Submitted:
14 June 2025
Posted:
16 June 2025
You are already at the latest version
Abstract
Keywords:
Introduction
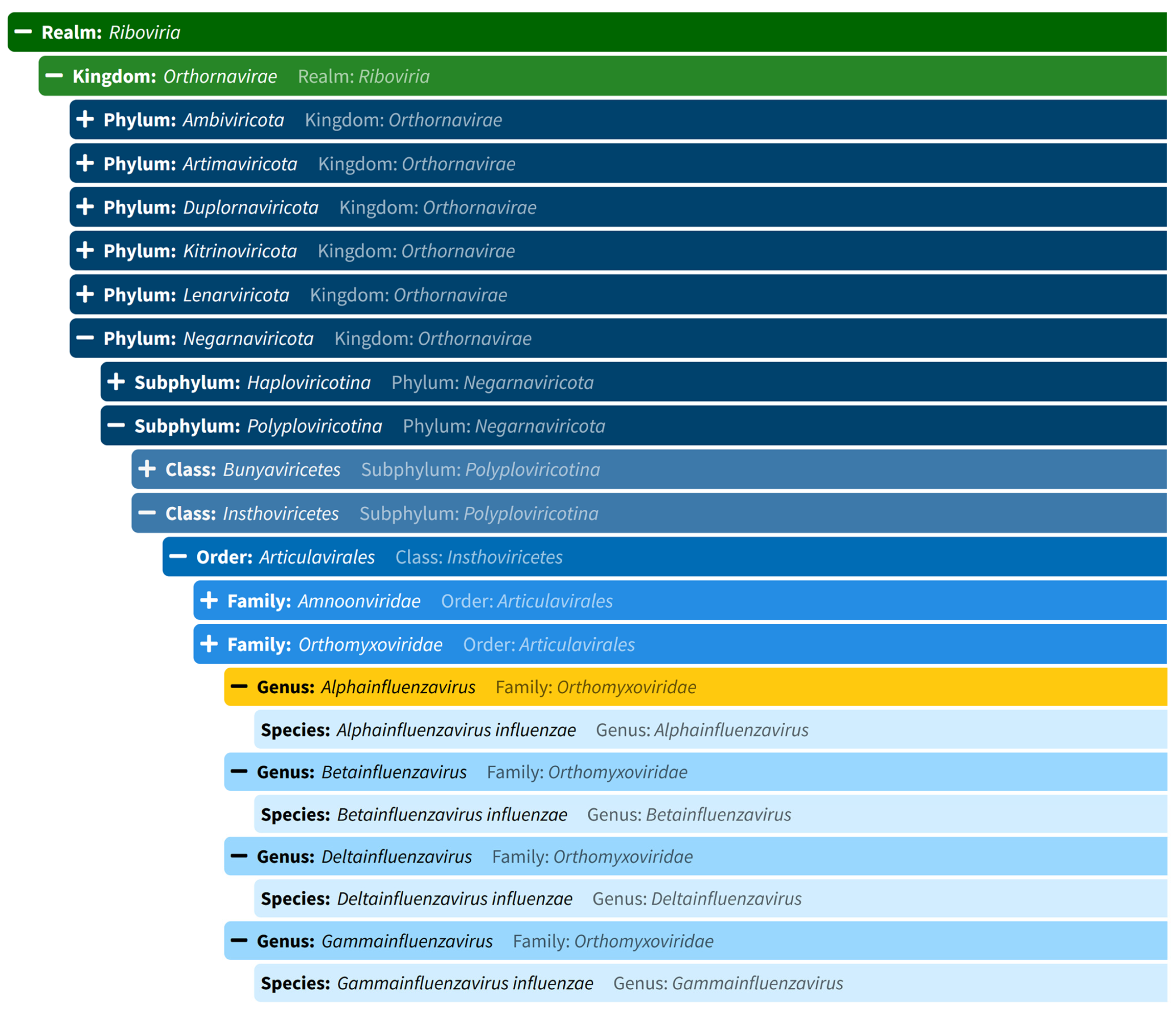
Nature and Classification of Influenzavirus
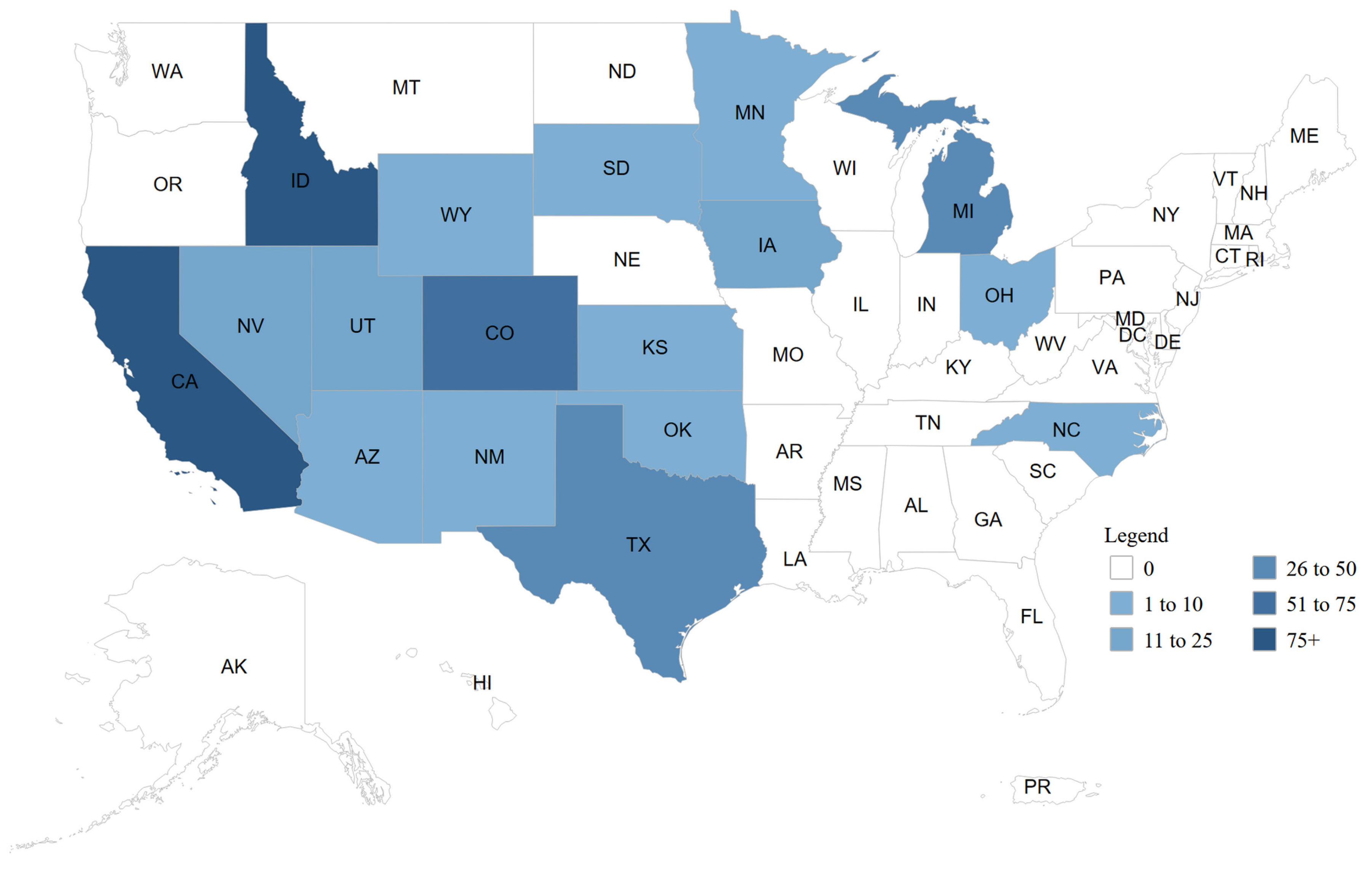
Worldwide Distribution of H5N1
History of Influenza Virus Infection in Cattle
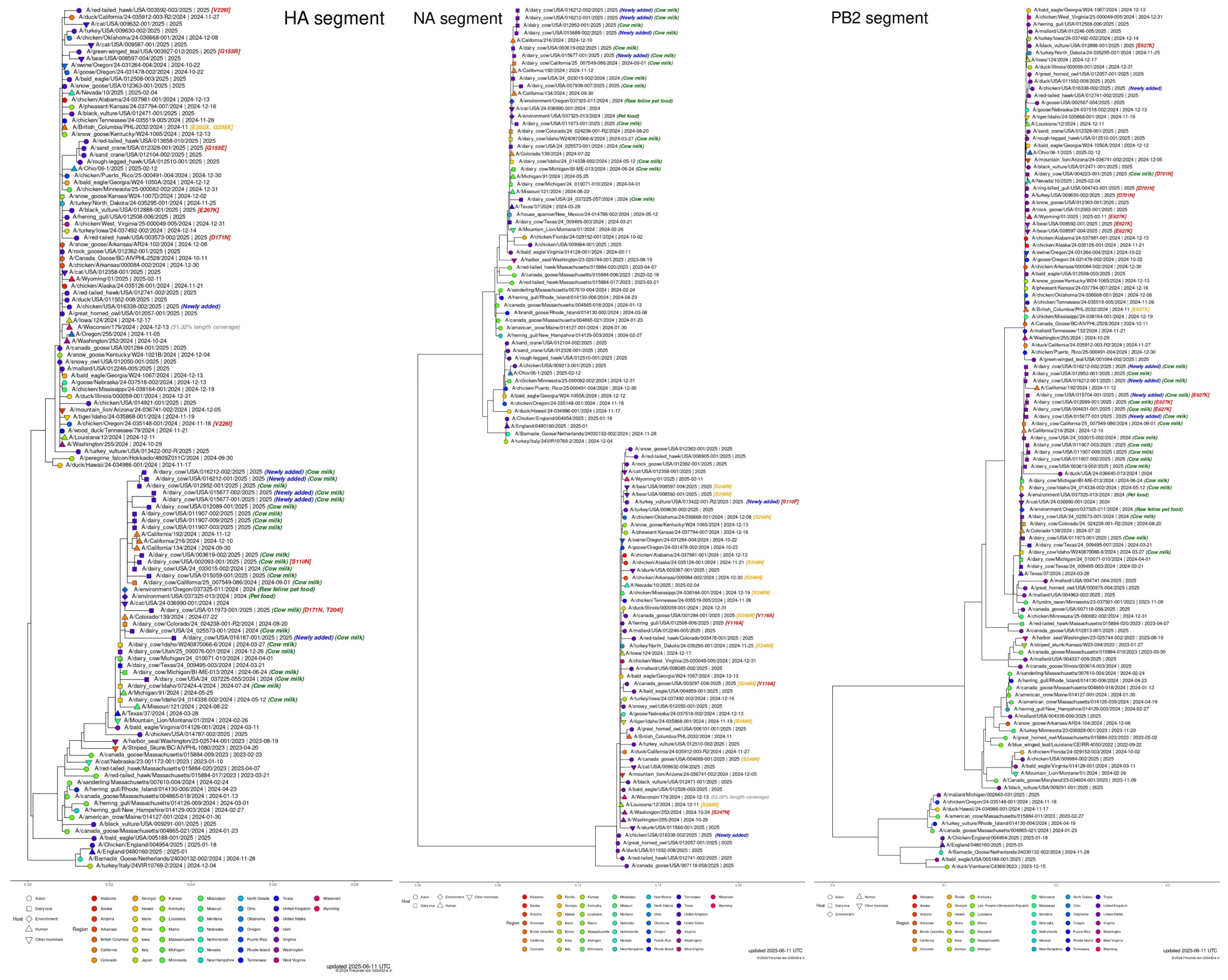
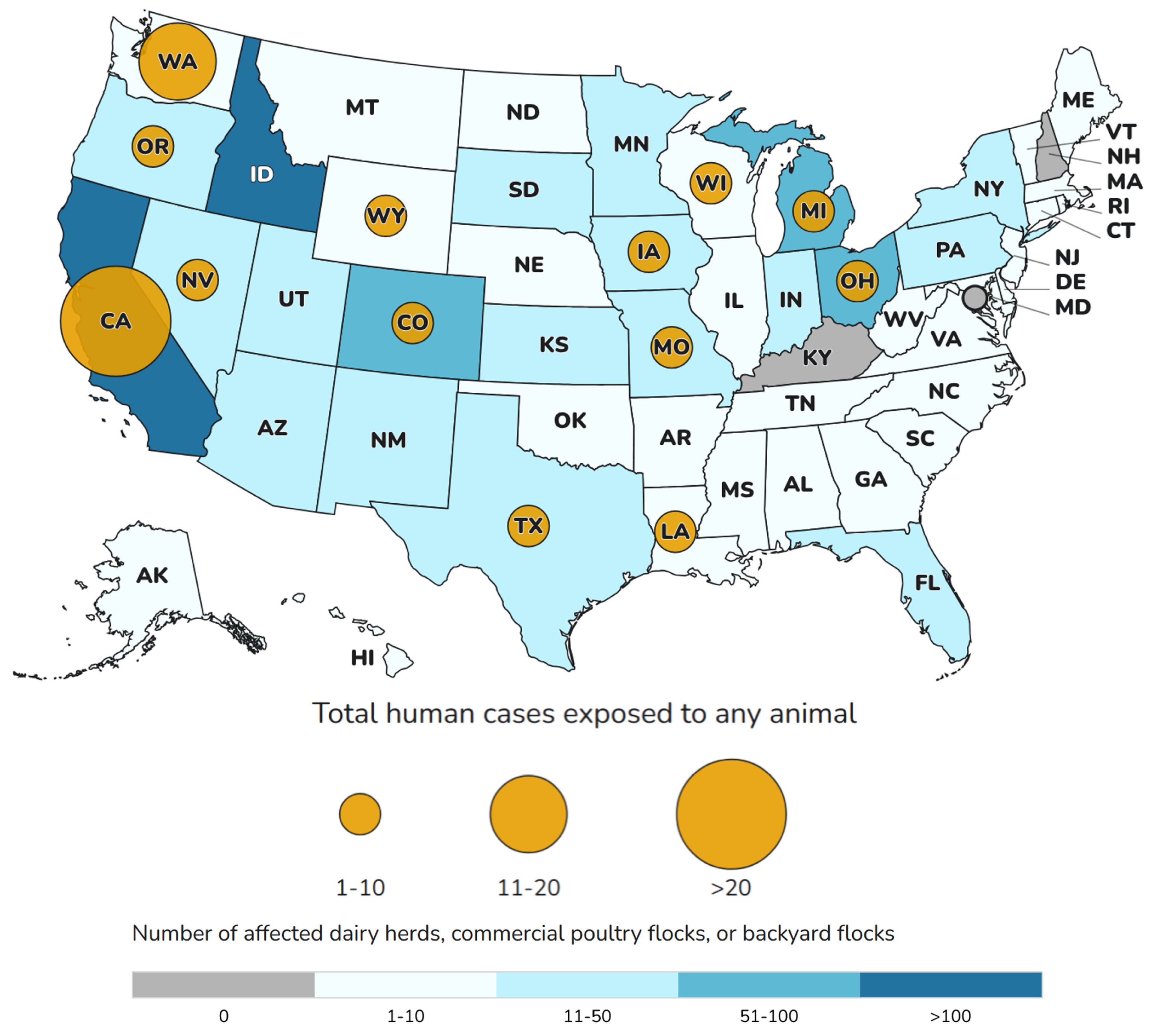
H5N1 in Cattle
H5N1 in Humans
Limitations
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Hutchinson, E.C. Influenza Virus. Trends Microbiol 2018, 26, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Jayaswal, N.; Kumar, S.; Rao, G.; Budha, R.R.; Mohanty, A.; Mehta, R.; Apostolopoulos, V.; Sah, S.; Bonilla-Aldana, D.K.; et al. Targeting H3N2 influenza: advancements in treatment and vaccine strategies. Expert Rev Anti Infect Ther 2025, 23, 5–18. [Google Scholar] [CrossRef]
- Park, J.E.; Ryu, Y. Transmissibility and severity of influenza virus by subtype. Infect Genet Evol 2018, 65, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, A.J.; Hui, D.S.; Ippolito, G.; Traore, T.; Satta, G.; Everett, D.B.; Zumla, A. Avian Influenza - The next travel-associated pandemic? Proactive One Health surveillance is required to reduce the risk of the spread. Travel Med Infect Dis 2025, 65, 102829. [Google Scholar] [CrossRef]
- Chaudhary, R.K.; L, A.; Patil, P.; Mateti, U.V.; Sah, S.; Mohanty, A.; Rath, R.S.; Padhi, B.K.; Malik, S.; Jassim, K.H.; et al. System Biology Approach to Identify the Hub Genes and Pathways Associated with Human H5N1 Infection. Vaccines (Basel) 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, *!!! REPLACE !!!*; Khandia, R.; Chopra, H.; Choudhary, O.P.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. The re-emergence of H3N2 influenza: An update on the risk and containment. New Microbes New Infect 2023, 53, 101147. [Google Scholar] [CrossRef]
- Peacock, T.P.; Moncla, L.; Dudas, G.; VanInsberghe, D.; Sukhova, K.; Lloyd-Smith, J.O.; Worobey, M.; Lowen, A.C.; Nelson, M.I. The global H5N1 influenza panzootic in mammals. Nature 2025, 637, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, E.D. Influenza pandemics of the 20th century. Emerg Infect Dis 2006, 12, 9–14. [Google Scholar] [CrossRef]
- Ankomah, A.A.; Moa, A.; Chughtai, A.A. The long road of pandemic vaccine development to rollout: A systematic review on the lessons learnt from the 2009 H1N1 influenza pandemic. Am J Infect Control 2022, 50, 735–742. [Google Scholar] [CrossRef]
- Charostad, J.; Rezaei Zadeh Rukerd, M.; Mahmoudvand, S.; Bashash, D.; Hashemi, S.M.A.; Nakhaie, M.; Zandi, K. A comprehensive review of highly pathogenic avian influenza (HPAI) H5N1: An imminent threat at doorstep. Travel Med Infect Dis 2023, 55, 102638. [Google Scholar] [CrossRef]
- Guan, Y.; Smith, G.J. The emergence and diversification of panzootic H5N1 influenza viruses. Virus Res 2013, 178, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Chavda, V.P.; Mehta, R.; Rodriguez-Morales, A.J.; Henao-MartÍnez, A.F.; Sah, R. Alert and surveillance on H5N1 influenza virus: risks to agriculture and public health. Ther Adv Infect Dis 2024, 11, 20499361241266521. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Bonilla-Aldana, D.K.; Paniz-Mondolfi, A.E. Concerns about influenza H5N8 outbreaks in humans and birds: Facing the next airborne pandemic? Travel Med Infect Dis 2021, 41, 102054. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Aldana, D.K.; Aguirre-Florez, M.; Villamizar-Peña, R.; Gutiérrez-Ocampo, E.; Henao-Martínez, J.F.; Cvetkovic-Vega, A.; Dhama, K.; Rabaan, A.; Sah, R.; Rodriguez-Morales, A.J.; et al. After SARS-CoV-2, will H5N6 and other influenza viruses follow the pandemic path? Infez Med 2020, 28, 475–485. [Google Scholar] [PubMed]
- Cruz, C.D.; Icochea, M.E.; Espejo, V.; Troncos, G.; Castro-Sanguinetti, G.R.; Schilling, M.A.; Tinoco, Y. Highly Pathogenic Avian Influenza A(H5N1) from Wild Birds, Poultry, and Mammals, Peru. Emerg Infect Dis 2023, 29, 2572–2576. [Google Scholar] [CrossRef]
