1. Introduction
Mitochondria are central to cellular energy metabolism, generating most of cellular ATP through oxidative phosphorylation (OXPHOS). In addition to energy production, these organelles play pivotal roles in regulating reactive oxygen species (ROS), apoptosis, calcium signaling, and various biosynthetic pathways, underscoring their essential role in maintaining cellular homeostasis. In cancer, mitochondrial function is often profoundly altered, reflecting the metabolic reprogramming that supports rapid cell proliferation, survival under stress, and resistance to therapy [
1].
Traditionally, cancer metabolism was characterized by the Warburg effect, which suggested that cancer cells favor glycolysis for ATP production even in the presence of oxygen [
2]. However, recent findings reveal that mitochondrial metabolism remains active and crucial in tumor growth and progression. Mitochondria in cancer cells exhibit remarkable metabolic plasticity and adaptability, enabling them to switch between various fuel sources, such as glucose, fatty acids and glutamine to meet the energy and biosynthetic demands. This metabolic flexibility allows tumor cells to thrive under metabolic stress and fluctuating nutrient availability [
2].
Proteomic and functional analyses across various cancer cell lines have uncovered distinct mitochondrial alterations, including enhanced spare respiratory capacity, increased basal respiration, and elevated ATP-linked respiration. These adaptations reflect the heightened bioenergetic and biosynthetic demands of cancer cells [
3]. Moreover, disruptions in mitochondrial dynamics, such as imbalances in fusion and fission, along with mutations in mitochondrial DNA (mtDNA), have been associated with oncogenesis and cancer progression. Structural and functional abnormalities in mitochondria are also implicated in altered redox signaling, fostering a pro-survival environment that supports malignant cell growth [
4].
Notably, reports from metabolic pathway profiling and real-time ATP production assays have shown that several types of cancers are highly dependent on mitochondrial ATP production. This dependence is particularly pronounced in cells that rely on oxidative metabolism for their growth and survival. For example, breast cancer cells with impaired mitochondrial function exhibit reduced tumorigenicity potential, suggesting the therapeutic promise of targeting mitochondrial pathways [
5]. Additionally, tumor cells can manipulate the surrounding stromal cells to supply mitochondrial fuels like lactate and ketone bodies, further emphasizing the complexity of the tumor metabolic network [
6].
As the understanding of cancer metabolism evolves, mitochondria emerge not only as key players in tumor biology but also as promising therapeutic targets. Investigating alterations in mitochondrial bioenergetics, fuel flexibility, and proteomic profiles in cancer cells can uncover the vulnerabilities that may be leveraged as the target for anti-cancer therapies. for anti-cancer strategies. This review explores the multifaceted roles of mitochondria in cancer cells, highlighting their functional adaptations, metabolic reprogramming, and potential as targets for therapeutic interventions.
2. Alteration of Mitochondrial Function in Cancer Cells
In healthy cells, mitochondria not only provide the majority of cellular energy but also regulate calcium homeostasis, redox signaling, and programmed cell death. In cancer cells, however, mitochondrial function is often reprogrammed to support the increased bioenergetic and biosynthetic demands of uncontrolled proliferation. Emerging evidence suggests that mitochondrial dysfunction, coupled with changes in mitochondrial morphology and dynamics, plays a critical role in tumor development and progression [
7]. The central role of mitochondria in energy metabolism is facilitated by the electron transport chain (ETC), which couples the transfer of electrons from NADH and FADH₂ to oxygen with the generation of a proton gradient across the inner mitochondrial membrane. This electrochemical gradient drives ATP synthesis via ATP synthase (Complex V). Despite the classic view stemming from the Warburg hypothesis, that cancer cells primarily rely on aerobic glycolysis; recent findings have demonstrated that mitochondria remain functional in many cancers and contribute significantly to cellular ATP pools. In some tumors, mitochondrial ATP production even surpasses that of glycolysis, underscoring the persistent importance of oxidative phosphorylation in cancer metabolism [
8]. In a study investigating the effects of K-Ras oncogene activation in mouse fibroblasts, Chiaradonna et al. (2006) demonstrated that transformed cells exhibited significant alterations in mitochondrial gene expression and morphology compared to their non-transformed counterparts. Transformed fibroblasts showed decreased ATP levels, reduced capacity to adapt mitochondrial morphology in response to glucose depletion, and partial mitochondrial uncoupling. These mitochondrial defects were directly linked to oncogenic Ras activation and were reversible upon expression of a dominant-negative GEF, highlighting a causal relationship between oncogene signaling and mitochondrial dysfunction. This finding suggests that in addition to glycolytic upregulation, mitochondrial impairment may be a direct consequence of oncogenic pathways, particularly those involving Ras family proteins [
9]. In another human model of mitochondrial dysfunction, Grünewald et al. (2010) investigated fibroblasts derived from Parkinson’s disease patients carrying mutations in the Parkin gene [
6]. Although not derived from cancer cells, this model provides key insights into how mitochondrial abnormalities manifest at the functional and morphological levels in disease states. The mutant cells demonstrated significantly lower ATP production, increased oxidative stress, and altered mitochondrial membrane potential under stress conditions. Morphological analysis revealed reduced mitochondrial branching following oxidative stress, indicating compromised mitochondrial dynamics. These findings parallel alterations often observed in cancer cells, such as diminished OXPHOS efficiency and fragmented mitochondrial networks, which may be adaptive responses to stress or reflect impaired mitophagy and quality control mechanisms [
6].
A critical aspect of mitochondrial dysfunction in cancer is the disruption of mitochondrial dynamics—the processes of mitochondrial fusion and fission that regulate mitochondrial shape, distribution, and function. In a comprehensive study, Srinivasan et al. (2017) examined the impact of mitochondrial DNA (mtDNA) depletion and oxidative stress on mitochondrial dynamics and retrograde signaling. Their results showed that cells with depleted mtDNA had disorganised cristae, reduced mitochondrial membrane potential, and an increased reliance on fission machinery such as DRP1. Simultaneously, mitochondrial fusion proteins such as OPA1 were downregulated. These structural changes correlated with a migratory phenotype and increased expression of stress response genes features commonly associated with tumor progression [
10]. Importantly, the study linked mitochondrial dysfunction to activation of retrograde signaling pathways, including calcium-calcineurin signaling and oxidative stress-mediated transcriptional programs. This signaling crosstalk between the mitochondria and the nucleus enables the cell to adapt its gene expression in response to mitochondrial damage but may also facilitate tumor survival and metastasis. For instance, increased mitochondrial ROS production can activate AMPK or stabilize HIF-1α, promoting angiogenesis, glycolysis, and resistance to apoptosis—traits that enhance tumor cell fitness in hypoxic environments [
10]. Moreover, the specific genetic alterations in mitochondrial enzymes have been implicated in hereditary and sporadic cancers. Mutations in components of Complex II (succinate dehydrogenase) and fumarate hydratase impair the tricarboxylic acid (TCA) cycle and contribute to the accumulation of oncometabolites such as succinate and fumarate. These metabolites inhibit prolyl hydroxylases, stabilising HIF-1α and promoting tumor angiogenesis and growth. Additional studies have identified mutations in Complex I subunits that enhance ROS production and activate pro-survival signaling cascades [
8,
9,
10]. Collectively, these findings challenge the outdated notion that mitochondria are largely irrelevant in cancer cell metabolism. Instead, they reveal that mitochondrial dysfunction manifested as impaired ATP production, increased oxidative stress, disrupted membrane potential, and altered dynamics, plays an active role in supporting tumor development and progression. The dual role of mitochondria in both energy supply and cell death regulation positions them as critical nodes in the balance between cancer cell survival and demise. In conclusion, mitochondrial function and dysfunction are intricately involved in the metabolic reprogramming of cancer cells. While cancer cells often upregulate glycolysis, many retain or even enhance mitochondrial oxidative phosphorylation to meet their high energy and biosynthetic demands. Mitochondrial abnormalities including altered dynamics, reduced ATP production, and retrograde signaling contribute to tumorigenesis and offer potential targets for therapy. A deeper understanding of these processes will be vital for developing novel and effective cancer treatments.
