Introduction
Ultra-high contrast (UHC) MRI is a term used to describe MR imaging that shows abnormalities with high contrast when little or no abnormality is seen using common conventional state-of-the-art MRI sequences. It is achieved without the use of increased static or gradient magnetic fields. For soft tissues, Xray CT can be regarded as high contrast, conventional state-of-the-art MRI as very high contrast and MRI which shows abnormalities with obvious contrast when they are not seen with conventional MRI as UHC [
1].
A common mechanism for achieving UHC MRI is synergistic contrast in which changes in a tissue property such as T
1 or T
2 are used twice or more in the same sequence to increase contrast rather than just using changes in a single property once which is the case with most conventional sequences [
2]. An example is the divided subtracted inversion recovery (dSIR) sequence in which the signals from two inversion recovery (IR) sequences with different inversion times (TIs) are subtracted to increase the contrast produced by small changes in T
1. This contrast is increased further by division of the subtraction by the sum of the signals from the two sequences [
3].
Another way of achieving UHC MRI is to use a high quality single tissue property map such as a T1 or T2 map and retrospectively apply a shaped filter to the map. The filter can increase contrast in one or more domains of tissue property values and maintain anatomical detail in the rest of the image. This is unlike narrow windowing of images which, when used to increase contrast, is accompanied by saturation of the display gray scale at the upper and lower signal thresholds. As a result, narrow windowing leads to loss of anatomical detail and limits the degree to which contrast can be increased by windowing.
The principal clinical focus of UHC MRI to date has been on small changes in tissue properties from normal. When normal and/or abnormal structures are already seen with high contrast using conventional sequences, there is no particular clinical gain in demonstrating them with even greater contrast. As a result, UHC MRI has been directed at normal or near normal appearing tissues seen with conventional state-of-the-art images where there is little or no apparent lesion contrast with the aim of demonstrating abnormalities with high contrast.
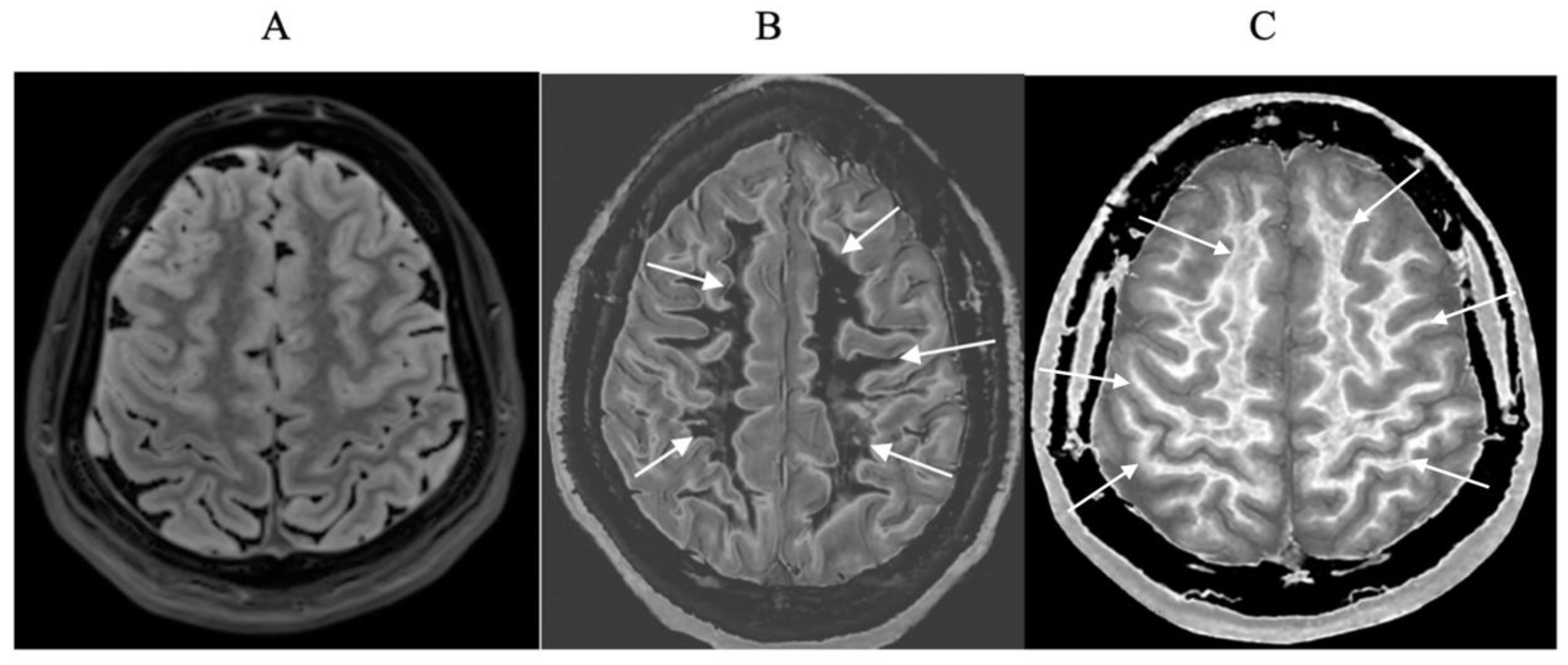
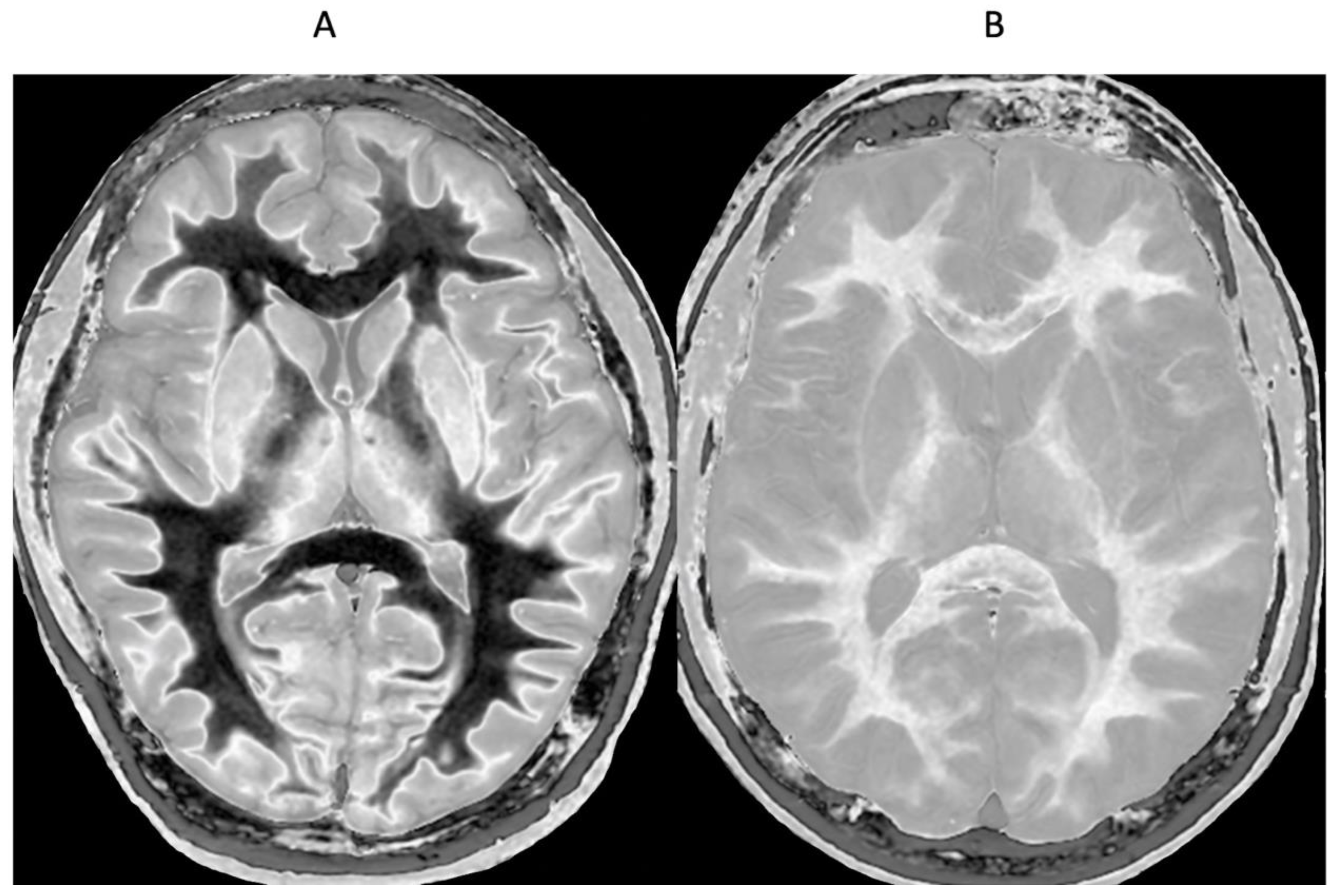
Small changes in T
1 may produce obvious abnormalities with the dSIR sequence as illustrated in a case of mild traumatic brain injury (mTBI) in an 24-year-old male patient (
Figure 1). No abnormality is seen on the T
2-FLAIR image in the patient (
Figure 1A). The dSIR sequence in a normal age matched control shows normal white matter in the cerebral hemispheres as low signal (dark) in
Figure 1B (arrows). The same dSIR sequence in the patient shows abnormal high signal in his entire white matter (
Figure 1C) (arrows). High signal (light) well defined boundaries are also seen between normal white and gray matter on the dSIR image in
Figure 1B. These are less obvious in
Figure 1C because of the high signal in the abnormal white matter.
High contrast abnormalities in white matter have been seen using dSIR T
1-BipoLAr Inversion Recovery (T
1-BLAIR) images of the type illustrated in
Figure 1 in areas where little or no abnormality has been seen on T
2-FLAIR or T
2-wSE images in cases of mTBI [
4], multiple sclerosis (MS) [
5], methamphetamine substance use disorder [
6] and Grinker’s myelinopathy [
7].
The purpose of this educational review is to describe the basic physics underlying four types of BLAIR images and illustrate their use in normal human subjects and patients.
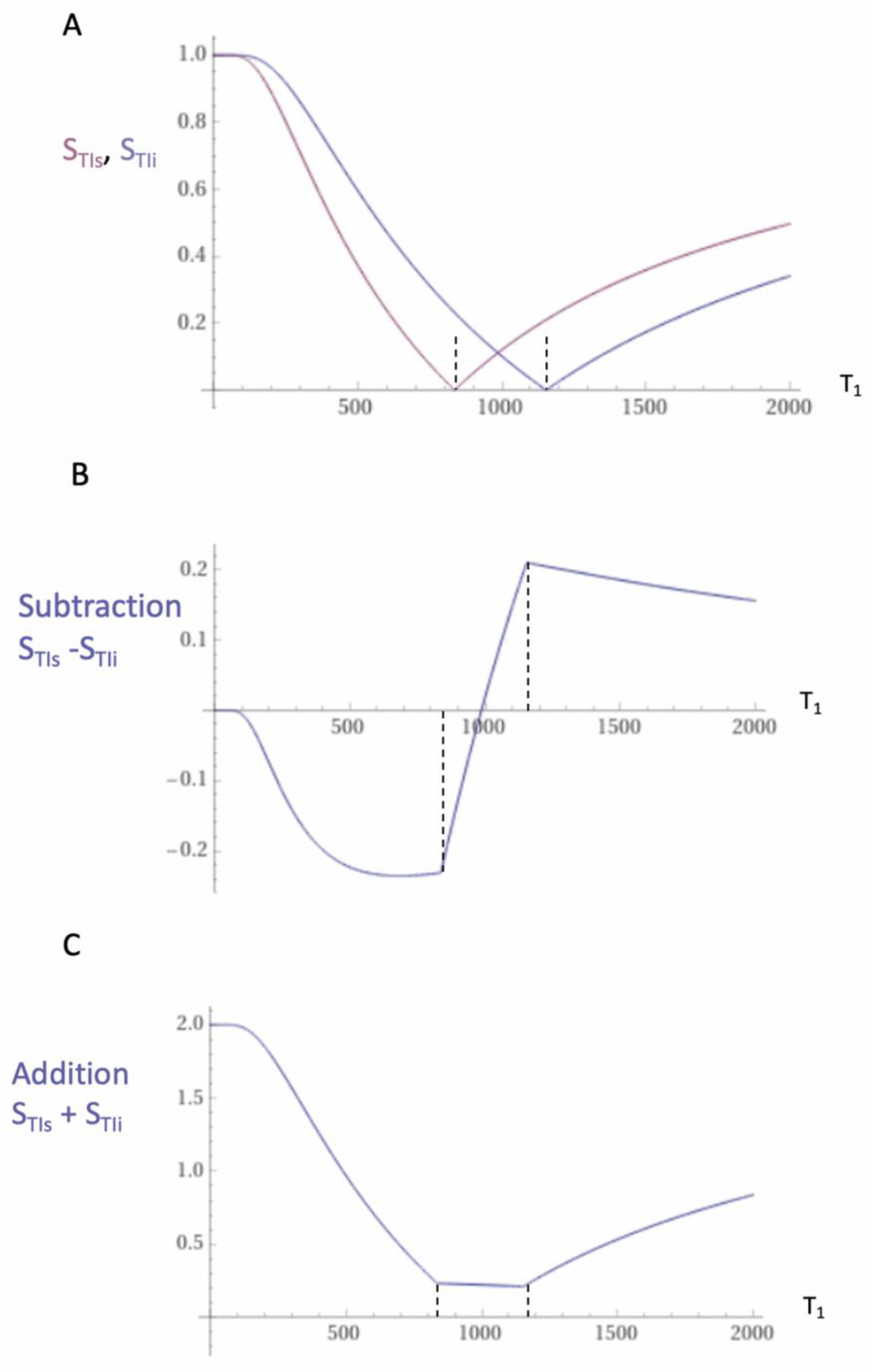
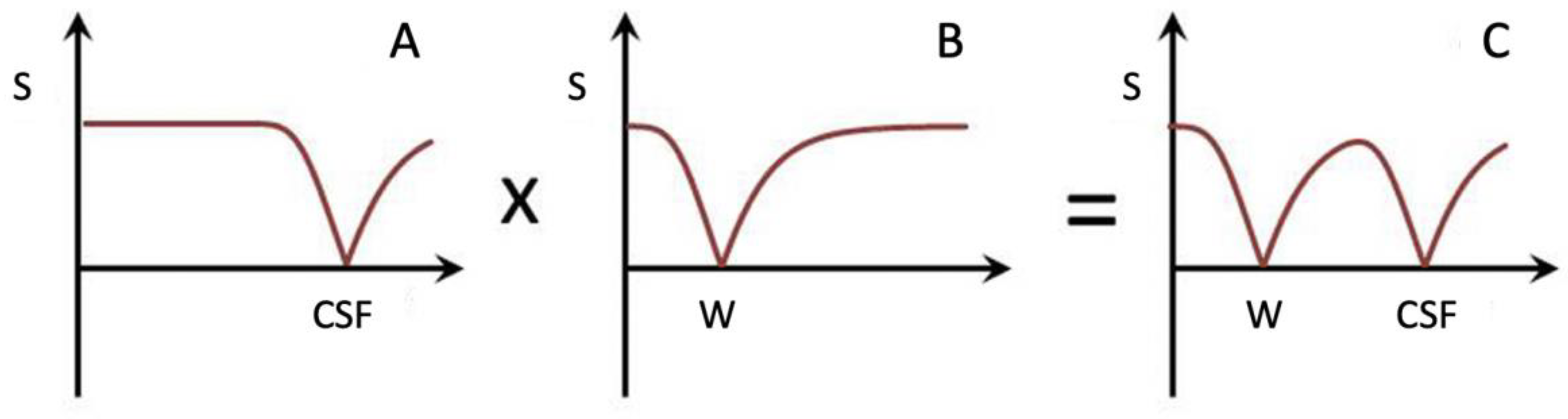
Subtracted IR (SIR) and divided Subtracted IR (dSIR) Bipolar Filters
Two conventional magnitude IR negative unipolar T
1-filters with different TIs, namely TI
short = TI
s and TI
intermediate = TI
i are shown in
Figure 5A. The subtraction: first TI
s T
1-filter minus the second TI
i T
1-filter produces the subtracted IR (SIR) bipolar T
1-filter shown in
Figure 5B. The vertical dashed lines at the null points of the two IR T
1-filters shown in
Figure 5A divide the X axis in
Figure 5B into the lowest Domain (lD), the middle Domain (mD) and the highest Domain (hD). In the mD in
Figure 5B (between the two dashed lines) the size of the slope of the SIR bipolar T
1-filter is about double that of the IR T
1-filters shown in
Figure 5A. This is because the slope of the SIR filter in its mD in
Figure 5B is the positive slope of the TI
s filter in the mD shown in
Figure 5A minus the negative slope of the TI
i filter in the mD also shown in
Figure 5A.
The two IR T
1-filters shown in
Figure 5A can also be added to give the Added IR (AIR) T
1-filter shown in
Figure 5C. In its mD, which is bounded by the vertical dashed lines, the signal is reduced to about 0.20 compared with its value of 2 at T
1 = 0 (i.e., about one tenth).
Figure 6A shows a divided SIR (dSIR) bipolar T
1-filter in which the SIR bipolar T
1-filter in
Figure 5B is divided by the AIR T
1-filter in
Figure 5C i.e.,
. The signal (S
dSIR) of the dSIR filter is given by:
where S
TIs is the signal from the shorter TI filter and S
TIi is the signal from the intermediate TI
i filter.
The resulting dSIR bipolar T
1-filter (
Figure 6A) shows a negative pole and a positive pole. Its mD shows a highly positive nearly linear slope which is about ten times greater than the slopes of the IR T
1-filters shown in
Figure 5A. The dSIR filter has maximum and minimum values of ±1. The contributions from r
m and T
2 in conventional IR sequences cancel out with dSIR sequences which are therefore T
1 maps. As a result, the whole sequence can be accurately represented by its bipolar T
1-filters.
Figure 6B compares the contrast produced by a conventional S
TIs IR unipolar T
1-filter (pink) to that from the SIR bipolar T
1-filter (blue) shown in
Figure 5B from the same increase in T
1 (horizontal positive green arrow, DT
1). DT
1 is multiplied by the slopes of the respective S
TI IR and SIR filters (red lines) to produce the differences in signal DS, i.e., the contrast from each of them. These are shown by the vertical pink and blue arrows on the right. The SIR bipolar T
1-filter generates about twice the contrast (blue arrow) of the S
TIs IR unipolar T
1-filter (pink arrow) from the same increase in T
1, DT
1.
Figure 6C compares the contrast produced by the S
TIs unipolar IR T
1-filter (pink) to that produced by the dSIR bipolar T
1-filter shown in
Figure 6A (blue). The increase in T
1 (horizontal positive green arrow, DT
1) is multiplied by the slopes of the respective S
TIs IR and dSIR filters (red lines) to produce the differences in signal DS, or contrast generated by the two filters. These are shown by the vertical pink and blue arrows on the right. For the same increase in T
1 (DT
1), the dSIR bipolar T
1-filter produces about ten times greater contrast than the S
TIs IR unipolar T
1-filter. The S
TIs IR T
1-filter is that of a conventional TI IR sequence such as MP-RAGE (magnetisation prepared-rapid acquisition gradient echo).
To produce the large increase in contrast shown in
Figure 6C, the dSIR sequence is typically targeted at the small increase in the T
1 of normal tissue produced by disease (shown by the horizontal green arrow) and this is positioned within the steeply sloping mD of the dSIR bipolar T
1-filter. This is done by choosing appropriate values of TI
s and TI
i. The target is often small increases in the T
1 of white matter. High contrast can also be produced by difference or change in T
1 within the lower part of the hD and the upper part of the lD where the dSIR bipolar T
1-filter has relatively steep slopes. More detail is available in reference [
3].