- Neumann, G.; Kawaoka, Y. Highly pathogenic H5N1 avian influenza virus outbreak in cattle: the knowns and unknowns. Nature Reviews Microbiology 2024, 22, 525–526. [Google Scholar] [CrossRef]
- Hu, X.; Saxena, A.; Magstadt, D.R.; Gauger, P.C.; Burrough, E.R.; Zhang, J.; Siepker, C.; Mainenti, M.; Gorden, P.J.; Plummer, P.J.; et al. Genomic characterization of highly pathogenic avian influenza A H5N1 virus newly emerged in dairy cattle. Emerg Microbes Infect 2024, 13, 2380421. [Google Scholar] [CrossRef]
- Cui, P.; Shi, J.; Wang, C.; Zhang, Y.; Xing, X.; Kong, H.; Yan, C.; Zeng, X.; Liu, L.; Tian, G.; et al. Global dissemination of H5N1 influenza viruses bearing the clade 2.3.4.4b HA gene and biologic analysis of the ones detected in China. Emerg Microbes Infect 2022, 11, 1693–1704. [Google Scholar] [CrossRef]
- Sreenivasan, C.C.; Li, F.; Wang, D. Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts. Viruses 2024, 16. [Google Scholar] [CrossRef]
- Ruiz-Saenz, J.; Martinez-Gutierrez, M.; Pujol, F.H. Multiple introductions of highly pathogenic avian influenza H5N1 clade 2.3.4.4b into South America. Travel Med Infect Dis 2023, 53, 102591. [Google Scholar] [CrossRef]
- Rimondi, A.; Vanstreels, R.E.T.; Olivera, V.; Donini, A.; Lauriente, M.M.; Uhart, M.M. Highly Pathogenic Avian Influenza A(H5N1) Viruses from Multispecies Outbreak, Argentina, August 2023. Emerg Infect Dis 2024, 30, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Haque, S.; Tawil, S.; Husni, R.; Bonilla-Aldana, D.K.; Montenegro-Idrogo, J.J.; Rodriguez-Morales, A.J. Avian influenza spillover to humans: Are we prepared to deal with another potential pandemic? Travel Med Infect Dis 2023, 55, 102634. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yeom, M.; Vu, T.T.H.; Do, H.Q.; Na, W.; Lee, M.; Jeong, D.G.; Cheon, D.S.; Song, D. Characterization of highly pathogenic avian influenza A (H5N1) viruses isolated from cats in South Korea, 2023. Emerg Microbes Infect 2024, 13, 2290835. [Google Scholar] [CrossRef]
- Mostafa, A.; Naguib, M.M.; Nogales, A.; Barre, R.S.; Stewart, J.P.; García-Sastre, A.; Martinez-Sobrido, L. Avian influenza A (H5N1) virus in dairy cattle: origin, evolution, and cross-species transmission. mBio 2024, 15, e0254224. [Google Scholar] [CrossRef] [PubMed]
- Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A.; et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 2024, 634, 669–676. [Google Scholar] [CrossRef]
- Burrough, E.R.; Magstadt, D.R.; Petersen, B.; Timmermans, S.J.; Gauger, P.C.; Zhang, J.; Siepker, C.; Mainenti, M.; Li, G.; Thompson, A.C.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg Infect Dis 2024, 30, 1335–1343. [Google Scholar] [CrossRef]
- Nelli, R.K.; Harm, T.A.; Siepker, C.; Groeltz-Thrush, J.M.; Jones, B.; Twu, N.C.; Nenninger, A.S.; Magstadt, D.R.; Burrough, E.R.; Piñeyro, P.E.; et al. Sialic Acid Receptor Specificity in Mammary Gland of Dairy Cattle Infected with Highly Pathogenic Avian Influenza A(H5N1) Virus. Emerg Infect Dis 2024, 30, 1361–1373. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Bonilla-Aldana, J.L.; Acosta-España, J.D.; Rodriguez-Morales, A.J. Highly Pathogenic Avian Influenza H5N1 in Cats (Felis catus): A Systematic Review and Meta-Analysis. Animals (Basel) 2025, 15. [Google Scholar] [CrossRef]
- Eisfeld, A.J.; Biswas, A.; Guan, L.; Gu, C.; Maemura, T.; Trifkovic, S.; Wang, T.; Babujee, L.; Dahn, R.; Halfmann, P.J.; et al. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature 2024, 633, 426–432. [Google Scholar] [CrossRef]
- Honein, M.A.; Olsen, S.J.; Jernigan, D.B.; Daskalakis, D.C. Challenges and Opportunities for Wastewater Monitoring of Influenza Viruses During the Multistate Outbreak of Highly Pathogenic Avian Influenza A(H5N1) Virus in Dairy Cattle and Poultry. Am J Public Health 2024, 114, 1309–1312. [Google Scholar] [CrossRef]