3. Alterations in ATP Production in Cancer Cells
A hallmark of cellular life is the ability to efficiently convert nutrients into usable energy. In eukaryotic cells, this is primarily accomplished through adenosine triphosphate (ATP) production via two major pathways: mitochondrial oxidative phosphorylation (OXPHOS) and cytosolic glycolysis. In normal cells, ATP production is predominantly driven by mitochondrial OXPHOS under normoxic conditions. However, cancer cells exhibit altered energy metabolism that often includes elevated glycolysis even in the presence of oxygen—a phenomenon widely known as the Warburg effect. Yet, modern research has challenged the oversimplification of cancer as exclusively glycolytic, revealing a more nuanced metabolic landscape where ATP production is flexible, dynamic, and heavily context-dependent [
11]. A foundational study by Louie et al. (2020) explored the total ATP production dynamics in MCF7 breast cancer cells under different nutrient conditions using Seahorse XF real-time analysis. They demonstrated that MCF7 cells could maintain significantly elevated ATP production when provided with a mixture of glucose, glutamine, and pyruvate, as opposed to single substrates. Interestingly, this enhancement in ATP production was mainly attributed to increased oxidative ATP production, not glycolytic flux, challenging the classical Warburg-centric perspective. The findings suggest that cancer cells retain a robust capacity for mitochondrial ATP synthesis and actively use it to meet high biosynthetic and proliferative demands [
12]. In stark contrast, non-cancerous myoblasts (C2C12 cells) exhibited no change in ATP production rate under varying substrate conditions, implying that cancer cells possess a unique metabolic plasticity that normal cells lack. This metabolic flexibility allows cancer cells to utilize multiple carbon sources to optimize ATP generation depending on availability. Importantly, the increase in oxidative ATP production occurred rapidly within minutes, suggesting that this was a functional response rather than one requiring transcriptional remodeling [
12].
The relevance of ATP levels in the survival and adaptability of cancer cells is further underscored by the study from Zhou et al. (2012), which investigated ATP production in chemoresistant colon cancer cell lines (HT29-OxR and HCT116-OxR). These cells exhibited defective mitochondrial ATP production but compensated for this deficit by upregulating aerobic glycolysis. Paradoxically, even with impaired mitochondria, the chemoresistant cells displayed higher intracellular ATP levels than their drug-sensitive parental counterparts. This increase in ATP supported enhanced signaling via hypoxia-inducible factor 1-alpha (HIF-1α), contributing to drug resistance. The study coined the term "ATP debt" to describe the additional energy required by cancer cells to maintain survival signaling under genotoxic stress. Intriguingly, direct ATP delivery into cells was sufficient to induce drug resistance in previously sensitive cells, while ATP depletion via glycolytic inhibition resensitized resistant cells to chemotherapy [
13]. These results highlight a critical distinction: although many cancer cells have dysfunctional mitochondria, they do not necessarily exhibit low ATP levels. Instead, they rewire metabolic pathways to maintain or even elevate ATP levels through enhanced glycolysis or auxiliary pathways. This capacity to uphold energy homeostasis under stress is likely a major factor behind cancer cell resilience and treatment resistance.
Another pathway contributing to ATP production in cancer cells—beyond glycolysis and classical OXPHOS is the serine, one-carbon, and glycine (SOG) metabolism pathway, as explored by Tedeschi et al. (2013). This pathway branches off from glycolysis and shuttles carbon units through folate metabolism and glycine cleavage, ultimately generating ATP, NADPH, and purine nucleotides. Using metabolic flux modeling and tracer analysis, the authors showed that the SOG pathway is significantly upregulated in a subset of breast, prostate, colorectal, and lung cancers. Notably, high expression of SOG pathway genes strongly correlated with increased proliferation and Myc oncogene activation—both indicators of aggressive tumor behavior [
14]. The SOG pathway was predicted to produce up to four molecules of ATP per glucose molecule via its unique integration of glycolytic intermediates with mitochondrial one-carbon metabolism. Inhibition of this pathway using methotrexate led to a marked decrease in ATP levels and activation of AMP-activated protein kinase (AMPK), confirming the pathway’s role in energy generation. These findings suggest that in some cancers, the SOG pathway acts as a third route for ATP production, complementing glycolysis and OXPHOS to ensure sufficient energy for growth and survival.
When comparing cancer cells to normal cells, a recurring theme is the flexibility of ATP production in tumors. While normal cells generally rely on OXPHOS under normoxic conditions and switch to glycolysis only under hypoxia, cancer cells often operate both pathways simultaneously or switch between them more readily. This adaptability allows them to maintain high ATP output even in hostile microenvironments where oxygen or nutrients may be limited [
11,
12]. In summary, cancer cells exhibit a dynamic and robust system for ATP production that surpasses that of normal cells in flexibility and capacity. While OXPHOS remains crucial in both cell types, cancer cells supplement mitochondrial ATP synthesis with enhanced glycolysis and alternative metabolic routes like the SOG pathway. Elevated intracellular ATP levels not only fuel biosynthesis and proliferation but also modulate survival signaling and therapy resistance. Understanding these mechanisms provides a strong rationale for the development of metabolic therapies aimed at disrupting ATP homeostasis in tumors.
4. Fuel Flexibility and Metabolic Reprogramming in Cancer: Utilization of Glucose, Glutamine, and Fatty Acids
Cancer cells undergo profound metabolic reprogramming to meet the demands of rapid proliferation, survival in harsh environments, and evasion of immune responses. A major feature of this metabolic shift is fuel flexibility—the capacity to switch between different energy substrates such as glucose, glutamine, and fatty acids. This section explores how cancer cells utilize each of these fuels and how Seahorse XF Analyzer-based bioenergetic profiling has illuminated these adaptive strategies [
15,
16].
4.1. Glucose Metabolism and the Warburg Effect
Glucose is a primary substrate for energy generation in both normal and cancerous cells. In cancer, however, glucose metabolism is frequently rerouted toward aerobic glycolysis, a phenomenon known as the Warburg effect [
15]. Despite the availability of oxygen, cancer cells convert glucose to lactate, sacrificing efficiency (less ATP per molecule of glucose) for speed and anabolic precursor generation. This metabolic phenotype supports the biosynthetic needs of rapidly proliferating cells. The Seahorse XF Analyzer has been instrumental in quantifying this glycolytic shift by measuring the extracellular acidification rate (ECAR), which correlates with lactate production and glycolytic flux [
15]. In the Seahorse Mito Stress Test, glycolytic activity is often assessed following inhibition of oxidative phosphorylation (OXPHOS) with drugs like oligomycin. In lupus-associated B and T cells, which show similarities to cancer metabolism, inhibition of glycolysis using 2-deoxyglucose (2DG) significantly reduces ECAR, indicating high glycolytic dependency [
15,
16]. In addition to providing energy, glucose metabolism affects intracellular pH. A 2020 study published in Cancer Research revealed that metabolic reprogramming in cancer also serves to regulate proton production. Across multiple cancer types, altered metabolism leads to increased intracellular acidification, which compensates for chronic alkaline stress driven by inflammation and iron overload [
17].
