Submitted:
14 May 2025
Posted:
16 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
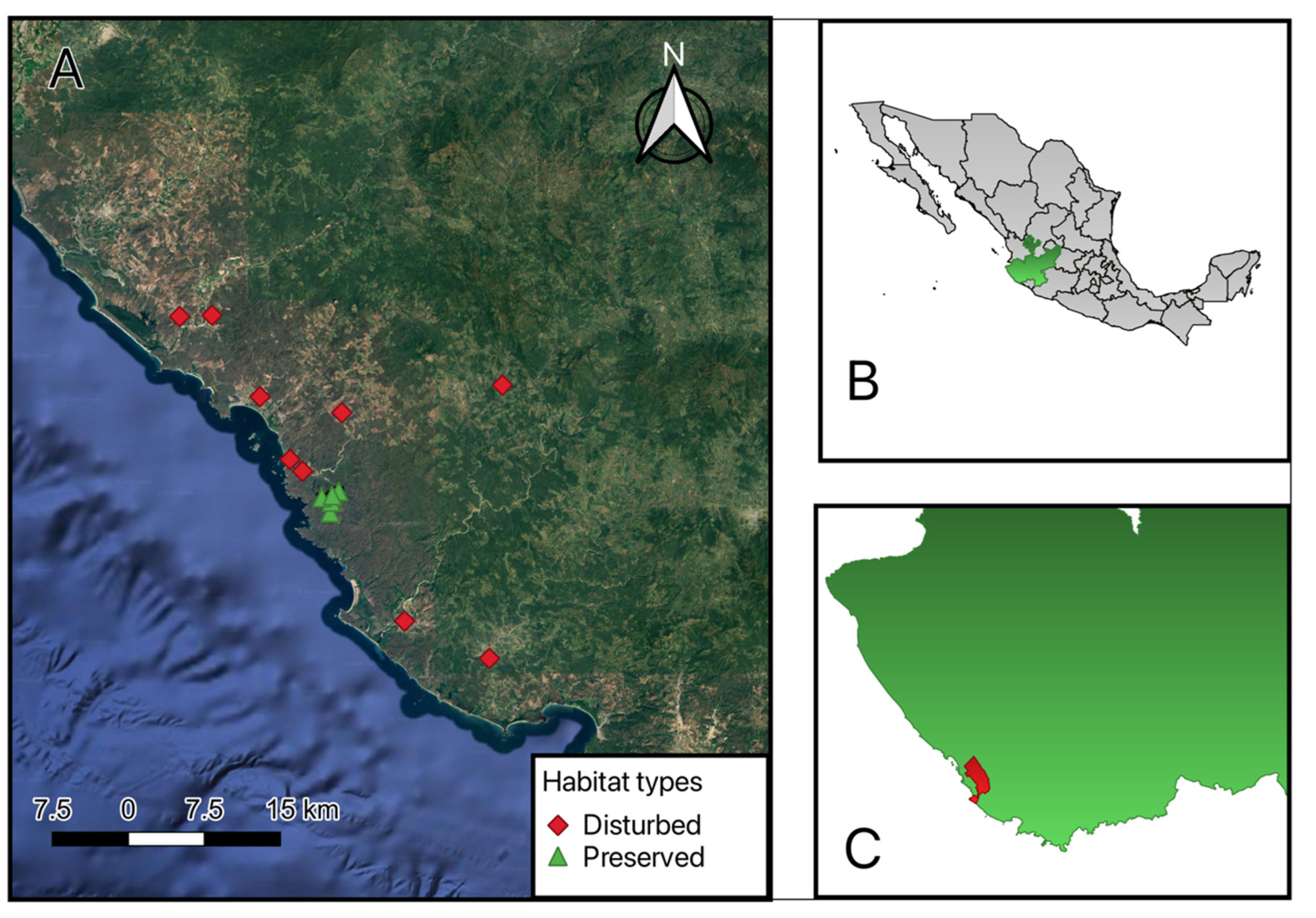
2.1. Study Site
2.2. Environmental and Body Temperature Recording
2.3. Thermographic Image Analysis and Thermal Stress Analysis
2.4. Energy Reserves Calculation
2.5. Statistical Analysis
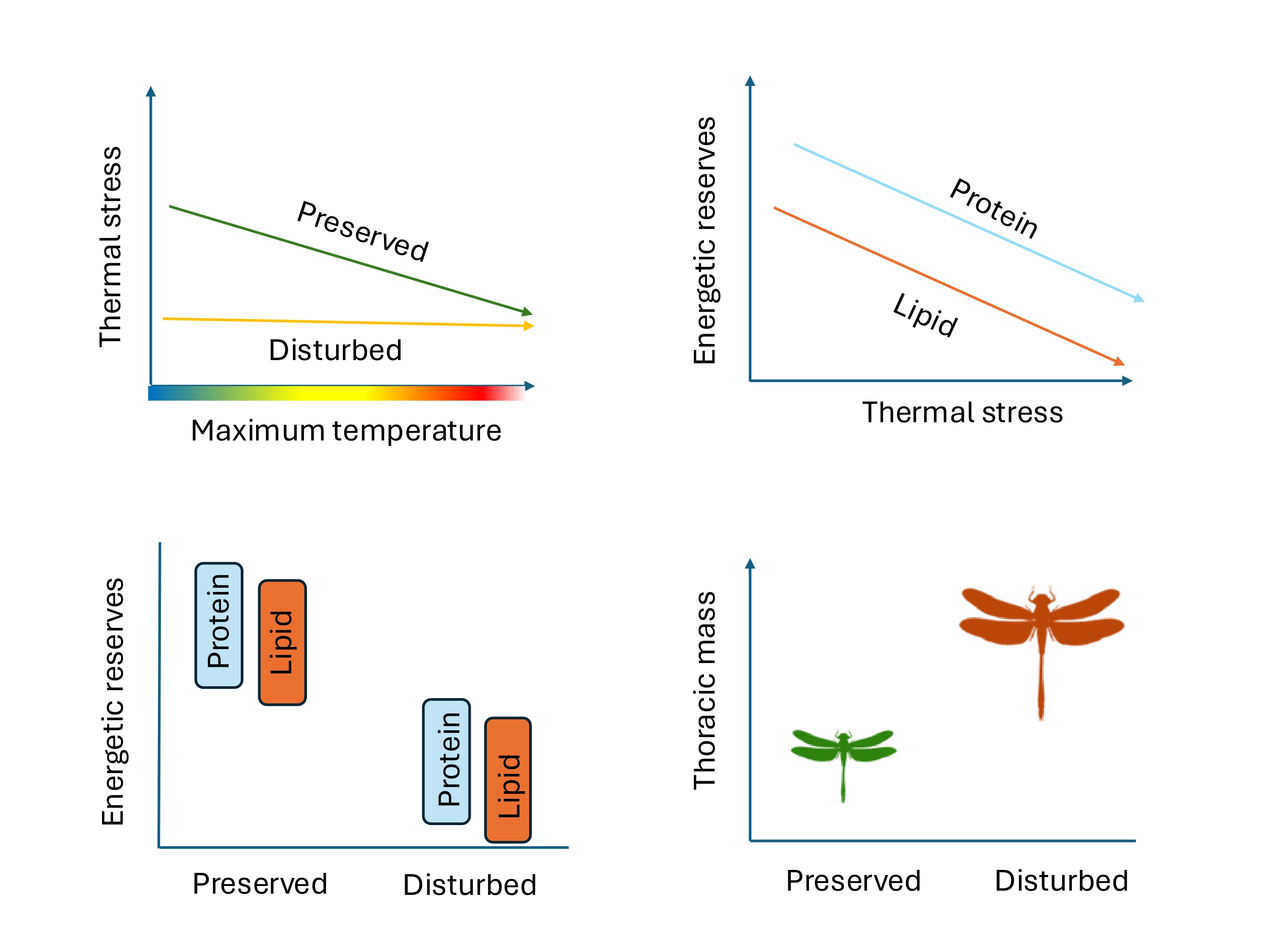
3. Results
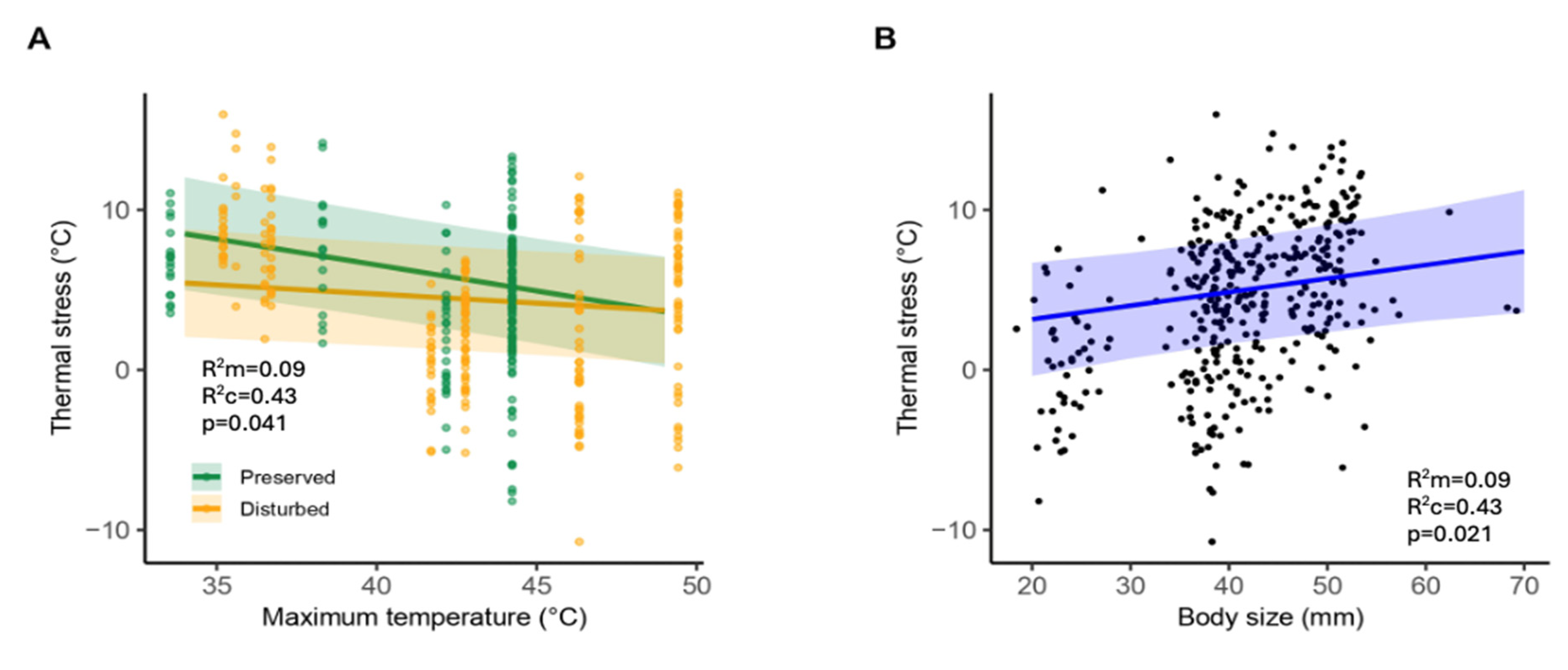
3.1. Environmental Temperatures
3.2. Thermal Stress
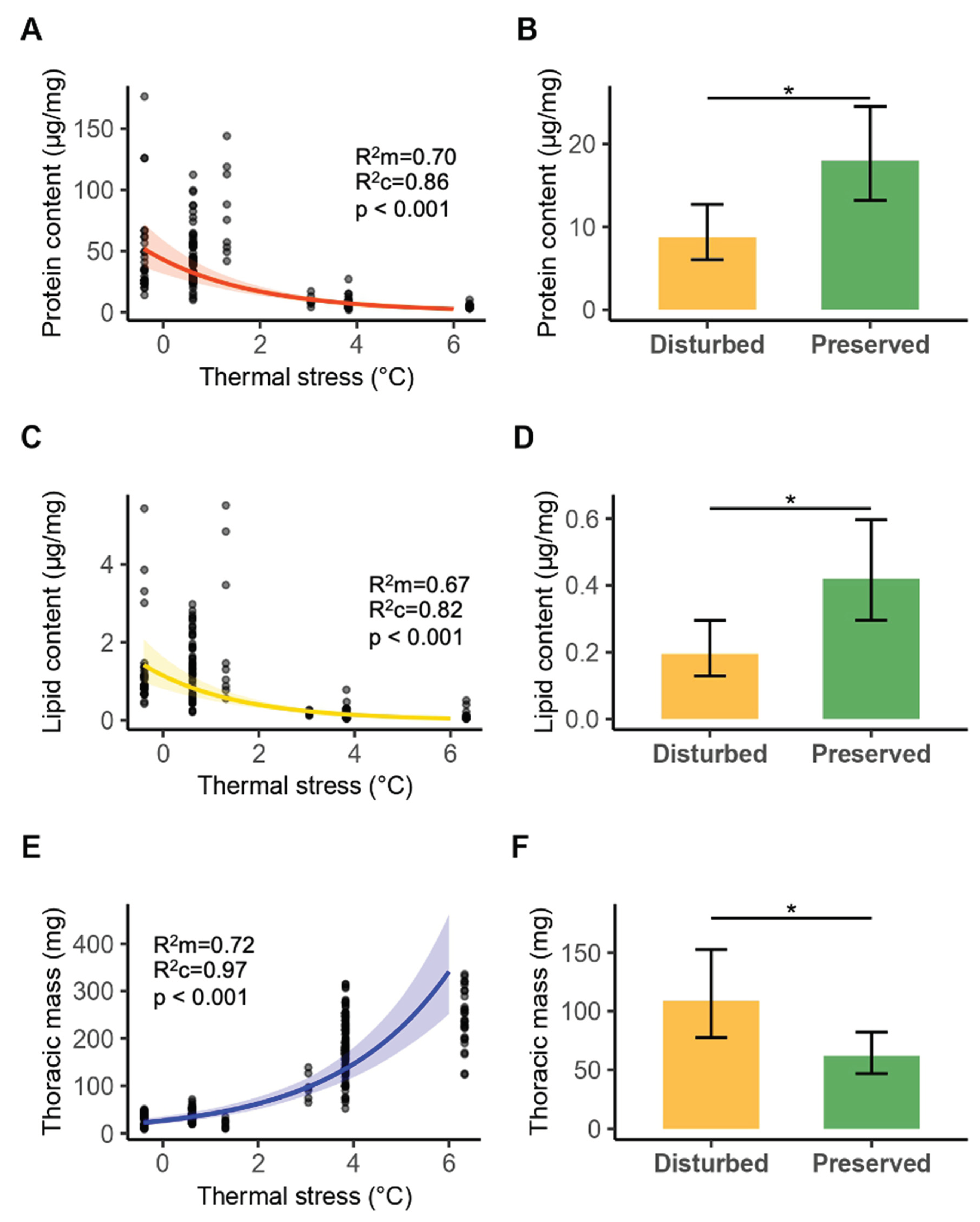
3.3. Energy Reserves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Püttker, T.; Crouzeilles, R.; Almeida-Gomes, M.; Schmoeller, M.; Maurenza, D.; Alves-Pinto, H.; Pardini, R.; Vieira, M. V; Banks-Leite, C.; Fonseca, C.R.; et al. Indirect Effects of Habitat Loss via Habitat Fragmentation: A Cross-Taxa Analysis of Forest-Dependent Species. Biol Conserv 2020, 241, 108368. [Google Scholar] [CrossRef]
- Piessens, K.; Adriaens, D.; Jacquemyn, H.; Honnay, O. Synergistic Effects of an Extreme Weather Event and Habitat Fragmentation on a Specialised Insect Herbivore. Oecologia 2009, 159, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Schowalter, T.D. Insect Responses to Major Landscape-Level Disturbance. Annu Rev Entomol 2012, 57, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Royer, P.D.; Cobb, N.S.; Clifford, M.J.; Huang, C.