Submitted:
20 June 2025
Posted:
24 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Description of the Crystal Structure of Thallium(I) Salinomycinate
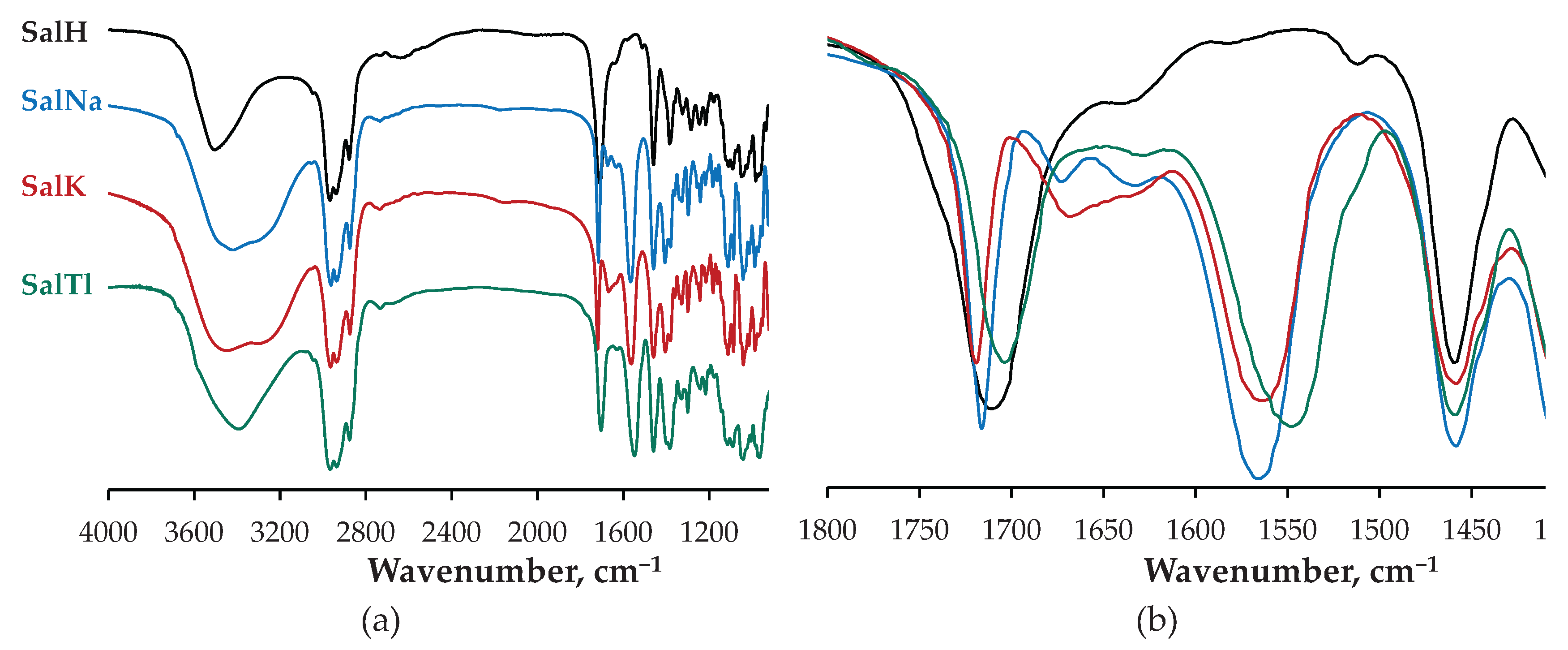
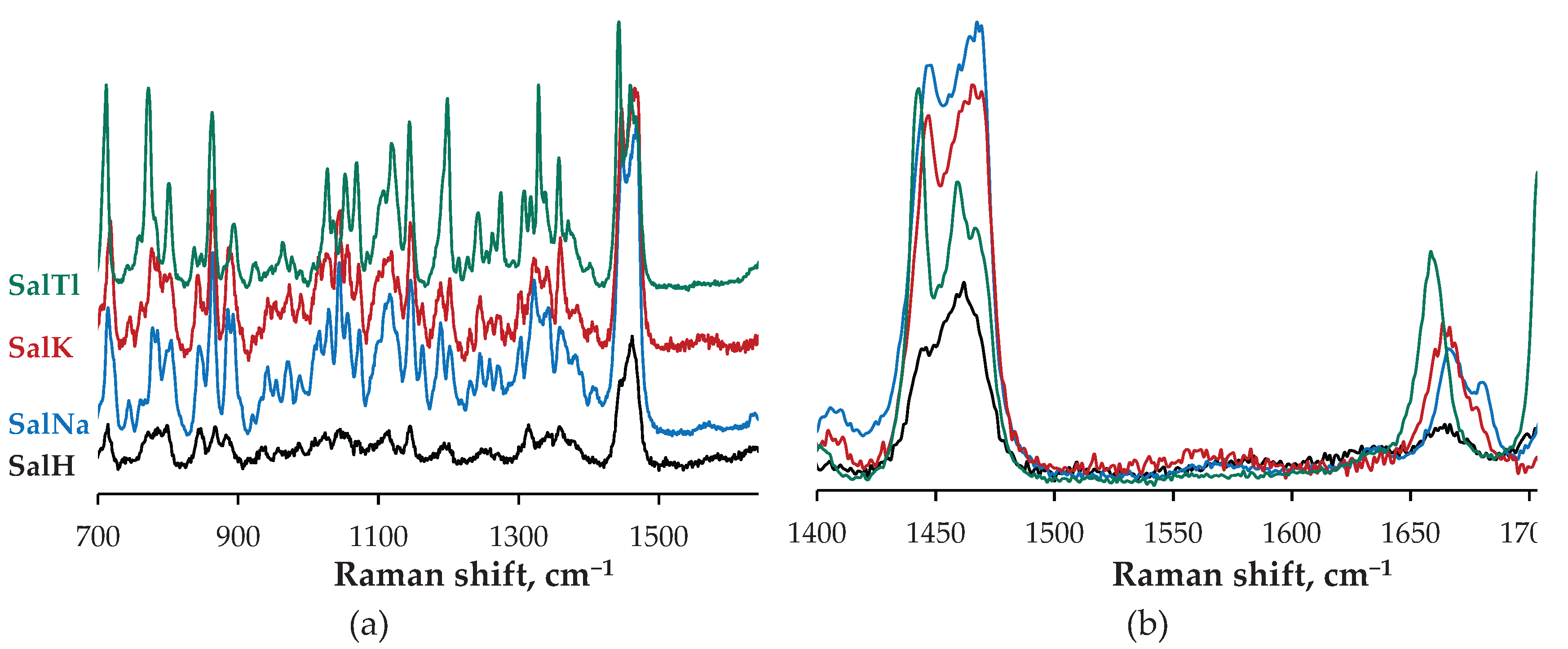
2.2. Spectral Characterization of SalTl
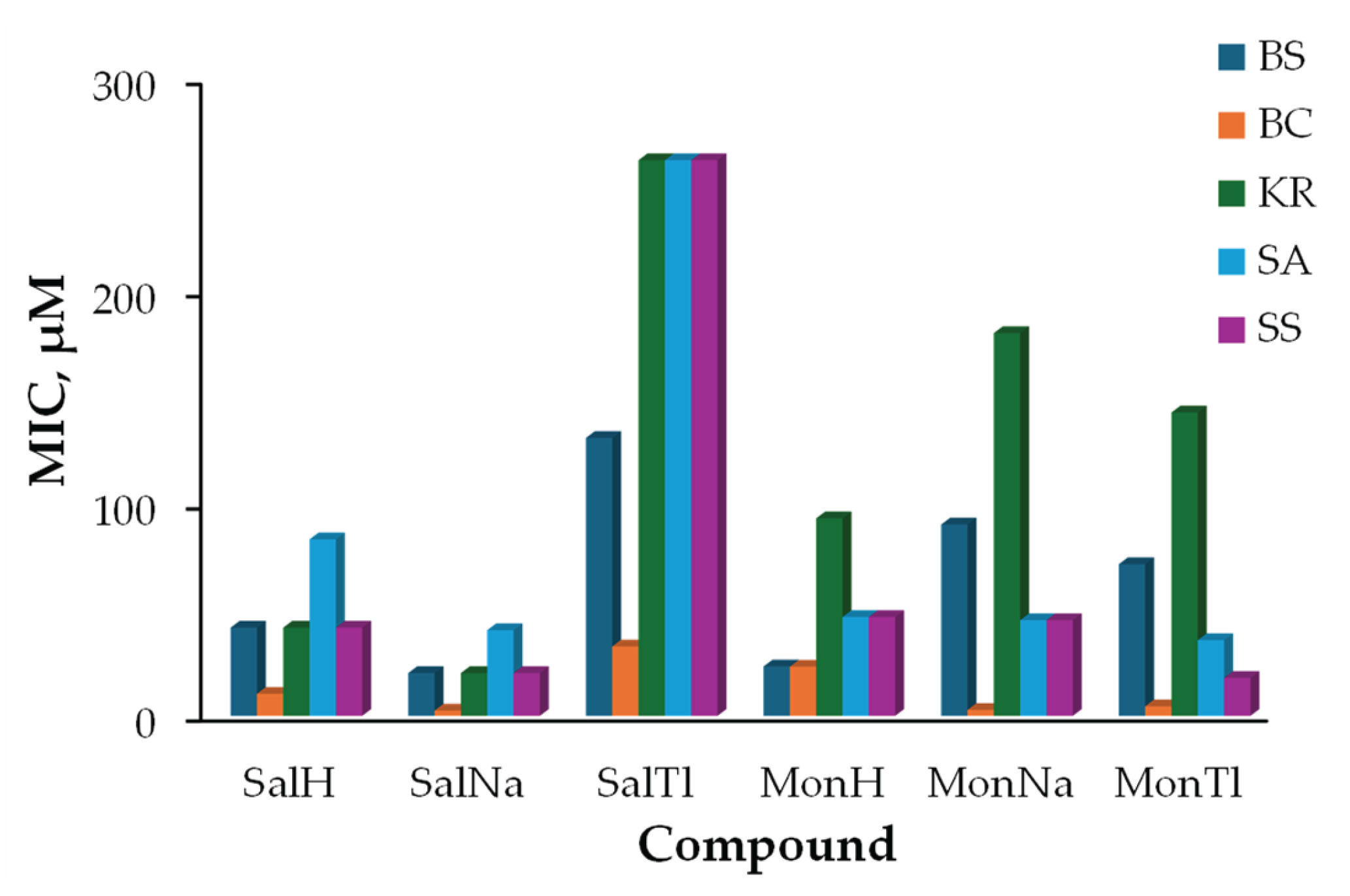
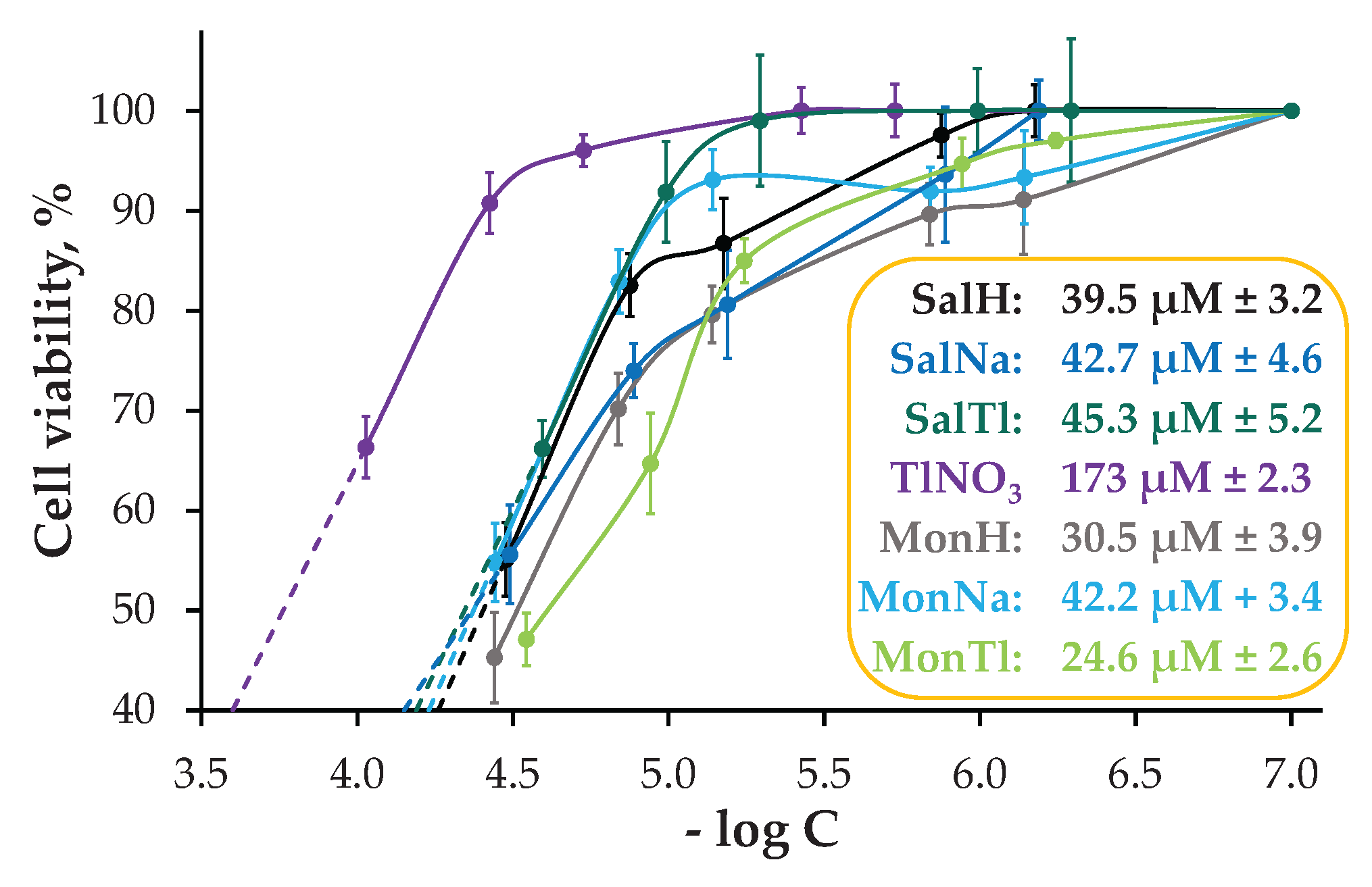
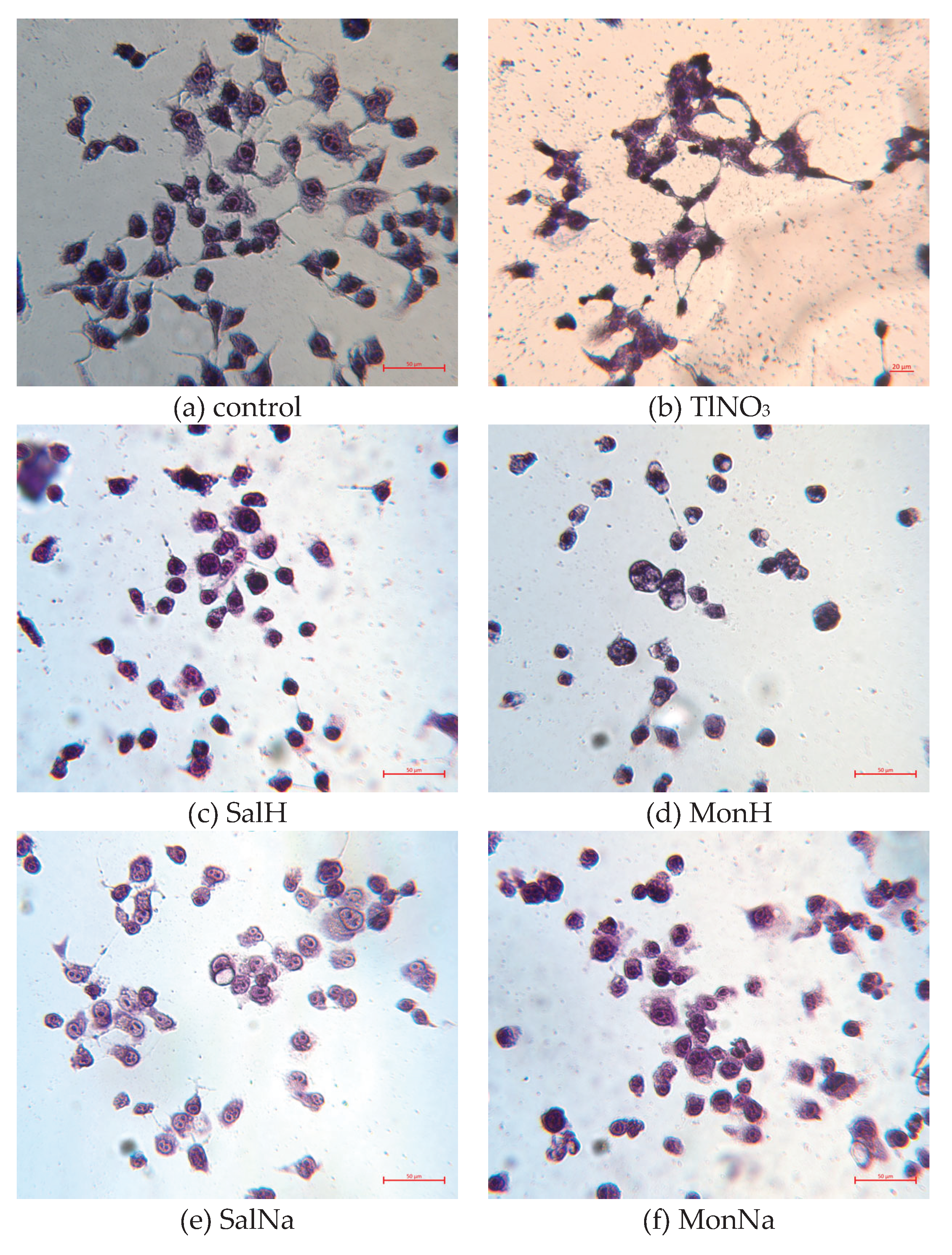
2.3. Biological Effect of SalTl and Related Compounds
3. Materials and Methods
3.1. Reagents and Materials
3.2. Synthesis of SalTl
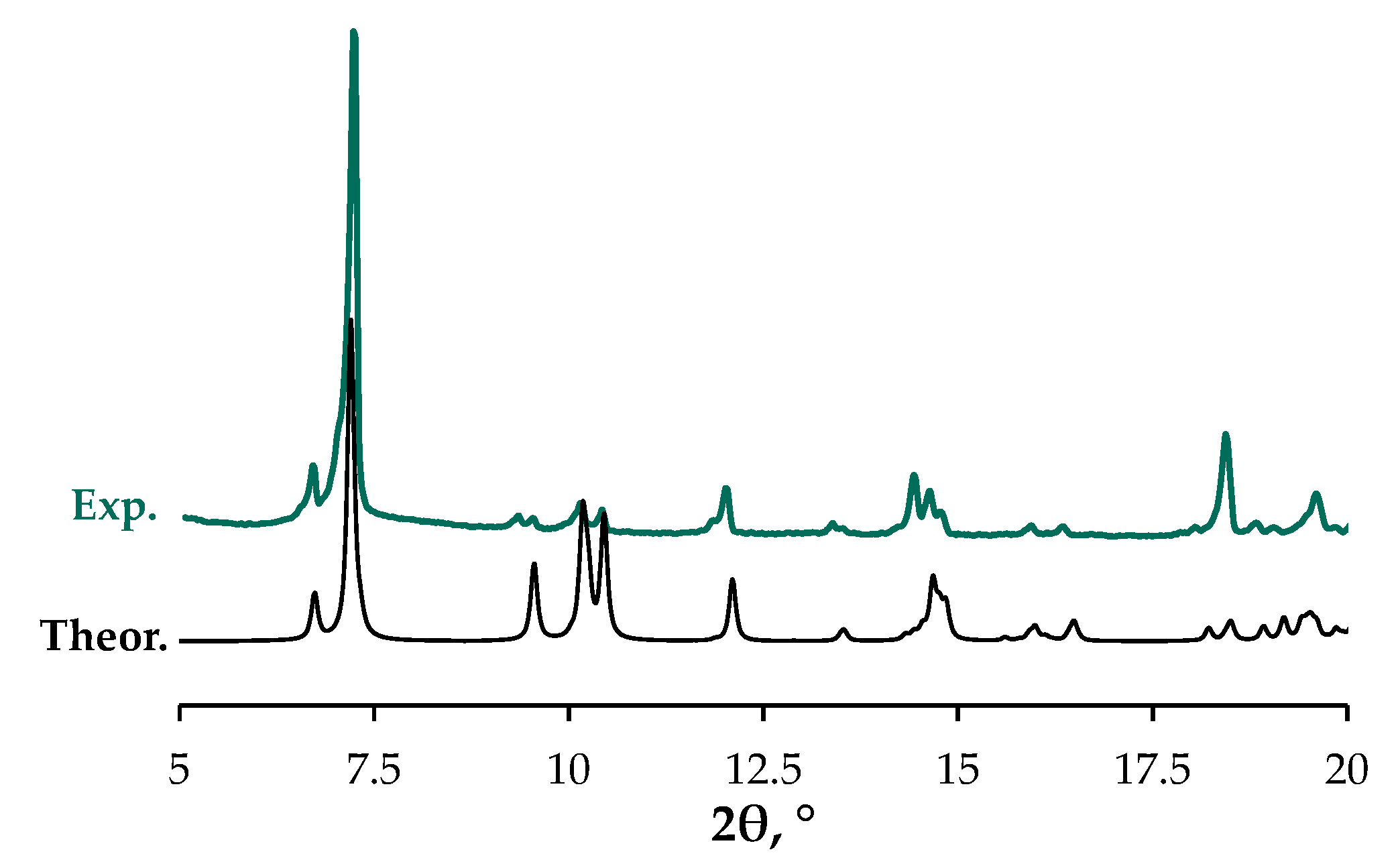
3.3. X-Ray Crystallography
3.4. Physical Measurements
3.5. Antibacterial Activity
3.6. Cell Cultivation and Cytotoxicity Assay
3.7. May-Gruenwald Giemsa (Pappenheim) Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Sample Availability
Acknowledgement
Conflicts of Interest
References
