Submitted:
22 May 2025
Posted:
23 May 2025
You are already at the latest version
Abstract
Keywords:
I. Introduction
III. the variety of elements in the periodic table

- Group 1 are the alkali metals (rubidium, sodium, potassium, lithium, cesium and francium): low melting points, low boiling points, low density, very reactive and good electrical conductivity.
- Group 2 are the alkaline earth metals (beryllium, radium, calcium, strontium, barium and magnesium): higher melting points than the alkali metals, higher boiling points than the alkali metals, higher densities compared to the alkali metals, lower reactivity than the alkali metals and good electrical conductivity.
- Group 13 is the boron family (gallium, aluminium, boron, indium, thallium and nihonium): low reactivity and lower conductivity than the metals.
- Group 14 is the carbon family (carbon, silicon, flerovium, tin, lead and germanium): very high melting point and high boiling point. • Group 15 is the nitrogen family (nitrogen, phosphorus, muscovy, antimony, bismuth and arsenic): low conductivity, low melting and boiling points
- Group 16 are the chalcogens (oxygen, liver, selenium, tellurium, polonium and sulfur): low melting and boiling points, a density that varies between some elements and low conductivity.
- Group 17 are the Halogens (fluorine, iodine, bromine, chlorine, astatine and tenessine): very low conductivity, melting point varies between them, boiling point depends on the element.
- Group 18 are the Noble Gases (helium, neon, argon, oganesson, xenon, radon and krypton): low melting point, low boiling point, low conductivity and low reactivity.
- Group 11 (copper, silver, gold, and roentgenium).
- Group 12 (zinc, cadmium, mercury, and copperium).
- Group 3 (scandium, yttrium, lanthanide series, and actinides).
- Group 4 (titanium, zirconium, hafnium, and rutherfordium).
- Group 5 (vanadium, niobium, tantalum, and dubnium).
- Group 6 (chromium, molybdenum, tungsten, and seaborgium).
- Group 7 (manganese, technetium, rhenium, and bohrium).
- Group 8 (iron, ruthenium, osmium, and hassium).
- Group 9 (cobalt, rhodium, iridium, and meitnerium).
- Group 10 (nickel, palladium, platinum, darmstadtium).
- Potassium: solid at room temperature, atomic number 19, belonging to the alkali metals, low density at 0.89 g/cm³, boiling point at 759 °C and melting point at 63.5 °C.
- Calcium: solid at room temperature, atomic number 20, belonging to the alkaline earth metals, calcium density at 1.55 g/cm³, boiling point at 1,484 °C and melting point at 842 °C.
- Aluminum: solid at room temperature, atomic number 13, belonging to the boron family, aluminum density at 2.7 g/cm³, boiling point at 2,470 °C and melting point at 660.3 °C.
- Tin: solid at room temperature, atomic number 50, part of the carbon family, the density of tin is 7.287 g/cm³, the boiling point is 2,602 °C and the melting point is 231.9 °C.
- Phosphorus: solid at room temperature, atomic number 15, part of the nitrogen family, the density of phosphorus is 1.82 g/cm³, the boiling point is 280.5 °C and the melting point is 44.1 °C.
- Oxygen: gas at room temperature, atomic number 8, part of the chalcogens, the density of oxygen is 1.429 g/L, the boiling point is -183 °C and the melting point is -218.8 °C.
- Bromine: is liquid at room temperature, has atomic number 35, is part of the halogens, the density of bromine is 3.110 g/cm³, the boiling point is 58.8 °C and the melting point is -7.2 °C.
- Helium: is a gas at room temperature, has atomic number 2, is part of the noble gases, the density of helium is 0.1785 g/L, the boiling point is -268.9 °C and the melting point is -272.2 °C.
- • Mercury: is liquid at room temperature, has atomic number 80. It is part of the transition metals, the density of mercury is 13.534 g/cm³, the boiling point is 356.7 °C and the melting point is -38.83 °C.
