Submitted:
12 June 2025
Posted:
16 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
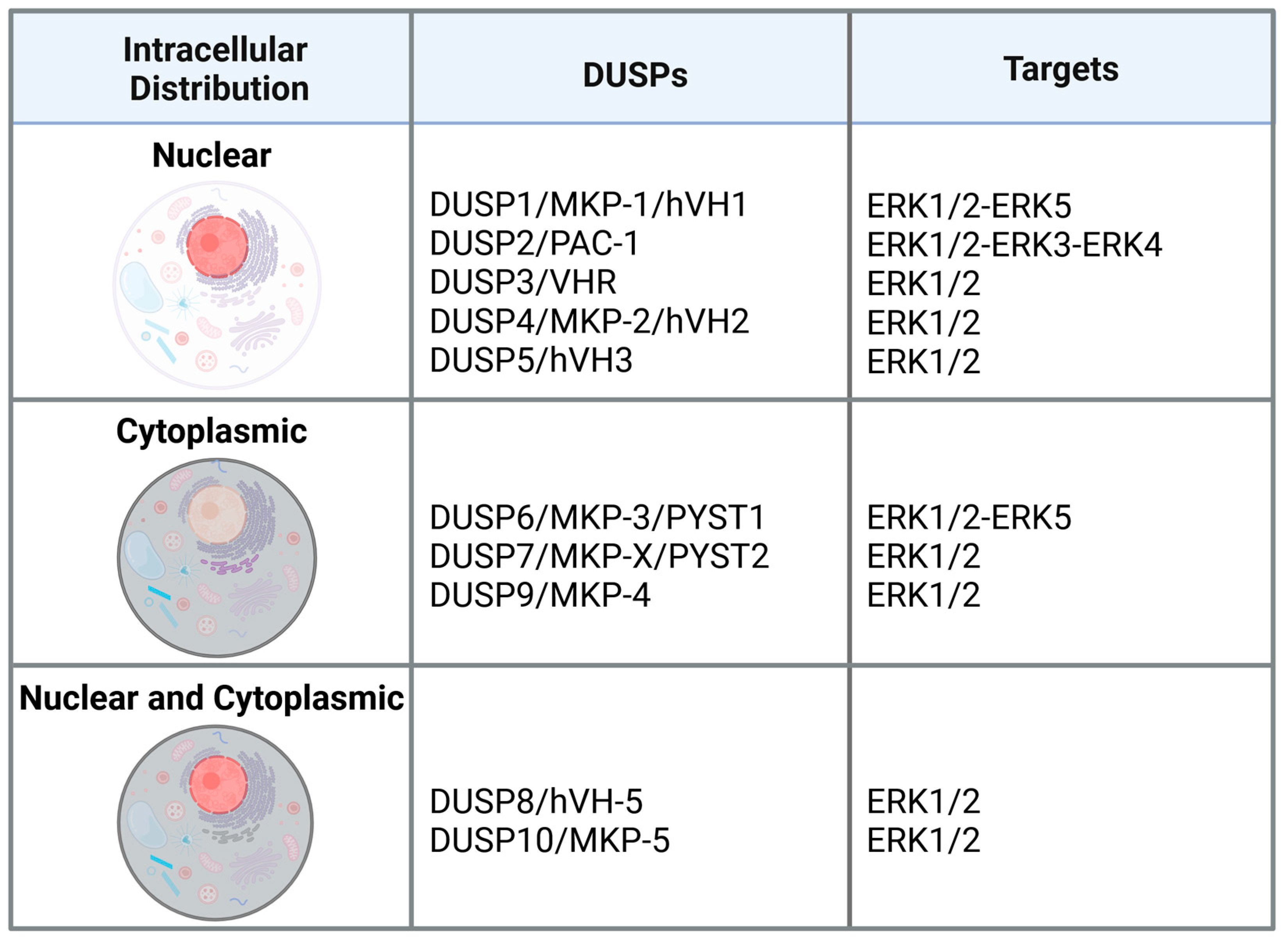
2. Role of ERK-Targeting DUSPs in Cancer
2.1. DUSP1
2.2. DUSP2
2.3. DUSP3
2.4. DUSP4
2.5. DUSP5
2.6. DUSP6
2.7. DUSP7
2.8. DUSP9
2.9. DUSP10
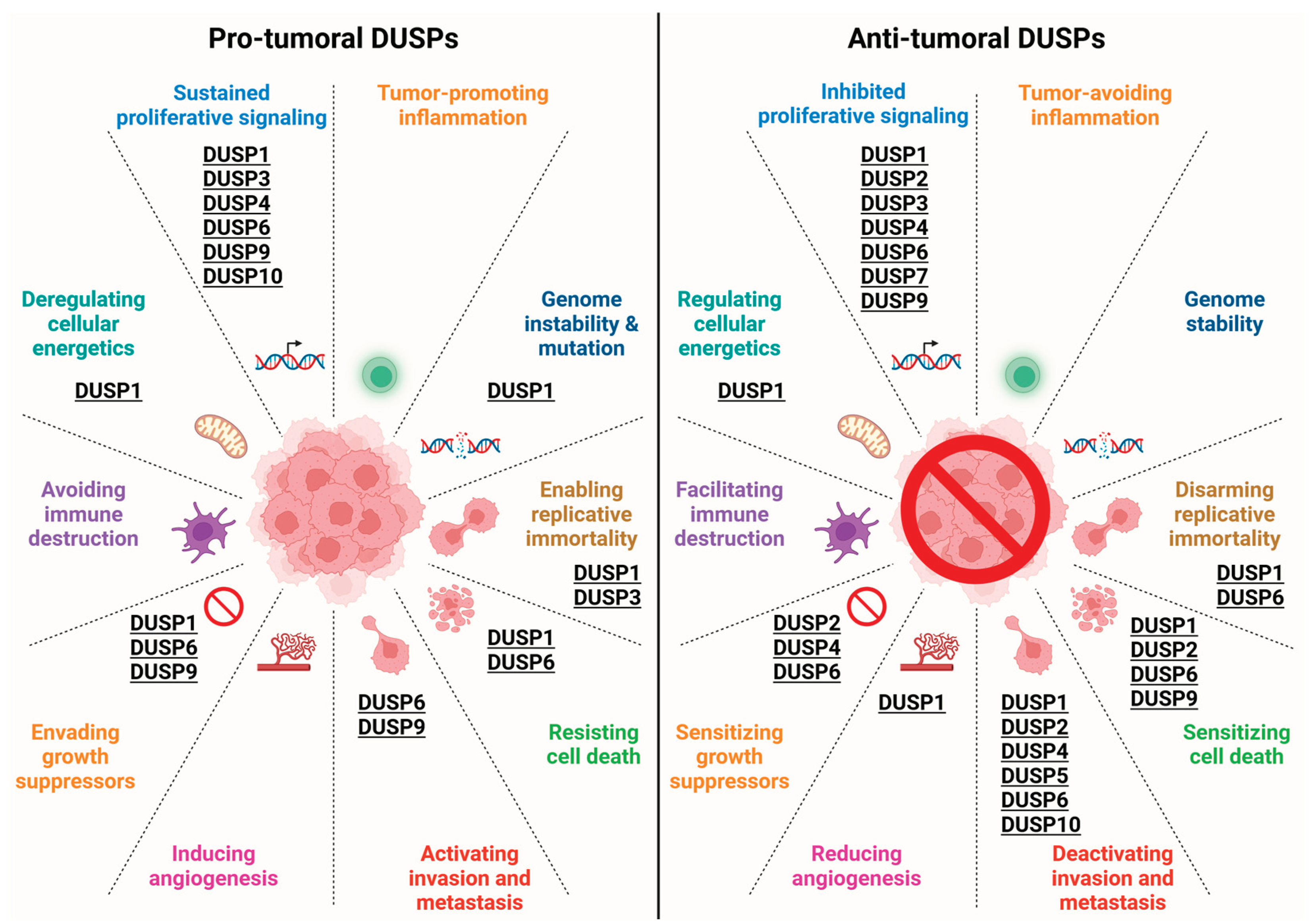
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2012, 76, 496. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-Activated Protein Kinase Pathways Mediated by ERK, JNK, and p38 Protein Kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Camps, M.; Nichols, A.; Arkinstall, S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000, 14, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Tan, T.-H. DUSPs, to MAP kinases and beyond. Cell Biosci. 2012, 2, 24. [Google Scholar] [CrossRef]
- Chen, H.-F.; Chuang, H.-C.; Tan, T.-H. Regulation of Dual-Specificity Phosphatase (DUSP) Ubiquitination and Protein Stability. Int. J. Mol. Sci. 2019, 20, 2668. [Google Scholar] [CrossRef]
- Kidger, A.M.; Keyse, S.M. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs). Semin. Cell Dev. Biol. 2016, 50, 125–132. [Google Scholar] [CrossRef]
- Liao, Q.; Guo, J.; Kleeff, J.; Zimmermann, A.; Büchler, M.W.; Korc, M.; Friess, H. Down-regulation of the dual-specificity phosphatase MKP-1 suppresses tumorigenicity of pancreatic cancer cells. Gastroenterology 2003, 124, 1830–1845. [Google Scholar] [CrossRef]
- Teng, F.; Xu, Z.; Chen, J.; Zheng, G.; Zheng, G.; Lv, H.; Wang, Y.; Wang, L.; Cheng, X. DUSP1 induces apatinib resistance by activating the MAPK pathway in gastric cancer. Oncol. Rep. 2018, 40, 1203–1222. [Google Scholar] [CrossRef]
- Chi, H.; Barry, S.P.; Roth, R.J.; Wu, J.J.; Jones, E.A.; Bennett, A.M.; Flavell, R.A. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl. Acad. Sci. 2006, 103, 2274–2279. [Google Scholar] [CrossRef]
- Liu, Y.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases--regulating the immune response. Nat Rev Immunol. 2007, 7, 202–12. [Google Scholar] [CrossRef]
- Boutros, T.; Chevet, E.; Metrakos, P. Mitogen-Activated Protein (MAP) Kinase/MAP Kinase Phosphatase Regulation: Roles in Cell Growth, Death, and Cancer. Pharmacol. Rev. 2008, 60, 261–310. [Google Scholar] [CrossRef] [PubMed]
- Loda, M.; Capodieci, P.; Mishra, R.; Yao, H.; Corless, C.; Grigioni, W.; Wang, Y.; Magi-Galluzzi, C.; Stork, P.J. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. . 1996, 149, 1553–64. [Google Scholar] [PubMed]
- Rojo, F.; González-Navarrete, I.; Bragado, R.; Dalmases, A.; Menéndez, S.; Cortes-Sempere, M.; Suárez, C.; Oliva, C.; Servitja, S.; Rodriguez-Fanjul, V.; et al. Mitogen-Activated Protein Kinase Phosphatase-1 in Human Breast Cancer Independently Predicts Prognosis and Is Repressed by Doxorubicin. Clin. Cancer Res. 2009, 15, 3530–3539. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Czerwenka, K.; Heinze, G.; Ryffel, M.; Schuster, E.; Witt, A.; Leodolter, S.; Zeillinger, R. Expression of KLF5 is a Prognostic Factor for Disease-Free Survival and Overall Survival in Patients with Breast Cancer. Clin. Cancer Res. 2006, 12, 2442–2448. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, H.-Q.; Zhou, Z.; Dong, J.-T.; Chen, C. KLF5 Promotes Breast Cell Survival Partially through Fibroblast Growth Factor-binding Protein 1-pERK-mediated Dual Specificity MKP-1 Protein Phosphorylation and Stabilization. J. Biol. Chem. 2009, 284, 16791–16798. [Google Scholar] [CrossRef]
- Liu, D.; Deng, Q.; Sun, L.; Wang, T.; Yang, Z.; Chen, H.; Guo, L.; Liu, Y.; Ma, Y.; Guo, N.; et al. A Her2-let-7-β2-AR circuit affects prognosis in patients with Her2-positive breast cancer. BMC Cancer 2015, 15, 832. [Google Scholar] [CrossRef]
- Molina, G.; Vogt, A.; Bakan, A.; Dai, W.; de Oliveira, P.Q.; Znosko, W.; E Smithgall, T.; Bahar, I.; Lazo, J.S.; Day, B.W.; et al. Zebrafish chemical screening reveals an inhibitor of Dusp6 that expands cardiac cell lineages. Nat. Chem. Biol. 2009, 5, 680–687. [Google Scholar] [CrossRef]
- Tuglu, M.M.; Bostanabad, S.Y.; Ozyon, G.; Dalkiliç, B.; Gurdal, H. The role of dual-specificity phosphatase 1 and protein phosphatase 1 in β2-adrenergic receptor-mediated inhibition of extracellular signal regulated kinase 1/2 in triple negative breast cancer cell lines. Mol Med Rep. 2018, 17, 2033–2043. [Google Scholar] [CrossRef]
- Kaltenmeier, C.T.; Vollmer, L.L.; Vernetti, L.A.; Caprio, L.; Davis, K.; Korotchenko, V.N.; Day, B.W.; Tsang, M.; Hulkower, K.I.; Lotze, M.T.; et al. A Tumor Cell-Selective Inhibitor of Mitogen-Activated Protein Kinase Phosphatases Sensitizes Breast Cancer Cells to Lymphokine-Activated Killer Cell Activity. J. Pharmacol. Exp. Ther. 2017, 361, 39–50. [Google Scholar] [CrossRef]
- Kim, M.-J.; Park, I.-J.; Yun, H.; Kang, I.; Choe, W.; Kim, S.-S.; Ha, J. AMP-activated Protein Kinase Antagonizes Pro-apoptotic Extracellular Signal-regulated Kinase Activation by Inducing Dual-specificity Protein Phosphatases in Response to Glucose Deprivation in HCT116 Carcinoma. 2010, 285, 14617–14627. [CrossRef]
- Venditto, V.J.; Simanek, E.E. Cancer Therapies Utilizing the Camptothecins: A Review of thein VivoLiterature. Mol. Pharm. 2010, 7, 307–349. [Google Scholar] [CrossRef]
