1. Introduction
Tinnitus is a common auditory condition characterized by the perception of ringing, buzzing, or other phantom sounds without an external auditory stimulus, affecting approximately 10–15% of the population [
1]. Tinnitus is not a disease itself; however, it is often associated with hearing loss, noise exposure, aging, or other underlying medical conditions resulting in a various degrees of severity impact on daily life [
2]. For some individuals, tinnitus remains a mild, occasional annoyance, on the contrary, others experience a chronic and distressing condition, significantly impairing sleep, concentration, mood, and overall well-being.
Despite extensive research, there is currently no universally effective treatment for tinnitus, and management strategies focus on symptom relief rather than a definite cure. Various therapeutic approaches, including sound therapy, cognitive-behavioral therapy (CBT), pharmacological interventions, and dietary supplements, have been explored to alleviate its burden [
3,
4,
5].
The subjective character of tinnitus among individuals necessitates the evaluation of its impact on quality of life through validated patient-reported outcome measures (PROMs). These standardized questionnaires provide valuable insights into the psychological, functional, and emotional burden of tinnitus, as well as the effectiveness of interventions. One of the most widely used instruments for tinnitus assessment is the Tinnitus Handicap Inventory (THI) [
6], a 25-item questionnaire designed to quantify the degree to which tinnitus affects a patient’s daily life. The THI is divided into three subclasses:
Functional: Measures difficulties in concentration, hearing, and daily activities.
Emotional: Evaluates frustration, stress, and emotional distress associated with tinnitus.
Catastrophic: Assesses extreme reactions such as feelings of helplessness or loss of control.
In addition to THI, other measures, such as sleep quality assessments, tinnitus severity scales, and treatment compliance tracking, are crucial in evaluating the effectiveness of interventions. Given the high prevalence of tinnitus-related insomnia, sleep disturbances are often a key factor in determining overall treatment success [
7,
8].
This study aims to provide valuable clinical insights into the potential therapeutic benefits of a dietary supplement, in alleviating tinnitus symptoms and improving patients’ quality of life. By employing validated assessment tools, such as the THI, this study seeks to provide objective evidence regarding the potential benefits of MEMOTIN® as a supportive intervention for individuals suffering from tinnitus.
2. Material and Methods
2.1. Study Design
This study is a prospective, non-interventional, non-placebo-controlled, multicenter clinical study conducted in private clinics. The study aims to evaluate the effectiveness and safety of MEMOTIN®, a dietary supplement formulated for individuals with subjective tinnitus. The study was carried out following the tenets principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonization. An informed consent was provided by all participants before inclusion in the study.
2.2. Study Product
The product under investigation is MEMOTIN®, which contains the following active ingredients per capsule:
Gingko Biloba—60 mg
Magnesium—220 mg
Zinc—10 mg
Vitamin B12—0.0025 mg
Melatonin—1.9 mg
Each component plays a distinct role in supporting neurological function, circulatory health, and sleep regulation, which are essential factors in tinnitus management [
1,
9,
10,
11,
12,
13].
| Component |
Action |
| Gingko Biloba |
May improve blood flow to the inner ear and reduce oxidative stress by altering the tone of blood vessels |
| Magnesium |
Prevent cochlear damage from noise-induced hearing loss by reducing calcium influx into hair cells |
| Zinc |
Protects the cochlea from free radical damage, maintaining cochlear integrity, modulating neurotransmission, and exhibiting antidepressant effects |
| Vitamin B12 |
Supports nerve function and may be beneficial for tinnitus linked to B12 deficiency |
| Melatonin |
Known for its sleep-regulating properties, engagement with the sleep-wake cycle and antioxidant activity |
2.3. Study Visits & Assessments
Patients were instructed to take one capsule daily, 30 minutes before bedtime, for a duration of 3 months. No additional recommendations regarding dietary modifications or exercise regiments were provided during the study.
The study was scheduled across two visits:

2.4. Study Population
The study will include adult patients diagnosed with subjective tinnitus persisting for more than six months.
