1. Introduction
Endometrial cancer (EC) is the most common malignancy of the female reproductive system in developed countries. Histopathologically, two main subtypes have been described: type I tumors (>80%), which are estrogen-dependent (and include grade 1 and 2 endometrioid adenocarcinomas) and type II tumors which are not estrogen-dependent (include the more clinically aggressive grade 3 endometrioid adenocarcinomas, serous papillary adenocarcinomas, clear cell adenocarcinomas and carcinosarcomas) [
1]. Type II tumors demonstrate a worse prognosis and are responsible for nearly half of the EC-related deaths [
2].
The 2016 European Society of Gynecological Oncology (ESGO) and the European Society for Radiotherapy and Oncology (ESTRO) consensus defined the high-risk group as endometrioid carcinomas of stage IB with a deep myometrial invasion (MI) >50% grade 3 or stage II–III any grade along with non-endometrioid carcinomas of stage I–III [
3,
4]. However, a molecular classification is recommended by ESGO, ESTRO and European Society of Pathology (ESP) guidelines, been fully integrated in the clinical routine for the risk classification of EC patients [
3,
4,
5,
6].
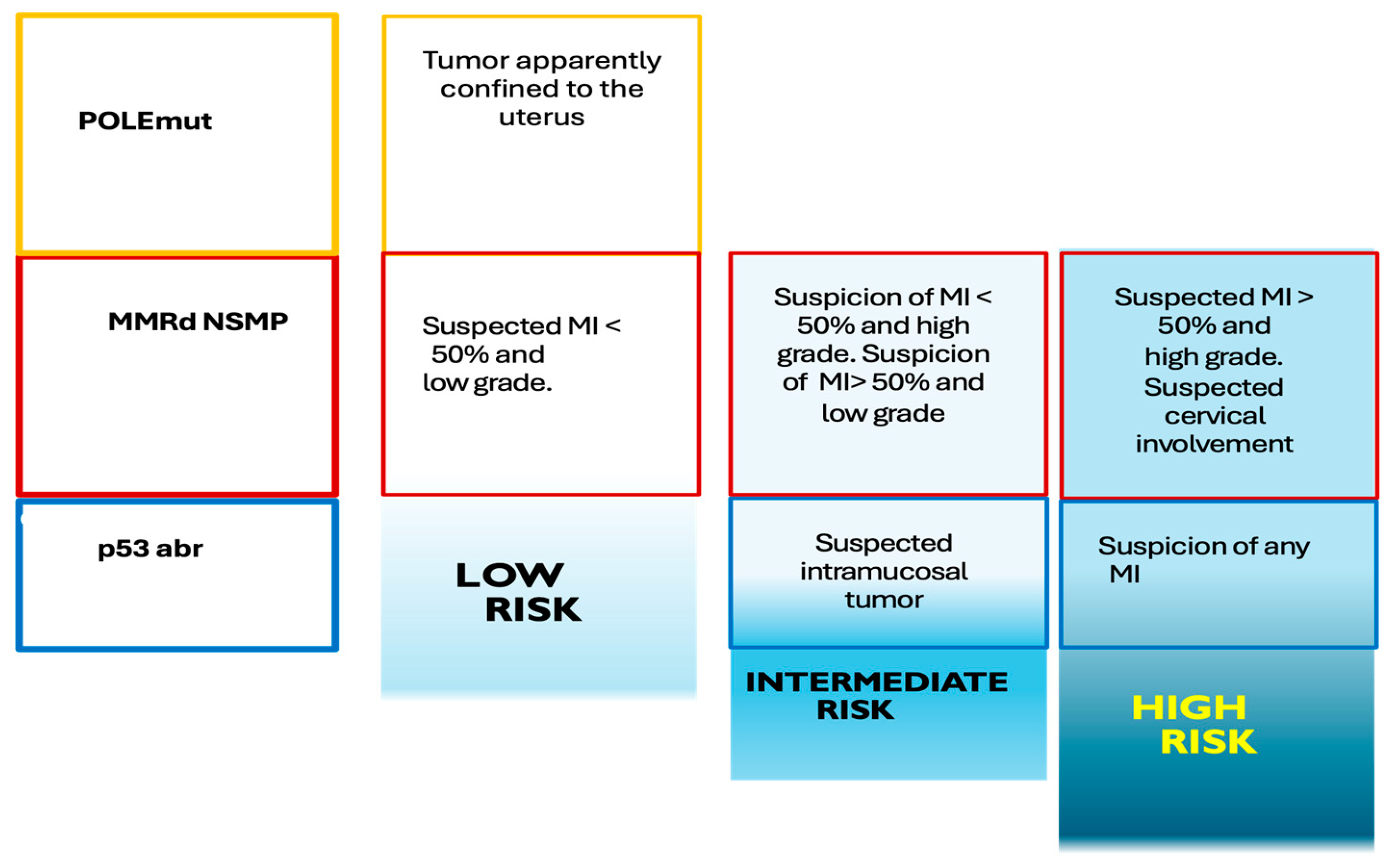
Figure 1 represents an adaptation of the molecular classification of EC.
The molecular characterization of EC increases the accuracy of the risk classification solely based on the key clinical histological parameters, such as histological subtype, grade, and MI [
5]. Thus, when molecular classification reveals an aberrant p53 or POLE mutation in stages I and II, this results in overstaging or downstaging of the disease, respectively [
3]. Published studies have confirmed that these molecular subtypes independently predict clinical outcomes and, as such, help inform risk stratification, particularly when considering whether to utilize adjuvant chemoradiotherapy (CRT) [
6].
Most patients with EC are confined to the uterine corpus at the time of diagnosis, with early stage and good prognosis. However, approximately 10%-15% of patients with EC present with advanced-stage and harbor disease beyond the uterus and abdominopelvic lymph nodes [
7].
Although the International Federation of Gynecology and Obstetrics (FIGO) staging system is primarily surgical, complementary presurgical imaging for estimating the preoperative stage adds a layer of precision to evaluate tumor size, myometrial, and cervical involvement, adnexal status, and lymph node involvement [
8]. Therefore, lymphadenectomy for staging purposes is usually reserved for patients with high-risk disease because of its associated perioperative complications and long-term morbidity.
Table 1 summarizes FIGO stage current classification.
Given the high specificity and positive predictive value (PPV), positron emission tomography/computed tomography (PET/CT) with fluorine-18-2-fluoro-2-deoxy-D- glucose ([¹⁸F]FDG). [¹⁸F]FDG-PET/CT is recommended be included in the staging evaluation. In addition, hybrid positron emission tomography/magnetic resonance imaging (PET/MRI) using [¹⁸F]FDG, has emerged as a promising imaging modality that combines metabolic and high-level anatomical information. [¹⁸F]FDG-PET/MRI is reported to have value in evaluating gynecological tumor staging, assessing treatment response, detecting recurrent lesions, and predicting prognosis.
The following sections present a review of the current state of PET/CT and PET/MRI using [¹⁸F]FDG and others radiotracers in EC.
2. Initial Staging
2.1. T Stage
Accurate assessment of the primary tumor (T stage), encompassing the depth of MI and potential extension to adjacent structures such as the cervix, is fundamental for therapeutic planning in EC.
Scientific evidence indicates that [¹⁸F]FDG-PET/CT is a technique that can facilitate improved diagnosis and, consequently, treatment of EC. Currently, [¹⁸F]FDG-PET/CT is used as an adjunct to conventional diagnostic imaging techniques. This is due to its limited spatial resolution, which hinders the precise assessment of small tumors and the exact delineation of local invasion; for this reason, the diagnostic performance of [¹⁸F]FDG-PET/CT for the primary tumor is inferior to that of MRI. Therefore, [¹⁸F]FDG-PET/CT is not recommended for screening or early diagnosis of primary lesions [
9]. Thus, for assessing the T stage of EC, MRI and, in certain contexts, transvaginal ultrasound performed by experts are recommended, as these modalities offer superior performance for these purposes [
9,
10].
Based on current evidence, [¹⁸F]FDG-PET/CT is not considered the imaging modality of choice for the detailed evaluation of T staging. Therefore, while [¹⁸F]FDG-PET/CT does not replace MRI for T stage determination, the metabolic information it provides could add additional biological characterization [
11,
12,
13,
14]. This implies providing functional information that could influence the interpretation of preoperative risk and, potentially, clinical decision-making.
[¹⁸F]FDG-PET/MRI has demonstrated high accuracy in the preoperative evaluation of EC, particularly in determining deep MI, with values around 80%, outperforming [¹⁸F]FDG-PET/CT [
15,
16]. Similarly, in a study by Yu et al. [
17], [¹⁸F]FDG-PET/MRI showed a sensitivity, specificity, and accuracy of 89%, 95%, and 93%, respectively, for detecting MI in a cohort of 57 patients with EC. Other study reported an accurate detection of cervical invasion with a sensitivity, specificity, and accuracy of 81%, 95%, and 91%, respectively. Kitajima et al. [
18] showed that [¹⁸F]FDG-PET/MRI had significantly higher accuracy than [¹⁸F]FDG-PET/CT for T staging (80% vs. 60%). Also, quantitative parameters, especially SUVmax/minimum apparent diffusion coefficient (ADCmin) index, were associated with tumor stage and other clinicopathological characteristics of aggressiveness [
17,
19].
2.2. N Stage
The detection of regional lymph node metastases (N stage) is a prognostic factor of great importance in EC and has direct implications for treatment planning.
Recent clinical studies support the use of [¹⁸F]FDG-PET/CT for nodal staging, as it possesses greater reliability in diagnosing pelvic and para-aortic lymph node metastases, compared to conventional imaging modalities such as CT or MRI alone [
9,
20].
Clinical guidelines such as those from the European Society for Medical Oncology (ESMO) indicate that [¹⁸F]FDG-PET/CT can be considered as an additional diagnostic test in the high-risk patient group to detect preoperative nodal extension in patients with EC [
10]. Likewise, the clinical guidelines of the National Comprehensive Cancer Network (NCCN) and the Spanish Society of Medical Oncology in collaboration with the Spanish Group for Ovarian Cancer Research (SEOM-GEICO) recommends that [¹⁸F]FDG-PET/CT should be considered if metastases are suspected, in selected cases in patients eligible for surgery or locoregional therapy [
21,
22].
Although there is currently no specific clinical guideline from the European Association of Nuclear Medicine (EANM) or the Society of Nuclear Medicine and Molecular Imaging (SNMMI) dedicated to the evaluation of EC, it is relevant to mention that the joint EANM/SNMMI guideline for cervical cancer highlighted that MRI is used for the evaluation of locoregional lymph node involvement, and CT can also provide essential information on nodal involvement. However, [¹⁸F]FDG-PET/CT exhibits higher sensitivity for the detection of metastases, including para-aortic nodal involvement, compared to MRI limited to the pelvis. Thus, this diagnostic approach could also be considered applicable, in certain clinical contexts, to the management of EC [
23].
A retrospective study that included patients with clinical stage I based on MRI and [¹⁸F]FDG-PET/CT, detected lymph node metastases at both pelvic and para-aortic levels in high-risk patients, as well as a higher recurrence rate. It is noteworthy that in this study, even patients in the low/intermediate-risk group presented lymph node metastases and nodal recurrence. This dual imaging assessment had a negative predictive value (NPV) of 0.945, although the low prevalence of lymph node involvement (5.5%) could bias the results. Therefore, it would be interesting to conduct prospective studies to confirm these findings, as it could help select a subgroup of low-risk patients in whom omitting complete surgical lymphadenectomy would lead to a significant reduction in the morbidity associated with these procedures, such as lymphedema. However, this approach requires robust validation and careful consideration of the inherent risk of not detecting micrometastases, which currently escapes the resolution of [¹⁸F]FDG-PET/CT [
24].
Corroborating the aforementioned information, Fasmer et al. [
14] evaluated a dual imaging approach using MRI in all patients and [¹⁸F]FDG-PET/CT only in selected cases based on MRI findings, which allowed the identification of preoperative risk groups with significant differences in survival. The approach selected approximately 54% of patients for [¹⁸F]FDG-PET/CT, demonstrating an improvement in the detection of lymph node metastases compared to MRI alone. Likewise, they demonstrated that imaging using MRI and [¹⁸F]FDG-PET/CT offers comparable diagnostic performance in low and high histological risk groups for predicting central parameters of the FIGO stage.
It is worth mentioning that the primary treatment for EC is hysterectomy with bilateral salpingo-oophorectomy, often accompanied by pelvic lymphadenectomy and, if necessary, para-aortic lymphadenectomy [
25]. Although the FIGO staging system is primarily surgical, complementary preoperative imaging to estimate the preoperative stage adds a level of precision to assess tumor size, myometrial and cervical involvement, adnexal status, and nodal involvement [
8]. Therefore, lymphadenectomy for staging purposes is usually reserved for patients with high-risk disease, due to its associated perioperative complications and long-term morbidity.
Likewise, [¹⁸F]FDG-PET/CT has demonstrated moderate sensitivity and high specificity for the detection of pelvic lymph node metastases. Sentinel lymph node (SLN) mapping technique along with thorough histopathological staging increased the identification of micrometastases in pelvic lymph nodes (incidence from 18.3% to 27.3%), which are not detectable by [¹⁸F]FDG-PET/CT due to its limited spatial resolution. That is, [¹⁸F]FDG-PET/CT may not detect micrometastases, which can be identified by thorough histopathological staging applied to surgically obtained sentinel nodes. Therefore, the combination of both modalities is promising for nodal staging purposes [
13,
26].
Fasmer et al. [
13] demonstrated that, although [¹⁸F]FDG-PET/CT has limitations in diagnosing micrometastases due to its reduced spatial resolution, an elevated metabolic tumor volume (MTV) could be associated with a higher risk of micrometastases, as it showed high diagnostic performance for predicting lymph node metastases and aggressive disease, representing a promising complement to conventional [¹⁸F]FDG-PET/CT interpretation.
