Submitted:
26 May 2025
Posted:
26 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction to Duchenne Muscular Dystrophy and Treatments
1.1. Duchenne Muscular Dystrophy (DMD) Pathology
1.2. Antisense DMD Treatments
2. Exon 44 Skipping Therapies
2.2. Mechanism and Targets
2.2. Current Pre-Clinical and Clinical Trial Results for AOC1044 and ENTR-601-44
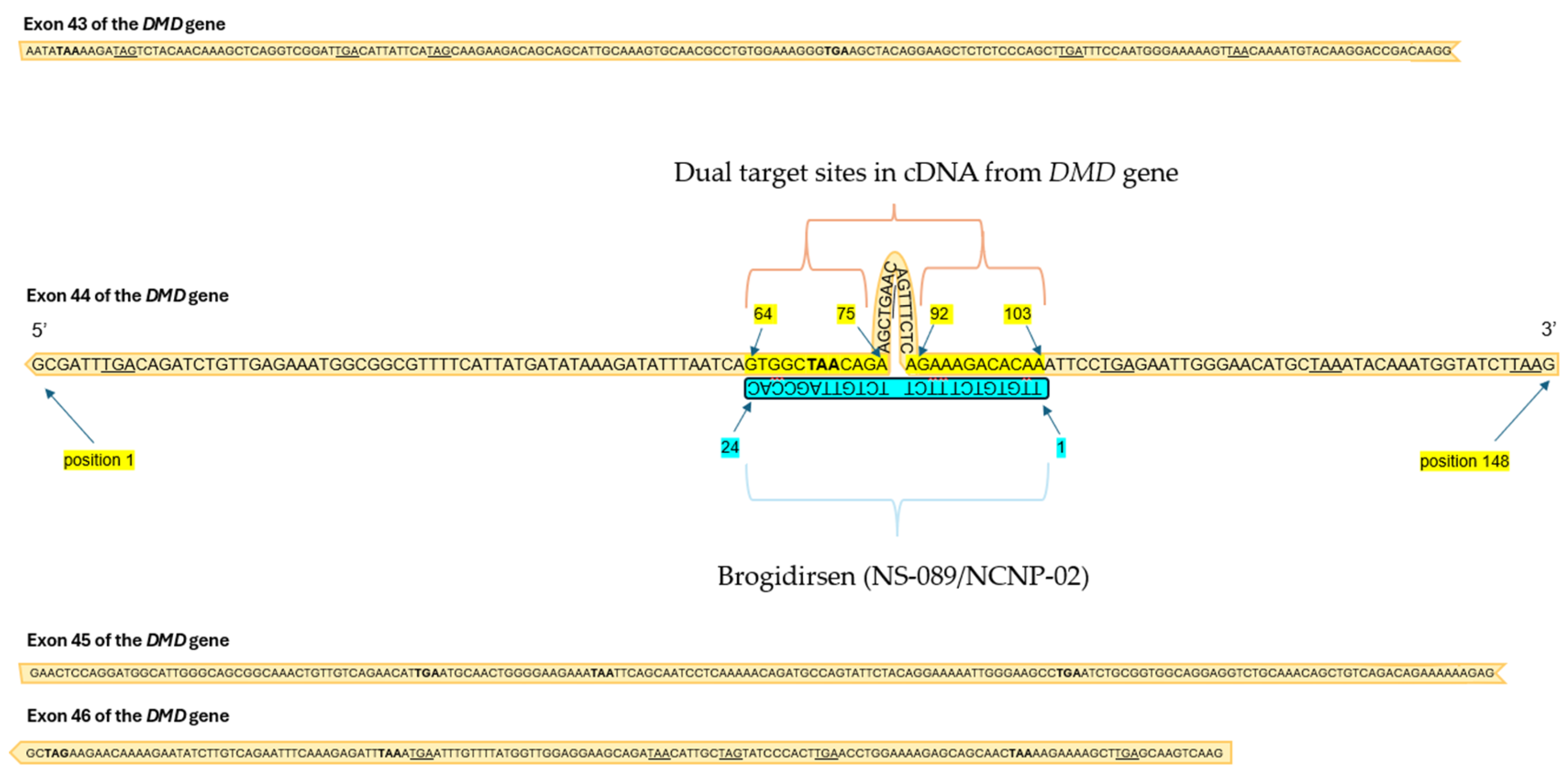
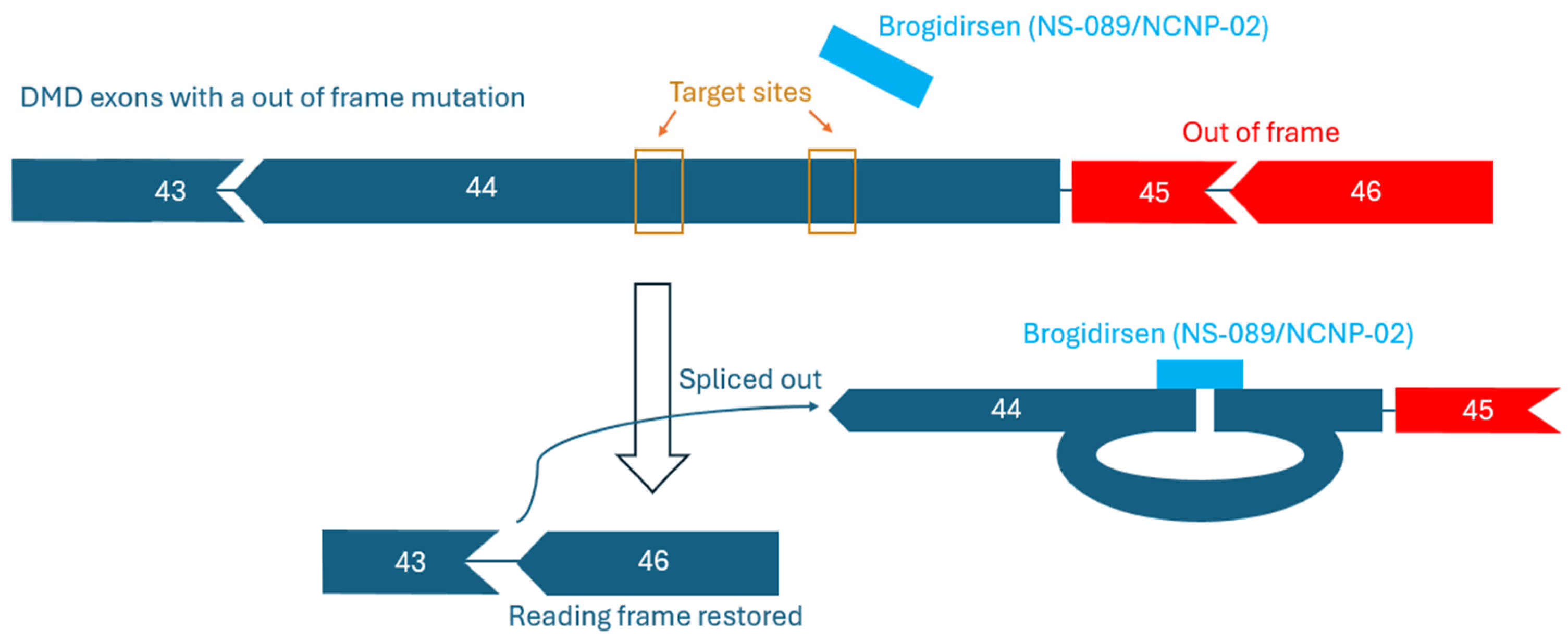
4. Brogidirsen Studies and Trials
4.1. Preclinical Study Results
4.2. Clinical Findings and Pharmacology
5. Comparison of Brogidirsen to Other Exon 44 Targeting Therapies
6. Addressing Challenges with Exon Skipping Therapies
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| DMD | Duchenne muscular dystrophy |
| ASO | Antisense oligonucleotide |
| PMO | Phosphorodiamidate morpholino oligomer |
| EEV | Endosomal Escape Vehicle |
| MTD | Maximum tolerated dose |
| TfR1 | transferrin receptor 1 |
| BMD | Becker muscular dystrophy |
References
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Trifirò, G. Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 1–20. [CrossRef]
- Duan D, Goemans N, Takeda S, et al. Duchenne muscular dystrophy. Nat Rev Dis Primers. 2021;7(1):13.
- Gao QQ, McNally EM. The dystrophin complex: Structure, function, and implications for therapy. Compr Physiol. 2015;5(3):1223–1239.
- Salari, N.; Fatahi, B.; Valipour, E.; Kazeminia, M.; Fatahian, R.; Kiaei, A.; Shohaimi, S.; Mohammadi, M. Global prevalence of Duchenne and Becker muscular dystrophy: a systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 1–12. [CrossRef]
- Sussman M. Duchenne Muscular Dystrophy . Journal of the American Academy of Orthopaedic Surgeons. 2002;10(2):138–151.
- Allen DG, Whitehead NP, Froehner SC. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol Rev. 2016;96(1):253–305.
- Shieh, P.B. Emerging Strategies in the Treatment of Duchenne Muscular Dystrophy. Neurotherapeutics 2018, 15, 840–848. [CrossRef]
- Bushby K, Finkel R, Birnkrant DJ, et al. Review Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol [Internet]. 2010;9:77–93.
