1. Introduction
The Forced Oscillation Technique (FOT) is a non-invasive, effort-independent method for assessing respiratory mechanics, particularly valuable in subjects who are unable to perform traditional pulmonary function tests like spirometry. [
1] Its simplicity and minimal cooperation requirements make it especially suitable in early childhood, where standard tests are often not feasible due to lack of coordination or understanding. By superimposing small pressure oscillations over normal tidal breathing, FOT provides detailed insights into lung function without requiring forced expiratory maneuvers. [
2]
The technique is increasingly recognized for its value in the diagnosis and monitoring of various pulmonary conditions, including asthma, bronchiolitis, cystic fibrosis, and interstitial lung diseases. It has been found especially effective in identifying early airway injury (before clinically relevant symptoms) and in tracking disease progression or treatment response over time. [
3,
4,
5,
6,
7]
FOT assesses respiratory mechanics by applying small pressure oscillations—usually through a mouthpiece—at varying frequencies to the respiratory system during tidal breathing. These oscillations allow the calculation of respiratory impedance, which consists of two main components: resistance (Rrs) and reactance (Xrs). Resistance reflects the opposition to airflow in the airways [
8,
9]., while reactance represents the elastic and inertial properties of the respiratory system. [
10,
11] Both parameters are sensitive to changes in airway caliber, lung compliance, and breathing frequency, making them valuable indicators of respiratory health. [
12]
However, accurate interpretation of FOT results requires comparison with reference values, which must be appropriate for the specific population and equipment used. This highlights the essential need for reference equations that take into account the main determinants such as age, height, gender, and population-specific characteristics. While several datasets of children have been published, they were using mainly impulse oscillometry (IOS). [
13,
14,
15,
16]
In recent years, multiple studies have focused on establishing reference values for children using pseudorandom noise as the forcing signal. Among the most widely used reference equations for respiratory impedance parameters are those developed by Calogero et al., based on a population of healthy Italian children. The results show a high degree of consistency with the equations published by Hall et al. in an Australian pediatric cohort. Both studies used measurements at 6, 8, and 10 Hz, corresponding to the frequencies applied in impulse oscillometry. [
17,
18,
19]
In 2022, Ducharme et al. introduced updated reference equations for Canadian children aged 3 to 17 years, based on oscillometry measurements performed at 5, 11, and 19 Hz, reflecting the frequency settings of their equipment. These equations expand the applicability across age ranges and facilitate comparisons across different technical protocols. [
20]
These types of studies are crucial for enabling accurate interpretation of FOT results and for distinguishing between normal and pathological findings in clinical settings.
Although FOT is recognized as a valuable tool for assessing respiratory function, its clinical application in Bulgaria remains relatively limited. Expanding access and standardizing protocols across centers would enhance diagnostic capabilities, especially in young children who are underserved by traditional testing methods.
This study aims to elicit the main determinants for resistance (Rrs) and reactance (Xrs) achieved through forced oscillation technique (FOT) in Bulgarian pediatric population aged 2 to 8.
2. Materials and Methods
Participants
In this study, we recruited a cohort of 100 healthy pre-school and young children who had no history of bronchial asthma, nor any previously reported episodes of wheezing or other chronic respiratory conditions. (
Table 1) To ensure the accuracy and reliability of the respiratory function measurements, we excluded children who had experienced any signs of respiratory tract infections or symptoms, such as coughing and nasal congestion, at least two weeks preceding the assessment.
All participants included in the final analysis were Caucasians and were enrolled from kindergartens and primary schools located in the Pazardzhik region of southern Bulgaria. The recruitment of children from the general population, rather than from clinical settings, was meant to establish statistically representative cohort of healthy children within this demographic group.
Prior to inclusion in the study, the purpose and procedures were explained in detail to the parents and legal guardians of each child. Efforts were made to ensure a child-friendly, stress-free testing environment during all measurements, particularly given the young age of the study population. Participation was strictly voluntary, and written informed consent was obtained for every child.
FOT Methodology
All participants were assessed on site - in kindergartens and primary schools located in Pazardzhik. The main parameters - Rrs and Xrs - were measured according to American Thoracic Society/European Respiratory Society (ATS/ERS) recommendation [
21], using a commercially available device implementing the FOT (Resmon PRO FULL (V3), RESTECH Srl, Milano, Italy), which generates an oscillatory sound signal at multiple frequencies - at 5, 11, and 19 Hz.
A daily calibration was performed as recommended prior to each testing session using a manufacturer-supplied calibration device to ensure accuracy and consistency of the measurements.