- Khurana, S.; King, L.R.; Manischewitz, J.; Posadas, O.; Mishra, A.K.; Liu, D.; Beigel, J.H.; Rappuoli, R.; Tsang, J.S.; Golding, H. Licensed H5N1 vaccines generate cross-neutralizing antibodies against highly pathogenic H5N1 clade 2.3.4.4b influenza virus. Nat Med 2024, 30, 2771–2776. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Reed, C.; Davis, C.T.; Uyeki, T.M.; Behravesh, C.B.; Kniss, K.; Budd, A.; Biggerstaff, M.; Adjemian, J.; Barnes, J.R.; et al. Outbreak of Highly Pathogenic Avian Influenza A(H5N1) Viruses in U.S. Dairy Cattle and Detection of Two Human Cases - United States, 2024. MMWR Morb Mortal Wkly Rep 2024, 73, 501–505. [Google Scholar] [CrossRef]
- Sah, R.; Srivastava, S.; Kumar, S.; Mehta, R.; Donovan, S.; Sierra-Carrero, L.; Luna, C.; Woc-Colburn, L.; Cardona-Ospina, J.A.; Hinestroza-Jordan, M.; et al. Concerns on H5N1 avian influenza given the outbreak in U.S. dairy cattle. Lancet Reg Health Am 2024, 35, 100785. [Google Scholar] [CrossRef]
- Lowen, A.C.; Baker, A.L.; Bowman, A.S.; García-Sastre, A.; Hensley, S.E.; Lakdawala, S.S.; Moncla, L.H.; Nelson, M.I.; Pekosz, A.; Poulson, R.L.; et al. Pandemic risk stemming from the bovine H5N1 outbreak: an account of the knowns and unknowns. J Virol 2025, e0005225. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M. Evolution and mutational landscape of highly pathogenic avian influenza strain A(H5N1) in the current outbreak in the USA and global landscape. Virology 2024, 600, 110246. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, C.; Jensen, H.E.; Trebbien, R.; Webby, R.J.; Larsen, L.E. Avian and Human Influenza A Virus Receptors in Bovine Mammary Gland. Emerg Infect Dis 2024, 30, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Oguzie, J.U.; Marushchak, L.V.; Shittu, I.; Lednicky, J.A.; Miller, A.L.; Hao, H.; Nelson, M.I.; Gray, G.C. Avian Influenza A(H5N1) Virus among Dairy Cattle, Texas, USA. Emerg Infect Dis 2024, 30, 1425–1429. [Google Scholar] [CrossRef]
- Cohen, J. Worries about bird flu in U.S. cattle intensify. Science 2024, 384, 12–13. [Google Scholar] [CrossRef]
- Kalthoff, D.; Hoffmann, B.; Harder, T.; Durban, M.; Beer, M. Experimental infection of cattle with highly pathogenic avian influenza virus (H5N1). Emerg Infect Dis 2008, 14, 1132–1134. [Google Scholar] [CrossRef]
- Halwe, N.J.; Cool, K.; Breithaupt, A.; Schön, J.; Trujillo, J.D.; Nooruzzaman, M.; Kwon, T.; Ahrens, A.K.; Britzke, T.; McDowell, C.D.; et al. H5N1 clade 2.3.4.4b dynamics in experimentally infected calves and cows. Nature 2025, 637, 903–912. [Google Scholar] [CrossRef]
- Perez-Acle, T.; Ravello, C.; Rosemblatt, M. Are we cultivating the perfect storm for a human avian influenza pandemic? Biol Res 2024, 57, 96. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Maemura, T.; Guan, L.; Eisfeld, A.J.; Biswas, A.; Kiso, M.; Uraki, R.; Ito, M.; Trifkovic, S.; Wang, T.; et al. A human isolate of bovine H5N1 is transmissible and lethal in animal models. Nature 2024, 636, 711–718. [Google Scholar] [CrossRef]
- WHO. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2024. 12 December 2024. Available online: https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who--2003-2024--20-december-2024 (accessed on 7 March 2025).
- CDC. First H5 Bird Flu Death Reported in United States. Available online: https://www.cdc.gov/media/releases/2025/m0106-h5-birdflu-death.html (accessed on 7 March 2025).
- Plaza, P.I.; Gamarra-Toledo, V.; Euguí, J.R.; Lambertucci, S.A. Recent Changes in Patterns of Mammal Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus Worldwide. Emerg Infect Dis 2024, 30, 444–452. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: Rapid Testing for the Avian Influenza A(H5N1) Virus is Urgently Required as Infections in Poultry and Dairy Cows are on the Rise, and so is Transmission to Humans. Med Sci Monit 2025, 31, e949109. [Google Scholar] [CrossRef]