- Ryder, S.; Leadley, R.M.; Armstrong, N.; Westwood, M.; De Kock, S.; Butt, T.; Jain, M.; Kleijnen, J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J. Rare Dis. 2017, 12, 79. [CrossRef]
- Mercuri E, Bönnemann CG, Muntoni F. Muscular dystrophies. The Lancet. 2019;394(10213):2025–2038.
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [CrossRef]
- Gatto, F.; Benemei, S.; Piluso, G.; Bello, L. The complex landscape of DMD mutations: moving towards personalized medicine. Front. Genet. 2024, 15, 1360224. [CrossRef]
- Godfrey C, Muses S, McClorey G, et al. How much dystrophin is enough: the physiological consequences of different levels of dystrophin in the mdx mouse. Hum Mol Genet. 2015;24(15):4225–4237.
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018, 17, 251–267. [CrossRef]
- Thomas, S.; Conway, K.M.; Fapo, O.; Street, N.; Mathews, K.D.; Mann, J.R.; Romitti, P.A.; Soim, A.; Westfield, C.; Fox, D.J.; et al. Time to diagnosis of Duchenne muscular dystrophy remains unchanged: Findings from the Muscular Dystrophy Surveillance, Tracking, and Research Network, 2000-2015. Muscle Nerve 2022, 66, 193–197. [CrossRef]
- Abbs, S.; Tuffery-Giraud, S.; Bakker, E.; Ferlini, A.; Sejersen, T.; Mueller, C.R. Best Practice Guidelines on molecular diagnostics in Duchenne/Becker muscular dystrophies. Neuromuscul. Disord. 2010, 20, 422–427. [CrossRef]
- Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for duchenne muscular dystrophy. Ann Neurol. 2012;71(3):304–313.
- E Emery, A. Clinical and molecular studies in Duchenne muscular dystrophy.. 1989, 306, 15–28.
- Andrews, J.G.; A Wahl, R. Duchenne and Becker muscular dystrophy in adolescents: current perspectives. Adolesc. Heal. Med. Ther. 2018, ume 9, 53–63. [CrossRef]
- Zambon, A.A.; Gupta, V.A.; Ridout, D.; Manzur, A.Y.; Baranello, G.; Trucco, F.; Muntoni, F.; Network, T.U.N.C. Peak functional ability and age at loss of ambulation in Duchenne muscular dystrophy. Dev. Med. Child Neurol. 2022, 64, 979–988. [CrossRef]
- Garg, S. Management of scoliosis in patients with Duchenne muscular dystrophy and spinal muscular atrophy: A literature review. J. Pediatr. Rehabilitation Med. 2016, 9, 23–29. [CrossRef]
- Hendriksen, J.G.M.; Vles, J.S.H. Neuropsychiatric Disorders in Males With Duchenne Muscular Dystrophy: Frequency Rate of Attention-Deficit Hyperactivity Disorder (ADHD), Autism Spectrum Disorder, and Obsessive—Compulsive Disorder. J. Child Neurol. 2008, 23, 477–481. [CrossRef]
- Banihani, R.; Smile, S.; Yoon, G.; Dupuis, A.; Mosleh, M.; Snider, A.; McAdam, L. Cognitive and Neurobehavioral Profile in Boys With Duchenne Muscular Dystrophy. J. Child Neurol. 2015, 30, 1472–1482. [CrossRef]
- Opstal, S.L.S.H.; Heutinck, L.; Jansen, M.; Krom, Y.D.; Cup, E.H.C.; Hendriksen, J.G.M.; Willemsen, M.A.A.P.; Verschuuren, J.J.G.M.; Niks, E.H.; de Groot, I.J.M. Occurrence of symptoms in different stages of Duchenne muscular dystrophy and their impact on social participation. Muscle Nerve 2021, 64, 701–709. [CrossRef]
- Mann JR, Zhang Y, McDermott S, et al. Racial and Ethnic Differences in Timing of Diagnosis and Clinical Services Received in Duchenne Muscular Dystrophy. Neuroepidemiology. 2023;57(2):90–99.
- Posner, N.; Manjelievskaia, J.; Talaga, A.K.; Richards, M.; Lew, C.R.; Merla, V.; Alvir, J.M.J.; Nelson, S.F. Real-world treatment and health care utilization among patients with Duchenne muscular dystrophy by race and ethnicity in a Medicaid population. J. Manag. Care Spéc. Pharm. 2025, 31, 205–213. [CrossRef]
- Sheikh, O.; Yokota, T. Developing DMD therapeutics: a review of the effectiveness of small molecules, stop-codon readthrough, dystrophin gene replacement, and exon-skipping therapies. Expert Opin. Investig. Drugs 2021, 30, 167–176. [CrossRef]
- Shirley, M. Casimersen: First Approval. Drugs 2021, 81, 875–879. [CrossRef]
- Syed, Y.Y. Eteplirsen: First Global Approval. Drugs 2016, 76, 1699–1704. [CrossRef]
- Anwar S, Yokota T. Golodirsen for Duchenne muscular dystrophy. Drugs of Today. 2020;56(8):491.