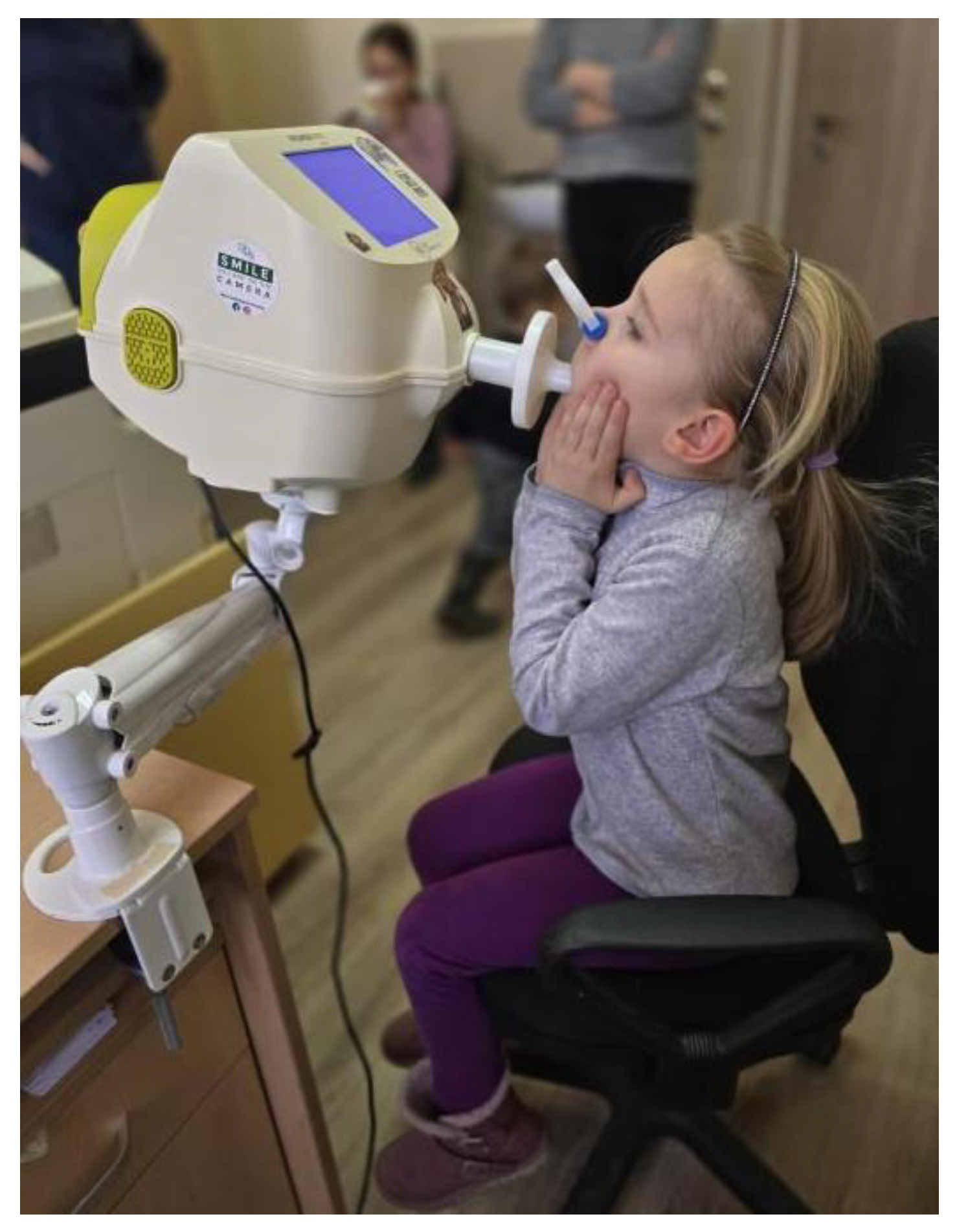
Each child performed the test in a seated position, with straight back and head held in a neutral or slightly extended position. To ensure proper airflow and minimize air leakage, a nose clip was placed on each child, and they were instructed to breathe quietly through a mouthpiece fitted with an antibacterial filter. To reduce upper airway shunting and improve measurement reliability, the cheeks and mouth floor were supported either by the examiner or, in the case of younger children, by a parent. (
Figure 1) [
1,
22,
23]
For each child, at least three acceptable measurements were performed. Each measurement included a minimum of six valid tidal breaths, following the manufacturer’s recommendations. Quality control was applied to all recordings. Data was reviewed and only recordings without artifacts—such as coughing, talking, or crying—were included. All accepted data met predefined technical criteria to ensure consistency and reliability.
From each recording, values for respiratory resistance (Rrs) and respiratory reactance (Xrs) were extracted at each of the three tested frequencies. These parameters served as the primary indicators for evaluating respiratory mechanics within the studied population.
Statistical Methods
The collected data were statistically analyzed using SPSS software, version 20.0. The analysis included descriptive statistics, as well as variance, correlation, regression, and comparative analyses to explore relationships between the Forced Oscillation Technique (FOT) parameters and anthropometric variables. Stepwise multiple linear regression models were applied to identify the most significant predictors/determinants of respiratory resistance and reactance, with height, weight, gender, and age entered as independent variables. These models helped determine the degree to which each factor contributed to the variation in respiratory mechanics across the pediatric population. A p-value of less than 0.05 was considered statistically significant.
The study protocol was reviewed and approved by the Ethics Committee at the Medical University of Plovdiv, Bulgaria. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki and all applicable national regulations concerning research involving human subjects.