- Rovida, E.; Stecca, B. Mitogen-activated protein kinases and Hedgehog-GLI signaling in cancer: A crosstalk providing therapeutic opportunities? Semin. Cancer Biol. 2015, 35, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Lee, M. ; Young, Kim, S. ; Kim, J.; Kim, H.S.; Kim, S.M.; Kim, E.J. Mitogen-activated protein kinase phosphatase-1 inhibition and sustained extracellular signal-regulated kinase 1/2 activation in camptothecin-induced human colon cancer cell death. Cancer Biol Ther. 2013, 14, 1007–15. [Google Scholar]
- Vogt, A.; McDonald, P.R.; Tamewitz, A.; Sikorski, R.P.; Wipf, P.; Skoko, J.J., 3rd; Lazo, J.S. A cell-active inhibitor of mitogen-activated protein kinase phosphatases restores paclitaxel-induced apoptosis in dexamethasone-protected cancer cells. Mol. Cancer Ther. 2008, 7, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.K.; Peng, B.Y.; Lin, C.M.; Wang, P.D.; Wang, J.R.; Chan, C.H.; Wei, H.J.; Deng, W.P. NSC 95397 Suppresses Proliferation and Induces Apoptosis in Colon Cancer Cells through MKP-1 and the ERK1/2 Pathway. Int J Mol Sci. 2018, 19, 1625. [Google Scholar] [CrossRef]
- Pan, J.; Lin, M.; Xu, Z.; Xu, M.; Zhang, J.; Weng, Z.; Lin, B.; Lin, X. CDKN2B antisense RNA 1 suppresses tumor growth in human colorectal cancer by targeting MAPK inactivator dual-specificity phosphatase 1. Carcinog. 2021, 42, 1399–1409. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Chaney, K.E.; Milewski, D.; Williams, K.B.; Williams, R.L.; Choi, K.; Miller, A.; Kalin, T.V.; Pressey, J.G.; Szabo, S.; et al. Targeted Inhibition of the Dual Specificity Phosphatases DUSP1 and DUSP6 Suppress MPNST Growth via JNK. Clin. Cancer Res. 2019, 25, 4117–4127. [Google Scholar] [CrossRef]
- He, W.; Liu, P.; Lei, Q.; Xu, J.; Liu, L. DUSP1 Promotes Osimertinib Drug-Tolerant Persistence by Inhibiting MAPK/ERK Signaling in Non-small Cell Lung Cancer. Mol. Biotechnol. 2024, 67, 1256–1268. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Zhou, J.-Y.; Liu, Y.; Wu, G.S. Mitogen-Activated Protein Kinase Phosphatase-1 Is Required for Cisplatin Resistance. Cancer Res. 2006, 66, 8870–8877. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.-Y.; Wu, G.S. ERK-Dependent MKP-1–Mediated Cisplatin Resistance in Human Ovarian Cancer Cells. Cancer Res. 2007, 67, 11933–11941. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.Y.; Kho, D.; Reiners, J.J. Jr.; Wu, G.S. Role for DUSP1 (dual-specificity protein phosphatase 1) in the regulation of autophagy. Autophagy. 2016, 12, 1791–1803. [Google Scholar] [CrossRef]
- Ma, M.; Wang, C.; Wu, M.; Gu, S.; Yang, J.; Zhang, Y.; Cheng, S.; Xu, S.; Zhang, M.; Wu, Y.; et al. CSGALNACT2 restricts ovarian cancer migration and invasion by modulating MAPK/ERK pathway through DUSP1. Cell. Oncol. 2023, 47, 897–915. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A.; Platt, F.M.; Ross, R.L.; Hurst, C.D. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009, 28, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Sathe, A.; Guerth, F.; Cronauer, M.V.; Heck, M.M.; Thalgott, M.; Gschwend, J.E.; Retz, M.; Nawroth, R. Mutant PIK3CA controls DUSP1-dependent ERK 1/2 activity to confer response to AKT target therapy. Br J Cancer. 2014, 111, 2103–13. [Google Scholar] [CrossRef] [PubMed]
- Pervin, S.; Singh, R.; A Freije, W.; Chaudhuri, G. MKP-1-induced dephosphorylation of extracellular signal-regulated kinase is essential for triggering nitric oxide-induced apoptosis in human breast cancer cell lines: implications in breast cancer. . 2003, 63, 8853–60. [Google Scholar]
- Wang, K.; Zhang, M.; Qian, Y.-Y.; Ding, Z.-Y.; Lv, J.-H.; Shen, H.-H. Imbalanced expression of mitogen-activated protein kinase phosphatase-1 and phosphorylated extracellular signal-regulated kinases in lung squamous cell carcinoma. J. Zhejiang Univ. B 2011, 12, 828–834. [Google Scholar] [CrossRef]
- Maraver, A.; Fernandez-Marcos, P.J.; Herranz, D.; Cañamero, M.; Muñoz-Martin, M.; Gómez-López, G.; Mulero, F.; Megías, D.; Sanchez-Carbayo, M.; Shen, J.; et al. Therapeutic Effect of γ-Secretase Inhibition in KrasG12V-Driven Non-Small Cell Lung Carcinoma by Derepression of DUSP1 and Inhibition of ERK. Cancer Cell 2012, 22, 222–234. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Q.; Cheng, Z.; Gu, J.; Feng, W.; Lei, T.; Huang, J.; Pu, J.; Chen, X.; Wang, Z. Long non-coding RNA CASC9 promotes gefitinib resistance in NSCLC by epigenetic repression of DUSP1. Cell Death Dis. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Liu, C.; Shi, Y.; Han, Z.; Pan, Y.; Liu, N.; Han, S.; Chen, Y.; Lan, M.; Qiao, T.; Fan, D. Suppression of the dual-specificity phosphatase MKP-1 enhances HIF-1 trans-activation and increases expression of EPO. Biochem. Biophys. Res. Commun. 2003, 312, 780–786. [Google Scholar] [CrossRef]
- Liu, C.; Shi, Y.; Du, Y.; Ning, X.; Liu, N.; Huang, D.; Liang, J.; Xue, Y.; Fan, D. Dual-specificity phosphatase DUSP1 protects overactivation of hypoxia-inducible factor 1 through inactivating ERK MAPK. Exp. Cell Res. 2005, 309, 410–418. [Google Scholar] [CrossRef]
- Calvisi, D.F.; Pinna, F.; Meloni, F.; Ladu, S.; Pellegrino, R.; Sini, M.; Daino, L.; Simile, M.M.; De Miglio, M.R.; Virdis, P.; Frau, M.; Tomasi, M.L.; Seddaiu, M.A.; Muroni, M.R.; Feo, F.; Pascale, R.M. Dual-specificity phosphatase 1 ubiquitination in extracellular signal-regulated kinase-mediated control of growth in human hepatocellular carcinoma. Cancer Res. 2008, 68, 4192–200. [Google Scholar] [CrossRef]
