Submitted:
30 May 2025
Posted:
30 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of HTNR
2.2.2. Analysis
2.2.3. Kinetic Model
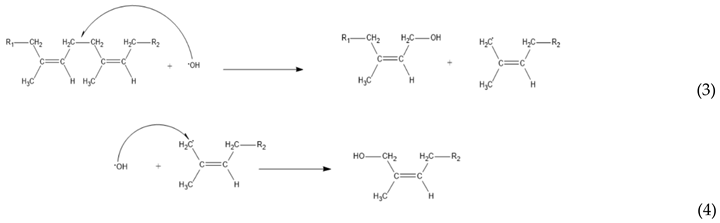
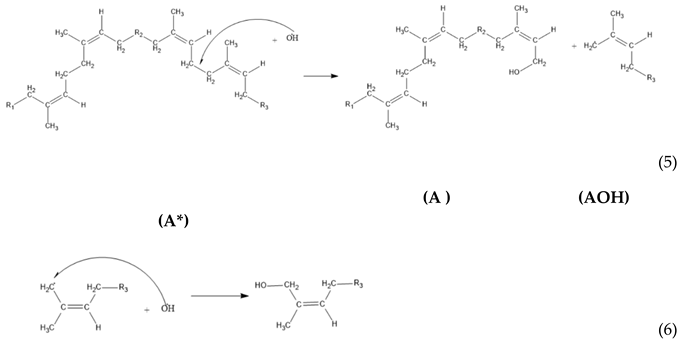
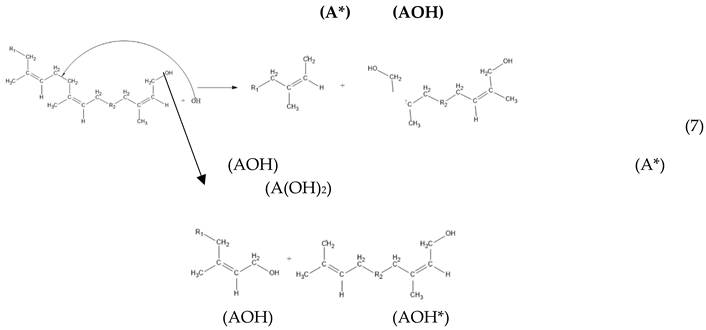
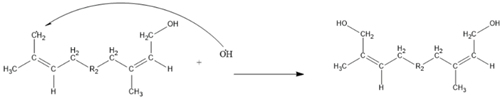
3. Results and Discussion
3.1. HTNR Characterization
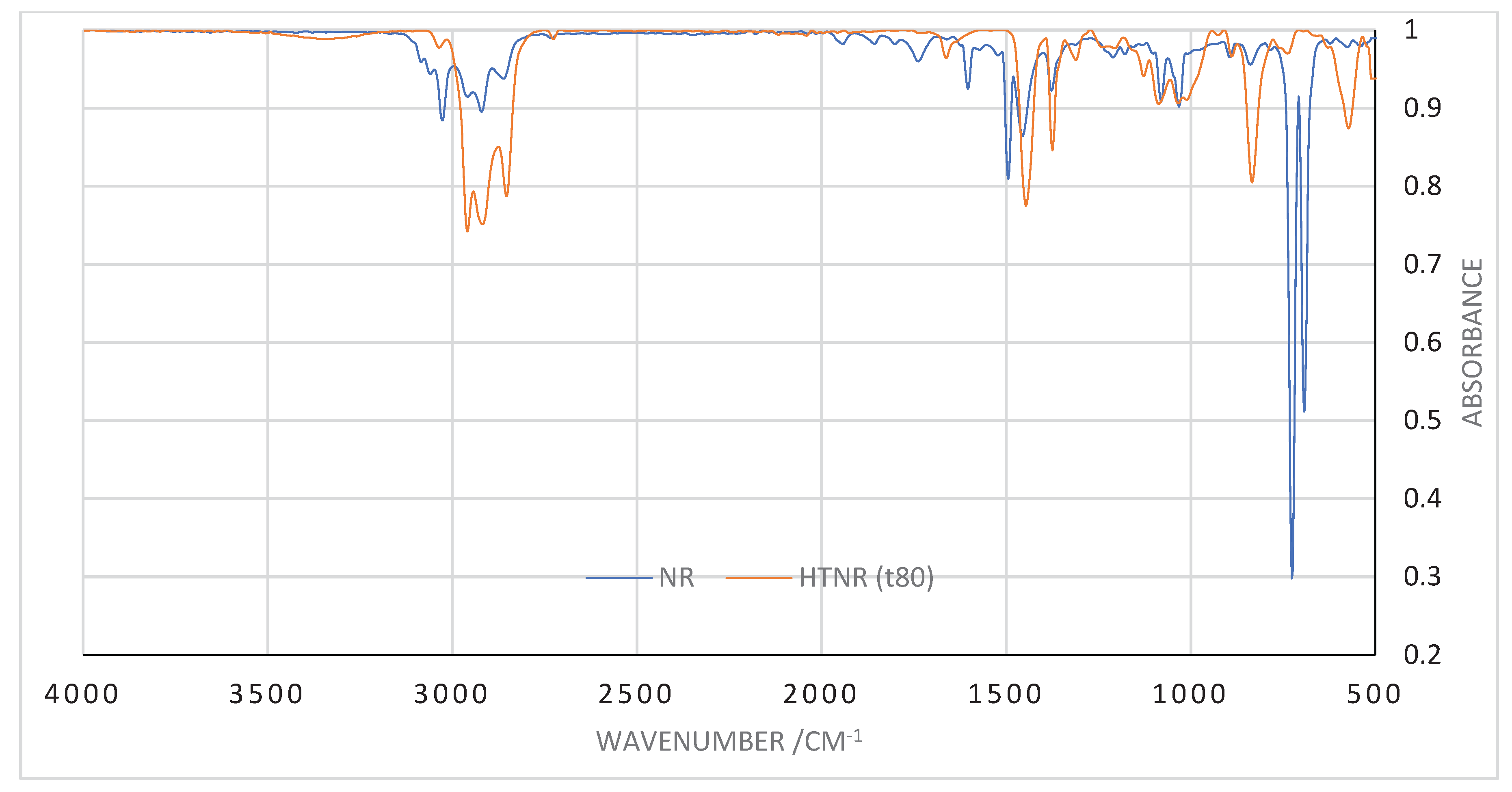
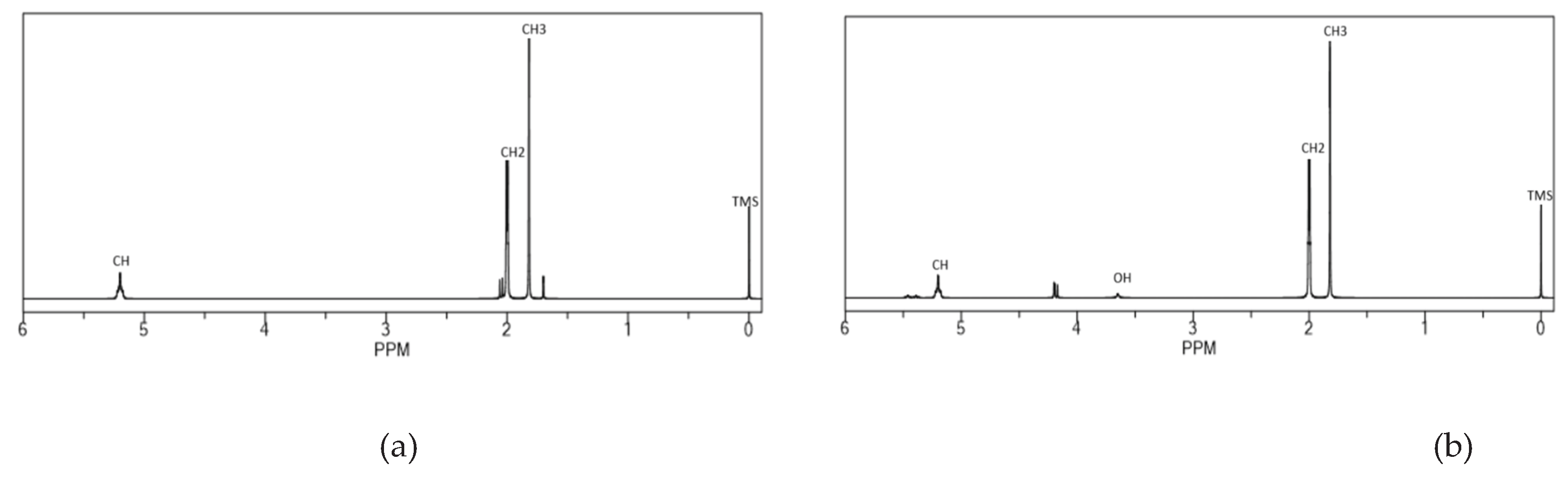
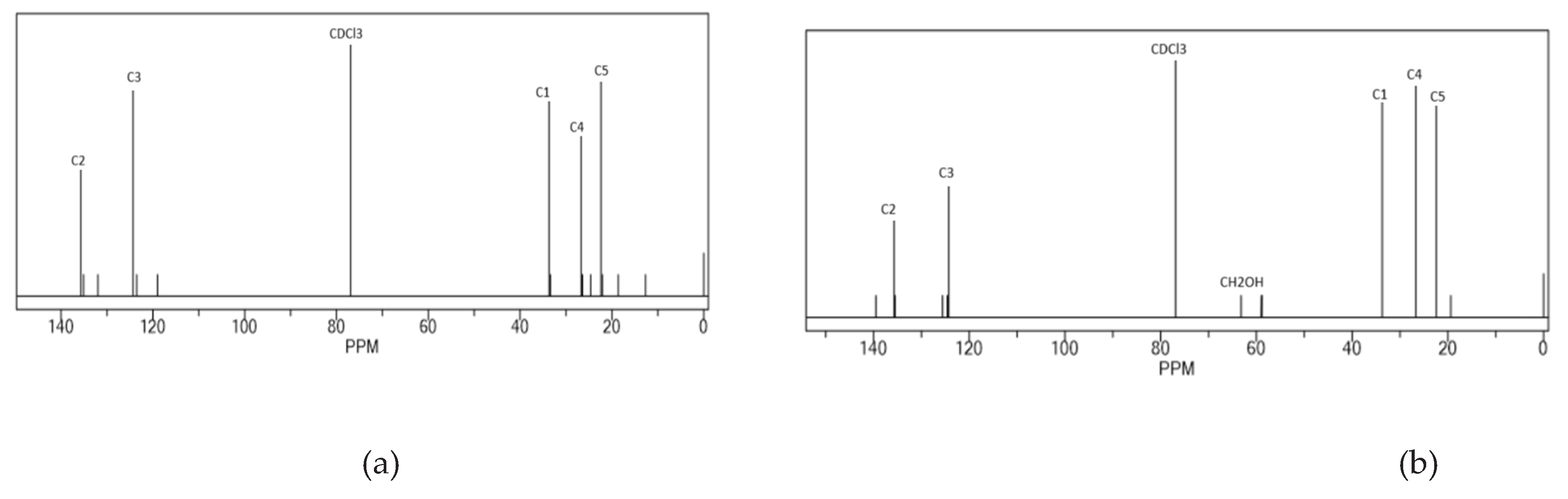
3.2. Penggunaan Serapan Infra Merah Untuk Analisis Depolimerisasi
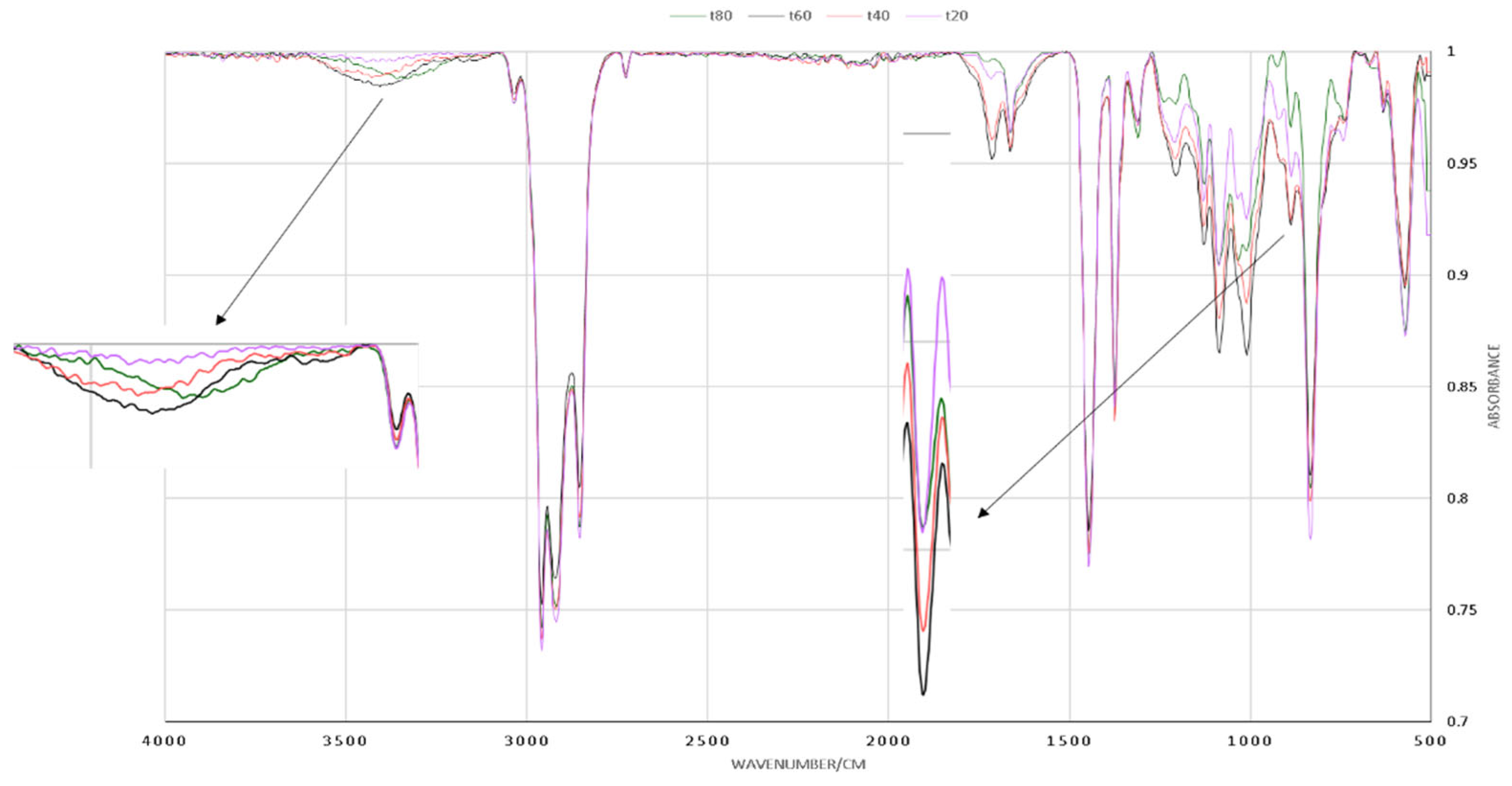
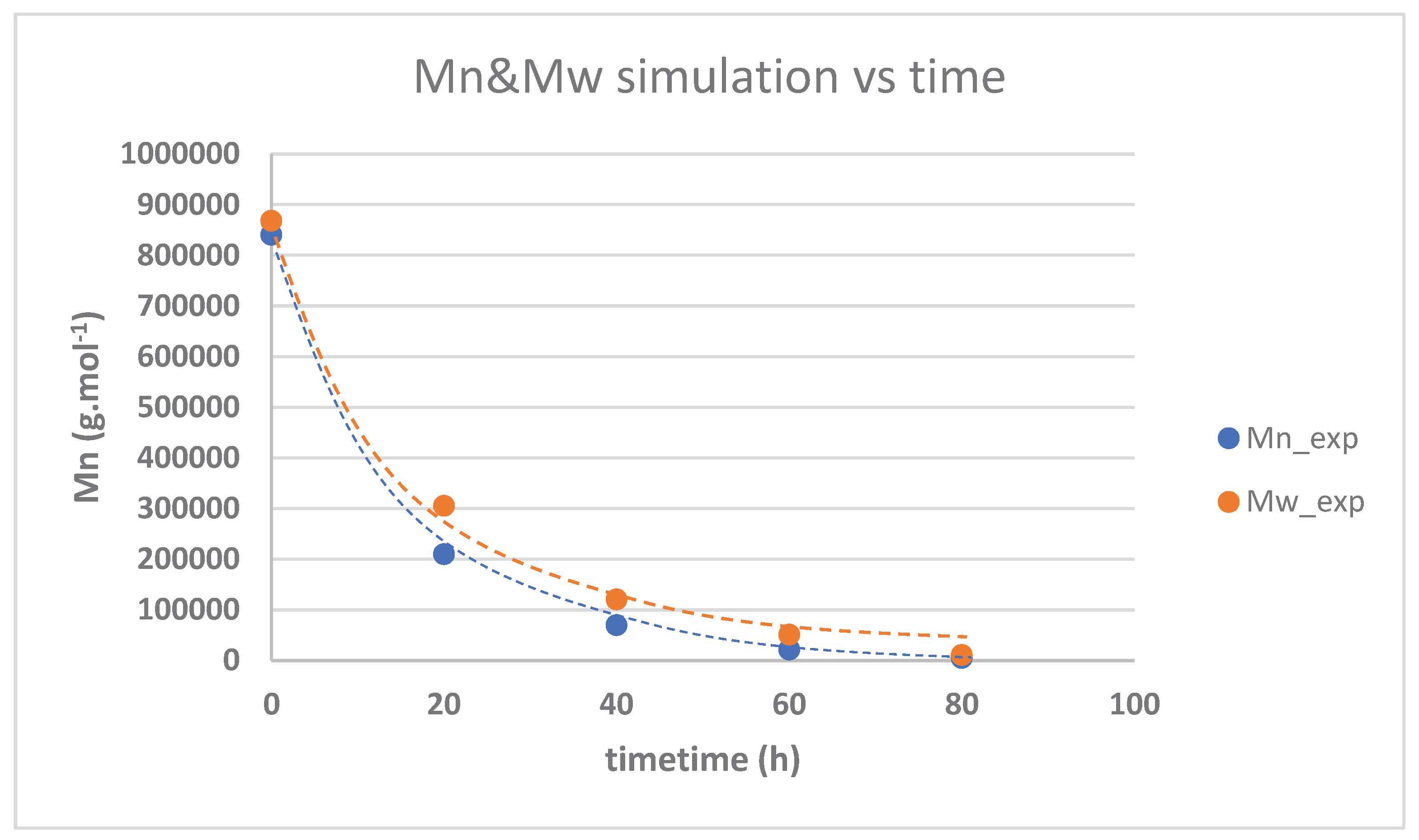
3.3. Kinetika reaksi pembentukan HTNR
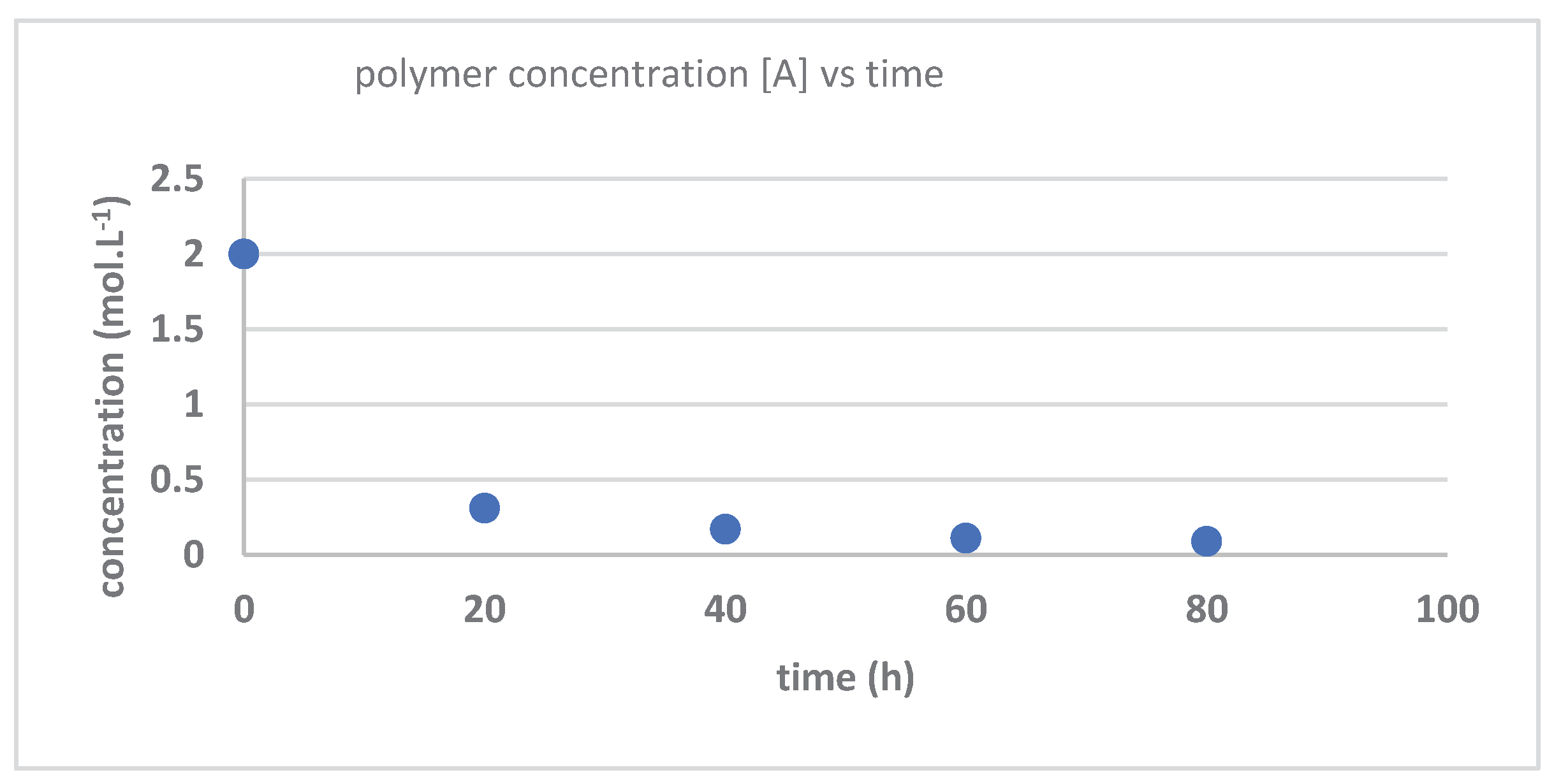
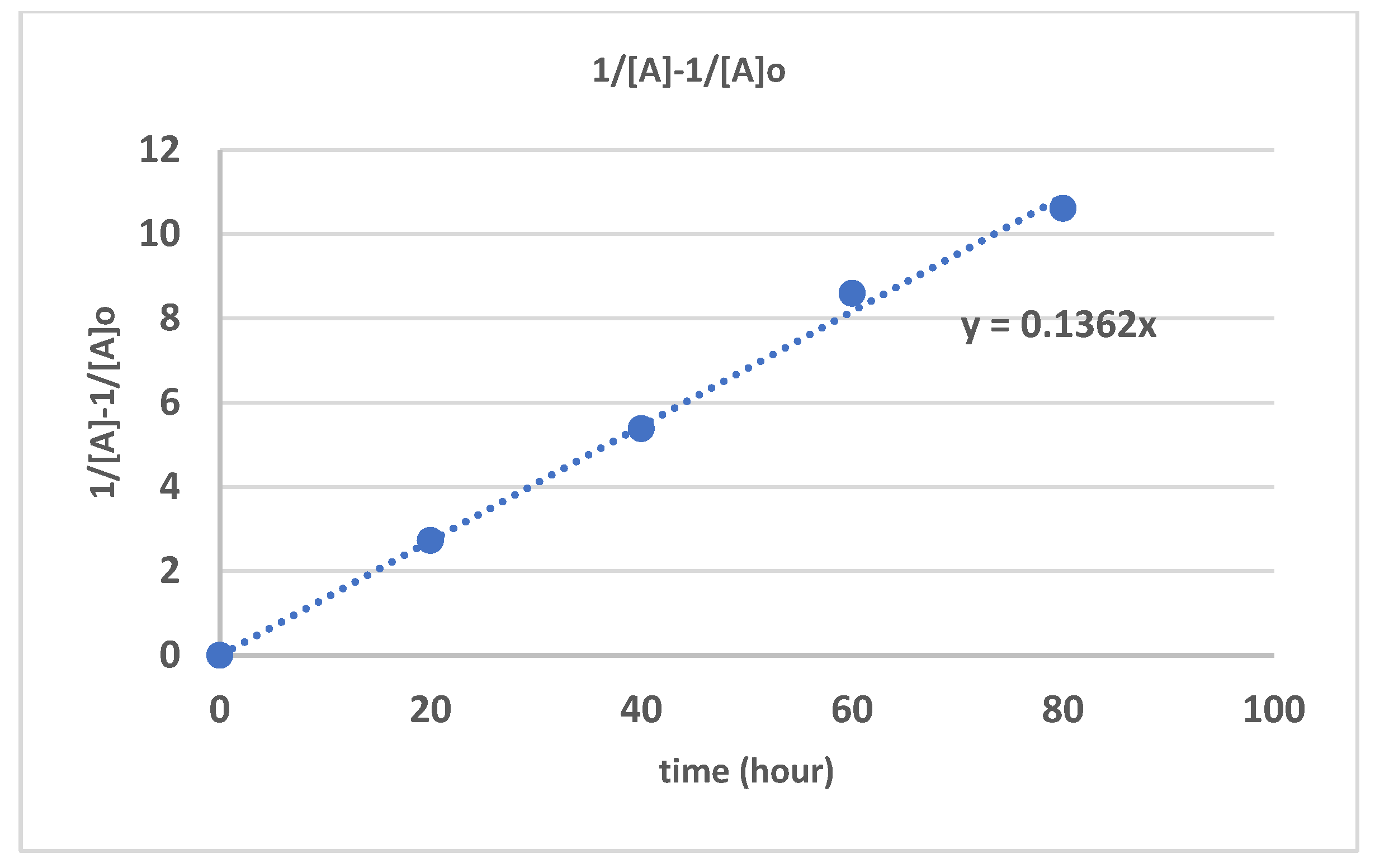
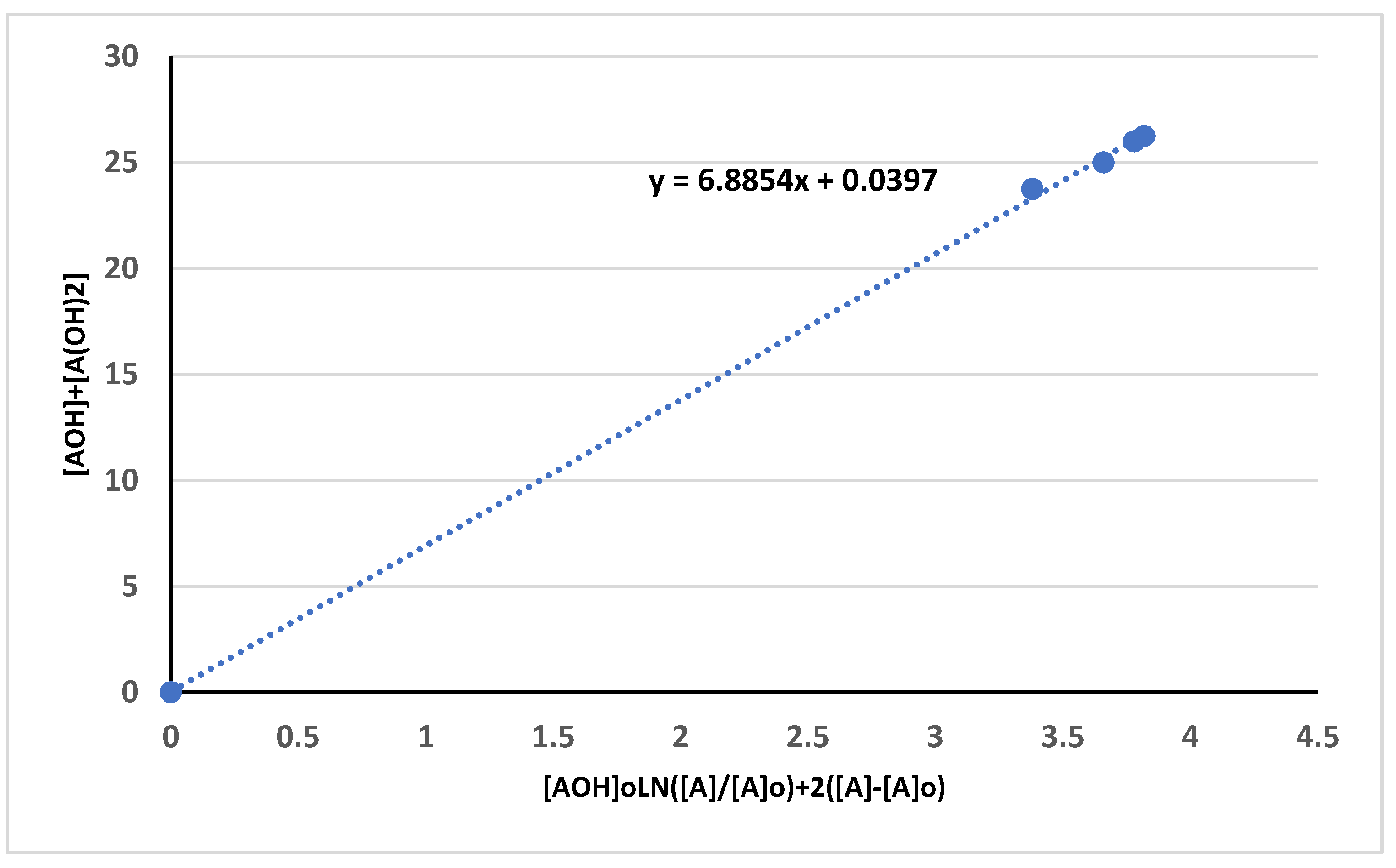
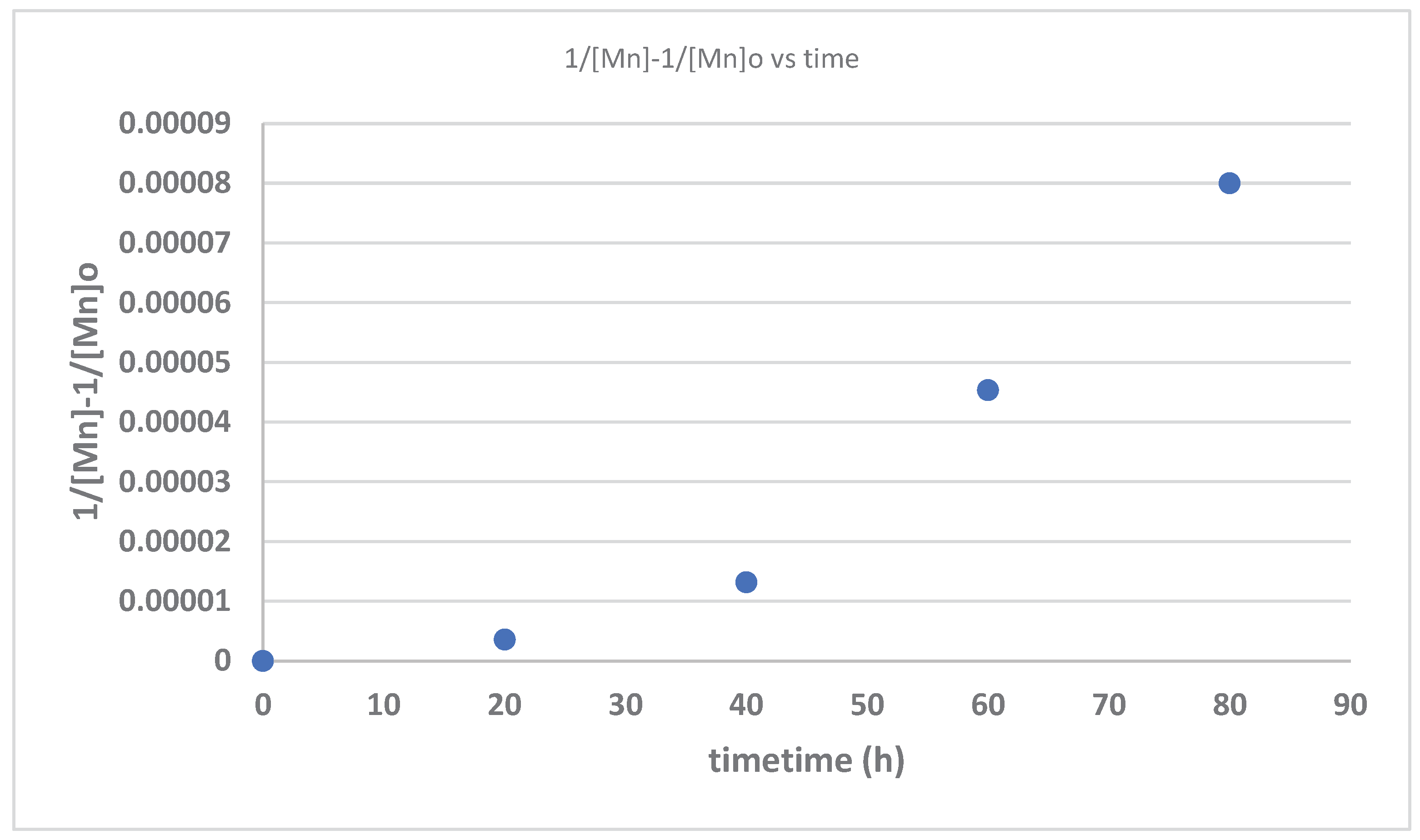
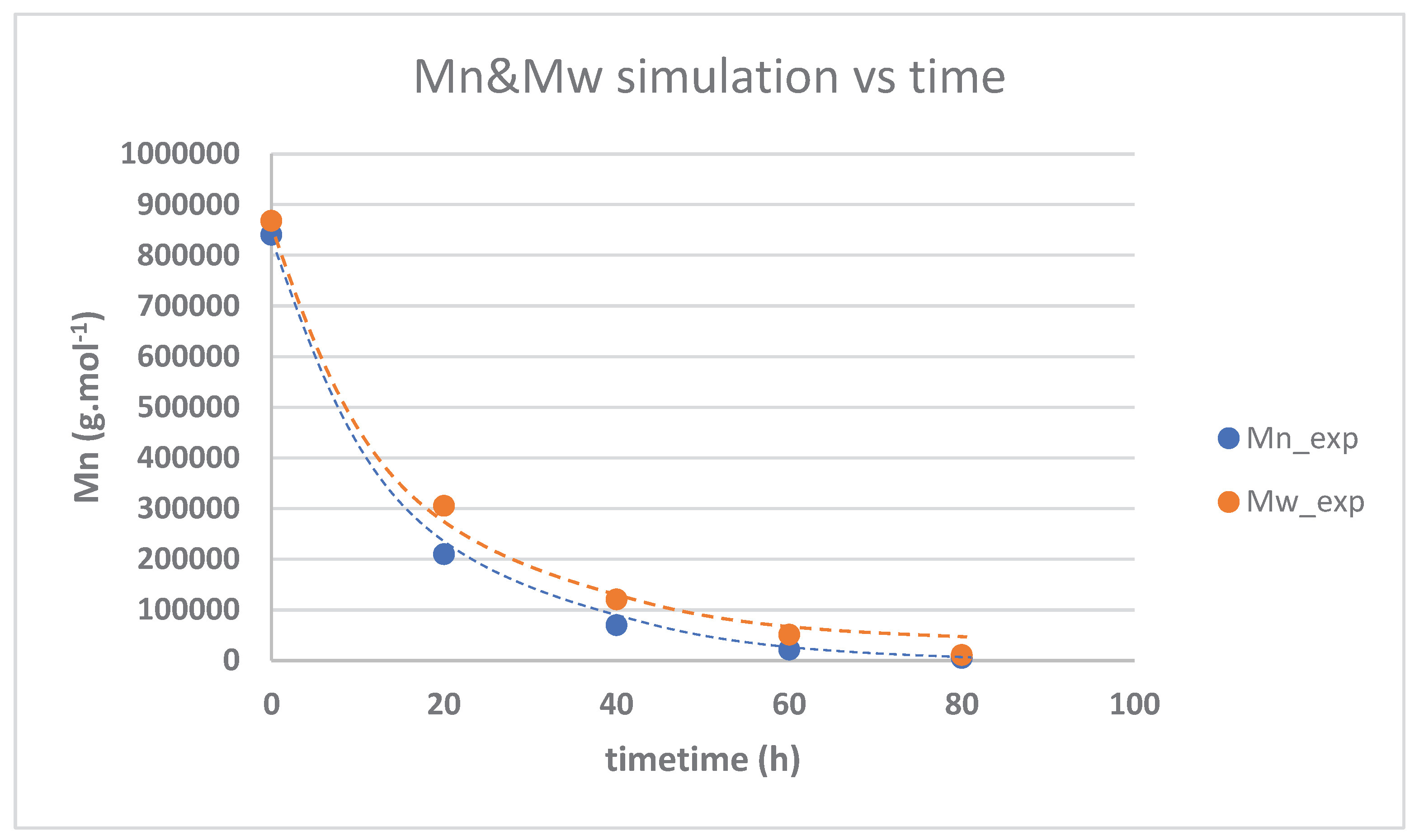
| Time (hours) | Mn_exp | Mw_exp | 1/[Mn}-1/[Mn]o | PDI |