- Guan, L.; Eisfeld, A.J.; Pattinson, D.; Gu, C.; Biswas, A.; Maemura, T.; Trifkovic, S.; Babujee, L.; Presler, R., Jr.; Dahn, R.; et al. Cow’s Milk Containing Avian Influenza A(H5N1) Virus - Heat Inactivation and Infectivity in Mice. N Engl J Med 2024, 391, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Louis, S.; Mark-Carew, M.; Biggerstaff, M.; Yoder, J.; Boehm, A.B.; Wolfe, M.K.; Flood, M.; Peters, S.; Stobierski, M.G.; Coyle, J.; et al. Wastewater Surveillance for Influenza A Virus and H5 Subtype Concurrent with the Highly Pathogenic Avian Influenza A(H5N1) Virus Outbreak in Cattle and Poultry and Associated Human Cases - United States, May 12-July 13, 2024. MMWR Morb Mortal Wkly Rep 2024, 73, 804–809. [Google Scholar] [CrossRef]
- Koopmans, M.P.G.; Barton Behravesh, C.; Cunningham, A.A.; Adisasmito, W.B.; Almuhairi, S.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Cediel Becerra, N.; Charron, D.F.; et al. The panzootic spread of highly pathogenic avian influenza H5N1 sublineage 2.3.4.4b: a critical appraisal of One Health preparedness and prevention. Lancet Infect Dis 2024, 24, e774–e781. [Google Scholar] [CrossRef]
- Spackman, E.; Jones, D.R.; McCoig, A.M.; Colonius, T.J.; Goraichuk, I.V.; Suarez, D.L. Characterization of highly pathogenic avian influenza virus in retail dairy products in the US. J Virol 2024, 98, e0088124. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; McVeigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford) 2020, 2020. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Kash, J.C. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 2010, 7, 440–451. [Google Scholar] [CrossRef]
- Liu, R.; Sheng, Z.; Huang, C.; Wang, D.; Li, F. Influenza D virus. Curr Opin Virol 2020, 44, 154–161. [Google Scholar] [CrossRef]
- Su, S.; Fu, X.; Li, G.; Kerlin, F.; Veit, M. Novel Influenza D virus: Epidemiology, pathology, evolution and biological characteristics. Virulence 2017, 8, 1580–1591. [Google Scholar] [CrossRef]
- Sonnberg, S.; Webby, R.J.; Webster, R.G. Natural history of highly pathogenic avian influenza H5N1. Virus Res 2013, 178, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nature Reviews Disease Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Carter, T.; Iqbal, M. The Influenza A Virus Replication Cycle: A Comprehensive Review. Viruses 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.; Fodor, E. Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis. Nat Rev Microbiol 2016, 14, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Lakadamyali, M.; Rust, M.J.; Zhuang, X. Endocytosis of influenza viruses. Microbes Infect 2004, 6, 929–936. [Google Scholar] [CrossRef]
- Kash, J.C.; Goodman, A.G.; Korth, M.J.; Katze, M.G. Hijacking of the host-cell response and translational control during influenza virus infection. Virus Res 2006, 119, 111–120. [Google Scholar] [CrossRef]
- Wagner, R.; Matrosovich, M.; Klenk, H.D. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol 2002, 12, 159–166. [Google Scholar] [CrossRef]
- Nayak, D.P.; Hui, E.K.; Barman, S. Assembly and budding of influenza virus. Virus Res 2004, 106, 147–165. [Google Scholar] [CrossRef]
- Luo, M. Influenza virus entry. Adv Exp Med Biol 2012, 726, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, S.; Blijleven, J.S.; Roos, W.H.; Onck, P.R.; van der Giessen, E.; van Oijen, A.M. Hemagglutinin-Mediated Membrane Fusion: A Biophysical Perspective. Annu Rev Biophys 2018, 47, 153–173. [Google Scholar] [CrossRef]
- Dadonaite, B.; Gilbertson, B.; Knight, M.L.; Trifkovic, S.; Rockman, S.; Laederach, A.; Brown, L.E.; Fodor, E.; Bauer, D.L.V. The structure of the influenza A virus genome. Nat Microbiol 2019, 4, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Pleschka, S. Overview of influenza viruses. Curr Top Microbiol Immunol 2013, 370, 1–20. [Google Scholar] [CrossRef] [PubMed]
- PAHO. La muerte púrpura: la gran gripe de 1918. Available online: https://www.paho.org/es/quienes-somos/historia-ops/muerte-purpura-gran-gripe-1918 (accessed on 11 March 2025).