- Kato, H.; Naiki-Ito, A.; Naiki, T.; Suzuki, S.; Yamashita, Y.; Sato, S.; Sagawa, H.; Kato, A.; Kuno, T.; Takahashi, S. Connexin 32 dysfunction promotes ethanol-related hepatocarcinogenesis via activation of Dusp1-Erk axis. Oncotarget 2015, 7, 2009–2021. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Zhou, H.; Liang, H.; Li, C. Regulation of ERK and AKT pathways by hepatitis B virus X protein via the Notch1 pathway in hepatocellular carcinoma. Int. J. Oncol. 2017, 51, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, J.-Y.; Zhao, L.-J.; Gao, B.-R.; Wan, X.-P.; Wang, J.-L. Dual-specificity Phosphatase 1 Deficiency Induces Endometrioid Adenocarcinoma Progression via Activation of Mitogen-activated Protein Kinase/Extracellular Signal-regulated Kinase Pathway. Chin. Med J. 2016, 129, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Rauhala, H.E.; Porkka, K.P.; Tolonen, T.T.; Martikainen, P.M.; Tammela, T.L.; Visakorpi, T. Dual-specificity phosphatase 1 and serum/glucocorticoid-regulated kinase are downregulated in prostate cancer. Int. J. Cancer 2005, 117, 738–745. [Google Scholar] [CrossRef]
- Gil-Araujo, B.; Lobo, M.-V.T.; Gutiérrez-Salmerón, M.; Gutiérrez-Pitalúa, J.; Ropero, S.; Angulo, J.C.; Chiloeches, A.; Lasa, M. Dual specificity phosphatase 1 expression inversely correlates with NF-κB activity and expression in prostate cancer and promotes apoptosis through a p38 MAPK dependent mechanism. Mol. Oncol. 2013, 8, 27–38. [Google Scholar] [CrossRef]
- Magi-Galluzzi, C.; Montironi, R.; Cangi, M.G.; Wishnow, K.; Loda, M. Mitogen-activated protein kinases and apoptosis in PIN. Virchows Arch. 1998, 432, 407–413. [Google Scholar] [CrossRef]
- Martínez-Martínez, D.; Toledo Lobo, M.-V.; Baquero, P.; Ropero, S.; Angulo, J.C.; Chiloeches, A.; Lasa, M. Downregulation of Snail by DUSP1 Impairs Cell Migration and Invasion through the Inactivation of JNK and ERK and Is Useful as a Predictive Factor in the Prognosis of Prostate Cancer. Cancers 2021, 13, 1158. [Google Scholar] [CrossRef]
- Ariano, C.; Costanza, F.; Akman, M.; Riganti, C.; Corà, D.; Casanova, E.; Astanina, E.; Comunanza, V.; Bussolino, F.; Doronzo, G. TFEB inhibition induces melanoma shut-down by blocking the cell cycle and rewiring metabolism. Cell Death Dis. 2023, 14, 1–21. [Google Scholar] [CrossRef]
- Wang, J.; Jia, Q.; Sun, J.; Wu, S.; Wei, L.; Yao, W. Arntl-induced upregulation of DUSP1 inhibits tumor progression in esophageal squamous cell carcinoma by inactivating ERK signaling. Cancer Biol. Ther. 2024, 25, 2408042. [Google Scholar] [CrossRef]
- Cheng, P.; Zhu, S.; Jun, L.; Huang, L.; Hong, Y. Production of DUSP1 protein using the baculovirus insect cell expression system and its in vitro effects on cancer cells. Int. J. Mol. Med. 2015, 35, 1715–1719. [Google Scholar] [CrossRef]
- Rohan, P.J.; Davis, P.; Moskaluk, C.A.; Kearns, M.; Krutzsch, H.; Siebenlist, U.; Kelly, K. PAC-1: a Mitogen-Induced Nuclear Protein Tyrosine Phosphatase. Science 1993, 259, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Hammer, M.; Mages, J. DUSP Meet Immunology: Dual Specificity MAPK Phosphatases in Control of the Inflammatory Response. J. Immunol. 2006, 177, 7497–7504. [Google Scholar] [CrossRef] [PubMed]
- Perander, M.; Al-Mahdi, R.; Jensen, T.C.; Nunn, J.A.L.; Kildalsen, H.; Johansen, B.; Gabrielsen, M.; Keyse, S.M.; Seternes, O.-M. Regulation of atypical MAP kinases ERK3 and ERK4 by the phosphatase DUSP2. Sci. Rep. 2017, 7, srep43471. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-W.; Lee, J.-C.; Yeh, Y.-C.; Hsu, K.-F.; Lai, Y.-Y.; Lin, S.-C.; Tsai, S.-J. Suppression of dual-specificity phosphatase–2 by hypoxia increases chemoresistance and malignancy in human cancer cells. J. Clin. Investig. 2011, 121, 1905–1916. [Google Scholar] [CrossRef]
- Karakashev, S.V.; Reginato, M.J. Hypoxia/HIF1α induces lapatinib resistance in ERBB2-positive breast cancer cells via regulation of DUSP2. Oncotarget 2015, 6, 1967–1980. [Google Scholar] [CrossRef]
- Hu, J.; Li, L.; Chen, H.; Zhang, G.; Liu, H.; Kong, R.; Chen, H.; Wang, Y.; Li, Y.; Tian, F.; et al. MiR-361-3p regulates ERK1/2-induced EMT via DUSP2 mRNA degradation in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, S.; Tan, Y.; Pan, S.; An, W.; Chen, Q.; Wang, X.; Xu, H. The SKA3-DUSP2 Axis Promotes Gastric Cancer Tumorigenesis and Epithelial-Mesenchymal Transition by Activating the MAPK/ERK Pathway. Front. Pharmacol. 2022, 13, 777612. [Google Scholar] [CrossRef]
- Zou, F.; Rao, T.; Chen, W.; Song, T.; Li, T.; Hu, W.; Li, L.; Yu, W.; Cheng, F. DUSP2 affects bladder cancer prognosis by down-regulating MEK/ERK and P38 MAPK signaling pathways through PTPN7. Cell. Signal. 2023, 112, 110893. [Google Scholar] [CrossRef]
- Todd, J.L.; Tanner, K.G.; Denu, J.M. Extracellular Regulated Kinases (ERK) 1 and ERK2 Are Authentic Substrates for the Dual-specificity Protein-tyrosine Phosphatase VHR. J. Biol. Chem. 1999, 274, 13271–13280. [Google Scholar] [CrossRef]
- Alonso, A.; Saxena, M.; Williams, S.; Mustelin, T. Inhibitory Role for Dual Specificity Phosphatase VHR in T Cell Antigen Receptor and CD28-induced Erk and Jnk Activation. J. Biol. Chem. 2001, 276, 4766–4771. [Google Scholar] [CrossRef]