| 0 | 840000 | 868000 | 0 | 1.033333 |
| 20 | 210000 | 305100 | 3.57E-06 | 1.452857 |
| 40 | 69700 | 120500 | 1.32E-05 | 1.728838 |
| 60 | 21500 | 51000 | 4.53E-05 | 2.372093 |
| 80 | 5300 | 11100 | 0.00008 | 2.09434 |
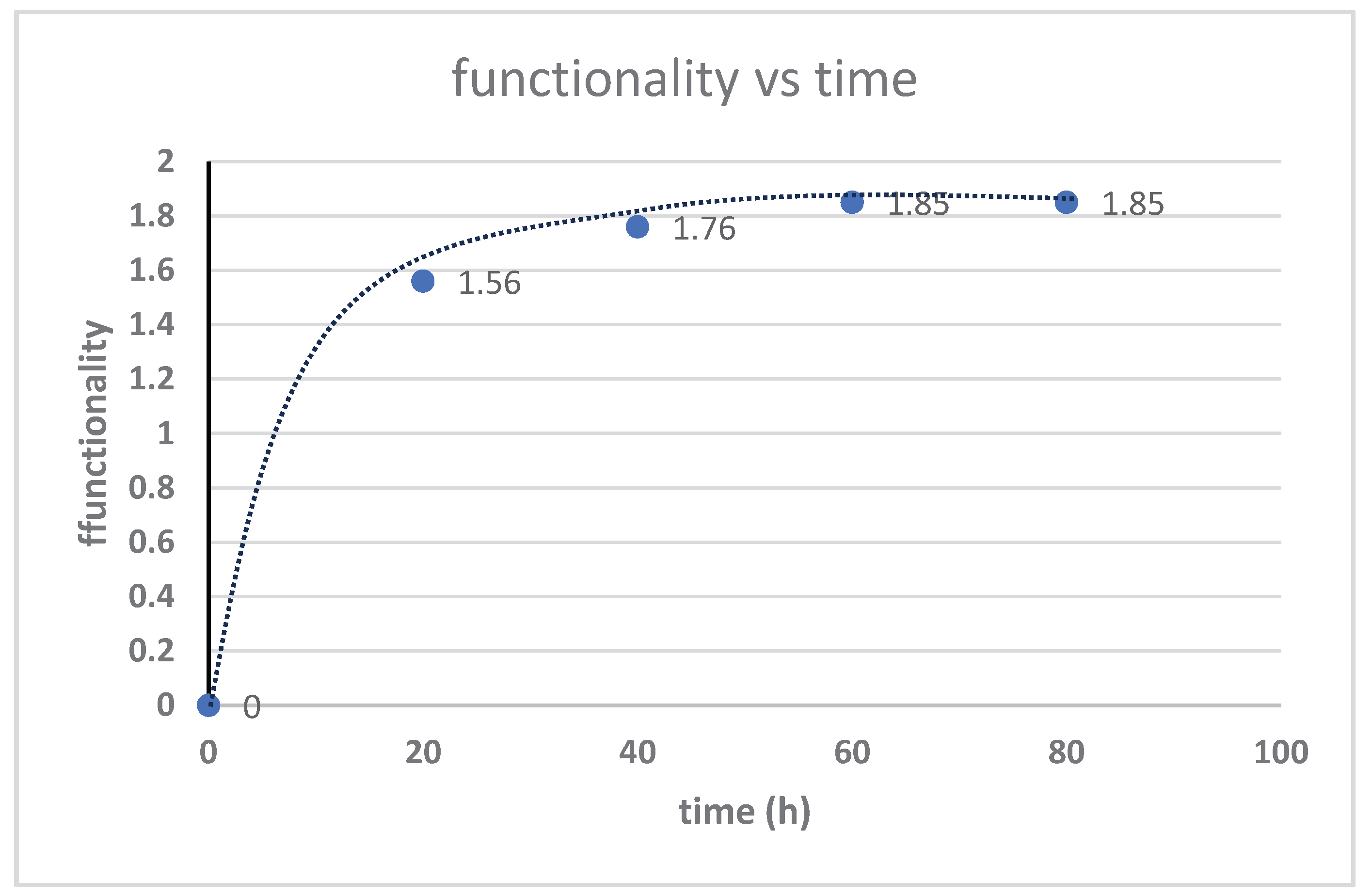
3.4. Pengaruh Suhu Reaksi
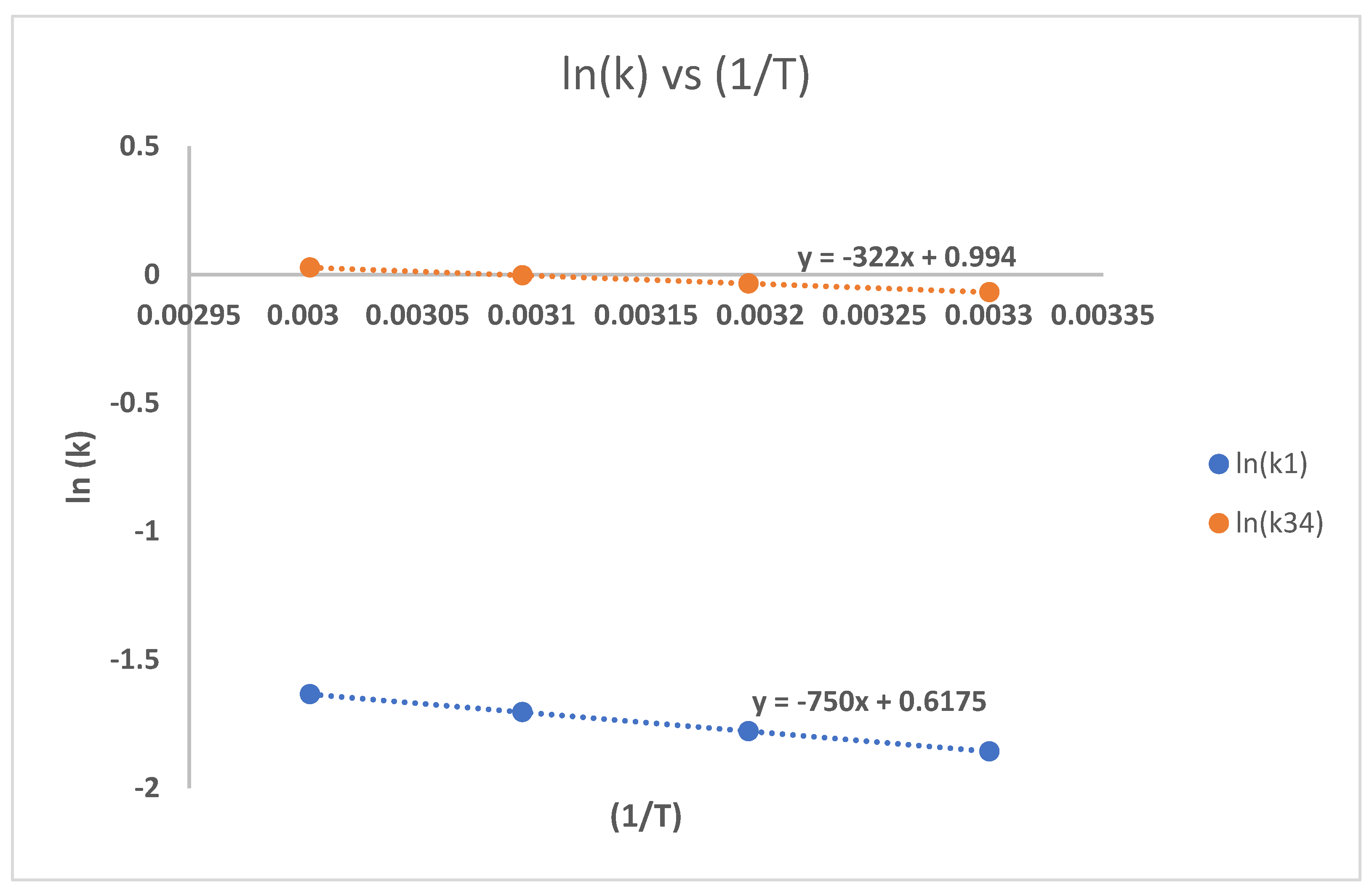
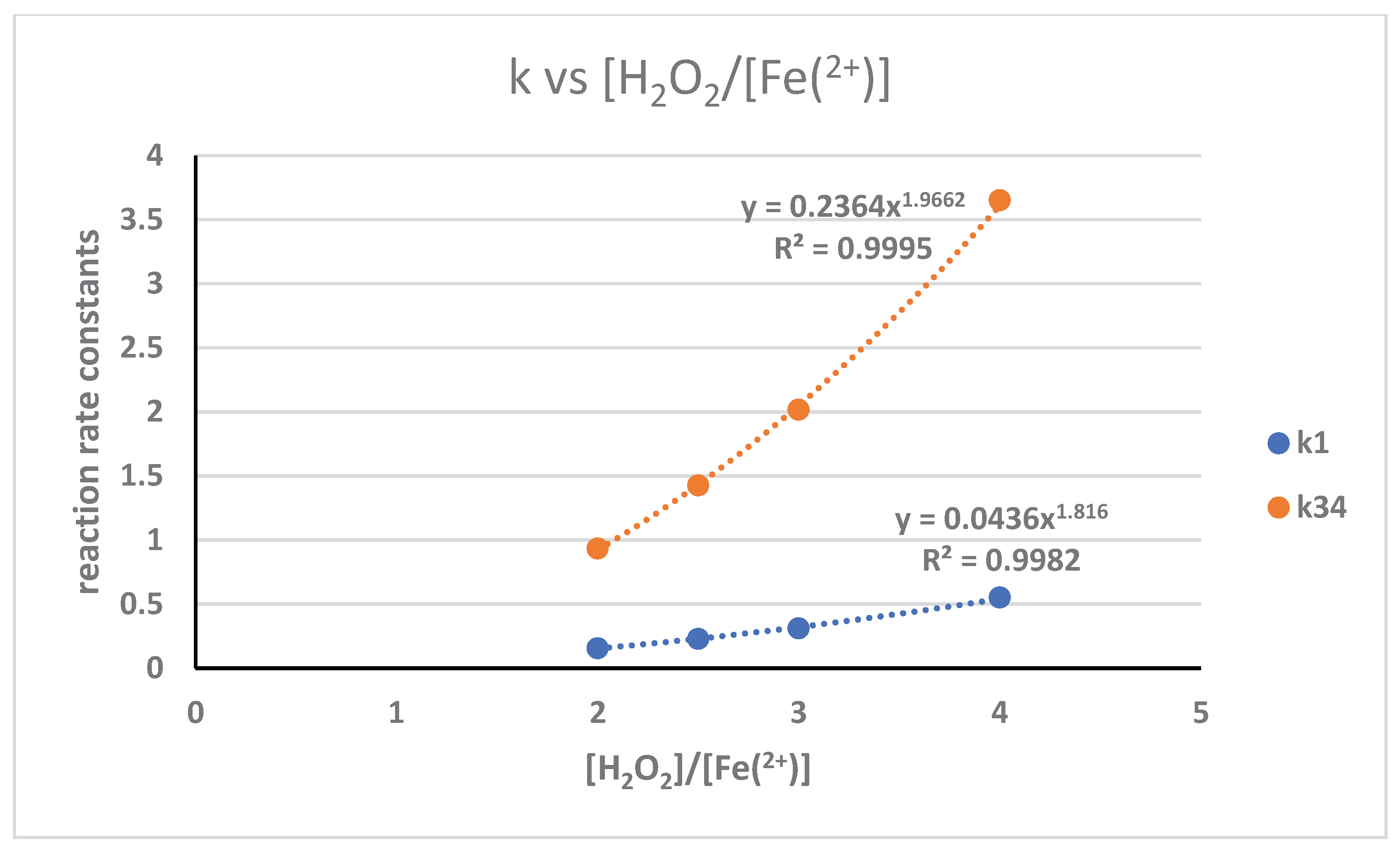
3.5. Pengaruh Rasio Reaktan Dengan Katalis
4. Conclusions
References
- Nor, H.M.; Ebdon, J.R. Telechelic Liquid Natural Rubber: A Review. Prog Polym Sci 1998, 23, 143–177. [CrossRef]
- Ly, P.H. Reinforcement of Natural Rubber from Hydroxyl-Terminated Liquid Natural Rubber Grafted Carbon Black. I. Grafting of Acyl Chloride Capped Liquid Natural Rubber onto Carbon Black. Journal of Macromolecular Science, Part A 1996, 33, 1931–1937. [CrossRef]
