred treatment strategy in pregnant women with T1DM [
1].
The CRISTAL study was the first randomized trial to evaluate off-label use of the MiniMed 780G system in automated mode during pregnancy. While no significant improvement in daytime TIR was observed, the AHCL system significantly increased overnight TIR and reduced the risk of hypoglycaemia, which was associated with improved glycaemic variability, reduced hypoglycaemia unawareness, and greater maternal satisfaction. Given that hypoglycaemia is a major barrier to tight glycaemic control during pregnancy, this finding is clinically important. The study also confirmed the safety of AHCL use during pregnancy. In comparison, the AiDAPT study demonstrated a significantly greater overall TIR (+10.5%) with the CamAPS FX system versus standard care. However, baseline characteristics differed: women in AiDAPT had higher initial HbA1c (7.7% vs 6.5%), lower baseline TIR and TBR, and most control participants were on BBT, unlike CRISTAL, where 77.5% used insulin pumps in manual mode. Furthermore, the CamAPS system targeted a lower glucose threshold for automated delivery (4.4 mmol/L) compared to MiniMed 780G (5.5 mmol/L), which may explain the greater glycaemic improvements observed in AiDAPT [
1].
The CopenFast study (Nørgaard et al.,
2023) was a single-centre, open-label, randomized controlled trial comparing faster-acting insulin aspart with conventional insulin aspart in pregnant women with pregestational diabetes (type 1 or type 2). A total of 208 women (133 with T1DM, 75 with T2DM) were enrolled before 14 weeks’ gestation and randomized 1:1 to receive either insulin as part of a basal-bolus regimen throughout pregnancy and 6 weeks postpartum. The primary outcome was 1-hour postprandial glucose, which was significantly lower with faster aspart (mean difference –0.37 mmol/L;
p=0.015). HbA1c, TIR, insulin requirements, and maternal weight gain were similar between groups. Neonatal outcomes, including rates of LGA infants (30% in both groups) and neonatal hypoglycaemia, did not differ significantly. These findings support its use as a safe and effective prandial insulin option during pregnancy in women with diabetes [
17].
Conclusions
The management of T1DM during pregnancy presents a unique and ongoing clinical challenge. Despite decades of progress in insulin therapy and glucose monitoring, women with T1DM remain at significantly increased risk for both maternal complications—such as preeclampsia, preterm labour, and caesarean section—and perinatal complications, including foetal overgrowth, neonatal hypoglycaemia, and admission to NICU. Achieving optimal glycaemic control throughout gestation, particularly maintaining a target HbA1c <6.5% and TIR ≥70% for glucose values between 3.5 and 7.8 mmol/L, is a critical determinant of pregnancy outcomes. However, these targets are rarely achieved in clinical practice, especially during the early and mid-second trimesters, and efforts to intensify glycaemic control are frequently complicated by an increased incidence of maternal hypoglycaemia, particularly in early pregnancy. Recent advancements in diabetes technology—most notably the emergence of CGM, SAP therapy, and AHCL systems—offer the potential to transform pregnancy care in women with T1DM. Evidence from landmark trials such as CONCEPTT, AiDAPT, and CRISTAL has consistently demonstrated that these technologies improve maternal glycaemic metrics, including HbA1c, TIR, and glycaemic variability. CGM use, even in the absence of AID, has been associated with improved neonatal outcomes, such as reduced incidence of LGA infants and neonatal hypoglycaemia, as shown in the CONCEPTT trial. SAP therapy, combining real-time CGM with programmable insulin delivery, offers enhanced flexibility and precision, with observational studies confirming significant benefits in maternal glucose control. AHCL systems represent the most advanced form of AID available to date. Trials such as AiDAPT (CamAPS FX) and CRISTAL (MiniMed 780G) provide robust data supporting their use in pregnancy. These systems significantly increased TIR, particularly overnight, without increasing the incidence of hypoglycaemia. While the CRISTAL study showed greater overnight benefits and safety, the AiDAPT study demonstrated larger overall improvements in glycaemic control—likely influenced by differences in baseline characteristics and system algorithms, including lower automated glucose targets in CamAPS FX. Importantly, neither study reported serious adverse events, affirming the safety profile of AHCL systems during pregnancy. Beyond metabolic parameters, the use of closed-loop technology has been shown to improve patient-reported outcomes, including reduced diabetes-related distress, improved sleep, greater confidence in glycaemic control, and a perception of normalcy in daily life. These psychosocial benefits, highlighted in the qualitative sub-study of AiDAPT (Lawton et al.), are particularly relevant during pregnancy, a period marked by heightened vulnerability and increased demands on self-care.
Implementation of these technologies, however, is not without barriers. As highlighted in the qualitative work by Rankin et al., healthcare providers recognize both the clinical potential and the challenges of integrating AHCL systems into antenatal care. These include device complexity, training needs, staffing limitations, and inequities in access. National health systems, including NICE in the UK, have begun to address these gaps. The 2023 NICE technology appraisal now recommends the use of HCL systems in all pregnant women with T1DM—regardless of HbA1c—provided that multidisciplinary support and patient education are available. Systems such as the MiniMed 780G, Tandem t: slim X2 with Control-IQ, and CamAPS FX fall under this recommendation. This represents a paradigm shift in clinical practice and signals institutional recognition of the value of AID systems. It is important to acknowledge that while AHCL systems substantially improve maternal glucose metrics, their impact on neonatal outcomes remains variable. Persistent rates of macrosomia and neonatal hypoglycaemia—even among women achieving near-normoglycaemia—suggest that additional pathophysiological mechanisms beyond maternal hyperglycaemia contribute to foetal overgrowth. These findings highlight the need for further research into the placental, hormonal, and metabolic drivers of perinatal outcomes in diabetes-complicated pregnancies.