- Rahmouni, S.; Cerignoli, F.; Alonso, A.; Tsutji, T.; Henkens, R.; Zhu, C.; Louis-dit-Sully, C.; Moutschen, M.; Jiang, W.; Mustelin, T. Loss of the VHR dual-specific phosphatase causes cell-cycle arrest and senescence. Nat Cell Biol. 2006, 8, 524–31. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.W.; Alam, H.; Dhar, S.S.; Giri, U.; Li, N.; Wei, Y.; Giri, D.; Cascone, T.; Kim, J.H.; Ye, Y.; Multani, A.S.; Chan, C.H.; Erez, B.; Saigal, B.; Chung, J.; Lin, H.K.; Wu, X.; Hung, M.C.; Heymach, J.V.; Lee, M.G. KDM2A promotes lung tumorigenesis by epigenetically enhancing ERK1/2 signaling. J Clin Invest. 2013, 123, 5231–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, C.; Wang, W.; Liu, Y.; He, H.; Chen, C.; Xiang, R.; Luo, Y.; Coleman, W.B. Identification of serum miR-1915-3p and miR-455-3p as biomarkers for breast cancer. PLOS ONE 2018, 13, e0200716. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Kabeer, S.W.; Sharma, S.; Tikoo, K. l-Methionine potentiates anticancer activity of Sorafenib by epigenetically altering DUSP3/ERK pathway in hepatocellular carcinoma. J Biochem Mol Toxicol. 2024, 38, e23663. [Google Scholar] [CrossRef]
- Keyse, S.M. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008, 27, 253–261. [Google Scholar] [CrossRef]
- Yip-Schneider, M.T.; Lin, A.; Marshall, M.S. Pancreatic Tumor Cells with Mutant K-ras Suppress ERK Activity by MEK-Dependent Induction of MAP Kinase Phosphatase-2. Biochem. Biophys. Res. Commun. 2001, 280, 992–997. [Google Scholar] [CrossRef]
- Hijiya, N.; Tsukamoto, Y.; Nakada, C.; Tung, Nguyen, L. ; Kai, T. ; Matsuura, K.; Shibata, K.; Inomata, M.; Uchida, T.; Tokunaga, A.; Amada, K.; Shirao, K.; Yamada, Y.; Mori, H.; Takeuchi, I.; Seto, M.; Aoki, M.; Takekawa, M.; Moriyama, M. Genomic Loss of DUSP4 Contributes to the Progression of Intraepithelial Neoplasm of Pancreas to Invasive Carcinoma. Cancer Res. 2016, 76, 2612–25. [Google Scholar]
- Li, K.-C.; Hua, K.-T.; Lin, Y.-S.; Su, C.-Y.; Ko, J.-Y.; Hsiao, M.; Kuo, M.-L.; Tan, C.-T. Inhibition of G9a induces DUSP4-dependent autophagic cell death in head and neck squamous cell carcinoma. Mol. Cancer 2014, 13, 172–172. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, G.; Zhang, P.F.; Zhang, J.; Huang, Y.X.; Lu, Y.M.; Da, W. ; Sun,Q. ; Zhu, J.S. Sanguinarine inhibits growth and invasion of gastric cancer cells via regulation of the DUSP4/ERK pathway. J Cell Mol Med. 2017, 21, 1117–1127. [Google Scholar]
- Balko, J.M.; Cook, R.S.; Vaught, D.B.; Kuba, M.G.; Miller, T.W.; E Bhola, N.; E Sanders, M.; Granja-Ingram, N.M.; Smith, J.J.; Meszoely, I.M.; et al. Profiling of residual breast cancers after neoadjuvant chemotherapy identifies DUSP4 deficiency as a mechanism of drug resistance. Nat. Med. 2012, 18, 1052–1059. [Google Scholar] [CrossRef]
- Balko, J.M.; Schwarz, L.J.; Bhola, N.E.; Kurupi, R.; Owens, P.; Miller, T.W.; Gómez, H.; Cook, R.S.; Arteaga, C.L. Activation of MAPK pathways due to DUSP4 loss promotes cancer stem cell-like phenotypes in basal-like breast cancer. Cancer Res. 2013, 73, :6346–58. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.A.; de Barros, A.L.B.; Leite, E.A. The potential use of simvastatin for cancer treatment: A review. Biomed. Pharmacother. 2021, 141, 111858. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H.; Lee, S.-H.; Kim, J.-Y.; Ahn, J.S.; Park, Y.H.; Im, Y.-H. Statins affect ETS1-overexpressing triple-negative breast cancer cells by restoring DUSP4 deficiency. Sci. Rep. 2016, 6, 33035. [Google Scholar] [CrossRef] [PubMed]
- Gaggianesi, M.; Turdo, A.; Chinnici, A.; Lipari, E.; Apuzzo, T.; Benfante, A.; Sperduti, I.; Di Franco, S.; Meraviglia, S.; Presti, E.L.; et al. IL4 Primes the Dynamics of Breast Cancer Progression via DUSP4 Inhibition. Cancer Res. 2017, 77, 3268–3279. [Google Scholar] [CrossRef]
- Haagenson, K.K.; Zhang, J.W.; Xu, Z.; Shekhar, M.P.; Wu, G.S. Erratum: Functional analysis of MKP-1 and MKP-2 in breast cancer tamoxifen sensitivity. Oncotarget 2018, 9, 35286–35286. [Google Scholar] [CrossRef]
- Klemm, S.; Evert, K.; Utpatel, K.; Muggli, A.; Simile, M.M.; Chen, X.; Evert, M.; Calvisi, D.F.; Scheiter, A. Identification of DUSP4/6 overexpression as a potential rheostat to NRAS-induced hepatocarcinogenesis. BMC Cancer. 2023, 23, 1086. [Google Scholar] [CrossRef]
- Cagnol, S.; Rivard, N. Oncogenic KRAS and BRAF activation of the MEK/ERK signaling pathway promotes expression of dual-specificity phosphatase 4 (DUSP4/MKP2) resulting in nuclear ERK1/2 inhibition. Oncogene 2012, 32, 564–576. [Google Scholar] [CrossRef]
- Ishibashi, T.; Bottaro, D.; Michieli, P.; A Kelley, C.; A Aaronson, S. A novel dual specificity phosphatase induced by serum stimulation and heat shock. J. Biol. Chem. 1994, 269, 29897–29902. [Google Scholar] [CrossRef]
- Patterson, K.I.; Brummer, T.; O'Brien, P.M.; Daly, R.J. Dual-specificity phosphatases: Critical regulators with diverse cellular targets. Biochem. J. 2009, 418, 475–489. [Google Scholar] [CrossRef]
- Mandl, M.; Slack, D.N.; Keyse, S.M. Specific Inactivation and Nuclear Anchoring of Extracellular Signal-Regulated Kinase 2 by the Inducible Dual-Specificity Protein Phosphatase DUSP5. Mol. Cell. Biol. 2005, 25, 1830–1845. [Google Scholar] [CrossRef]
- Shin, S.-H.; Park, S.-Y.; Kang, G.H. Down-Regulation of Dual-Specificity Phosphatase 5 in Gastric Cancer by Promoter CpG Island Hypermethylation and Its Potential Role in Carcinogenesis. Am. J. Pathol. 2013, 182, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, L.K.; Kidger, A.M.; Delavaine, L.; Stewart, G.; van Schelven, S.; Davidson, J.; Bryant, C.J.; Caddye, E.; East, P.; Caunt, C.J.; et al. Dual-specificity phosphatase 5 regulates nuclear ERK activity and suppresses skin cancer by inhibiting mutant Harvey-Ras (HRas Q61L )-driven SerpinB2 expression. Proc. Natl. Acad. Sci. 2014, 111, 18267–18272. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Chen, J.-Y.; Han, Z.-D.; He, H.-C.; Chen, J.-H.; Chen, Y.-R.; Yang, S.-B.; Wu, Y.-D.; Zeng, Y.-R.; Zou, J.; et al. Down-regulation of dual-specificity phosphatase 5 predicts poor prognosis of patients with prostate cancer. Int J Clin Exp Med. 2015, 8, 4186–94. [Google Scholar] [PubMed]
