Submitted:
14 May 2025
Posted:
16 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Rheological Study of Different “Ink” Composition
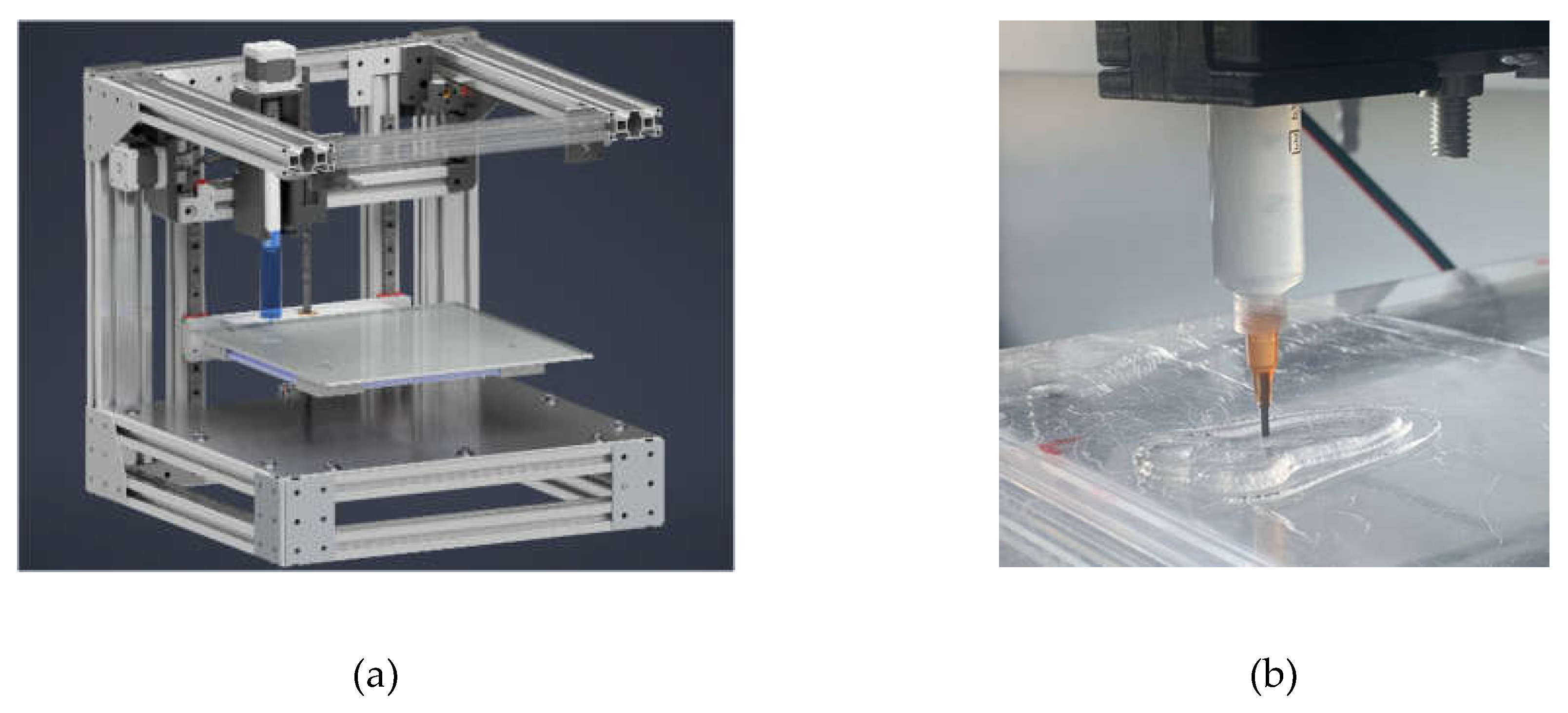
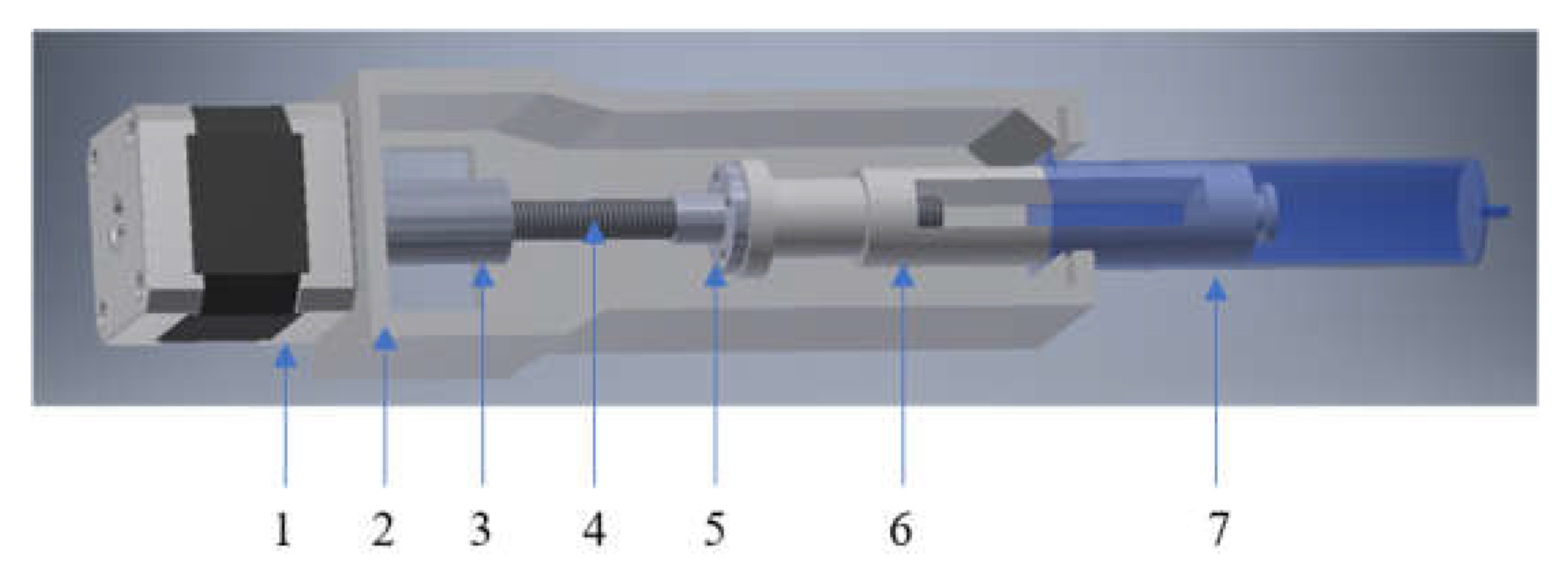
2.2. Investigation of 3D-Printing Process
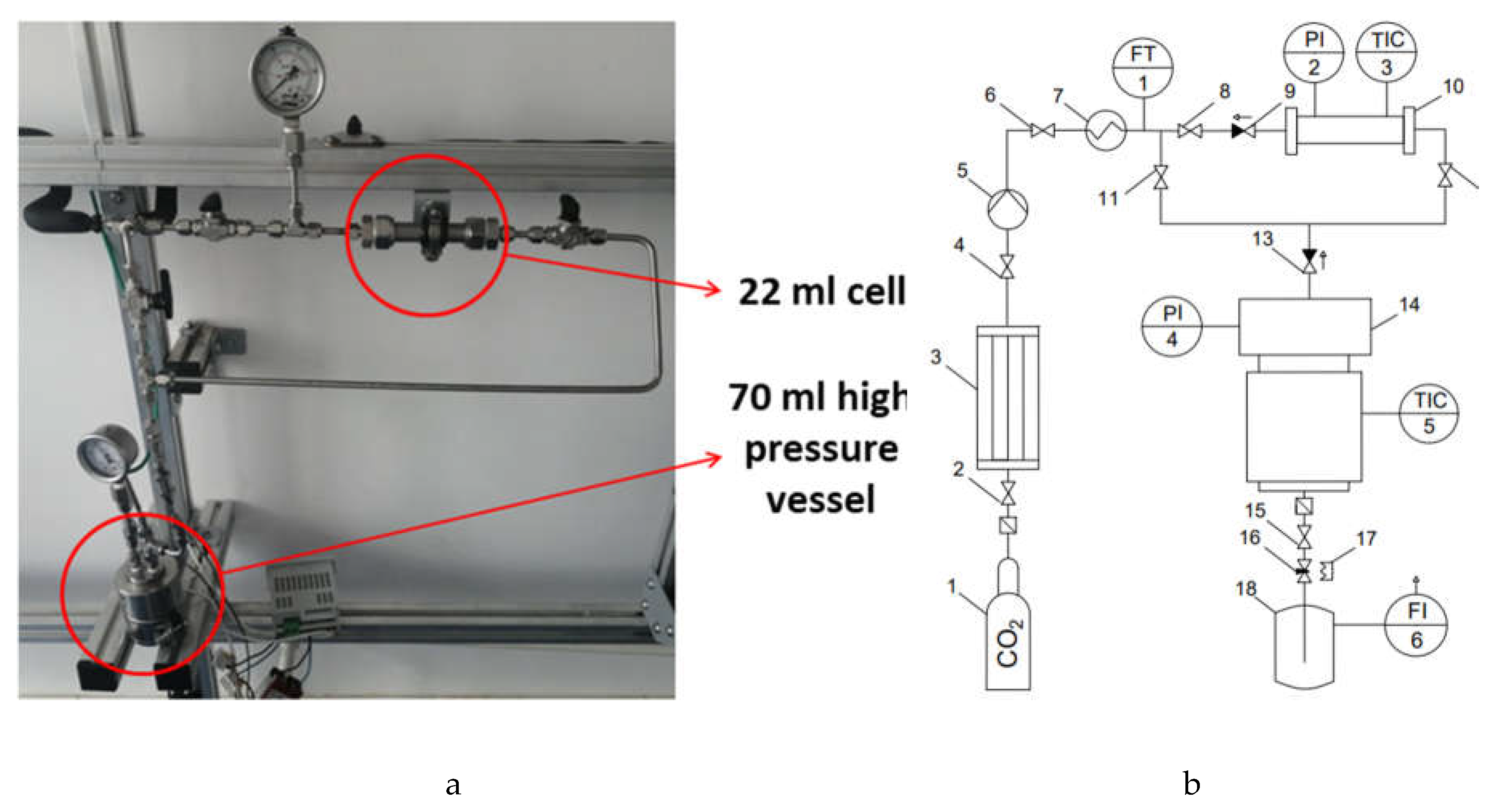
2.3. Integrated Supercritical Drying and Sterilization Processes
2.4. Analytical Study of Hybryd Aerogel Based on Polyelectrolyte Complex
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods of “Ink” and Hetrophsae Sysytem Preparation
4.2.1. 3D Printing Process Utilizing Heterophase System
4.2.2. Direct Ink Writing (DIW)
4.2.3. Heterophase System Preparation
4.3. Implementation of 3D Printing Process
4.4. Integrated Supercritical Drying and Sterilization Processes
4.5. Analytical Study
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smirnova I., Gurikov P. Aerogel production: Current status, research directions, and future opportunities // The Journal of Supercritical Fluids. ‒ 2018. ‒ Vol. 134. ‒ Pp. 228-233.
- Zhao S., Malfait W. J., Guerrero-Alburquerque N., Koebel M. M., Nyström G. Biopolymer aerogels and foams: Chemistry, properties, and applications // Angewandte Chemie International Edition. ‒ 2018. ‒ Vol. 57, 26. ‒ Pp. 7580-7608.