- WHO. Number of COVID-19 deaths reported to WHO (cumulative total). Available online: https://data.who.int/dashboards/covid19/deaths (accessed on 11 March 2025).
- Hermann, E.; Krammer, F. Clade 2.3.4.4b H5N1 neuraminidase has a long stalk, which is in contrast to most highly pathogenic H5N1 viruses circulating between 2002 and 2020. mBio 1128, e0398924. [Google Scholar] [CrossRef]
- Bennett-Laso, B.; Berazay, B.; Muñoz, G.; Ariyama, N.; Enciso, N.; Braun, C.; Krüger, L.; Barták, M.; González-Aravena, M.; Neira, V. Confirmation of highly pathogenic avian influenza H5N1 in skuas, Antarctica 2024. Front Vet Sci 2024, 11, 1423404. [Google Scholar] [CrossRef]
- Banyard, A.C.; Bennison, A.; Byrne, A.M.P.; Reid, S.M.; Lynton-Jenkins, J.G.; Mollett, B.; De Silva, D.; Peers-Dent, J.; Finlayson, K.; Hall, R.; et al. Detection and spread of high pathogenicity avian influenza virus H5N1 in the Antarctic Region. Nat Commun 2024, 15, 7433. [Google Scholar] [CrossRef]
- Lisovski, S.; Günther, A.; Dewar, M.; Ainley, D.; Aldunate, F.; Arce, R.; Ballard, G.; Bauer, S.; Belliure, J.; Banyard, A.C.; et al. Unexpected Delayed Incursion of Highly Pathogenic Avian Influenza H5N1 (Clade 2.3.4.4b) Into the Antarctic Region. Influenza Other Respir Viruses 2024, 18, e70010. [Google Scholar] [CrossRef]
- Fasanmi, O.G.; Odetokun, I.A.; Balogun, F.A.; Fasina, F.O. Public health concerns of highly pathogenic avian influenza H5N1 endemicity in Africa. Vet World 2017, 10, 1194–1204. [Google Scholar] [CrossRef]
- Tassoni, L.; Fusaro, A.; Milani, A.; Lemey, P.; Awuni, J.A.; Sedor, V.B.; Dogbey, O.; Commey, A.N.; Meseko, C.; Joannis, T.; et al. Genetically Different Highly Pathogenic Avian Influenza A(H5N1) Viruses in West Africa, 2015. Emerg Infect Dis 2016, 22, 2132–2136. [Google Scholar] [CrossRef]
- Abolnik, C.; Phiri, T.; Peyrot, B.; de Beer, R.; Snyman, A.; Roberts, D.; Ludynia, K.; Jordaan, F.; Maartens, M.; Ismail, Z.; et al. The Molecular Epidemiology of Clade 2.3.4.4B H5N1 High Pathogenicity Avian Influenza in Southern Africa, 2021-2022. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Abolnik, C.; Roberts, L.C.; Strydom, C.; Snyman, A.; Roberts, D.G. Outbreaks of H5N1 High Pathogenicity Avian Influenza in South Africa in 2023 Were Caused by Two Distinct Sub-Genotypes of Clade 2.3.4.4b Viruses. Viruses 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Leguia, M.; Garcia-Glaessner, A.; Muñoz-Saavedra, B.; Juarez, D.; Barrera, P.; Calvo-Mac, C.; Jara, J.; Silva, W.; Ploog, K.; Amaro, L.; et al. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nat Commun 2023, 14, 5489. [Google Scholar] [CrossRef]
- Bruno, A.; Alfaro-Núñez, A.; de Mora, D.; Armas, R.; Olmedo, M.; Garcés, J.; Vaca, M.S.; De la Torre, E.; Jarrin, D.; Burbano, L.; et al. Phylogenetic analysis reveals that the H5N1 avian influenza A outbreak in poultry in Ecuador in November 2022 is associated with the highly pathogenic clade 2.3.4.4b. Int J Infect Dis 2023, 133, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Paternina, D.; Herazo, R.; Oviedo, M.; Mattar, S. Dramatic re-emergence of avian influenza in Colombia and Latin America. Travel Med Infect Dis 2024, 59, 102711. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.A.; Brock, N.; Belser, J.A.; Sun, X.; Pappas, C.; Kieran, T.J.; Basu Thakur, P.; Zeng, H.; Cui, D.; Frederick, J.; et al. Highly pathogenic avian influenza A(H5N1) virus of clade 2.3.4.4b isolated from a human case in Chile causes fatal disease and transmits between co-housed ferrets. Emerg Microbes Infect 2024, 13, 2332667. [Google Scholar] [CrossRef]
- Kandeil, A.; Patton, C.; Jones, J.C.; Jeevan, T.; Harrington, W.N.; Trifkovic, S.; Seiler, J.P.; Fabrizio, T.; Woodard, K.; Turner, J.C.; et al. Rapid evolution of A(H5N1) influenza viruses after intercontinental spread to North America. Nat Commun 2023, 14, 3082. [Google Scholar] [CrossRef]
- Ly, H. Highly pathogenic avian influenza H5N1 virus infections of dairy cattle and livestock handlers in the United States of America. Virulence 2024, 15, 2343931. [Google Scholar] [CrossRef] [PubMed]
- Tawidian, P.; Torchetti, M.K.; Killian, M.L.; Lantz, K.; Dilione, K.E.; Ringenberg, J.M.; Bevins, S.N.; Lenoch, J.B.; Ip, H.S. Genotypic Clustering of H5N1 Avian Influenza Viruses in North America Evaluated by Ordination Analysis. Viruses 2024, 16. [Google Scholar] [CrossRef]
- Pabbaraju, K.; Tellier, R.; Wong, S.; Li, Y.; Bastien, N.; Tang, J.W.; Drews, S.J.; Jang, Y.; Davis, C.T.; Fonseca, K.; et al. Full-genome analysis of avian influenza A(H5N1) virus from a human, North America, 2013. Emerg Infect Dis 2014, 20, 887–891. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Fouchier, R.A.M.; Lewis, N. Highly Pathogenic Avian Influenza Viruses at the Wild-Domestic Bird Interface in Europe: Future Directions for Research and Surveillance. Viruses 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Engelsma, M.; Heutink, R.; Harders, F.; Germeraad, E.A.; Beerens, N. Multiple Introductions of Reassorted Highly Pathogenic Avian Influenza H5Nx Viruses Clade 2.3.4.4b Causing Outbreaks in Wild Birds and Poultry in The Netherlands, 2020-2021. Microbiol Spectr 2022, 10, e0249921. [Google Scholar] [CrossRef]
- Muzyka, D.; Rula, O.; Tkachenko, S.; Muzyka, N.; Köthe, S.; Pishchanskyi, O.; Stegniy, B.; Pantin-Jackwood, M.; Beer, M. Highly Pathogenic and Low Pathogenic Avian Influenza H5 Subtype Viruses in Wild Birds in Ukraine. Avian Dis 2019, 63, 219–229. [Google Scholar] [CrossRef]
- King, J.; Harder, T.; Conraths, F.J.; Beer, M.; Pohlmann, A. The genetics of highly pathogenic avian influenza viruses of subtype H5 in Germany, 2006-2020. Transbound Emerg Dis 2021, 68, 1136–1150. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Calle-Hernández, D.M.; Ulloque-Badaracco, J.R.; Alarcón-Braga, E.A.; Hernández-Bustamante, E.A.; Cabrera-Guzmán, J.C.; Quispe-Vasquez, S.M.; Huayta-Cortez, M.A.; Benites-Zapata, V.A.; Rodriguez-Morales, A.J. Highly pathogenic avian influenza A(H5N1) in animals: A systematic review and meta-analysis. New Microbes New Infect 2024, 60-61, 101439. [Google Scholar] [CrossRef]
- Ariyama, N.; Pardo-Roa, C.; Muñoz, G.; Aguayo, C.; Ávila, C.; Mathieu, C.; Almonacid, L.I.; Medina, R.A.; Brito, B.; Johow, M.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Wild Birds, Chile. Emerg Infect Dis 2023, 29, 1842–1845. [Google Scholar] [CrossRef]