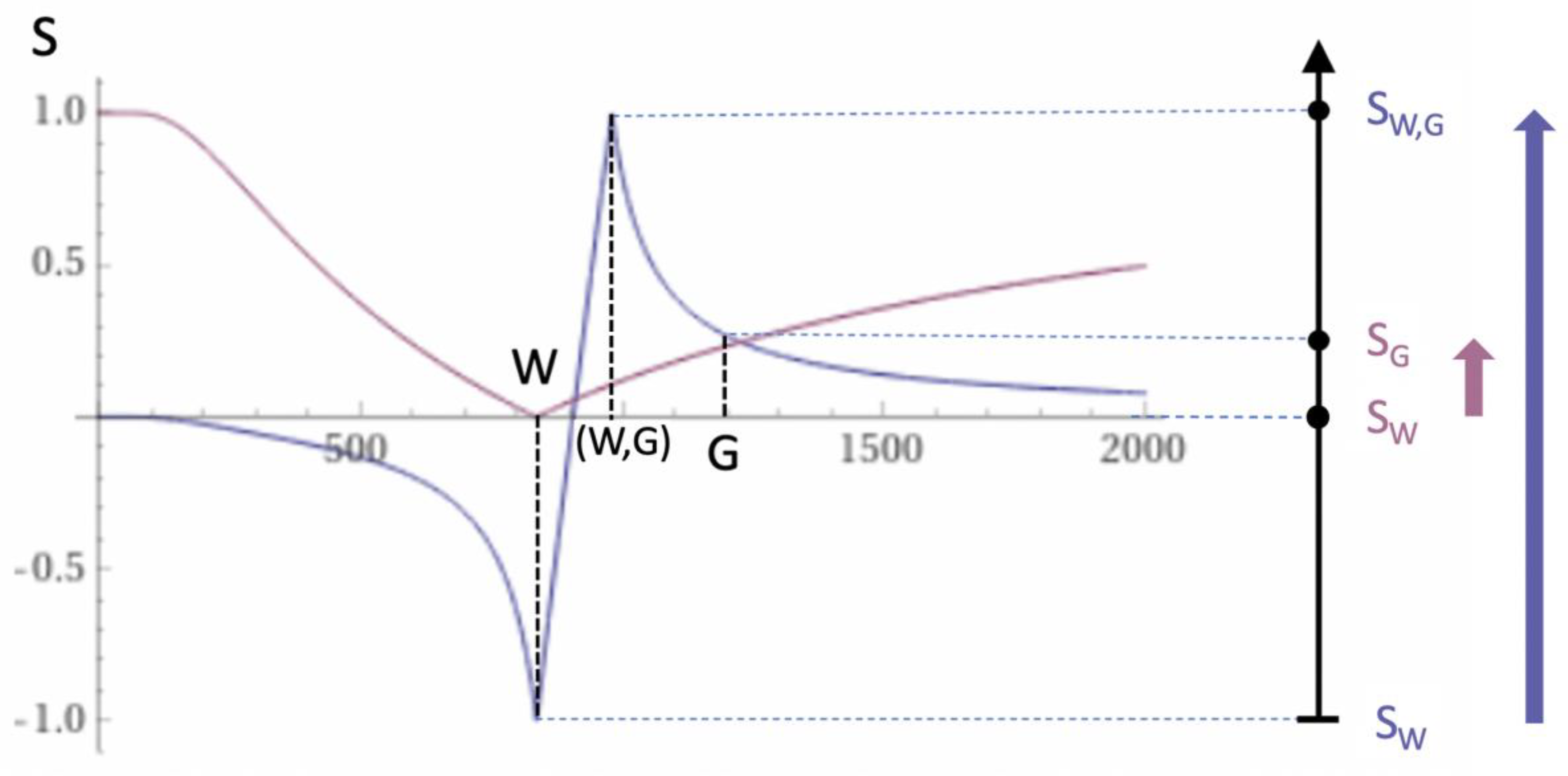
Contrast at Tissue Boundaries
MRI tissue boundaries take different forms such as a gradual change in signal from one tissue to another as well as sharply defined high and low signal boundaries between tissues. In the boundary region between two pure tissues (such as between white and gray matter) the T
1s of voxels which contain mixtures of the two tissues typically span the range of T
1 values between those of the two pure tissues. If a narrow mD dSIR bipolar T
1-filter (e.g., with TI
s nulling normal white matter, and TI
i longer than TI
s but less than that needed to null gray matter) is used there are mixtures of white and gray matter in voxels in the boundary region between the two tissues which have intermediate T
1 values (T
1W,G) that correspond with the peak of the bipolar T
1-filter as shown in
Figure 7. This produces a high signal boundary between white matter and gray matter. The dSIR bipolar T
1-filter shows much higher contrast than that produced between white matter and gray matter by a conventional white matter nulled IR sequence such as MP-RAGE (
Figure 7). The mechanism shown in
Figure 7 produces the high signal S
W,G at the boundary between normal white and normal gray matter shown in
Figure 1B. High signal boundaries are also seen at the junction between CSF and white matter at ventricular boundaries, and can be seen at the boundary between gray matter and CSF with wide mD sequences [
3].
T1 Maps and Qualitative—Quantitative MRI
To better understand the dSIR bipolar T
1-filter, a linear equation of the form y = mx + c can be used to approximate the filter in its mD. The equation is produced by fitting a straight line between the first and last points of the mD (i.e., first point x =
and y = -1, and last point x =
and y = +1). In the mD, the signal of the dSIR sequence S
dSIR, is given by:
where ΔTI = TI
i − TI
s (i.e., second TI minus first TI) which is positive, and ΣTI = TI
s + TI
i which is also positive. Note that because DTI is positive, the slope
is positive. The offset is negative.
The expression in Equation (2) illustrates four key features of the dSIR bipolar T1-filter, firstly, the near linear change in signal (i.e., SdSIR) with T1 in the mD, secondly, the filter has a slope in the mD equal to , thirdly the filter shows high sensitivity to small changes in T1 when the size of ΔTI is small. When DT1 is small, the size of DTI can be decreased to match it and so scale up the sensitivity of the filter. The reduction in DTI increases the steepness of the T1-filter in the mD and thus the amplification of contrast produced from DT1. This compensates for the small value of DT1 until the sequence becomes SNR and/or artefact limited. This is not the case with conventional IR sequences where, if DT1 is decreased, contrast decreases.
Fourthly, Equation (3) can be used to calculate T
1 values in the mD so that:
The linear approximation is only valid in the mD. Also, it is assumed that TR is long compared to tissue T1 values. An equivalent expression that allows for incomplete recovery of longitudinal magnetisation during TR can be formulated.
Outside of the mD in the lD and hD, the magnitude of the dSIR bipolar T1-filter signal decreases towards zero at minimum and maximum values of T1. T1s in the mD occupy the full range of the display gray scale from black to white. T1s from the shortest and longest T1s in the lD and hD respectively only occupy parts of the display gray scale.
Magnetisation transfer (MT) (see later) may have a substantial effect on T1 values which can be regarded as observed T1s reflecting the process of signal acquisition as well as T1 as an intrinsic property of tissue.
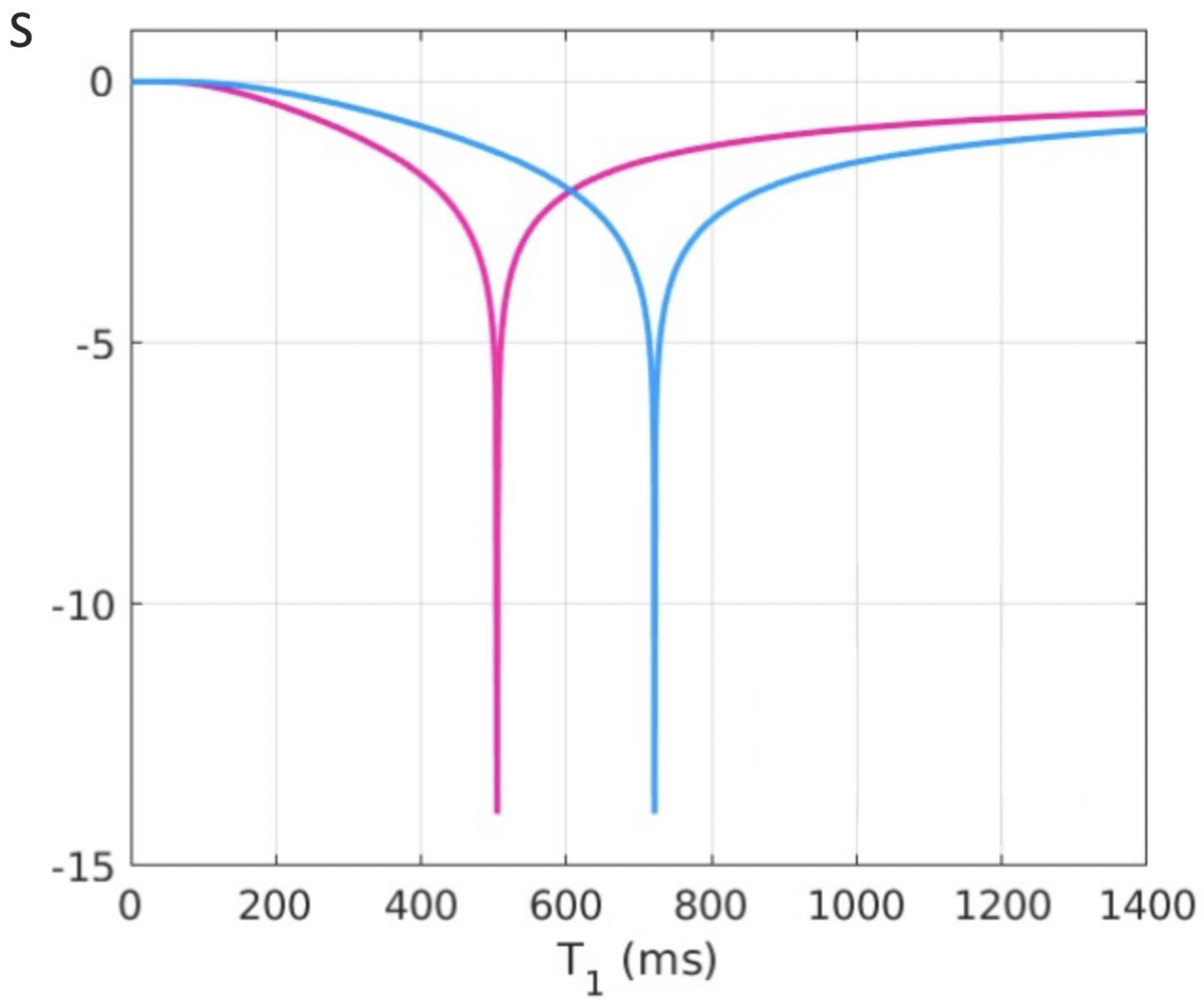
Log Then Subtracted Inversion Recovery (lSIR) Sequences
Another bipolar T
1-filter is the natural logarithm (ln) then subtracted inversion recovery (lSIR) filter [
10]. The signal of this filter (S
lSIR) is half of the subtraction: ln short S
TIs T
1-filter minus ln intermediate S
TIi T
1-filter. The ln S
TIs and ln S
TIi negative unipolar T
1-filters are shown in
Figure 8 and have sharper negative poles than those of unipolar S
TIs and S
TIi T
1-filters shown in
Figure 5A.
Figure 9 shows a dSIR bipolar T
1-filter in blue and the corresponding lSIR bipolar T
1-filter with the same TIs in orange. The two filters appear similar in the lowest region of the lD and in the highest region of the hD as well as in the central region of the mD. The slope of the dSIR filter is essentially constant and equal to
. The magnitude of the slope of the lSIR filter is the same that of the dSIR filter at the centre of the mD but greater around the lower and higher nulling TI values. The lSIR filter asymptotically approaches negative and positive infinity at the two corresponding T
1s. The lSIR filter increases its contrast amplification as the change in T
1 from normal becomes smaller. Like the dSIR filter, the lSIR filter essentially eliminates the r
m and T
2 dependence of the full IR sequence and so can be used to model the full behaviour of the sequence.
The lSIR filter is an inverse hyperbolic tangent of the dSIR filter in the mD (SlSIR = atanh SdSIR), and an inverse hyperbolic cotangent of the dSIR filter in the lD and hD.
From a practical point of view, the lSIR T1-filter has 2-3 times the slope of the dSIR T1-filter for very small differences or changes in T1 around the null points and thus has 20-30 times greater contrast than conventional IR sequences for the same very small differences or changes in T1. The maximum and minimum values of the lSIR T1-filter are usually shown as ±2-3 (rather than ±infinity). This compares with values of ±1 for the dSIR T1-filter. The low and high signal boundaries of the lSIR T1-filter are narrower than those of the corresponding dSIR filter. The lSIR T1-filter shows a selective increase in slope, or sharpening, close to the nullpoints.
Composite (c) Bipolar Filters (T1 as well as T2, T2*, D*, c and/or MT)
It is possible to introduce an attenuation filter S
OF (other filter) and apply it to one of the two IR filters used for dSIR bipolar T
1-filter imaging in
Figure 5 and
Figure 6. S
OF may equal e
–DTE/T2, e
–DTE/T2* or e
–DbD* which introduce T
2, T
2* and D* dependence respectively. This provides additional contrast to the T
1 contrast of the dSIR bipolar T
1-filter. When DTE equals zero, or Db equals zero, S
OF = 1. If S
OF is set to 0.5 and 0.25, the curves shown in
Figure 10A,B result when using a narrow mD dSIR sequence (TIs = 350 ms and 500 ms). The nulling times are the same for all values of S
OF.
In
Figure 10A attenuation of the first IR filter (with TI
s) is shown with S
OF set at 1 (blue), 0.5 (orange) and 0.25 (yellow). The yellow and orange curves are wider around the outside of the first (negative) pole at the first TI
s and are narrower and inside the second (positive) pole at TI
i. This results in a broadening and loss of contrast and lower signal white matter around the first negative pole as well as increased contrast and narrower boundaries between white and gray matter around the positive pole. S
OF can be set negative values to reverse the sign of the relevant contrast and make it synergistic with the T
1 contrast.
In
Figure 10B, attenuation of the second IR filter (with TI
i) is shown. As S
OF is decreased from 1.0 to 0.5 and 0.25, the filter narrows around the first (negative) pole and widens around the second (positive) pole. The negative contrast produced for the same difference in T
1 is increased around the first negative pole. Composite (c) forms of the dSIR and lSIR bipolar T
1-filters can be designated as cdSIR, clSIR etc.
Susceptibility and MT can also be used to attenuate signals and produce supplementary contrast. The combination of T1 and T2* contrast may be of value in demonstrating the central vein sign and paramagnetic rim sign in multiple sclerosis (MS) as well as late sequelae of haemorrhage and fMRI in combination with perfusion.
Methods
With approval from the New Zealand Health and Disability Ethics Committee 20/CEN/246 (26 June 2020), 21/CEN/246 (1 May 2021); 20/NTB/14 (21 May 2020); UAHPEC 018466 (20 Dec 2019); and the Northern B Health and Disability Ethics Committee 2022 EXP 13106 (18 Nov 2022) as well as informed consent from each subject, MR scans were performed on four adult normal controls, seven adult patients with mild traumatic brain injury (mTBI), MS, methamphetamine substance use disorder, Grinker’s myelinopathy, Parkinson’s disease and white matter in a patient with a glioma. The research was conducted in accordance with the Declaration of Helsinki.
3T scanners (General Electric Healthcare Premier and Siemens Healthineers Skyra) were used. 2D IR FSE sequences were performed with a TI
s chosen to null the shortest T
1 normal white matter (or slightly shorter) and a longer TI
i chosen to produce narrow mD dSIR images targeted at small increases in the T
1 of white matter from normal as illustrated in
Figure 6C. 3D wide mD dSIR images with a short TI
s and a long TI
l were also obtained [
5]. Longer TE 2D IR images were acquired to produce cdSIR images. Positionally matched 2D and 3D T
2-FLAIR images were obtained as well as susceptibility weighted filtered images as described in
Table 1.
Illustrative Cases
Mild Traumatic Brain Injury (mTBI)
Conventional MRI images usually show little or no change in mTBI but narrow mD dSIR images frequently show extensive high signal areas in white matter (the whiteout sign) (
Figure 1). This may resolve within as little as two days or persist over years. It is generally attributed to neuroinflammation but oedema, demyelination and degeneration may also have a role.
In addition to the whiteout sign, there may be loss of contrast in the gray matter of the thalamus due to an increase in T
1. This is manifest as a low contrast appearance between the medial and lateral thalamus (
Figure 12A) (arrows) (a grayout sign). After remission normal high gray white matter contrast is restored (
Figure 12B) (arrows). Contrast between the lateral thalamus (arrows) and the posterior limb of the internal capsule (PLIC) is low in (
Figure 12A) but very high in (
Figure 12B). The low contrast between the thalamus and the PLIC in (A) results from a reduction in signal in the gray matter of the lateral thalamus due to an increase in its T
1 in the hD as well as an increase in signal in the white matter of the PLIC due to an increase in its T
1 in the mD. This is reversed in (
Figure 12B) where the normal high contrast boundary is restored.
Figure 13A shows a normal control with obvious contrast between the heads of the caudate nuclei and the adjacent CSF as well as between the cortex and CSF.
Figure 13B shows a patient with mTBI who has a whiteout sign. There are grayout signs in the thalamus and putamen. In addition, contrast is lost between the heads of the caudate nuclei and CSF as well as between cortex and CSF which appear isointense. These are grayout signs. Thus, the patient has a combination of whiteout and grayout signs.
There may also be gray matter changes elsewhere in mTBI. The normal red nuclei have relatively short T
1s resulting in their normal signal being mid-gray in the mD (arrows) (
Figure 14A). They shows a higher signal due to increase in T
1 in a case of mTBI (arrows) (
Figure 14B).
Multiple Sclerosis (MS)
Focal lesions, as well as patchy white matter changes, may be seen in areas that appear normal on T
2-wFSE images in a patient in remission (
Figure 15) (compare with normal low signal appearance of normal peripheral white matter in
Figure 1B). In another case during a relapse, one lesion is seen on the T
2-FLAIR image in
Figure 16A (long arrow). This lesion is also seen in
Figure 16B (long arrow). There are an additional six lesions (small arrows) in
Figure 16B. Five of the lesions in
Figure 16B have high signal boundaries. This can be due to a large increase in T
1 in the lesion beyond the peak of the bipolar T
1-filter or a decrease in the abnormal T
1 in the lesion due to the presence of paramagnetic free iron. Two of the lesions with high signal boundaries shown in
Figure 16B have paramagnetic rim signs on the filtered susceptibility weighted images (arrows,
Figure 16C). The high signal boundaries seen on some MS lesions may be a sign of disease activity.
In addition, there are widespread abnormal areas in white matter which are only seen on the dSIR images (
Figure 16). These changes are typically bilateral, symmetrical and have an increased signal. They have the features of a whiteout sign (
Figure 16B) and to date have only been seen in patients with MS during a relapse.
Composite T
1 and T
2 cdSIR images may show higher contrast than corresponding dSIR images (
Figure 17A,B).
In the spinal cord in another patient diagnosed with MS, the T
2-FLAIR image only shows an ill-defined smudge (arrow) (
Figure 18A). The corresponding wide mD dSIR image (
Figure 18B) shows an extensive, well defined, high contrast abnormality (arrows). Another lesion is seen in the medulla on the dSIR image (arrow) but not on the T
2-FLAIR image.
Methamphetamine Substance Use Disorder
Patients with methamphetamine substance use disorder may show a heterogenous pattern of white matter abnormalities with dSIR sequences but they may also show clearly defined whiteout signs and these can remit with continued abstinence (
Figure 19) (compare with normal appearance in
Figure 1B). The situation may be confounded in the case shown by a previous mTBI.
Delayed Post-Hypoxic Leukoencephalopathy (Grinker’s Myelinopathy)
This is thought to be a rare syndrome in which classically patients make an initial recovery after a hypoxic and/or ischaemic episode, and then deteriorate with severe neurological signs such as Parkinsonism and akinetic mutism. Conventional MRI shows widespread abnormalities in the white matter of the cerebral hemispheres. In post-hypoxic patients, it is possible to see extensive abnormalities in white matter with dSIR sequences where no abnormalities are seen with conventional sequences [
7]. The patients typically have cognitive symptoms of less severity than those included in classical descriptions. This less severe form of Grinker’s myelinopathy may be much more common than the classical form but not be recognised radiologically using conventional MRI sequences.
Parkinson’s Disease
Parkinson’s disease may show more obvious bilaminar signs in the cortex than in age matched controls (i.e., an increase in signal in the outer layer of the cortex) with narrow mD dSIR sequences (white arrows) (
Figure 20). The inner higher signal is from the boundary between white matter and gray matter and the next layer outwards is lower signal in the inner cortex. The next outer layer beyond this is the higher signal layer in the outer cortex.
Bubble signs in which circular areas of similar size are seen in the gray matter in the basal ganglia and thalamus can also be seen (e.g., black arrows) (
Figure 20).
White Matter Associated with Cerebral Tumours
In cases of cerebral tumours, extensive white matter changes have been seen after chemotherapy in areas that appeared normal on T
2-FLAIR images (
Figure 21A,B). When typical radiation or chemotherapy changes have been seen on T
2-FLAIR images, additional changes have been observed in the form of whiteout signs.
Normal Control and MS Patient with dSIR and lSIR Images
dSIR and lSIR images (
Figure 22A,B) are compared in a 46-year-old male normal control. White matter-gray matter boundaries and the bilaminar cortex sign (arrows) are seen with higher contrast and higher spatial resolution on the lSIR image. Bubble signs are also better seen in the thalamus and putamen on the lSIR image. A small decrease in T
1 through the sharp peak of the lSIR bipolar T
1-filter results in finer boundaries and narrower margin bubble signs with the lSIR bipolar T
1-filter compared to the dSIR bipolar T
1-filter.