- Agtarap, A.; Chamberlin, J.W.; Pinkerton, M.; Steinrauf, L. (1967) The structure of monensic acid, a new biologically active compound. J. Am. Chem. Soc. 1967, 89, 5737–5739. [CrossRef]
- Agtarap, A.; Chamberlin, J.W. Monensin, a new biologically active compound. IV. Chemistry. Antimicrob. Agents Chemother. (Bethesda) 1967, 7, 359−362. https://pubmed.ncbi.nlm.nih.gov/5596160/.
- Miyazaki, Y.; Shibuya, M.; Sugawara, H.; Kawaguchi, O.; Hirsoe, C. Salinomycin, a new polyether antibiotic. J. Antibiotics 1974, 27, 814–821. [CrossRef]
- Paulus, E.F.; Kurz, M.; Matter, H.; Vértesy, L. Solid-state and solution structure of the salinomycin−sodium complex: stabilization of different conformers for an ionophore in different environments. J. Am. Chem. Soc. 1998, 120, 8209–8221. [CrossRef]
- Patel, D.J.; Shen, C. Structural and kinetic studies of lasalocid A (X537A) and its silver, sodium, and barium salts in nonpolar solvents. Proc. Natl. Acad. Sci. USA 1976, 73, 1786−1790. [CrossRef]
- Erwin, G.S.; Heikkinen, J.; Pauliina Halima, P.; Haber, C.L. Streptomyces lasalocidi sp. nov. (formerly “Streptomyces lasaliensis”), an actinomycete isolated from soil which produces the polyether antibiotic lasalocid. Intern. J. System. Evolut. Microbiol. 2020, 70, 3076−3083. [CrossRef]
- Berg, D.H.; Hamil, R.L. The isolation and characterization of narasin, a new polyether antibiotic. J. Antibiot. 1978, 31, 1−6. [CrossRef]
- Jeffers, T.K.; Tonkinson, L.V.; Callender, M.E. Anticoccidial efficacy of narasin in battery cage trials. Poult. Sci. 1988, 67, 1043–1049. [CrossRef]
- Liu, C.M.; Hermann, T.E.; Downey, A.; Prosser, B.L.; Schildknecht, E.; Palleroni, N.J.; Westley, J.W.; Miller. P.A. Novel polyether antibiotics X-14868A, B, C, and D produced by a Nocardia. Discovery, fermentation, biological as well as ionophore properties and taxonomy of the producing culture. J. Antibiot. 1983, 36, 343–350. [CrossRef]
- McDougald, L.R.; Wang, G.T.; Kantor, S.; Schenkel, R.; Quarles, C. Efficacy of maduramicin against ionophore-tolerant field isolates of coccidia in broilers. Avian Dis. 1987, 31, 302−308. https://pubmed.ncbi.nlm.nih.gov/3619823/.
- Tynan, E.J.3rd; Nelson, T.H.; Davies, R.A.; Wernau, W.C. The production of semduramicin by direct fermentation. J. Antibiot. (Tokyo) 1992, 45, 813–815. [CrossRef]
- Callaway, T.R.; Edrington, T.S.; Rychlik, J.L.; Genovese, K. J.; Poole, T.L.; Jung, Y.S.; Bischoff, K.M.; Anderson, R.C; Nisbet, D.J. Ionophores: their use as ruminant growth promotants and impact on food safety. Curr. Issues Intest. Microbiol. 2003, 4, 43−51. https://pubmed.ncbi.nlm.nih.gov/14503688/.