- Jimenez-Bluhm, P.; Siegers, J.Y.; Tan, S.; Sharp, B.; Freiden, P.; Johow, M.; Orozco, K.; Ruiz, S.; Baumberger, C.; Galdames, P.; et al. Detection and phylogenetic analysis of highly pathogenic A/H5N1 avian influenza clade 2.3.4.4b virus in Chile, 2022. Emerg Microbes Infect 2023, 12, 2220569. [Google Scholar] [CrossRef] [PubMed]
- Kutkat, O.; Gomaa, M.; Moatasim, Y.; El Taweel, A.; Kamel, M.N.; El Sayes, M.; GabAllah, M.; Kandeil, A.; McKenzie, P.P.; Webby, R.J.; et al. Highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b in wild rats in Egypt during 2023. Emerg Microbes Infect 2024, 13, 2396874. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: Concerns as Highly Pathogenic Avian Influenza (HPAI) Virus of the H5N1 Subtype is Identified in Dairy Cows and Other Mammals. Med Sci Monit 2024, 30, e945315. [Google Scholar] [CrossRef]
- Szaluś-Jordanow, O.; Golke, A.; Dzieciątkowski, T.; Chrobak-Chmiel, D.; Rzewuska, M.; Czopowicz, M.; Sapierzyński, R.; Kardas, M.; Biernacka, K.; Mickiewicz, M.; et al. A Fatal A/H5N1 Avian Influenza Virus Infection in a Cat in Poland. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Briand, F.X.; Souchaud, F.; Pierre, I.; Beven, V.; Hirchaud, E.; Hérault, F.; Planel, R.; Rigaudeau, A.; Bernard-Stoecklin, S.; Van der Werf, S.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Domestic Cat, France, 2022. Emerg Infect Dis 2023, 29, 1696–1698. [Google Scholar] [CrossRef] [PubMed]
- Chothe, S.K.; Srinivas, S.; Misra, S.; Nallipogu, N.C.; Gilbride, E.; LaBella, L.; Mukherjee, S.; Gauthier, C.H.; Pecoraro, H.L.; Webb, B.T.; et al. Marked neurotropism and potential adaptation of H5N1 clade 2.3.4.4.b virus in naturally infected domestic cats. Emerg Microbes Infect 2025, 14, 2440498. [Google Scholar] [CrossRef] [PubMed]
- Naraharisetti, R.; Weinberg, M.; Stoddard, B.; Stobierski, M.G.; Dodd, K.; Wineland, N.; Beal, M.; Morse, J.; Hatter, S.; Sledge, D.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection of Indoor Domestic Cats Within Dairy Industry Worker Households - Michigan, May 2024. MMWR. Morbidity and mortality weekly report 2025, 74, 61–65. [Google Scholar] [CrossRef]
- Brown, J.D.; Black, A.; Haman, K.H.; Diel, D.G.; Ramirez, V.E.; Ziejka, R.S.; Fenelon, H.T.; Rabinowitz, P.M.; Stevens, L.; Poulson, R.; et al. Antibodies to Influenza A(H5N1) Virus in Hunting Dogs Retrieving Wild Fowl, Washington, USA. Emerg Infect Dis 2024, 30, 1271–1274. [Google Scholar] [CrossRef]
- Maas, R.; Tacken, M.; Ruuls, L.; Koch, G.; van Rooij, E.; Stockhofe-Zurwieden, N. Avian influenza (H5N1) susceptibility and receptors in dogs. Emerg Infect Dis 2007, 13, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhong, G.; Wang, G.; Deng, G.; Li, Y.; Shi, J.; Zhang, Z.; Guan, Y.; Jiang, Y.; Bu, Z.; et al. Dogs are highly susceptible to H5N1 avian influenza virus. Virology 2010, 405, 15–19. [Google Scholar] [CrossRef]
- Kwon, T.; Trujillo, J.D.; Carossino, M.; Lyoo, E.L.; McDowell, C.D.; Cool, K.; Matias-Ferreyra, F.S.; Jeevan, T.; Morozov, I.; Gaudreault, N.N.; et al. Pigs are highly susceptible to but do not transmit mink-derived highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b. Emerg Microbes Infect 2024, 13, 2353292. [Google Scholar] [CrossRef]
- Imai, M.; Herfst, S.; Sorrell, E.M.; Schrauwen, E.J.; Linster, M.; De Graaf, M.; Fouchier, R.A.; Kawaoka, Y. Transmission of influenza A/H5N1 viruses in mammals. Virus Res 2013, 178, 15–20. [Google Scholar] [CrossRef]
- Yu, J.; Li, T.; Wen, Z.; Wu, S.; Wang, Z.; Zheng, J.; Chen, M.; Chen, F.; Wei, W.K.; Zhai, S.L.; et al. Identification of D/Yama2019 Lineage-Like Influenza D Virus in Chinese Cattle. Front Vet Sci 2022, 9, 939456. [Google Scholar] [CrossRef]
- Sreenivasan, C.; Thomas, M.; Sheng, Z.; Hause, B.M.; Collin, E.A.; Knudsen, D.E.; Pillatzki, A.; Nelson, E.; Wang, D.; Kaushik, R.S.; et al. Replication and Transmission of the Novel Bovine Influenza D Virus in a Guinea Pig Model. J Virol 2015, 89, 11990–12001. [Google Scholar] [CrossRef]
- Ferguson, L.; Eckard, L.; Epperson, W.B.; Long, L.P.; Smith, D.; Huston, C.; Genova, S.; Webby, R.; Wan, X.F. Influenza D virus infection in Mississippi beef cattle. Virology 2015, 486, 28–34. [Google Scholar] [CrossRef]
- Flynn, O.; Gallagher, C.; Mooney, J.; Irvine, C.; Ducatez, M.; Hause, B.; McGrath, G.; Ryan, E. Influenza D Virus in Cattle, Ireland. Emerg Infect Dis 2018, 24, 389–391. [Google Scholar] [CrossRef]
- Collin, E.A.; Sheng, Z.; Lang, Y.; Ma, W.; Hause, B.M.; Li, F. Cocirculation of two distinct genetic and antigenic lineages of proposed influenza D virus in cattle. J Virol 2015, 89, 1036–1042. [Google Scholar] [CrossRef]
- Silveira, S.; Falkenberg, S.M.; Kaplan, B.S.; Crossley, B.; Ridpath, J.F.; Bauermann, F.B.; Fossler, C.P.; Dargatz, D.A.; Dassanayake, R.P.; Vincent, A.L.; et al. Serosurvey for Influenza D Virus Exposure in Cattle, United States, 2014-2015. Emerg Infect Dis 2019, 25, 2074–2080. [Google Scholar] [CrossRef] [PubMed]
- Quast, M.; Sreenivasan, C.; Sexton, G.; Nedland, H.; Singrey, A.; Fawcett, L.; Miller, G.; Lauer, D.; Voss, S.; Pollock, S.; et al. Serological evidence for the presence of influenza D virus in small ruminants. Vet Microbiol 2015, 180, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Ducatez, M.F.; Pelletier, C.; Meyer, G. Influenza D virus in cattle, France, 2011-2014. Emerg Infect Dis 2015, 21, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, C.J.; Oliva, J.; Pauly, M.; Losch, S.; Wildschutz, F.; Muller, C.P.; Hübschen, J.M.; Ducatez, M.F. Influenza D Virus Circulation in Cattle and Swine, Luxembourg, 2012-2016. Emerg Infect Dis 2018, 24, 1388–1389. [Google Scholar] [CrossRef]
- da Silva, M.S.; Mosena, A.C.S.; Baumbach, L.; Demoliner, M.; Gularte, J.S.; Pavarini, S.P.; Driemeier, D.; Weber, M.N.; Spilki, F.R.; Canal, C.W. Cattle influenza D virus in Brazil is divergent from established lineages. Arch Virol 2022, 167, 1181–1184. [Google Scholar] [CrossRef]
- CDC. Situación actual: influenza aviar en vacas lecheras. Available online: https://espanol.cdc.gov/bird-flu/situation-summary/mammals.html#:~:text=El%201%20de%20abril%2C%20los,(H5N1)%20de%20la%20HPAI (accessed on 13 March 2025).