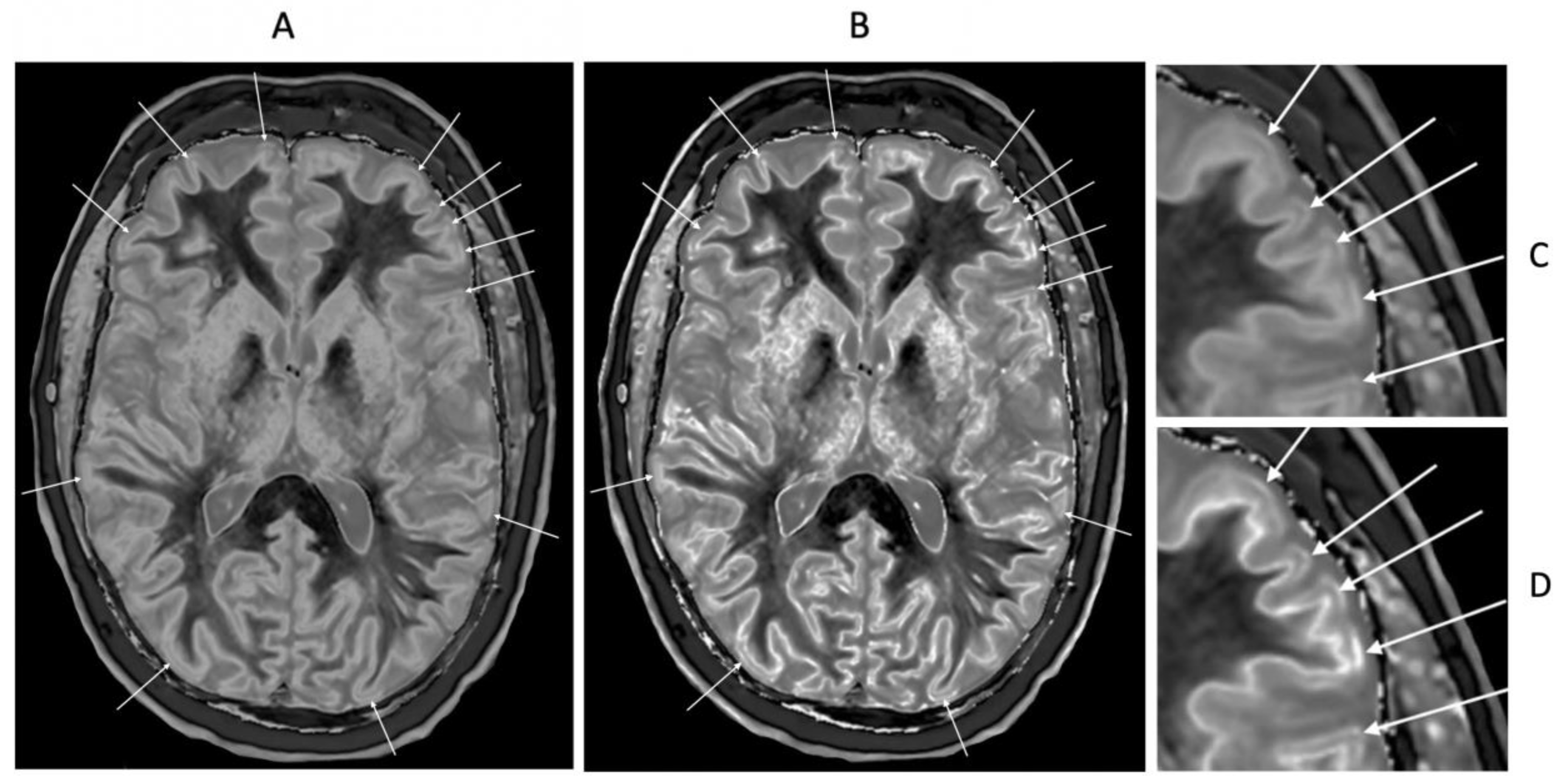
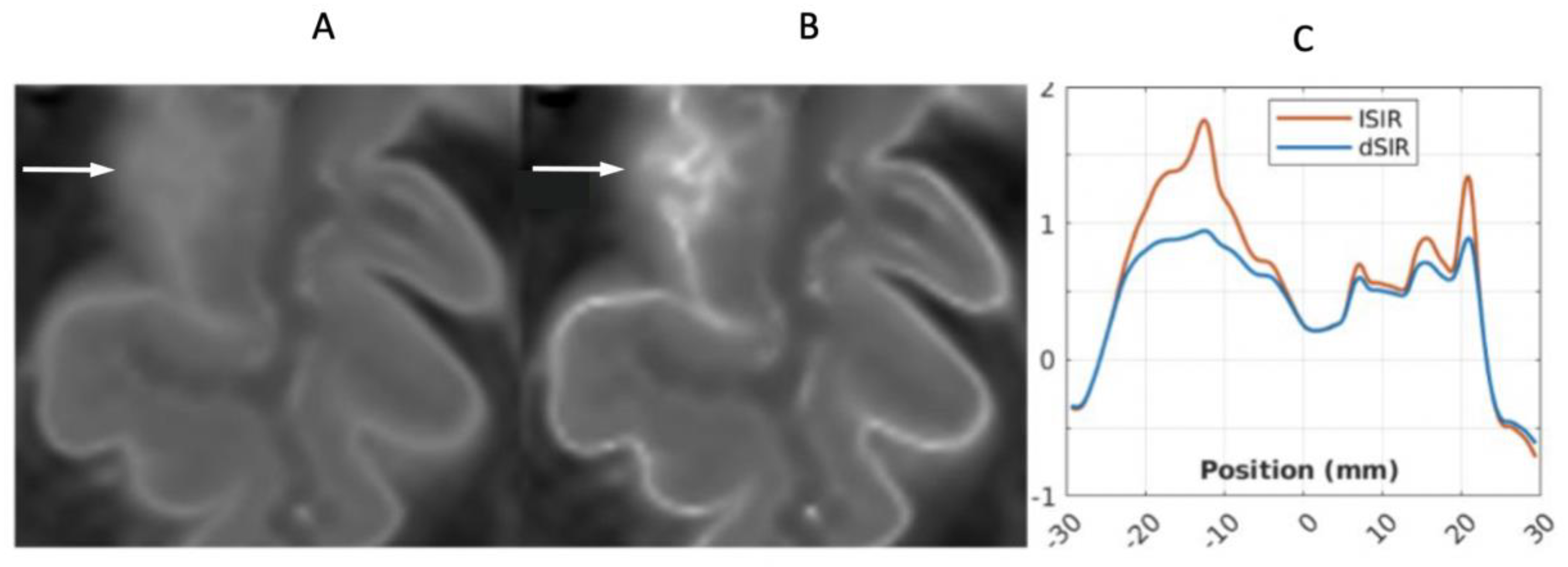
In a 41-year-old female patient with MS, the dSIR image (
Figure 23A) shows a blurred leukocortical lesion in the right medial frontal region with no evidence of a white matter-gray matter boundary within it. The matched lSIR image (
Figure 23B) shows a disrupted high signal boundary between white matter and gray matter within the lesion. Profiles (plots of signal against position in mm) through the lesion at the level shown by the arrows for the dSIR (blue) and lSIR (orange) images shows a higher signal and steeper slope for the lSIR image (
Figure 23C). The spatial resolution of the contrast i.e., change in signal with change in position is generally higher on the lSIR image.
Discussion
UHC MRI using Bipolar Filters
UHC MRI is a term used to describe MRI techniques which show much greater contrast than conventional state-of-the-art MRI images while having similar spatial resolutions and comparable acquisition times. This is achieved with no increase in static or gradient magnetic field. UHC MRI using bipolar filters employs sequences which exist on most MRI machines together with minimal image processing. It can be implemented in a very short time at very little cost.
UHC MRI is distinguished from ultra-high field MRI which uses static magnetic field strengths of 7T or higher to improve image signal-to-noise ratio. This improvement may be used to increase contrast, spatial resolution and/or the speed of MRI scans [
11,
12,
13,
14,
15].
UHC MRI is also distinguished from ultra-high spatial resolution MRI where typically increased gradient strength is used to improve spatial resolution [
16,
17].
The four groups of BLAIR sequences discussed in this paper are summarised in
Table 2 including some of their variants and the dominant source(s) of contrast for each sequence. The sequence signal equations and other relevant functions are included in
Table 3. The Tables also provide information for instituting synthetic imaging although relevant MT effects may need to be added.
While the emphasis in this paper has been on white matter in the mD and grey matter in the hD, very high contrast can also be seen in the central grey matter of the brain when this is in the mD rather than in the hD. In addition, iron containing tissues have only been studied with dSIR using the single tissue property T1, not the composite sequence cdSIR using the two tissue properties T1 and T2*.
Understanding the contrast produced by bipolar TP-filter sequences is greatly helped by use of their TP-filters. These filters relate differences or changes in tissue properties to contrast using their slopes and explain signal and contrast patterns that can otherwise appear inexplicable using conventional approaches [
8,
9].
Artefacts from too high a value of TI
i, misregistration, partial volume effects and other causes are described in reference [
5]. Means of avoiding these and/or reducing their effects are included in reference [
5].
Tissue and Fluid Boundaries
dSIR and lSIR images are characterised by sharply defined high contrast, higher spatial resolution boundaries between white matter and gray matter as well as between white matter and CSF, and between gray matter and CSF. The site of the boundaries as well as their width can be changed by varying the TIs of the sequence [
5]. The boundaries are specific and located at iso-T
1 contours. They are not part of a generalised pattern of edge enhancement.
In addition, there are well defined low signal (dark) boundaries between white matter, gray matter and CSF as modelled by bipolar TP-filters.
High signal in-plane boundaries on dSIR images usually have a uniform and consistent appearance although these are subject to partial volume effects and may simulate lesions particularly with 2D images acquired with relatively thick slices (e.g., 2-4 mm). Through-plane partial volume effects also produce high signal from boundaries and these are more prominent with thick slices.
A systematic treatment of boundaries including variation in signal with position using TP-filters, the partial derivative change in T
1 with tissue fraction and the partial derivative change of tissue fraction with position is described in reference [
5]. Using this approach, differences in signal (i.e., contrast) can be related to differences in position within the boundary region.
Hybrid Quantitative and Qualitative Imaging
dSIR sequences produce single images that can be used both quantitatively and qualitatively. The images show high contrast and this can be used for conventional qualitative image interpretation. The dSIR images are also T1 maps so they can be used to directly measure T1 values in regions of interest placed on them for quantitative studies. In comparison with the conventional approaches to T1 quantitation which use separate images for qualitative interpretation and for T1 mapping, dSIR images: (i) do not require working with images of two different types; (ii) allow precise placement of ROIs in relation to lesions on the images used for radiological interpretation, and (iii) require no extra time for additional acquisitions.
T
1 values of dSIR sequences have shown comparable accuracy to conventional T
1 mapping in the mD but, as expected, differences in the lD and hD [
18,
19,
20].
Magnetisation Transfer (MT)
With 2D multislice acquisitions, there may be incidental magnetisation transfer (MT) effects from off-resonance 180° or lower flip angle pulses used during FSE acquisitions. Using two pool modelling, MT decreases the r
m observed (r
mobs) in the free pool but also decreases the observed T
1 (T
1obs) of the free pool in proportion to the reduction in r
mobs [
21,
22,
23,
24,
25,
26,
27]. This reduction may be quite substantial and decrease normal r
mobs and T
1obs by as much as 50%. In general, MT effects are decreased in disease so the reduction in r
mobs and T
1obs in diseased tissue due to MT is generally less than that in normal tissue. This is manifest as an apparent increase in r
mobs and T
1obs in diseased tissue relative to normal tissue. This is in addition to any increases in r
mobs and T
1 in diseased tissue for other reasons such as pathological change due to oedema.
2D FSE images with higher echo train lengths (ETLs) require more 180° or similar pulses and thus produce a corresponding increase in MT effects during multislice acquisitions. These produce a greater reduction in values of T1obs and so need shorter nulling TIs. MT effects are less with gradient echo acquisitions as often used with 3D acquisitions. As a result, T1obs may be longer with 3D gradient echo acquisitions than with 2D FSE acquisitions and so their nulling TIs may need to be correspondingly longer. The contrast between normal and abnormal tissues may also be lower with gradient echo acquisitions.
MT can be used intentionally either before or after preparation inversion pulses, as well as both before and after preparation inversion pulses in IR sequences [
21]. MT can also be used as an attenuating filter S
OF in composite bipolar filters.
Synthetic Bipolar T1-filters
Synthetic bipolar T
1-filters can be of two types as shown in
Table 2. The first uses Equation 1 and the relationships shown in
Figure 6,
Figure 8 and
Figure 9 to synthesise dSIR and lSIR images with different TIs from two directly acquired images. The second type uses a synthetic bipolar TP-filter which may be the same or similar to the dSIR and lSIR filters and applies these to tissue property maps. This approach has two aspects: (i) acquiring the tissue property map which should be of high quality in the tissue property Domains or parts of Domains of interest, and (ii) using a visualization function such as a bipolar filter to increase contrast without saturating voxel values. These two aspects are incorporated in directly acquired dSIR and lSIR imaging.
Signs Produced with dSIR Sequences
The Whiteout Sign
This is a generalised bilateral symmetrical increase in signal in white matter of the cerebral and cerebellar hemispheres as well as the brainstem [
1]. The increase in signal follows the pattern of normal white matter signals and can involve all, or almost all, of the white matter in a particular slice. It has specific features such as sparing of the anterior and posterior central corpus callosum (genu and splenium) as well as peripheral white matter in the cerebral hemispheres (
Figure 1,
Figure 12,
Figure 13,
Figure 14,
Figure 19 and
Figure 21). The white matter signal can increase up to that of the high signal boundary between white and gray matter and can be divided into five grades with grades 1 and 2 normal and grades 3,4,5 abnormal signs, or alternatively grade 3 as indeterminate and grades 4 and 5 as abnormal white out signs [
1]. It is due to widespread, small increases in T
1 and is thought to be particularly associated with neuroinflammation but may also be due to oedema, demyelination, degeneration and other causes. It has also been seen in mTBI, hypoxic-ischaemic injury to the brain, multiple sclerosis and white matter associated with tumours.
Grayout Signs
With grayout signs there is a loss of contrast in gray matter such as is seen in the thalamus and basal ganglia after mTBI (
Figure 12 and
Figure 13), or a loss of contrast between gray matter and white matter, or between gray matter and CSF (
Figure 13B). Grayout signs are generally more subtle than the whiteout sign. They are associated with an increase in T
1 in gray matter.
In young subjects, the normal lateral thalamus gray matter (TG
lat) tends to have a shorter T
1 than the normal medial thalamus gray matter (TG
med) (upper horizontal negative solid green arrow). This leads to higher signal in the lateral thalamus with a narrow mD dSIR sequence (first vertical blue arrow) (
Figure 24). The T
1 of the lateral thalamus may increase in disease (middle horizontal positive green striped arrow in
Figure 24) which produces a loss of contrast with the medial thalamus (blue circle). With reversion back to normal on recovery, the T
1 may decrease back to normal (lowest horizontal negative green arrow) restoring contrast (second vertical blue arrow).
The whiteout sign may be accompanied by grayout signs in conditions such as mTBI as described in this paper. The term post-insult leukoencephalopathy syndromes (PILS) described in reference [
1] refers to whiteout signs after mTBI, Grinker’s myelinopathy, etc. A term that includes both the whiteout sign and grayout signs as well as the possibility of ongoing effects (rather than just post-insult effects) is reactive encephalopathy (RE). Depending on the observed features (presence/absence of grayout signs), the pattern may be described as PILS/RE. Experimental studies to determine histological origins of the whiteout and grayout signs are in progress.
The Bilaminar Cortex Sign
This is an increase in signal in the outer layer of the cortex which is often barely seen in young adults but becomes more obvious with increasing age as well as in Parkinson’s disease. It is seen with narrow mD dSIR images in which there is a high signal at the white matter gray matter junction, a lower signal layer in the inner cortex and a higher signal layer in the outer cortex (
Figure 20 and
Figure 22). The higher signal generally corresponds to layers I-III and the lower signal to layers IV-VI of the cortex. The increase in signal is usually most obvious at the crest of gyri and to a lesser extent in the banks of sulci. It may be obscured by partial volume effects particularly on 2D images of 4 mm thickness compared with the estimated 2-4 mm thickness of the cortex. The sign may be due to increased free iron content with a decrease in T
1 of the normal cortex with increase in age, with an exaggeration of this in Parkinson’s disease [
28]. It is distinguished from the double cortex sign [
29] which is due to iron deposited in a subcortical location. A bilaminar cortical appearance has been seen with MP2RAGE sequences at 7T after detailed image processing and has been attributed to differences in myelin [
30]. The bilaminar cortex sign differs from the high signal that can be seen at the cortical margin on T
2-FLAIR images. This signal is attributed to increased T
2 relative to brain in cortical veins/pia arachnoid (as opposed to a decreased T
1 in the bilaminar cortex sign). It is usually more evident on 2D T
2-FLAIR images with longer TIs and TRs than 3D T
2-FLAIR images though the latter may have very long effective TEs. It can be present in young subjects who do not show a bilaminar cortex sign.
Bubble Signs
With the narrow mD dSIR sequence nulling white matter, bubble signs are seen with an increase in T
1 in white matter that crosses the high signal boundary (“overshoots”) and enters the hD (
Figure 16B) [
5]. There is an outer high signal margin and a lower signal central region [
3]. Bubble signs may also be seen in the gray matter of the thalamus and basal ganglia with narrow mD dSIR sequences when the T
1 in the gray matter decreases and crosses the bipolar T
1-filter peak. Bubble signs of this type may become more obvious with age, and are seen in Parkinson’s disease. The decrease in T
1 in gray matter sufficient to cross the high signal boundary of narrow mD dSIR sequences is illustrated in
Figure 20 and probably reflects heterogeneous distribution of free iron. Bubble signs may also be produced in MS by release of free iron at the rim of lesions decreasing the T
1 in abnormally increased T
1 lesions.
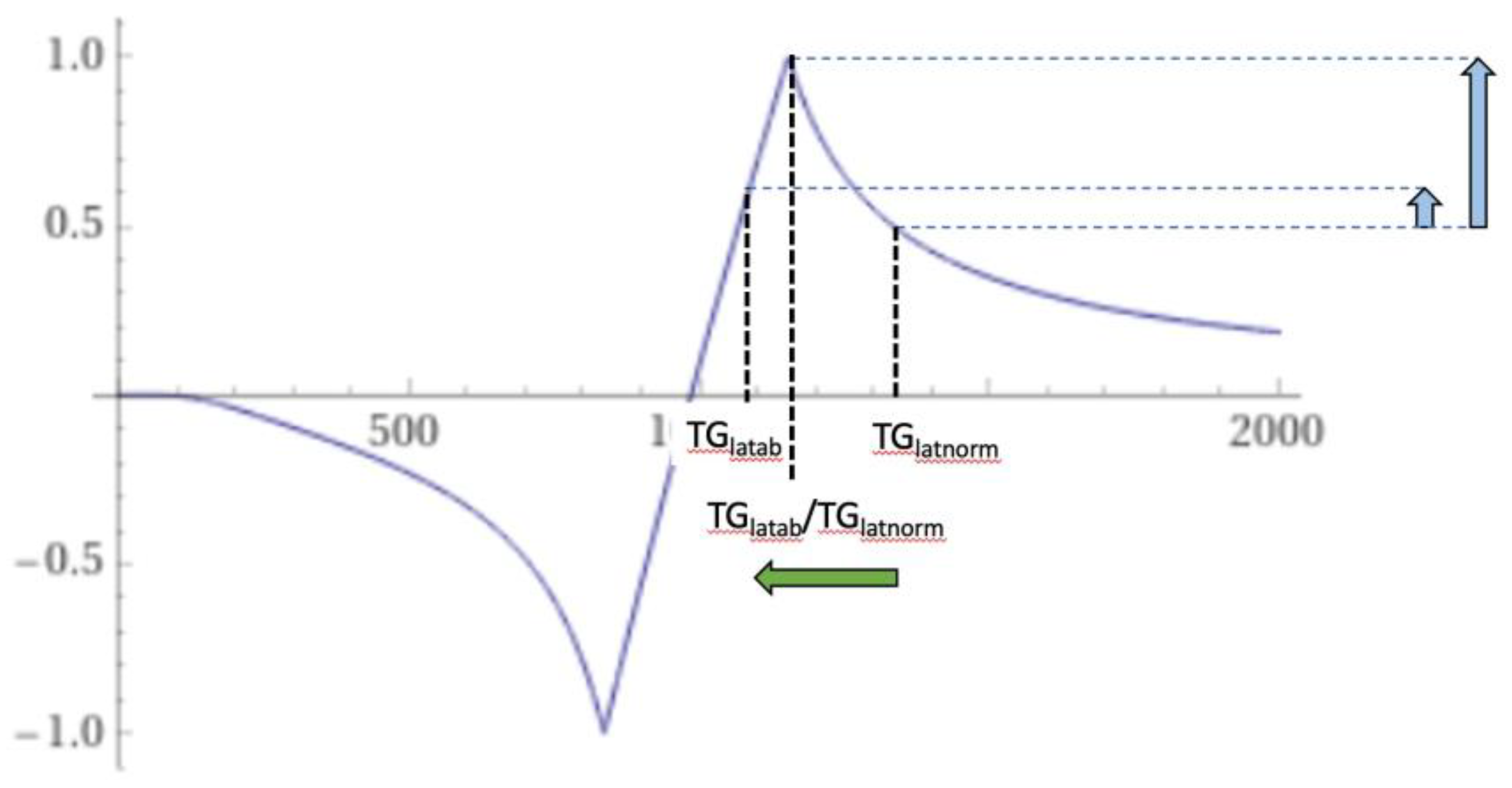
Figure 25 illustrates the origin of the bubble sign in the thalamus. The T
1 of lateral gray matter is decreased from normal (TG
latnorm) to abnormal (TG
latab). In doing so, it passes through the peak signal where there is a partial volume effect between TG
latnorm and TG
latab. This results in a high signal on the periphery and a lower signal centrally in the bubble as shown by the vertical blue arrows in
Figure 25.
Primary Cerebral Hemisphere Cortices
The primary motor, somato-sensory and visual cortices layers IV-VI have relatively greater myelin than elsewhere in the cortex, and this results in a shorter T1 and so the deep cortical signal is lower than the superficial signal from layers I-III when using narrow mD dSIR sequences compared with cortex elsewhere.