Previous studies comparing [¹⁸F]FDG-PET/MRI and [¹⁸F]FDG-PET/CT for N staging have reported contradictory results with comparable performance for both [
18] vs higher accuracies for [¹⁸F]FDG-PET/MRI [
15,
16].
As disease extension is often unclear before surgery. Weissinger et al. [
27], published a prospective study to evaluate [¹⁸F]FDG-PET/MRI as a minimal invasive technique to assess N-staging. [¹⁸F]FDG-PET/MRI demonstrated high specificity in detecting para-aortic lymph nodes (99%), but moderate sensitivity (67%). The results were comparable in terms of pelvic lymph nodes with a specificity of 98% and a sensitivity of 73%. Other authors support the excellent results of [¹⁸F]FDG-PET/MRI for N-staging, showing high accuracy in detecting lymph node involvement (sensitivity: 86%, specificity: 93%, accuracy: 91%), making it especially effective for nodal staging [
16].
Figure 2.
2.3. M Stage
The identification of distant metastases (M stage) at the time of initial diagnosis is a crucial prognostic factor that significantly alters the therapeutic approach, generally orienting it towards systemic treatments.
[¹⁸F]FDG-PET/CT is considered the most sensitive and useful non-invasive imaging modality for the detection of distant metastases in patients with EC, especially in high-risk cases or those with aggressive histological subtypes (clear cell/serous/dedifferentiated carcinomas and carcinosarcoma), surpassing CT and MRI in the detection of extraregional metastatic disease. Its early use in the diagnosis of high-risk patients could optimize the selection of candidates for cytoreductive surgery versus primary systemic therapy, avoiding unnecessary surgeries in patients with disseminated disease [
28].
The ESMO clinical guideline suggests considering [¹⁸F]FDG-PET/CT as an additional diagnostic test in high-risk patients to detect preoperative extrapelvic extension and postoperative recurrence. In addition, a chest CT is recommended as part of the initial evaluation to rule out pulmonary metastases in patients with high-risk EC [
10]. The NCCN clinical guideline recommends considering whole-body CT to assess the presence of metastatic disease in patients with high-grade carcinomas, in those with an incidental finding of cancer or incomplete staging who present significant risk factors, or if there is a well-founded clinical suspicion of metastases or in the context of suspected recurrence or subsequently developed metastatic disease, noting that [¹⁸F]FDG-PET/CT can be considered in patients with suspected metastases in selected patients [
21,
29]. The SEOM-GEICO clinical guideline indicates that, at a minimum, a whole-body CT should be performed to rule out distant metastases, and that [¹⁸F]FDG-PET/CT can also be used for this purpose in selected patients eligible for surgery or locoregional therapy [
22].
As previously mentioned, although there is no specific SNMMI or EANM guideline for EC, the joint EANM/SNMMI guideline for cervical cancer mentions a higher sensitivity of [¹⁸F]FDG-PET/CT for the detection of metastases, an approach that could also be considered applicable in certain clinical contexts of EC [
23]. For example, peritoneal dissemination has been observed in 10-20% of patients with high histological risk [
30]. Likewise, Hong et al. [
31] demonstrated that [¹⁸F]FDG-PET/CT significantly identified bone metastases in patients with type II endometrial carcinoma, especially with serous histology, which is associated with aggressive biology and poorer prognosis. Furthermore, they noted that [¹⁸F]FDG-PET/CT is superior to CT for detecting bone metastases and other non-visible distant metastases. Therefore, in these high-risk patients, [¹⁸F]FDG-PET/CT seem to be justified.
Figure 3.
Data published in the literature defines that [¹⁸F]FDG-PET/MRI has a high specificity and accuracy in assessing metastatic disease. A retrospective study reported a sensitivity, specificity and accuracy of M-staging of 33%, 100% and 82%, respectively for [¹⁸F]FDG-PET/MRI, despite that in this study [¹⁸F]FDG-PET/MRI was realized without gadolium-based contrast [
32]. In a prospective study including 35 patients with pathologically proven EC, [¹⁸F]FDG-PET/MRI revealed hepatic metastasis in one patient, which was histologically confirmed by biopsy [
27]. In the metaanalysis of Bezzi et al. [
19] compared to other imaging techniques, [¹⁸F]FDG-PET/MRI seems to show a higher diagnostic accuracy in detecting soft tissue invasion and abdominopelvic metastases from primary EC.
Beyond its diagnostic performance, [¹⁸F]FDG-PET/MRI presents several additional advantages that enhance its clinical value. The combination of PET and MRI adds unique benefits when analyzing endometrial and menstrual changes, particularly in reproductive-age women and complex staging cases. Moreover, [¹⁸F]FDG-PET/MRI represents a viable alternative to conventional imaging methods, especially for patients who are unable to receive iodinated or gadolinium-based contrast agents. Another benefit is the lower radiation exposure associated with [¹⁸F]FDG-PET/MRI compared to [¹⁸F]FDG-PET/CT or CT imaging [
19].
3. Image-Guided Surgery and Radiotherapy
Gynaecological cancers have particularly benefitted from the increasing use of imaging to guide radiation treatment planning both for external beam radiotherapy and brachytherapy [
33].
The main treatment options for EC include surgery with or without radiotherapy, brachytherapy and chemotherapy [
34]. In addition, for localized EC the standard treatment is hysterectomy with bilateral salpingo-oophorectomy, often accompanied by pelvic lymphadenectomy and, if necessary, para-aortic lymphadenectomy [
25].
In locally advanced EC, CRT is routinely administered as definitive therapy and can be used alone or in combination with surgery in the remaining of stages [
35]. The recent identification of molecular EC subtypes has led to the development of targeted therapies, particularly for early recurrent EC subtypes [
36].
Although imaging is not a part of the staging system for EC it does influence treatment selection [
37,
38]. MRI is the preferred imaging modality for preoperative assessment of key prognostic factors, specifically tumor size, depth of MI, cervical stromal involvement, extrauterine disease, and nodal involvement. Thus, MRI is vital to planning definitive, neoadjuvant, adjuvant, and salvage radiation for EC because it enables visualization of tumor myometrial depth and local extent, as well as adjacent organ involvement [
33].
In cases of local recurrence with invasion of adjacent organs or the pelvic sidewall, MRI should be used to assess the likelihood of surgical resectability and to plan salvage therapy. Moreover [¹⁸F]FDG-PET/CT is helpful to exclude nodal and distant metastases.
[¹⁸F]FDG-PET/CT is considered a valuable tool for the detection of lymph node metastases, as it offers greater accuracy than CT or MRI alone, especially for the evaluation of para-aortic lymph nodes [
12]. In addition, the high positive predictive value of [¹⁸F]FDG-PET/CT is useful for referring patients to appropriate cytoreductive surgery [
12]. However, its main limitation lies in the detection of micrometastases or involvement in normal-sized lymph nodes, situations in which SLN mapping followed by exhaustive histopathological analysis may offer superior performance. Furthermore, [¹⁸F]FDG-PET/CT can be particularly useful in patients classified as high-risk to identify nodal disease not suspected by other techniques, which may influence the extent of lymphadenectomy to be performed or the planning of adjuvant radiotherapy.
Therefore, the optimization of N staging likely resides in the combination of metabolic-anatomical information from [¹⁸F]FDG-PET/CT with the biological profile of the tumor. The issue of whether [¹⁸F]FDG-PET/CT or PET/MRI may reduce the need for systematic lymphadenectomy in low-risk patients by improving nodal assessment, need to be demonstrated in prospective studies.
4. Response to Treatment
4.1. Locorregional Disease
Following the initiation of therapy, monitoring tumour response is critical for guiding further clinical management. Unlike anatomical imaging, [¹⁸F]FDG-PET/CT enables functional assessment in EC by detecting early changes in glucose metabolism that typically precede morphological alterations. These metabolic changes may serve as early indicators of therapeutic efficacy, providing valuable information on tumour biology before the structural response becomes evident [
39]. However, the clinical use of [¹⁸F]FDG-PET/CT in the evaluation of locoregional response to treatment in EC remains a developing field.
Viable tumor is more likely to show hypermetabolism and can therefore be differentiated from post-therapeutic changes on [¹⁸F]FDG-PET/CT. A key challenge lies in determining the optimal timing of post-therapy imaging, as metabolic changes caused by chemotherapy or radiotherapy—particularly inflammation—can result in false-positive findings. Current evidence suggests that [¹⁸F]FDG-PET/CT should generally be delayed for 8–12 weeks after radiotherapy and at least 6 weeks after surgery to minimize interpretation errors. However, inflammatory changes may persist and interfere with interpretation even up to 6 months post-treatment in some cases. Despite these limitations, post-radiation changes seem to play a minimal impact of on [¹⁸F]FDG-PET’s diagnostic accuracy [
40,
41].
Few studies have investigated the utility of [¹⁸F]FDG-PET/CT in the assessment of EC response [
42,
43]. In one cohort of 21 patients undergoing [¹⁸F]FDG-PET/CT before and after chemotherapy, significant changes in [¹⁸F]FDG uptake were strongly correlated with treatment response, achieving a sensitivity of 90% and a specificity of 80% in distinguishing responders from non-responders, although the relatively small cohort size and the moderate rate of false positives highlight the need for further validation in larger prospective studies [
42]. Moreover, the degree of metabolic response demonstrated on [¹⁸F]FDG-PET/CT may carry prognostic value, as greater reductions in glucose uptake have been associated with improved long-term outcomes [
43].
[¹⁸F]FDG-PET/MRI is a promising imaging tool for monitoring treatment response in EC due to its ability to provide both metabolic and anatomical information in a single scan supporting personalized follow-up strategies.
However, the scarce literature for both hybrid diagnostic techniques explains the current lack of consensus among international societies for their routine use in this setting [
3,
4,
5].
4.2. Distant Metastasic Disease
In patients with unresectable or metastatic EC, [¹⁸F]FDG-PET/CT plays a critical role in assessing response to systemic therapy [
44]. Its incorporation into post-treatment surveillance protocols has demonstrated both diagnostic validity and clinical utility, particularly for early identification of disease progression or treatment response when anatomical imaging is limited by post-therapeutic changes or low sensitivity for metabolically active lesions [
45].
Figure 4.
Thus, in selected advanced or high-risk cases, [¹⁸F]FDG-PET/CT provides a robust tool for evaluating systemic therapy response and informing management, especially in oligometastatic settings or when conventional imaging is inconclusive. In addition, early and significant metabolic response to therapy, as measured by [¹⁸F]FDG-PET/CT, has been associated with improved overall survival (OS) in patients with metastatic gynecological malignancies, including EC [
40,
46].
5. Recurrence Detection
Although the overall treatment effect of EC is good, up to 15-20% of patients have relapsed occurring within the first 2 years after treatment. This percentage may be even higher in patients with aggressive histologic subtypes (e.g., serous or clear cell carcinoma) [
40,
47].
EC tends to recur in the pelvis, especially in the vaginal vault and pelvic lymph nodes, followed by para-aortic lymph nodes [
48]. The vagina, particularly the vaginal apex, is the most common site of local recurrence in EC (42% of recurrences). Reported 5-year cure rates in patients with isolated vaginal apex recurrence range from 40% to 60%, underscoring the importance of early detection through functional imaging modalities such as [¹⁸F]FDG-PET/CT for guiding rescue therapies [
44].
Figure 5 and
Figure 6.
Extrapelvic recurrence commonly involves the peritoneum and lungs. Atypical metastatic sites include extra-abdominal lymph nodes, liver, adrenals, brain, bones, and soft tissue [
49].
Figure 7.
A meta-analysis of 11 studies, including 541 patients, confirmed the high diagnostic accuracy of [¹⁸F]FDG-PET/CT for detecting EC recurrence, with pooled sensitivity and specificity of 95.8% and 92.5%, respectively. Notably, [¹⁸F]FDG-PET/CT findings altered clinical management in 22–35% of cases [
50].
While [¹⁸F]FDG-PET/CT offers high sensitivity for metabolically active lesions, MRI provides superior soft tissue contrast, which is particularly useful for assessing local recurrences in the pelvis, lymph nodes, and peritoneum. MRI appears as an intermediate or heterogeneous T2 signal intensity mass, with associated restricted diffusion and enhancement [
37]. Some authors described that [¹⁸F]FDG-PET/MRI is equivalent or outperforms [¹⁸F]FDG-PET/CT in recurrence detection [
51,
52,
53].
Chao et al. [
54] aimed to assess the value of integrating [¹⁸F]FDG-PET/CT into the management of EC in comparison with conventional imaging alone. The combination [¹⁸F]FDG-PET with MRI/CT significantly outperformed MRI/CT alone for overall lesion detection (AUC 0.949 vs. 0.872, respectively; p = 0.004), including both pelvic and extrapelvic metastases.
Current guidelines recommend [¹⁸F]FDG-PET/CT when recurrence is suspected [
3,
21,
22]. In follow-up, although intensive surveillance is not generally recommended, an individualized scheme based on symptomatology and clinical aspects is proposed, with periodic physical examinations and transvaginal ultrasounds. Additional imaging (whole-body CT, PET/CT, or MRI) is considered useful in the presence of suspicious findings of recurrence or in patients with stage III-IV disease, suggesting in the latter scenario a whole-body CT every 6 months for the first 3 years and then every 6-12 months for an additional 2 years to detect asymptomatic recurrences [
29,
55].
[¹⁸F]FDG-PET/CT should not be used for standard surveillance after treatment. However, some authors reported that this technique was able in detecting recurrence in up to 12% of asymptomatic patients, influencing treatment decisions in 21.9% of cases [
56,
57]. Thus, although routine use of [¹⁸F]FDG-PET/CT in unselected EC populations is unlikely to be cost-effective, prospective studies focusing on well-defined high-risk groups are warranted to better define its potential role in personalized post-treatment surveillance strategies.
Figure 8.