- Tögel, L.; Nightingale, R.; Wu, R.; Chüeh, A.C.; Al-Obaidi, S.; Luk, I.; Dávalos-Salas, M.; Chionh, F.; Murone, C.; Buchanan, D.D.; et al. DUSP5 is methylated in CIMP-high colorectal cancer but is not a major regulator of intestinal cell proliferation and tumorigenesis. Sci. Rep. 2018, 8, 1767. [Google Scholar] [CrossRef]
- Boonruang, K.; Kim, I.; Kwag, C.; Ryu, J.; Baek, S.J. Quercetin induces dual specificity phosphatase 5 via serum response factor. BMB Rep. 2023, 56, 508–513. [Google Scholar] [CrossRef]
- Wang, R.; Bao, H.; Du, W.; Chen, X.; Liu, H.; Han, D.; Wang, L.; Wu, J.; Wang, C.; Yang, M.; et al. P68 RNA helicase promotes invasion of glioma cells through negatively regulating DUSP5. Cancer Sci. 2019, 110, 107–117. [Google Scholar] [CrossRef]
- Wang, R.; Jiao, Z.; Li, R.; Yue, H.; Chen, L. p68 RNA helicase promotes glioma cell proliferation in vitro and in vivo via direct regulation of NF- B transcription factor p50. Neuro-Oncology 2012, 14, 1116–1124. [Google Scholar] [CrossRef]
- Nokin, M.-J.; Bellier, J.; Durieux, F.; Peulen, O.; Rademaker, G.; Gabriel, M.; Monseur, C.; Charloteaux, B.; Verbeke, L.; van Laere, S.; et al. Methylglyoxal, a glycolysis metabolite, triggers metastasis through MEK/ERK/SMAD1 pathway activation in breast cancer. Breast Cancer Res. 2019, 21, 1–19. [Google Scholar] [CrossRef]
- Chen, P.; Wang, J.; Yao, Y.; Qu, Y.; Ji, M.; Hou, P. Targeting DUSP5 suppresses malignant phenotypes of BRAF-mutant thyroid cancer cells and improves their response to sorafenib. Endocrine 2024, 85, 1268–1277. [Google Scholar] [CrossRef]
- Caunt, C.J.; Keyse, S.M. Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling. FEBS J. 2013, 280, 489–504. [Google Scholar] [CrossRef]
- Keyse, S.M.; Ginsburg, M. Amino acid sequence similarity between CL100, a dual-specificity MAP kinase phosphatase and cdc25. Trends Biochem. Sci. 1993, 18, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, K.; Nishida, E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2007, 1773, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.M.; Keyse, S.M. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 2007, 26, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Stecca, B.; Rovida, E. Impact of ERK5 on the Hallmarks of Cancer. Int. J. Mol. Sci. 2019, 20, 1426. [Google Scholar] [CrossRef]
- Ahmad, M.K.; Abdollah, N.A.; Shafie, N.H.; Yusof, N.M.; Razak, S.R.A. Dual-specificity phosphatase 6 (DUSP6): a review of its molecular characteristics and clinical relevance in cancer. Cancer Biol Med. 2018, 15, 14–28. [Google Scholar]
- Ekerot, M.; Stavridis, M.P.; Delavaine, L.; Mitchell, M.P.; Staples, C.; Owens, D.M.; Keenan, I.D.; Dickinson, R.J.; Storey, K.G.; Keyse, S.M. Negative-feedback regulation of FGF signalling by DUSP6/MKP-3 is driven by ERK1/2 and mediated by Ets factor binding to a conserved site within the DUSP6/MKP-3 gene promoter. Biochem J. 2008, 412, 287–98. [Google Scholar] [CrossRef]
- Bermudez, O.; Jouandin, P.; Rottier, J.; Bourcier, C.; Pagès, G.; Gimond, C. Post-transcriptional regulation of the DUSP6/MKP-3 phosphatase by MEK/ERK signaling and hypoxia. J. Cell. Physiol. 2010, 226, 276–284. [Google Scholar] [CrossRef]
- Zeliadt, N.A.; Mauro, L.J.; Wattenberg, E.V. Reciprocal regulation of extracellular signal regulated kinase 1/2 and mitogen activated protein kinase phosphatase-3. Toxicol Appl Pharmacol. 2008, 232, 408–17. [Google Scholar] [CrossRef]
- Pratilas, C.A.; Taylor, B.S.; Ye, Q.; Viale, A.; Sander, C.; Solit, D.B.; Rosen, N. V600E BRAF is associated with disabled feedback inhibition of RAF–MEK signaling and elevated transcriptional output of the pathway. Proc. Natl. Acad. Sci. 2009, 106, 4519–4524. [Google Scholar] [CrossRef]
- Moncho-Amor, V.; Pintado-Berninches, L.; de Cáceres, I.I.; Martín-Villar, E.; Quintanilla, M.; Chakravarty, P.; Cortes-Sempere, M.; Fernández-Varas, B.; Rodriguez-Antolín, C.; de Castro, J.; et al. Role of Dusp6 Phosphatase as a Tumor Suppressor in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2019, 20, 2036. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.M.; Huang, S.-M.; Park, S.-K.; Kim, D.-H.; Kim, D.H.; Lee, C.S.; Suh, K.S.; Yi, E.S.; Kim, K.-H. Differential expression of DUSP6 with expression of ERK and Ki-67 in non-small cell lung carcinoma. Pathol. - Res. Pr. 2011, 207, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Unni, A.M.; Harbourne, B.; Oh, M.H.; Wild, S.; Ferrarone, J.R.; Lockwood, W.W.; Varmus, H. Hyperactivation of ERK by multiple mechanisms is toxic to RTK-RAS mutation-driven lung adenocarcinoma cells. eLife 2018, 7. e33718. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Unni, A.M.; Lockwood, W.W.; Zejnullahu, K.; Lee-Lin, S.-Q.; Varmus, H. Evidence that synthetic lethality underlies the mutual exclusivity of oncogenic KRAS and EGFR mutations in lung adenocarcinoma. eLife 2015, 4, e06907. [Google Scholar] [CrossRef]
- Zhang, Z.; Kobayashi, S.; Borczuk, A.C.; Leidner, R.S.; LaFramboise, T.; Levine, A.D.; Halmos, B. Dual specificity phosphatase 6 (DUSP6) is an ETS-regulated negative feedback mediator of oncogenic ERK signaling in lung cancer cells. Carcinog. 2010, 31, 577–586. [Google Scholar] [CrossRef]