4.2. Glutamine as a Carbon and Nitrogen Source
When glucose availability is limited, cancer cells often rely on glutamine—, an amino acid that feeds into the tricarboxylic acid (TCA) cycle via conversion to α-ketoglutarate. Glutamine serves as both a carbon donor for mitochondrial respiration and a nitrogen source for biosynthesis [
16]. The enzyme glutaminase (GLS), which catalyzes the conversion of glutamine to glutamate, is critical for glutaminolysis. Inhibition of GLS using BPTES has been shown to reduce mitochondrial oxygen consumption rate (OCR), indicating the importance of glutamine in fueling OXPHOS [
16]. Seahorse Fuel Flex assays, which track changes in OCR following sequential addition of inhibitors like BPTES, enable researchers to quantify this dependency in real time [
15]. In human corneal endothelial cells (cHCECs), a model for mitochondrial bioenergetics, mature differentiated cells demonstrated elevated OCR after glutamine supplementation, while immature or stressed cell populations did not show the same capacity for glutaminolysis. This finding highlights the link between mitochondrial function, cell differentiation, and glutamine utilization [
18].
4.3. Fatty Acid Oxidation (FAO) as a Backup Fuel Source
Fatty acids are another key energy source, especially in nutrient-deprived environments. Fatty acid oxidation (FAO) supplies acetyl-CoA for the TCA cycle, supporting ATP production and redox homeostasis. In cancer, FAO contributes to metastatic potential and survival under oxidative stress [
16]. Etomoxir, an inhibitor of carnitine palmitoyl transferase 1 (CPT1), is used in Seahorse assays to block FAO and assess its contribution to OCR. For instance, in glioblastoma tumor spheres, etomoxir reduced both OCR and invasive properties, illustrating the critical role of FAO in maintaining aggressive phenotypes [
16]. The Seahorse Fuel Flex test also allows evaluation of spare respiratory capacity by observing OCR after FAO inhibition. This capacity reflects the cell’s ability to respond to increased energy demand and has been linked to tumor resilience and therapy resistance [
15].
4.4. Metabolic Redundancy and Adaptation
Cancer cells often exhibit metabolic redundancy, enabling them to switch between glucose, glutamine, and FAO pathways depending on nutrient availability or therapy pressure. This adaptability is a hallmark of treatment resistance and tumor progression. Seahorse XF Fuel Flex assays have revealed that some cancer cells display primary dependence on glucose, while others show a compensatory increase in glutamine or fatty acid utilization when one pathway is inhibited [
15]. Such metabolic switching is influenced by oncogenes like MYC and transcription factors like HIF-1α, which regulate genes involved in glycolysis and glutaminolysis (16,17). Furthermore, studies in cHCECs have shown that cells with higher "effector cell" ratios—indicators of maturity and functionality—prefer OXPHOS and utilize glutamine and fatty acids more efficiently, while stressed or undifferentiated cells default to glycolysis [
18].
5. Molecular Dysregulation of Mitochondrial Dynamics in Cancer
5.1. Alterations in Mitochondrial Dynamics in Cancer
Mitochondria are highly dynamic organelles, constantly undergoing fission and fusion to maintain proper morphology, distribution, and function. This process, known as mitochondrial dynamics, plays a critical role in maintaining cellular energy homeostasis, bioenergetics, signaling, and apoptosis. In healthy cells, a balanced cycle of fission and fusion ensures mitochondrial quality control and adapts mitochondrial networks to metabolic needs [
19]. However, in cancer cells, these dynamics are profoundly altered, contributing to tumorigenesis, progression, metastasis, and resistance to therapies. The disruption of this balance can reshape mitochondrial structure and localization, leading to metabolic reprogramming and altered cellular behavior [
20]. The two major mitochondrial dynamics processes—fusion and fission—are mediated by specific sets of proteins: mitofusin 1 and 2 (MFN1, MFN2) and optic atrophy protein 1 (OPA1) regulate fusion, while fission is primarily controlled by dynamin-related protein 1 (Drp1), mitochondrial fission 1 protein (FIS1), mitochondrial fission factor (MFF), and other regulators such as mitochondrial fission process protein 1 (MTFP1). Alterations in the expression or activity of these proteins are commonly observed in a variety of cancer types and are increasingly recognized as critical modulators of malignant behavior [
19,
20,
21].
Genomic analyses show that genes encoding mitochondrial dynamics regulators are recurrently amplified in multiple cancers, including breast, lung, pancreatic, and head and neck cancers. Specifically, amplifications of OPA1, MFN1, and DNM1L (encoding Drp1) were found in more than 5% of high-grade serous ovarian cancers (HGSOC), breast carcinomas, and lung adenocarcinomas [
19]. These genetic alterations can modify the mitochondrial morphology towards either excessive fragmentation or hyperfusion, influencing tumor behavior.
In a study by Zhao et al. (2013), metastatic breast cancer cells were found to exhibit increased mitochondrial fragmentation compared to non-metastatic cells. This was due to elevated Drp1 levels and decreased Mfn1 expression. Drp1 not only mediated mitochondrial fission but also facilitated the redistribution of mitochondria to the lamellipodial regions of cancer cells, where energy is most needed for motility. Knockdown of Drp1 or overexpression of Mfn1 resulted in elongated mitochondria and significantly reduced cell migration and invasion in vitro. Conversely, silencing Mfn1 and Mfn2 promoted fragmentation and enhanced metastatic behavior [
20]. Mutant oncogenes also modulate mitochondrial dynamics. For instance, in pancreatic ductal adenocarcinoma (PDAC), mutant KRAS signaling promotes mitochondrial fragmentation via enhanced Drp1 activity, which is essential for KRAS-driven tumor growth [
19]. These changes in mitochondrial shape are not merely structural but play a central role in regulating apoptosis, cell metabolism, and adaptation to stress, which collectively promote malignancy. These findings emphasize the connection between mitochondrial dynamics and cancer cell motility. Mitochondrial fission appears necessary for redistributing mitochondria to cellular protrusions, such as lamellipodia, where energy-demanding activities like actin remodeling and focal adhesion turnover occur. Moreover, pharmacological inhibition of Drp1 using Mdivi-1 suppressed migration, offering a potential therapeutic angle.
In a separate investigation into oncocytic thyroid tumors by Ferreira-da-Silva et al. (2015), abnormal mitochondrial accumulation and morphology were observed as hallmarks of these cancers. Oncocytic tumors are known for their high mitochondrial content, and this study demonstrated upregulation of both fusion (Mfn1, Mfn2, Opa1) and fission (Drp1, Fis1) proteins. Notably, Drp1 overexpression was specifically associated with malignant transformation and increased migration potential in thyroid tumor cells [
21]. Genetic and pharmacologic inhibition of Drp1 reduced the migratory ability of oncocytic thyroid cancer cells, suggesting that Drp1 plays a role beyond mitochondrial morphology—it may directly impact metastatic capacity. This study reinforces the concept that imbalanced mitochondrial dynamics, favoring fission, is linked with increased tumor aggressiveness. Interestingly, these alterations occurred despite similar levels of mitochondrial content (as normalized by SDHA staining), indicating that it is not mitochondrial mass but dynamics and structure that determine cancer cell behavior [
21].