- Dhillon, S. Viltolarsen: First Approval. Drugs 2020, 80, 1027–1031. [CrossRef]
- Clemens PR, Previtera ML, Crozier RA, et al. Brogidirsen, an investigational exon 44 skipping agent for the treatment of Duchenne muscular dystrophy: Clinical trial design (Phase 2). Chicago, IL ; 2024.
- Findlay, A.R.; Wein, N.; Kaminoh, Y.; Taylor, L.E.; Dunn, D.M.; Mendell, J.R.; King, W.M.; Pestronk, A.; Florence, J.M.; Mathews, K.D.; et al. Clinical phenotypes as predictors of the outcome of skipping aroundDMDexon 45. Ann. Neurol. 2015, 77, 668–674. [CrossRef]
- Anwar, S.; Yokota, T. The Dysferlinopathies Conundrum: Clinical Spectra, Disease Mechanism and Genetic Approaches for Treatments. Biomolecules 2024, 14, 256. [CrossRef]
- Tsoumpra, M.K.; Fukumoto, S.; Matsumoto, T.; Takeda, S.; Wood, M.J.; Aoki, Y. Peptide-conjugate antisense based splice-correction for Duchenne muscular dystrophy and other neuromuscular diseases. EBioMedicine 2019, 45, 630–645. [CrossRef]
- Echigoya, Y.; Lim, K.R.Q.; Melo, D.; Bao, B.; Trieu, N.; Mizobe, Y.; Maruyama, R.; Mamchaoui, K.; Tanihata, J.; Aoki, Y.; et al. Exons 45–55 Skipping Using Mutation-Tailored Cocktails of Antisense Morpholinos in the DMD Gene. Mol. Ther. 2019, 27, 2005–2017. [CrossRef]
- Min, Y.-L.; Chemello, F.; Li, H.; Rodriguez-Caycedo, C.; Sanchez-Ortiz, E.; Mireault, A.A.; McAnally, J.R.; Shelton, J.M.; Zhang, Y.; Bassel-Duby, R.; et al. Correction of Three Prominent Mutations in Mouse and Human Models of Duchenne Muscular Dystrophy by Single-Cut Genome Editing. Mol. Ther. 2020, 28, 2044–2055. [CrossRef]
- Nakamura A, Fueki N, Shiba N, et al. Deletion of exons 3−9 encompassing a mutational hot spot in the DMD gene presents an asymptomatic phenotype, indicating a target region for multiexon skipping therapy. J Hum Genet. 2016;61(7):663–667.
- Aartsma-Rus, A.; Fokkema, I.; Verschuuren, J.; Ginjaar, I.; Van Deutekom, J.; Van Ommen, G.-J.; Den Dunnen, J.T. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum. Mutat. 2009, 30, 293–299. [CrossRef]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [CrossRef]
- Haque, U.S.; Kohut, M.; Yokota, T. Comprehensive review of adverse reactions and toxicology in ASO-based therapies for Duchenne Muscular Dystrophy: From FDA-approved drugs to peptide-conjugated ASO. Curr. Res. Toxicol. 2024, 7, 100182. [CrossRef]
- Devi, G.R. Delivery of Phosphorodiamidate Morpholino Antisense Oligomers in Cancer Cells. 2009. p. 351–361.
- Summerton, J.; Weller, D. Morpholino Antisense Oligomers: Design, Preparation, and Properties. Antisense Nucleic Acid Drug Dev. 1997, 7, 187–195. [CrossRef]
- Maksudov, F.; Kliuchnikov, E.; Pierson, D.; Ujwal, M.; Marx, K.A.; Chanda, A.; Barsegov, V. Therapeutic phosphorodiamidate morpholino oligonucleotides: Physical properties, solution structures, and folding thermodynamics. Mol. Ther. - Nucleic Acids 2023, 31, 631–647. [CrossRef]
- Hudziak, R.M.; Barofsky, E.; Barofsky, D.F.; Huang, S.-B.; Weller, D.D. Resistance of Morpholino Phosphorodiamidate Oligomers to Enzymatic Degradation. Antisense Nucleic Acid Drug Dev. 1996, 6, 267–272. [CrossRef]
- Moulton, J.D.; Jiang, S. Gene Knockdowns in Adult Animals: PPMOs and Vivo-Morpholinos. Molecules 2009, 14, 1304–1323. [CrossRef]
- Palacio-Castañeda, V.; Brock, R.; Verdurmen, W.P.R. Generation of Protein-Phosphorodiamidate Morpholino Oligomer Conjugates for Efficient Cellular Delivery via Anthrax Protective Antigen. 2022. p. 129–141.