2.5. Exclusion Criteria
Patients meeting any of the following criteria will be excluded from the study:
Use of similar tinnitus treatments in the past three months.
Current treatment with anticoagulant medications.
History of alcohol or substance abuse within the last six months.
Presence of serious otologic conditions, including Ménière’s disease, Otosclerosis, Otitis media, Eardrum perforation
Diagnosed psychiatric disorders, including depression, psychosis, or other severe mental health conditions.
Pregnant or lactating women.
Patients with renal failure.
Know hypersensitivity or allergy to any of the supplement’s ingredients.
Patients with scheduled surgery during the study period.
2.6. Study Objectives
The primary objective was to assess the effectiveness of MEMOTIN® in reducing the impact of tinnitus on patients’ quality of life over a 3-month treatment period.
Changes in THI total score and emotional subscale from baseline to 3 months were used as primary endpoints. Moreover, the evaluation of safety and tolerability of MEMOTIN® during the study period was secondary assessed.
2.7. Assessment Tool: THI Score
The THI questionnaire is a widely recognized and validated instrument for quantifying the impact of tinnitus on an individual’s daily life [
6,
14]. It consists of 25 items, categorized into three subscales:
Functional (13 items)—Evaluates the impact of tinnitus on daily activities, including concentration and sleep.
Emotional (7 items)—Assesses tinnitus-related psychological distress, such as frustration, anxiety, and depression.
Catastrophic (5 items)—Measures extreme negative reactions to tinnitus, such as feelings of helplessness or loss of control.
In this study, the validated Greek translation of the THI questionnaire was utilized [
15].
In conclusion, effectiveness outcomes were analyzed in the per-protocol (PP) population, which included 161 patients. The PP population consisted of all participants who did not have any major protocol deviations. Safety data were analyzed in the safety population, which included 172 patients who received at least one dose of the treatment. Effectiveness outcomes were expressed as mean ± standard deviation (SD) and compared between visits using paired statistical tests. A p-value of less than 0.05 was considered statistically significant. The treatment was deemed effective if the Tinnitus Handicap Inventory (THI) score was reduced by at least 20%.
The study was approved by the Ethics Committee of the Iaso General Clinic 37-39, Kifissias Avenue, 151 23 Maroussi, Athens, Greece +30 210 61 84 000. All data was encrypted before further analysis.
3. Results
3.1. Study Population
A total of 174 patients were included, of whom 161 (92.5%) completed the study. Two patients were lost to follow up, nine patients had symptoms for less than 6 months and two patients received the product less than a month (Table 1).
| Demographics |
Ν=161 |
| Age |
58.17 ± 12.64 |
| Age of Tinnitus onset |
55 ± 11.12 |
| Sex |
|
| Female |
77 (47.8%) |
| Male |
83 (51.6%) |
| Hearing Loss |
|
| Yes |
66 (41%) |
| No |
87 (54%) |
3.2. Reduction in Total THI Score
Following the administration of MEMOTIN
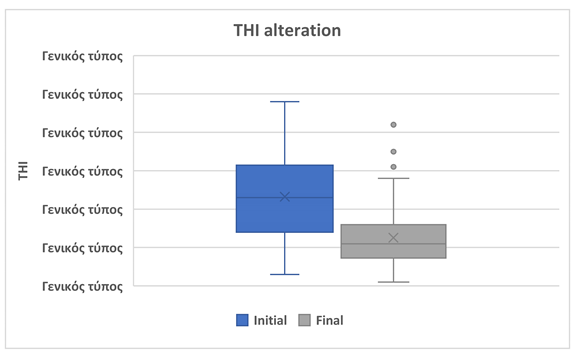
®, the mean THI total score significantly decreased from 46.67 ± 21.33 at baseline to 25.11 ± 14.49 after 90 days of treatment. This corresponds to a mean reduction of 21.56 points (p = 0.0001), which exceeds both the clinically significant threshold of 7 points and the 20% reduction criterion (9.33 points relative to baseline). (Table 2, Figure 1).