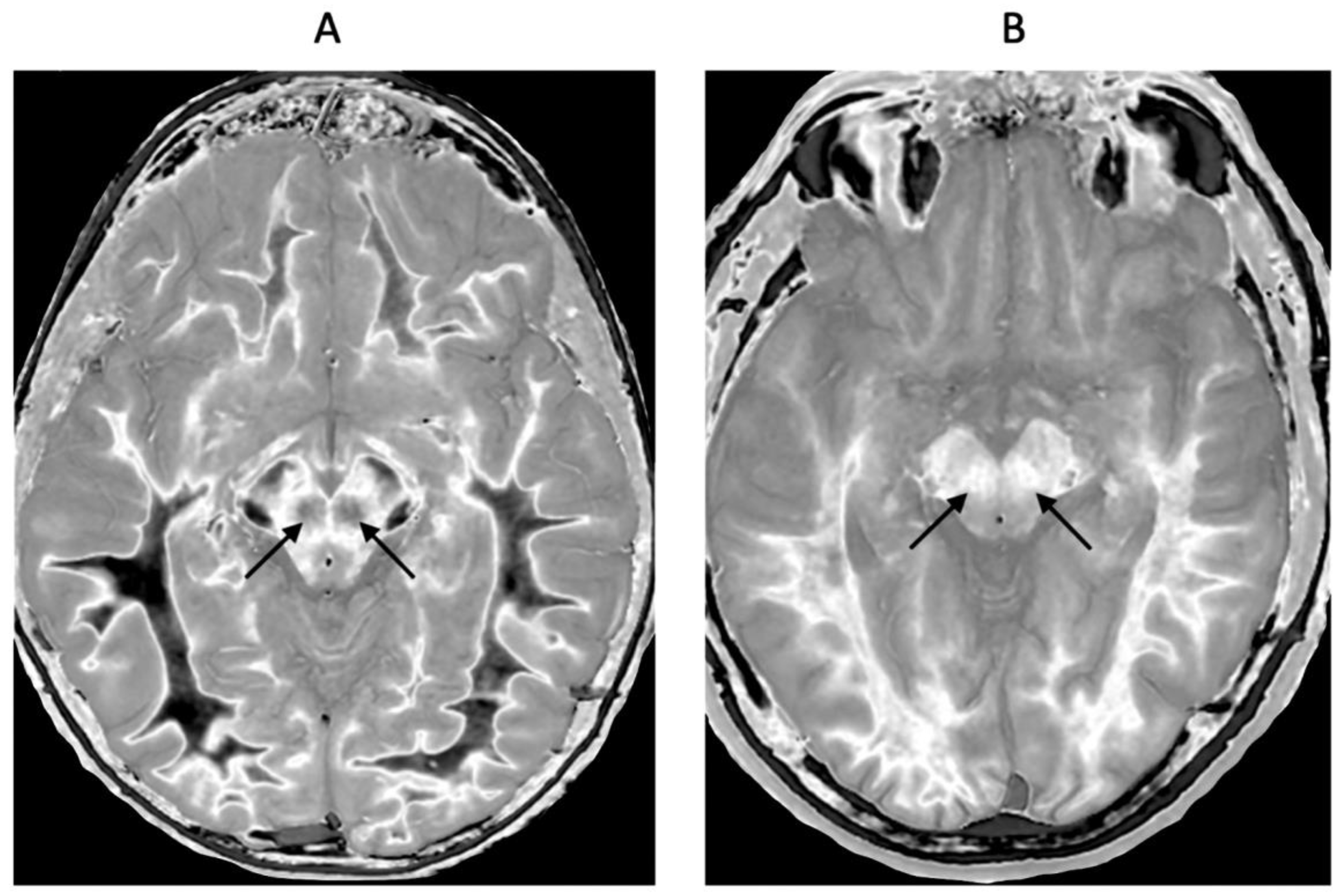
Figure 1.
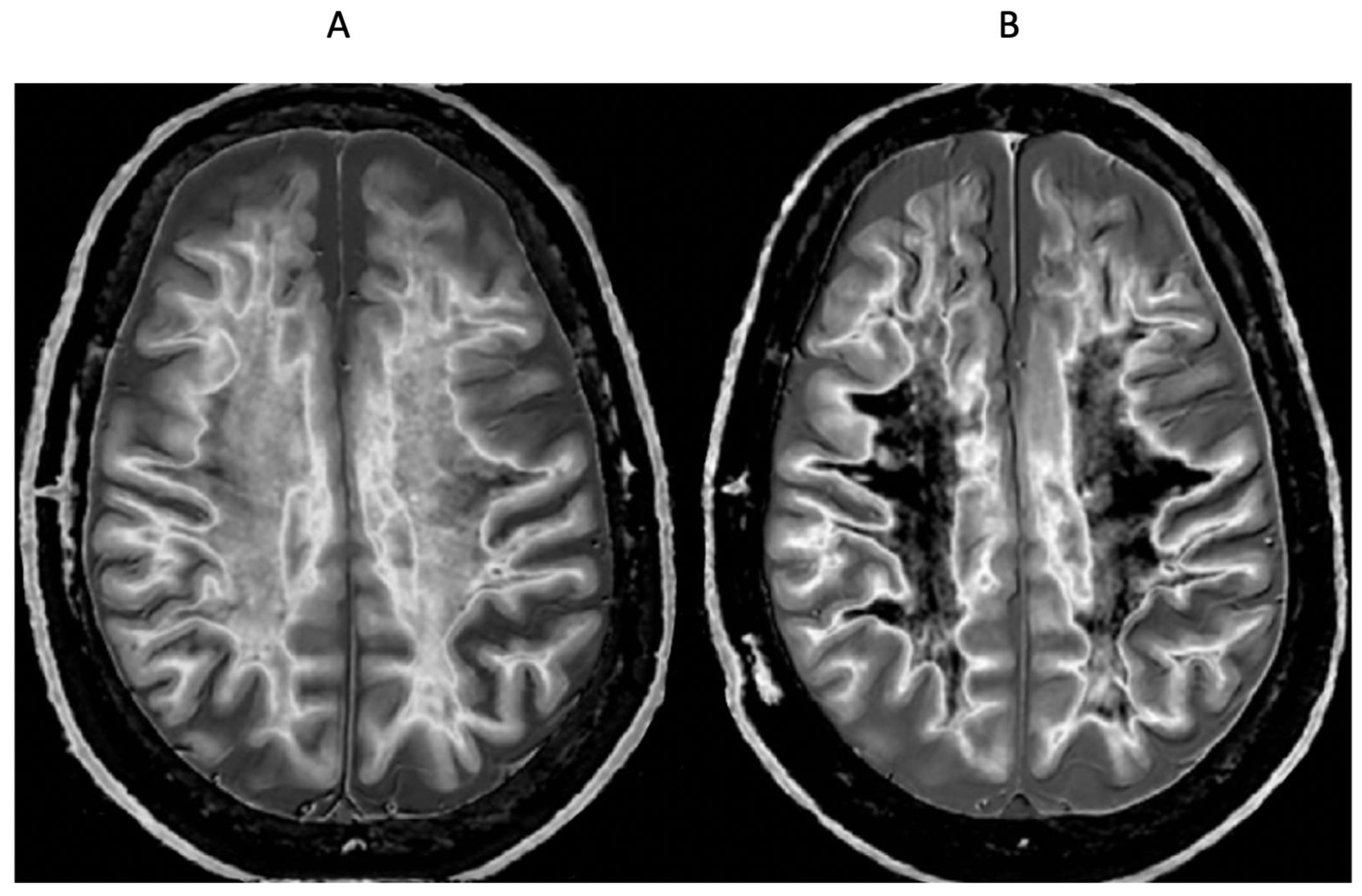
Positionally matched images of the brain in a 24-year-old male patient with mTBI (A and C) and a normal control (B). (A) is a T2-FLAIR image in the patient which shows no abnormality. (B) is a narrow mD dSIR (T1-BLAIR) image of the brain in the normal control. The white matter in the central region of this image has a normal low signal (dark) appearance (arrows). (C) is a narrow mD dSIR (T1-BLAIR) image performed in the patient with the same sequence as in the control. This image shows all the patient’s white matter with abnormal high signal (light) (arrows) rather than the normal low signal (dark) appearance in the control (arrows) (B). There is a night and day difference in signal between normal and abnormal white matter in (B) and (C). High signal boundaries are seen between normal white matter and normal gray matter in (B). These are less obvious in (C) because of the high signal in the abnormal white matter.
Figure 1.
Positionally matched images of the brain in a 24-year-old male patient with mTBI (A and C) and a normal control (B). (A) is a T2-FLAIR image in the patient which shows no abnormality. (B) is a narrow mD dSIR (T1-BLAIR) image of the brain in the normal control. The white matter in the central region of this image has a normal low signal (dark) appearance (arrows). (C) is a narrow mD dSIR (T1-BLAIR) image performed in the patient with the same sequence as in the control. This image shows all the patient’s white matter with abnormal high signal (light) (arrows) rather than the normal low signal (dark) appearance in the control (arrows) (B). There is a night and day difference in signal between normal and abnormal white matter in (B) and (C). High signal boundaries are seen between normal white matter and normal gray matter in (B). These are less obvious in (C) because of the high signal in the abnormal white matter.
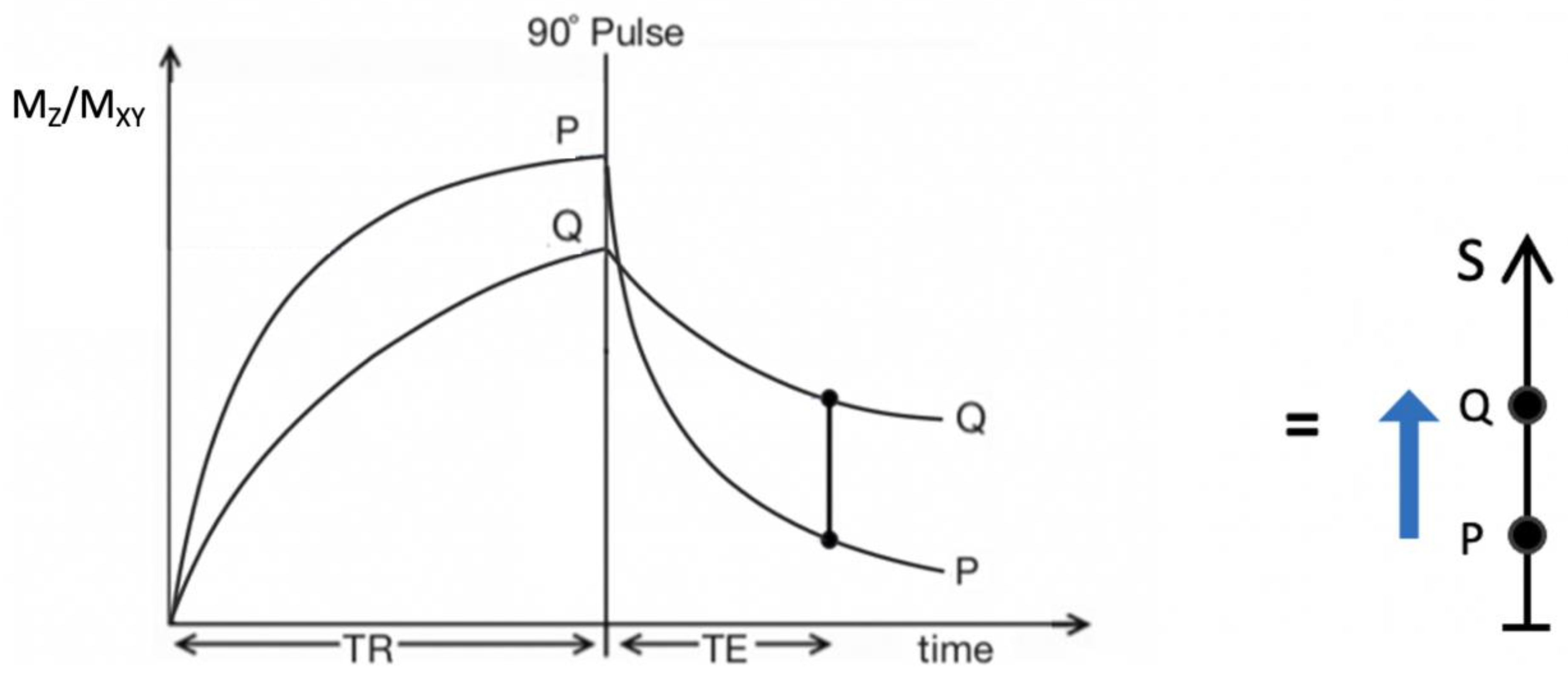
Figure 2.
Plot of MZ/MXY against time for a T2-weighted version of the SE sequence for two tissues P (with a shorter T1 and T2) and Q (with a longer T1 and T2). Overall T1 and T2 dependent contrast between P and Q is shown with the positive blue arrow on the right.
Figure 2.
Plot of MZ/MXY against time for a T2-weighted version of the SE sequence for two tissues P (with a shorter T1 and T2) and Q (with a longer T1 and T2). Overall T1 and T2 dependent contrast between P and Q is shown with the positive blue arrow on the right.
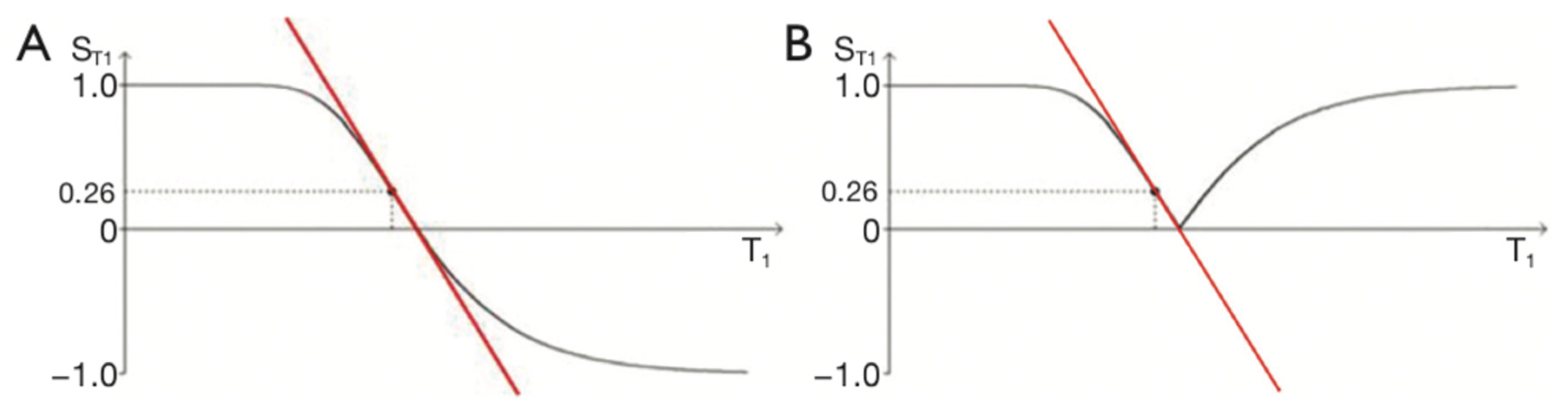
Figure 3.
IR T1-filters with plots of signal ST1 against T1 in phase-sensitive (A) and magnitude forms (B). (A) is a low pass filter and (B) is a negative unipolar filter. (A) shows both positive and negative values for ST1 whereas (B) shows negative values “reflected” across the X axis so they become positive. The maximum size of the slopes of the two T1-filters which are tangential to the slope are shown as red lines. The slopes are negative in both cases.
Figure 3.
IR T1-filters with plots of signal ST1 against T1 in phase-sensitive (A) and magnitude forms (B). (A) is a low pass filter and (B) is a negative unipolar filter. (A) shows both positive and negative values for ST1 whereas (B) shows negative values “reflected” across the X axis so they become positive. The maximum size of the slopes of the two T1-filters which are tangential to the slope are shown as red lines. The slopes are negative in both cases.
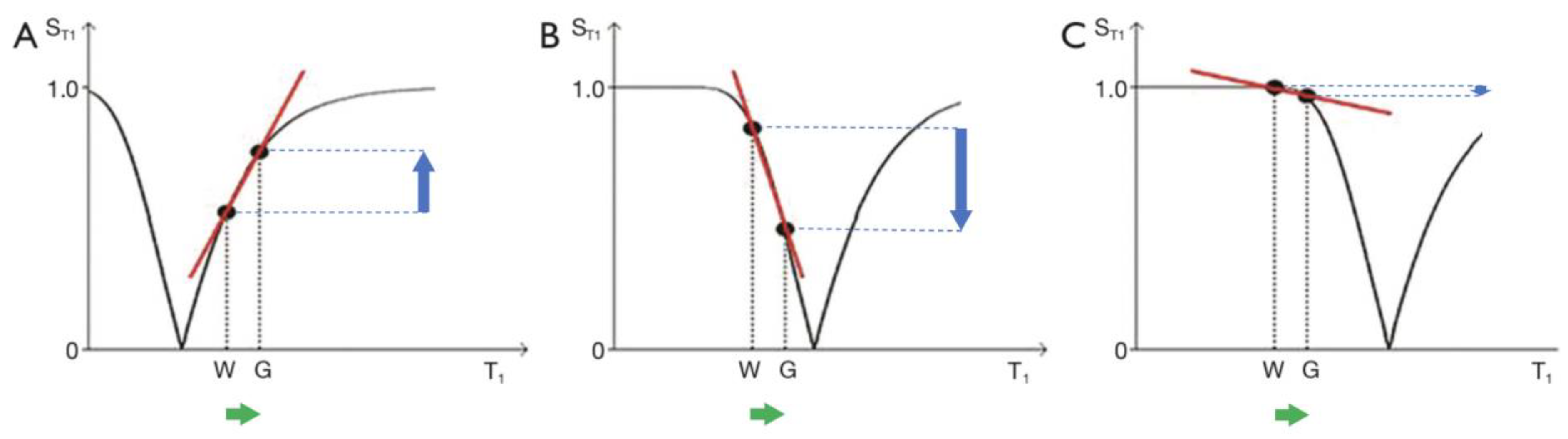
Figure 4.
The long TR IR sequence. Negative unipolar T1-filters for short TIs (A, left), intermediate TIi (B, centre) and long TIl (C, right) including white (W) and gray (G) matter. Signal ST1 is plotted against T1 in each case (black curves). The positions of W and G are the same at each of the three TIs. TI is increased from TIs (left) to TIi (centre) and increased further to TIl (right). In (A), (B) and (C), the increase in T1 from W to G (horizontal positive green arrows) is multiplied by the relevant slopes of the filters (red lines) to produce contrast. This is moderately positive, strongly negative, and mildly negative contrast in (A), (B) and (C) respectively (vertical blue arrows).
Figure 4.
The long TR IR sequence. Negative unipolar T1-filters for short TIs (A, left), intermediate TIi (B, centre) and long TIl (C, right) including white (W) and gray (G) matter. Signal ST1 is plotted against T1 in each case (black curves). The positions of W and G are the same at each of the three TIs. TI is increased from TIs (left) to TIi (centre) and increased further to TIl (right). In (A), (B) and (C), the increase in T1 from W to G (horizontal positive green arrows) is multiplied by the relevant slopes of the filters (red lines) to produce contrast. This is moderately positive, strongly negative, and mildly negative contrast in (A), (B) and (C) respectively (vertical blue arrows).
Figure 5.
Unipolar IR (A), subtracted IR (SIR) (B) and Added IR (AIR) (C) T1-filters. T1 is shown along the X axes in ms. (A) shows a TIs unipolar T1-filter (pink) and a TIi unipolar T1-filter (blue), (B) shows the subtraction (STIs − STIi) IR or SIR bipolar T1-filter, and (C) shows the addition (STIs + STIi) IR or AIR T1-filter. The vertical lines divide the X axes into lowest, middle and highest Domains (lD, mD and hD). The mD contains T1 values between the vertical dashed lines. In (B) the slope of the curve of the SIR T1-filter in the mD is about double that of the STIs T1-filter (pink in [A]). In (C) the signal at T1=0 is doubled to 2.0, and the signal in the mD is reduced to about 0.20 in the nearly linear, slightly downward sloping central part of the AIR T1-filter (i.e., in the mD).
Figure 5.
Unipolar IR (A), subtracted IR (SIR) (B) and Added IR (AIR) (C) T1-filters. T1 is shown along the X axes in ms. (A) shows a TIs unipolar T1-filter (pink) and a TIi unipolar T1-filter (blue), (B) shows the subtraction (STIs − STIi) IR or SIR bipolar T1-filter, and (C) shows the addition (STIs + STIi) IR or AIR T1-filter. The vertical lines divide the X axes into lowest, middle and highest Domains (lD, mD and hD). The mD contains T1 values between the vertical dashed lines. In (B) the slope of the curve of the SIR T1-filter in the mD is about double that of the STIs T1-filter (pink in [A]). In (C) the signal at T1=0 is doubled to 2.0, and the signal in the mD is reduced to about 0.20 in the nearly linear, slightly downward sloping central part of the AIR T1-filter (i.e., in the mD).
Figure 6.
Figure 6A shows division of the SIR bipolar T
1-filter in
Figure 5B by the addition T
1-filter AIR in
Figure 5C to give the dSIR bipolar T
1-filter. T
1 is shown along the X axis in ms.
Figure 6B shows comparison of the conventional IR S
TIs unipolar T
1-filter (pink) with the SIR bipolar T
1-filter (blue) for a small increase in T
1 (horizontal positive green arrow, DT
1).
