Submitted:
13 June 2025
Posted:
16 June 2025
You are already at the latest version
Abstract
Keywords:
- Mechanisms of Epileptogenesis
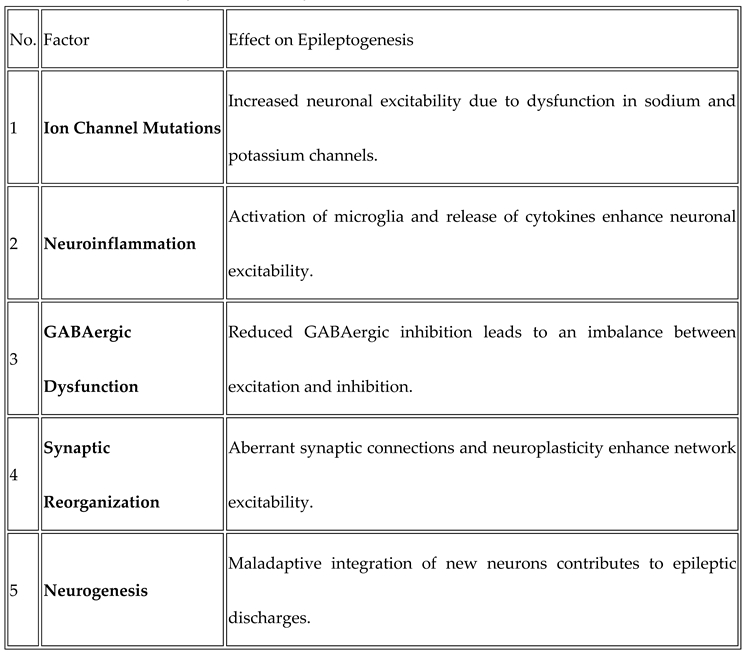
- Synaptic Reorganization and Neuroplasticity: The earliest event in epileptogenesis is synaptic reorganization, which results from an injury or some other triggering factor. Following an insult, the brain undergoes neuroplastic changes and they include both excitatory and inhibitory synaptic transmission. Aberrant excitatory synapse development augments neuronal excitability, along with increased synaptic strength…
- Dysfunction of Ion Channels: Ion channels are essential to controlling the electrical activities of neurons. Abnormal ion channel function can lead to abnormal neuronal firing, which is characteristic of epileptic seizures. Changes in these channels may enhance neuronal excitability, thereby causing epilepsy…
- GABAergic System Dysfunction: The GABAergic system provides the main inhibitory control in the brain and is responsible for maintaining the balance between excitation and inhibition. Lack of effective GABAergic signaling is considered one of the primary factors responsible for initiating epileptic activity…
- Immune Response and Neuroinflammation: There is increasing evidence that the neuroinflammatory processes contribute to the development of epilepsy. Following an injury, infection, or sustained seizures, the brain’s resident immune cells microglia and astrocytes activate and release pro-inflammatory cytokines…
- Cell Death and Neurogenesis: In several types of epilepsy and especially following brain injury, there is neuronal cell death in several important structures, including the hippocampus. However, some regions, such as the dentate gyrus, have the ability to undergo neurogenesis, which is the generation of new neurons in response to injury…
- Overview
- Mechanisms of Epileptogenesis
- 1.
- Synaptic Reseorganization and Neuroplasticity
- One of the earliest alterations in the process of epileptogenesis is the reorganization of synapses, which occurs due to an injury or another initiating factor. When the brain experiences an insult, it neuroplastically adapts both excitatory and inhibitory synaptic circuits. Aberrant activation of synapses can arise from strengthened synaptic pathways, potentially leading to increased neuronal excitability. The abnormal development of synaptic circuitry in specific brain areas, such as the hippocampus, is commonly observed in temporal lobe epilepsy (TLE), one of the most prevalent types of epilepsy. The hippocampal circuits associated with temporal lobe epilepsy ignore the negative feedback signals that regulate and inhibit the system, instead focusing on amplification, which results in random seizures ([1]).
- In particular, long-term potentiation (LTP) of synapses is often observed, and it can become excessively abnormal after a brain injury or other kind of insult, adding to the hyperactivity ([2]). Also, neurogenesis — the formation of new nerve cells within certain brain areas like the hippocampus — may enhance the level of synaptic modifications that occur after a brain injury, involving some New neurons in a way that increases hyperexcitability ([3]).
- 2.