| THI total score |
| |
Ν=161 |
| Initial, mean ± SD |
46.67 ± 21.33 |
| Final, mean ± SD |
25.11 ± 14.49 |
| Difference |
-21.56 |
| % of reduction |
46.19% |
| p-value |
0,0001 |
3.3. THI Emotional Subscale
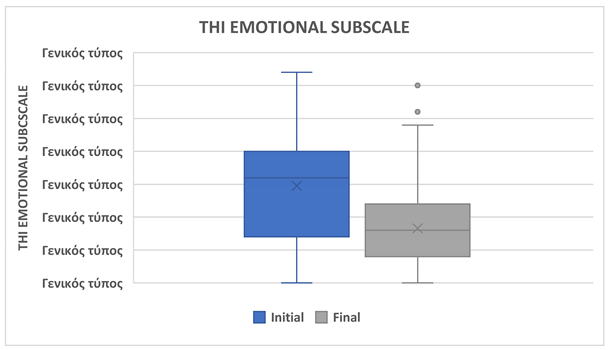
A statistically significant reduction was observed in the THI Emotional Subscale score, which decreased from 14.83 ± 8.21 at baseline to 8.30 ± 5.73 after treatment (p = 0.0001). This suggests a meaningful improvement in patients’ emotional responses to tinnitus. (Table 3, Figure 2).
| THI Emotional Subscale |
| |
Ν=161 |
| Initial, mean ± SD |
14.83 ± 8.21 |
| Final, mean ± SD |
8.30 ± 5.73 |
| Difference |
-6.53 |
| % of reduction |
44.03% |
| p-value |
0,0001 |
3.4. Impact on Insomnia
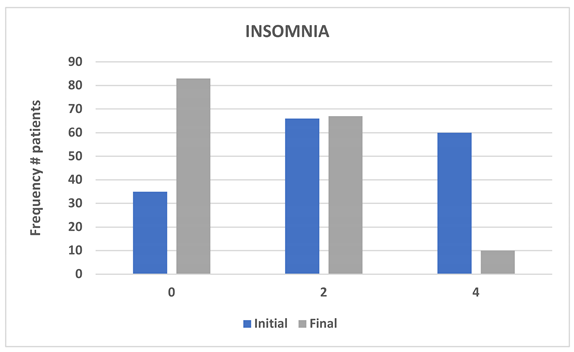
Analysis of insomnia-related responses showed that Category 2 (indicating that tinnitus sometimes affects sleep) was the most frequently reported category at baseline. Following the administration of MEMOTIN
®, there was a statistically significant reduction in the impact of tinnitus on sleep (p < 0.05), demonstrating an improvement in sleep quality. (Table 4, Figure 3).
| |
INITIAL |
FINAL |
| |
FREQUENCY |
(%) |
FREQUENCY |
(%) |
| 0 |
35 |
21.7 |
83 |
51.6 |
| 2 |
66 |
41.0 |
67 |
41.6 |
| 4 |
60 |
37.3 |
10 |
6.2 |
| Missing values |
|
|
1 |
0.6 |
| Total |
161 |
|
161 |
|
3.5. Tinnitus Severity Scale
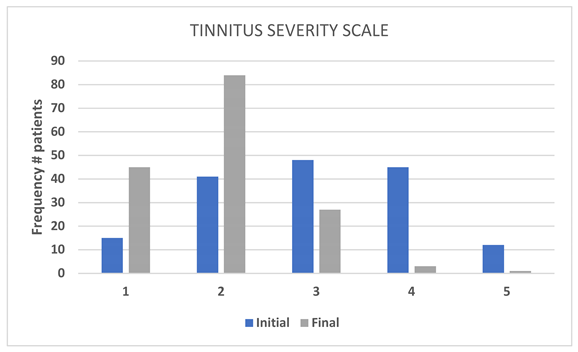
A descriptive analysis of the Tinnitus Severity Scale indicated a statistically significant reduction in tinnitus severity following MEMOTIN
® treatment (p < 0.05). This further supports the effectiveness of the supplement in alleviating tinnitus symptoms. (Table 5, Figure 4).