- Novilla, M.N. Ionophores. In Veterinary Toxicology; Gupta, R.C., Ed.; Academic press: MA, USA, 2007, pp. 1021−1041. [CrossRef]
- Dutton, C.J., Banks, B.J., Cooper, C.B. Polyether ionophores. Nat. Prod. Rep. 1995, 12, 165−181. [CrossRef]
- Riddell, F.G. Structure, conformation, and mechanism in the membrane transport of alkali metal ions by ionophoric antibiotics. Chirality 2002, 14, 121–125. [CrossRef]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019, 118, 2009–2026. [CrossRef]
- Pinkerton, M.; Steinrauf, L.K. Molecular structure of monovalent metal cation complexes of monensin. J. Mol. Biol. 1970, 49, 533−546. [CrossRef]
- Duax, W.L.; Smith, G.D.; Strong, P.D. Complexation of metal ions by monensin. Crystal and molecular structure of hydrated and anhydrous crystal forms of sodium monensin. J. Am. Chem. Soc. 1980, 102, 6725−6729. [CrossRef]
- Pangborn, W.; Duax, W.L.; Langs, D. The hydrated potassium complex of the ionophore monensin A. J. Am. Chem. Soc. 1987, 109, 2163−2165. [CrossRef]
- Paz, F.A.A.; Gates, P.J.; Fowler, S.; Gallimore, A.; Harvey, B.; Lopes, N.P.; Stark, C.B.W.; Staunton, J.l Klinowski, J.; Spencer, J.B. Sodium monensin dihydrate. Acta Cryst. 2003, E59, m1050−m1052. [CrossRef]
- Paulus, E.F.; Vértesy, L. Crystal structure of the antibiotic SY-1 (20-deoxy-salinomycin): sodium 2-(6-[2-(5-ethyl-5-hydroxy-6-methyl-tetrahydro-pyran-2-yl)-2,10,12-trimethyl-1,6,8-trioxa-dispiro[4.1.5.3]pentadec-13-en-9-yl]-2-hydroxy-1,3-dimethyl-4-oxo-heptyl-5-methyl-tetrahydro-pyran-2-yl)-butyrate—methanolsolvate (1:0.69), C42H69NaO10 · 0.69CH3OH. Z. Kristallogr. NCS 2003, 218, 575–577. [CrossRef]
- Paulus, E.F.; Vértesy, L. Crystal structure of 2-(6-[2-(5-ethyl-5-hydroxy-6-methyl-tetrahydro-pyran-2-yl)-15-oxo-2,10,12-trimethyl-1,6,8-trioxa-dispiro[4.1.5.3]pentadec-13-en-9-yl]-2-hydroxy-1,3-dimethyl-4-oxo-heptyl-5-methyl-tetrahydro-pyran-2-yl)-butyrate sodium, Na(C42H67O11), SY-9 – antibiotic 20-oxo-salinomycin. Z. Kristallogr. NCS 2004, 219, 184–186. [CrossRef]
- Huczynski, A.; Ratajczak-Sitarz, M.; Katrusiak, A.; Brzezinski, B. Molecular structure of the 1:1 inclusion complex of monensin A sodium salt with acetonitrile. J. Mol. Struct. 2007, 832, 84−89. [CrossRef]
- Huczynski, A.; Ratajczak-Sitarz, M.; Katrusiak, A.; Brzezinski, B. Molecular structure of the 1:1 inclusion complex of monensin A lithium salt with acetonitrile. J. Mol. Struct. 2007, 871, 92−97. [CrossRef]
- Yildirim, S.O.; McKee, V.; Khardli, F.Z.; Mimouni, M.; Hadda, H.B. Rubidium(I) monensinate dihydrate. Acta Cryst. 2008, E64, m154-m155. [CrossRef]
- Huczynski, A.; Ratajczak-Sitarz, M.; Katrusiak, A.; Brzezinski, B. Molecular structure of rubidium six-coordinated dihydrate complex with monensin A. J. Mol. Struct. 2008, 888, 224−229. [CrossRef]
- Mehlhorn, H.; Pooch, H.; Raether, W. (1983) The action of polyether ionophorous antibiotics (monensin, salinomycin, lasalocid) on developmental stages of Eimeria tenella (Coccidia, Sporozoa) in vivo and in vitro: study by light and electron microscopy. Ztsch. Parasitenk. 1983, 69, 457–471. [CrossRef]
- Wang, Z.; Suo,X.; Xia, X.; Shen, J. Influence of monensin on cation influx and Na+-K+-ATPase activity of Eimeria tenella sporozoites in vitro. J. Parasitol. 2006, 92, 1092–1096. [CrossRef]
- Gough, L.P.; Shacklette, H.T.; Case, A.A. Element concentrations toxic to plants, animals, and man. Geol. Survey. Bull. 1979, 1466, 1−78. [CrossRef]
- Karbowska, B. Presence of thallium in the environment: sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess 2016, 188, art. 640. [CrossRef]
- Gagne, O.C.; Hawthorne, F.C. Bond-length distributions for ions bonded to oxygen: metalloids and post-transition metals. Acta Cryst. 2018, B74, 63–78. [CrossRef]
- Aoki, K.; Suh, H.; Nagashima, H.; Uzawa, J.; Yamazaki, H. Crystal structures of two polymorphic thallium( I) salts of the antibiotic lasalocid A: a polymeric form involving metal-phenyl π-bonding and a monomeric form involving the “half-naked” metal ion. J. Am. Chem. Soc. 1992, 114, 5722−5729. [CrossRef]
- Akkurt, M.; Yıldırım, S.Ö.; Khardli, F.-Z.; Mimouni, M.; McKee, V.; Haddab, T.B. Crystal structure of a new polymeric thallium-lasalocid complex: lasalocide anion-thallium(I) containing aryl-Tl interactions. ARKIVOC 2008, xv, 121−132. [CrossRef]
- Alleaume, M.; Hickel, D. Crystal structure of the thallium salt of the antibiotic grisorixin. Chem. Comm. 1972, 175−176. [CrossRef]
- Sakurai, T.; Kobayashi, K.; Nakamura, G.; Isono, K. Structure of the thallium salt of cationomycin. Acta Cryst. 1982, B38, 2471−2473. [CrossRef]
- Riche, C.; Pascard-Billy, C. Emericid-thallium(I). In Proceedings of the 3rd European Crystallographic Meeting, Zurich, Switzerland 6-10 September 1976.