- Ion Channel Dysfunction
- Ion channels have a fundamental function in the regulation of the electrical activity of neurons. Abnormal functioning of these channels leads to abnormal neuronal firing, which is the hallmark of epileptic seizures. The voltage-gated sodium, potassium, and calcium channels have a critical role in the proper functioning and maintenance of neural circuits. Their alteration can lead to increased neuronal excitability and epilepsy.
- For instance, sodium channels SCN1A and SCN2A are known to cause generalized epilepsies through their mutations ([4]). Sodium channel dysfunctions result in decreased action potential propagation and abnormal neuronal firing, which underlie seizure activity. Mutations in repolarization potassium channels can also increase their excitability; thus, hyperexcitability ([5]). In calcium channels, malfunctioning may lead to excessive release of neurotransmitters, exacerbating the pathophysiology of epilepsy ([6]).
- 3.
- GABAergic Dysfunction
- The GABAergic system, which provides the primary inhibitory control in the brain, plays a critical role in maintaining the excitation-inhibition balance. Dysfunction in GABAergic signaling is considered one of the most significant issues in understanding the mechanisms involved in epileptogenesis. Gamma-aminobutyric acid (GABA) binds to GABA receptors, opening chloride channels that generate inhibitory postsynaptic potentials (IPSPs) to inhibit neurons. However, in many epilepsy syndromes, the GABAergic system becomes compromised, leading to reduced inhibition and a relative increase in excitatory signaling.
- Changes in GABA receptor expression and GABA synthesis have been noted in animal models of epilepsy ([7]). Furthermore, the loss of GABAergic interneurons, which control network activity within the brain, leads to a breakdown of inhibitory control, contributing to the development of seizures ([8]). These data indicate that impaired GABAergic function significantly contributes to the processes underlying epileptic conditions.
- 4.
- Neuroinflammation and Immune Response
- The relationship of neuroinflammation with the pathology of epilepsy is increasingly gaining attention. Microglia and astrocytes – the brain’s immune cells – become activated and release pro-inflammatory cytokines, chemokines, and other signaling molecules in response to injury, infection, or prolonged seizures ([9]). These molecules can further aggravate neuronal injury, excitability, and change synaptic plasticity, thereby contributing to the epileptogenic process.
- Chronic activation of glial cells is associated with the progression from brain injury to epilepsy. Inflammatory cytokines like TNF-α, IL-1β, and IL-6 are documented to enhance neuronal excitability and lower the threshold for seizure generation ([10]). Also, the role of blood-brain barrier (BBB) disruption is crucial in this scenario. BBB rupture permits the infiltration of immune cells into the brain parenchyma, which causes more neuroinflammation and supports epileptogenesis ([11]).
- 5.
- Neurogenesis and Cell Death
- In certain types of epilepsy, especially post traumatic epilepsy, there is neuronal cell death in critical areas such as the hippocampus. On the other hand, the dentate gyrus is able to perform neurogenesis, where new neurons are formed in response to injury ([12]). Although neurogenesis may help to some extent by replacing lost neurons, failure to integrate properly into the existing circuitry can be detrimental.
- The inappropriate integration of newly formed neurons has been associated with the onset of temporal lobe epilepsy (TLE). These neurons are theorized to form abnormal connections that promote the spread of epileptic activity ([13]). In addition to this, there is a contribution to excessive neurogenesis in the formation of granule cells, which have been demonstrated to increase epileptic activity ([14]).