In the context of [¹⁸F]FDG-PET/MRI, Sawicki et al. [
58]. reported that this hybrid technique identified a higher number of cancer recurrences compared to MRI alone (100% vs. 83.6%, respectively; p < 0.01). [¹⁸F]FDG-PET/MRI also offers the advantage of distinguishing post-therapeutic tissue changes from true local relapse and detecting small lymph node metastases that may appear morphologically benign on conventional imaging. Moreover, [¹⁸F]FDG-PET/MRI has demonstrated superior performance in identifying bone lesions and distant metastases compared to PET/CT during restaging [
19].
6. Radiomics: Molecular Imaging Biomarkers in Clinicopathologic and Prognosis Prediction
Radiomics is an evolving field that enables high-throughput extraction of quantitative features from medical images, such as [¹⁸F]FDG-PET/CT, transforming them into structured, analyzable data for predictive modelling and clinical decision support. In EC, radiomics offers a non-invasive approach to characterize tumor biology and when integrated with clinicopathologic data and multi-omics frameworks, radiomic analysis may enhance risk stratification, treatment response prediction, and personalized prognosis [
59].
6.1. Clinicopathological Association of 18F-FDG PET/CT-Derived Parameters
[¹⁸F]FDG-PET/CT has been evaluated for its ability to predict clinicopathologic risk preoperatively. Several studies have demonstrated that the semiquantitative maximum standardized uptake value (SUVmax) and the MTV of the primary tumor, calculated with [¹⁸F]FDG-PET/CT, can provide indirect prognostic information, reflecting the biological aggressiveness of the tumor. SUVmax, SUVmean, MTV, and TLG have been linked with adverse features including deep of MI, lymphovascular invasion (LVI), larger tumor diameter, cervical stromal involvement, and lymph node metastases [
12,
60,
61]. For example, Sudo et al. [
62] reported a SUVmax threshold of 16 to predict LVI in patients with MI, with an accuracy of 88.2%. Thus, these parameters offer insights into tumor aggressiveness and metabolic burden. An overview of the principal quantitative parameters derived from [¹⁸F]FDG-PET/CT, used as imaging biomarkers, is summarized on
Table 2.
According to parameters indicative of metabolic tumor burden, some groups suggested that MTV and TLG might be promising markers for LN involvement [
63]. Erdogan et al. [
64] proposed MTV cut-offs of 19.6, 14.3, and 29.7 mL for early FIGO, MI, and lymph node metastasis, respectively. Mapelli et al. [
65] found TLG values at 40-60% thresholds to correlate with both FIGO and pathological tumor stage and MTV60 ≥7.8 mL and TLG40 ≥77.6 showed high specificity for advanced disease.
Regarding risk stratification, [¹⁸F]FDG-PET/CT might serve as a predictive tool for p53 overexpression being recognized as a relevant prognostic factor in EC [
66]. Several studies have defined SUVmax cut-offs for this purpose. The available literature reports that SUVmax of primary tumor is significantly higher in high-risk patients compared to low-risk ones, with sensitivities and specificities moderate to high [
12]. Lee et al. [
67] reported a cut-off of 8.7, achieving 75.6% sensitivity, 89.5% specificity, and 81.7% accuracy. Özgü et al. [
68] proposed a threshold of 6.7, with sensitivity as high as 92.9% for identifying low-risk patients. However, overlapping values between high- and low-risk groups limit the reliability of SUVmax alone.
Volumetric PET parameters, such as MTV and TLG, have demonstrated greater potential for preoperative risk stratification. Kitajima et al. [
69] identified a TLG threshold of 70.2, with 72% sensitivity and 74.2% specificity for identifying high-risk patients. Similarly, Mapelli et al. [
65] reported TLG cut-off values at different percentage thresholds (40%, 50%, and 60%) of 83.69, 61.81, and 41.32, respectively, with corresponding specificity of 71.43% and sensitivity around 60%. Moreover, a retrospective study reported that TLG had the highest AUCs for predicting the high-risk group. A TLG cut-off of 52.7, determined by ROC analysis, showed 74.1% sensitivity and 91.7% specificity for risk stratification [
62].
For [¹⁸F]FDG-PET/MRI, Shih et al. [
70], reported the associations between imaging biomarkers and pathological prognostic factors. The authors reported association of ADCmin and SUVmax with tumor grade, stage, deep MI, cervical invasion, lymphovascular space involvement, and lymph node metastasis in 69 patients with newly diagnosed EC.
6.2. Prognostic Value of 18F-FDG PET/CT-Derived Parameters
SUVmax of the primary tumor has been associated with OS, disease-free survival (DFS), and progression-free survival (PFS). Previous authors have suggested that preoperative SUVmax could act as an independent prognostic marker for recurrence and mortality in EC [
13,
71]. Fasmer et al. demonstrated that a high MTV can predict aggressive disease in EC [
13]. Nakamura et al. [
71] reported that high SUVmax independently predicted poorer DFS and OS in a cohort of 131 patients. However, other studies reported weak associations or no significant relationship with prognosis [
72,
73,
74].
Volumetric parameters such as MTV and TLG are emerging as stronger prognostic indicators. Shim et al. [
75] reported that lower MTV (HR = 1.010; 95% CI: 1.002–1.018; P = 0.010) and lower TLG (HR = 1.001; 95% CI: 1.000–1.002; P = 0.024) were significantly associated with prolonged DFS. Liu et al. [
72] demonstrated that MTV > 450 mL and TLG > 2700 were associated with adverse outcomes in FIGO stage IV EC.
Using [¹⁸F]FDG-PET/MRI, Shih et al. [
70] showed that derived biomarkers, particularly MTV and TLG, were associated with PFS and OS in patients with endometrial cancer and MTV was an independent predictor of PFS.
Metabolic activity in lymph nodes, particularly nodal SUV (SUVN), has also demonstrated prognostic relevance. Kim et al. [
76] reported that both tumor SUV (SUVT) and SUVN predicted OS, with higher SUVN linked to poorer outcomes. In line with these findings, Chung et al. [
77] proposed the SUVN/SUVT ratio as a novel marker, identifying a cut-off that predicted recurrence with a moderate sensitivity and specificity.
A recent systematic review by Noriega-Alvarez et al. [
78], analyzing 26 studies involving 1,918 patients, supported the utility of preoperative SUVmax as a non-invasive prognostic marker of recurrence and survival in EC. Both MTV and TLG also showed potential as independent predictors of outcome, appearing more accurate than SUVmax alone for risk stratification. However, the variability in cut-off values likely reflects methodological differences between studies, including variations in PET thresholding techniques, FIGO stage distributions, and inconsistent definitions of high- and low-risk categories. Thus, further validation through large, multicenter prospective studies with sufficient follow-up is essential to validate their clinical utility.
In addition, some limitations regarding technical issues may interfere with accurate quantification of metabolic parameters, such as partial volume effects in small or necrotic lesions or physiologic [¹⁸F]FDG activity in the bladder and ureters. Advanced radiomic approaches, including voxel-by-voxel tumor analysis, may improve reproducibility and capture intratumoral heterogeneity more effectively. However, despite these limitations, the consistent association of MTV and TLG with prognosis across studies supports their potential as robust imaging biomarkers.
As summary, preoperative [¹⁸F]FDG-PET/CT FDG-PET/CT, particularly the SUVmax of the primary tumor, may provide valuable clinical and prognostic information in EC, including associations with MI histological grade, lymph node metastasis, and overall risk classification. SUVmax has been proposed as a feasible non-invasive biomarker for risk assessment and treatment planning, with potential utility as an independent prognostic indicator for recurrence and mortality. Moreover, preoperative MTV and TLG have shown promise as independent prognostic factors for predicting recurrence in EC. These volumetric parameters are believed to better reflect the overall hypermetabolic tumor burden compared to SUVmax, supporting their potential role in risk stratification.
7. Future Directions
7.1. Non-FDG Radiotracers
16a-18F-fluoro17b-estradiol ([¹⁸F]-FES) binds to estrogen receptor alpha (ERa) showing to correlate well with the biopsy-determined ER status in EC [
79,
80]. Thus, [¹⁸F]-FES PET/CT enables noninvasive assessment of in vivo ERa status across the whole tumor or even to confirm the presence of ERa-positive metastases [
81]. Some studies have reported the prognostic value of ERa expression in EC being identified a higher level of ERa as a predictive factor for favorable survival [
82,
83,
84]. These data suggest that [¹⁸F]-FES PET/CT might be a biomarker for predicting poor-prognosis factors of EC before surgery being useful in determining therapeutic strategies and might improve the prognosis for patients with EC [
85].
In addition, a combined use of [¹⁸F]FDG-PET/CT and [¹⁸F]-FES PET/CT seems to discriminate between low- and high-grade EC showing the latter significantly greater [¹⁸F]-FDG/[¹⁸F]-FES ratios than low-risk carcinomas or hyperplasia. Thus, this index may serve as a promising PET biomarker for distinguishing histological subtypes and prognosis [
86,
87]. Previous authors described that ¹⁸F]-FDG/[¹⁸F]-FES tumor uptake ratio also correlated well with PFS and OS in more aggressive EC as uterine sarcomas [
88,
89].
Despite those successes, [¹⁸F]-FES PET/CT has some shortcomings, including slow blood clearance and rapid metabolization, both of which are factors increasing nonspecific signal and hence reducing tumor detectability [
90,
91]. To palliate the main weaknesses of [¹⁸F]-FES, an alternative ER-targeting molecule, 4-fluoro-11b-methoxy-16a-18 F-fluoroestradiol ([¹⁸F]-4FMFES) has been developed, showing to resist hepatic metabolism in humans [
92,
93]. Preliminary reports indicates that tumor-to-background ratio is significantly higher for [¹⁸F]-4FMFES than for [¹⁸F]FDG-PET/CT in EC [
94].
[68Ga]-labeled fibroblast activation protein inhibitors ([68Ga]-FAPI) are novel PET radiopharmaceuticals that target FAPs. Cancer-associated fibroblasts demonstrate FAP expression, unlike the normal fibroblasts from which they differentiate [
95]. FAP expression has been reported in various types of cancers, including gynecologic malignancies [
96,
97].
The utility of [68Ga]-FAPI PET/CT in EC is limited by physiological uptake in normal uterine tissue [
98]. However, in special subtypes, as clear-cell EC, [68Ga]-FAPI-04 PET/CT outperforms [¹⁸F]FDG, because of its poor uptake [
99,
100].
3 0-Deoxy-3’-[18F]fluorothymidine (FLT) PET has been reported as a valuable diagnostic tool for differentiating uterine leiomyosarcoma from leiomyoma, although the experience is limited [
101].
7.2. Machine Learning
The use of machine learning (ML) models in preoperatively predicting several features of EC aggressiveness is being investigated.
In a retrospective study including 123 EC patients who underwent [¹⁸F]FDG-PET/CT for preoperative staging, several PET variables as SUVmax, SUVmean, MTV and TLG were computed on the primary tumor. Histotype, MI, risk group, lymph-nodal involvement, and p53 expression were retrieved from histology. ML-based classification using conventional [¹⁸F]FDG-PET/CT parameters and clinical data demonstrated ability to characterize the investigated features of EC aggressiveness, providing a non-invasive way to support preoperative stratification of EC patients [
102].
8. Conclusions
Scientific literature indicates that [¹⁸F]FDG-PET/CT is a valuable technique that can facilitate improved diagnosis and treatment of EC. Due to its limited spatial resolution, the diagnostic performance of [¹⁸F]FDG-PET/CT in the primary tumor is low; however, it exhibits greater reliability in the diagnosis of lymph node metastases, distant metastases, and recurrence of EC than conventional imaging modalities.
The functional imaging capability of [¹⁸F]FDG-PET/CT provides important prognostic information and may guide clinical decisions regarding the continuation, modification, or escalation of therapy. In addition, although the utility of [¹⁸F]FDG-PET/CT in monitoring response is not yet standardized in clinical guidelines, emerging evidence suggests that it can offer valuable insights into metabolic response post-therapy.
[18F]-FDG PET/MRI enhances the accuracy of TNM staging in EC, offering superior preoperative evaluation of tumor invasion, lymph node involvement, and distant spread, with respect to the rest of imaging techniques, which supports better risk stratification and surgical planning. This modality is especially helpful in cases with ambiguous findings from other imaging methods and may be critical for planning fertility-preserving or minimally invasive strategies.
The evidence strengthens the clinical relevance of [¹⁸F]FDG-PET-based metabolic parameters for predicting tumor biology, treatment outcomes and prognosis in EC. The incorporation of these molecular imaging biomarkers into risk stratification models holds promise for refining personalized treatment strategies and guiding clinical decision-making.
Regarding non-[18F]-FDG radiotracers, further research is required to evaluate their role in the management of EC.
Author Contributions
AMGV, MPPJ, SAGO and ENA collected and review literature and wrote paper; MTR, ASM and MCR designed figures; AMGV and ENA integrated and reviewed the paper. All the authors read and edited the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest. The authors have no relevant financial or non-financial interests to disclose. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article
Abbreviations
The following abbreviations are used in this manuscript:
| EC |
Endometrial cancer |
| PET/CT |
Positron emission tomography/computed tomography |
| [¹⁸F]FDG |
fluorine-18-2-fluoro-2-deoxy-D- glucose |
| MRI |
Magnetic resonance imaging |
References
- Maheshwari, E.; Nougaret, S.; Stein, E.B.; Rauch, G.M.; Hwang, K.-P.; Stafford, R.J.; Klopp, A.H.; Soliman, P.T.; Maturen, K.E.; Rockall, A.G.; et al. Update on MRI in Evaluation and Treatment of Endometrial Cancer. Radiographics 2022, 42, 2112–2130. [CrossRef]
-
WHO Classification of Tumours of Female Reproductive Organs; Kurman, R.J., International Agency for Research on Cancer, World Health Organization, Eds.; World Health Organization classification of tumours; 4th ed.; International Agency for Research on Cancer: Lyon, 2014; ISBN 978-92-832-2435-8.