- Ghaddar, N.; Wang, S.; Woodvine, B.; Krishnamoorthy, J.; van Hoef, V.; Darini, C.; Kazimierczak, U.; Ah-Son, N.; Popper, H.; Johnson, M.; Officer, L.; Teodósio, A.; Broggini, M.; Mann, K.K.; Hatzoglou, M.; Topisirovic, I.; Larsson, O.; Le Quesne, J.; Koromilas, A.E. The integrated stress response is tumorigenic and constitutes a therapeutic liability in KRAS-driven lung cancer. Nat Commun. 2021, 12, 4651. [Google Scholar] [CrossRef]
- An, B.C. ; Choi, Y,D. ; Oh, I.J.; Kim, J.H.; Park, J.I.; Lee, S.W. GPx3-mediated redox signaling arrests the cell cycle and acts as a tumor suppressor in lung cancer cell lines. PLoS One. 2018, 13, e0204170. [Google Scholar]
- Zhong, C.; Chen, C.; Yao, F.; Fang, W. ZNF251 promotes the progression of lung cancer by activating ERK signaling. Cancer Sci. 2020, 111, 3236–3244. [Google Scholar] [CrossRef]
- Li, W.; Song, L.; Ritchie, A.; Melton, D.W. Increased levels of DUSP6 phosphatase stimulate tumourigenesis in a molecularly distinct melanoma subtype. Pigment. Cell Melanoma Res. 2012, 25, 188–199. [Google Scholar] [CrossRef]
- Wei, K.C.; Chen, R.F.; Chen, Y.F.; Lin, C.H. Hinokitiol suppresses growth of B16 melanoma by activating ERK/MKP3/proteosome pathway to downregulate survivin expression. Toxicol Appl Pharmacol. 2019, 366, 35–45. [Google Scholar] [CrossRef]
- Li, W.; Melton, D.W. Cisplatin regulates the MAPK kinase pathway to induce increased expression of DNA repair gene ERCC1 and increase melanoma chemoresistance. Oncogene 2012, 31, 2412–2422. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H.; Kim, S.H.; Trousil, S.; Frederick, D.T.; Piris, A.; Yuan, P.; Cai, L.; Gu, L.; Li, M.; Lee, J.H.; et al. Loss of cohesin complex components STAG2 or STAG3 confers resistance to BRAF inhibition in melanoma. Nat. Med. 2016, 22, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Tusa, I.; Gagliardi, S.; Tubita, A.; Pandolfi, S.; Urso, C.; Borgognoni, L.; Wang, J.; Deng, X.; Gray, N.S.; Stecca, B.; et al. ERK5 is activated by oncogenic BRAF and promotes melanoma growth. Oncogene 2018, 37, 2601–2614. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sahoo, A.; Sawada, J.; Marchica, J.; Sahoo, S.; Layng, F.I.; Finlay, D.; Mazar, J.; Joshi, P.; Komatsu, M.; et al. MicroRNA-211 Modulates the DUSP6-ERK5 Signaling Axis to Promote BRAFV600E-Driven Melanoma Growth In Vivo and BRAF/MEK Inhibitor Resistance. J. Investig. Dermatol. 2020, 141, 385–394. [Google Scholar] [CrossRef]
- Chan, D.W.; Liu, V.W.; Tsao, G.S.; Yao, K.-M.; Furukawa, T.; Chan, K.K.; Ngan, H.Y. Loss of MKP3 mediated by oxidative stress enhances tumorigenicity and chemoresistance of ovarian cancer cells. Carcinog. 2008, 29, 1742–1750. [Google Scholar] [CrossRef]
- Tong, X.; Mu, P.; Zhang, Y.; Zhao, J.; Wang, X. TRIM59, amplified in ovarian cancer, promotes tumorigenesis through the MKP3/ERK pathway. J Cell Physiol. 2020, 235, 8236–8245. [Google Scholar] [CrossRef]
- Furukawa, T.; Sunamura, M.; Motoi, F.; Matsuno, S.; Horii, A. Potential Tumor Suppressive Pathway Involving DUSP6/MKP-3 in Pancreatic Cancer. Am. J. Pathol. 2003, 162, 1807–1815. [Google Scholar] [CrossRef]
- Beaudry, K.; Langlois, M.; Montagne, A.; Cagnol, S.; Carrier, J.C.; Rivard, N. Dual-specificity phosphatase 6 deletion protects the colonic epithelium against inflammation and promotes both proliferation and tumorigenesis. J. Cell. Physiol. 2019, 234, 6731–6745. [Google Scholar] [CrossRef]
- Jiang, H.; Deng, L.; Lin, Z.; Yang, K.; Yang, J.; Zhao, W.; Gong, W. GSDMB interacts with IGF2BP1 to suppress colorectal cancer progression by modulating DUSP6-ERK pathway. Int. Immunopharmacol. 2024, 143, 113280. [Google Scholar] [CrossRef]
- Xu, N.; Dao, F.; Shi, Z.; Sun, K.; Qin, Y. WT1 together with RUNX1::RUNX1T1 targets DUSP6 to dampen ERK activity in acute myeloid leukaemia. Br. J. Haematol. 2024, 205, 1848–1859. [Google Scholar] [CrossRef]
- Wong, V.C.L.; Chen, H.; Ko, J.M.Y.; Chan, K.W.; Chan, Y.P.; Law, S.; Chua, D.; Kwong, D.L.; Lung, H.L.; Srivastava, G.; et al. Tumor suppressor dual-specificity phosphatase 6 (DUSP6) impairs cell invasion and epithelial-mesenchymal transition (EMT)-associated phenotype. Int. J. Cancer 2011, 130, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.-J.; Liang, S.-M.; He, P.-J.; Zhao, X.-B.; Li, M.-J.; Geng, F. Dusp6 inhibits epithelial-mesenchymal transition in endometrial adenocarcinoma via ERK signaling pathway. Radiol. Oncol. 2019, 53, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Chiappinelli, K.B.; Rimel, B.; Massad, L.S.; Goodfellow, P.J. Infrequent methylation of the DUSP6 phosphatase in endometrial cancer. Gynecol. Oncol. 2010, 119, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, M.; Jiang, P.; Zhou, Y.; Yan, X.; Zhou, C.; Mu, Y.; Xiao, S.; Ji, G.; Wu, N.; et al. RPL22L1 fosters malignant features of cervical cancer via the modulation of DUSP6-ERK axis. J. Transl. Med. 2025, 23, 1–14. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Shi, X.; Yin, L.; Zhai, W.; Gao, S.; Chen, Y.; Zhang, T. Calcium saccharate/DUSP6 suppresses renal cell carcinoma glycolytic metabolism and boosts sunitinib efficacy via the ERK-AKT pathway. Biochem. Pharmacol. 2024, 224, 116247. [Google Scholar] [CrossRef]