| |
INITIAL |
FINAL |
| |
FREQUENCY |
(%) |
FREQUENCY |
(%) |
| 1 |
15 |
9.3 |
45 |
28.0 |
| 2 |
41 |
25.5 |
84 |
52.2 |
| 3 |
48 |
29.8 |
27 |
16.8 |
| 4 |
45 |
28.0 |
3 |
1.9 |
| 5 |
12 |
7.5 |
1 |
0.6 |
| Missing values |
|
|
1 |
0.6 |
| Total |
161 |
|
161 |
|
3.6. Comparison of THI Score with Hearing Loss and Age
A comparison between hearing loss and THI score improvement revealed no statistically significant association (p = 0.637). This suggests that pre-existing hearing loss does not influence the degree of improvement in THI scores. Similarly, a comparison between age and THI score improvement showed no statistically significant difference (p = 0.694). This indicates that age does not affect the extent of improvement in tinnitus symptoms.
3.7. Patient Compliance and safety
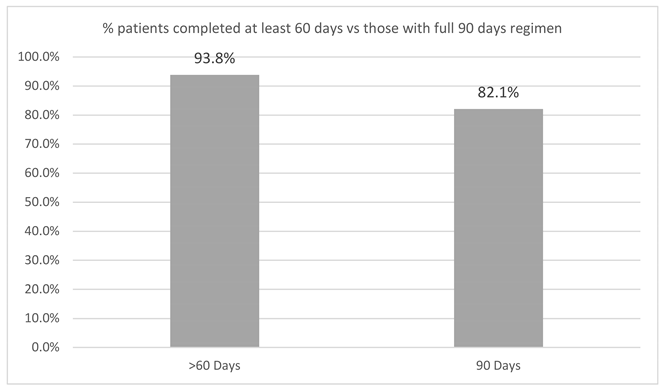
Patient adherence to treatment was high, with most participants completing the 90-day treatment period. A graphical representation (below) illustrates the percentage of patients who completed at least 60 days versus those who completed the full 90-day regimen (Figure 5). Regarding safety, a total of four adverse events (AEs) were reported (2.3%), all of which were mild in intensity. These included one case, each of headache, dizziness, overstimulation, and gastric discomfort. No participants discontinued the study due to adverse events.
4. Discussion
Tinnitus remains a complex condition with no universally accepted pharmacological treatment, leading researchers to explore alternative therapeutic strategies, including dietary supplements. MEMOTIN®, a supplement containing Gingko Biloba, Magnesium, Zinc, Vitamin B12, and Melatonin, was evaluated in this study for its potential to alleviate tinnitus symptoms. The combination of these ingredients addresses vascular health, neuronal protection, and sleep regulation, all of which are crucial factors in tinnitus pathophysiology.
While dietary supplements are often considered a complementary approach, they may offer a non-invasive and well-tolerated alternative for individuals seeking relief from tinnitus-related distress by improving emotional affectation combined with a good safety profile16. The results of this study provide evidence that MEMOTIN® significantly reduced tinnitus severity and improved quality of life, particularly in the THI total and emotional subscale scores. However, despite these promising findings, further research with larger sample sizes and long-term follow-ups is necessary to establish the consistency and reproducibility of these effects in broader populations.
4.1. Reduction in THI Score: Clinical Relevance and Interpretation
A key outcome of this study was the significant reduction in the Tinnitus Handicap Inventory (THI) total score following 90 days of MEMOTIN
® supplementation. The mean THI score decreased by 21.56 points, exceeding the clinically meaningful threshold of 7 points and the 20% improvement criterion (9.33 points relative to baseline). A prospective, interventional study with a similar dietary supplement showed a decrease in THI score by 15.7 points [
16], whereas Cima et al. [
17] presented a mean reduction of 22.3 points in THI scores among individuals who were treated according to the Cognitive Behavioral Therapy’s (CBT) tenets, with this improvement lasting for 12 months.