-Y.; Breshears, D.D.; Adams, H.D.; Villegas, J.C. Extreme Climatic Event-Triggered Overstorey Vegetation Loss Increases Understorey Solar Input Regionally: Primary and Secondary Ecological Implications. Journal of Ecology 2011, 99, 714–723. [Google Scholar] [CrossRef]
- Tuff, K.T.; Tuff, T.; Davies, K.F. A Framework for Integrating Thermal Biology into Fragmentation Research. Ecol Lett 2016, 19, 361–374. [Google Scholar] [CrossRef]
- Zlotnick, O.B.; Musselman, K.N.; Levy, O. Deforestation Poses Deleterious Effects to Tree-Climbing Species under Climate Change. Nat Clim Chang 2024, 14, 289–295. [Google Scholar] [CrossRef]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in Fluctuating Thermal Environments. Annu Rev Entomol 2015, 60, 123–140. [Google Scholar] [CrossRef]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and Climate Change Are Reshaping Insect Biodiversity Worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu Rev Entomol 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Giménez Gómez, V.C.; Verdú, J.R.; Zurita, G.A. Thermal Niche Helps to Explain the Ability of Dung Beetles to Exploit Disturbed Habitats. Sci Rep 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Rocha, T.S.; Calvão, L.B.; Juen, L.; Oliveira-Junior, J.M.B. Effect of Environmental Integrity on the Functional Composition of the Odonata (Insecta) Community in Streams in the Eastern Amazon. Front Ecol Evol 2023, 11. [Google Scholar] [CrossRef]
- Castillo-Pérez, E.U.; Rivera-Duarte, J.D.; Abellán, P.; del-Val, E.; González-Tokman, D.; Córdoba-Aguilar, A. Thriving in the Heat: How High Temperatures and Habitat Disturbance Shape Odonate Taxonomic and Functional Diversity in the Tropics. Insect Conserv Divers 2024. [Google Scholar] [CrossRef]
- Suárez-Tovar, C.M.; Ulises Castillo-Pérez, E.; Antonio Sandoval-García, I.; Schondube, J.E.; Cano-Santana, Z.; Córdoba-Aguilar, A. Resilient Dragons: Exploring Odonata Communities in an Urbanization Gradient. Ecol Indic 2022, 141, 109134. [Google Scholar] [CrossRef]
- Castillo-Pérez, U.; May, M.L.; Córdoba-Aguilar, A. Thermoregulation in Odonata. Dragonflies and Damselflies 2022, 101–112. [Google Scholar]