3. Results
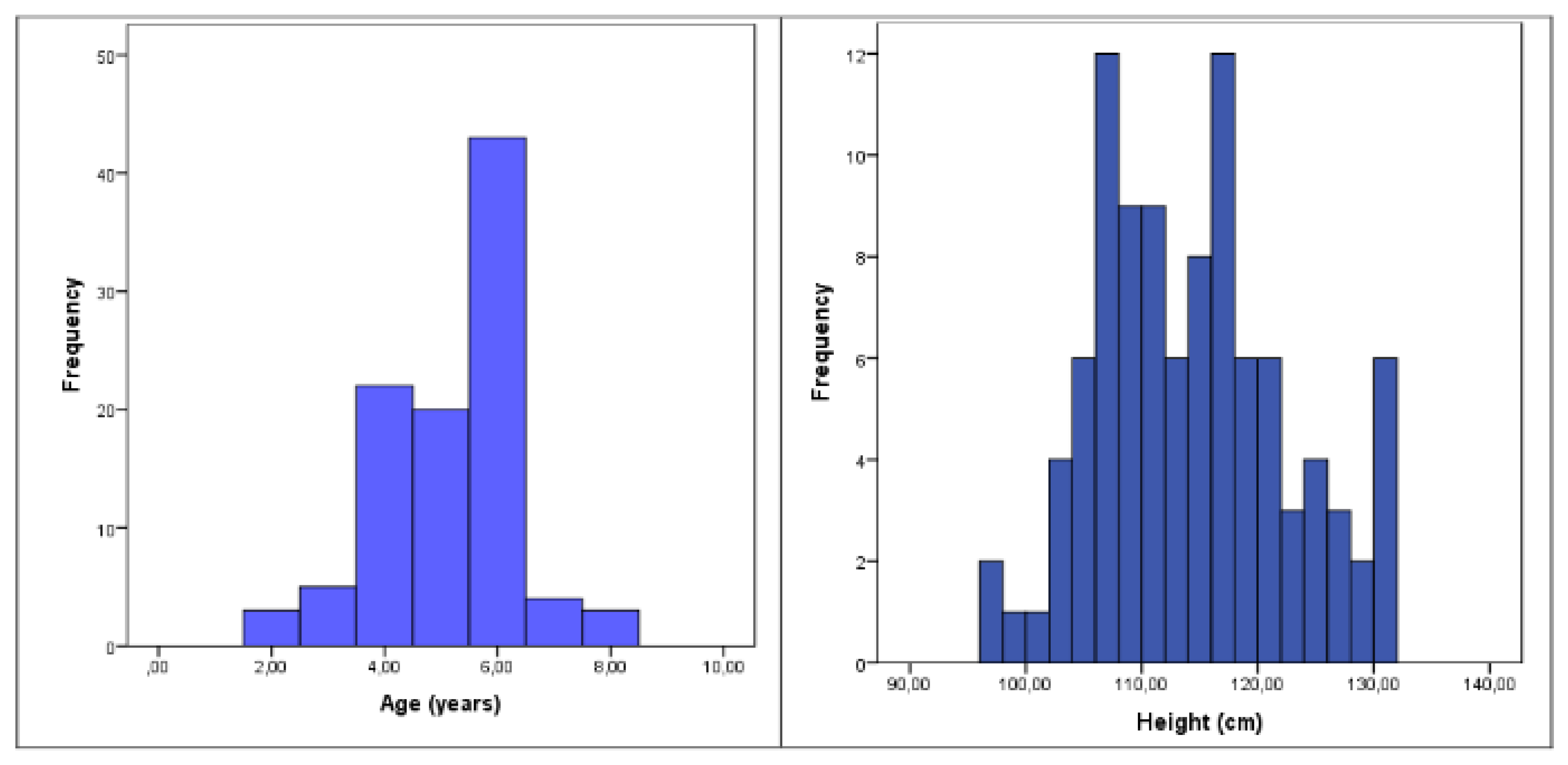
A total of 100 children, ranging in age from 2 to 8 years, were included in the study sample. There was a slight predominance of boys, who made up 56% of the sample, compared to 44% girls. The distributions of age and height within the cohort are illustrated in
Figure 2, providing a clear overview of the developmental spread across the sample and the demographic and anthropometric characteristics of the study participants are illustrated on
Table 1.
Correlation coefficients between resistance and reactance and the anthropometric variables—age, height and weight are presented on
Table 2. The parameters are analyzed separately for boys and girls. Thе breakdown shows clearly how the FOT measures relate to growth parameters across sexes.
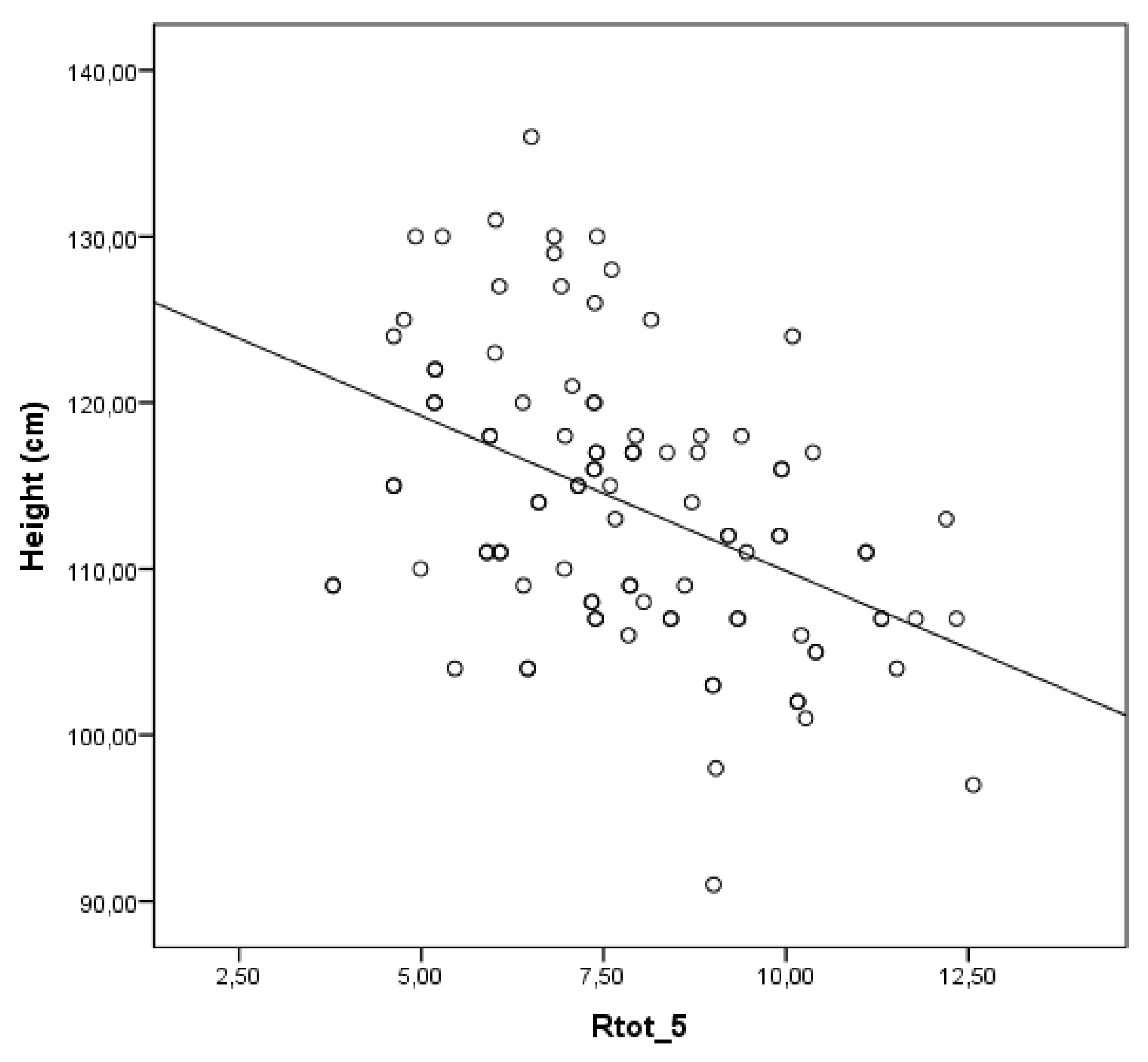
Moderate inverse correlation exists between the mean whole breath resistance at 5 Hz (Rtot 5) and height in children (r = -0.446; p < 0.001) –
Figure 3.