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227−250. [CrossRef]
- Rokitskaya, T.I.; Firsov, A.M.; Khailova, L.S.; Kotova, E.A.; Antonenko, Y.N. Selectivity of cation transport across lipid membranes by the antibiotic salinomycin. Biochim Biophys Acta Biomembr. 2023, 1865, 184182. [CrossRef]
- Gerasimova, T.P.; Katsyuba, S.A. Infrared and Raman bands of cyclopentadienyl ligands as indicators of electronic configuration of metal centers in metallocenes. J. Organom. Chem. 2015, 776, 30e34. [CrossRef]
- Saleh, F.; Kheirandish, F.; Azizi, H.; Azizi, M. Molecular diagnosis and characterization of Bacillus subtilis isolated from burn wound in Iran. Res. Mol. Med. 2014, 2, 40−44. [CrossRef]
- Loggenberg, S.R.; Twilley, D.; de Cahna, M,N.; Lall, N. Medicinal plants used in South Africa as antibacterial agents for wound healing. In Medicinal Plants as Anti-Infectives. Chassagne, F., Ed.; Academic Press: MA, USA, 2022; pp. 139−182. [CrossRef]
- Anany, H.; Brovko, L.Y.; El Arabi, T.; Griffiths, M.W. Bacteriophages as antimicrobials in food products: aApplications against particular pathogens. In Handbook of Natural Antimicrobials for Food Safety and Quality; Taylor, T.M., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 89−116. [CrossRef]
- Zaheen, Z.; War, A.F.; Ali, F.; Yatoo, A.M.; Ali, M.N.; Ahmad, S.B.; Rehman, M.U.; Paray, B.A. Common bacterial infections affecting freshwater fish fauna and impact of pollution and water quality characteristics on bacterial pathogenicity. Bact. Fish Dis. 2022, 133−154. [CrossRef]
- Hajam, Y.A.; Kumar, R.; Rani, R.; Sharma, P., Diksha. Efficacy of different treatments available against bacterial pathogens in fish. Bact. Fish Dis. 2022, 379−398. [CrossRef]
- Becker, K.; Rutsch, F.; Uekötter, A.; Kipp, F.; König, J.; Marquardt, T.; Peters, G., von Eiff, C. Kocuria rhizophila adds to the emerging spectrum of micrococcal species involved in human infections. J. Clin. Microbiol. 2008, 46, 3537−3539. [CrossRef]
- Castro, A.; Silva, J.; Teixeira, P. Staphylococcus aureus, a food pathogen: virulence factors and antibiotic resistance. In Handbook of Food Bioengineering, Foodborne Diseases; Holban, A.M.; Grumezescu, A.M., Eds.; Academic Press: MA, USA, 2018; pp. 213−238. [CrossRef]
- Azimi, T.; Mirzadeh, M.; Sabour, A., Nasser, A.; Fallah, F.; Pourmand, M.R. Coagulase-negative staphylococci (CoNS) meningitis: a narrative review of the literature from 2000 to 2020. New Microbes New Infect. 2020, 37, 100755. [CrossRef]