- May, M.L. Thermoregulation and Adaptation to Temperature in Dragonflies (Odonata : Anisoptera). Ecol Monogr 1976, 46, 1–32. [Google Scholar] [CrossRef]
- Polcyn, D.M. Thermoregulation During Summer Activity in Mojave Desert Dragonflies (Odonata: Anisoptera). Funct Ecol 1994, 8, 441–449. [Google Scholar] [CrossRef]
- Lahondère, C. Recent Advances in Insect Thermoregulation. Journal of Experimental Biology 2023, 226. [Google Scholar] [CrossRef]
- May, M. Insect Thermoregulation. Annu Rev Entomol 1979, 24, 313–349. [Google Scholar] [CrossRef]
- Ma, G.; Ma, C. Sen Effect of Acclimation on Heat-Escape Temperatures of Two Aphid Species: Implications for Estimating Behavioral Response of Insects to Climate Warming. J Insect Physiol 2012, 58, 303–309. [Google Scholar] [CrossRef]
- Klepsatel, P.; Gáliková, M.; Xu, Y.; Kühnlein, R.P. Thermal Stress Depletes Energy Reserves in Drosophila. Sci Rep 2016, 6, 33667. [Google Scholar] [CrossRef]
- García-Oliva, F.; Camou, A.; Maass, J.M. El Clima de La Región Central de La Costa Del Pacífico Mexicano. In Historia Natural de Chamela; Noguera-Alderte, A.N., Vega-Rivera, J.H., García-Aldrete, A.N., Quesada, M., Eds.; Instituto de Biología, UNAM: México, 2002; pp. 3–10. [Google Scholar]