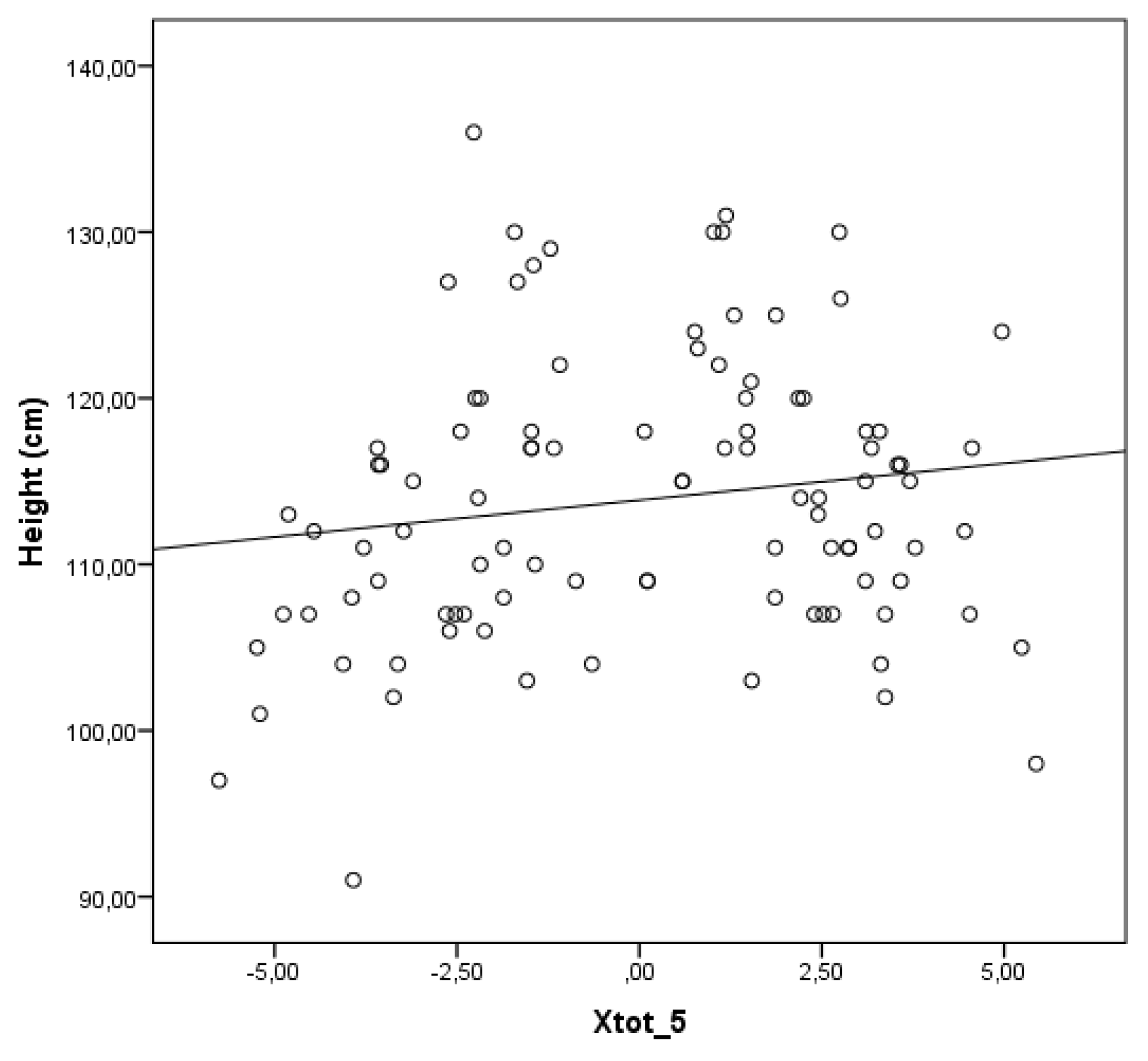
In contrast to the findings related to the mean whole breath resistance at 5 Hz (Rtot 5), the correlation presented in
Figure 4 demonstrates a weak but statistically significant positive relationship between the mean whole breath reactance at 5 Hz (Xtot 5) and height (r = 0.153; p = 0.014). This suggests that, with increasing height, there is a slight upward trend in Xtot 5 values.
There was no significant association between Rtot 5 or Xtot 5 and sex in our population of children aged 2 to 8 years, indicating that these respiratory impedance parameters are not influenced by gender within this age group.