- Aartsma-Rus, A.; Straub, V.; Hemmings, R.; Haas, M.; Schlosser-Weber, G.; Stoyanova-Beninska, V.; Mercuri, E.; Muntoni, F.; Sepodes, B.; Vroom, E.; et al. Development of Exon Skipping Therapies for Duchenne Muscular Dystrophy: A Critical Review and a Perspective on the Outstanding Issues. Nucleic Acid Ther. 2017, 27, 251–259. [CrossRef]
- Havens, M.A.; Hastings, M.L. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res. 2016, 44, 6549–6563. [CrossRef]
- Tang A, Yokota T. Is Duchenne gene therapy a suitable treatment despite its immunogenic class effect? Expert Opin Drug Saf. 2025;24(4):395–411.
- Ersöz, E.; Demir-Dora, D. Unveiling the potential of antisense oligonucleotides: Mechanisms, therapies, and safety insights. Drug Dev. Res. 2024, 85, e22187. [CrossRef]
- Bladen, C.L.; Salgado, D.; Monges, S.; Foncuberta, M.E.; Kekou, K.; Kosma, K.; Dawkins, H.; Lamont, L.; Roy, A.J.; Chamova, T.; et al. The TREAT-NMD DMD Global Database: Analysis of More than 7,000 Duchenne Muscular Dystrophy Mutations. Hum. Mutat. 2015, 36, 395–402. [CrossRef]
- Stahl M, Zhu Y, Goel V, et al. AOC 1044 as a Novel Therapeutic Approach for DMD Patients Amenable to Exon 44 Skipping: EXPLORE44TM Phase 1/2 Healthy Volunteer Data [Internet]. Orlando, FL; 2024. Available from: https://clinicaltrials.gov/ct2/show/NCT05670730.
- Cochran, M.; Marks, I.; Albin, T.; Arias, D.; Kovach, P.; Darimont, B.; Huang, H.; Etxaniz, U.; Kwon, H.W.; Shi, Y.; et al. Structure–Activity Relationship of Antibody–Oligonucleotide Conjugates: Evaluating Bioconjugation Strategies for Antibody–Phosphorodiamidate Morpholino Oligomer Conjugates for Drug Development. J. Med. Chem. 2024, 67, 14868–14884. [CrossRef]
- Wilton-Clark, H.; Yokota, T. Recent Trends in Antisense Therapies for Duchenne Muscular Dystrophy. Pharmaceutics 2023, 15, 778. [CrossRef]
- Etxaniz, U.; Marks, I.; Albin, T.; Diaz, M.; Bhardwaj, R.; Anderson, A.; Tyaglo, O.; Hoang, T.; Missinato, M.A.; Svensson, K.; et al. AOC 1044 induces exon 44 skipping and restores dystrophin protein in preclinical models of Duchenne muscular dystrophy. Nucleic Acids Res. 2025, 53. [CrossRef]
- Nguyen, Q.Q.; Yokota, T. Antisense oligonucleotides for the treatment of cardiomyopathy in Duchenne muscular dystrophy. 2019, 11, 1202–1218.
- Entrada Therapeutics. Entrada Therapeutics Announces FDA Removal of Clinical Hold on ENTR-601-44 [Internet]. 2025. Available from: www.entradatx.com.
- Qian, Z.; Martyna, A.; Hard, R.L.; Wang, J.; Appiah-Kubi, G.; Coss, C.; Phelps, M.A.; Rossman, J.S.; Pei, D. Discovery and Mechanism of Highly Efficient Cyclic Cell-Penetrating Peptides. Biochemistry 2016, 55, 2601–2612. [CrossRef]
- Sahni, A.; Qian, Z.; Pei, D. Cell-Penetrating Peptides Escape the Endosome by Inducing Vesicle Budding and Collapse. ACS Chem. Biol. 2020, 15, 2485–2492. [CrossRef]
- Oldham M, Estrella N, Kumar A, et al. 433P Therapeutic potential of ENTR-601-44, an Endosomal Escape Vehicle (EEVTM) - Oligonucleotide Conjugate for the treatment of exon 44 skip amenable DMD. Neuromuscular Disorders. 2024;43:104441.304.
- Wahab E. A study to investigate how ENTR-601-44 behaves in the body, the safety and how well tolerated different increasing amounts of the drug ENTR-601-44 are when given to healthy male volunteers. http://isrctn.com/. London, UK: MAC Clinical Research; 2024.
- Komaki H, Takeshita E, Kunitake K, et al. Phase 1/2 trial of brogidirsen: Dual-targeting antisense oligonucleotides for exon 44 skipping in Duchenne muscular dystrophy. Cell Rep Med. 2025;6(1):101901.