- Castillo-Pérez, E.U.; Suárez-Tovar, C.M.; González-Tokman, D.; Schondube, J.E.; Córdoba-Aguilar, A. Insect Thermal Limits in Warm and Perturbed Habitats: Dragonflies and Damselflies as Study Cases. J Therm Biol 2022, 103, 103164. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, B. Insect Thermoregulation. Endeavour 1995, 19, 28–33. [Google Scholar] [CrossRef]
- Verdú, J.R.; Arellano, L.; Numa, C. Thermoregulation in Endothermic Dung Beetles (Coleoptera: Scarabaeidae): Effect of Body Size and Ecophysiological Constraints in Flight. J Insect Physiol 2006, 52, 854–860. [Google Scholar] [CrossRef]
- Bota-Sierra, C.A.; Cordero-Rivera, A.; Novelo-Gutiérrez, R.; Sánchez-Herrera, M.; Londoño, G.A. Can High Temperatures Affect Body Size in Insects? The Case of Rubyspot Damselflies in the Colombian Western Andes. Diversity (Basel) 2024, 16, 743. [Google Scholar] [CrossRef]
- Klepsatel, P.; Wildridge, D.; Gáliková, M. Temperature Induces Changes in Drosophila Energy Stores. Sci Rep 2019, 9, 5239. [Google Scholar] [CrossRef]