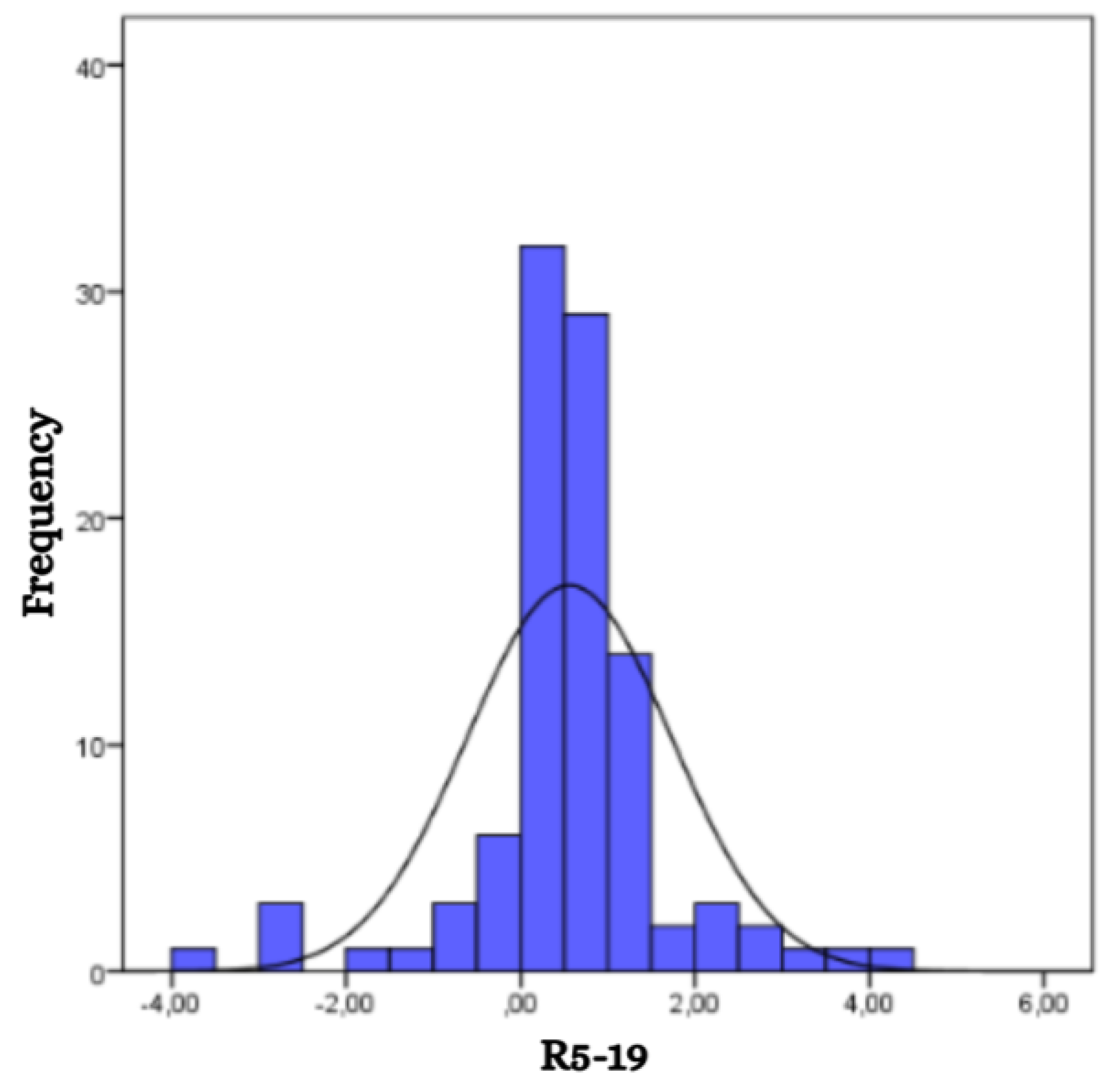
The mean value of the difference in respiratory resistance between 5 Hz and 19 Hz (R5-19) for the studied group was 0.55, with a range from -3.82 to 4.46 (
Figure 5). No significant association was found between R5-19 and either sex or age of the children, although slight variations in mean values were observed between boys (0.57) and girls (0.53).
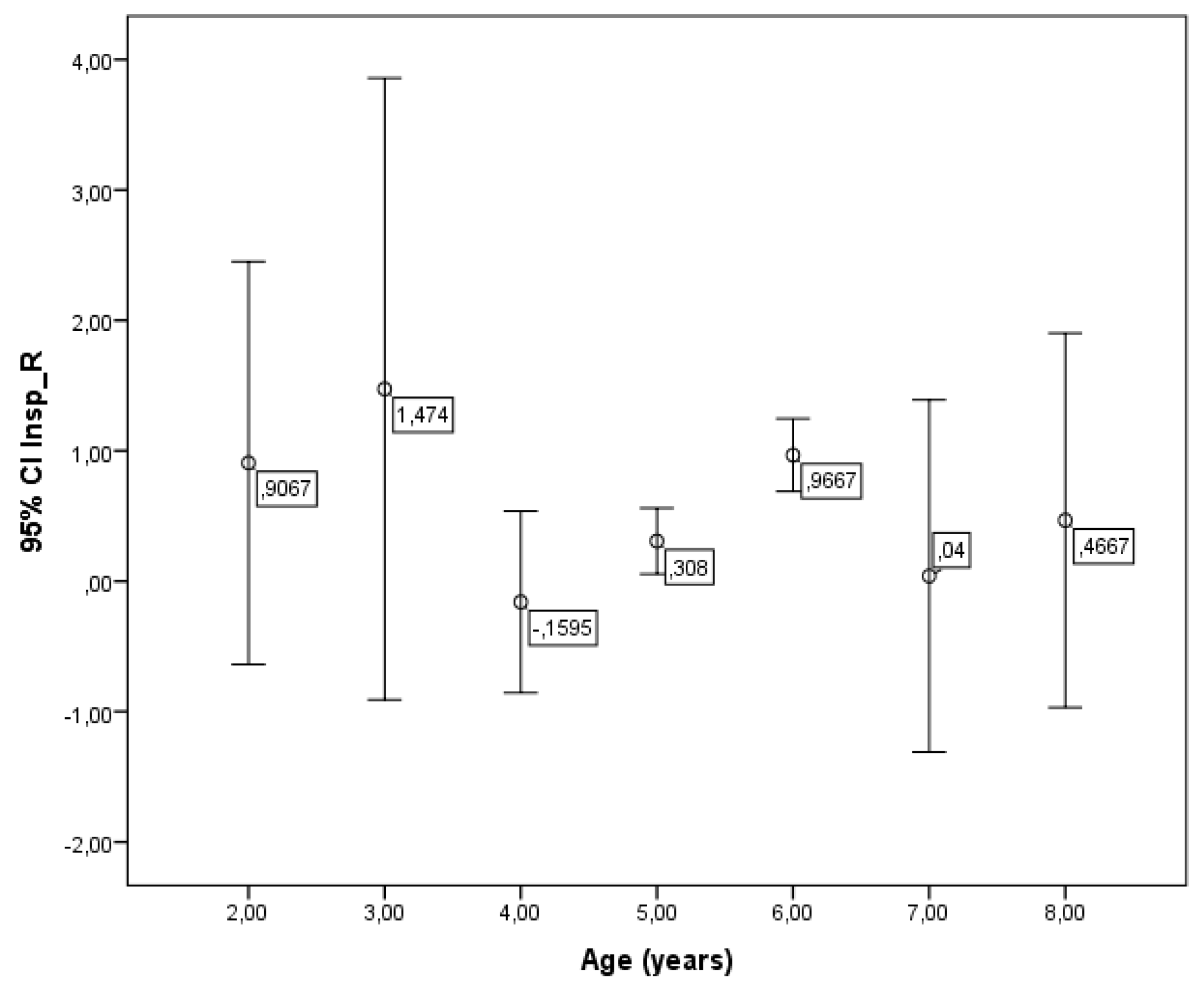
Although no overall correlation between R5-19 and age was found, a significant difference in R5-19 values was observed across different age groups (p = 0.003) (
Figure 6). However, no significant associations were detected between R5-19 and either weight or height in the children studied.
A multiple stepwise regression analysis was performed using the lowest recorded value for each child, considering both natural and logarithmic transformations of the variables: height, weight, age, and sex. The analysis revealed that height was the only significant predictor across all measured parameters, with one exception - reactance at an oscillating frequency of 5 Hz - where both height and weight emerged as significant predictors. For all other variables, neither sex nor age contributed meaningfully to the predictive models. These findings suggest that height plays a central role in influencing the physiological outcomes examined, whereas age and sex appear to have minimal predictive value in this context. A summary of the regression results is provided in
Table 3.
4. Discussion
This study represents the first comprehensive effort to identify the key determinants for respiratory impedance parameters - resistance (Rrs) and reactance (Xrs) - obtained via the forced oscillation technique (FOT) in the pediatric population of Bulgaria. Height was identified as the primary determinant of both respiratory resistance and reactance across all tested frequencies and is a superior predictor of respiratory impedance compared to other commonly used parameters such as age, weight, or sex. [
17,
19,
24,
25,
26] Although these additional factors are often included in lung function models, their incorporation did not significantly enhance the predictive accuracy of our regression analyses.
These findings are in line with well-documented physiological patterns, where somatic growth, particularly increases in height, corresponds with the expansion of airway caliber and lung volumes during childhood. As the lungs and airways grow, airway resistance tends to decrease, while elastic recoil and overall compliance (reflected in reactance) change accordingly. These developmental trends are especially important in early life when lung growth is rapid and non-linear. [
12]
In the absence of region-specific normative data, this study fills a critical gap by providing population-relevant reference values for respiratory impedance measured via FOT. The results offer a valuable foundation for clinical interpretation and future research in pediatric respiratory assessment.