- Ambrosi A., Pumera M. 3D-printing technologies for electrochemical applications // Chemical Society Reviews. ‒ 2016. ‒ Vol. 45, 10. ‒ Pp. 2740-2755.
- Maleki H., Hüsing N. Current status, opportunities and challenges in catalytic and photocatalytic applications of aerogels: Environmental protection aspects // Applied Catalysis B: Environmental. ‒ 2018. ‒ Vol. 221. ‒ Pp. 530-555.
- Shaari N., Kamarudin S. K. Current status, opportunities, and challenges in fuel cell catalytic application of aerogels // International Journal of Energy Research. ‒ 2019. ‒ Vol. 43, 7. ‒ Pp. 2447-2467.
- Shin J. H., Heo J.-H., Jeon S., Park J. H., Kim S., Kang H.-W. Bio-inspired hollow PDMS sponge for enhanced oil–water separation // Journal of hazardous materials. ‒ 2019. ‒ Vol. 365. ‒ Pp. 494-501.
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review // Chemical Engineering Journal. ‒ 2016. ‒ Vol. 300. ‒ Pp. 98-118.
- Xu X., Zhang Q., Hao M., Hu Y., Lin Z., Peng L., Wang T., Ren X., Wang C., Zhao Z. Double-negative-index ceramic aerogels for thermal superinsulation // Science. ‒ 2019. ‒ Vol. 363, 6428. ‒ Pp. 723-727.
- Muñoz-Ruíz, A. Synthesis and Characterization of a New Collagen-Alginate Aerogel for Tissue Engineering / Muñoz-Ruíz A., Escobar-García D.M., Quintana M., Pozos-Guillén A., Flores H. // Journal of Nanomaterials. – 2019. – Т. 2019. [CrossRef]
- Obaidat, R.M. Drying Using Supercritical Fluid Technology as a Potential Method for Preparation of Chitosan Aerogel Microparticles / Obaidat R.M., Tashtoush B.M., Bayan M.F., T. Al Bustami R., Alnaief M. // AAPS PharmSciTech. – 2015. – Т. 16, № 6. – С. 1235–1244. [CrossRef]
- Santos-López, G. Aerogels from Chitosan Solutions in Ionic Liquids / Santos-López G., Argüelles-Monal W., Carvajal-Millan E., López-Franco Y.L., Recillas-Mota M.T., Lizardi-Mendoza J. // Polymers. – 2017. – Т. 9, № 12. – С. 722. [CrossRef]
- Mecwan, M. Recent advances in biopolymer-based hemostatic materials / Mecwan M., Li J., Falcone N., Ermis M., Torres E [и др.] // Regenerative Biomaterials. – 2022. – Т. 9. [CrossRef]
- Kushwaha, R. Biopolymers as Topical Haemostatic Agents: Current Trends and Technologies / Kushwaha R., Sharma S., Kumar S., Kumar A. // Materials Chemistry Horizons. – 2023. – Т. 2, № 1. – С. 11–39. [CrossRef]
- Lovskaya, D.D. Aerogels as drug delivery systems: In vitro and in vivo evaluations / Lovskaya D.D., Lebedev A.E., Menshutina N. V. // The Journal of Supercritical Fluids. – 2015. –Т. 106. – С. 115–121. [CrossRef]
- Okutucu, B. The medical applications of biobased aerogels: ‘Natural aerogels for medical usage’ / Okutucu B. // Medical Devices & Sensors. – 2021. – Т. 4, № 1. – С. e10168. [CrossRef]
- Singh, A.K. Fabrication and investigation of physicochemical and biological properties of 3D printed sodium alginate-chitosan blend polyelectrolyte complex scaffold for bone tissue engineering application / Singh A.K., Pramanik K. // Journal of Applied Polymer Science. – 2023. – Т. 140, № 12. – С. e53642. [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications / Rinaudo M. // Progress in Polymer Science. – 2006. – Т. 31, № 7. – С. 603–632. [CrossRef]