- Nippon Shinyaku Co. Ltd. FDA Grants Breakthrough Therapy Designation to NS-089/NCNP-02 for the Treatment of Duchenne Muscular Dystrophy. Nippon Shinyaku Co, Ltd. [Internet]. 2023 Jul 28; Available from: https://www.ncnp.go.jp/topics/2022/20220317e.html.
- Komaki H, Takeshita E, Kunitake K, et al. P.123 A Phase I/II study of NS-089/NCNP-02, Exon 44 skipping drug, in patients with Duchenne muscular dystrophy. Neuromuscular Disorders. 2022;32:S99–S100.
- Shimo, T.; Maruyama, R.; Yokota, T. Designing Effective Antisense Oligonucleotides for Exon Skipping [Internet]. Bernardini C, ediTor. Duchenne Muscular D. New York: Humana Press; 2018. Available from: http://www.springer.com/series/7651.
- Watanabe, N.; Tone, Y.; Nagata, T.; Masuda, S.; Saito, T.; Motohashi, N.; Takagaki, K.; Aoki, Y.; Takeda, S. Exon 44 skipping in Duchenne muscular dystrophy: NS-089/NCNP-02, a dual-targeting antisense oligonucleotide. Mol. Ther. - Nucleic Acids 2023, 34, 102034. [CrossRef]
- Komaki H, Takeshima Y, Matsumura T, et al. Viltolarsen in Japanese Duchenne muscular dystrophy patients: A phase 1/2 study. Ann Clin Transl Neurol. 2020;7(12):2393–2408.
- Komaki H, Takeshita E, Kunitake K, et al. Phase 1/2 trial of brogidirsen: Dual-targeting antisense oligonucleotides for exon 44 skipping in Duchenne muscular dystrophy [supplemental information]. Cell Rep Med [Internet]. 2025;101901.
- Broomfield, J.; Abrams, K.; Latimer, N.; Guglieri, M.; Rutherford, M.; Crowther, M. Natural history of Duchenne muscular dystrophy in the United Kingdom: A descriptive study using the Clinical Practice Research Datalink. Brain Behav. 2023, 13, e3331. [CrossRef]
- Tulangekar, A.; Sztal, T.E. Inflammation in Duchenne Muscular Dystrophy–Exploring the Role of Neutrophils in Muscle Damage and Regeneration. Biomedicines 2021, 9, 1366. [CrossRef]
- Ishizuka, T.; Komaki, H.; Asahina, Y.; Nakamura, H.; Motohashi, N.; Takeshita, E.; Shimizu-Motohashi, Y.; Ishiyama, A.; Yonee, C.; Maruyama, S.; et al. Systemic administration of the antisense oligonucleotide NS-089/NCNP-02 for skipping of exon 44 in patients with Duchenne muscular dystrophy: Study protocol for a phase I/II clinical trial. Neuropsychopharmacol. Rep. 2023, 43, 277–286. [CrossRef]
- Holland, A.; Lonkar, P.; Sweeney, C.; Zhang, H.; Svenstrup, N.; Gibbons, C.; Xu, L.; Foy, J.; Goyal, J. P27 Three novel enhanced delivery Oligonucleotide candidates for Duchenne muscular dystrophy mediate high levels of exon 53, 45, and 44 skipping. Neuromuscul. Disord. 2023, 33, S103–S104. [CrossRef]
- Mellion, M.; Larkindale, J.; Lonkar, P.; Goyal, J.; Holland, A.; Foy, J.; Garg, B.; Yu, S.; Frank, A.; Abbott, C.; et al. PGN-EDO51, an Enhanced Delivery Oligonucleotide (EDO) for the Treatment of Duchenne Muscular Dystrophy (DMD): Results of a Phase 1 Study in Healthy Volunteers (P3-8.004). Neurology 2023, 100. [CrossRef]
- Shah, N.A.; Wilton-Clark, H.; Haque, F.; Powell, B.; Sutanto, L.E.; Maradiya, R.; Zhabyeyev, P.; Roshmi, R.R.; Anwar, S.; Aslesh, T.; et al. DG9 boosts PMO nuclear uptake and exon skipping to restore dystrophic muscle and cardiac function. Nat. Commun. 2025, 16, 1–16. [CrossRef]
- Shang, M.; Wu, Y.; Wang, Y.; Cai, Y.; Jin, J.; Yang, Z. Dual antisense oligonucleotide targeting miR-21/miR-155 synergize photodynamic therapy to treat triple-negative breast cancer and inhibit metastasis. Biomed. Pharmacother. 2022, 146, 112564. [CrossRef]
- Moulton, H.M.; Moulton, J.D. Morpholinos and their peptide conjugates: Therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim. et Biophys. Acta (BBA) - Biomembr. 2010, 1798, 2296–2303. [CrossRef]
- Haque, U.S.; Yokota, T. Enhancing Antisense Oligonucleotide-Based Therapeutic Delivery with DG9, a Versatile Cell-Penetrating Peptide. Cells 2023, 12, 2395. [CrossRef]