In summary, AID systems, particularly AHCL technologies, offer a clinically effective, safe, and patient-centred approach to managing T1DM in pregnancy. Their integration into routine prenatal care has the potential to improve both maternal and neonatal outcomes, reduce the psychological burden of diabetes management, and enhance quality of life. Ongoing efforts should focus on ensuring equitable access, provider training, and personalized system optimization, alongside continued research into long-term neonatal effects. With appropriate implementation and support, AHCL systems should be considered a cornerstone of modern diabetes care in pregnancy.
List of Abbreviations
ADA - American Diabetes Association
AHCL – Advanced Hybrid Closed-Loop System
AID – Automated Insulin Delivery
ATTD – Advanced Technologies & Treatments for Diabetes
CGM – Continuous Glucose Monitoring
CSII – Continuous Subcutaneous Insulin Infusion
T1DM – Type 1 Diabetes Mellitus
T2DM – Type 2 Diabetes Mellitus
HbA1c – Glycated Haemoglobin
LGA – Large for Gestational Age
MDI – Multiple Day Injections
NICE – National Institute for Health and Care Excellence
NICU – Neonatal Intensive Care Unit
SAP – Sensor-Augmented Pump
SGA – Small for Gestational Age
SMBG – Self-Monitoring Blood Glucose
TAR – Time Above Range
TBR – Time Below Range
TIR – Time in Range
References
- Benhalima K, Beunen K, Van Wilder N, et al. Comparing advanced hybrid closed-loop therapy and standard insulin therapy in pregnant women with type 1 diabetes (CRISTAL): a parallel-group, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2024;12(6):390-403.
- Imafuku, H.; Tanimura, K.; Masuko, N.; Tomimoto, M.; Shi, Y.; Uchida, A.; Deguchi, M.; Fujioka, K.; Yamamoto, A.; Yoshino, K.; et al. Advantages of sensor-augmented insulin pump therapy for pregnant women with type 1 diabetes mellitus. J. Diabetes Investig. 2023, 14, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.S.; E Donovan, L.; Corcoy, R.; E Murphy, K.; A Amiel, S.; Hunt, K.F.; Asztalos, E.; Barrett, J.F.R.; Sanchez, J.J.; de Leiva, A.; et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017, 390, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.R.; Howgate, C.; O’Keefe, J.; Myers, J.; Morgan, M.; A Coleman, M.; Jolly, M.; Valabhji, J.; Scott, E.M.; Knighton, P.; et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. Lancet Diabetes Endocrinol. 2021, 9, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Benhalima K, Jendle J, Beunen K, et al. Automated insulin delivery for pregnant women with type 1 diabetes: where do we stand? J Diabetes Sci Technol. 2024 Jan 10:19322968231223934.
- Lee, T.T.M.; Collett, C.; Man, M.-S.; Hammond, M.; Shepstone, L.; Hartnell, S.; Gurnell, E.; Byrne, C.; Scott, E.M.; Lindsay, R.S.; et al. AiDAPT: automated insulin delivery amongst pregnant women with type 1 diabetes: a multicentre randomized controlled trial – study protocol. BMC Pregnancy Childbirth 2022, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Medtronic Diabetes. MiniMed™ 780G System. Available at: https://www.medtronic-diabetes.
- Tandem Diabetes Care. t:slim X2 with Control-IQ technology. Available at: https://www.tandemdiabetes.
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.R. Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5% time in range matters. Diabetologia 2019, 62, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Stewart, Z.A.; Wilinska, M.E.; Hartnell, S.; Temple, R.C.; Rayman, G.; Stanley, K.P.; Simmons, D.; Law, G.R.; Scott, E.M.; Hovorka, R.; et al. Closed-Loop Insulin Delivery during Pregnancy in Women with Type 1 Diabetes. New Engl. J. Med. 2016, 375, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Lason, I.; Cyganek, K.; Witek, P.; Matejko, B.; Malecki, M.T.; Skupien, J. Continuous glucose monitoring and insulin pump therapy in pregnant women with type 1 diabetes mellitus. Ginekol. Polska 2021, 92, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Lee TTM, Collett C, Bergford S, et al. AiDAPT Collaborative Group. Automated Insulin Delivery in Women with Pregnancy Complicated by Type 1 Diabetes. N Engl J Med. 2023 Oct 26;389(17):1566-1578.
- Rankin, D.; Hart, R.I.; Kimbell, B.; Barnard-Kelly, K.; Brackenridge, A.; Byrne, C.; Collett, C.; Dover, A.R.; Hartnell, S.; Hunt, K.F.; et al. Rollout of Closed-Loop Technology to Pregnant Women with Type 1 Diabetes: Healthcare Professionals' Views About Potential Challenges and Solutions. Diabetes Technol. Ther. 2023, 25, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.; Kimbell, B.; Closs, M.; Hartnell, S.; Lee, T.T.; Dover, A.R.; Reynolds, R.M.; Collett, C.; Barnard-Kelly, K.; Hovorka, R.; et al. Listening to Women: Experiences of Using Closed-Loop in Type 1 Diabetes Pregnancy. Diabetes Technol. Ther. 2023, 25, 845–855. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Hybrid closed-loop systems for managing blood glucose levels in type 1 diabetes. 2023.
- Nørgaard, S.K.; Søholm, J.C.; Mathiesen, E.R.; Nørgaard, K.; Clausen, T.D.; Holmager, P.; Do, N.C.; Damm, P.; Ringholm, L. Faster-acting insulin aspart versus insulin aspart in the treatment of type 1 or type 2 diabetes during pregnancy and post-delivery (CopenFast): an open-label, single-centre, randomised controlled trial. Lancet Diabetes Endocrinol. 2023, 11, 811–821. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).