The observed reduction suggests that MEMOTIN
® effectively alleviates both the perceived intensity of tinnitus and its impact on daily life. More notably, the significant improvement in the THI emotional subscale (p = 0.0001) highlights its potential role in reducing tinnitus-related emotional distress. Emotional burden is a critical factor influencing patients’ well-being, often leading to frustration, anxiety, and in some cases, depression. The observed improvements indicate that MEMOTIN
® may not only reduce tinnitus perception but also enhance emotional resilience and coping mechanisms. Interestingly, similar evidence shows that tricyclic antidepressants (e.g., nortriptyline) can reduce emotional distress, especially in patients with comorbid depression but some studies present low grade of quality as a result of the failure to separate the effects on tinnitus from the effects on symptoms of anxiety and depression. Contrariwise, selective serotonin reuptake inhibitors (SSRIs) may not provide overall improvement in any of the validated tinnitus-related parameters except for a possible benefit associated with higher doses of the drug [
18]. However, in any case of antidepressants therapy, effects on tinnitus loudness were limited. Notable reduction in emotional distress, including anxiety and catastrophic thinking, was also observed among patients underwent Mindfulness-based cognitive therapy for chronic tinnitus [
19].
4.2. Impact on Quality of Life: Sleep and Psychological Well-Being
Tinnitus can concurrently coexist with other conditions such as anxiety, depression, dysfunction of the temporomandibular joint, hyperacusis and headache, difficulty concentrating, sleep disturbances, and even emotional distress [
20], resulting in a severely impacted quality of life. Wang et al. [
21] found the mediating effect of sleep disorders between tinnitus severity and anxiety accounted for 27.88% of the total effect size. In this study, insomnia-related complaints showed a statistically significant reduction (p < 0.05), indicating that MEMOTIN
® may help mitigate the sleep disturbances frequently associated with tinnitus. Given that melatonin is a key component of MEMOTIN
®, its well-documented role in sleep regulation and antioxidant protection may contribute to this positive outcome.
Furthermore, improvements in tinnitus severity scores (p < 0.05) reinforce the potential benefits of MEMOTIN® in reducing tinnitus-related distress. However, it is important to recognize inter-individual variability, as tinnitus perception and response to treatment can be influenced by psychological factors, lifestyle, and underlying medical conditions. The findings our study suggest that incorporating dietary supplementation as part of a holistic tinnitus management plan may provide meaningful relief for certain individuals.
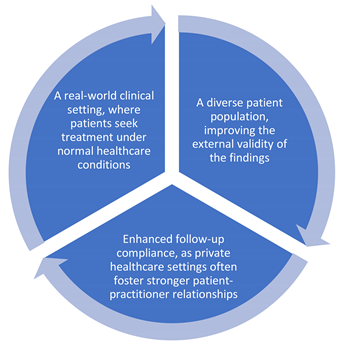
4.3. The Value of a Multicenter Clinical Study in Private Clinics
One of the strengths of this study is its rigorous multicenter design, which increases the generalizability of the results. Conducting the study in private clinics provided several advantages, including:
Additionally, centralized data collection and validation across multiple sites reduce biases and enhance the credibility of the results. While randomized controlled trials (RCTs) remain the gold standard for clinical research, real-world studies conducted in private practice settings offer valuable insights into patient adherence, treatment efficacy, and tolerability in everyday scenarios. Nonetheless, a carefully conducted multicenter study with systematic data collection and strong statistical analysis can yield clinically significant and widely applicable results. Various observational study designs may be utilized, such as cross-sectional, cohort, and case-control approaches [
22].
4.4. Comparison with Previous Studies
The results of this study align with findings from previous research investigating dietary supplements for tinnitus management. Gingko Biloba, one of the key components of MEMOTIN
®, has been studied for its potential role in improving circulatory function and reducing oxidative stress in the auditory system. Some studies have reported modest improvements in tinnitus severity, while others suggest no significant benefit compared to placebo. Clearly, the available literature shows mixed results [
1]. Both Krauss et al. [
23] and von Boetticher et al. [
24] highlighted the beneficial outcomes of Gingko Biloba, while a review of 12 studies including 1,915 participants held by Sereda et al. [
25], showed that Ginkgo biloba may have little to no effect in THI scores at three to six months compared to placebo.