- Portela Salomão, R.; González-Tokman, D.; Dáttilo, W.; López-Acosta, J.C.; Favila, M.E. Landscape Structure and Composition Define the Body Condition of Dung Beetles (Coleoptera: Scarabaeinae) in a Fragmented Tropical Rainforest. Ecol Indic 2018, 88, 144–151. [Google Scholar] [CrossRef]
- Martínez-Ibarra, J.A.; Martínez-Hernández, F.; Villalobos, G.; Vences-Blanco, M.O.; Salazar-Schettino, P.M. Update on the Distribution of Triatoma Bolivari and Triatoma Brailovskyi (Hemiptera: Reduviidae: Triatominae) in Western Mexico. Journal of Vector Ecology 2010, 35, 432–434. [Google Scholar] [CrossRef]
- Takano-Rojas, H.; Murray-Tortarolo, G.; Maass, M.; Castillo, A. Characterization, Variability and Long-Term Trends on Local Climate in a Mexican Tropical Dry Forest. International Journal of Climatology 2023, 43, 5077–5091. [Google Scholar] [CrossRef]
- Flores-Casas, R.; Ortega-Huerta, M.A. Modelling Land Cover Changes in the Tropical Dry Forest Surrounding the Chamela-Cuixmala Biosphere Reserve, Mexico. Int J Remote Sens 2019, 40, 6948–6974. [Google Scholar] [CrossRef]
- IBUNAM Datos Climáticos Estación Chamela Available online:. Available online: http://www.ibiologia.unam.mx/ebchamela/www/clima.html (accessed on 1 November 2019).