These results align with broader findings across cancers where fragmented mitochondrial networks are linked to malignancy. For instance, mitochondrial fragmentation has also been observed in aggressive renal cell carcinomas and glioblastomas, supporting the idea that enhanced fission is a conserved feature of metastatic tumors.
5.2. p53, Drp1, and Metastatic Potential
The tumor suppressor p53, widely known for its roles in DNA repair and apoptosis, has been shown to regulate mitochondrial dynamics. p53 suppresses the expression of MTFP1, a protein that promotes mitochondrial fission by enhancing Drp1 phosphorylation at Ser616. Loss of p53 leads to exaggerated mitochondrial fragmentation, which correlates with aggressive cancer phenotypes, epithelial-to-mesenchymal transition (EMT), and enhanced metastatic capacity [
22]. Mechanistically, the absence of p53 activates mTORC1 signaling, upregulating MTFP1, which then facilitates Drp1-driven mitochondrial fission. This pathway, termed the mTORC1/MTFP1/Drp1 axis, culminates in increased ERK1/2 activation, MMP9 expression, and cell migration. Interfering with this axis, either genetically or pharmacologically, inhibits these metastatic phenotypes, suggesting therapeutic potential. Furthermore, wild-type p53 enhances mitochondrial fusion and elongation, thereby suppressing invasive cell migration. This highlights the dual role of p53 as both a genomic and mitochondrial gatekeeper in tumor progression [
22].
5.3. Dynamic Interplay and Context-Specific Effects
Despite the overarching trends, the biological outcomes of mitochondrial fission or fusion vary based on cancer type and cellular context. For example, promoting mitochondrial fusion using P-Mito compounds was shown to suppress pancreatic tumor growth and induce chromosomal instability due to persistent hyperfusion [
23]. On the other hand, inhibiting fission via Drp1 downregulation diminished tumor proliferation and migration, pointing to its potential as a therapeutic target. In estrogen receptor-positive breast cancers, fusion proteins OPA1 and MFN2 were found to be downregulated in comparison to normal mammary epithelial cells, suggesting a predisposition to a fragmented mitochondrial state. This fragmented state is often associated with poor prognosis and drug resistance [
23]. However, the complexity arises from paradoxical findings such as mitochondrial fission reducing metastasis in certain cancers like triple-negative breast cancer (TNBC). This context-dependent behavior suggests that therapeutic strategies must consider tumor subtype and mitochondrial status before modulation [
22].
5.4. Mitochondrial Trafficking and Cortical Localization
Mitochondrial positioning within the cell is another emerging theme in cancer dynamics. A study by Caino et al. (2016) revealed that metastatic tumor cells co-opt a neuronal mitochondrial trafficking network, typically active in neurons, to redistribute mitochondria to the cortical cytoskeleton. This network includes syntaphilin (SNPH), kinesin motor proteins (like KIF5B), and GTPases such as Miro1 and Miro2 [
24]. The authors identified SNPH as a critical brake on mitochondrial motility. In tumors, SNPH is frequently downregulated, allowing mitochondria to move more rapidly and accumulate in cell protrusions. This redistribution fuels localized ATP production, facilitating chemotaxis, invasion, and metastasis. Silencing SNPH increased both mitochondrial fission rates and fusion events, suggesting that hyperactive mitochondrial dynamics contribute to metastatic capacity. Importantly, restoring SNPH expression suppressed invasion and cell migration in multiple cancer models [
24]. Their findings suggest that mitochondrial fission in cancer is not just a structural change but part of a coordinated system that facilitates energy delivery to the leading edge of migrating tumor cells. This spatial regulation of mitochondrial function may be especially important in cells undergoing epithelial-to-mesenchymal transition (EMT), which requires dynamic cytoskeletal remodeling and significant energy expenditure.
Mitochondrial dynamics represent a vital layer of regulation in cancer biology, linking bioenergetics with cell structure, signaling, and motility. The imbalance between fission and fusion—particularly through the upregulation of Drp1 and downregulation of MFN1/MFN2—facilitates mitochondrial fragmentation and subcellular relocalization, a feature strongly associated with metastatic potential [
23,
24]. In several cancer types, including breast and thyroid tumors, this shift in dynamics correlates with increased invasion and poorer prognosis. Additionally, cancer cells exploit neuronal-like mitochondrial trafficking mechanisms to position mitochondria at sites of high energy demand, such as the cortical cytoskeleton, further enhancing their invasive capabilities and highlighting the intricate nature of mitochondrial behavior in malignancy [
23,
26].
These alterations in mitochondrial dynamics reflect a finely tuned imbalance regulated by core proteins including Drp1, OPA1, MFN1/MFN2, and MTFP1 [
25]. The resulting structural and functional changes influence cancer cell survival, proliferation, and migration, often involving critical oncogenic pathways such as p53 and mTORC1. Importantly, this dysregulation introduces specific therapeutic vulnerabilities that can be targeted using pharmacological agents designed to modulate mitochondrial morphology and function. Inhibiting excessive fission, restoring fusion balance, or blocking mitochondrial trafficking may all disrupt the energetic and structural support systems that drive tumor aggressiveness [
22].
Given the diversity in mitochondrial behavior across cancer types, future research should prioritize the identification of biomarkers that reflect mitochondrial dynamic states and predict therapeutic responses. Integrating mitochondrial-targeted therapies with apoptosis-inducing agents may offer synergistic benefits, particularly in treatment-resistant cancers characterized by p53 mutations or aberrant KRAS signaling. Understanding and targeting mitochondrial dynamics not only enhances our knowledge of cancer progression but also opens the door to novel anti-metastatic strategies that disrupt the cellular energy architecture underpinning tumor invasion and dissemination.
6. Mitochondrial Morphology in Cancer Cells
Mitochondria, commonly known as the cell's powerhouse, display a dynamic and flexible morphology that mirrors both physiological and pathological conditions. Their shape results from the finely tuned balance between mitochondrial fission and fusion, processes that are crucial for sustaining cellular energy production, redox balance, and cell survival [
25]. In cancer cells, this delicate equilibrium is frequently disrupted, leading to profound alterations in mitochondrial shape, distribution, and ultrastructure. These morphological changes are increasingly recognised as not only hallmarks of metabolic reprogramming and tumor progression, but also as active drivers of drug resistance and adaptation to the tumor microenvironment. Influenced by factors such as oxidative stress, altered membrane potential, and oncogenic signaling, mitochondrial remodeling contributes significantly to the malignant phenotype and represents a critical feature of cancer cell biology [
25,
26,
27].