The observed inverse relationship between Rtot 5 and height is physiologically consistent with normal lung growth and airway development during early childhood. [
27,
28] This developmental trajectory is well-documented and aligns with established pediatric respiratory physiology. The trend for decreasing Rtot 5 as height increases supports the reliability of this parameter as a sensitive and developmentally appropriate marker for assessing airway function in young children. [
12]
Moreover, these findings highlight the clinical utility of FOT in pediatric populations, particularly in younger children who may not reliably perform conventional spirometry due to age or cognitive limitations. [
1,
29] This technique provides a non-invasive, effort-independent assessment of respiratory mechanics, allowing for objective monitoring of airway resistance even in minimally cooperative patients. These attributes make FOT especially valuable for early detection and longitudinal tracking of respiratory conditions such as asthma, bronchopulmonary dysplasia, or post-infectious airway obstruction in preschool-aged children. [
8,
9,
30,
31]
Indeed, height was the only statistically significant predictor for Rrs across all frequencies tested. This finding is consistent with many other articles that also reported inverse correlations between height and resistance in healthy children. [
32,
33,
34,
35,
36,
37,
38] Although our regression intercepts differ slightly from those reported by Klug and Bisgaard [
39], this discrepancy primarily reflects differences in baseline values (offsets), likely due to variations in measurement techniques, equipment calibration, or sample characteristics. Importantly, the slope of the resistance–height relationship in our study closely aligns with their results, supporting the universality of the developmental trend across different cohorts.
In contrast to the inverse relationship observed for Rtot 5, a weak but statistically significant positive correlation was found between Xtot 5 and height. This suggests that as children grow taller, Xtot 5 values become less negative, reflecting improved compliance and reduced peripheral airway resistance—expected features of normal lung development. This trend is supported by prior research showing that reactance increases (becomes less negative) with lung growth in children, which identifies height as key determinant of reactance values in pediatric populations. [
13,
40]
Our findings align with previous studies reporting no significant sex-related differences in respiratory impedance parameters measured via FOT in young children. For instance, Dencker et al. [
13] found that gender did not significantly influence resistance or reactance values in children under 10 years of age. This consistency is likely due to the minimal physiological differences in lung size, thoracic structure, and airway mechanics between boys and girls prior to puberty.