- Sukhawipat, N.; Saetung, N.; Pasetto, P.; Pilard, J.-F.; Bistac, S.; Saetung, A. A Novel High Adhesion Cationic Waterborne Polyurethane for Green Coating Applications. Prog Org Coat 2020, 148, 105854. [CrossRef]
- Gopakumar, S.; Nair, M.R.G. Natural Rubber–Polyurethane Block Copolymers: Nonlinear Structural Variations with NCO/OH Ratio. Polym Eng Sci 2006, 46, 1812–1821. [CrossRef]
- Saetung, A.; Kaenhin, L.; Klinpituksa, P.; Rungvichaniwat, A.; Tulyapitak, T.; Munleh, S.; Campistron, I.; Pilard, J. Synthesis, Characteristic, and Properties of Waterborne Polyurethane Based on Natural Rubber. J Appl Polym Sci 2012, 124, 2742–2752. [CrossRef]
- Tsupphayakorn-aek, P.; Suwan, A.; Tulyapitak, T.; Saetung, N.; Saetung, A. A Novel UV-Curable Waterborne Polyurethane-Acrylate Coating Based on Green Polyol from Hydroxyl Telechelic Natural Rubber. Prog Org Coat 2022, 163, 106585. [CrossRef]
- Panwiriyarat, W.; Tanrattanakul, V.; Pilard, J.-F.; Pasetto, P.; Khaokong, C. Preparation and Properties of Bio-Based Polyurethane Containing Polycaprolactone and Natural Rubber. J Polym Environ 2013, 21, 807–815. [CrossRef]
- Kandil, H.; Abo-Salem, H.M. A Novel Thiourea Based Interfacial Modifier for Silica-filled Natural Rubber Composites. J Appl Polym Sci 2023, 140. [CrossRef]
- Katueangngan, K.; Tulyapitak, Tulyapong.; Saetung, A.; Soontaranon, S.; Nithi-uthai, N. Renewable Interfacial Modifier for Silica Filled Natural Rubber Compound. Procedia Chem 2016, 19, 447–454. [CrossRef]
- Srisuwan, S.; Ruksakulpiwat, Y.; Chumsamrong, P. Physical Properties of Poly(Lactic Acid)/Hydroxyl Terminated Natural Rubber Blends. Macromol Symp 2015, 354, 118–124. [CrossRef]
- Baharulrazi, N.; Mohd Nor, H.; Wan Ali, W.K. Hydroxyl Terminated Natural Rubber (HTNR) as a Binder in Solid Rocket Propellant. Applied Mechanics and Materials 2014, 695, 174–178. [CrossRef]