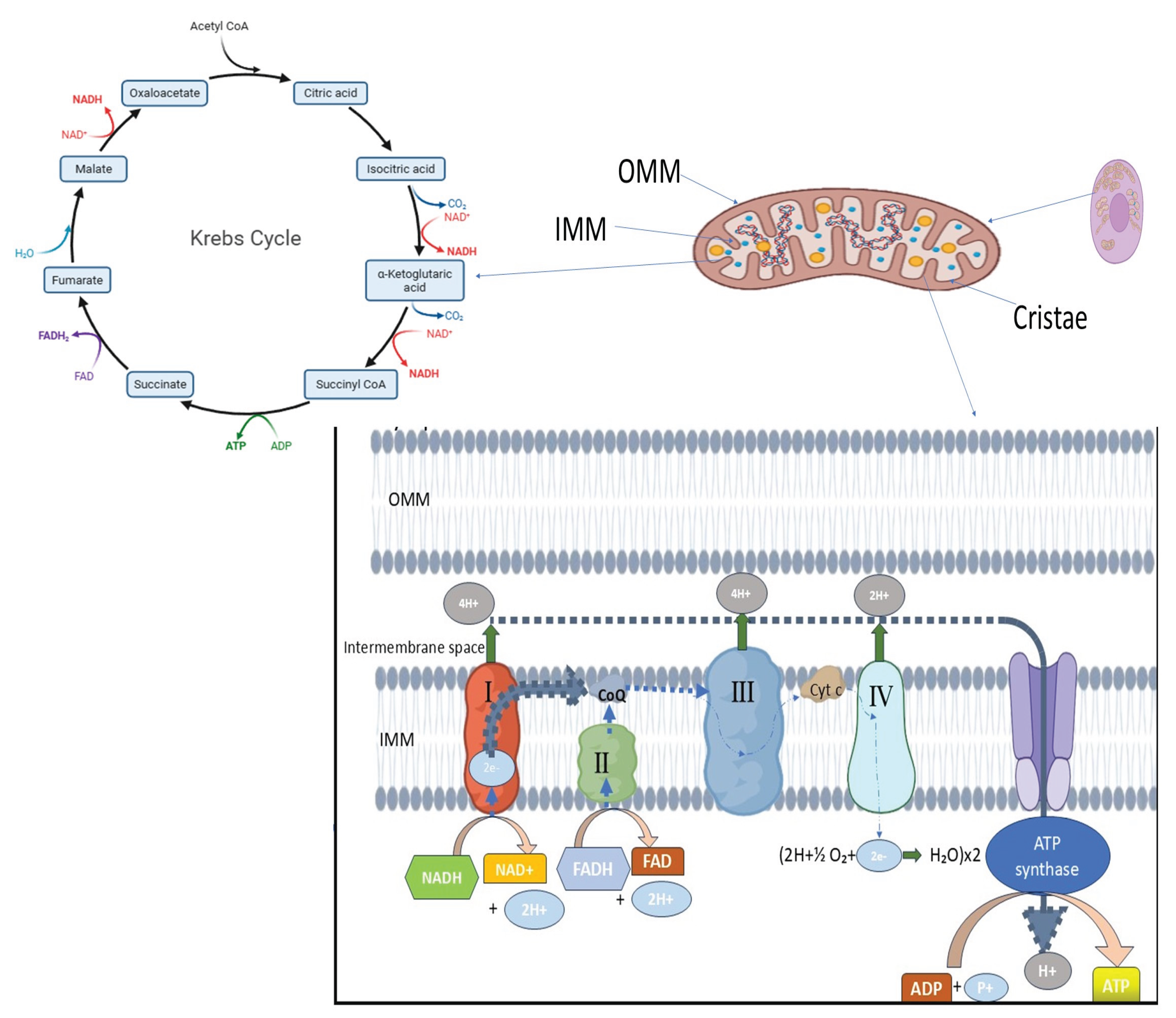
For optimal bioenergetic function and cellular homeostasis, mitochondria in healthy eukaryotic cells maintain a dynamic and tightly controlled morphology. Usually found in metabolically active cells, mitochondria are long, tubular organelles that form an interconnected network. This shape is dynamic; rather, the size, number, and distribution of mitochondria are constantly balanced by fusion and fission events, which are collectively referred to as mitochondrial dynamics (
Figure 1). Complementation between partially damaged organelles is facilitated by mitochondrial fusion, which permits the mixing of mitochondrial contents such as proteins, metabolites, and mitochondrial DNA (mtDNA). On the other hand, the ability to isolate damaged mitochondrial segments for removal through mitophagy relies on fission. This process facilitates quality control and the redistribution of mitochondria during cell division or in response to changes in metabolic demand. Together, these mechanisms preserve mitochondrial flexibility and integrity across various physiological conditions [
24]. Healthy mitochondria possess a double-membrane structure, with an inner mitochondrial membrane (IMM) that folds inward to form cristae and an outer mitochondrial membrane (OMM) that encloses the organelle (
Figure 1). By containing vital elements of the electron transport chain (ETC) and ATP synthase required for oxidative phosphorylation, these cristae greatly expand the membrane surface area. The maintenance of cristae architecture is crucial for controlling reactive oxygen species (ROS), regulating calcium homeostasis, and ensuring efficient ATP synthesis. Healthy mitochondria display intact membranes and well-defined, closely spaced cristae under electron microscopy, indicating robust bioenergetic capacity [
26]. Mitochondrial dysfunction is frequently linked to changes in this typical morphology, such as excessive fragmentation, swelling, or loss of cristae structure. These alterations have been noted in diseases such as cancer, neurodegeneration, and infertility. For example, aberrant fission that results in mitochondrial fragmentation has been connected to increased production of ROS and impaired oxidative phosphorylation, both of which contribute to the development of disease and cellular damage [
25,
26] Additionally, because structural disruptions are directly linked to metabolic abnormalities, sophisticated imaging studies have emphasized the significance of preserving mitochondrial morphology in live-cell contexts [
28].
Therefore, mitochondria's distinctive shape—a balance of linked tubules with preserved cristae—reflects their functional state. A crucial factor in comprehending both normal cell function and the etiology of different disorders is the proper regulation of mitochondrial shape, which is not only structural but also closely related to mitochondrial and cellular physiology.