In our cohort of children aged 2 to 8 years, the absence of sex-specific trends in Rrs and Xrs supports the idea that sexual dimorphism in lung function typically becomes evident only during and after puberty, driven by divergent growth patterns and hormonal changes. [
13] Therefore, the lack of significant sex-related variation in this age group justifies the application of shared reference equations for both sexes in clinical and research settings.
The frequency-dependent change in resistance (R5–19), reflecting small airway resistance, showed no significant correlation with sex, age, height, or weight in our cohort, consistent with findings from De et al. [
41] In contrast, however, De et al. reported a clear decrease in R5–19 values with increasing age, suggesting a more pronounced age-related decline in small airway resistance. This difference may reflect variations in study populations, measurement techniques, or age distribution. Overall, these findings underscore the complexity of interpreting R5–19 in early childhood and the potential influence of subtle developmental factors.
5. Conclusions
This study confirms that height is the most significant anthropometric determinant of respiratory resistance, aligning with established physiological understanding and previous research. While sex and age showed no consistent linear relationship with Rrs and Xrs, significant group differences suggest developmental influences, particularly in early childhood. The development of population-specific reference equations is essential for accurate interpretation of FOT results in both clinical and research settings.
Author Contributions
Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing and visualization are done by all three authors - P.S., S.M., B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the Medical University of Plovdiv (protocol code № 5 / 06.06.2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this article are not readily available due to ethical personal data sharing restrictions. Requests to access the datasets should be directed to the corresponding author.
Acknowledgments
We gratefully acknowledge the support of the Medical University of Plovdiv for providing the institutional framework and resources that made this study possible.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FOT |
Forced oscillation technique |
| Rrs |
Respiratory resistance |
| Xrs |
Respiratory reactance |
| R5–19 |
Difference in respiratory resistance between 5 Hz and 19 Hz |
| Rtot 5 |
Mean resistance of the whole breath at 5 Hz |
| Xtot 5 |
Mean reactance of the whole breath at 5 Hz |
References
- Oostveen, E. MacLeod D.; Lorino H.; et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003, 22, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Stoimenova, P.; Mandadzhieva, S.; Marinov, B. Clinical applications of forced oscillation technique (FOT) for diagnosis and management of obstructive lung diseases in children. Folia Medica 2024, 66, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Stockley, J.; Cooper, B.; Stockley, R.; Sapey, E. Small airways disease: time for a revisit? International Journal of Chronic Obstructive Pulmonary Disease 2017, 12, 2343–2353. [Google Scholar] [CrossRef]
- Ribeiro CO, Faria ACD, Lopes AJ et al Forced oscillation technique for early detection of the effects of smoking and COPD: contribution of fractional-order modeling. Int J Chron Obstruct Pulmon Dis. 2018 Oct 11;13:3281-3295.
- Bhattarai, P.; Myers, S.; Chia, C.; et al. Clinical Application of Forced Oscillation Technique (FOT) in Early Detection of Airway Changes in Smokers. J Clin Med. 2020, 9, 2778. [Google Scholar] [CrossRef] [PubMed]
- Borrill, Z.L.; Roy, K.; Vessey, R.S.; Woodcock, A.A.; Singh, D. Non-invasive biomarkers and pulmonary function in smokers. Int. J. Chronic Obstr. Pulm. Dis. 2008, 3, 171–183. [Google Scholar] [CrossRef]
- Schivinski, C.; De Assumpção, M.; De Figueiredo, F.; Wamosy, R.; Ferreira, L.; Ribeiro, J.D. Impulse oscillometry, spirometry, and passive smoking in healthy children and adolescents. Rev. Port. Pneumol. (Engl. Ed.) 2017, 23, 311–316. [Google Scholar] [CrossRef]
- Brashier, B.; & Salvi, S. Measuring lung function using sound waves: role of the forced oscillation technique and impulse oscillometry system. Breathe 11, 57–65. [CrossRef]
- Smith H., J.; Eichler, R.; Vogel, J.; & Arnold, J. Technische Adaption der Impuls-Oszillometrie an spezielle Untersuchungsbedingungen [Technical adaptation of impulse oscillometry to special research conditions]. Pneumologie 1997 (Stuttgart, Germany), 51 Suppl 2, 465–468.; while reactance represents the elastic and inertial properties of the respiratory system.
- Desai, U.; & Joshi J., M. Impulse Oscillometry. Advances in Respiratory Medicine. 2019; 87, 235–238. [Google Scholar] [CrossRef]
- Liang, X.; Zheng, J.; Gao, Y.; Zhang, Z.; Han, W.; Du, J.; Lu, Y.; Chen, L.; Wang, T.; Liu, J.; Huang, G.; Zhao, B.; Zhao, G.; Zhang, X.; Peng, Y.; Chen, X.; & Zhou, N. Clinical application of oscillometry in respiratory diseases: an impulse oscillometry registry. ERJ open research 2022, 8, 00080–2022. [Google Scholar] [CrossRef]
- Kostorz-Nosal, S.; Jastrzębski, D.; Błach, A.; & Skoczyński, S. Window of opportunity for respiratory oscillometry: A review of recent research. Respiratory physiology & neurobiology 2023, 316, 104135. [Google Scholar] [CrossRef]
- Dencker M, Malmberg LP, Valind S, Thorsson O,Karlsson MK,Pelkonen A, Pohjanpalo A, Haahtela T, Turpeinen M, Wollmer P. Reference values for respiratory system impedance by using impulse oscillometry in children aged 2–11 years. Clin Physiol Funct Imaging 2006, 26, 247–250. [CrossRef] [PubMed]
- Amra B, Soltaninejad F, Golshan M. Respiratory resistance by impulse oscillometry in healthy Iranian children aged 5–19 years. Iran J Allergy Asthma Immunol 2008, 7, 25–29.
- Frei J, Jutla J, Kramer G, Hatzakis GE, Ducharme FM, Davis GM. Impulse oscillometry: reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest 2005, 128, 1266–1273.