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee, FIGO Women’s Cancer Committee FIGO Staging of Endometrial Cancer: 2023. Int J Gynaecol Obstet 2023, 162, 383–394. [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-Up. Ann Oncol 2016, 27, 16–41. [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Endometrial Carcinoma. International Journal of Gynecological Cancer 2021, 31, 12–39. [CrossRef]
- Cosgrove, C.M.; Tritchler, D.L.; Cohn, D.E.; Mutch, D.G.; Rush, C.M.; Lankes, H.A.; Creasman, W.T.; Miller, D.S.; Ramirez, N.C.; Geller, M.A.; et al. An NRG Oncology/GOG Study of Molecular Classification for Risk Prediction in Endometrioid Endometrial Cancer. Gynecol Oncol 2018, 148, 174–180. [CrossRef]
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current Recommendations and Recent Progress in Endometrial Cancer. CA Cancer J Clin 2019, 69, 258–279. [CrossRef]
- Wu, C.-Y.; Tai, Y.-J.; Shih, I.-L.; Chiang, Y.-C.; Chen, Y.-L.; Hsu, H.-C.; Cheng, W.-F. Preoperative Magnetic Resonance Imaging Predicts Clinicopathological Parameters and Stages of Endometrial Carcinomas. Cancer Med 2022, 11, 993–1004. [CrossRef]
- Civan, C.; Kuyumcu, S. PET Imaging of Endometrial Cancer. nts 2022, 8, 167–173. [CrossRef]
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Endometrial Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Annals of Oncology 2022, 33, 860–877. [CrossRef]
- Shibuya, H.; Kobayashi, Y.; Watanabe, M.; Momomura, M.; Matsumoto, H.; Morisada, T. The Prognostic Value of Maximum Standardized Uptake Value (SUVmax) of 18F-FDG PET/CT for Risk Stratification and Outcome Prediction in Endometrial Cancer: A Retrospective Analysis. Cureus. 2025, 17, 80109.
- Ghooshkhanei, H.; Treglia, G.; Sabouri, G.; Davoodi, R.; Sadeghi, R. Risk Stratification and Prognosis Determination Using (18)F-FDG PET Imaging in Endometrial Cancer Patients: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2014, 132, 669–676. [CrossRef]
- Fasmer, K.E.; Gulati, A.; Dybvik, J.A.; Ytre-Hauge, S.; Salvesen, Ø.; Trovik, J.; Krakstad, C.; Haldorsen, I.S. Preoperative 18F-FDG PET/CT Tumor Markers Outperform MRI-Based Markers for the Prediction of Lymph Node Metastases in Primary Endometrial Cancer. Eur Radiol 2020, 30, 2443–2453. [CrossRef]
- Fasmer, K.E.; Gulati, A.; Dybvik, J.A.; Wagner-Larsen, K.S.; Lura, N.; Salvesen, Ø.; Forsse, D.; Trovik, J.; Pijnenborg, J.M.A.; Krakstad, C.; et al. Preoperative Pelvic MRI and 2-[18F]FDG PET/CT for Lymph Node Staging and Prognostication in Endometrial Cancer-Time to Revisit Current Imaging Guidelines? Eur Radiol 2023, 33, 221–232. [CrossRef]
- Bian, L.; Wang, M.; Gong, J.; Liu, H.; Wang, N.; Wen, N.; Fan, W.; Xu, B.; Wang, M.; Ye, M.; et al. Comparison of Integrated PET/MRI with PET/CT in Evaluation of Endometrial Cancer: A Retrospective Analysis of 81 Cases. PeerJ 2019, 7, e7081. [CrossRef]
- Ironi, G.; Mapelli, P.; Bergamini, A.; Fallanca, F.; Candotti, G.; Gnasso, C.; Taccagni, G.L.; Sant’Angelo, M.; Scifo, P.; Bezzi, C.; et al. Hybrid PET/MRI in Staging Endometrial Cancer: Diagnostic and Predictive Value in a Prospective Cohort. Clin Nucl Med 2022, 47, e221–e229. [CrossRef]
- Yu, Y.; Zhang, L.; Sultana, B.; Wang, B.; Sun, H. Diagnostic Value of Integrated 18F-FDG PET/MRI for Staging of Endometrial Carcinoma: Comparison with PET/CT. BMC Cancer 2022, 22, 947. [CrossRef]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Takahashi, S.; Ebina, Y.; Miyahara, Y.; Yamada, H.; Sugimura, K. Value of Fusion of PET and MRI for Staging of Endometrial Cancer: Comparison with 18F-FDG Contrast-Enhanced PET/CT and Dynamic Contrast-Enhanced Pelvic MRI. Eur J Radiol 2013, 82, 1672–1676. [CrossRef]
- Bezzi, C.; Zambella, E.; Ghezzo, S.; Fallanca, F.; Samanes Gajate, A.M.; Franchini, A.; Ironi, G.; Bergamini, A.; Monaco, L.; Evangelista, L.; et al. 18F-FDG PET/MRI in Endometrial Cancer: Systematic Review and Meta-Analysis. Clin Transl Imaging 2022, 10, 45–58. [CrossRef]
- Vahidfar, N.; Farzanefar, S.; Ahmadzadehfar, H.; Molloy, E.N.; Eppard, E. A Review of Nuclear Medicine Approaches in the Diagnosis and the Treatment of Gynecological Malignancies. Cancers (Basel) 2022, 14, 1779. [CrossRef]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2023, 21, 181–209. [CrossRef]
- Barretina-Ginesta, M.P.; Quindós, M.; Alarcón, J.D.; Esteban, C.; Gaba, L.; Gómez, C.; Fidalgo, J.A.P.; Romero, I.; Santaballa, A.; Rubio-Pérez, M.J. SEOM-GEICO Clinical Guidelines on Endometrial Cancer (2021). Clin Transl Oncol 2022, 24, 625–634. [CrossRef]
- Adam, J.A.; Loft, A.; Chargari, C.; Delgado Bolton, R.C.; Kidd, E.; Schöder, H.; Veit-Haibach, P.; Vogel, W.V. EANM/SNMMI Practice Guideline for [18F]FDG PET/CT External Beam Radiotherapy Treatment Planning in Uterine Cervical Cancer v1.0. Eur J Nucl Med Mol Imaging 2021, 48, 1188–1199. [CrossRef]
- Seon, K.E.; Shin, Y.; Lee, J.-Y.; Nam, E.J.; Kim, S.; Kim, Y.T.; Kim, S.W. Is Presumed Clinical Stage I Endometrial Cancer Using PET-CT and MRI Accurate in Predicting Surgical Staging? J Gynecol Oncol 2025, 36, e25. [CrossRef]
- Otero-García, M.M.; Mesa-Álvarez, A.; Nikolic, O.; Blanco-Lobato, P.; Basta-Nikolic, M.; de Llano-Ortega, R.M.; Paredes-Velázquez, L.; Nikolic, N.; Szewczyk-Bieda, M. Role of MRI in Staging and Follow-up of Endometrial and Cervical Cancer: Pitfalls and Mimickers. Insights Imaging 2019, 10, 19. [CrossRef]
- Signorelli, M.; Crivellaro, C.; Buda, A.; Guerra, L.; Fruscio, R.; Elisei, F.; Dolci, C.; Cuzzocrea, M.; Milani, R.; Messa, C. Staging of High-Risk Endometrial Cancer With PET/CT and Sentinel Lymph Node Mapping. Clinical Nuclear Medicine 2015, 40, 780–785. [CrossRef]
- Weissinger, M.; Bala, L.; Brucker, S.Y.; Kommoss, S.; Hoffmann, S.; Seith, F.; Nikolaou, K.; la Fougère, C.; Walter, C.B.; Dittmann, H. Additional Value of FDG-PET/MRI Complementary to Sentinel Lymphonodectomy for Minimal Invasive Lymph Node Staging in Patients with Endometrial Cancer: A Prospective Study. Diagnostics (Basel) 2024, 14, 376. [CrossRef]
- Şahin, G.; HazırBulan, A.; Sözen, I.; Kocadal, N.Ç.; Alkış, İ.; Yardımcı, A.H.; Akkaş, B.E.; Arslan, H.S. Optimizing Final Pathology Determination in Endometrial Cancer: The Role of PET/CT, MRI, and Biopsy in Serous, Mixed Cell, Clear Cell, and Grade 3 Endometrioid Subtypes. Diagnostics 2025, 15, 731. [CrossRef]
- Restaino, S.; Paglietti, C.; Arcieri, M.; Biasioli, A.; Della Martina, M.; Mariuzzi, L.; Andreetta, C.; Titone, F.; Bogani, G.; Raimondo, D.; et al. Management of Patients Diagnosed with Endometrial Cancer: Comparison of Guidelines. Cancers (Basel) 2023, 15, 1091. [CrossRef]
- Bogani, G.; Gostout, B.S.; Dowdy, S.C.; Multinu, F.; Casarin, J.; Cliby, W.A.; Frigerio, L.; Kim, B.; Weaver, A.L.; Glaser, G.E.; et al. Clinical Utility of Preoperative Computed Tomography in Patients With Endometrial Cancer. Int J Gynecol Cancer 2017, 27, 1685–1693. [CrossRef]
- Hong, L.; Cristiano, L.; Peters, E.; Ioffe, Y. Detection of Bone Metastases in Uterine Cancer: How Common Are They and Should PET/CT Be the Standard for Diagnosis? Gynecol Oncol Rep 2021, 35, 100698. [CrossRef]
- Tsuyoshi, H.; Tsujikawa, T.; Yamada, S.; Okazawa, H.; Yoshida, Y. Diagnostic Value of 18F-FDG PET/MRI for Staging in Patients with Endometrial Cancer. Cancer Imaging 2020, 20, 75. [CrossRef]
- Kidd, E.A. Imaging to Optimize Gynecological Radiation Oncology. Int J Gynecol Cancer 2022, 32, 358–365. [CrossRef]
- Van Den Heerik, A.S.V.M.; Horeweg, N.; De Boer, S.M.; Bosse, T.; Creutzberg, C.L. Adjuvant Therapy for Endometrial Cancer in the Era of Molecular Classification: Radiotherapy, Chemoradiation and Novel Targets for Therapy. International Journal of Gynecological Cancer 2021, 31, 594–604. [CrossRef]
- Koskas, M.; Amant, F.; Mirza, M.R.; Creutzberg, C.L. Cancer of the Corpus Uteri: 2021 Update. Int J Gynaecol Obstet 2021, 155 Suppl 1, 45–60. [CrossRef]
- Jamieson, A.; Huvila, J.; Thompson, E.F.; Leung, S.; Chiu, D.; Lum, A.; McConechy, M.; Grondin, K.; Aguirre-Hernandez, R.; Salvador, S.; et al. Variation in Practice in Endometrial Cancer and Potential for Improved Care and Equity through Molecular Classification. Gynecol Oncol 2022, 165, 201–214. [CrossRef]
- Jacobsen, M.C.; Maheshwari, E.; Klopp, A.H.; Venkatesan, A.M. Image-Guided Radiotherapy for Gynecologic Malignancies: What the Radiologist Needs to Know. Radiol Clin North Am 2023, 61, 725–747. [CrossRef]
- Conway, J.L.; Lukovic, J.; Laframboise, S.; Ferguson, S.E.; Han, K. Brachy-Ing Unresectable Endometrial Cancers with Magnetic Resonance Guidance. Cureus 2018, 10, e2274. [CrossRef]
-
Nuclear Medicine Manual on Gynaecological Cancers and Other Female Malignancies; Collarino, A., Vidal-Sicart, S., Valdés Olmos, R.A., Eds.; Springer International Publishing: Cham, 2022; ISBN 978-3-031-05496-9.