Figure 6C is a comparison of the S
TIs unipolar T
1-filter (pink) with the dSIR bipolar T
1-filter (blue) for the same small increase in T
1. In 6B the contrast produced by the SIR bipolar T
1-filter is about twice that produced by the IR unipolar T
1-filter (blue and pink arrows). In 6C the contrast produced by the dSIR bipolar T
1-filter is about ten times greater than that produced by the IR unipolar T
1-filter (blue and pink arrows). The display gray scale has no negative values so white matter appears on it as zero signal (black) at the lowest part of the display scale when using both the T
1-filters shown in each of 6B and 6C.
Figure 6.
Figure 6A shows division of the SIR bipolar T
1-filter in
Figure 5B by the addition T
1-filter AIR in
Figure 5C to give the dSIR bipolar T
1-filter. T
1 is shown along the X axis in ms.
Figure 6B shows comparison of the conventional IR S
TIs unipolar T
1-filter (pink) with the SIR bipolar T
1-filter (blue) for a small increase in T
1 (horizontal positive green arrow, DT
1).
Figure 6C is a comparison of the S
TIs unipolar T
1-filter (pink) with the dSIR bipolar T
1-filter (blue) for the same small increase in T
1. In 6B the contrast produced by the SIR bipolar T
1-filter is about twice that produced by the IR unipolar T
1-filter (blue and pink arrows). In 6C the contrast produced by the dSIR bipolar T
1-filter is about ten times greater than that produced by the IR unipolar T
1-filter (blue and pink arrows). The display gray scale has no negative values so white matter appears on it as zero signal (black) at the lowest part of the display scale when using both the T
1-filters shown in each of 6B and 6C.
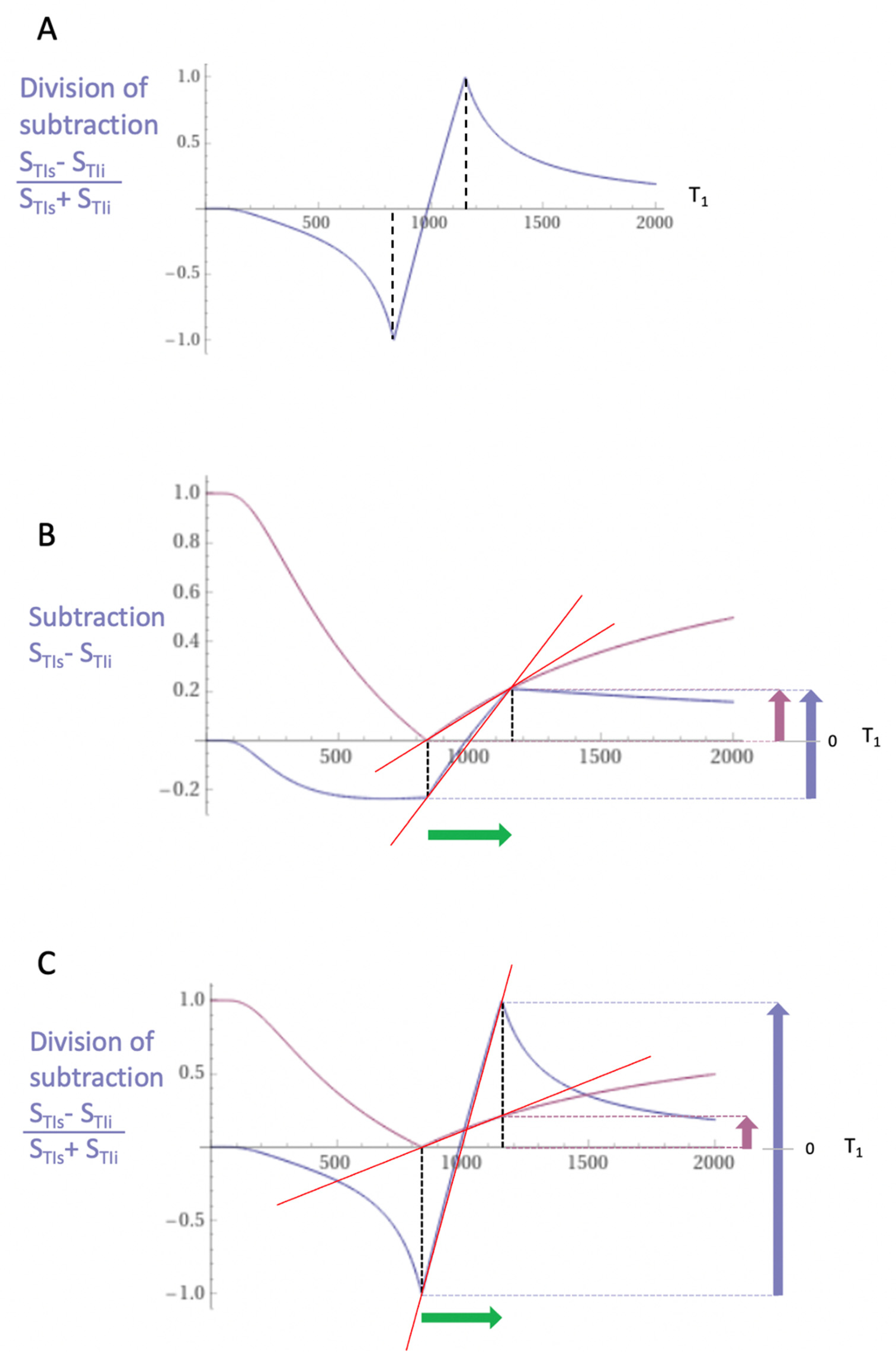
Figure 7.
Boundaries. This image shows a narrow mD dSIR bipolar T1-filter with a mD extending from white matter (W) to a T1W,G between the TIs of W and gray matter (G) (blue curve) as well as a white matter nulled conventional IR unipolar T1-filter e.g., MP-RAGE (pink curve). The X axis is shown in ms. The contrast produced from the difference in signal between W and G by the unipolar T1-filter is (SG minus SW, pink curve) and is shown by the vertical pink arrow. With the bipolar T1-filter (blue curve) there is a partial volume effect between W and G producing a T1W,G between the T1s of W and G which results in the high signal, SW,G shown on the bipolar T1-filter (blue curve). The contrast between this high signal and white matter is the difference (SW,G minus SW, in blue) and is shown by the vertical blue arrow. The contrast produced by the bipolar T1-filter at the boundary between W and G is far greater than that produced at the boundary between W and G by the unipolar T1-filter.
Figure 7.
Boundaries. This image shows a narrow mD dSIR bipolar T1-filter with a mD extending from white matter (W) to a T1W,G between the TIs of W and gray matter (G) (blue curve) as well as a white matter nulled conventional IR unipolar T1-filter e.g., MP-RAGE (pink curve). The X axis is shown in ms. The contrast produced from the difference in signal between W and G by the unipolar T1-filter is (SG minus SW, pink curve) and is shown by the vertical pink arrow. With the bipolar T1-filter (blue curve) there is a partial volume effect between W and G producing a T1W,G between the T1s of W and G which results in the high signal, SW,G shown on the bipolar T1-filter (blue curve). The contrast between this high signal and white matter is the difference (SW,G minus SW, in blue) and is shown by the vertical blue arrow. The contrast produced by the bipolar T1-filter at the boundary between W and G is far greater than that produced at the boundary between W and G by the unipolar T1-filter.
Figure 8.
Plots of ln S
TIs (TI
s = 350 ms) vs T
1 (pink), and ln S
TIi (TI
i = 500 ms) vs T
1 (blue). The plots have a negative unipolar form with steeply sloping signal gradients around their negative poles. The pink and blue ln S
TIs and ln S
TIi curves are steeper than the corresponding magnitude curves shown in
Figure 5A for conventional IR T
1-filters. The lSIR filter S
lSIR = ½(ln S
TIs − ln S
TIi) reverses the sign of the S
TIi filter so it becomes positive as seen in the following figure (
Figure 9) (orange curve).
Figure 8.
Plots of ln S
TIs (TI
s = 350 ms) vs T
1 (pink), and ln S
TIi (TI
i = 500 ms) vs T
1 (blue). The plots have a negative unipolar form with steeply sloping signal gradients around their negative poles. The pink and blue ln S
TIs and ln S
TIi curves are steeper than the corresponding magnitude curves shown in
Figure 5A for conventional IR T
1-filters. The lSIR filter S
lSIR = ½(ln S
TIs − ln S
TIi) reverses the sign of the S
TIi filter so it becomes positive as seen in the following figure (
Figure 9) (orange curve).
Figure 9.
Plot of signal vs T1 in ms for a dSIR bipolar T1-filter (blue curve) and the corresponding lSIR bipolar T1-filter with the same null points (orange curve) (TIs = 350 ms and TIi = 500 ms, DTI = 150 ms). The dSIR filter shows the usual pattern with maximum values of ±1. It has an essentially constant slope in the mD. The lSIR filter has similar values to the dSIR filter in the regions of lowest and highest values of T1 as well as in the centre of the mD. However around the lower and upper T1 nulling values it has much steeper slopes and proceeds asymptotically to minus and plus infinity respectively (values of S along the Y axis in the range of ± 2 are shown). From a practical point of view, the contrast for very small differences or changes in T1 is 2-3 times greater with the lSIR filter compared with the corresponding dSIR filter. For the same difference in T1 in the regions close to the null points the lSIR filter is steeper than the dSIR filter. This results in greater contrast and spatial resolution.
Figure 9.
Plot of signal vs T1 in ms for a dSIR bipolar T1-filter (blue curve) and the corresponding lSIR bipolar T1-filter with the same null points (orange curve) (TIs = 350 ms and TIi = 500 ms, DTI = 150 ms). The dSIR filter shows the usual pattern with maximum values of ±1. It has an essentially constant slope in the mD. The lSIR filter has similar values to the dSIR filter in the regions of lowest and highest values of T1 as well as in the centre of the mD. However around the lower and upper T1 nulling values it has much steeper slopes and proceeds asymptotically to minus and plus infinity respectively (values of S along the Y axis in the range of ± 2 are shown). From a practical point of view, the contrast for very small differences or changes in T1 is 2-3 times greater with the lSIR filter compared with the corresponding dSIR filter. For the same difference in T1 in the regions close to the null points the lSIR filter is steeper than the dSIR filter. This results in greater contrast and spatial resolution.
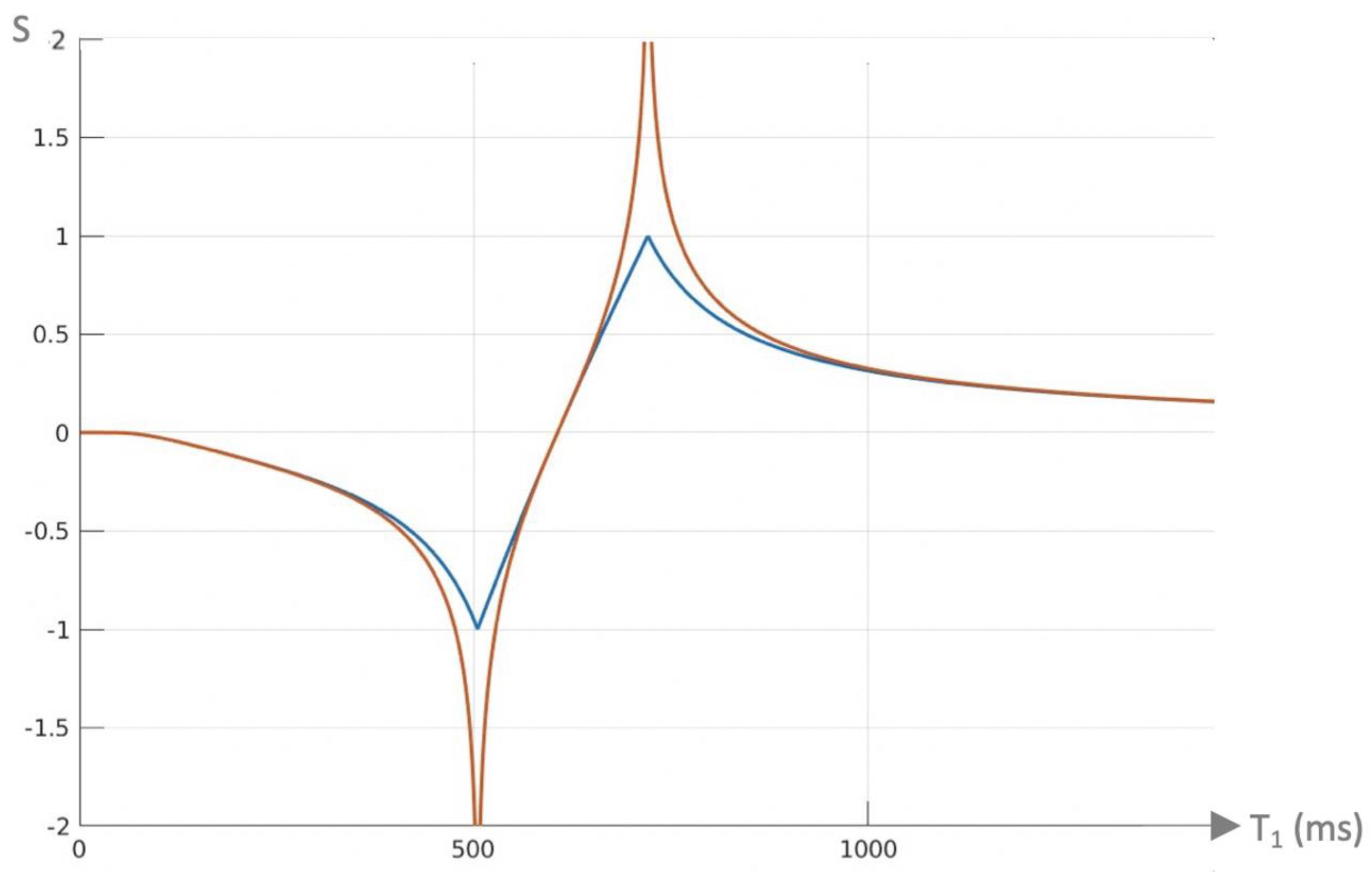
Figure 10.
Composite dSIR filters (cdSIRs with TIs = 350 ms and TIi = 500 ms). Attenuated first IR T1-filter (TIs) in (A) and attenuated second IR T1-filter (TIi) in (B). (A) shows the conventional bipolar T1-filter (blue) with SOF = 1, an attenuated first TIs filter (SOF = 0.5) (red) and a further attenuated first TIs filter (SOF = 0.25) (yellow). In (A), as SOF is decreased to 0.5 and 0.25 the filters around the first negative pole become wider and are positioned outside the blue filter. The attenuated filters around the second positive pole show higher contrast and become narrower. They are located inside the blue filter. (B) shows the conventional bipolar T1-filter (blue) with SOF = 1, and attenuated second TIi filter SOF = 0.5 (red) and a more attenuated second TIs filter (SOF = 0.25) (yellow). As SOF is decreased, the filters around the first negative pole become narrower and show higher contrast. They are positioned within the blue filter. The attenuated filters around the second positive pole become wider and show lower contrast. They are positioned outside of the blue filter. For T2, a short T2 increases attenuation relative to a longer T2 and this corresponds to the narrower and sharper peak with greater negative contrast and spatial resolution of the contrast (A).
Figure 10.
Composite dSIR filters (cdSIRs with TIs = 350 ms and TIi = 500 ms). Attenuated first IR T1-filter (TIs) in (A) and attenuated second IR T1-filter (TIi) in (B). (A) shows the conventional bipolar T1-filter (blue) with SOF = 1, an attenuated first TIs filter (SOF = 0.5) (red) and a further attenuated first TIs filter (SOF = 0.25) (yellow). In (A), as SOF is decreased to 0.5 and 0.25 the filters around the first negative pole become wider and are positioned outside the blue filter. The attenuated filters around the second positive pole show higher contrast and become narrower. They are located inside the blue filter. (B) shows the conventional bipolar T1-filter (blue) with SOF = 1, and attenuated second TIi filter SOF = 0.5 (red) and a more attenuated second TIs filter (SOF = 0.25) (yellow). As SOF is decreased, the filters around the first negative pole become narrower and show higher contrast. They are positioned within the blue filter. The attenuated filters around the second positive pole become wider and show lower contrast. They are positioned outside of the blue filter. For T2, a short T2 increases attenuation relative to a longer T2 and this corresponds to the narrower and sharper peak with greater negative contrast and spatial resolution of the contrast (A).
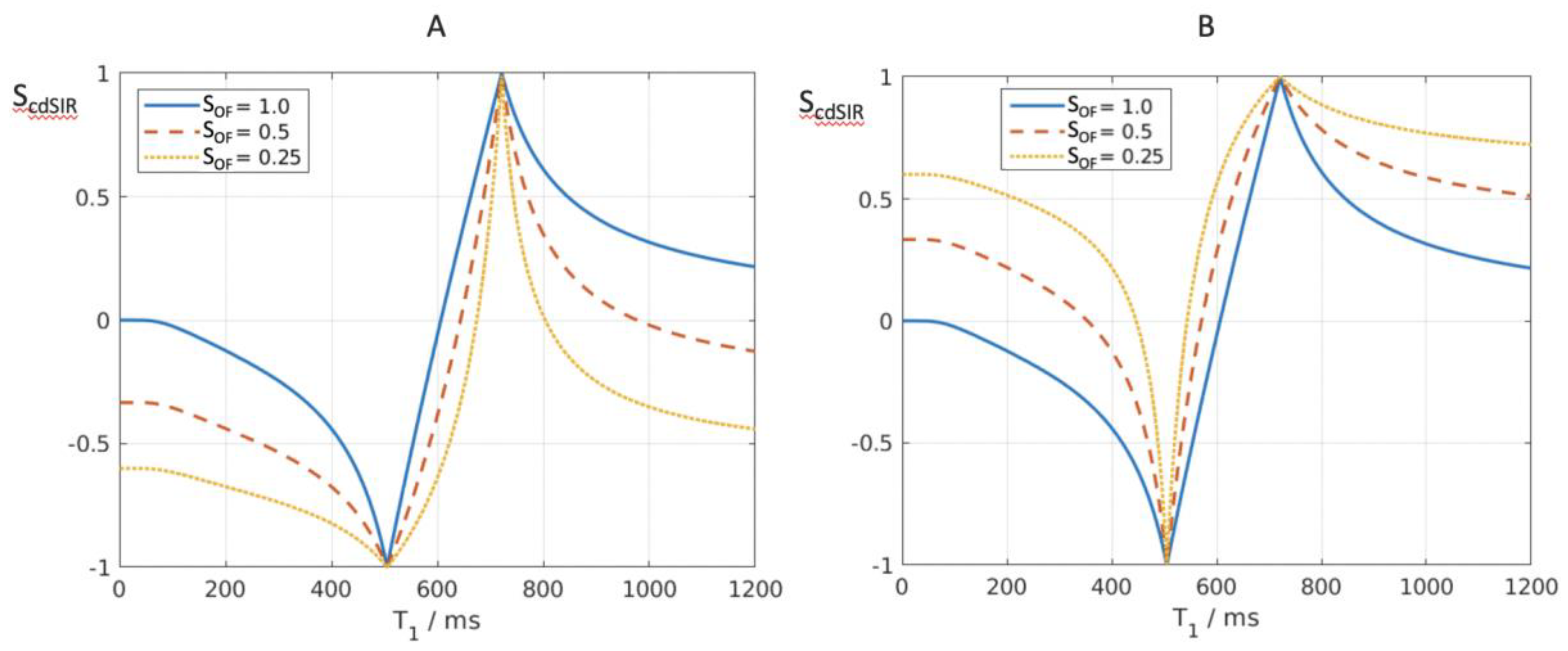
Figure 11.
Comparison of conventional windowing (A and B) and a dSIR bipolar T1-filter (C). (A) plots the display signal SD against image signal S for two tissues P and Q over the full image gray scale range shown along the Y axis. There is a linear relationship between SD and S over the full gray scale range. In (B) the image is narrowly windowed to place the signals from P and Q at the lower and upper ends of the SD range shown on the Y axis. The contrast between P and Q (difference in signal level shown on the Y axis) is increased in (B) compared with (A). In (B) signal values less than P on the X axis are all shown at the lowest signal level on the Y axis, and signal values along the X axis greater than Q are all shown at the highest signal level on the Y axis (horizontal lines). In these regions, voxels have the same signals and show no contrast between them. They form isointense blocks and anatomical detail is lost within the blocks. (C) plots signal against T1 using a dSIR bipolar T1-filter. Contrast between P and Q is high in the mD as in (B). Values of T1 less than P and greater than Q do not form plateaux as in (B) but follow the bipolar T1-filter in the lD and hD. There are therefore differences in signal between voxels so contrast is maintained and anatomical detail is preserved outside of the mD bounded by P and Q.
Figure 11.
Comparison of conventional windowing (A and B) and a dSIR bipolar T1-filter (C). (A) plots the display signal SD against image signal S for two tissues P and Q over the full image gray scale range shown along the Y axis. There is a linear relationship between SD and S over the full gray scale range. In (B) the image is narrowly windowed to place the signals from P and Q at the lower and upper ends of the SD range shown on the Y axis. The contrast between P and Q (difference in signal level shown on the Y axis) is increased in (B) compared with (A). In (B) signal values less than P on the X axis are all shown at the lowest signal level on the Y axis, and signal values along the X axis greater than Q are all shown at the highest signal level on the Y axis (horizontal lines). In these regions, voxels have the same signals and show no contrast between them. They form isointense blocks and anatomical detail is lost within the blocks. (C) plots signal against T1 using a dSIR bipolar T1-filter. Contrast between P and Q is high in the mD as in (B). Values of T1 less than P and greater than Q do not form plateaux as in (B) but follow the bipolar T1-filter in the lD and hD. There are therefore differences in signal between voxels so contrast is maintained and anatomical detail is preserved outside of the mD bounded by P and Q.