- Evidence Synthesis
- Interpretations & Implications
- Conclusion:
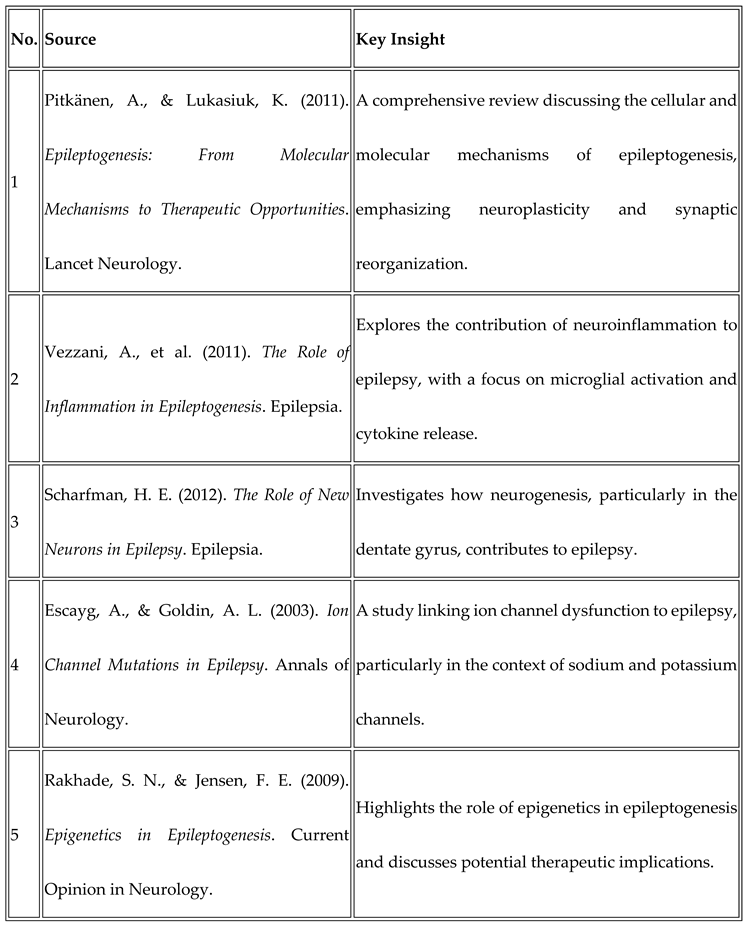
Source Analysis
Factors Contributing to Epileptogenesis:
Funding
NOTE
Conflicts of Interest
Ethical Approval
Data Availability
References
- Pitkänen, A., & Lukasiuk, K. (2011). Epileptogenesis: From Molecular Mechanisms to Therapeutic Opportunities. Lancet Neurology. 2011; 10(1): 33-43.
- Vezzani, A., et al. (2011). The Role of Inflammation in Epileptogenesis. Epilepsia. 2011; 52(6): 1201-1207.
- Scharfman, H. E. (2012). The Role of New Neurons in Epilepsy. Epilepsia. 2012; 53(10): 1675-1686.
- Gouder, A. A., et al. (2017). Neurogenesis and Synaptic Reorganization in Epilepsy. Epilepsia. 2017; 58(5): 763-774.
- Schwaller, B., et al. (2002). Reduced GABA Receptor Expression in Epileptic Animals. Neuroscience. 2002; 118(3): 753-759.
- Hübner, C., et al. (2001). Loss of GABAergic Interneurons in Epileptic Brains. Brain Research. 2001; 914(1): 59-66.
- Biervert, C., et al. (1998). Potassium Channel Mutations in Epileptic Syndromes. Annals of Neurology. 1998; 44(1): 51-56.
- Riazi, K., et al. (2008). Inflammation and Seizures: Cytokine Modulation of Epileptic Brain. Epilepsia. 2008; 49(6): 1005-1012.
- Pekcec, A., et al. (2015). Blood-Brain Barrier Disruption in Epileptic Brain Injury. Brain Research. 2015; 1623: 105-112.
- Parent, J. M., et al. (1997). Neurogenesis in Epileptic Brain. Science. 1997; 275(5302): 1415-1418.
- Jessberger, S., et al. (2007). Aberrant Neurogenesis in Temporal Lobe Epilepsy. Neurobiology of Disease. 2007; 26(3): 628-634.
- Scharfman, H. E. (2012). Abnormal Neurogenesis and Epilepsy. Frontiers in Neurology. 2012; 3: 32.
- Vezzani, A., et al. (2011). Neuroinflammation in Epileptogenesis. Epilepsia. 2011; 52(7): 1094-1101.
- Escayg, A., & Goldin, A. L. (2003). Ion Channel Mutations in Epilepsy. Annals of Neurology. 2003; 54(2): 233-248.
- Ficker, D. M., et al. (2010). Ion Channelopathies in Epileptic Syndromes. Journal of Neurology. 2010; 257(7): 1055-1061.
- McNamara, J. O. (2006). Epilepsy: A Disorder of Brain Excitability. The Lancet Neurology. 2006; 5(5): 309-316.
- Wilke, C. A., et al. (2016). GABAergic Dysfunction in Epileptogenesis: A Therapeutic Target. Neurochemistry International. 2016; 99: 105-110.
- Sahoo, S. K., et al. (2014). Mechanisms of GABAergic Dysfunction in Epileptic Syndromes. Neuroscientific Review. 2014; 23(1): 62-70.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