- Amit, A.; Person, O.; Keidar, Z. FDG PET/CT in Monitoring Response to Treatment in Gynecological Malignancies. Current Opinion in Obstetrics & Gynecology 2013, 25, 17–22. [CrossRef]
- Zukotynski, K.; Grant, F.D.; Curtis, C.; Micheli, L.; Treves, S.T. Skeletal Scintigraphy in Pediatric Sports Medicine. AJR Am J Roentgenol 2010, 195, 1212–1219. [CrossRef]
- Nishiyama, Y.; Yamamoto, Y.; Kanenishi, K.; Ohno, M.; Hata, T.; Kushida, Y.; Haba, R.; Ohkawa, M. Monitoring the Neoadjuvant Therapy Response in Gynecological Cancer Patients Using FDG PET. Eur J Nucl Med Mol Imaging 2008, 35, 287–295. [CrossRef]
- Chung, H.H.; Lee, I.; Kim, H.S.; Kim, J.W.; Park, N.-H.; Song, Y.S.; Cheon, G.J. Prognostic Value of Preoperative Metabolic Tumor Volume Measured by 18F-FDG PET/CT and MRI in Patients with Endometrial Cancer. Gynecologic Oncology 2013, 130, 446–451. [CrossRef]
- Faria, S.; Devine, C.; Viswanathan, C.; Javadi, S.; Korivi, B.R.; Bhosale, P.R. FDG-PET Assessment of Other Gynecologic Cancers. PET Clinics 2018, 13, 203–223. [CrossRef]
- Tripathy, S.; Parida, G.K.; Kumar, R. Quantitative Assessment of Gynecologic Malignancies. PET Clin 2018, 13, 269–288. [CrossRef]
- Khessib, T.; Jha, P.; Davidzon, G.A.; Iagaru, A.; Shah, J. Nuclear Medicine and Molecular Imaging Applications in Gynecologic Malignancies: A Comprehensive Review. Seminars in Nuclear Medicine 2024, 54, 270–292. [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial Cancer. The Lancet 2005, 366, 491–505. [CrossRef]
- Sohaib, S.A.; Houghton, S.L.; Meroni, R.; Rockall, A.G.; Blake, P.; Reznek, R.H. Recurrent Endometrial Cancer: Patterns of Recurrent Disease and Assessment of Prognosis. Clin Radiol 2007, 62, 28–34; discussion 35-36. [CrossRef]
- Kurra, V.; Krajewski, K.M.; Jagannathan, J.; Giardino, A.; Berlin, S.; Ramaiya, N. Typical and Atypical Metastatic Sites of Recurrent Endometrial Carcinoma. Cancer Imaging 2013, 13, 113–122. [CrossRef]
- Kadkhodayan, S.; Shahriari, S.; Treglia, G.; Yousefi, Z.; Sadeghi, R. Accuracy of 18-F-FDG PET Imaging in the Follow up of Endometrial Cancer Patients: Systematic Review and Meta-Analysis of the Literature. Gynecol. Oncol. 2013, 128, 397–404. [CrossRef]
- Nie, J.; Zhang, J.; Gao, J.; Guo, L.; Zhou, H.; Hu, Y.; Zhu, C.; Li, Q.; Ma, X. Diagnostic Role of 18F-FDG PET/MRI in Patients with Gynecological Malignancies of the Pelvis: A Systematic Review and Meta-Analysis. PLoS One 2017, 12, e0175401. [CrossRef]
- Kirchner, J.; Sawicki, L.M.; Suntharalingam, S.; Grueneisen, J.; Ruhlmann, V.; Aktas, B.; Deuschl, C.; Herrmann, K.; Antoch, G.; Forsting, M.; et al. Whole-Body Staging of Female Patients with Recurrent Pelvic Malignancies: Ultra-Fast 18F-FDG PET/MRI Compared to 18F-FDG PET/CT and CT. PLoS One 2017, 12, e0172553. [CrossRef]
- Zheng, M.; Xie, D.; Pan, C.; Xu, Y.; Yu, W. Diagnostic Value of 18F-FDG PET/MRI in Recurrent Pelvis Malignancies of Female Patients: A Systematic Review and Meta-Analysis. Nucl Med Commun 2018, 39, 479–485. [CrossRef]
- Chao, A.; Chang, T.-C.; Ng, K.-K.; Hsueh, S.; Huang, H.-J.; Chou, H.-H.; Tsai, C.-S.; Yen, T.-C.; Wu, T.-I.; Lai, C.-H. 18F-FDG PET in the Management of Endometrial Cancer. Eur J Nucl Med Mol Imaging 2006, 33, 36–44. [CrossRef]
- Queiroz, M.A.; Kubik-Huch, R.A.; Hauser, N.; Freiwald-Chilla, B.; von Schulthess, G.; Froehlich, J.M.; Veit-Haibach, P. PET/MRI and PET/CT in Advanced Gynaecological Tumours: Initial Experience and Comparison. Eur Radiol 2015, 25, 2222–2230. [CrossRef]
- Belhocine, T.; De Barsy, C.; Hustinx, R.; Willems-Foidart, J. Usefulness of 18F-FDG PET in the Post-Therapy Surveillance of Endometrial Carcinoma. Eur J Nucl Med 2002, 29, 1132–1139. [CrossRef]
- Park, J.-Y.; Kim, E.N.; Kim, D.-Y.; KiM, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. Clinical Impact of Positron Emission Tomography or Positron Emission Tomography/Computed Tomography in the Posttherapy Surveillance of Endometrial Carcinoma: Evaluation of 88 Patients. International Journal of Gynecological Cancer 2008, 18, 1332–1338. [CrossRef]
- Sawicki, L.M.; Kirchner, J.; Grueneisen, J.; Ruhlmann, V.; Aktas, B.; Schaarschmidt, B.M.; Forsting, M.; Herrmann, K.; Antoch, G.; Umutlu, L. Comparison of 18F–FDG PET/MRI and MRI Alone for Whole-Body Staging and Potential Impact on Therapeutic Management of Women with Suspected Recurrent Pelvic Cancer: A Follow-up Study. Eur J Nucl Med Mol Imaging 2018, 45, 622–629. [CrossRef]
- Lin, G.; Lai, C.-H.; Yen, T.-C. Emerging Molecular Imaging Techniques in Gynecologic Oncology. PET Clinics 2018, 13, 289–299. [CrossRef]
- Torizuka, T.; Nakamura, F.; Takekuma, M.; Kanno, T.; Ogusu, T.; Yoshikawa, E.; Okada, H.; Maeda, M.; Ouchi, Y. FDG PET for the Assessment of Myometrial Infiltration in Clinical Stage I Uterine Corpus Cancer. Nuclear Medicine Communications 2006, 27.
- Walentowicz-Sadłecka, M.; Sadłecki, P.; Małecki, B.; Walentowicz, P.; Marszałek, A.; Domaracki, P.; Grabiec, M. [SUVmax measured by 18F FDG PET/CT in the primary tumor in relation to clinical and pathological features of endometrial cancer]. Ginekol. Pol. 2013, 84, 748–753. [CrossRef]
- Sudo, S.; Hattori, N.; Manabe, O.; Kato, F.; Mimura, R.; Magota, K.; Sugimori, H.; Hirata, K.; Sakuragi, N.; Tamaki, N. FDG PET/CT Diagnostic Criteria May Need Adjustment Based on MRI to Estimate the Presurgical Risk of Extrapelvic Infiltration in Patients with Uterine Endometrial Cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 676–684. [CrossRef]
- Crivellaro, C.; Landoni, C.; Elisei, F.; Buda, A.; Bonacina, M.; Grassi, T.; Monaco, L.; Giuliani, D.; Gotuzzo, I.; Magni, S.; et al. Combining Positron Emission Tomography/Computed Tomography, Radiomics, and Sentinel Lymph Node Mapping for Nodal Staging of Endometrial Cancer Patients. Int J Gynecol Cancer 2020, 30, 378–382. [CrossRef]
- Erdogan, M.; Erdemoglu, E.; Evrimler, Ş.; Hanedan, C.; Şengül, S.S. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis Assessed by 18F-FDG PET/CT in Endometrial Cancer. Nucl Med Commun 2019, 40, 1099–1104. [CrossRef]
- Mapelli, P.; Bergamini, A.; Fallanca, F.; Rancoita, P.M.V.; Cioffi, R.; Incerti, E.; Rabaiotti, E.; Petrone, M.; Mangili, G.; Candiani, M.; et al. Prognostic Role of FDG PET-Derived Parameters in Preoperative Staging of Endometrial Cancer. Rev Esp Med Nucl Imagen Mol 2019, 38, 3–9. [CrossRef]
- Perrone, E.; De Felice, F.; Capasso, I.; Distefano, E.; Lorusso, D.; Nero, C.; Arciuolo, D.; Zannoni, G.F.; Scambia, G.; Fanfani, F. The Immunohistochemical Molecular Risk Classification in Endometrial Cancer: A Pragmatic and High-Reproducibility Method. Gynecol Oncol 2022, 165, 585–593. [CrossRef]
- Lee, H.J.; Ahn, B.-C.; Hong, C.M.; Song, B.I.; Kim, H.W.; Kang, S.; Jeong, S.Y.; Lee, S.-W.; Lee, J. Preoperative Risk Stratification Using (18)F-FDG PET/CT in Women with Endometrial Cancer. Nuklearmedizin 2011, 50, 204–213. [CrossRef]
- Özgü, E.; Öz, M.; Yıldız, Y.; Özgü, B.S.; Erkaya, S.; Güngör, T. Prognostic Value of 18F-FDG PET/CT for Identifying High- and Low-Risk Endometrial Cancer Patients. Ginekol. Pol. 2016, 87, 493–497. [CrossRef]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Maeda, T.; Ebina, Y.; Yamada, H.; Okunaga, T.; Kubo, K.; Sofue, K.; Kanda, T.; et al. Preoperative Risk Stratification Using Metabolic Parameters of (18)F-FDG PET/CT in Patients with Endometrial Cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1268–1275. [CrossRef]
- Shih, I.-L.; Yen, R.-F.; Chen, C.-A.; Cheng, W.-F.; Chen, B.-B.; Zheng, Q.-Y.; Cheng, M.-F.; Chen, J.L.-Y.; Shih, T.T.-F. PET/MRI in Endometrial Cancer: Imaging Biomarkers Are Associated with Disease Progression and Overall Survival. Acad Radiol 2024, 31, 939–950. [CrossRef]
- Nakamura, K.; Joja, I.; Fukushima, C.; Haruma, T.; Hayashi, C.; Kusumoto, T.; Seki, N.; Hongo, A.; Hiramatsu, Y. The Preoperative SUVmax Is Superior to ADCmin of the Primary Tumour as a Predictor of Disease Recurrence and Survival in Patients with Endometrial Cancer. Eur J Nucl Med Mol Imaging 2013, 40, 52–60. [CrossRef]
- Liu, F.-Y.; Chao, A.; Lai, C.-H.; Chou, H.-H.; Yen, T.-C. Metabolic Tumor Volume by 18F-FDG PET/CT Is Prognostic for Stage IVB Endometrial Carcinoma. Gynecol. Oncol. 2012, 125, 566–571. [CrossRef]
- Kitajima, K.; Kita, M.; Suzuki, K.; Senda, M.; Nakamoto, Y.; Sugimura, K. Prognostic Significance of SUVmax (Maximum Standardized Uptake Value) Measured by [18F]FDG PET/CT in Endometrial Cancer. Eur J Nucl Med Mol Imaging 2012, 39, 840–845. [CrossRef]
- Yanarateş, A.; Budak, E. Prognostic Role of PET/CT in Endometrial Cancer. Ginekol. Pol. 2019, 90, 491–495. [CrossRef]
- Shim, S.-H.; Kim, D.-Y.; Lee, D.-Y.; Lee, S.-W.; Park, J.-Y.; Lee, J.; Kim, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. Metabolic Tumour Volume and Total Lesion Glycolysis, Measured Using Preoperative 18 F-FDG PET/CT, Predict the Recurrence of Endometrial Cancer. BJOG: Int J Obstet Gy 2014, 121, 1097–1106. [CrossRef]
- Kim, H.J.; Choi, J.; Jeong, Y.H.; Jo, K.H.; Lee, J.-H.; Cho, A.; Yun, M.; Lee, J.D.; Kim, Y.T.; Kang, W.J. Prognostic Value of Metabolic Activity Measured by (18)F-FDG PET/CT in Patients with Advanced Endometrial Cancer. Nucl Med Mol Imaging 2013, 47, 257–262. [CrossRef]
- Chung, H.H.; Cheon, G.J.; Kim, J.-W.; Park, N.-H.; Song, Y.S. Prognostic Value of Lymph Node-to-Primary Tumor Standardized Uptake Value Ratio in Endometrioid Endometrial Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 47–55. [CrossRef]
- Noriega-Álvarez, E.; García Vicente, A.M.; Jiménez Londoño, G.A.; Martínez Bravo, W.R.; González García, B.; Soriano Castrejón, Á.M. A Systematic Review about the Role of Preoperative 18F-FDG PET/CT for Prognosis and Risk Stratification in Patients with Endometrial Cancer. Revista Española de Medicina Nuclear e Imagen Molecular (English Edition) 2023, 42, 24–32. [CrossRef]
- Tsujikawa, T.; Yoshida, Y.; Kiyono, Y.; Kurokawa, T.; Kudo, T.; Fujibayashi, Y.; Kotsuji, F.; Okazawa, H. Functional Oestrogen Receptor α Imaging in Endometrial Carcinoma Using 16α-[18F]Fluoro-17β-Oestradiol PET. Eur J Nucl Med Mol Imaging 2011, 38, 37–45. [CrossRef]
- Zhao, Z.; Yoshida, Y.; Kurokawa, T.; Kiyono, Y.; Mori, T.; Okazawa, H. 18F-FES and 18F-FDG PET for Differential Diagnosis and Quantitative Evaluation of Mesenchymal Uterine Tumors: Correlation with Immunohistochemical Analysis. J Nucl Med 2013, 54, 499–506. [CrossRef]
- Mori, T.; Kasamatsu, S.; Mosdzianowski, C.; Welch, M.J.; Yonekura, Y.; Fujibayashi, Y. Automatic Synthesis of 16α-[18F]Fluoro-17β-Estradiol Using a Cassette-Type [18F]Fluorodeoxyglucose Synthesizer. Nuclear Medicine and Biology 2006, 33, 281–286. [CrossRef]
- Gehrig, P.A.; Van Le, L.; Olatidoye, B.; Geradts, J. Estrogen Receptor Status, Determined by Immunohistochemistry, as a Predictor of the Recurrence of Stage I Endometrial Carcinoma. Cancer 1999, 86, 2083–2089.