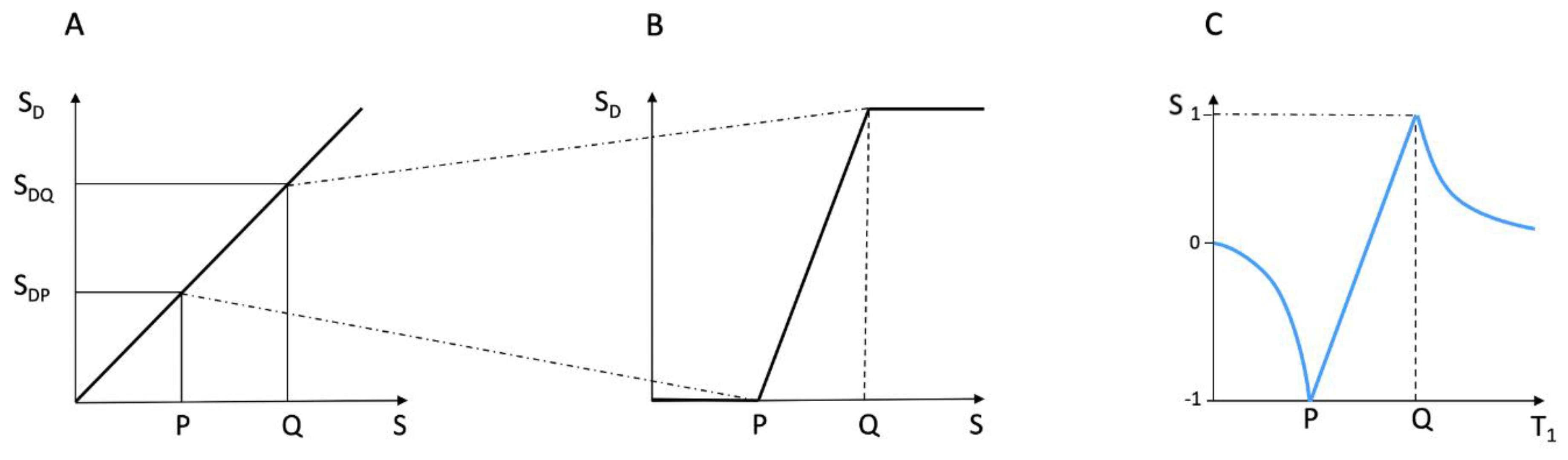
Figure 12.
18-year-old patient with mTBI 21h (A) and 64h (B) post injury imaged with the same narrow mD dSIR (T1-BLAIR) sequence. In (A) the patient shows a whiteout sign (grade 4 out of 5) with high signal in most of the white matter in the cerebral hemispheres except for the anterior and posterior central corpus callosum. The posterior limb of the internal capsule is high signal. The thalami show low internal contrast from medial to lateral (arrows on lateral margins of the thalami). There is also low external contrast between the lateral margins of the thalami and the adjacent posterior limbs of the internal capsule.On the follow up examination at 64h (B) the whiteout sign has resolved with low signal now in the white matter including the posterior limbs of the internal capsule (except for the corticospinal tracts). The thalami now show high internal contrast from medial to lateral (arrows on lateral margins of the thalamus) which is the normal appearance at this age. There is now also very high external contrast between the lateral margin of the thalamus and the adjacent posterior limb of the internal capsule. Image (A) shows the grayout sign which is a reduction in the high contrast between the medial and lateral gray matter of the thalamus. The high contrast is restored in (B). No abnormality was seen on the corresponding T2-FLAIR images obtained at both time points.
Figure 12.
18-year-old patient with mTBI 21h (A) and 64h (B) post injury imaged with the same narrow mD dSIR (T1-BLAIR) sequence. In (A) the patient shows a whiteout sign (grade 4 out of 5) with high signal in most of the white matter in the cerebral hemispheres except for the anterior and posterior central corpus callosum. The posterior limb of the internal capsule is high signal. The thalami show low internal contrast from medial to lateral (arrows on lateral margins of the thalami). There is also low external contrast between the lateral margins of the thalami and the adjacent posterior limbs of the internal capsule.On the follow up examination at 64h (B) the whiteout sign has resolved with low signal now in the white matter including the posterior limbs of the internal capsule (except for the corticospinal tracts). The thalami now show high internal contrast from medial to lateral (arrows on lateral margins of the thalamus) which is the normal appearance at this age. There is now also very high external contrast between the lateral margin of the thalamus and the adjacent posterior limb of the internal capsule. Image (A) shows the grayout sign which is a reduction in the high contrast between the medial and lateral gray matter of the thalamus. The high contrast is restored in (B). No abnormality was seen on the corresponding T2-FLAIR images obtained at both time points.
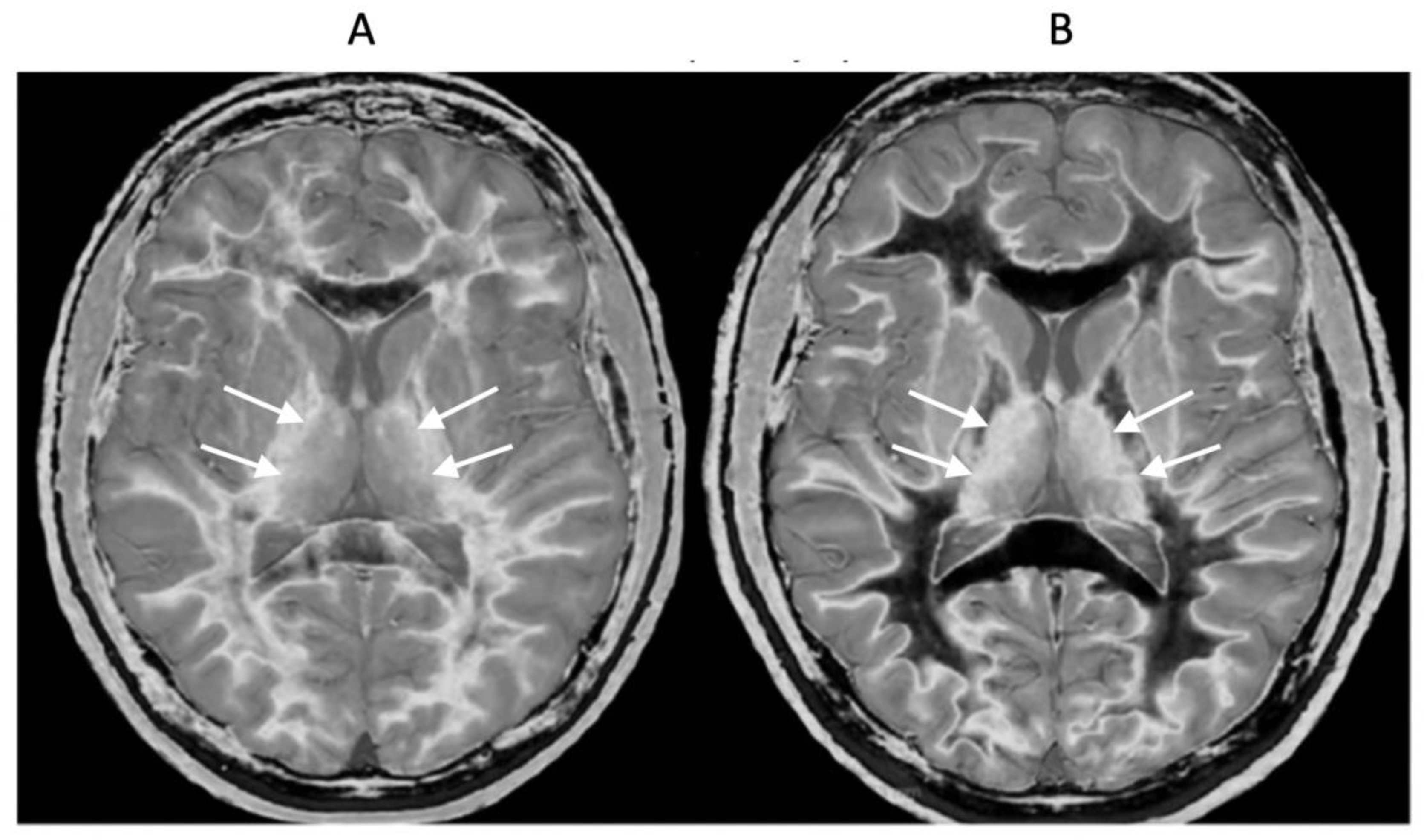
Figure 13.
Normal control (A) and patient with mTBI (B) showing a whiteout sign and grayout signs (narrow mD dSIR [T1-BLAIR] images). The normal control shows the heads of the caudate nuclei with higher signal than the adjacent CSF. Contrast is also seen between the cortex and CSF. In (B), the patient shows a whiteout sign. There are grayout signs in the thalami and putamina. In addition, contrast between the heads of the caudate nuclei and CSF is reduced and there is little or no contrast between cortex and CSF which are isointense. These are also grayout signs. No abnormality was seen on the T2-FLAIR images in the normal control or patient.
Figure 13.
Normal control (A) and patient with mTBI (B) showing a whiteout sign and grayout signs (narrow mD dSIR [T1-BLAIR] images). The normal control shows the heads of the caudate nuclei with higher signal than the adjacent CSF. Contrast is also seen between the cortex and CSF. In (B), the patient shows a whiteout sign. There are grayout signs in the thalami and putamina. In addition, contrast between the heads of the caudate nuclei and CSF is reduced and there is little or no contrast between cortex and CSF which are isointense. These are also grayout signs. No abnormality was seen on the T2-FLAIR images in the normal control or patient.
Figure 14.
Red nuclei in an 18-year-old normal control (A) and an 18-year-old male patient with mTBI (B). 2D narrow mD dSIR (T1-BLAIR) images. In (A) the normal control shows low signal in the white matter of the cerebral hemisphere, cortico-spinal tracts and the ascending sensory tracts. The red nuclei (arrows) have an intermediate mid-gray signal. In (B) the patient shows high signal in the cerebral white matter, the corticospinal tracts and the ascending sensory tracts (whiteout sign, grade 4 out of 5). In addition, the red nuclei are higher signal than in (A) (arrows). No abnormality was seen on the T2-FLAIR images in the normal control or patient.
Figure 14.
Red nuclei in an 18-year-old normal control (A) and an 18-year-old male patient with mTBI (B). 2D narrow mD dSIR (T1-BLAIR) images. In (A) the normal control shows low signal in the white matter of the cerebral hemisphere, cortico-spinal tracts and the ascending sensory tracts. The red nuclei (arrows) have an intermediate mid-gray signal. In (B) the patient shows high signal in the cerebral white matter, the corticospinal tracts and the ascending sensory tracts (whiteout sign, grade 4 out of 5). In addition, the red nuclei are higher signal than in (A) (arrows). No abnormality was seen on the T2-FLAIR images in the normal control or patient.
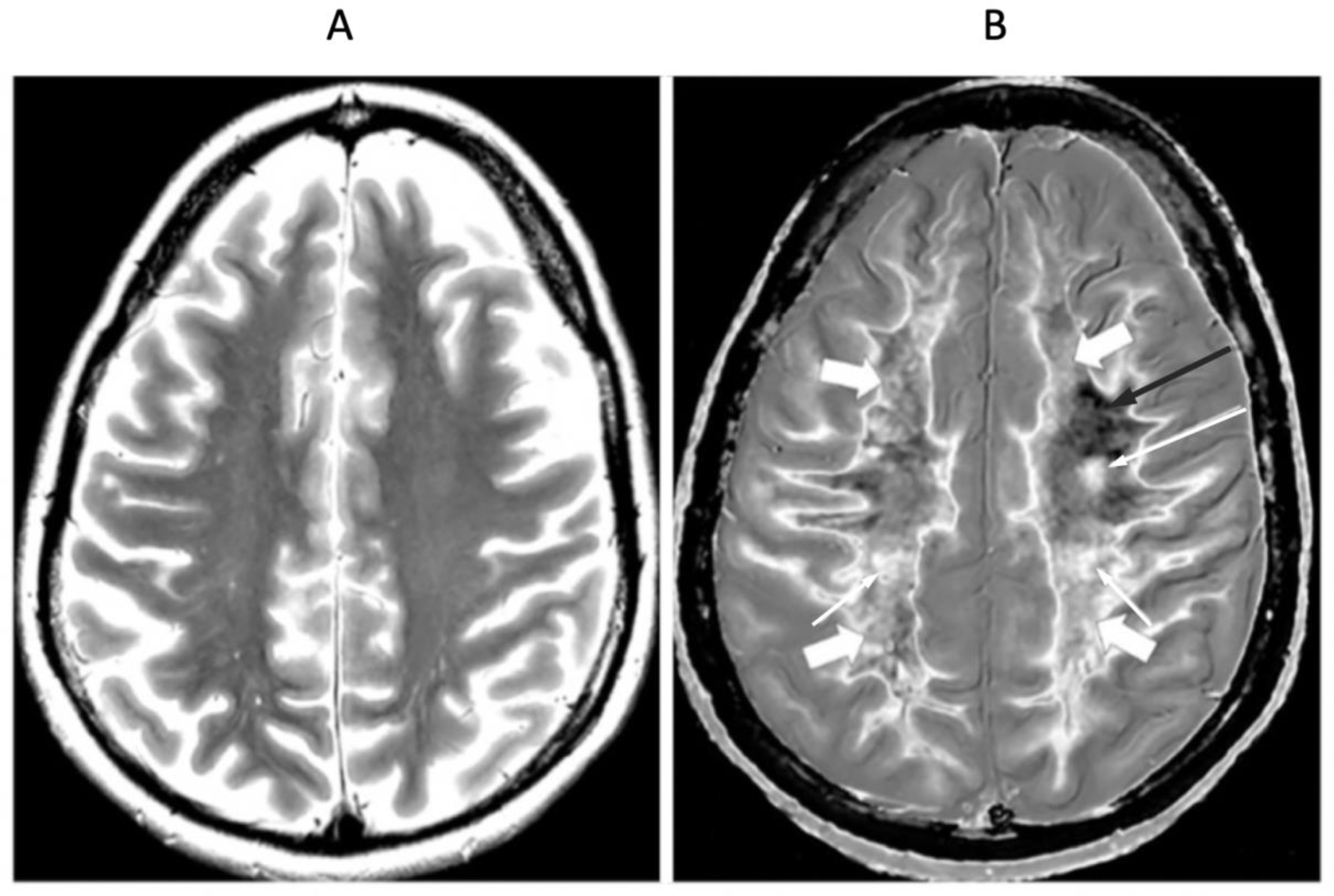
Figure 15.
41-year-old female patient with MS in remission. 2D T2-wSE (A) and narrow mD dSIR (T1-BLAIR) (B) images at the same level. No abnormality is seen in (A). A focal lesion is seen in (B) (long narrow white arrow) and the cortico-spinal tracts show a high signal (short narrow white arrows). In addition there is patchy increased signal in white matter (short thick white arrows) with only a small region showing a normal or near normal low signal appearance (long black arrow). High contrast and high spatial resolution contrast are seen at the boundaries between normal white matter and normal gray matter in (B). These features are less obvious in areas where the white matter has abnormal high signal.
Figure 15.
41-year-old female patient with MS in remission. 2D T2-wSE (A) and narrow mD dSIR (T1-BLAIR) (B) images at the same level. No abnormality is seen in (A). A focal lesion is seen in (B) (long narrow white arrow) and the cortico-spinal tracts show a high signal (short narrow white arrows). In addition there is patchy increased signal in white matter (short thick white arrows) with only a small region showing a normal or near normal low signal appearance (long black arrow). High contrast and high spatial resolution contrast are seen at the boundaries between normal white matter and normal gray matter in (B). These features are less obvious in areas where the white matter has abnormal high signal.
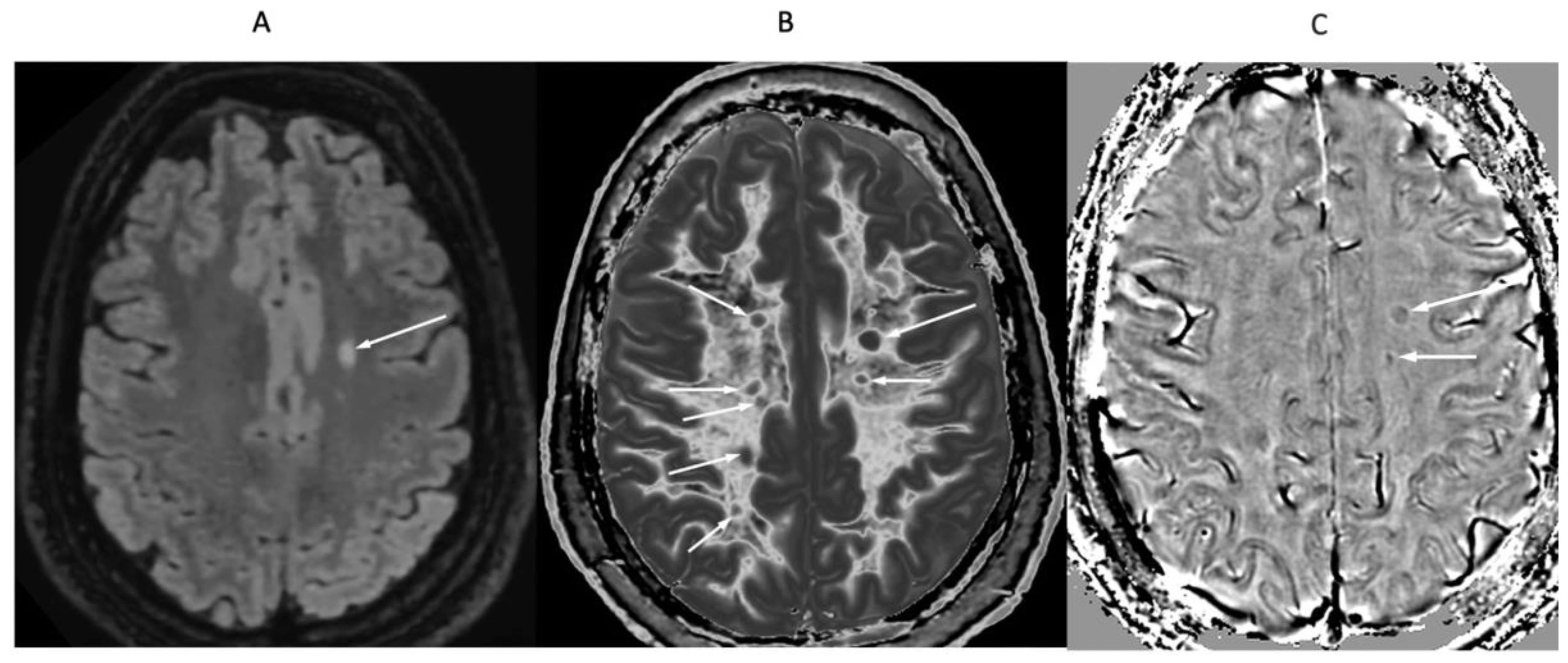
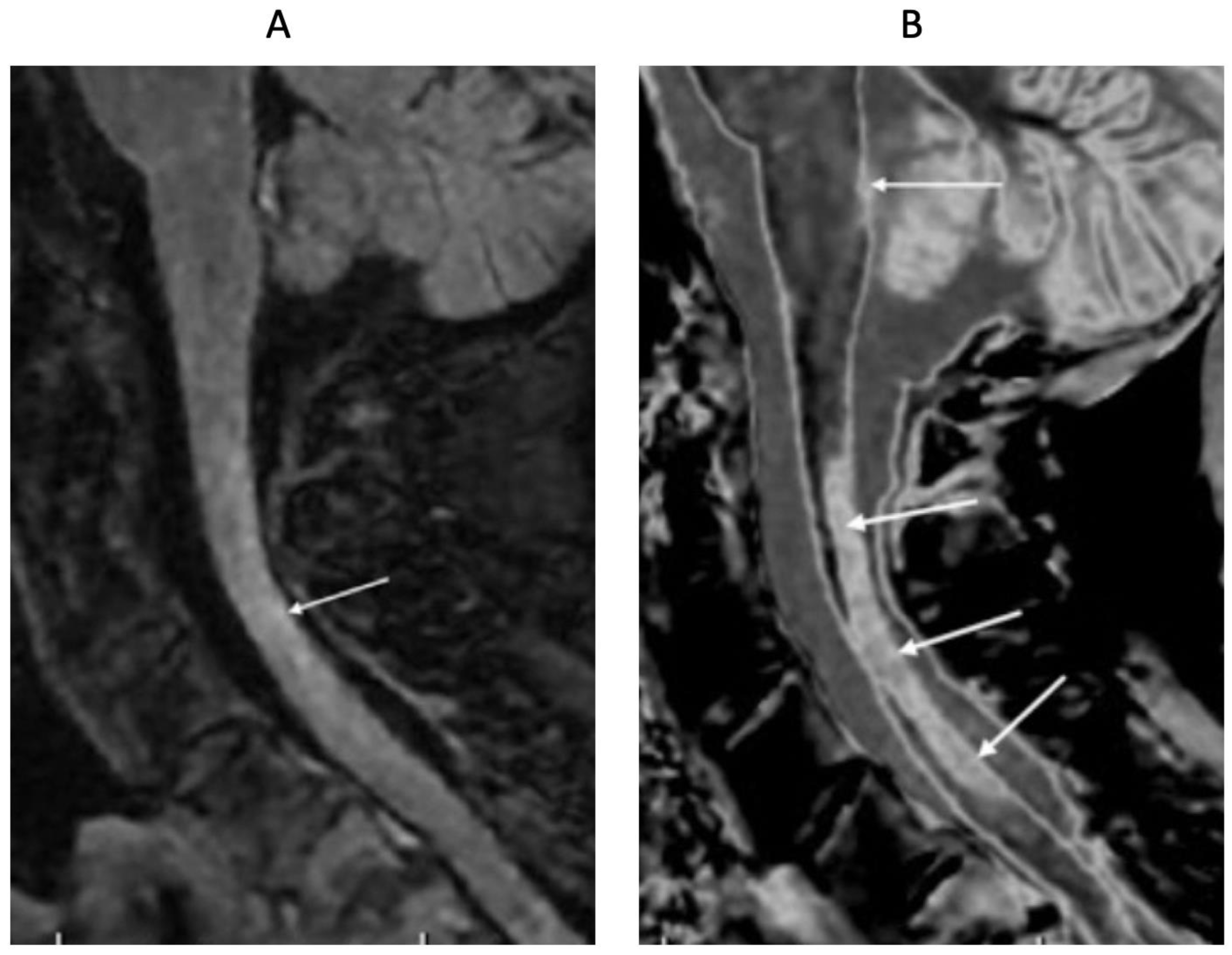
Figure 16.