- Nowowiejska B, Tomalak W, Radlinski J, Siergiejko G, Latawiec W, Kaczmarski M. Transient reference values for impulse oscillometry for children aged 3–18 years. Pediatr Pulmonol 2008, 43, 1193–1197. [CrossRef] [PubMed]
- Calogero C, Simpson SJ, Lombardi E, et al. Respiratory impedance and bronchodilator responsiveness in healthy children aged 2-13 years. Pediatr Pulmonol. 2013, 48, 707–715. [CrossRef] [PubMed]
- Calogero C, Parri N, Baccini A, et al. Respiratory impedance and bronchodilator response in healthy Italian preschool children. Pediatr Pulmonol. 2010, 45, 1086–1094. [CrossRef]
- Hall GL, Sly PD, Fukushima T, Kusel MM, Franklin PJ, Horak F, Jr.; Patterson H, Gangell C, Stick SM. Respiratory function in healthy young children using forced oscillations. Thorax 2007, 62, 521–526.
- Ducharme FM, Smyrnova A, Lawson CC, et al. Reference values for respiratory sinusoidal oscillometry in children aged 3 to 17 years. Pediatr Pulmonol 2022, 57, 2092–2102. [CrossRef]
- King GG, Bates J, Berger KI, et al. Technical standards for respiratory oscillometry. Eur Respir J 2020, 55, 1900753. [CrossRef]
- Kaminsky DA, Irvin CG, Lundblad L et al. Oscillation mechanics of the human lung periphery in asthma. J Appl Physiol 2004, 97, 1849–58. [CrossRef]
- Bickel S, Popler J, Lesnick B et al. Impulse oscillometry: interpretation and practical applications. Chest 2014, 146, 841–847.
- Malmberg, L.P.; Pelkonen, A.; Poussa, T.; Pohianpalo, A.; Haahtela, T.; Turpeinen, M. Determinants of respiratory system input impedance and bronchodilator response in healthy Finnish preschool children. Clin Physiol Funct Imaging 2002, 22, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yoon, J.W.; Shin, Y.H.; Jee, H.M.; Wee, Y.S.; Chang, S.J.; Sim, J.H.; Yum, H.Y.; Han, M.Y. Reference values for respiratory system impedance using impulse oscillometry in healthy preschool children. Korean J Pediatr 2011, 54, 64–68. [Google Scholar] [CrossRef]
- Frei, J.; Jutla, J.; Kramer, G.; Hatzakis, G.E.; Ducharme, F.M.; Davis, G.M. Impulse oscillometry: reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest 2005, 128, 1266–1273. [Google Scholar] [CrossRef]
- Zapletal, A.; Paul, T.; & Samanek, M. Pulmonary function in children and adolescents: Methods, reference values. Progress in Respiratory Research 1976, 9, 1–210. [Google Scholar]
- Eigen, H.; Bieler, H.; Grant, D.; Christoph, K.; Terrill, D.; & Heilman, D. K. Spirometric pulmonary function in healthy preschool children. American Journal of Respiratory and Critical Care Medicine 2001, 163(3 Pt 1), 619–623. [Google Scholar] [CrossRef]
- Beydon, N.; Davis S., D.; Lombardi, E.; Allen J., L.; Arets H., G.; Aurora, P.; et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. American Journal of Respiratory and Critical Care Medicine 2007, 175, 1304–1345. [Google Scholar] [CrossRef]
- Hall G., L.; Reinero C., R.; Bates J. H., T.; & King G., G. Oscillometry: An alternative and complementary respiratory function test to spirometry. Thorax 2021, 76, 738–744. [Google Scholar]
- Desai, U.; & Joshi J., M. Joshi J. M. Impulse Oscillometry. Advances in Respiratory Medicine. 2019; 87, 235–238. [Google Scholar] [CrossRef]
- Liang, X.L.; Gao, Y.; Guan, W.J. , et al. Reference values of respiratory impedance with impulse oscillometry in healthy Chinese adults. J Thorac Dis. 2021;13, 3680-3691. [CrossRef]
- Zhao, M.; Han, A.; Fang, J. Determination of respiratory impedance of healthy adults by pulsating pulmonary function. Chin J Tuberc Respir Dis 2002, 25, 636. [Google Scholar]
- Oostveen E, Boda K, van der Grinten CP, et al. Respiratory impedance in healthy subjects: baseline values and bronchodilator response. Eur Respir J 2013, 42, 1513–23. [CrossRef]
- Zhang X, Zheng J, Chen Y, et al. Investigations of impulse oscillation technique and predictive equations in Macao healthy adults. Journal of Practical Internal Medicine 2015, 35, 431–5.
- Kalchiem-Dekel O, Hines SE. Forty years of reference values for respiratory system impedance in adults: 1977-2017. Respir Med 2018, 136, 37–47. [CrossRef] [PubMed]
- Li F, Wang X, Wan Y, et al. Analysis of the normal Values of pulmonary function by impulse oscillometry in healthy adults in Lanzhou. Prog Microbiol Immunol 2012, 40, 34–7.
- Hellinckx J, De Boeck K, Bande-Knops J, et al. Bronchodilator response in 3–6.5 years old healthy and stable asthmatic children. Eur Respir J 1998, 12, 438–443. [CrossRef]
- Klug B, Bisgaard H. Specific airway resistance, interrupter resistance, and respiratory impedance in healthy children aged 2–7 years. Pediatr Pulmonol 1998, 25, 322–331. [CrossRef]
- de Assumpção MS, Gonçalves RM, Martins R, Bobbio TG, Schivinski CI. Reference Equations for Impulse Oscillometry System Parameters in Healthy Brazilian Children and Adolescents. Respir Care. 2016, 61, 1090–1099. [CrossRef]
- De S, Banerjee N, Tiwari RR. Regression Equations of Respiratory Impedance Measured by Forced Oscillation Technique for Indian Children. Indian J Pediatr. 2020;87, 192-199. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).