- Sánchez-Azofeifa, G.A.; Quesada, M.; Cuevas-Reyes, P.; Castillo, A.; Sánchez-Montoya, G. Land Cover and Conservation in the Area of Influence of the Chamela-Cuixmala Biosphere Reserve, Mexico. For Ecol Manage 2009, 258, 907–912. [Google Scholar] [CrossRef]
- Villa-Galaviz, E.; Boege, K.; Del-Val, E. Resilience in Plant-Herbivore Networks during Secondary Succession. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Johansson, F.; Crowley, P.H.; Brodin, T. Sexual Size Dimorphism and Sex Ratios in Dragonflies (Odonata). Biological Journal of the Linnean Society 2005, 86, 507–513. [Google Scholar] [CrossRef]
- Foray, V.; Pelisson, P.-F.; Bel-Venner, M.-C.; Desouhant, E.; Venner, S.; Menu, F.; Giron, D. ; Rey Benjamin A Handbook for Uncovering the Complete Energetic Budget in Insects: The van Handel’s Method (1985) Revisited. Physiol Entomol 2012, 37, 295–302. [Google Scholar] [CrossRef]
- Marden, J.H. Dragonfly Flight Performance: A Model System for Biomechanics, Physiological Genetics, and Animal Competitive Behaviour. Dragonflies and Damselflies. Model Organisms for Ecological and Evolutionary Research. Oxford University Press, Oxford 2008, 249–261.
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Wagenmakers, E.-J.; Farrell, S.; Wagenmakers, -J AIC Model Selection Using Akaike Weights; 2004.
- Lüdecke, D.; Ben-Shachar, M.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J Open Source Softw 2021, 6, 3139. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models 2024.
- R Core Team R: A Language and Environment for Statistical Computing 2023.
- Chown, S.L.; Nicolson, S.W. Thermoregulation. In Insect Physiological Ecology: Mechanisms and Patterns; Oxford University Press: Oxford, 2004. [Google Scholar]
- Corbet, P.S. Dragonflies: Behaviour and Ecology of Odonata; Colchester,UK, 1999; ISBN 0-8014-2592-1.
- Heinrich, B.C.; Casey, T.M. Heat Transfer in Dragonflies: ‘Fliers’ and ‘Perchers. ’ Journal of Experimental Biology 1978, 74, 17–36. [Google Scholar] [CrossRef]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect Responses to Heat: Physiological Mechanisms, Evolution and Ecological Implications in a Warming World. Biological Reviews 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Córdoba-Aguilar, A.; Rocha-Ortega, M. Damselfly (Odonata: Calopterygidae) Population Decline in an Urbanizing Watershed. Journal of Insect Science 2019, 19, 30. [Google Scholar] [CrossRef]
- Tüzün, N.; Stoks, R. Lower Bioenergetic Costs but Similar Immune Responsiveness under a Heat Wave in Urban Compared to Rural Damselflies. Evol Appl 2021, 14, 24–35. [Google Scholar] [CrossRef]
- Corbet, P.S.; May, M.L. Fliers and Perchers among Odonata: Dichotomy or Multidimensional Continuum? A Provisional Reappraisal. International Journal of Odonatology 2008, 11, 155–171. [Google Scholar] [CrossRef]
- Candy, D.J.; Becker, A.; Wegener, G. Coordination and Integration of Metabolism in Insect Flight*. Comp Biochem Physiol B Biochem Mol Biol 1997, 117, 497–512. [Google Scholar] [CrossRef]
- Janssens, M. Hormonal Control of Flight Metabolism in Odonata?, 1995.