32-year-old female with MS during a relapse. T
2-FLAIR (A), synthetic narrow mD dSIR (T
1-BLAIR) (B) and filtered gradient echo (C) images. On the T
2-FLAIR image (A), one lesion is seen (long arrow). The surrounding white matter appears normal. On the T
1-BLAIR image (B), the lesion shown on the T
2-FLAIR image is seen with a high signal boundary (long arrow) as well as six other lesions four of which also show high signal boundaries or bubble signs (short arrows). Two of the five high signal lesions show paramagnetic rim signs on the filtered gradient echo image (arrows) in (C). In addition, most of the white matter in (B) is high signal corresponding to a high grade 4/5 whiteout sign [
1]. No whiteout sign is seen on the T
2-FLAIR image (A).
Figure 16.
32-year-old female with MS during a relapse. T
2-FLAIR (A), synthetic narrow mD dSIR (T
1-BLAIR) (B) and filtered gradient echo (C) images. On the T
2-FLAIR image (A), one lesion is seen (long arrow). The surrounding white matter appears normal. On the T
1-BLAIR image (B), the lesion shown on the T
2-FLAIR image is seen with a high signal boundary (long arrow) as well as six other lesions four of which also show high signal boundaries or bubble signs (short arrows). Two of the five high signal lesions show paramagnetic rim signs on the filtered gradient echo image (arrows) in (C). In addition, most of the white matter in (B) is high signal corresponding to a high grade 4/5 whiteout sign [
1]. No whiteout sign is seen on the T
2-FLAIR image (A).
Figure 17.
24-year-old female patient with MS in remission (TI
s = 350 ms, TI
i = 500 ms). Narrow mD dSIR (T
1-BLAIR) (A) and composite (T
1 and T
2) cdSIR (TI
s/TE
s = 350/7 and 80 ms; TI
i/TE
i = 500/7 ms) (T
1,T
2-BLAIR) (B) images. A leukocortical lesion is seen on the T
1-BLAIR image (A) and with higher contrast on the T
1,T
2-BLAIR image in (B) (arrows). White matter and gray matter boundaries are higher contrast and narrower on the composite filter cdSIR image (B). Also white matter is more uniformly low signal in (B). These features are consistent with the attenuation of the first IR TI
s filter signal increasing contrast and narrowing boundaries at the positive pole and decreasing these at the negative pole as shown in
Figure 10A.
Figure 17.
24-year-old female patient with MS in remission (TI
s = 350 ms, TI
i = 500 ms). Narrow mD dSIR (T
1-BLAIR) (A) and composite (T
1 and T
2) cdSIR (TI
s/TE
s = 350/7 and 80 ms; TI
i/TE
i = 500/7 ms) (T
1,T
2-BLAIR) (B) images. A leukocortical lesion is seen on the T
1-BLAIR image (A) and with higher contrast on the T
1,T
2-BLAIR image in (B) (arrows). White matter and gray matter boundaries are higher contrast and narrower on the composite filter cdSIR image (B). Also white matter is more uniformly low signal in (B). These features are consistent with the attenuation of the first IR TI
s filter signal increasing contrast and narrowing boundaries at the positive pole and decreasing these at the negative pole as shown in
Figure 10A.
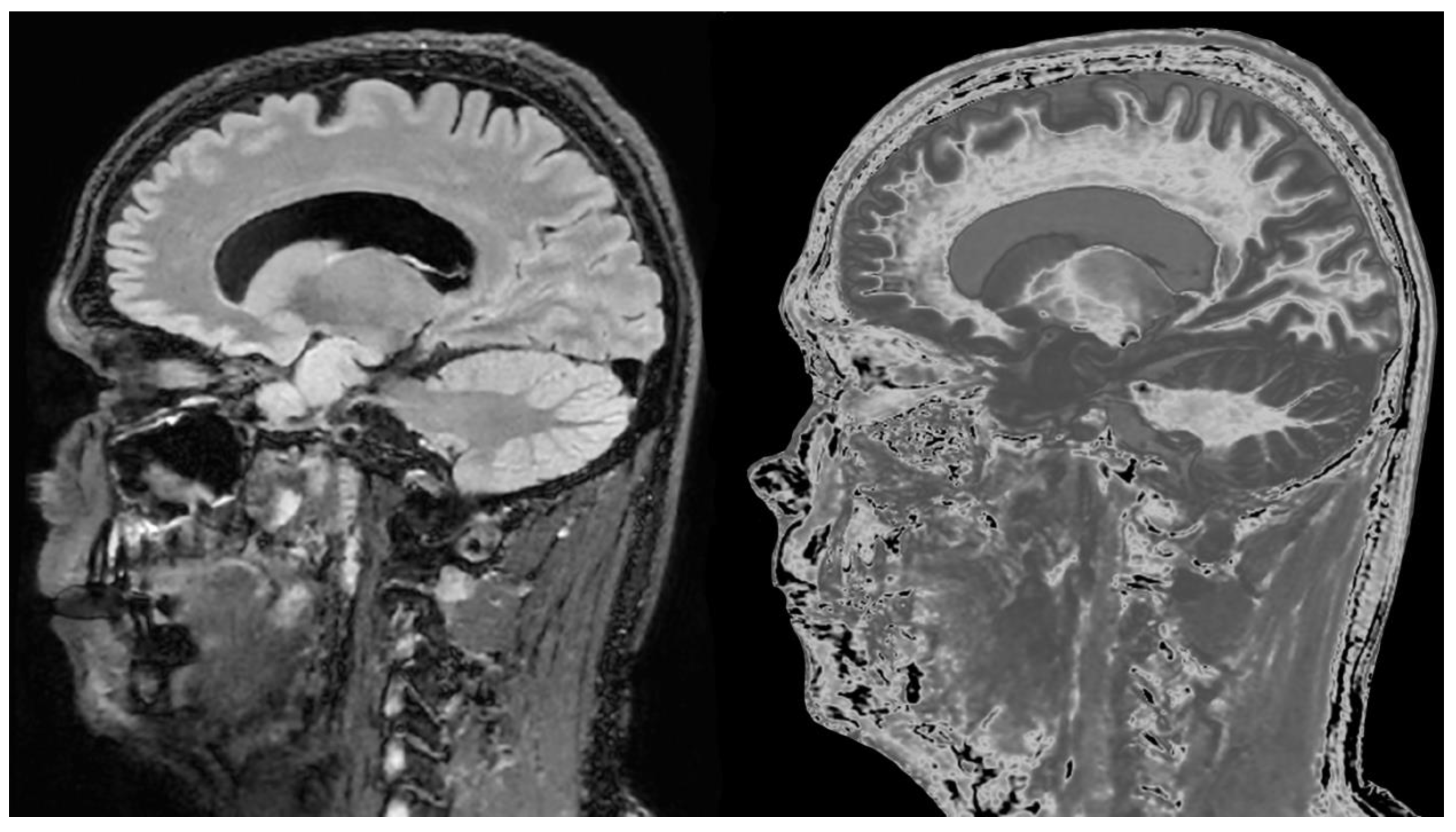
Figure 18.
76-year-old female patient in remission with a diagnosis of MS. Sagittal 3D T2-FLAIR (A) and 3D wide mD dSIR (T1-BLAIR) (B) images. The T2-FLAIR image shows a poorly defined area of increased signal in the cervical cord (arrow). The T1-BLAIR image shows a high contrast lesion with sharply defined (“punched out”) boundaries in the cervical cord (lower three arrows). This is much more extensive than in (A). An additional lesion is seen in the medulla on the T1-BLAIR image in the region of the area postrema (highest arrow) (B) but not on the T2-FLAIR image (A). The extended lesion in the cervical cord and the lesion in the area postrema raise the possibility of neuromyelitis optica spectrum disorder. Other conventional sequences such as T2-wSE or STIR may perform better than T2-FLAIR in the cervical cord.
Figure 18.
76-year-old female patient in remission with a diagnosis of MS. Sagittal 3D T2-FLAIR (A) and 3D wide mD dSIR (T1-BLAIR) (B) images. The T2-FLAIR image shows a poorly defined area of increased signal in the cervical cord (arrow). The T1-BLAIR image shows a high contrast lesion with sharply defined (“punched out”) boundaries in the cervical cord (lower three arrows). This is much more extensive than in (A). An additional lesion is seen in the medulla on the T1-BLAIR image in the region of the area postrema (highest arrow) (B) but not on the T2-FLAIR image (A). The extended lesion in the cervical cord and the lesion in the area postrema raise the possibility of neuromyelitis optica spectrum disorder. Other conventional sequences such as T2-wSE or STIR may perform better than T2-FLAIR in the cervical cord.
Figure 19.
51-year-old male patient with methamphetamine substance use disorder after one month’s abstinence (A) and after nine months’ abstinence (B). Positionally matched narrow mD dSIR (T1-BLAIR) images. In (A) there is extensive high signal in the white matter of the cerebral hemispheres with only small areas of normal or near normal white matter (dark) at the periphery of the white matter (whiteout sign, grade 4 out of 5). After nine months’ abstinence (B), the high signal areas in (A) have markedly regressed. There is some intermediate signal in the more central white matter and lower signal in the peripheral white matter (whiteout sign grade 2 out of 5, where grade 1 is normal). No abnormality was seen in either examination on the corresponding T2-FLAIR images. A previous mTBI in the patient may have been a confounder.
Figure 19.
51-year-old male patient with methamphetamine substance use disorder after one month’s abstinence (A) and after nine months’ abstinence (B). Positionally matched narrow mD dSIR (T1-BLAIR) images. In (A) there is extensive high signal in the white matter of the cerebral hemispheres with only small areas of normal or near normal white matter (dark) at the periphery of the white matter (whiteout sign, grade 4 out of 5). After nine months’ abstinence (B), the high signal areas in (A) have markedly regressed. There is some intermediate signal in the more central white matter and lower signal in the peripheral white matter (whiteout sign grade 2 out of 5, where grade 1 is normal). No abnormality was seen in either examination on the corresponding T2-FLAIR images. A previous mTBI in the patient may have been a confounder.
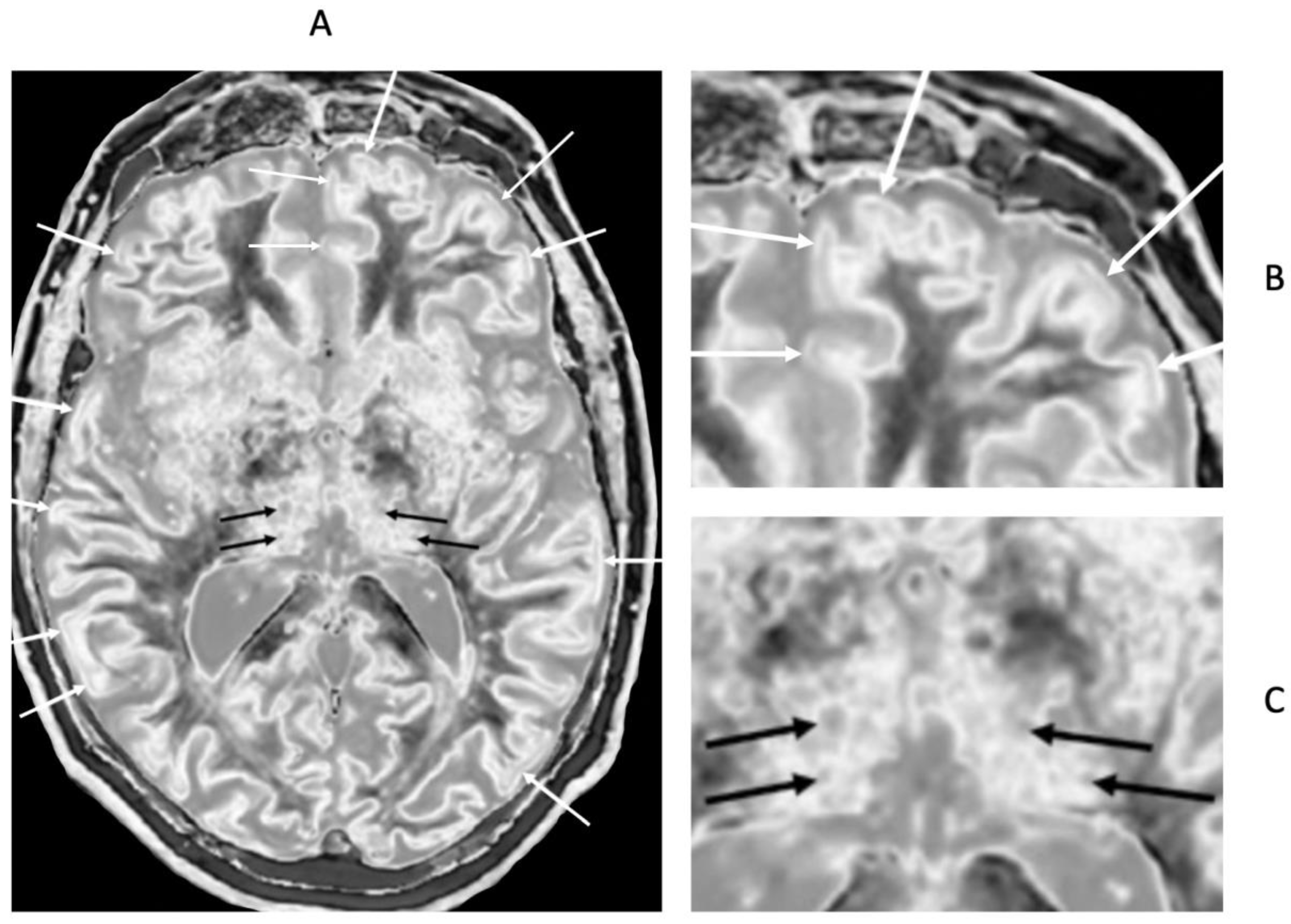
Figure 20.
72-year-old male patient with Parkinson’s disease. 2D narrow mD dSIR (T
1-BLAIR) image (A) with insets of anterior left cortex (B) and thalami (C). There is high signal in the superficial layer of the cortex at multiple sites (bilaminar cortex signs) which are more obvious on the inset (white arrows) (B). There are circular appearances in the thalami, putamen and heads of the caudate nuclei (bubble signs) which are more obvious on the inset (e.g., black arrows) (C). The bubble sign is due to focal reductions in T
1 probably from the presence of free iron. The thalamic signal is slightly higher medially compared with laterally unlike the appearance of the normal thalamus in the 18-year-old patient shown in
Figure 12B.
Figure 20.
72-year-old male patient with Parkinson’s disease. 2D narrow mD dSIR (T
1-BLAIR) image (A) with insets of anterior left cortex (B) and thalami (C). There is high signal in the superficial layer of the cortex at multiple sites (bilaminar cortex signs) which are more obvious on the inset (white arrows) (B). There are circular appearances in the thalami, putamen and heads of the caudate nuclei (bubble signs) which are more obvious on the inset (e.g., black arrows) (C). The bubble sign is due to focal reductions in T
1 probably from the presence of free iron. The thalamic signal is slightly higher medially compared with laterally unlike the appearance of the normal thalamus in the 18-year-old patient shown in
Figure 12B.
Figure 21.
65-year-old female with a glioma (not shown) post chemotherapy. Parasagittal T2-FLAIR (A) and synthetic narrow mD dSIR (T1-BLAIR) (B) images. On the T2-FLAIR image no abnormality is seen. On the T1-BLAIR image (B), the white matter has a high signal corresponding to a high grade whiteout sign. This includes the cerebellar white matter which is similar in intensity to the cerebral white matter. There is some sparing of the peripheral white matter in (B).
Figure 21.
65-year-old female with a glioma (not shown) post chemotherapy. Parasagittal T2-FLAIR (A) and synthetic narrow mD dSIR (T1-BLAIR) (B) images. On the T2-FLAIR image no abnormality is seen. On the T1-BLAIR image (B), the white matter has a high signal corresponding to a high grade whiteout sign. This includes the cerebellar white matter which is similar in intensity to the cerebral white matter. There is some sparing of the peripheral white matter in (B).
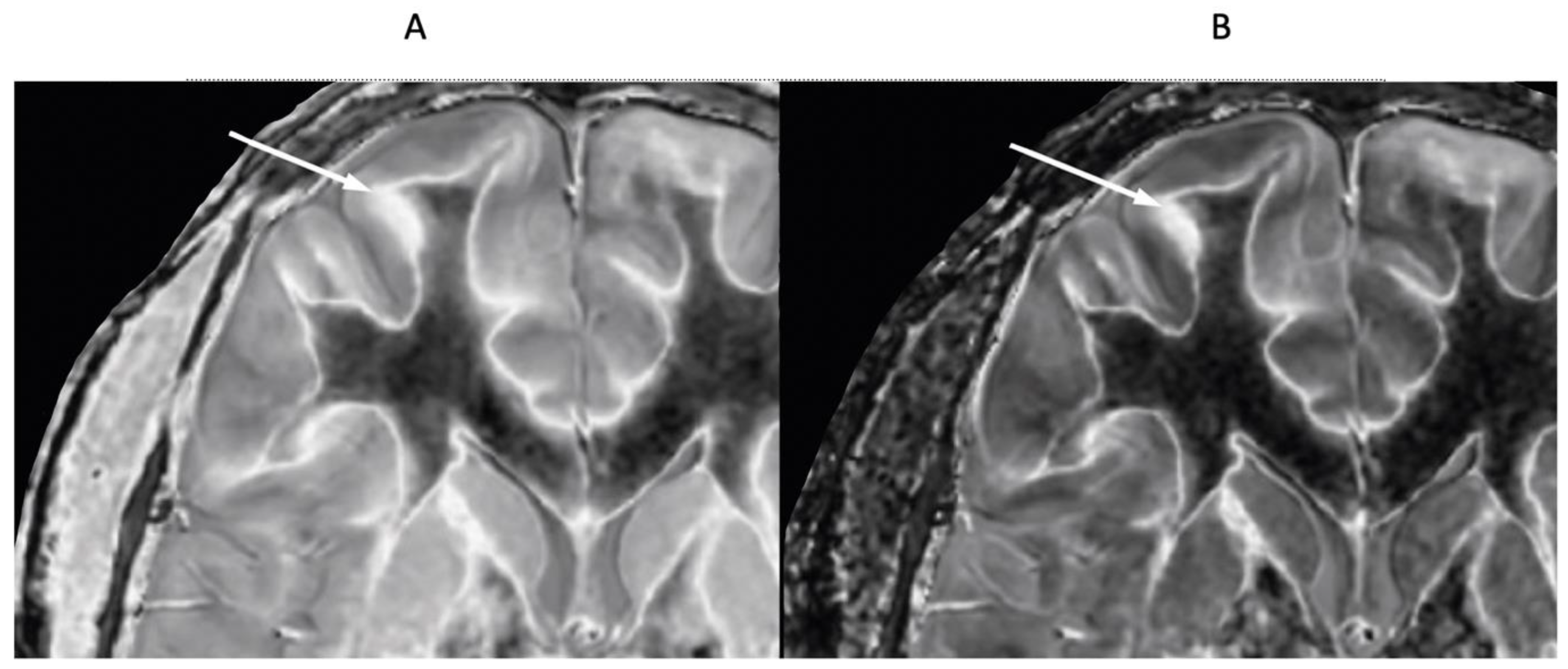
Figure 22.
46-year-old normal control. Narrow mD dSIR (A) and narrow mD lSIR (B) T1-BLAIR images with insets of anterior left cortex dSIR in (C) and anterior left cortex lSIR in (D). The boundaries between white matter and gray matter are seen with higher contrast and the contrast is seen with higher spatial resolution in (B) and (D) compared with (A) and (C). Bilaminar cortex signs are also seen with higher contrast and spatial resolution in (B) and (D) (white arrows). There are also small bubble signs in the putamen and medial thalamus which are better seen on the lSIR image (B) compared with the dSIR image (A).
Figure 22.