- Wegrzynowska-Drzymalska, K. Crosslinking of Chitosan with Dialdehyde Chitosan as a New Approach for Biomedical Applications / Wegrzynowska-Drzymalska K., Grebicka P., Mlynarczyk D.T. [и др.] // Materials. – 2020. – Т. 13, № 15. – С. 3413. [CrossRef]
- Gabriele, F. Ionic and covalent crosslinking in chitosan-succinic acid membranes: Effect on physicochemical properties / Gabriele F., Donnadio A., Casciola M., Germani R., Spreti N // Carbohydrate Polymers. – 2021. – Т. 251. – С. 117106. [CrossRef]
- Kulig, D. Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio / Kulig D., Zimoch-Korzycka A., Jarmoluk A., Marycz K. // Polymers 2016, Vol. 8, Page 167. – 2016. – Т. 8, № 5. – С. 167. [CrossRef]
- Gierszewska, M. pH-responsive chitosan/alginate polyelectrolyte complex membranes reinforced by tripolyphosphate / Gierszewska M., Ostrowska-Czubenko J., Chrzanowska E. // European Polymer Journal. – 2018. – Т. 101. – С. 282–290. [CrossRef]
- Li, Z. Chitosan–alginate hybrid scaffolds for bone tissue engineering / Li Z., Ramay H.R., Hauch K.D., Xiao D., Zhang M. // Biomaterials. – 2005. – Т. 26, № 18. – С. 3919–3928. [CrossRef]
- Morozov AM, Morozova AD, Belyak MA, Zamana YuA, Zhukov SV Infections associated with the provision of health care. A modern view of the problem // Bulletin of new medical technologies. 2022. No. 4. P. 107-116.
- Garmanov S. Yu., Nurislamova GR, Fatkhullin RR, Goryunova SM Cross-contamination in chemical and pharmaceutical production: problems of standardization and unification of requirements // Actual problems of our time. 2006. No. 6. P. 294-305.
- Fedotov AE Production of sterile drugs // Pharmaceutical technologies and packaging. 2014. No. 9 (247). P. 38-41.
- Burak, L.Ch. Modern methods of preservation used in the food industry // The scientific her-itage. 2022. No. 89. P. 106-124.
- Tapal’skiy D.V., Osipov V.A., Sukhaya G.N., Yarmoolenko M.A., Rogachev A.A., Rogachev A.V. Biocompatible composite antibacterial coatings for protecting implants from microbial biofilms // Problems of health and ecology. 2013. Vol. 36. No. 2. P. 129-134.
- Nemoikina A.L., Babkina O.V., Alekseenko K.V., Vaitulevich E.A. Study of the influence of ethylene oxide sterilization modes on the properties of glycolide-lactide threads // Bulletin of Tomsk State University. 2014. No. 382. P. 230-233.
- Vorobyov V., Bozhkova S.A., Tikhilov R.M., Cherny A.Zh. Modern methods of processing and sterilization of allogenic bone tissues (literature review) // Traumatology and Orthopedics of Russia. 2017. Vol. 23. No. 3. P. 134-147.
- Hossain Md.S., Nik Norulaini №.A., Banana A.A., Mohd Zulkhairi A.R., Ahmad Naim A.Y., Mohd Omar A.K. Modeling the supercritical carbon dioxide inactivation of Staphylococcus aureus, Escherichia coli and Bacillus subtilis in human body fluids clinical waste // Chemical Engineering Journal. 2016. V. 296. P. 173-181.
- Furukawa S., Watanabe T., Koyama T., Hirata J., Narisawa №., Ogihara H., Yamasaki M. Inactivation of food poisoning bacteria and Geobacillus stearothermophilus spores by high pressure carbon dioxide treatment // Food Control. 2009. V. 20. № 1. P. 53-58.