- Waldrop, M.A.; Filnemus; Ben Yaou, R.; Lucas, K.K.; Martin, A.S.; O’rourke, E.; Ferlini, A.; Muntoni, F.; Leturcq, F.; Tuffery-Giraud, S.; et al. Clinical Phenotypes of DMD Exon 51 Skip Equivalent Deletions: A Systematic Review. J. Neuromuscul. Dis. 2020, 7, 217–229. [CrossRef]
- Lim, K.R.Q.; Maruyama, R.; Yokota, T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des. Dev. Ther. 2017, ume11, 533–545. [CrossRef]
- Lim, K.R.Q.; Woo, S.; Melo, D.; Huang, Y.; Dzierlega, K.; Shah, N.A.; Aslesh, T.; Roshmi, R.R.; Echigoya, Y.; Maruyama, R.; et al. Development of DG9 peptide-conjugated single- and multi-exon skipping therapies for the treatment of Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. 2022, 119. [CrossRef]
- Béroud, C.; Tuffery-Giraud, S.; Matsuo, M.; Hamroun, D.; Humbertclaude, V.; Monnier, N.; Moizard, M.-P.; Voelckel, M.-A.; Calemard, L.M.; Boisseau, P.; et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum. Mutat. 2006, 28, 196–202. [CrossRef]
- Echigoya, Y.; Lim, K.R.Q.; Melo, D.; Bao, B.; Trieu, N.; Mizobe, Y.; Maruyama, R.; Mamchaoui, K.; Tanihata, J.; Aoki, Y.; et al. Exons 45–55 Skipping Using Mutation-Tailored Cocktails of Antisense Morpholinos in the DMD Gene. Mol. Ther. 2019, 27, 2005–2017. [CrossRef]
- Van Deutekom JCT, Van Ommen GJB. Advances in Duchenne muscular dystrophy gene therapy. Nat Rev Genet. 2003. p. 774–783.
- Findlay AR, Wein N, Kaminoh Y, et al. Clinical phenotypes as predictors of the outcome of skipping around DMD exon 45. Ann Neurol. 2015;77(4):668–674.
- Nakamura A, Fueki N, Shiba N, et al. Deletion of exons 3−9 encompassing a mutational hot spot in the DMD gene presents an asymptomatic phenotype, indicating a target region for multiexon skipping therapy. J Hum Genet. 2016;61(7):663–667.
- Anthony, K.; Cirak, S.; Torelli, S.; Tasca, G.; Feng, L.; Arechavala-Gomeza, V.; Armaroli, A.; Guglieri, M.; Straathof, C.S.; Verschuuren, J.J.; et al. Dystrophin quantification and clinical correlations in Becker muscular dystrophy: implications for clinical trials. Brain 2011, 134, 3547–3559. [CrossRef]
- Heo, Y.A. Golodirsen: First Approval. Drugs 2020, 80, 329–333. [CrossRef]
- Relizani, K.; Griffith, G.; Echevarría, L.; Zarrouki, F.; Facchinetti, P.; Vaillend, C.; Leumann, C.; Garcia, L.; Goyenvalle, A. Efficacy and Safety Profile of Tricyclo-DNA Antisense Oligonucleotides in Duchenne Muscular Dystrophy Mouse Model. Mol. Ther. - Nucleic Acids 2017, 8, 144–157. [CrossRef]
- McDonald, C.M.; Shieh, P.B.; Abdel-Hamid, H.Z.; Connolly, A.M.; Ciafaloni, E.; Wagner, K.R.; Goemans, N.; Mercuri, E.; Khan, N.; Koenig, E.; et al. Open-Label Evaluation of Eteplirsen in Patients with Duchenne Muscular Dystrophy Amenable to Exon 51 Skipping: PROMOVI Trial. J. Neuromuscul. Dis. 2021, 8, 989–1001. [CrossRef]
- Servais, L.; Mercuri, E.; Straub, V.; Guglieri, M.; Seferian, A.M.; Scoto, M.; Leone, D.; Koenig, E.; Khan, N.; Dugar, A.; et al. Long-Term Safety and Efficacy Data of Golodirsen in Ambulatory Patients with Duchenne Muscular Dystrophy Amenable to Exon 53 Skipping: A First-in-human, Multicenter, Two-Part, Open-Label, Phase 1/2 Trial. Nucleic Acid Ther. 2022, 32, 29–39. [CrossRef]
- Tang, A.; Yokota, T. Duchenne muscular dystrophy: promising early-stage clinical trials to watch. Expert Opin. Investig. Drugs 2024, 33, 201–217. [CrossRef]
- Dovari, A.; Inuganti, B.; Nadimpally, J.; Vatturi, S.; Hyderboini, R.; Goyal, R. PRO38 Twenty Years of Clinical Trials in Duchenne Muscular Dystrophy: A Low Clinical Drug Development Success. Value Heal. 2021, 24, S204. [CrossRef]