- Goujgoulova, G.; Koev, K. Risk Assessment of Spread of the Influenza A Virus in Cows in South Bulgaria. Viruses 2025, 17. [Google Scholar] [CrossRef]
- Rubin, E.J.; Baden, L.R.; Goldstein, R.; Shuford, J.A.; Morrissey, S. NEJM Outbreaks Update - H5N1: A View from the States. N Engl J Med 2025. [Google Scholar] [CrossRef]
- Hawman, D.W.; Tipih, T.; Hodge, E.; Stone, E.T.; Warner, N.; McCarthy, N.; Granger, B.; Meade-White, K.; Leventhal, S.; Hatzakis, K.; et al. Clade 2.3.4.4b but not historical clade 1 HA replicating RNA vaccine protects against bovine H5N1 challenge in mice. Nat Commun 2025, 16, 655. [Google Scholar] [CrossRef] [PubMed]
- Alexakis, L.; Buczkowski, H.; Ducatez, M.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Ståhl, K.; Staubach, C.; Svartström, O.; Terregino, C.; et al. Avian influenza overview September-December 2024. Efsa j 2025, 23, e9204. [Google Scholar] [CrossRef]
- Mostafa, A.; Barre, R.S.; Allué-Guardia, A.; Escobedo, R.A.; Shivanna, V.; Rothan, H.; Castro, E.M.; Ma, Y.; Cupic, A.; Jackson, N.; et al. Replication kinetics, pathogenicity and virus-induced cellular responses of cattle-origin influenza A(H5N1) isolates from Texas, United States. Emerg Microbes Infect 2025, 14, 2447614. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-Q.; Hutter, C.R.; Markin, A.; Thomas, M.; Lantz, K.; Killian, M.L.; Janzen, G.M.; Vijendran, S.; Wagle, S.; Inderski, B.; et al. Emergence and interstate spread of highly pathogenic avian influenza A(H5N1) in dairy cattle in the United States. Science 2025, 388, eadq0900. [Google Scholar] [CrossRef]
- Song, H.; Hao, T.; Han, P.; Wang, H.; Zhang, X.; Li, X.; Wang, Y.; Chen, J.; Li, Y.; Jin, X.; et al. Receptor binding, structure, and tissue tropism of cattle-infecting H5N1 avian influenza virus hemagglutinin. Cell 2025, 188, 919–929.e919. [Google Scholar] [CrossRef]
- Imai, M.; Ueki, H.; Ito, M.; Iwatsuki-Horimoto, K.; Kiso, M.; Biswas, A.; Trifkovic, S.; Cook, N.; Halfmann, P.J.; Neumann, G.; et al. Highly pathogenic avian H5N1 influenza A virus replication in ex vivo cultures of bovine mammary gland and teat tissues. Emerg Microbes Infect 2025, 14, 2450029. [Google Scholar] [CrossRef] [PubMed]
- Crossley, B.M.; Miramontes, C.C.; Rejmanek, D.; Gallardo, R.; Pereira, R. In-laboratory inactivation of H5N1 in raw whole milk through milk acidification: Results from a pilot study. J Dairy Sci 2025, 108, 2264–2275. [Google Scholar] [CrossRef]
- Kaiser, F.; Cardenas, S.; Yinda, K.C.; Mukesh, R.K.; Ochwoto, M.; Gallogly, S.; Wickenhagen, A.; Bibby, K.; de Wit, E.; Morris, D.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Stability in Irradiated Raw Milk and Wastewater and on Surfaces, United States. Emerg Infect Dis 2025, 31. [Google Scholar] [CrossRef]
- Le Sage, V.; Campbell, A.J.; Reed, D.S.; Duprex, W.P.; Lakdawala, S.S. Persistence of Influenza H5N1 and H1N1 Viruses in Unpasteurized Milk on Milking Unit Surfaces. Emerg Infect Dis 2024, 30, 1721–1723. [Google Scholar] [CrossRef]
- Schafers, J.; Warren, C.J.; Yang, J.; Zhang, J.; Cole, S.J.; Cooper, J.; Drewek, K.; Kolli, B.R.; McGinn, N.; Qureshi, M.; et al. Pasteurisation temperatures effectively inactivate influenza A viruses in milk. Nat Commun 2025, 16, 1173. [Google Scholar] [CrossRef]
- Falender, R.; Radniecki, T.S.; Kelly, C.; Cieslak, P.; Mickle, D.; Hall, H.; Scholz, R.; Sutton, M. Avian Influenza A(H5) Subtype in Wastewater - Oregon, September 15, 2021-July 11, 2024. MMWR Morb Mortal Wkly Rep 2025, 74, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Kumar, M.; Murugkar, H.V.; Nagarajan, S.; Tosh, C.; Namdeo, P.; Singh, R.; Mishra, S.; Senthilkumar, D.; Singh, V.P.; et al. Highly pathogenic avian influenza (H5N1) infection in crows through ingestion of infected crow carcasses. Microb Pathog 2023, 183, 106330. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.S.; Franson, J.C.; Gill, R.E.; Meteyer, C.U.; TeSlaa, J.L.; Nashold, S.; Dusek, R.J.; Ip, H.S. Experimental challenge and pathology of highly pathogenic avian influenza virus H5N1 in dunlin (Calidris alpina), an intercontinental migrant shorebird species. Influenza Other Respir Viruses 2011, 5, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Takadate, Y.; Tsunekuni, R.; Kumagai, A.; Mine, J.; Kikutani, Y.; Sakuma, S.; Miyazawa, K.; Uchida, Y. Different Infectivity and Transmissibility of H5N8 and H5N1 High Pathogenicity Avian Influenza Viruses Isolated from Chickens in Japan in the 2021/2022 Season. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Vahlenkamp, T.W.; Teifke, J.P.; Harder, T.C.; Beer, M.; Mettenleiter, T.C. Systemic influenza virus H5N1 infection in cats after gastrointestinal exposure. Influenza Other Respir Viruses 2010, 4, 379–386. [Google Scholar] [CrossRef]