- Wu, Q.-N.; Liao, Y.-F.; Lu, Y.-X.; Wang, Y.; Lu, J.-H.; Zeng, Z.-L.; Huang, Q.-T.; Sheng, H.; Yun, J.-P.; Xie, D.; et al. Pharmacological inhibition of DUSP6 suppresses gastric cancer growth and metastasis and overcomes cisplatin resistance. Cancer Lett. 2018, 412, 243–255. [Google Scholar] [CrossRef]
- Yang, B.; Tan, Y.; Sun, H.; Fan, J.; Tang, Z.; Ji, Y. Higher intratumor than peritumor expression of DUSP6/MKP-3 is associated with recurrence after curative resection of hepatocellular carcinoma. Chin Med J (Engl). 2014, 127, 1211–7. [Google Scholar]
- Lee, J.U.; Huang, S.; Lee, M.H.; Lee, S.E.; Ryu, M.J.; Kim, S.J.; Kim, Y.K.; Kim, S.Y.; Joung, K.H.; Kim, J.M.; et al. Dual specificity phosphatase 6 as a predictor of invasiveness in papillary thyroid cancer. Eur. J. Endocrinol. 2012, 167, 93–101. [Google Scholar] [CrossRef]
- Buffet, C.; Hecale-Perlemoine, K.; Bricaire, L.; Dumont, F.; Baudry, C.; Tissier, F.; Bertherat, J.; Cochand-Priollet, B.; Raffin-Sanson, M.-L.; Cormier, F.; et al. DUSP5 and DUSP6, two ERK specific phosphatases, are markers of a higher MAPK signaling activation in BRAF mutated thyroid cancers. PLOS ONE 2017, 12, e0184861. [Google Scholar] [CrossRef]
- Messina, S.; Frati, L.; Leonetti, C.; Zuchegna, C.; Di Zazzo, E.; Calogero, A.; Porcellini, A. Dual-specificity phosphatase DUSP6 has tumor-promoting properties in human glioblastomas. Oncogene 2011, 30, 3813–3820. [Google Scholar] [CrossRef]
- Lin, Y.K.; Wu, W.; Ponce, R.K.; Kim, J.W.; Okimoto, R.A. Negative MAPK-ERK regulation sustains CIC-DUX4 oncoprotein expression in undifferentiated sarcoma. Proc. Natl. Acad. Sci. 2020, 117, 20776–20784. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Parra, I.; Zhang, M.; Hilsenbeck, S.G.; Tsimelzon, A.; Furukawa, T.; Horii, A.; Zhang, Z.-Y.; Nicholson, R.I.; Fuqua, S.A. Elevated Expression of Mitogen-Activated Protein Kinase Phosphatase 3 in Breast Tumors: A Mechanism of Tamoxifen Resistance. Cancer Res. 2006, 66, 5950–5959. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wu, C.; Wei, C.; Li, D.; Hua, K.; Song, J.; Xu, H.; Chen, L.; Fang, L. Silencing of DUSP6 gene by RNAi-mediation inhibits proliferation and growth in MDA-MB-231 breast cancer cells: an in vitro study. . 2015, 8, 10481–90. [Google Scholar] [PubMed]
- Bergholz, J.; Zhang, Y.; Wu, J.; Meng, L.; Walsh, E.M.; Rai, A.; Sherman, M.Y.; Xiao, Z.X. ΔNp63α regulates Erk signaling via MKP3 to inhibit cancer metastasis. Oncogene. 2014, 33, 212–24. [Google Scholar] [CrossRef]
- Kong, T.; Laranjeira, A.B.A.; Yang, K.; Fisher, D.A.C.; Yu, L.; De La Frégonnière, L.P.; Wang, A.Z.; Ruzinova, M.B.; Fowles, J.S.; Fulbright, M.C.; et al. DUSP6 mediates resistance to JAK2 inhibition and drives leukemic progression. Nat. Cancer 2023, 4, 108–127. [Google Scholar] [CrossRef]
- Tischer, T.; Schuh, M. The Phosphatase Dusp7 Drives Meiotic Resumption and Chromosome Alignment in Mouse Oocytes. Cell Rep. 2016, 17, 1426–1437. [Google Scholar] [CrossRef]
- Castro-Sánchez, P.; Ramirez-Munoz, R.; Lamana, A.; Ortiz, A.; González-Álvaro, I.; Roda-Navarro, P. mRNA profilin identifies low levels of phosphatases dual-specific phosphatase-7 (DUSP7) and cell division cycle-25B (CDC25B) in patients with early arthritis. Clin. Exp. Immunol. 2017, 189, 113–119. [Google Scholar] [CrossRef]
- Chuang, H.-C.; Tan, T.-H. MAP4K Family Kinases and DUSP Family Phosphatases in T-Cell Signaling and Systemic Lupus Erythematosus. Cells 2019, 8, 1433. [Google Scholar] [CrossRef]
- Peng, W.-X.; Huang, J.-G.; Yang, L.; Gong, A.-H.; Mo, Y.-Y. Linc-RoR promotes MAPK/ERK signaling and confers estrogen-independent growth of breast cancer. Mol. Cancer 2017, 16, 1–11. [Google Scholar] [CrossRef]
- Ikegawa, S. Expression, Regulation and Function of Asporin, A Susceptibility Gene in Common Bone and Joint Diseases. Curr. Med. Chem. 2008, 15, 724–728. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Z.; Li, H.; Zhao, Y.; Guo, Q.; Yu, Y.; Zhu, S.; Zhang, S.; Min, L. Asporin promotes cell proliferation via interacting with PSMD2 in gastric cancer. Front. Biosci. 2019, 24, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, M.; Feng, W.; Rao, H.; Zhang, W.; Liu, C.; Xu, Y.; Wang, Z.; Teng, Y.; Yang, X.; et al. Loss of SETD2 aggravates colorectal cancer progression caused by SMAD4 deletion through the RAS/ERK signalling pathway. Clin. Transl. Med. 2023, 13, e1475. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhou, L.; Li, S.; He, W.; Zheng, J.; Hou, Z.; He, P. Let-7c-5p Down Regulates the Proliferation of Colorectal Cancer Through the MAPK-ERK-Signaling Pathway. Biochem. Genet. 2023, 62, 3231–3243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lagowski, J.; Sundholm, A.; Sundberg, A.; Kulesz-Martin, M. Microtubule Disruption and Tumor Suppression by Mitogen-Activated Protein Kinase Phosphatase 4. Cancer Res. 2007, 67, 10711–10719. [Google Scholar] [CrossRef]
- Qiu, Z.; Liang, N.; Huang, Q.; Sun, T.; Xue, H.; Xie, T.; Wang, X.; Wang, Q. Downregulation of DUSP9 Promotes Tumor Progression and Contributes to Poor Prognosis in Human Colorectal Cancer. Front. Oncol. 2020, 10. 547011. [Google Scholar] [CrossRef]
- Imajo, M.; Kondoh, K.; Yamamoto, T.; Nakayama, K.; Nakajima-Koyama, M.; Nishida, E. Antagonistic Interactions between Extracellular Signal-Regulated Kinase Mitogen-Activated Protein Kinase and Retinoic Acid Receptor Signaling in Colorectal Cancer Cells. Mol. Cell. Biol. 2017, 37, e00012–17. [Google Scholar] [CrossRef]