- Lek, A.; Wong, B.; Keeler, A.; Blackwood, M.; Ma, K.; Huang, S.; Sylvia, K.; Batista, A.R.; Artinian, R.; Kokoski, D.; et al. Death after High-Dose rAAV9 Gene Therapy in a Patient with Duchenne’s Muscular Dystrophy. New Engl. J. Med. 2023, 389, 1203–1210. [CrossRef]
- Bönnemann, C.G.; Belluscio, B.A.; Braun, S.; Morris, C.; Singh, T.; Muntoni, F. Dystrophin Immunity after Gene Therapy for Duchenne’s Muscular Dystrophy. New Engl. J. Med. 2023, 388, 2294–2296. [CrossRef]
| ClinicalTrials.gov ID | NCT04129294 | NCT05135663 | NCT05996003 |
| Phase | I/II | II | II |
| Start date | Dec 2019 | June 2021 | Feb 2024 |
| (Estimated) End date | May 2022 | July 2026 | Nov 2025 |
| Description | Dose-escalation, open-label | Open-label, extension study | Open-label, multi-center |
| Dose | 80 mg/kg, 40 mg/kg | ||
| Primary Endpoint | Safety, tolerability | Safety | Safety, pharmacokinetics |
| Secondary Endpoints | Pharmacokinetics, efficacy | Efficacy | Efficacy |
| Enrollment numbers | 6 | 6 | 20 (6 in Cohort 1, 14 in Cohort 2) |
| Route | Intravenous | Intravenous | Intravenous |
| Life expectancy | At least one year | Same participants as NCT04129294 | N/A |
| Age Range | 8-17 | 8-17 | 4-15 |
| Design | 24 weeks treatment, 12 weeks follow up period | Administer once weekly for 216 weeks | Once weekly for 4 weeks at 1 of 3 doses, 24 weeks at MTD1 after |
| Corticosteroid use | None, or at least 6 months of stable use | Same participants as NCT04129294 | Stable dose for at least 3 months |
| Ambulation Requirements | Ambulant | Same participants as NCT04129294 | Able to walk independently without devices |
| Exclusion Criteria | No DNA polymorphisms that could compromise therapy and pre-mRNA binding, FVC1 < 50% of predicted, EF1 < 40%, FS1 < 25% based on ECHO1, current infections, cardiomyopathy, liver/renal disease, previous severe drug allergy, continuous use of artificial respirator, previous use of investigational therapies | Same participants as NCT04129294 | Body weight of <20 kg, cardiomyopathy symptoms, use of anabolic steroids, use of other investigational drugs in the past three months, surgery in last 3 months, taken gene therapy or another exon skipping drug |
| Other Criteria | Adequate intact muscles for biopsy, able to give written informed consent, QTc1 <450 msec (<480 msec for subject with Bundle Branch Block) | Must have participated in NCT04129294 | Able to complete the TTSTAND1 without assistance in <20 seconds, |
| Test Timing | At end of treatment period (24 weeks) | Up to Week 243 | Baseline, Week13, Week25 |
| Genotype | Out of frame deletion(s) amenable to exon 44 skipping | Out of frame deletion(s) amenable to exon 44 skip-ping | Amenable to exon 44 skipping |
| Reference | [63] | [72] |
| Exon skipping therapies | AOC1044 (delpacibart zotadirsen) | ENTR-601-44 | NS-089/NCNP-02 (brogidirsen) |
| Modification | Antibody-conjugated | Peptide-conjugated | Unmodified |
| Details | 4 PMOs conjugated to anti-TfR1 | PMO conjugated to EEV | No conjugations |
| Targeting Mechanism | TfR1 for targeted delivery | EEV to escape endosomes | Natural biodistribution |
| Target site(s) | Single | Single | Dual |
| Regulatory State | No clinical holds | FDA hold lifted in 2025 | No clinical holds |
| Current Clinical Stage | Phase II (Part B of EXPLORE44-OLE, enrolling DMD patients) | Phase Ib (ELEVATE-44-102 trial, enrolling DMD patients) | Phase II (NCT05996003, recruiting DMD patients) |
| Age | 7 to 27 years old | ||
| Reported clinical trial design(s) | 40 healthy volunteers in double-blind, placebo-controlled trial | 4 dose levels tested in healthy males | 4 dose levels tested in 6 DMD patients |
| 25 patients receiving AOC1044 | 32 patients to be enrolled | 20 patients to be enrolled |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