- Toxicological review of thallium and compounds. U.S. Environmental Protection Agency, Washington, DC. EPA/635/R-08/001F; www.epa.gov/iris (accessed on April 26, 2025).
- https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on April 24, 2025).
- Yahya, M.A.; Sharon, S.M.; Hantisteanu, S.; Hallak, M.; Bruchim, I. The role of the insulin-like growth factor 1 pathway in immune tumor microenvironment and its clinical ramifications in gynecologic malignancies. Front. Endocrinol. 2018, 9, 297. [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc. 2016, 2016(4):pdb.prot087379. [CrossRef]
- Wang, H.; Zhang, H.; Zhu, Y.; Wu, Z.; Cui, C.; Cai, F. Anticancer mechanisms of salinomycin in breast cancer and its clinical applications. Front. Oncol. 2021, 11, 654428. [CrossRef]
- Zhou, Y.; Deng, Y.; Wang, J.; Yan, Z.; Wei, Q.; Ye, J.; Zhang, J.; He, T.-C.; Qiao, M. Effect of antibiotic monensin on cell proliferation and IGF1R signaling pathway in human colorectal cancer cells. Annals Med. 2023, 55, 954–964. [CrossRef]
- SADABS, v 2016/2 Bruker Analytical X-Ray Systems; Bruker AXS Inc.: Madison, WI, USA, 2016.
- SAINTV8.38A Bruker Analytical X-Ray Systems; Bruker AXS Inc.: Madison, WI, USA, 2018.
- SHELXTL 2018, Bruker Analytical X-Ray Systems; Bruker AXS Inc.: Madison, WI, USA, 2018.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Cryst. B 2013, 69, 249–259. [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1−A3.B.3. [CrossRef]
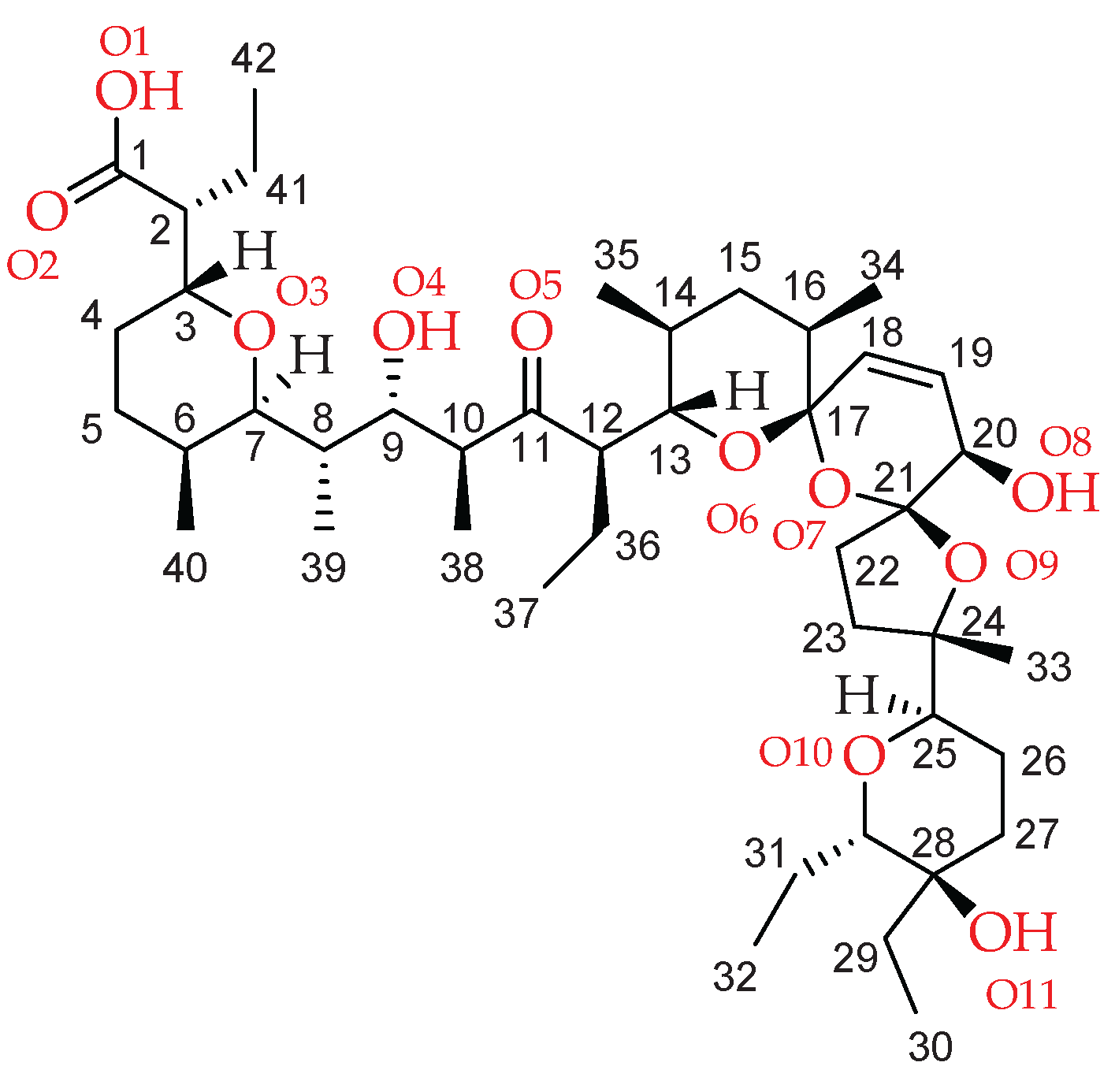
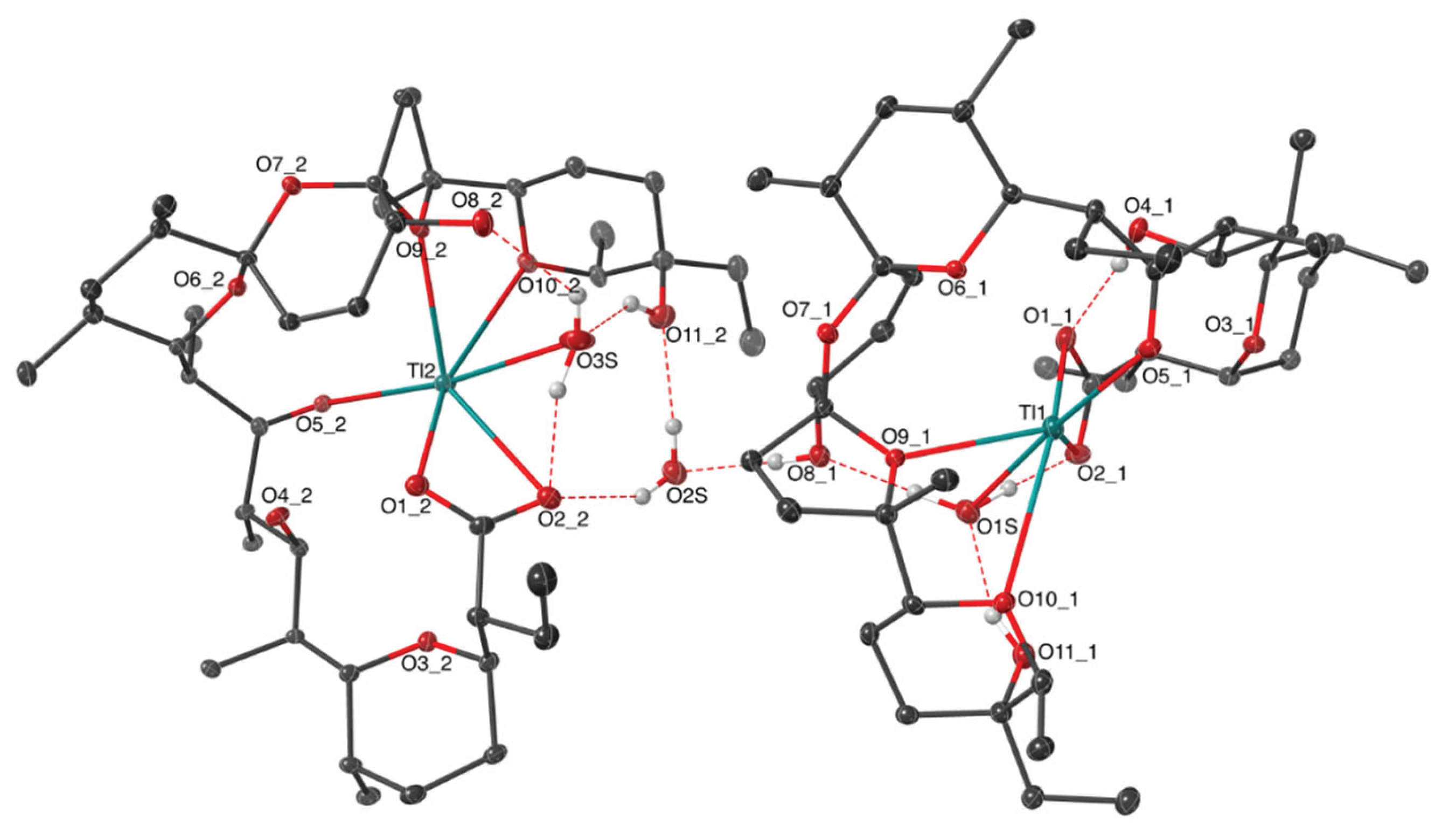
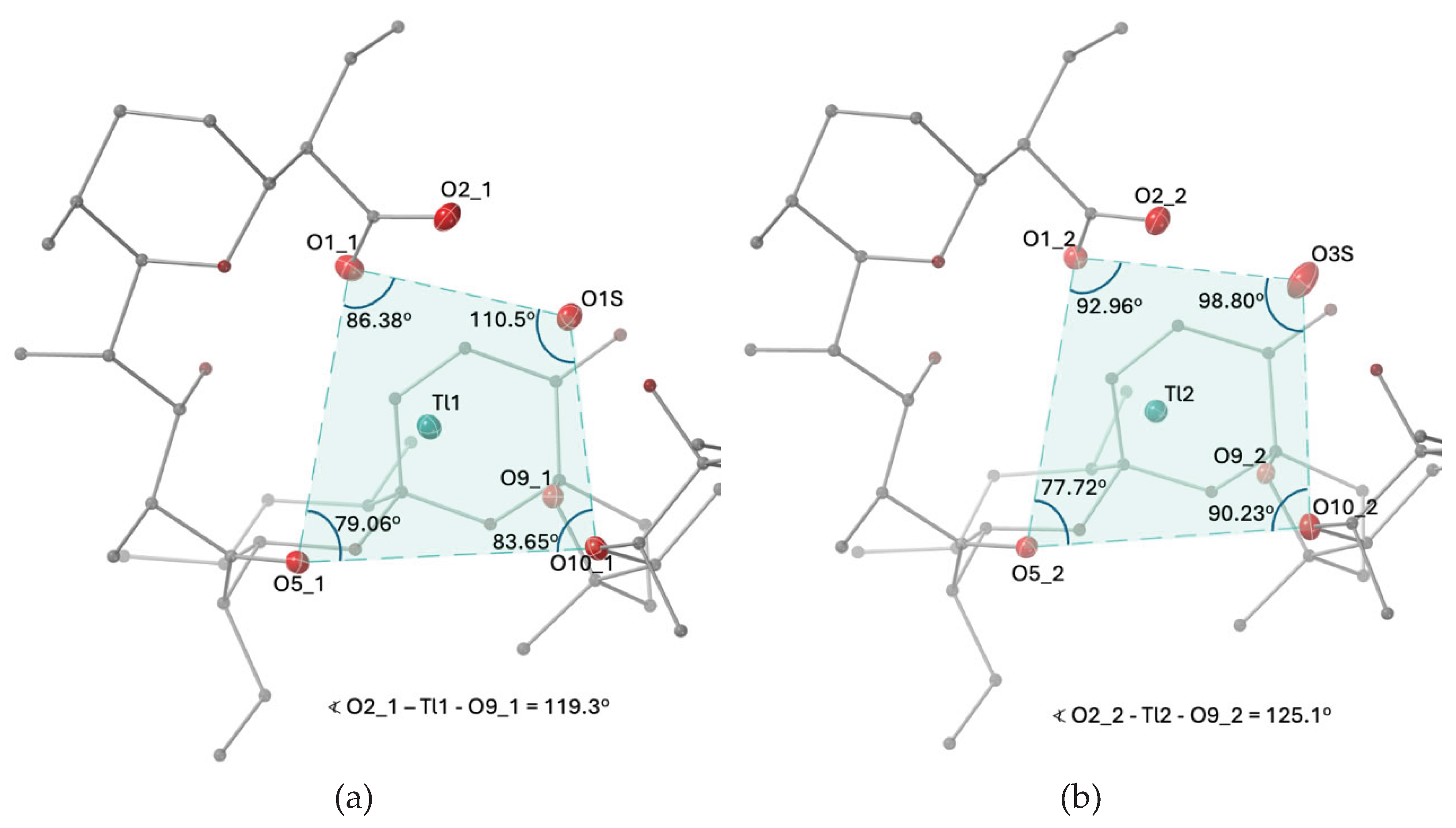

| Bond lenght | SalTl1 | SalTl2 |
| Tl − O1_n | 2.742(7) | 2.741(6) |
| Tl − O2_n | 3.330(6) | 3.138(6) |
| Tl − O5_n | 2.866(5) | 2.897(5) |
| Tl − O9_n | 2.788(5) | 2.724(5) |
| Tl − O10_n | 3.266(5) | 3.041(6) |
| Tl − O1S | 2.699(6) | - |
| Tl − O3S | - | 3.029(9) |
| Bond angle | SalTl1 | SalTl2 |
| O1_n – Tl – O5_n | 109.5(2) | 107.3(2) |
| O1_n – Tl – O10_n | 152.6(2) | 155.3(2) |
| O1_n – Tl – O1S | 78.5(2) | - |
| O1_n – Tl – O3S | - | 76.1(2) |
| O1_n – Tl – O2_n | 41.9(2) | 43.6(2) |
| O2_n – Tl – O1S | 59.8(2) | - |
| O2_n – Tl – O3S | - | 51.4(2) |
| O9_n – Tl – O5_n | 103.6(1) | 96.9(1) |
| O9_n – Tl – O10_n | 56.4(1) | 58.3(2) |
| Bond | SalTl1 | SalTl2 |
| O1_n − O4_n | 2.680(9) | 2.720(8) |
| O2_n − O1S | 2.602(8) | − |
| O2_n − O3S | − | 2.675(9) |
| O8_n − O1S | 2.718(8) | − |
| O8_n − O3S | − | 2.684(9) |
| O11_n − O1S | 2.672(8) | − |
| O11_n − O3S | − | 2.651(10) |
| O8_n − O2S | 2.714(9) | − |
| O2_n − O2S | − | 2.848(9) |