Additionally, melatonin, a naturally occurring indoleamine that helps regulate circadian and circannual rhythms, has been previously studied for its effects on tinnitus-related sleep disturbances, with positive findings indicating its role in reducing tinnitus perception, improving sleep quality, and enhancing overall well-being. Based on research findings melatonin has demonstrated its protective role against cochlear damage caused by acoustic trauma and ototoxic substances, while clinical studies have shown that it can reduce the severity of tinnitus symptoms. As a result, melatonin is considered a potential beneficial treatment option for individuals with tinnitus, in the context of its influence on neural plasticity, oxidative and nitrosative stress, apoptosis, and autophagy [
26]. Our study supports these earlier findings by demonstrating significant improvements in THI scores and sleep-related distress.
However, not all studies investigating dietary supplements for tinnitus have yielded consistent results. Variability in study designs, dosages, treatment durations, and patient populations may contribute to these discrepancies. Compared to previous trials, the present study demonstrates a larger and statistically significant reduction in THI scores, reinforcing the potential role of MEMOTIN® as a supportive intervention for tinnitus sufferers.
Future research should focus on larger, placebo-controlled randomized trials to confirm these findings. More long-term follow-up studies are necessary to assess the sustainability of improvements. Comparative studies evaluating MEMOTIN® against other tinnitus management strategies, including sound therapy, behavioral interventions, and pharmacological options.
5. Conclusions
This study provides compelling evidence that MEMOTIN® supplementation may lead to statistically and clinically significant improvements in tinnitus severity, emotional burden, and sleep quality. The results underscore the potential role of dietary supplements as an adjunctive therapy for tinnitus management. Moreover, the multicenter design in private clinics enhances the validity and applicability of the findings in real-world clinical practice. While the findings are promising, further research through larger, controlled trials with extended follow-up is warranted to establish MEMOTIN® as a reliable and standardized approach for tinnitus relief. Such results will contribute to understanding the supplement’s efficacy and safety profile, offering guidance for future clinical applications.
Conflict of Interest: The authors have no conflicts of interest to declare.
Funding
This study was supported by bioAxess Pharmaceuticals Ltd., Pentelis Avenue 1, 15235 Vrilissia, Athens, Greece. The funder had no role in the design, data collection, data analysis, and reporting of this study.
Acknowledgments
The authors would like to acknowledge and thank the participants and the private practice health care providers for their kind collaboration.
| |
Name |
Surname |
Private Clinic |
| 1 |
Grigorios |
Alexopoulos |
Kapodistriou 10,Attiki |
| 2 |
Maria |
Apostolidou |
Egnatia 90, Thessaloniki |
| 3 |
Maria |
Askiti |
A.Thrakis 48, Thessaloniki |
| 4 |