- Xu, C.; Dong, L.; Xin, L.; Lan, Y.; Chen, Y.; Yang, L.; Shu, Y. Human avian influenza A (H5N1) virus infection in China. Sci China C Life Sci 2009, 52, 407–411. [Google Scholar] [CrossRef]
- Galli, M.; Giacomelli, A.; Lai, A.; Zehender, G. H5N1 influenza A virus: lessons from past outbreaks and emerging threats. Infez Med 2025, 33, 76–89. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Milton, S.; Abdul Hamid, C.; Reinoso Webb, C.; Presley, S.M.; Shetty, V.; Rollo, S.N.; Martinez, D.L.; Rai, S.; Gonzales, E.R.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection in a Dairy Farm Worker. N Engl J Med 2024, 390, 2028–2029. [Google Scholar] [CrossRef]
- Le Sage, V.; Werner, B.D.; Merrbach, G.A.; Petnuch, S.E.; O’Connell, A.K.; Simmons, H.C.; McCarthy, K.R.; Reed, D.S.; Moncla, L.H.; Bhavsar, D.; et al. Influenza A(H5N1) Immune Response among Ferrets with Influenza A(H1N1)pdm09 Immunity. Emerg Infect Dis 2025, 31, 477–487. [Google Scholar] [CrossRef]
- Leonard, J.; Harker, E.J.; Szablewski, C.M.; Margrey, S.F.; II, K.F.G.; Crossley, K.; Fletcher, E.; McCreavy, C.J.; Weis-Torres, S.; Wang, D.; et al. Seroprevalence of Highly Pathogenic Avian Influenza A(H5) Virus Infections Among Bovine Veterinary Practitioners — United States, September 2024. MMWR Morb Mortal Wkly Rep 2025 2025, 74, 50–52. [Google Scholar] [CrossRef]
- Mahase, E. Bird flu: US reports first human death in person infected with H5N1. Bmj 2025, 388, r28. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.; Coyle, J.; Mikesell, L.; Stoddard, B.; Eckel, S.; Weinberg, M.; Kuo, J.; Riner, D.; Margulieux, K.; Stricklen, J.; et al. Influenza A(H5N1) Virus Infection in Two Dairy Farm Workers in Michigan. N Engl J Med 2024, 391, 963–964. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Penaloza, J.A.; Belser, J.A.; Brock, N.; Kieran, T.J.; Sun, X.; Pappas, C.; Zeng, H.; Carney, P.; Chang, J.; Bradley-Ferrell, B.; et al. Transmission of a human isolate of clade 2.3.4.4b A(H5N1) virus in ferrets. Nature 2024, 636, 705–710. [Google Scholar] [CrossRef] [PubMed]
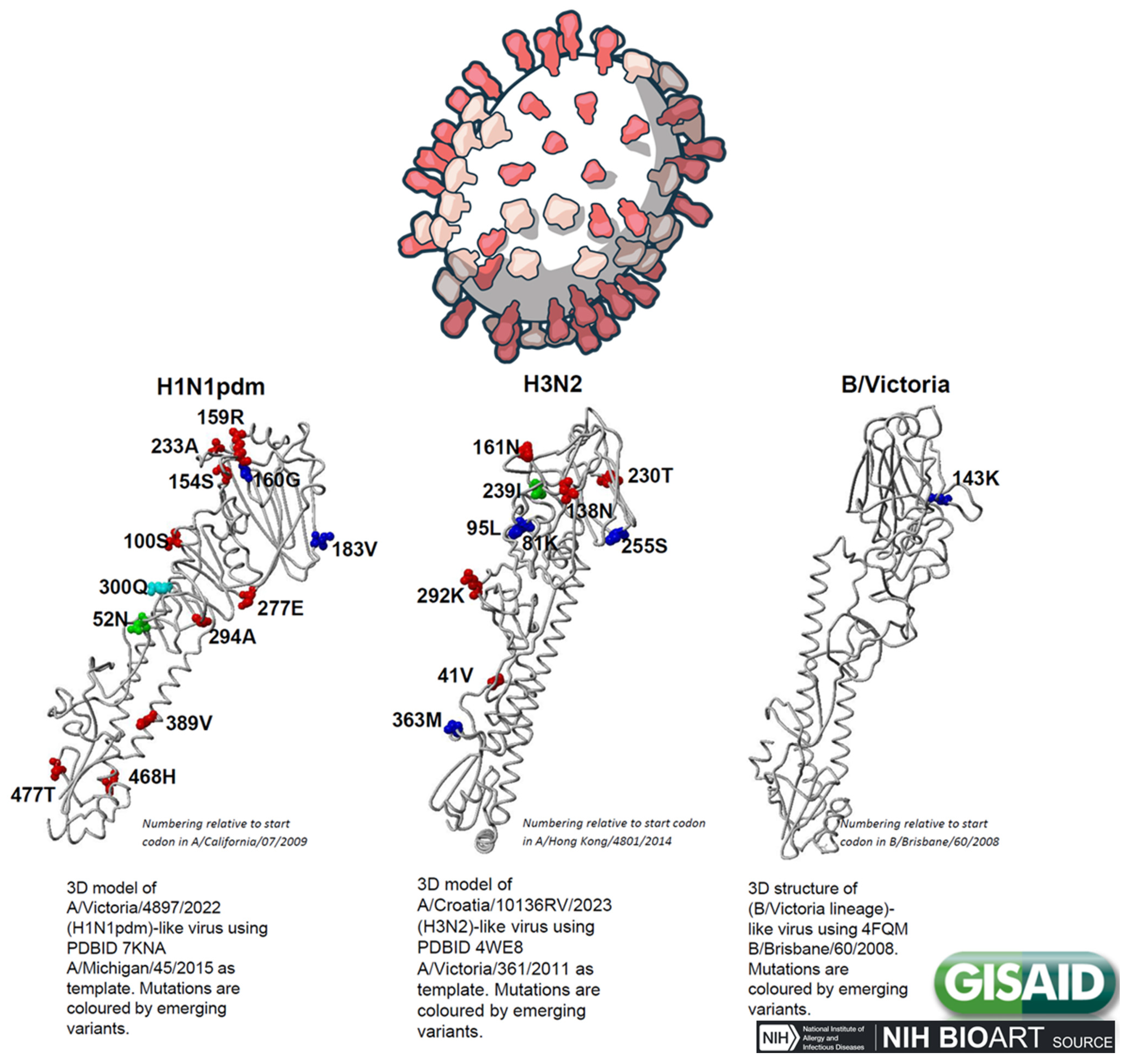

| Country | 2003-2009 | 2010-2014 | 2015-2019 | 2020 | 2021 | 2022 | 2023 | 2024 | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Deaths | Cases | Deaths | Cases | Deaths | Cases | Deaths | Cases | Deaths | Cases | Deaths | Cases | Deaths | Cases | Deaths | Cases | Deaths | |
| Australia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Azerbaijan | 8 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 5 |
| Bangladesh | 1 | 0 | 6 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 1 |
| Cambodia | 9 | 7 | 47 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 4 | 10 | 2 | 72 | 43 |
| Canada | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 |
| Chile | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| China | 38 | 25 | 9 | 5 | 6 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 56 | 32 |
| Djibouti | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Ecuador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Egypt | 90 | 27 | 120 | 50 | 149 | 43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 359 | 120 |
| India | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Indonesia | 162 | 134 | 35 | 31 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 200 | 168 |
| Iraq | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 |
| Lao | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 |
| Myanmar | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Nepal | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Nigeria | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Pakistan | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 |
| Spain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Thailand | 25 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 17 |
| Turkey | 12 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 4 |
| UK | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 5 | 0 |
| USA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 58 | 0 | 59 | 0 |
| Vietnam | 112 | 57 | 15 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 129 | 65 |
| Total | 468 | 282 | 233 | 125 | 160 | 48 | 1 | 0 | 2 | 1 | 6 | 1 | 12 | 4 | 72 | 3 | 954 | 464 |
| Domain | Challenges | Strategies |
|---|---|---|
| Surveillance |
|
|
| Diagnostics |
|
|
| Control |
|
|
| Prevention |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