- Jongen, V.; Briët, J.; De Jong, R.; Ten Hoor, K.; Boezen, M.; Van Der Zee, A.; Nijman, H.; Hollema, H. Expression of Estrogen Receptor-Alpha and -Beta and Progesterone Receptor-A and -B in a Large Cohort of Patients with Endometrioid Endometrial Cancer. Gynecologic Oncology 2009, 112, 537–542. [CrossRef]
- Trovik, J.; Wik, E.; Werner, H.M.J.; Krakstad, C.; Helland, H.; Vandenput, I.; Njolstad, T.S.; Stefansson, I.M.; Marcickiewicz, J.; Tingulstad, S.; et al. Hormone Receptor Loss in Endometrial Carcinoma Curettage Predicts Lymph Node Metastasis and Poor Outcome in Prospective Multicentre Trial. Eur J Cancer 2013, 49, 3431–3441. [CrossRef]
- Yamada, S.; Tsuyoshi, H.; Yamamoto, M.; Tsujikawa, T.; Kiyono, Y.; Okazawa, H.; Yoshida, Y. Prognostic Value of 16α-18F-Fluoro-17β-Estradiol PET as a Predictor of Disease Outcome in Endometrial Cancer: A Prospective Study. J Nucl Med 2021, 62, 636–642. [CrossRef]
- Tsujikawa, T.; Yoshida, Y.; Mori, T.; Kurokawa, T.; Fujibayashi, Y.; Kotsuji, F.; Okazawa, H. Uterine Tumors: Pathophysiologic Imaging with 16alpha-[18F]Fluoro-17beta-Estradiol and 18F Fluorodeoxyglucose PET--Initial Experience. Radiology 2008, 248, 599–605. [CrossRef]
- Tsujikawa, T.; Yoshida, Y.; Kudo, T.; Kiyono, Y.; Kurokawa, T.; Kobayashi, M.; Tsuchida, T.; Fujibayashi, Y.; Kotsuji, F.; Okazawa, H. Functional Images Reflect Aggressiveness of Endometrial Carcinoma: Estrogen Receptor Expression Combined with 18F-FDG PET. Journal of Nuclear Medicine 2009, 50, 1598–1604. [CrossRef]
- Yamamoto, M.; Tsujikawa, T.; Yamada, S.; Kurokawa, T.; Shinagawa, A.; Chino, Y.; Mori, T.; Kiyono, Y.; Okazawa, H.; Yoshida, Y. 18F-FDG/18F-FES Standardized Uptake Value Ratio Determined Using PET Predicts Prognosis in Uterine Sarcoma. Oncotarget 2017, 8, 22581–22589. [CrossRef]
- Van Kruchten, M.; Hospers, G.A.P.; Glaudemans, A.W.J.M.; Hollema, H.; Arts, H.J.G.; Reyners, A.K.L. Positron Emission Tomography Imaging of Oestrogen Receptor-Expression in Endometrial Stromal Sarcoma Supports Oestrogen Receptor-Targeted Therapy: Case Report and Review of the Literature. European Journal of Cancer 2013, 49, 3850–3855. [CrossRef]
- Mankoff, D.A.; Tewson, T.J.; Eary, J.F. Analysis of Blood Clearance and Labeled Metabolites for the Estrogen Receptor Tracer [F-18]-16 Alpha-Fluoroestradiol (FES). Nucl Med Biol 1997, 24, 341–348. [CrossRef]
- Tewson, T.J.; Mankoff, D.A.; Peterson, L.M.; Woo, I.; Petra, P. Interactions of 16alpha-[18F]-Fluoroestradiol (FES) with Sex Steroid Binding Protein (SBP). Nucl Med Biol 1999, 26, 905–913. [CrossRef]
- Seimbille, Y.; Ali, H.; Van Lier, J.E. Synthesis of 2,16α- and 4,16α-Difluoroestradiols and Their 11β-Methoxy Derivatives as Potential Estrogen Receptor-Binding Radiopharmaceuticals. J. Chem. Soc., Perkin Trans. 1 2002, 657–663. [CrossRef]
- Seimbille, Y.; Rousseau, J.; Bénard, F.; Morin, C.; Ali, H.; Avvakumov, G.; Hammond, G.L.; Van Lier, J.E. 18F-Labeled Difluoroestradiols: Preparation and Preclinical Evaluation as Estrogen Receptor-Binding Radiopharmaceuticals. Steroids 2002, 67, 765–775. [CrossRef]
- Paquette, M.; Espinosa-Bentancourt, E.; Lavallee, E.; Phoenix, S.; Lapointe-Milot, K.; Bessette, P.; Guerin, B.; Turcotte, E.E. 18 F-4FMFES and18 F-FDG PET/CT in ER+ Endometrial Carcinomas: Preliminary Report. J Nucl Med 2021, jnumed.121.262617. [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J Nucl Med 2019, 60, 386–392. [CrossRef]
- Dendl, K.; Koerber, S.A.; Finck, R.; Mokoala, K.M.G.; Staudinger, F.; Schillings, L.; Heger, U.; Röhrich, M.; Kratochwil, C.; Sathekge, M.; et al. 68Ga-FAPI-PET/CT in Patients with Various Gynecological Malignancies. Eur J Nucl Med Mol Imaging 2021, 48, 4089–4100. [CrossRef]
- Li, T.; Zhang, J.; Yan, Y.; Tan, M.; Chen, Y. Applications of FAPI PET/CT in the Diagnosis and Treatment of Breast and the Most Common Gynecologic Malignancies: A Literature Review. Front Oncol 2024, 14, 1358070. [CrossRef]
- Usmani, S.; Al Riyami, K.; Ahmad, N.; Burney, I.A. The Role of 68Ga-FAPI PET/CT Imaging in Gynecological Cancers: An Integrative Review. Nuclear Medicine Communications 2025, 46, 585–591. [CrossRef]
- Wu, C.; Chen, R.; Zhou, X.; Xia, Q.; Liu, J. Preoperative Evaluation of Residual Tumor in Patients with Endometrial Carcinoma by Using 18F-FDG PET/CT. J Cancer 2020, 11, 2283–2288. [CrossRef]
- Kiran, M.Y.; Has Simsek, D.; Sanli, Y.; Kuyumcu, S. 18F-FDG and 68Ga-FAPI-04 PET/CT in Evaluating Clear-Cell Endometrial Cancer. Clin Nucl Med 2023, 48, e87–e88. [CrossRef]
- Yamane, T.; Takaoka, A.; Kita, M.; Imai, Y.; Senda, M. 18F-FLT PET Performs Better than 18F-FDG PET in Differentiating Malignant Uterine Corpus Tumors from Benign Leiomyoma. Ann Nucl Med 2012, 26, 478–484. [CrossRef]
- Bezzi, C.; Bergamini, A.; Mathoux, G.; Ghezzo, S.; Monaco, L.; Candotti, G.; Fallanca, F.; Gajate, A.M.S.; Rabaiotti, E.; Cioffi, R.; et al. Role of Machine Learning (ML)-Based Classification Using Conventional 18F-FDG PET Parameters in Predicting Postsurgical Features of Endometrial Cancer Aggressiveness. Cancers 2023, 15, 325. [CrossRef]
Figure 1.
Preoperative risk groups apparently confined to the uterus with molecular classification. MI: myometrial invasion. POLEmut: Molecular subgroup with mutation in the POLE gene. MMRd: Molecular subgroup with mutation in DNA repair proteins. NSMP: Molecular subgroup that does not have mutation in POLE, p53 or DNA repair proteins p53abn: Molecular subgroup with mutation in p53. The loss of expression of DNA repair proteins (MLH1, PMS2, MSH2, MSH6) reflects a high probability that the tumor presents high microsatellite instability, which leads to an intermediate prognosis. Similarly, an abnormal pattern of p53 staining is associated with alterations (mutations) in the TP53 gene, which confers a worse prognosis, while the detection of mutations in the POLE gene is associated with tumors with a good prognosis with a lower risk of recurrence. Adaptation from SEGO (Spanish Society of Ginecological Oncology) Oncoguide: Endometrial cancer 2023”. ISBN: 978-84-09-40278-6.
Figure 1.
Preoperative risk groups apparently confined to the uterus with molecular classification. MI: myometrial invasion. POLEmut: Molecular subgroup with mutation in the POLE gene. MMRd: Molecular subgroup with mutation in DNA repair proteins. NSMP: Molecular subgroup that does not have mutation in POLE, p53 or DNA repair proteins p53abn: Molecular subgroup with mutation in p53. The loss of expression of DNA repair proteins (MLH1, PMS2, MSH2, MSH6) reflects a high probability that the tumor presents high microsatellite instability, which leads to an intermediate prognosis. Similarly, an abnormal pattern of p53 staining is associated with alterations (mutations) in the TP53 gene, which confers a worse prognosis, while the detection of mutations in the POLE gene is associated with tumors with a good prognosis with a lower risk of recurrence. Adaptation from SEGO (Spanish Society of Ginecological Oncology) Oncoguide: Endometrial cancer 2023”. ISBN: 978-84-09-40278-6.
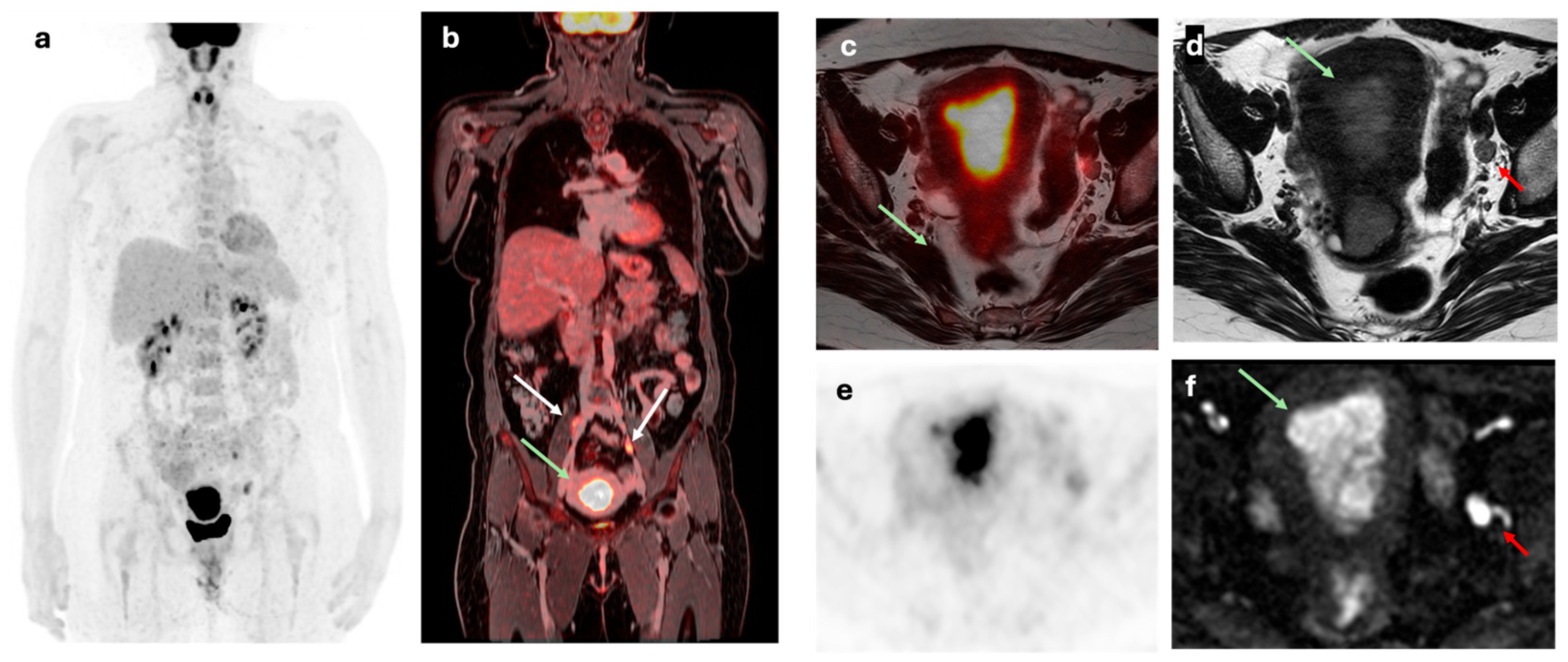
Figure 2.
A 50-year-old woman with a FIGO IIIC1 EC. (a) Maximum intensity projection (MIP) and (b) coronal fused [18F]F-FDG PET/MRI staging images showing primary endometrial tumor with bilateral common iliac lymph node involvement (white arrows) and left external iliac node metastasis. (c) Axial fused [18F]F-FDG PET/MRI, (d) axial T2-weighted, (e) axial [18F]F-FDG PET and (f) DWI images showing a heterogeneous on T2 with diffusion restriction of primary tumor (green arrow) and a 9 mm left external iliac lymph node with pathological FDG uptake (red arrow).
Figure 2.
A 50-year-old woman with a FIGO IIIC1 EC. (a) Maximum intensity projection (MIP) and (b) coronal fused [18F]F-FDG PET/MRI staging images showing primary endometrial tumor with bilateral common iliac lymph node involvement (white arrows) and left external iliac node metastasis. (c) Axial fused [18F]F-FDG PET/MRI, (d) axial T2-weighted, (e) axial [18F]F-FDG PET and (f) DWI images showing a heterogeneous on T2 with diffusion restriction of primary tumor (green arrow) and a 9 mm left external iliac lymph node with pathological FDG uptake (red arrow).
Figure 3.
A 70-year-old woman with a diagnosis of stage IV serous EC. The maximum intensity projection PET image (a) shows extensive disease with uterine involvement, supra- and infradiaphragmatic adenopathy, and peritoneal involvement (red arrows). Fused axial CT and [18F]F-FDG PET/CT slices (b, c, and d) show foci of pathologic hypermetabolism in the uterus, pelvic lymph nodes, and peritoneum (b); peritoneal implants in the greater omentum (c); and mediastinal adenopathy in the right paratracheal region and the aortopulmonary window (d).
Figure 3.
A 70-year-old woman with a diagnosis of stage IV serous EC. The maximum intensity projection PET image (a) shows extensive disease with uterine involvement, supra- and infradiaphragmatic adenopathy, and peritoneal involvement (red arrows). Fused axial CT and [18F]F-FDG PET/CT slices (b, c, and d) show foci of pathologic hypermetabolism in the uterus, pelvic lymph nodes, and peritoneum (b); peritoneal implants in the greater omentum (c); and mediastinal adenopathy in the right paratracheal region and the aortopulmonary window (d).