6.1. Morphological Shifts During Tumor Progression
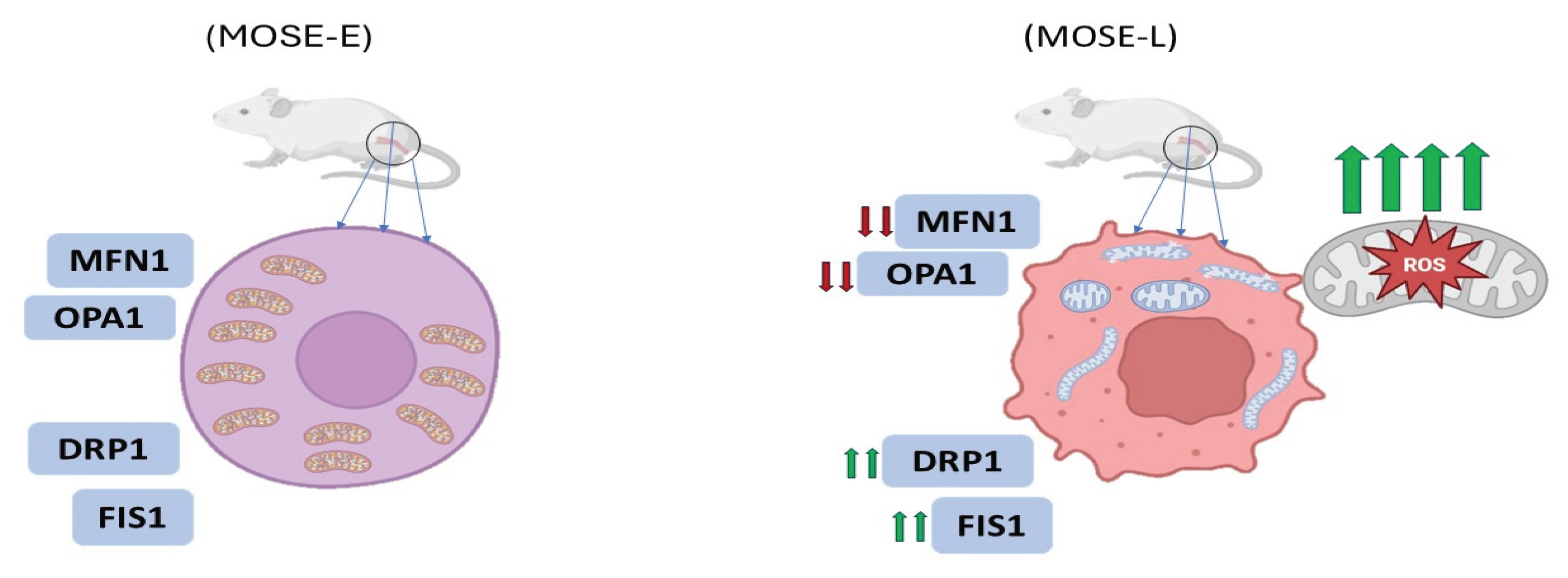
One of the most compelling illustrations of morphological transformation occurs in ovarian cancer progression. In a study utilising the mouse ovarian surface epithelial (MOSE) model, mitochondrial morphology transitioned from a filamentous network in benign cells (MOSE-E) to fragmented and enlarged spherical structures in highly aggressive tumor-initiating cells (MOSE-LTICv) (
Figure 2) [
27]. These changes were attributed to an imbalance in the expression of mitochondrial dynamics proteins: mitofusin 1 (MFN1) and OPA1 (fusion mediators) were downregulated, while fission-related proteins such as DRP1 and FIS1 were upregulated (
Figure 2). The transformation coincided with adaptation to hypoxic environments, where fragmented mitochondria facilitated enhanced autophagy and ROS regulation, supporting cell survival under stress [
27].
Another study has shown that mitochondrial morphology varies greatly among different cancer types and can be used to characterize the bioenergetic and therapeutic response profiles of tumor cells. In a study by Giedt et al. (2016), mitochondrial phenotypes in both cancer cell lines and patient-derived xenografts were categorized into three primary morphological classes: punctate (fragmented), intermediate, and filamentous (elongated). Each phenotype correlated with specific metabolic features. For example, cells displaying a predominantly punctate mitochondrial network tended to have lower oxidative phosphorylation (OXPHOS) activity and a higher reliance on glycolysis, indicating a metabolic reprogramming consistent with the Warburg effect.
Their imaging-based computational pipeline provided robust quantification of mitochondrial morphology and showed that morphological phenotypes remain consistent over time within a cell population, even across different cancer origins. Furthermore, treatments like ABT-263 and cisplatin shifted mitochondrial networks toward a more punctate state in drug-sensitive cancers, implying that mitochondrial morphology is sensitive to therapeutic intervention and may serve as an early biomarker of drug efficacy [
29]. Also, Grieco et al.2021 study demonstrated that mitochondrial fragmentation was localized around the nucleus in malignant cells, with decreased branching, shorter mean branch length, and increased circularity. This contrasted starkly with the elongated, well-distributed mitochondrial networks of benign cells. Interestingly, while hypoxia had a modest effect on mitochondrial morphology, the degree of malignancy was the primary determinant of mitochondrial form.
6.2. Ultrastructural Deformities and Functional Implications
Transmission electron microscopy (TEM) studies provide further insight into mitochondrial architecture at the nanoscale. In retinoblastoma specimens, poorly differentiated tumors exhibited mitochondria with markedly swollen appearances, disorganized or dissolved cristae (cristolysis) (
Figure 3), and even mitochondrial ghost structures—remnants without discernible internal membranes [
30]. These changes were more pronounced in tumors exhibiting necrosis or high invasiveness, implying a correlation between structural degradation and tumor aggressiveness. Additionally, retinoblastoma cells demonstrated signs of early apoptosis and necroptosis alongside mitochondrial deformation. Swollen mitochondria with dense matrices, disrupted outer membranes, and cristae loss reflect severe dysfunction in bioenergetic output and protein import systems. These abnormalities likely arise from impaired oxidative phosphorylation (OXPHOS), consistent with a shift toward glycolytic metabolism, characteristic of the Warburg phenotype (
Figure 3) [
31].
6.3. Quantitative Assessment Using Fractal Analysis
To complement qualitative classification, Lennon et al. (2016) utilized fractal geometry analysis to objectively assess mitochondrial morphology in malignant mesothelioma (MM). Using fractal dimension and lacunarity, they quantified the complexity and texture of mitochondrial networks in different MM cell lines and compared them with control mesothelial cells. Control MeT-5A cells displayed elongated and highly interconnected mitochondria, characterized by high fractal dimension and low lacunarity, representing a well-organized mitochondrial network. In contrast, mesothelioma cells such as H513 and H2596 showed lower fractal dimension and higher lacunarity, indicative of more fragmented, less space-filling networks [
30]. Interestingly, these morphological changes also correlated with therapeutic response. MM cells with lower fractal dimension and more fragmented mitochondria were more sensitive to metformin, an OXPHOS inhibitor, and mdivi-1, a Drp1 inhibitor targeting mitochondrial fission. Meanwhile, mitochondrial morphology was a better predictor of drug sensitivity than OCR (oxygen consumption rate) or ECAR (extracellular acidification rate), commonly used metabolic readouts. This highlights that structural assessments of mitochondria may have more predictive value in certain contexts than metabolic assays alone. A more granular look into mitochondrial morphology was presented by Picard et al.2012, who conducted a comprehensive electron microscopy study of skeletal muscle mitochondria, comparing intermyofibrillar (IMF) and subsarcolemmal (SS) populations. Although their study focused on muscle, the methods and descriptors—circularity, aspect ratio, form factor, and membrane contact points—are highly applicable to cancer cell analysis. Picard et al. found that mitochondrial morphology is not static; it exists on a spectrum influenced by metabolic activity, with more elongated and interconnected mitochondria observed in metabolically active regions. The presence of electron-dense contact points between adjacent mitochondria (putative fusion/fission sites) suggests dynamic interactions even in post-mitotic tissues. In cancer, these metrics often shift toward increased circularity and reduced aspect ratio, indicating fragmentation and functional compartmentalization. In the context of cancer, such morphometric tools offer a non-invasive approach to assess mitochondrial health and predict treatment responses. For instance, higher circularity and reduced branching may be predictive of cells reliant on glycolysis and prone to oxidative stress [
32].