Dimitris |
Chochlidakis |
Favierou 14, Evia |
| 5 |
Pantelis |
Chouridis |
A.Papanastasiou 56, Thessaloniki |
| 6 |
Konstantinos |
Daskolias |
Sarantaporou 59 Attiki |
| 7 |
Athanasios |
Filentas |
28 Octovriou 8, Thessaloniki |
| 8 |
Olga |
Kesidou |
Lenorman 242, Attiki |
| 9 |
Panagiotis |
Kontopoulos |
Mikras Asias 9, Attiki |
| 10 |
Ioannis |
Koulentis |
Trikoupi 1-3, Attiki |
| 11 |
Vasilis |
Miliakis |
Dimarchiou 8, Attiki |
| 12 |
Dimitris |
Nepkas |
Anaximandrou 32, Thessaloniki |
| 13 |
Eleni |
Pantzopoulou |
V.Pavlou 161, Attiki |
| 14 |
Evagelos |
Papadopoulos |
Praxitelous 137, Attiki |
| 15 |
Elina |
Papadopoulou |
V.Konstantinou 181, Attiki |
| 16 |
Christos |
Patras |
Antheon 38, Thessaloniki |
| 17 |
Georgios |
Peltekis |
Komninon 94, Thessaloniki |
| 18 |
Dimitris |
Sfikas |
Alexandras Av 91, Attiki |
| 19 |
Nikolaos |
Sotiropoulos |
Dimokratias Av 19, Attiki |
| 20 |
Silvia |
Testoni |
Ermou 20, Attiki |
| 21 |
Augoustos |
Theodosis |
Irakleiou Av 66, Attiki |
| 22 |
Georgios |
Tirelis |
Thivon 510, Attiki |
| 23 |
Melina |
Tsea Politi |
M.Chrisostomou 6, Kavala |
| 24 |
Antonis |
Vasileiadis |
O.Patapiou 17, Veria |
| 25 |
Adriana |
Vasiliou |
Ellis 6, Attiki |
| 26 |
Anna |
Yakinthou |
P.Mela 37, Thessaloniki |
References
- Kalentakis, Z.; Feretzakis, G.; Baxevani, G.; Dritsas, G.; Papatheodorou, E. The Efficacy of a Food Supplement in the Treatment of Tinnitus with Comorbid Headache: A Statistical and Machine Learning Analysis with a Literature Review. Audiol. Neurotol. 2024, 1–12. [CrossRef]
- Mazurek, B.; Hesse, G.; Dobel, C.; Kratzsch, V.; Lahmann, C.; Sattel, H. Clinical practice guideline: Chronic tinnitus—diagnosis and treatment. Dtsch. Aerzteblatt Online 2022, 119, 219. [CrossRef]
- The Tinnitus Retraining Therapy Trial Research Group; Scherer, R.W.; Formby, C. Effect of Tinnitus Retraining Therapy vs Standard of Care on Tinnitus-Related Quality of Life. Arch. Otolaryngol. Neck Surg. 2019, 145, 597–608. [CrossRef]
- Wegner, I.; A Hall, D.; Smit, A.L.; McFerran, D.; Stegeman, I. Betahistine for tinnitus. Cochrane Database Syst. Rev. 2018, 2018, CD013093. [CrossRef]
- Sereda, M.; Xia, J.; Scutt, P.; Hilton, M.P.; El Refaie, A.; Hoare, D.J. Ginkgo biloba for tinnitus. Cochrane Database Syst. Rev. 2022, 2023. [CrossRef]
- Newman, C.W.; Jacobson, G.P.; Spitzer, J.B. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Neck Surg. 1996, 122, 143–148. [CrossRef]
- Cima, R.F.; van Breukelen, G.; Vlaeyen, J.W. Tinnitus-related fear: Mediating the effects of a cognitive behavioural specialised tinnitus treatment. Hear. Res. 2018, 358, 86–97. [CrossRef]
- Fernández, M.; Cuesta, M.; Sanz, R.; Cobo, P. Comparison of Tinnitus Handicap Inventory and Tinnitus Functional Index as Treatment Outcomes. Audiol. Res. 2022, 13, 23–31. [CrossRef]
- Li, M.-Z.; Zhang, Y.; Zou, H.-Y.; Ouyang, J.-Y.; Zhan, Y.; Yang, L.; Cheng, B.C.-Y.; Wang, L.; Zhang, Q.-X.; Lei, J.-F.; et al. Investigation of Ginkgo biloba extract (EGb 761) promotes neurovascular restoration and axonal remodeling after embolic stroke in rat using magnetic resonance imaging and histopathological analysis. Biomed. Pharmacother. 2018, 103, 989–1001. [CrossRef]