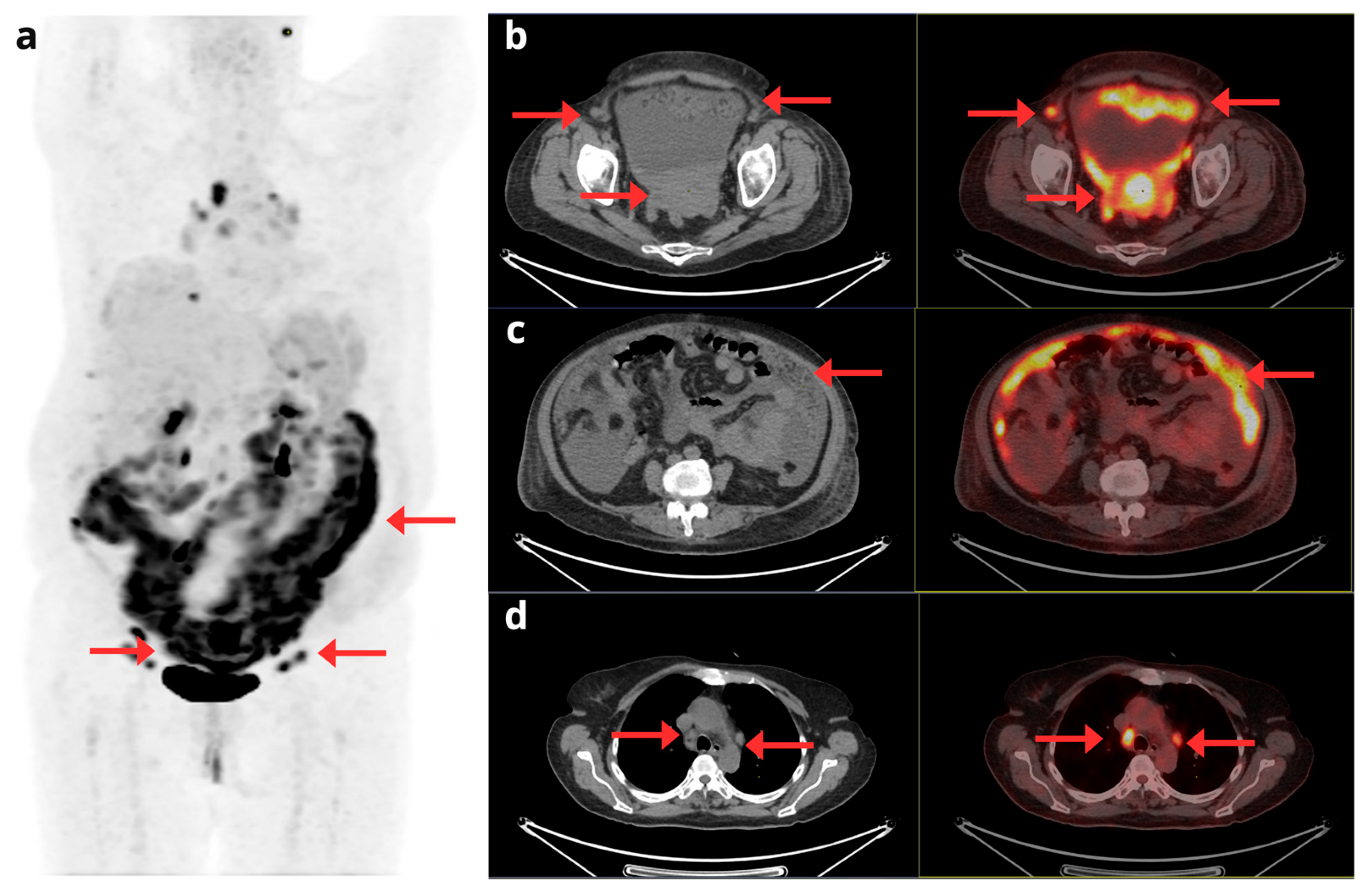
Figure 4.
A 58-year-old woman diagnosed with stage IV clear cell EC, undergoing treatment with Pembrolizumab and Lenvatinib. A comparison is presented between the pre-treatment [18F]F-FDG PET/CT (top row) and follow-up study (bottom row). The maximum intensity projection PET images (a) provide a global view confirming clear disease progression. This progression is evident through both an increase in size and metabolism of known lesions and the appearance of multiple new metastatic foci (red arrows). Axial slices detail these findings, showing increased pelvic involvement (b), enlarged supra and infradiaphragmatic lymphadenopathies (c, d), and the appearance of new bilateral pulmonary nodules (e).
Figure 4.
A 58-year-old woman diagnosed with stage IV clear cell EC, undergoing treatment with Pembrolizumab and Lenvatinib. A comparison is presented between the pre-treatment [18F]F-FDG PET/CT (top row) and follow-up study (bottom row). The maximum intensity projection PET images (a) provide a global view confirming clear disease progression. This progression is evident through both an increase in size and metabolism of known lesions and the appearance of multiple new metastatic foci (red arrows). Axial slices detail these findings, showing increased pelvic involvement (b), enlarged supra and infradiaphragmatic lymphadenopathies (c, d), and the appearance of new bilateral pulmonary nodules (e).
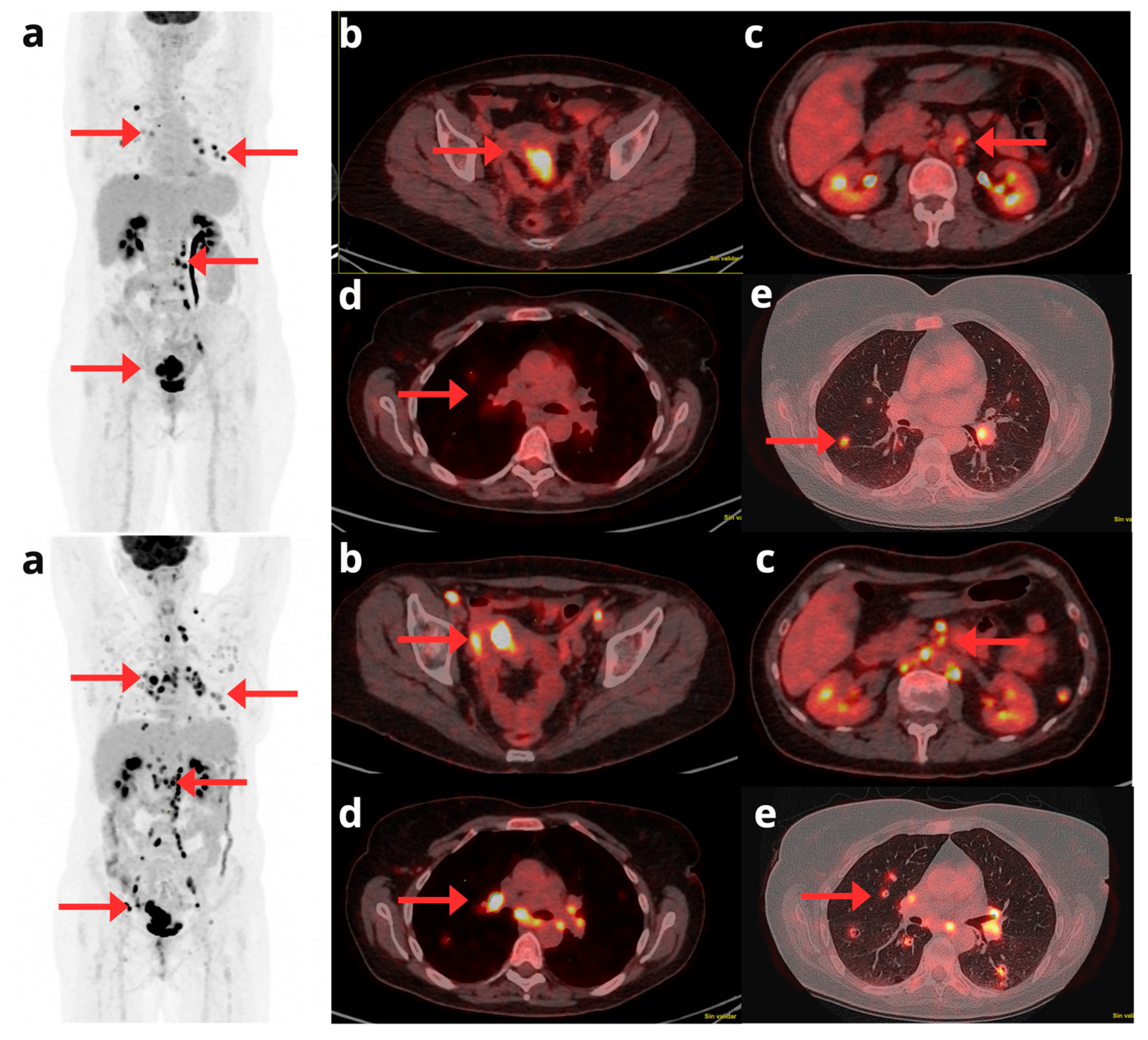
Figure 5.
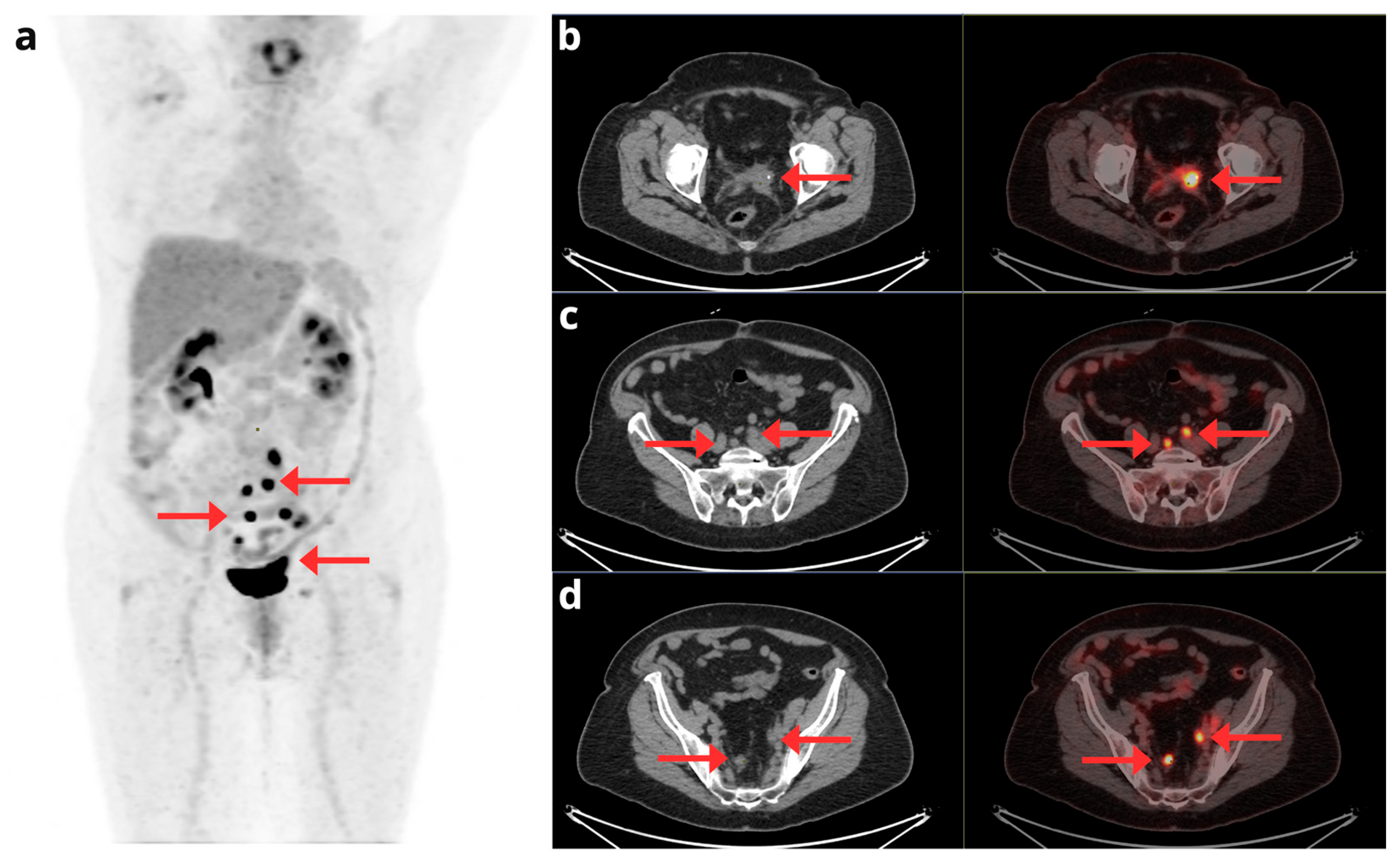
A 72-year-old woman with a history of stage IA G2 endometrioid EC, treated in October 2014 with laparoscopic hysterectomy and bilateral salpingo-oophorectomy. In May 2019, a follow-up CT scan revealed a suspicious nodular image suggestive of recurrence in the vaginal cuff, prompting a [18F]F-FDG PET/CT request in June 2019. The [18F]F-FDG PET/CT confirmed disease recurrence; the maximum intensity projection PET image (a) shows focal involvement in the vaginal cuff and pelvic lymph nodes (red arrows). Axial CT and PET/CT slices (b, c, and d) localize the recurrence to the left-sided vaginal cuff (b) and additionally demonstrate metastatic lymphadenopathies in the common iliac chains (c) and bilateral internal iliac chains (d).
Figure 5.
A 72-year-old woman with a history of stage IA G2 endometrioid EC, treated in October 2014 with laparoscopic hysterectomy and bilateral salpingo-oophorectomy. In May 2019, a follow-up CT scan revealed a suspicious nodular image suggestive of recurrence in the vaginal cuff, prompting a [18F]F-FDG PET/CT request in June 2019. The [18F]F-FDG PET/CT confirmed disease recurrence; the maximum intensity projection PET image (a) shows focal involvement in the vaginal cuff and pelvic lymph nodes (red arrows). Axial CT and PET/CT slices (b, c, and d) localize the recurrence to the left-sided vaginal cuff (b) and additionally demonstrate metastatic lymphadenopathies in the common iliac chains (c) and bilateral internal iliac chains (d).