IV. The use of the specific physical concept intensity in elements of the periodic table
V. The variety of particles
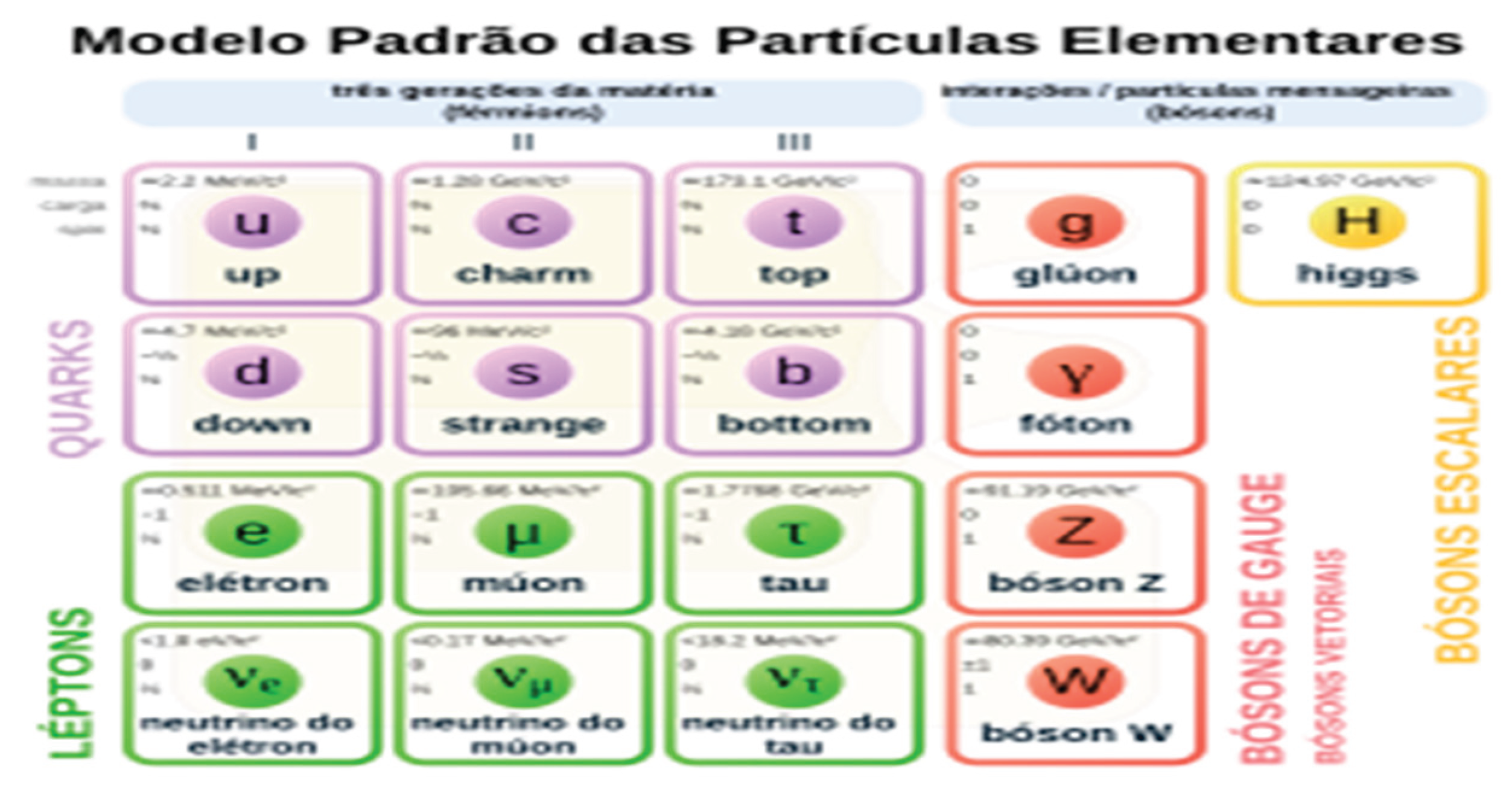
- Up (u): have an electric charge of +2/3, their mass is 2.2 MeV/c², it is the lightest of the six flavors, it is stable matter, they are present in protons and neutrons.
- Down (d): have an electric charge of -1/3, their mass is 4.7 MeV/c², it is stable matter, present in protons and neutrons.
- Charme (c) has an electric charge of +2/3, their mass is 1.28 GeV/c², it is made of unstable material and presents meson particles.
- Strange(s): Have an electric charge of -1/3, their mass is 96 MeV/c², it is unstable matter and present in mesons or bariums.
- Top (t): Have an electric charge of +2/3, their mass is 173 GeV/c², it is unstable material and deteriorates quickly.
- Bottom part (b): They have an electric charge of -1/3, their mass is 4.18 GeV/c², it is unstable matter.
- Electron (e⁻): has an electric charge of -1, an electron mass of 0.511 MeV/c², is stable, is present in atoms and participates in electromagnetic, weak and gravitational interactions.
- Muon (μ⁻): has an electric charge of -1, a muon mass of 105.7 MeV/c², is not stable in nature and participates in electromagnetic, weak and gravitational interactions.
- Tau (τ⁻): has an electric charge of -1, a tau mass of 1.777 MeV/c², is not stable in nature and participates in electromagnetic, weak and gravitational interactions.
- Electron neutrino (νₑ): has an electric charge of zero, an electron neutrino mass of almost zero, is a stable particle and participates in weak interactions and gravity.
- Muon neutrino (ν_μ): has zero electric charge, zero mass, is a stable particle in nature, important for the balance of decay processes and present in weak and gravitational force interactions.