6.4. Mitochondrial Morphology and Drug Resistance
The relationship between mitochondrial morphology and chemoresistance is particularly evident in pancreatic ductal adenocarcinoma (PDAC). In a study by Dash et al. (2024), PDAC cells deficient in deoxycytidine kinase (DCK), a key enzyme involved in activating gemcitabine, exhibited remarkable upregulation of mitochondrial genes associated with OXPHOS. These DCK-knockout (KO) cells had higher mitochondrial respiration and ATP production, correlating with enhanced resistance to gemcitabine. Notably, electron microscopy revealed abnormal mitochondrial morphology in DCK KO cells, providing strong evidence for the connection between morphological changes and metabolic reprogramming [
33,
34]. Mitochondria in DCK-deficient cells were described as more compact and structurally altered, and these changes were accompanied by reduced ROS levels, upregulation of antioxidant genes (SOD1, SOD2), and increased anti-apoptotic BCL2 expression. The morphological alterations were functionally significant, as targeting these mitochondria with complex I inhibitors (e.g., IACS-010759) or BCL2 inhibitors (venetoclax) sensitized the resistant PDAC cells to treatment. Therefore, mitochondrial shape not only reflects bioenergetic adaptations but also has the potential to influence the therapeutic vulnerabilities. Mitochondrial morphology also holds promise as a diagnostic biomarker. In retinoblastoma, poorly differentiated tumors consistently displayed fewer and less structured mitochondria compared to their well-differentiated counterparts [
30]. These morphological traits could potentially be used to stratify patients by prognosis or to guide therapy intensity. From a therapeutic standpoint, interventions targeting mitochondrial dynamics or morphology-modifying pathways (e.g., fission inhibitors like mDivi-1 or fusion promoters) could restore bioenergetic balance and trigger apoptosis. Furthermore, drugs targeting OXPHOS complexes may preferentially affect cancer cells with disrupted mitochondrial architecture and function.
7. Conclusions
Mitochondria are recognized as crucial regulators in cancer biology, controlling a wide range of cellular processes including metabolism, redox balance, apoptosis, and stress adaptation. They are no longer considered passive organelles solely responsible for ATP production. This review emphasizes the structural reprogramming and functional plasticity of mitochondria, which contribute to the malignant phenotype across various tumor types. While the Warburg effect—suggesting that cancer cells prefer glycolysis even in the presence of oxygen—has long been a focal point, substantial evidence now shows that mitochondrial oxidative phosphorylation (OXPHOS) remains active and essential in many cancers. Rather than simply shutting down mitochondrial respiration, cancer cells reprogram it to support growth, resist apoptosis, and survive in hypoxic or nutrient-deprived environments. In fact, in tumors with high metabolic demands, ATP synthesis via mitochondrial respiration often surpasses that of glycolysis. Additionally, the role of mitochondria in tumor energy metabolism is further extended by other bioenergetic pathways, such as fatty acid oxidation and the serine-one carbon-glycine (SOG) pathway.
One of the most striking hallmarks of cancer cell mitochondria is metabolic flexibility. This enables tumor cells to switch between using fatty acid oxidation, glucose, and glutamine as fuel sources based on the surrounding environment. Such flexibility supports biosynthesis and redox homeostasis in addition to energy production. This metabolic reprogramming is closely related to mitochondrial respiration rates, substrate usage patterns, and even drug responses, underscoring its diagnostic and therapeutic significance. The review highlights the importance of mitochondrial dynamics—the ongoing cycle of fission and fusion—in cancer development and metastasis, in addition to its role in metabolism. Mitochondrial fragmentation, compromised OXPHOS, and an increased propensity for migration and invasion result from disturbances in this balance, especially through overactive Drp1-mediated fission and loss of fusion regulators such as MFN1, MFN2, and OPA1. Since mitochondria are redistributed to the leading edge of migrating cells, providing localized ATP for motility, these alterations not only reflect tumor aggressiveness but also actively contribute to the potential of metastasis.
Crucially, genetic changes in regulators of mitochondrial dynamics, like downregulation of p53, which typically inhibits fission, and amplifications of DNM1L (Drp1), further entrench cancer cells in a state that is resistant to apoptosis and pro-metastatic. Since not all tumors exhibit the same level of mitochondrial remodeling, context-specific assessment is crucial when thinking about mitochondrial-targeted treatments. In certain situations, suppressing fission blunts invasion and improves the therapeutic response, whereas encouraging fusion sometimes prevents proliferation.
From filamentous to punctate forms, mitochondrial morphology provides a real-time readout of bioenergetic states and the course of disease. Fractal analysis of mitochondrial networks and high-resolution imaging have shown that elongated, interconnected networks of mitochondria are usually found in benign or well-differentiated cells, whereas fragmented, spherical mitochondria are common in aggressive and drug-resistant tumors. In addition to serving as markers of the severity of the disease, these morphological characteristics may also forecast how well a treatment will work. Research conducted on retinoblastoma and pancreatic models has demonstrated a correlation between morphological disruption and ROS levels, OXPHOS activity, and therapeutic outcomes, thereby confirming mitochondria as both therapeutic targets and biomarkers.
Given these diverse functions, cancer cells' mitochondria offer a special therapeutic opportunity. It may be possible to specifically reduce tumor viability while preserving normal cells by targeting mitochondrial ATP synthesis, changing dynamics through Drp1 or fusion protein modulation, or interfering with mitochondrial trafficking and positioning. In tumors with a high degree of mitochondrial dependency, combined approaches that combine metabolic inhibitors with pro-apoptotic agents or oxidative stress inducers may be especially successful.
In summary, mitochondria in cancer cells are not just malfunctioning metabolic remnants; rather, they are morphologically modified, functionally optimized, and strategically rewired to promote malignancy. We can discover new pathways for precise, mitochondria-targeted cancer therapies by further deconstructing their structural, metabolic, and regulatory complexity. This will ultimately improve prognosis and overcome treatment resistance.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| OXPHOS |
Oxidative phosphorylation |
| ROS |
Reactive oxygen species |
| mtDNA |
Mitochondrial DNA |
| ETC |
Electron transport chain |
| TCA |
Tricarboxylic acid |
| OXPHOS |
Oxidative phosphorylation |
| AMPK |
AMP-activated protein kinase |
| OCR |
Oxygen consumption rate |
| ECAR |
Extracellular acidification rate |
| cHCECs |
Human corneal endothelial cells |
| HGSOC |
High-grade serous ovarian cancers |
| PDAC |
Pancreatic ductal adenocarcinoma |
| EMT |
Epithelial-to-mesenchymal transition |
| DCK |
Deoxycytidine kinase |
References
- Espinosa, J. A., Pohan, G., Arkin, M. R., & Markossian, S. (2021). Real-time assessment of mitochondrial toxicity in HepG2 cells using the seahorse extracellular flux analyzer. Current Protocols, 1(3), e75. [CrossRef]
- Cheng, G., Zielonka, J., McAllister, D., Tsai, S., Dwinell, M. B., & Kalyanaraman, B. (2014). Profiling and targeting of cellular bioenergetics: inhibition of pancreatic cancer cell proliferation. British journal of cancer, 111(1), 85-93. [CrossRef]
- Campioni, G., Pasquale, V., Busti, S., Ducci, G., Sacco, E., & Vanoni, M. (2022). An optimized workflow for the analysis of metabolic fluxes in cancer spheroids using Seahorse technology. Cells, 11(5), 866. [CrossRef]
- Fonseca, J., Moradi, F., Valente, A. J., & Stuart, J. A. (2018). Oxygen and glucose levels in cell culture media determine resveratrol’s effects on growth, hydrogen peroxide production, and mitochondrial dynamics. Antioxidants, 7(11), 157.
- Sanchez-Alvarez, R., Martinez-Outschoorn, U. E., Lamb, R., Hulit, J., Howell, A., Gandara, R., ... & Sotgia, F. (2013). Mitochondrial dysfunction in breast cancer cells prevents tumor growth: understanding chemoprevention with metformin. Cell Cycle, 12(1), 172-182.