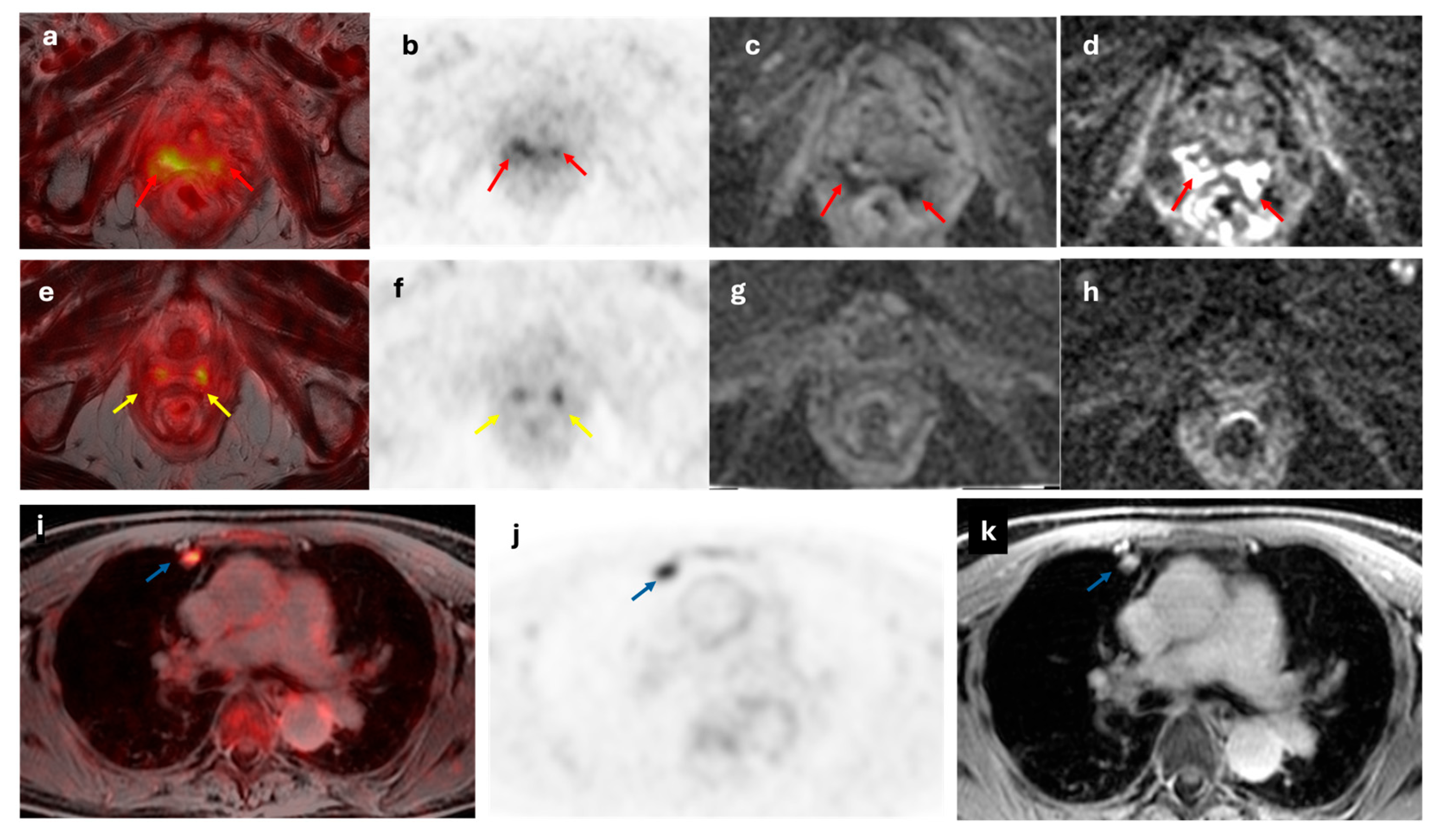
Figure 6.
A 64-year-old woman with recurrent EC at vaginal stump (red arrow). The lesion demonstrated increased metabolic activity on axial fused [18F]F-FDG PET/MRI and axial [18F]F-FDG PET images (a and b), along with diffusion restriction on axial ADC and DWI images (c and d). The lateral vaginal walls show diffuse increased metabolic activity (yellow arrows; e and f) with no diffusion restriction (g and h) consistent with inflammatory changes secondary to radiotherapy. Axial fused [18F]F-FDG PET/MRI, axial [18F]F-FDG PET and axial T2-weighted MRI images (i,j and k, respectively) show a hypermetabolic nodule in the right upper lung lobe (blue arrow), suggestive of malignancy.
Figure 6.
A 64-year-old woman with recurrent EC at vaginal stump (red arrow). The lesion demonstrated increased metabolic activity on axial fused [18F]F-FDG PET/MRI and axial [18F]F-FDG PET images (a and b), along with diffusion restriction on axial ADC and DWI images (c and d). The lateral vaginal walls show diffuse increased metabolic activity (yellow arrows; e and f) with no diffusion restriction (g and h) consistent with inflammatory changes secondary to radiotherapy. Axial fused [18F]F-FDG PET/MRI, axial [18F]F-FDG PET and axial T2-weighted MRI images (i,j and k, respectively) show a hypermetabolic nodule in the right upper lung lobe (blue arrow), suggestive of malignancy.
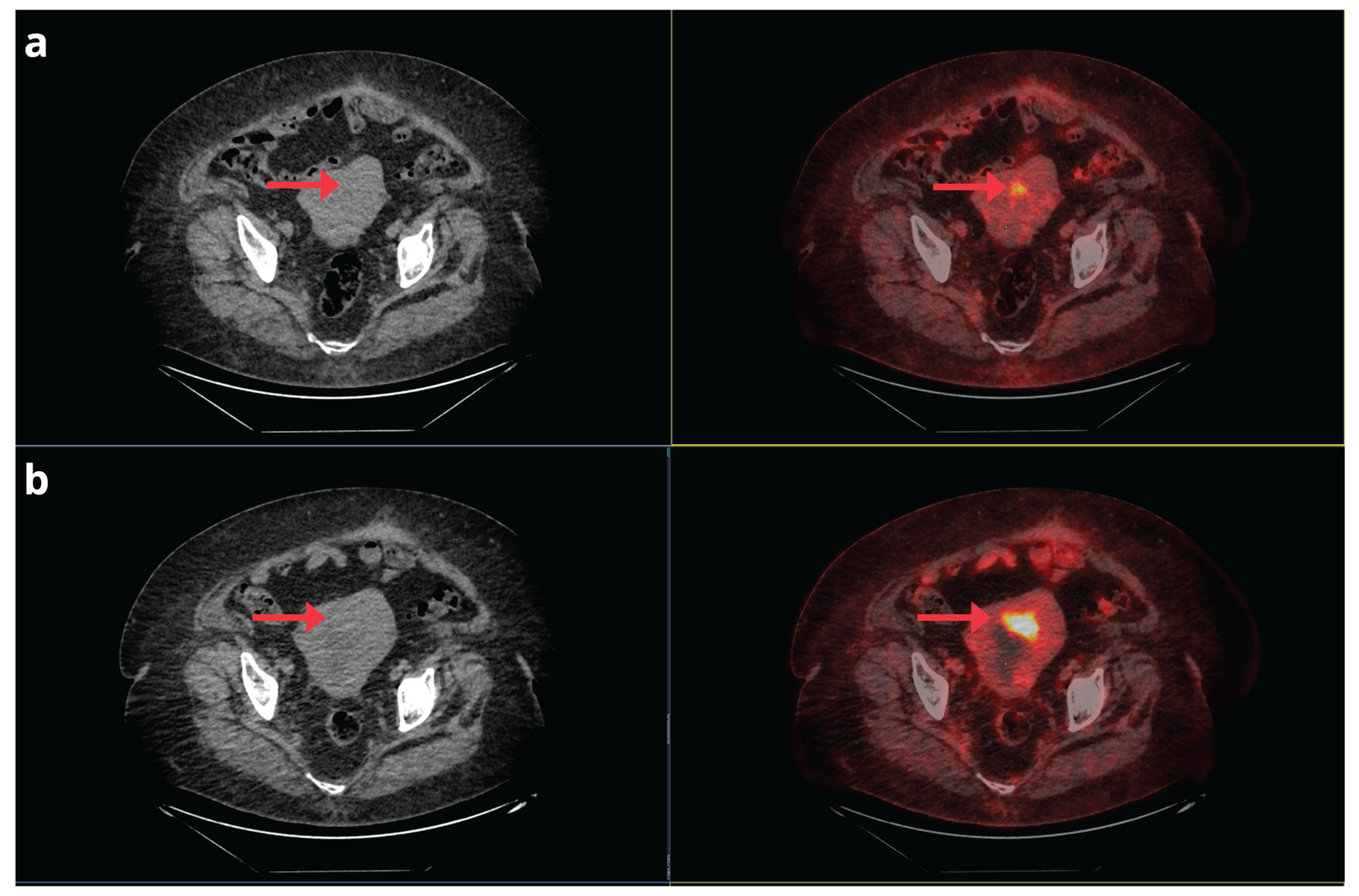
Figure 7.
A 75-year-old woman with a history of Stage IB FIGO clear cell EC, treated in April 2022 with total hysterectomy, bilateral salpingo-oophorectomy, pelvic and aortocaval lymphadenectomy, and omentectomy, followed by adjuvant chemo-radiotherapy and brachytherapy. Due to the appearance of pulmonary nodules on a follow-up CT scan, a [18F]F-FDG PET/CT was requested. The study revealed distant disease recurrence, with the maximum intensity projection PET image (a) demonstrating multiple pulmonary nodules, pleural implants, and bone involvement (red arrows). Axial CT and PET/CT slices (b, c, and d) confirmed foci of pathological hypermetabolism corresponding to bilateral pulmonary nodules (b), an implant in the right costal pleura (c), a mediastinal lymphadenopathy in a prevascular location, a subpleural pulmonary nodule in the right lower lobe, and the 4th right anterior costal arch (d).
Figure 7.
A 75-year-old woman with a history of Stage IB FIGO clear cell EC, treated in April 2022 with total hysterectomy, bilateral salpingo-oophorectomy, pelvic and aortocaval lymphadenectomy, and omentectomy, followed by adjuvant chemo-radiotherapy and brachytherapy. Due to the appearance of pulmonary nodules on a follow-up CT scan, a [18F]F-FDG PET/CT was requested. The study revealed distant disease recurrence, with the maximum intensity projection PET image (a) demonstrating multiple pulmonary nodules, pleural implants, and bone involvement (red arrows). Axial CT and PET/CT slices (b, c, and d) confirmed foci of pathological hypermetabolism corresponding to bilateral pulmonary nodules (b), an implant in the right costal pleura (c), a mediastinal lymphadenopathy in a prevascular location, a subpleural pulmonary nodule in the right lower lobe, and the 4th right anterior costal arch (d).
Figure 8.
A 65-year-old female with a history of stage IB FIGO serous EC, treated with radiotherapy and brachytherapy. A comparison of two [18F]F-FDG PET/CT studies is presented. The axial fused CT and PET/CT slices in the top row correspond to the first post-treatment study, showing residual focal uptake in the anterior uterine wall (a). The axial fused CT and PET/CT slices in the bottom row, from the follow-up study 6 months later, demonstrate an increase in both the intensity and extent of this uptake suggestive of progression (b).
Figure 8.
A 65-year-old female with a history of stage IB FIGO serous EC, treated with radiotherapy and brachytherapy. A comparison of two [18F]F-FDG PET/CT studies is presented. The axial fused CT and PET/CT slices in the top row correspond to the first post-treatment study, showing residual focal uptake in the anterior uterine wall (a). The axial fused CT and PET/CT slices in the bottom row, from the follow-up study 6 months later, demonstrate an increase in both the intensity and extent of this uptake suggestive of progression (b).
Table 1.
TNM stage and description of FIGO classification of endometrial cancer.
Table 1.
TNM stage and description of FIGO classification of endometrial cancer.
| FIGO stage |
FIGO description |
T |
N |
M |
| I |
Tumor confined to the body of the uterus |
T1 |
N0 |
M0 |
| IA |
No myometrial invasion or less than half |
T1a |
N0 |
M0 |
| IB |
Myometrial invasion equal to or greater than half |
T1b |
N0 |
M0 |
| II |
Tumor invading cervical stroma without extending beyond the uterus |
T2 |
N0 |
M0 |
| III |
Local and/or regional extension of the tumor |
T3 |
N0-N1 |
M0 |
| IIIA |
Tumor invading the serosa of the uterine body and/or adnexa |
T3a |
N0 |
M0 |
| IIIB |
Vaginal and/or parametrial involvement |
T3b |
N0 |
M0 |
| IIIC1 |
Positive pelvic nodes |
T1-T3 |
N1 |
M0 |
| IIIC2 |
Positive aortic nodes with or without positive pelvic nodes |
T1-T3 |
N1 |
M0 |
| IVA |
Tumor invading the lining of the bladder and/or rectum |
T4 |
Any N |
M0 |
| IVB |
Distant metastases, including intra-abdominal and/or inguinal node metastases |
Any T |
Any N |
M1 |
Table 2.
Quantitative ¹⁸F-FDG PET/CT biomarkers.
Table 2.
Quantitative ¹⁸F-FDG PET/CT biomarkers.
| Biomarker |
Description |
|
SUV
|
Measure the uptake of the radioactive tracer in a specific ROI to assess the activity and metabolism of tissues:
SUV = Tracer concentration in ROI (kBq/mL)/Injected dose per body weight (kBq/g)
|
|
SUVmean
|
Calculating the average tracer uptake in the selected ROI
A comprehensive assessment of the overall tracer uptake within the ROI, useful for areas with varying tracer uptake (e.g., tumors)
|
|
SUVmax
|
Indicating the highest level of tracer uptake within a defined ROI
|
|
MTV
|
The metabolically active volume of the tumor (i.e., the portion of the tumor with a high SUV)
|
|
TLG
|
Provides a more comprehensive measure of tumor activity than SUVmax or SUVmean alone. TLG = SUVmean × MTV
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).