- Tau neutrino (ν_τ): has zero electric charge, very small mass, is a stable particle in nature, present in weak and gravitational force interactions.
- Photon (γ): related to the electromagnetic force, a particle without rest mass, has a high speed, has no electric charge and behaves as both a wave and a particle, has spin 1.
- Gluon (g): related to the strong force that holds protons and neutrons together, has spin 1 and has no mass in reserve.
- Bosons W⁺, W⁻ and Z⁰: related to the weak force, W⁺ has an electric charge +1 and mass 80.4 GeV/c², W⁻ has an electric charge -1 and mass 80.4 GeV/c², Z⁰ has an electric charge 0 and mass 91.2 GeV/c².
- Graviton (maybe): may or may not exist, has no mass, has no electric charge, acts in the gravitational force, has spin 2 and has a high speed.
- Higgs boson (H): is responsible for the mass of particles by the Higgs field, has a mass of 125 GeV/c², has zero electric charge and has zero spin.
- Hadrons: are composite particles formed by quarks and gluons, and interact through the strong force
- Proton: is a particle composed of 2 up quarks and 1 down quark, has a positive electric charge, mass of 1.6726 × 10⁻²⁷ kg, spin ½, is stable by nature and participates in the strong force, weak force and electromagnetic force interactions.
- Neutron: is a particle composed of 1 up quark and 2 down quarks, has zero electric charge, mass of 1.6749 × 10⁻²⁷ kg, spin ½, and participates in the weak and strong force interactions.
- Pion (π⁺, π⁰, π⁻): π⁰ has zero electric charge and a fast lifetime, π⁰ has a mass of 135.0 MeV/c², π⁺ has 1 up quark plus a down antiquark and electric charge +1, with a mass of 139.6 MeV/c², π⁻ has an electric charge of -1 and a mass of 139.6 MeV/c², and π⁻ has a down quark plus an up antiquark.
- strong>∙ Kaon (K⁺, K⁻, K⁰): K⁺ is composed of an up quark plus a strange antiquark with electric charge +1, with a mass of 493.7 MeV/c², K⁻ is composed of a strange quark plus an up antiquark. with an electric charge of -1, the mass of K⁻ is 493.7 MeV/c², K⁰ is composed of down quark plus strange antiquark with zero electric charge and K⁰ has a mass of 497.6 MeV/c².
VI. The use of the specific physical concept of intensity in particles
VII. Variety of molecules
- Water: its formula contains two hydrogen atoms and one oxygen atom, its molecular geometry is angular, its boiling point is 100 °C, its melting point is 0 °C, its density is 997 kg/m³, and it has numerous applications in nature.
- Carbon dioxide: its formula contains one carbon atom and two oxygen atoms, its density is 1.98 kg/m³, its molecular geometry is linear, its boiling point is -78.5 °C, its melting point is -56.6 °C, and it participates in cellular respiration and photosynthesis.
- Glucose: it contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. It is highly soluble in water. Its ideal pH is 7. It is colorless (colorless), its melting point is 146 °C, and its density is 1.54 g/cm³.
- Methane: has a carbon atom bonded to four hydrogen atoms, molecular geometry is tetrahedral, it is a gas in nature, the melting point is -182.5, the boiling point is -161.5 °C, the density of methane is 0.717 kg/m³.
VIII. The use of the intensity of the specific physical concept in the variety of molecules
IX. Conclusion
References
- CARDOSO, Carlos Eduardo Ramos. Obligatory Necessity Theory: Elements and Facts Influenced by the intensity of the specific physical concept. 2024.
- CARDOSO, Carlos Eduardo Ramos. Theory of Differences between Elements: explanation of the differences between quantum physics and the theory of general relativity. 2024.
- CARDOSO, Carlos Eduardo Ramos. As equações e seus efeitos. Em outras palavras, v. 200, n. 300, p. 500, 2025.
- TOLENTINO, Mario; ROCHA-FILHO, Romeu C.; CHAGAS, Aécio Pereira. Alguns aspectos históricos da classificação periódica dos elementos químicos. Química nova, v. 20, p. 103-117, 1997.
- MOREIRA, Marco Antonio. O modelo padrão da física de partículas. Revista Brasileira de Ensino de Física, v. 31, p. 1306.1-1306.11, 2009.
- CLARO, Paulo Ribeiro. Astroquímica. Revista de Ciência Elementar, v. 5, n. 3, 2017.
- SILVA, MARA LÚCIA RODRIGUES; MARTIN, VERA APARECIDA FERNANDES. SISTEMA SOLAR, ELEMENTOS QUÍMICOS, SUBSTÂNCIAS, TERRA E VIDA.
- RODRIGUES, Cláudia Vilega. O sistema solar. Introdução à astronomia e astrofísica. São José dos Campos: INPE, p. 1-45, 2003.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