46-year-old normal control. Narrow mD dSIR (A) and narrow mD lSIR (B) T1-BLAIR images with insets of anterior left cortex dSIR in (C) and anterior left cortex lSIR in (D). The boundaries between white matter and gray matter are seen with higher contrast and the contrast is seen with higher spatial resolution in (B) and (D) compared with (A) and (C). Bilaminar cortex signs are also seen with higher contrast and spatial resolution in (B) and (D) (white arrows). There are also small bubble signs in the putamen and medial thalamus which are better seen on the lSIR image (B) compared with the dSIR image (A).
Figure 23.
41-year-old female patient with MS. A leukocortical lesion in the right medial frontal region is shown on the narrow mD dSIR (T1-BLAIR) image (A) and a matching lSIR image (B) (arrows). There are also left to right profiles with signal plotted against position (in mm) for the dSIR (blue) and lSIR (orange) images (C) at the level of the horizontal arrows shown in (A) and (B). No boundary between white matter and gray matter is seen within the lesion in (A). A disrupted high signal boundary between white matter and gray matter is seen in the lesion in (B). The lSIR profile (orange) has higher signal and steeper slopes than the dSIR profile (blue) in (C). The difference in signal (or contrast) achieved for the same change in position is generally greater with the lSIR filter i.e., the contrast shown on the lSIR image generally has a higher spatial resolution.
Figure 23.
41-year-old female patient with MS. A leukocortical lesion in the right medial frontal region is shown on the narrow mD dSIR (T1-BLAIR) image (A) and a matching lSIR image (B) (arrows). There are also left to right profiles with signal plotted against position (in mm) for the dSIR (blue) and lSIR (orange) images (C) at the level of the horizontal arrows shown in (A) and (B). No boundary between white matter and gray matter is seen within the lesion in (A). A disrupted high signal boundary between white matter and gray matter is seen in the lesion in (B). The lSIR profile (orange) has higher signal and steeper slopes than the dSIR profile (blue) in (C). The difference in signal (or contrast) achieved for the same change in position is generally greater with the lSIR filter i.e., the contrast shown on the lSIR image generally has a higher spatial resolution.
Figure 24.
Thalamus. dSIR narrow mD (TIs = 350 ms and TIi = 500 ms) bipolar T1-filter for normal appearance, abnormal increase in T1 in lateral thalamic gray matter within the hD with loss of contrast with medial thalamic gray matter (striped green arrow) and subsequent decrease in T1 to normal with restoration of contrast with medial thalamic gray matter (solid green arrow). The normal thalamus gray matter shows a decrease in T1 from medial (TGmed) to lateral (TGlat) (highest horizontal negative solid green arrow) and corresponding positive contrast (first vertical positive blue arrow). With disease, there is an increase in T1 in the lateral thalamus (middle horizontal positive striped green arrow) with a loss of contrast with the medial thalamus (blue circle). With later regression of this and return of the T1 difference between the lateral and medial thalamus to normal (lowest negative solid green arrow), normal positive contrast is seen between the medial and lateral thalamus (second vertical positive blue arrow).
Figure 24.
Thalamus. dSIR narrow mD (TIs = 350 ms and TIi = 500 ms) bipolar T1-filter for normal appearance, abnormal increase in T1 in lateral thalamic gray matter within the hD with loss of contrast with medial thalamic gray matter (striped green arrow) and subsequent decrease in T1 to normal with restoration of contrast with medial thalamic gray matter (solid green arrow). The normal thalamus gray matter shows a decrease in T1 from medial (TGmed) to lateral (TGlat) (highest horizontal negative solid green arrow) and corresponding positive contrast (first vertical positive blue arrow). With disease, there is an increase in T1 in the lateral thalamus (middle horizontal positive striped green arrow) with a loss of contrast with the medial thalamus (blue circle). With later regression of this and return of the T1 difference between the lateral and medial thalamus to normal (lowest negative solid green arrow), normal positive contrast is seen between the medial and lateral thalamus (second vertical positive blue arrow).
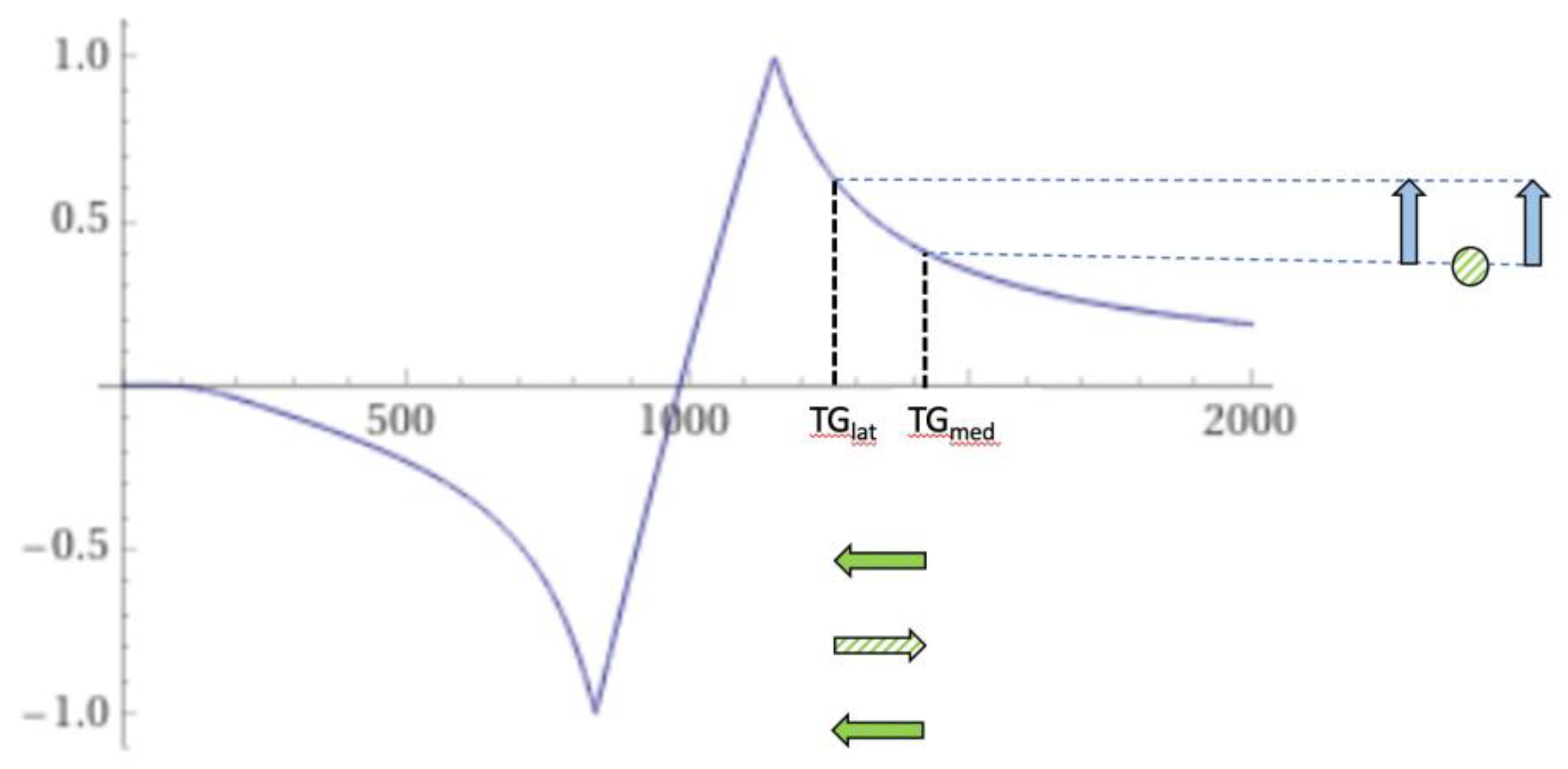
Figure 25.
Thalamus. Narrow mD (TIs = 350 ms and TIi = 500 ms). dSIR bipolar T1-filter for a larger abnormal decrease in T1 in lateral thalamic gray matter from normal (TGlatnorm) to abnormal (TGlatab) extending from the hD through the signal peak to the mD (horizontal negative green arrow). Signal in the lateral thalamus is highest where there is partial volume effect between abnormal and normal gray matter of the thalamus (TGlatab/TGlatnorm) (longer vertical blue arrow). Signal from abnormal lateral thalamus in the middle Domain (TGlatab) (shorter blue arrow) is less than the signal in the lateral thalamus at the boundary between normal and abnormal thalamus (longer blue arrow). The higher and lower signals (vertical blue arrows) result in a bubble sign with higher signal in the periphery where there are partial volume effects and lower signal centrally where there is only abnormal gray matter. The same argument applies to the medial thalamus.
Figure 25.
Thalamus. Narrow mD (TIs = 350 ms and TIi = 500 ms). dSIR bipolar T1-filter for a larger abnormal decrease in T1 in lateral thalamic gray matter from normal (TGlatnorm) to abnormal (TGlatab) extending from the hD through the signal peak to the mD (horizontal negative green arrow). Signal in the lateral thalamus is highest where there is partial volume effect between abnormal and normal gray matter of the thalamus (TGlatab/TGlatnorm) (longer vertical blue arrow). Signal from abnormal lateral thalamus in the middle Domain (TGlatab) (shorter blue arrow) is less than the signal in the lateral thalamus at the boundary between normal and abnormal thalamus (longer blue arrow). The higher and lower signals (vertical blue arrows) result in a bubble sign with higher signal in the periphery where there are partial volume effects and lower signal centrally where there is only abnormal gray matter. The same argument applies to the medial thalamus.
Figure 26.
A DIR sequence bipolar T1-filter for imaging the brain. (A) is the IR T1-filter nulling CSF, (B) is the IR T1-filter nulling white matter (W), and (C) is (A) multiplied by (B) in which both W and CSF are nulled. (C) is the DIR bipolar T1-filter. It has two negative poles.
Figure 26.
A DIR sequence bipolar T1-filter for imaging the brain. (A) is the IR T1-filter nulling CSF, (B) is the IR T1-filter nulling white matter (W), and (C) is (A) multiplied by (B) in which both W and CSF are nulled. (C) is the DIR bipolar T1-filter. It has two negative poles.
Figure 27.
Plots of signal vs T
1 (T
1-filters) for the FLAWS-uni/MP2RAGE (blue), FLAWS-min (yellow), FLAWS-hc (green) and FLAWS-hco (orange) T
1-filters from Beaumont et al.
Figure 1B in reference [
24].The FLAWS-uni/MP2RAGE T
1-filter (blue) is a negative unipolar T
1-filter for brain and CSF with a moderate negative slope between white matter (WM) and gray matter (GM), and a moderate positive slope between GM and CSF at 4000 ms. The CSF and WM signals are similar. The display range is from GM to WM. (GP is the globus pallidus).The FLAWS-min T
1-filter (yellow) is a positive unipolar T
1-filter with a moderate positive slope from WM to beyond GM and a moderate negative slope from this point to CSF with CSF low signal. The display range is from WM and CSF to just higher than GM.The FLAWS-hc T
1-filter (green) is monotonic for brain and CSF. It has a higher positive slope than the magnitude of the FLAWS-min/MP2RAGE slope from WM to GM and CSF has a very high signal. WM is at the lowest level on the display range and CSF is at the highest level. The FLAWS-hco T
1-filter is monotonic with a higher size of slope than FLAWS-uni/MP2RAGE between WM and GM and a very low signal for CSF. The display range is from CSF to white matter.
Reproduced with permission: from Beaumont et al. [
24])
Figure 27.
Plots of signal vs T
1 (T
1-filters) for the FLAWS-uni/MP2RAGE (blue), FLAWS-min (yellow), FLAWS-hc (green) and FLAWS-hco (orange) T
1-filters from Beaumont et al.
Figure 1B in reference [
24].The FLAWS-uni/MP2RAGE T
1-filter (blue) is a negative unipolar T
1-filter for brain and CSF with a moderate negative slope between white matter (WM) and gray matter (GM), and a moderate positive slope between GM and CSF at 4000 ms. The CSF and WM signals are similar. The display range is from GM to WM. (GP is the globus pallidus).The FLAWS-min T
1-filter (yellow) is a positive unipolar T
1-filter with a moderate positive slope from WM to beyond GM and a moderate negative slope from this point to CSF with CSF low signal. The display range is from WM and CSF to just higher than GM.The FLAWS-hc T
1-filter (green) is monotonic for brain and CSF. It has a higher positive slope than the magnitude of the FLAWS-min/MP2RAGE slope from WM to GM and CSF has a very high signal. WM is at the lowest level on the display range and CSF is at the highest level. The FLAWS-hco T
1-filter is monotonic with a higher size of slope than FLAWS-uni/MP2RAGE between WM and GM and a very low signal for CSF. The display range is from CSF to white matter.
Reproduced with permission: from Beaumont et al. [
24])
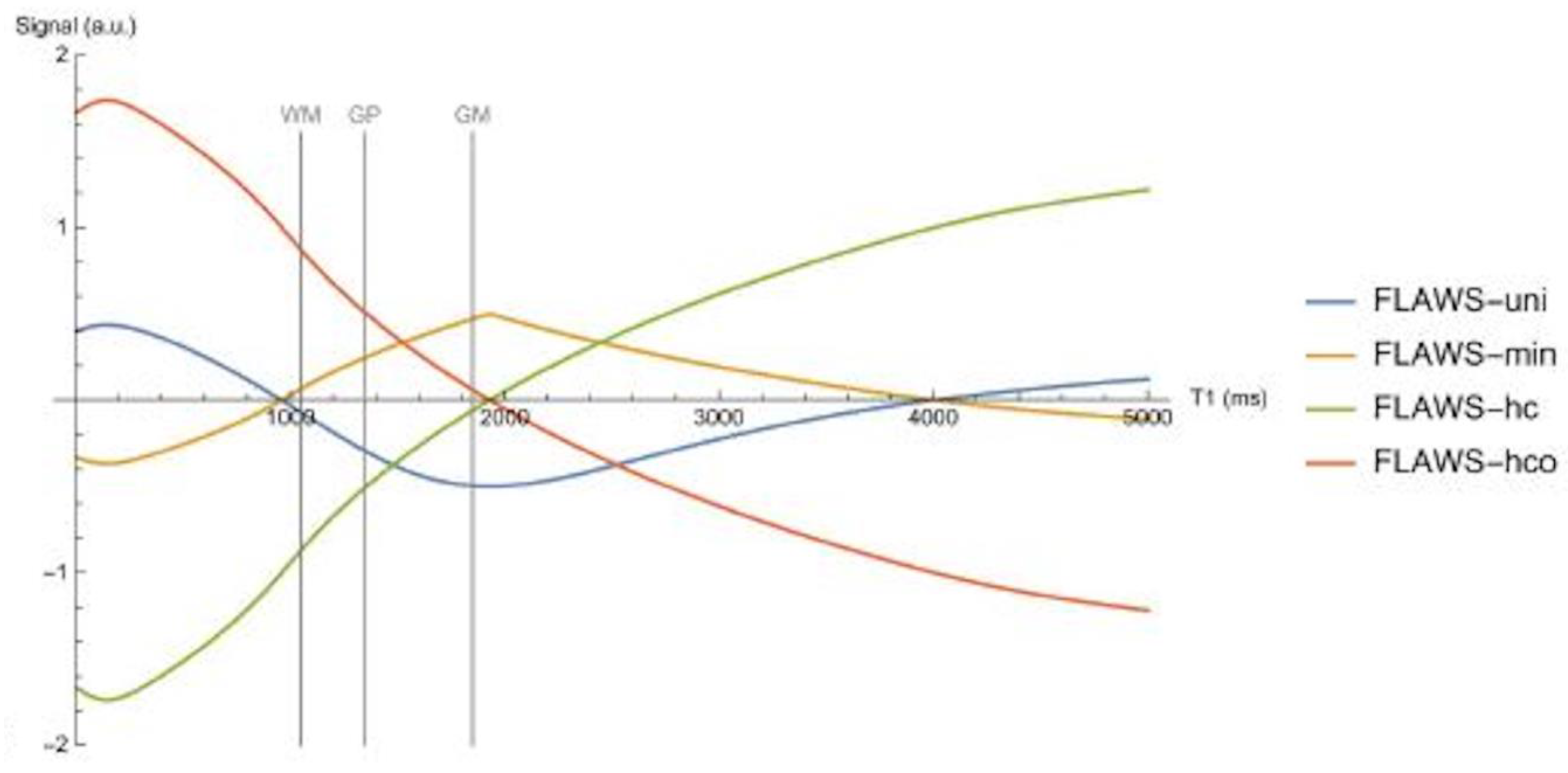
Table 1.
Pulse sequences and pulse sequence parameters. Only 2D scans were performed on the Siemens Healthineers Skyra system.
Table 1.
Pulse sequences and pulse sequence parameters. Only 2D scans were performed on the Siemens Healthineers Skyra system.
| # |
Sequence |
TR (ms) |
TI (ms) |
TE (ms) |
Matrix size Voxel size
(mm) |
Number of slices |
Slice thickness (mm) |
| 1* |
2D IR FSE (for white nulling matter) |
5,796 |
350 |
7 |
256 × 224
0.9 × 1.0
Z512 0.4 × 0.4 |
26 |
4 |
| 2* |
2D IR FSE (used with #1 for narrow mD dSIR) |
5,796 |
500 |
7 |
256 × 224
0.9 × 1.0
Z512 0.4 × 0.4 |
26 |
4 |
| 3* |
2D IR FSE (for longer T1 nulling) |
5,796 |
600 |
7 |
256 × 224
0.9 × 1.0
Z512 0.4 × 0.4 |
26 |
4 |
| 4* |
2D IR FSE (used with #1 for wide mD dSIR) |
5,796 |
800 |
7 |
256 × 224
0.9 × 1.0
Z512 0.4 × 0.4 |
26 |
4 |
| 5 |
3D BRAVO (for white matter nulling) |
2,000 |
400 |
6 |
256 × 256
0.8 × 0.8
Z512 |
220 |
0.8 |
| 6 |
3D BRAVO (used with #5 for wide mD dSIR) |
2,000 |
800 |
6 |
256 × 256
0.8 × 0.8
Z512 |
220 |
0.8 |
| 7* |
2D T2-FLAIR |
6,300 |
1,851 |
102 |
320 × 240
0.7 × 0.7
Z512 0.4 × 0.4 |
26 |
4 |
| 8 |
3D T2-FLAIR |
6,300 |
1,850 |
102 |
256 × 256
0.8 × 0.8
Z512 0.6 × 0.6 |
252 |
0.8 |
| 9 |
3D susceptibility weighted |
40 |
- |
32 |
300 × 300
0.8 × 0.8
Z512 |
110 |
2 |
Table 2.
Four types of directly acquired BLAIR sequences and their tissue properties. The fourth column shows the dominant source(s) of contrast for each sequence in their common mode(s) of use.
Table 2.
Four types of directly acquired BLAIR sequences and their tissue properties. The fourth column shows the dominant source(s) of contrast for each sequence in their common mode(s) of use.
| BLAIR sequence |
Reverse subtracted BLAIR sequence |
Tissue properties |
Dominant source(s) of contrast—BLAIR |
†DIR
lDIR |
-
- |
rm, T1, T2, ±MT
rm, T1, T2, ±MT |
T1,T2-BLAIR
T1,T2-BLAIR |
†SIR
ENVI |
rSIR
- |
rm, T1, T2, ±MT
rm, T1, T2, ±MT |
T1,T2-BLAIR
T1,T2-BLAIR |
†dSIR
cdSIR |
drSIR
cdrSIR |
T1, ±MT
T1, ±T2, ±T2*, ±D*, ±c, ±MT |
T1-BLAIR
T1,T2-BLAIR, etc |
†lSIR
clSIR |
lrSIR
clrSIR |
T1, ±MT
T1, ±T2, ±T2*, ±D*, ±c, ±MT |
T1-BLAIR
T1,T2-BLAIR, etc |
Table 3.
Signal equations for bipolar filters and other functions.
Table 3.
Signal equations for bipolar filters and other functions.
| # |
Filter, Other Functions |
Signal Equation |
Fig. # |
| 1 |
IR, TIs
|
STIs = 1−2e−TIs/T1
|
3,4,5 |
| 2 |
IR, TIi
|
STIi = 1−2e−TIi/T1
|
3,4,5 |
| 3 |
DIR, TIl, TIs
|
SDIR = rm (1−2e−TIl/T1) (1−2e−TIs/T1) e−TE/T2 |
26 |
| 4 |
SIR |
SSIR = STIs − STIi
|
5 |
| 5 |
dSIR |
|
6,9 |
| 6 |
dSIR |
(in lD and hD)
ΔTI = TIi − TIs
(in mD) |
6,9 |
| 7 |
cdSIR |
|
10 |
| 8 |
cdSIR, SOF
|
SOF = ±e−DTE/T2, ± e−DTE/T2*, ±e−DbD*, etc |
10 |
| 9 |
dSIR, SdSIR
|
(in mD) |
6 |
| 10 |
dSIR, T1
|
(in mD) |
6 |
| 11 |
lSIR |
SlSIR = ½(ln STIs − ln STIi) |
8,9 |
| 12 |
clSIR |
|
|
| 13 |
clSIR, lSIR |
, , ±DbD *, etc |
|
| 14 |
lSIR, dSIR † |
SlSIR = atanh SdSIR (in mD) |
9 |
| 15 |
dSIR †† |
(in mD) |
6,9 |
| 16 |
lSIR ††† |
(in mD) |
9 |