- Lu, H.; Tran, L.; Park, Y.; Chen, I.; Lan, J.; Xie, Y.; Semenza, G.L. Reciprocal Regulation of DUSP9 and DUSP16 Expression by HIF1 Controls ERK and p38 MAP Kinase Activity and Mediates Chemotherapy-Induced Breast Cancer Stem Cell Enrichment. Cancer Res. 2018, 78, 4191–4202. [Google Scholar] [CrossRef]
- Mishra, A.; Oulès, B.; Pisco, A.O.; Ly, T.; Liakath-Ali, K.; Walko, G.; Viswanathan, P.; Tihy, M.; Nijjher, J.; Dunn, S.-J.; et al. A protein phosphatase network controls the temporal and spatial dynamics of differentiation commitment in human epidermis. eLife 2017, 6. e27356. [Google Scholar] [CrossRef]
- Nomura, M.; Shiiba, K.; Katagiri, C.; Kasugai, I.; Masuda, K.; Sato, I.; Sato, M.; Kakugawa, Y.; Nomura, E.; Hayashi, K.; Nakamura, Y.; Nagata, T.; Otsuka, T.; Katakura, R.; Yamashita, Y.; Sato, M.; Tanuma, N.; Shima, H. Novel function of MKP-5/DUSP10, a phosphatase of stress-activated kinases, on ERK-dependent gene expression, and upregulation of its gene expression in colon carcinomas. Oncol Rep. 2012, 28, 931–6. [Google Scholar]
- Ng, K.Y.; Chan, L.H.; Chai, S.; Tong, M.; Guan, X.Y.; Lee, N.P. , Yuan, Y. ; Xie, D.; Lee, T.K.; Dusetti, N.J., Carrier, A.; Ma, S. TP53INP1 Downregulation Activates a p73-Dependent DUSP10/ERK Signaling Pathway to Promote Metastasis of Hepatocellular Carcinoma. Cancer Res. 2017, 77, 4602–4612. [Google Scholar]
- Hou, J.; He, Z.; Liu, T.; Chen, D.; Wang, B.; Wen, Q.; Zheng, X. Evolution of Molecular Targeted Cancer Therapy: Mechanisms of Drug Resistance and Novel Opportunities Identified by CRISPR-Cas9 Screening. Front. Oncol. 2022, 12, 755053. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Brown, J.S. The Evolution and Ecology of Resistance in Cancer Therapy. Cold Spring Harb. Perspect. Med. 2020, 10, a040972. [Google Scholar] [CrossRef] [PubMed]
- Tubita, A.; Tusa, I.; Rovida, E. Playing the Whack-A-Mole Game: ERK5 Activation Emerges Among the Resistance Mechanisms to RAF-MEK1/2-ERK1/2- Targeted Therapy. Front. Cell Dev. Biol. 2021, 9. 647311. [Google Scholar] [CrossRef] [PubMed]
| Gene/MKP | Tumour type (xenografted cell line/animal model) | Treatment/genetic manipulation (observed secondary effect on DUSPs) | Biological outcome | Effect on ERKs | Ref |
|---|---|---|---|---|---|
| DUSP1 | HCC | Cx32 k/o (Reduced DUSP1) |
Increased ethanol-induced HCC | Increased pERK1/2 | [42] |
| DUSP1 | CRC (HCT116 and SW480) |
DUSP1 overexpression | Increased tumour growth | Reduced pERK1/2 | [26] |
| DUSP1 | BLCA (HT1197 and RT112 |
AKTi (Increased DUSP1) |
Reduced tumour growth | Reduced pERK1/2 | [34] |
| DUSP1 | ESCC (TE-1) |
ARNTL overexpression (Increased DUSP1) |
Reduced tumour growth | Reduced pERK1/2 | [50] |
| DUSP2 | PDAC (PANC-1, SW1990, BxPC-3, CFPAC-1) |
Mir-361-3p overexpression (Reduced DUSP2) |
Increased metastasis | Increased pERK1/2 | [57] |
| DUSP2 | GC (MGC-803, HGC-27) |
SKA3 KD (Increased DUSP2) |
Reduced tumour growth | Reduced pERK1/2 | [58] |
| DUSP2 | BLCA | DUSP2 overexpression | Reduced tumor growth | Reduced pERK1/2 | [59] |
| DUSP3 | NSCLC (H1792) |
KDM2A KD (Increased DUSP3) |
Reduced tumour growth | Reduced pERK1/2 |
[63] |
| DUSP3 | HCC (diethylnitrosamine) |
DUSP3 overexpression | Reduced tumour growth/Increased sorafenib sensitivity | Reduced pERK1/2 | [65] |
| DUSP4 | BC (MCF7) |
DUSP4 KD | Increased tumour growth | Increased pERK1/2 | [75] |
| DUSP4 | PDAC (PANC-1) |
DUP4 overexpression | Reduced tumour growth | Reduced pERK1/2 | [68] |
| DUSP5 | Skin cancer (HRasQ61L mice) |
DUSP5 KO | Increased papilloma formation | Increased pERK1/2 | [83] |
| DUSP5 | Thyroid Cancer (K1) |
DUSP5 KO | Reduced tumour growth | Reduced pERK1/2 | [90] |
| DUSP6 | Ovarian Cancer (A2780) |
DUSP6 overexpression | Reduced tumour growth/increased cisplatin sensitivity | Reduced pERK1/2 | [116] |
| DUSP6 | Melanoma (A375) |
DUSP6 overexpression | Reduced tumour growth | Reduced pERK5 | [115] |
| DUSP6 | CRC (Apc Min/+ mice) |
DUSP6 KO |
Increased intestinal tumorigenesis | Increased pERK1/2 | [119] |
| DUSP6 | GC (BGC823, SGC7901, SGC7901/DDP on PDX and xenografts) |
DUSP6 KO BCI |
Reduced tumour growth | Increased pERK1/2 | [127] |
| DUSP6 | Melanoma (A375) |
STAG2 or STAG3 KD (Reduced DUSP6) |
Reduced BRAFi sensitivity | Increased pERK1/2 | [113] |
| DUSP6 | Cervical Cancer (HeLa) |
RPL22L1 KD (Increased DUSP6) |
Reduced tumour growth | Reduced pERK1/2 | [125] |
| DUSP6 | CRC (GSDMB transgenic mice) |
GSDMB overexpression (Increased DUSP6) |
Reduced tumor formation | Reduced pERK1/2 | [120] |
| DUSP6 | AML (Kasumi-1 and HL60) |
WT1 overexpression/KD (Increased/reduced DUSP6) |
Reduced/increased tumour growth | Reduced/increased pERK1/2 | [121] |
| DUSP6 | Renal Cell Carcinoma (786-O) | Sunitinib and Calcium saccharate (Increased DUSP6) |
Reduced tumour growth | Reduced pERK1/2 | [126] |
| DUSP6 | ESCC (SLMT-1) NPC (HONE1) |
DUSP6 overexpression | Reduced tumour growth | Reduced pERK1/2 | [122] |
| DUSP6 | NSCLC (H460) |
DUSP6 KD | Increased tumour growth | Increased pERK5 | [101] |
| DUSP6 | AML (CD45.1+ C57BL/6J mice) |
BCI | Reduced tumour progression | Reduced pERK1/2 | [136] |
| DUSP7 | CRC | SETD2/SMAD4 KO (Reduced DUSP7) | Increased tumour growth | Reduced pERK1/2 | [143] |
| DUSP1 and DUSP6 | MPNSTs (S462.TY PDX and xenografts) |
DUSP1 and/or DUSP6 KD BCI |
Reduced tumour growth | Increased pERK1/2 | [27] |
| DUSP10 | HCC (MHCC97L) |
TP53INP1 KD (Reduced DUSP10) |
Increased metastasis | Increased pERK1/2 | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