- Gupta, S.K.; Kurup, M.R.; Devadoss, E.; Muthiah, Rm.; Thomas, S. Development and Evaluation of a Novel Binder Based on Natural Rubber and High-Energy Polyurethane/Composite Propellants. J Appl Polym Sci 1985, 30, 1095–1112. [CrossRef]
- Krishnamurthy, V.N.; Thomas, S. ISRO Polyol- The Versatile Binder for Composite Solid Propellants for Launch Vehicles and Missiles. Def Sci J 1987, 37, 29–37.
- Onn, M.; Md Nor, H.; Wan Ali, W.K. Development of Solid Rocket Propellant Based on Isophorone Diisocyanate – Hydroxyl Terminated Natural Rubber Binder. J Teknol 2014, 69. [CrossRef]
- Thomas, S.; Varghese, T.L.; Gupta, S.K.; Ram, T.S.; Krishnamurthy, V.N. Natural Rubber Based Fuel Rich Propellant for Ramjet Rocket. Def Sci J 1992, 42, 141.
- Ravindran, T.; Nayar, M.R.G.; Francis, J.D. A Novel Method for the Preparation of Hydroxyl Terminated Liquid Natural Rubber. Die Makromolekulare Chemie, Rapid Communications 1986, 7, 159–163. [CrossRef]