| O11_n − O2S | − | 2.738(9) |
| Compound | Vibration | IR, cm−1 | Raman, cm−1 |
| SalH | νC=O (isolated) | 1704 | 1705 |
| νC=O (COOH) | 1716 | − | |
| νC=C | “hidden” | 1664 | |
| SalNa | νC=O (isolated) | 1716 | 1718, 1706 |
| νC=O (COO−, asym) | 1567 | 1570 | |
| νC=O (COO−, sym) | 1406 | − | |
| νC=C | “hidden” | 1681 / 1667 | |
| SalK | νC=O (isolated) | 1719 | 1722 |
| νC=O (COO−, asym) | 1564 | 1560 | |
| νC=O (COO−, sym) | 1405 | − | |
| νC=C | “hidden” | 1665 | |
| SalTl | νC=O (isolated) | 1703 | 1703 |
| νC=O (COO−, asym) | 1549 | 1555 | |
| νC=O (COO−, sym) | 1398 | − | |
| νC=C | “hidden” | 1659 |
| Band | C17−C18 | C18=C19 | C19−C20 | C20−C21 | C21−O7 | O7−C17 |
| SalNa1 | 1.485 | 1.344 | 1.492 | 1.556 | 1.429 | 1.433 |
| SalNa2 | 1.472 | 1.311 | 1.420 | 1.566 | 1.435 | 1.429 |
| average | 1.479 | 1.328 | 1.456 | 1.561 | 1.432 | 1.431 |
| SalTl1 | 1.507 | 1.318 | 1.497 | 1.522 | 1.419 | 1.431 |
| SalTl2 | 1.484 | 1.328 | 1.494 | 1.527 | 1.422 | 1.425 |
| average | 1.500 | 1.323 | 1.500 | 1.525 | 1.421 | 1.428 |
| Angle | C17−C18−C19 | C18=C19−C20 | C19−C20−C21 | C20−C21−O7 | C21−O7−C17 | O7−C17−C18 |
| SalNa1 | 122.1 | 121.0 | 112.4 | 105.7 | 122.4 | 113.0 |
| SalNa2 | 123. 0 | 124.7 | 111.1 | 109.1 | 120.7 | 112.2 |
| average | 122.6 | 122.9 | 111.8 | 107.4 | 121.6 | 112.6 |
| SalTl1 | 122.7 | 121.2 | 108.9 | 110.5 | 121.4 | 112.7 |
| SalTl2 | 122.7 | 120.2 | 109.0 | 109.0 | 120.3 | 113.6 |
| average | 122.7 | 120.7 | 109.0 | 109.8 | 120.9 | 113.2 |
| Crystal data | SalTl |
| Chemical formula | C42H69O11Tl×1.5H2O |
| Mr | 981.36 |
| Crystal system, space group | Monoclinic, P1 |
| Temperature (K) | 107 |
| a (Å) | 12.2005(7) |
| b (Å) | 13.7304(8) |
| c (Å) | 14.7041(8) |
| α (°) | 116.623(2) |
| β (°) | 93.382(2) |
| γ (°) | 90.454(2) |
| V (Å3) | 2196.6(2) |
| Z | 2 |
| Radiation type, λ [Å] | Cu Kα, 1.54178 |
| µ (mm−1) | 7.559 |
| Crystal size (mm3) | 0.09 × 0.15 × 0.19 |
| Data collection | |
| Diffractometer | Bruker APEX-II CCD |
| Absorption correction | Multi-scan |
| Tmin, Tmax | 0.482, 0.753 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 40202, 14134, 14042 |
| Rint | 0.0527 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.0360, 0.0918, 1.061 |
| No. of parameters | 1033 |
| No. of restraints | 3 |
| Δρmax, Δρmin (e Å−3) | 2.8 (around the very heavy Tl atom)/−0.8 |
| Absolute structure | Flack x determined using quotients [(I+)-(I–)] / [(I+)+(I–)] [59] |
| 0.086(7) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