- Grünewald, A., Voges, L., Rakovic, A., Kasten, M., Vandebona, H., Hemmelmann, C., ... & Klein, C. (2010). Mutant Parkin impairs mitochondrial function and morphology in human fibroblasts. PloS one, 5(9), e12962. [CrossRef]
- Aminzadeh-Gohari, S., Weber, D. D., Catalano, L., Feichtinger, R. G., Kofler, B., & Lang, R. (2020). Targeting mitochondria in melanoma. Biomolecules, 10(10), 1395.
- Desousa, B. R., Kim, K. K., Jones, A. E., Ball, A. B., Hsieh, W. Y., Swain, P., ... & Divakaruni, A. S. (2023). Calculation of ATP production rates using the Seahorse XF Analyzer. EMBO reports, 24(10), e56380.
- Chiaradonna, F., Gaglio, D., Vanoni, M., & Alberghina, L. (2006). Expression of transforming K-Ras oncogene affects mitochondrial function and morphology in mouse fibroblasts. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1757(9-10), 1338-1356.
- Srinivasan, S., Guha, M., Kashina, A., & Avadhani, N. G. (2017). Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1858(8), 602-614. [CrossRef]
- Mikirova, N. A., Casciari, J., Gonzalez, M. J., Miranda-Massari, J. R., Riordan, N., & Duconge, J. (2017). Bioenergetics of human cancer cells and normal cells during proliferation and differentiation. Cancer Ther OncolInt J, 3, 1-8.
- Louie, M. C., Ton, J., Brady, M. L., Le, D. T., Mar, J. N., Lerner, C. A., ... & Mookerjee, S. A. (2020). Total cellular ATP production changes with primary substrate in MCF7 breast cancer cells. Frontiers in Oncology, 10, 1703.
- Zhou, Y., Tozzi, F., Chen, J., Fan, F., Xia, L., Wang, J., ... & Weihua, Z. (2012). Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer research, 72(1), 304-314. [CrossRef]
- Tedeschi, P. M., Markert, E. K., Gounder, M., Lin, H., Dvorzhinski, D., Dolfi, S. C., ... & Vazquez, A. (2013). Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell death & disease, 4(10), e877-e877.
- Van der Windt, G. J., Chang, C. H., & Pearce, E. L. (2016). Measuring bioenergetics in T cells using a seahorse extracellular flux analyzer. Current protocols in immunology, 113(1), 3-16.
- Mayberry, C. L., Wilson, J. J., Sison, B., & Chang, C. H. (2024). Protocol to assess bioenergetics and mitochondrial fuel usage in murine autoreactive immunocytes using the Seahorse Extracellular Flux Analyzer. STAR protocols, 5(2), 102971. [CrossRef]
- Sun, H., Zhou, Y., Skaro, M. F., Wu, Y., Qu, Z., Mao, F., ... & Xu, Y. (2020). Metabolic reprogramming in cancer is induced to increase proton production. Cancer Research, 80(5), 1143-1155.
- Numa, K., Ueno, M., Fujita, T., Ueda, K., Hiramoto, N., Mukai, A., ... & Hamuro, J. (2020). Mitochondria as a platform for dictating the cell fate of cultured human corneal endothelial cells. Investigative Ophthalmology & Visual Science, 61(14), 10-10.
- Anderson, G. R., Wardell, S. E., Cakir, M., Yip, C., Ahn, Y. R., Ali, M., ... & Wood, K. C. (2018). Dysregulation of mitochondrial dynamics proteins are a targetable feature of human tumors. Nature Communications, 9(1), 1677.
- Zhao, J., Zhang, J., Yu, M., Xie, Y., Huang, Y., Wolff, D. W., ... & Tu, Y. (2013). Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene, 32(40), 4814-4824.
- Ferreira-da-Silva, A., Valacca, C., Rios, E., Pópulo, H., Soares, P., Sobrinho-Simoes, M., ... & Campello, S. (2015). Mitochondrial dynamics protein Drp1 is overexpressed in oncocytic thyroid tumors and regulates cancer cell migration. PloS one, 10(3), e0122308.
- Phan, T. T., Lin, Y. C., Chou, Y. T., Wu, C. W., & Lin, L. Y. (2022). Tumor suppressor p53 restrains cancer cell dissemination by modulating mitochondrial dynamics. Oncogenesis, 11(1), 26.
- Chang, J. C., Chang, H. S., Wu, Y. C., Cheng, W. L., Lin, T. T., Chang, H. J., ... & Liu, C. S. (2019). Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. Journal of Experimental & Clinical Cancer Research, 38, 1-16.
- Caino, M. C., Seo, J. H., Aguinaldo, A., Wait, E., Bryant, K. G., Kossenkov, A. V., ... & Altieri, D. C. (2016). A neuronal network of mitochondrial dynamics regulates metastasis. Nature communications, 7(1), 13730.
- Zhang, Z., Wakabayashi, N., Wakabayashi, J., Tamura, Y., Song, W. J., Sereda, S., ... & Sesaki, H. (2011). The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Molecular biology of the cell, 22(13), 2235-2245. [CrossRef]
- Dong, F., Zhu, M., Zheng, F., & Fu, C. (2022). Mitochondrial fusion and fission are required for proper mitochondrial function and cell proliferation in fission yeast. The FEBS journal, 289(1), 262-278.
- Raad, G., Bakos, H. W., Bazzi, M., Mourad, Y., Fakih, F., Shayya, S., ... & Fakih, C. (2021). Differential impact of four sperm preparation techniques on sperm motility, morphology, DNA fragmentation, acrosome status, oxidative stress, and mitochondrial activity: A prospective study. Andrology, 9(5), 1549-1559.
- Grieco, J. P., Allen, M. E., Perry, J. B., Wang, Y., Song, Y., Rohani, A., ... & Schmelz, E. M. (2021). Progression-mediated changes in mitochondrial morphology promotes adaptation to hypoxic peritoneal conditions in serous ovarian cancer. Frontiers in oncology, 10, 600113.
- Lefebvre, A. E., Ma, D., Kessenbrock, K., Lawson, D. A., & Digman, M. A. (2021). Automated segmentation and tracking of mitochondria in live-cell time-lapse images. Nature methods, 18(9), 1091-1102. [CrossRef]
- Giedt, R. J., Fumene Feruglio, P., Pathania, D., Yang, K. S., Kilcoyne, A., Vinegoni, C., ... & Weissleder, R. (2016). Computational imaging reveals mitochondrial morphology as a biomarker of cancer phenotype and drug response. Scientific reports, 6(1), 32985.
- Singh, L., Nag, T. C., & Kashyap, S. (2016). Ultrastructural changes of mitochondria in human retinoblastoma: correlation with tumor differentiation and invasiveness. Tumor Biology, 37, 5797-5803.
- Lennon, F. E., Cianci, G. C., Kanteti, R., Riehm, J. J., Arif, Q., Poroyko, V. A., ... & Salgia, R. (2016). Unique fractal evaluation and therapeutic implications of mitochondrial morphology in malignant mesothelioma. Scientific reports, 6(1), 24578.
- Picard, M., White, K., & Turnbull, D. M. (2013). Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: a quantitative three-dimensional electron microscopy study. Journal of applied physiology, 114(2), 161-171. [CrossRef]
- Dash, S., Ueda, T., Komuro, A., Honda, M., Sugisawa, R., & Okada, H. (2024). Deoxycytidine kinase inactivation enhances gemcitabine resistance and sensitizes mitochondrial metabolism interference in pancreatic cancer. Cell Death & Disease, 15(2), 131.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).