- Ravindran, T.; Nayar, M.R.G.; Francis, D.J. Production of Hydroxyl-terminated Liquid Natural Rubber—Mechanism of Photochemical Depolymerization and Hydroxylation. J Appl Polym Sci 1988, 35, 1227–1239. [CrossRef]
- Pham, H.L.; Do, B.T.; Pham, T.S.; Le, D.G. Synthesis and Characterisation of Hydroxyl-Terminated Liquid Natural Rubber by Photo-Fenton Reaction. ASEAN Journal on Science and Technology for Development 2017, 30, 29–36. [CrossRef]
- Giang, L.D.; Thao, D.L.M.; Huong, H.T.; Thu Hiep, L.T. Synthesis of Hydroxyl Terminated Liquid Natural Rubber by Oxidative Depolymerization of Deproteinized Natural Rubber. Vietnam J Sci Technol 2016, 54, 340. [CrossRef]
- Azhar, N.H.A.; Md Rasid, H.; M. Yusoff, S.F. Epoxidation and Hydroxylation of Liquid Natural Rubber. Sains Malays 2017, 46, 485–491. [CrossRef]
- Flory, P.J. Statistical Thermodynamics of Random Networks. Proceedings of the Royal Society of London. A. Mathematical and Physical Sciences 1976, 351, 351–380. [CrossRef]
- Wibowo, H.B.; Dharmawan, W.C.; Wibowo, R.S.M. Bulk Polymerization Kinetics of Hydroxy Terminated Polybutadiene and Toluene Diisocyanate with Infrared Spectroscopy. Indonesian Journal of Chemistry 2018, 18, 552. [CrossRef]
- Wibowo, H.; Sitompul, H.; Budi, R.; Hartaya, K.; Abdillah, L.; Ardianingsih, R.; Wibowo, R. Hexogen Coating Kinetics with Polyurethane-Based Hydroxyl-Terminated Polybutadiene (HTPB) Using Infrared Spectroscopy. Polymers (Basel) 2022, 14, 1184. [CrossRef]
- Nogueira, R.F.P.; Trovó, A.G.; Silva, M.R.A. da; Villa, R.D.; Oliveira, M.C. de Fundamentos e Aplicações Ambientais Dos Processos Fenton e Foto-Fenton. Quim Nova 2007, 30, 400–408.
- dos Santos, K.J.L.; dos Santos, G.E. de S.; de Sá, Í.M.G.L.; Ide, A.H.; Duarte, J.L. da S.; de Carvalho, S.H.V.; Soletti, J.I.; Meili, L. Wodyetia Bifurcata Biochar for Methylene Blue Removal from Aqueous Matrix. Bioresour Technol 2019, 293, 122093. [CrossRef]
- Krause, A.; Lange, A.; Ezrin, M.; Ruby, K. Plastics Analysis Guide: Chemical and Instrumental Methods; Plastics Analysis Guide: Chemical and Instrumental Methods; Hauser Publishers, Munich, 1983; ISBN 9783446135871.
- Wisetkhamsai, K.; Patthaveekongka, W.; Arayapranee, W. Study on Degradation of Natural Rubber Latex Using Hydrogen Peroxide and Sodium Nitrite in the Presence of Formic Acid. Polymers (Basel) 2023, 15, 1031. [CrossRef]
- Wibowo, H.B. KAJIAN PROGRAM PENINGKATAN KINERJA PROPELAN KOMPOSIT BERBASIS AP/HTPB/AL. Jurnal Teknologi Dirgantara 2019, 16. [CrossRef]
- Ibrahim, S.; Daik, R.; Abdullah, I. Functionalization of Liquid Natural Rubber via Oxidative Degradation of Natural Rubber. Polymers (Basel) 2014, 6, 2928–2941. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