- Sacktor, B. Biochemistry of Insect Flight. In Insect Biochemistry and Function; Candy, D.J., Kilby, B.A., Eds.; Springer US: Boston, MA, 1975; ISBN 978-1-4899-3204-4. [Google Scholar]
- Kallapur, V.L.; George, C.J. Fatty Acid Oxidation by the Flight Muscles of the Dragonfly, Pantala Flavescens. J Insect Physiol 1973, 19, 1035–1040. [Google Scholar] [CrossRef]
- Suárez-Tovar, C.M.; Rocha-Ortega, M.; Córdoba-Aguilar, A. Is Body Condition of Mexican Rubyspot (Odonata:Zygoptera) Associated with Urbanization? J Insect Conserv 2023, 27, 961–969. [Google Scholar] [CrossRef]
- Salomão, R.P.; Alvarado, F.; Baena-Díaz, F.; Favila, M.E.; Iannuzzi, L.; Liberal, C.N.; Santos, B.A.; Villegas-Guzmán, G.A.; González-Tokman, D. Negative Effects of Urbanisation on the Physical Condition of an Endemic Dung Beetle from a Neotropical Hotspot. Ecol Entomol 2020, 45, 886–895. [Google Scholar] [CrossRef]
- Córdoba-Aguilar, A.; González-Tokman, D.M. The Behavioral and Physiological Ecology of Adult Rubyspot Damselflies (Hetaerina, Calopterygidae, Odonata). Adv Study Behav 2014, 46, 311–341. [Google Scholar] [CrossRef]
- Roeder, K.A.; Behmer, S.T. Lifetime Consequences of Food Protein-Carbohydrate Content for an Insect Herbivore. Funct Ecol 2014, 28, 1135–1143. [Google Scholar] [CrossRef]
- Suárez-Tovar, C.M.; Rocha-Ortega, M.; González-Voyer, A.; González-Tokman, D.; Córdoba-Aguilar, A. The Larger the Damselfly, the More Likely to Be Threatened: A Sexual Selection Approach. J Insect Conserv 2019, 23, 535–545. [Google Scholar] [CrossRef]
- Attiwilli, S.; Karmakar, T.; Isvaran, K.; Kunte, K. Habitat Preference and Functional Traits Influence Responses of Tropical Butterflies to Varied Habitat Disturbance. Int J Trop Insect Sci 2022, 42, 855–864. [Google Scholar] [CrossRef]
- Eggenberger, H.; Frey, D.; Pellissier, L.; Ghazoul, J.; Fontana, S.; Moretti, M. Urban Bumblebees Are Smaller and More Phenotypically Diverse than Their Rural Counterparts. Journal of Animal Ecology 2019, 88, 1522–1533. [Google Scholar] [CrossRef]
- O’Donnell, M.J. A Perspective on Insect Water Balance. Journal of Experimental Biology 2022, 225, jeb242358. [Google Scholar] [CrossRef]
- Rocha-Ortega, M.; Rodríguez, P.; Córdoba-Aguilar, A. Spatial and Temporal Effects of Land Use Change as Potential Drivers of Odonate Community Composition but Not Species Richness. Biodivers Conserv 2019, 28, 451–466. [Google Scholar] [CrossRef]
- Rocha-Ortega, M.; Rodríguez, P.; Córdoba-Aguilar, A. Can Dragonfly and Damselfly Communities Be Used as Bioindicators of Land Use Intensification? Ecol Indic 2019, 107. [Google Scholar] [CrossRef]
- Heinrich, B. Thermoregulation in Endothermic Insects. Science (1979) 1974, 185, 747–756. [Google Scholar] [CrossRef] [PubMed]
- May, M.L. Odonata: Who They Are and What They Have Done for Us Lately: Classification and Ecosystem Services of Dragonflies. Insects 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.B.; Chumchal, M.M.; Drenner, R.W.; Kennedy, J.H. Seasonality of Odonate-mediated Methylmercury Flux from Permanent and Semipermanent Ponds and Potential Risk to Red-winged Blackbirds (Agelaius Phoeniceus). Environ Toxicol Chem 2017, 36, 2833–2837. [Google Scholar] [CrossRef]
- Córdoba-Aguilar, A.; San Miguel-Rodríguez, M.; Rocha-Ortega, M.; Lanz-Mendoza, H.; Cime-Castillo, J.; Benelli, G. Adult Damselflies as Possible Regulators of Mosquito Populations in Urban Areas. Pest Manag Sci 2021, 77, 4274–4287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