- Abaamrane, L.; Raffin, F.; Gal, M.; Avan, P.; Sendowski, I. Long-term administration of magnesium after acoustic trauma caused by gunshot noise in guinea pigs. Hear. Res. 2009, 247, 137–145. [CrossRef]
- Pirodda, A.; Raimondi, M.C.; Ferri, G.G. Exploring the reasons why melatonin can improve tinnitus. Med Hypotheses 2010, 75, 190–191. [CrossRef]
- Coelho, C.B.; Tyler, R.; Hansen, M. Zinc as a Possible Treatment for Tinnitus. Prog Brain Res 2007, 166, 279–285. [CrossRef]
- Singh, C.; Kawatra, R.; Gupta, J.; Awasthi, V.; Dungana, H. Therapeutic role of Vitamin B12 in patients of chronic tinnitus: A pilot study. Noise Heal. 2016, 18, 93–7. [CrossRef]
- Shin, S.-H.; Byun, S.W.; Park, Y.; Lee, H.Y. The Tinnitus Handicap Inventory is a better indicator of the overall status of patients with tinnitus than the Numerical Rating Scale. Am. J. Otolaryngol. 2023, 44, 103719. [CrossRef]
- Papitsi, I.; Balatsouras, D.G.; Makris, I.; Koukoutsis, G.; Kaberos, A.; Tzavara, C.; Nikolopoulos, T.; Sarafis, P. Validation of the Greek version of Tinnitus Handicap Inventory. Audiol. Res. 2020, 10, 21–43. [CrossRef]
- Knäpper, J.; Girauta, M.V.; Coromina, J. Effectiveness of Tinnitan Duo® in Subjective Tinnitus with Emotional Affectation: A Prospective, Interventional Study. J. Diet. Suppl. 2021, 20, 1–14. [CrossRef]
- Cima, R.F.; Maes, I.H.; A Joore, M.; Scheyen, D.J.; El Refaie, A.; Baguley, D.M.; Anteunis, L.J.; van Breukelen, G.J.; Vlaeyen, J.W. Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: a randomised controlled trial. Lancet 2012, 379, 1951–1959. [CrossRef]
- Baldo, P.; Doree, C.; Molin, P.; McFerran, D.; Cecco, S. Antidepressants for patients with tinnitus. Cochrane Database Syst. Rev. 2012, 2012, CD003853. [CrossRef]
- McKenna, L.; Marks, E.M.; Hallsworth, C.A.; Schaette, R. Mindfulness-Based Cognitive Therapy as a Treatment for Chronic Tinnitus: A Randomized Controlled Trial. Psychother. Psychosom. 2017, 86, 351–361. [CrossRef]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [CrossRef]
- Wang, S.; Cha, X.; Li, F.; Li, T.; Wang, T.; Wang, W.; Zhao, Z.; Ye, X.; Liang, C.; Deng, Y.; et al. Associations between sleep disorders and anxiety in patients with tinnitus: A cross-sectional study. Front. Psychol. 2022, 13, 963148. [CrossRef]
- Ramgopal, S.; Benedetti, J.; Cotter, J.M. Performing a Multicenter Retrospective Study. Hosp. Pediatr. 2025, 15, e77–e82. [CrossRef]
- Krauss, P.; Tziridis, K.; Metzner, C.; Schilling, A.; Hoppe, U.; Schulze, H. Stochastic Resonance Controlled Upregulation of Internal Noise after Hearing Loss as a Putative Cause of Tinnitus-Related Neuronal Hyperactivity. Front. Neurosci. 2016, 10, 597. [CrossRef]
- von Boetticher, A. Ginkgo biloba extract in the treatment of tinnitus: a systematic review. Neuropsychiatr. Dis. Treat. 2011, 7, 441–447. [CrossRef]
- Sereda, M.; Xia, J.; Scutt, P.; Hilton, M.P.; El Refaie, A.; Hoare, D.J. Ginkgo biloba for tinnitus. Cochrane Database Syst. Rev. 2022, 2023. [CrossRef]
- Hosseinzadeh, A.; Kamrava, S.K.; Moore, B.C.; Reiter, R.J.; Ghaznavi, H.; Kamali, M.; Mehrzadi, S. Molecular Aspects of Melatonin Treatment in Tinnitus: A Review. Curr. Drug Targets 2019, 20, 1112–1128. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).