- Garcia-Gonzalez L., Geeraerd A.H., Spilimbergo S., Elst K., Van Ginneken L., Debevere J., Van Impe J.F., Devlieghere F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future // International Journal of Food Microbiology. 2007. V. 117. № 1. P. 1–28.
- Tomasko D.L., Li H., Liu D., Han X., Wingert M.J., Lee L.J., Koelling K.W. A Review of CO2 Applications in the Processing of Polymers // Industrial & Engineering Chemistry Research. 2003. V. 42. № 25. P. 6431-6456.
- Menshutina, N.; Abramov, A.; Tsygankov, P.; Lovskaya, D. Extrusion-based 3D printing for highly porous alginate materials production. Gels 2021, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Menshutina, N.; Abramov, A.; Okisheva, M.; Tsygankov, P. Investigation of the 3D Printing Process Utilizing a Heterophase System. Gels 2023, 9, 566. [Google Scholar] [CrossRef]
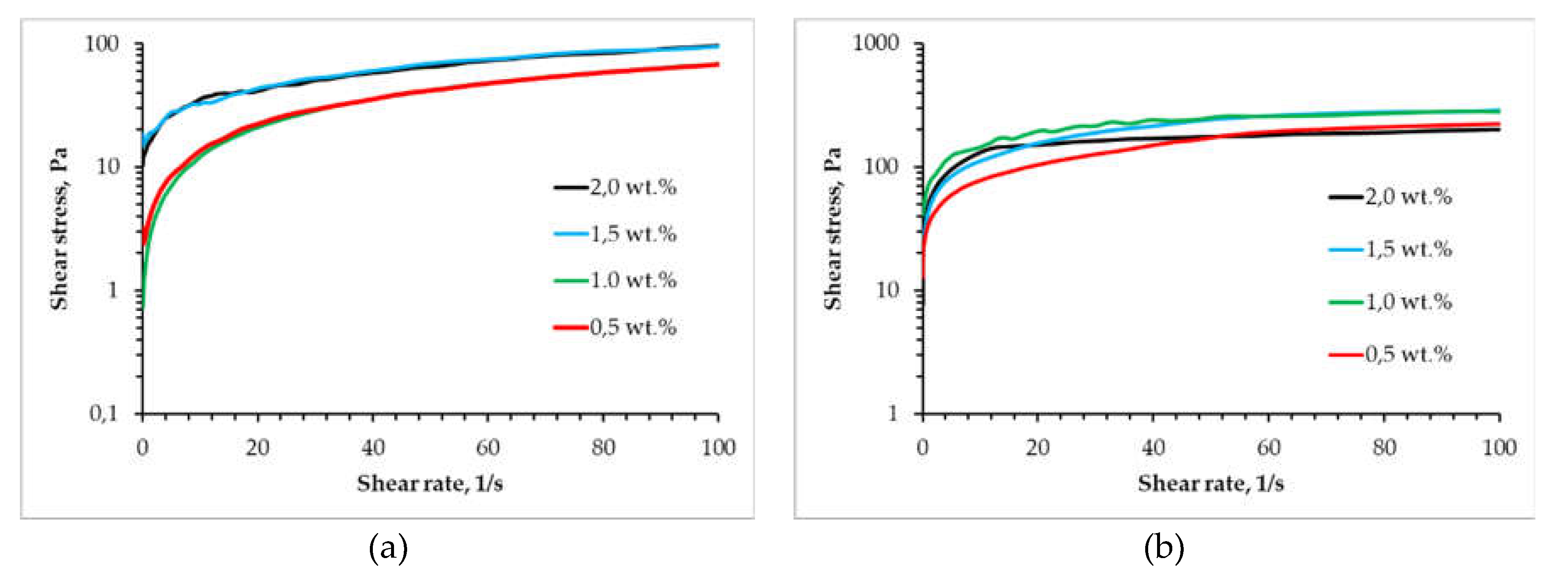
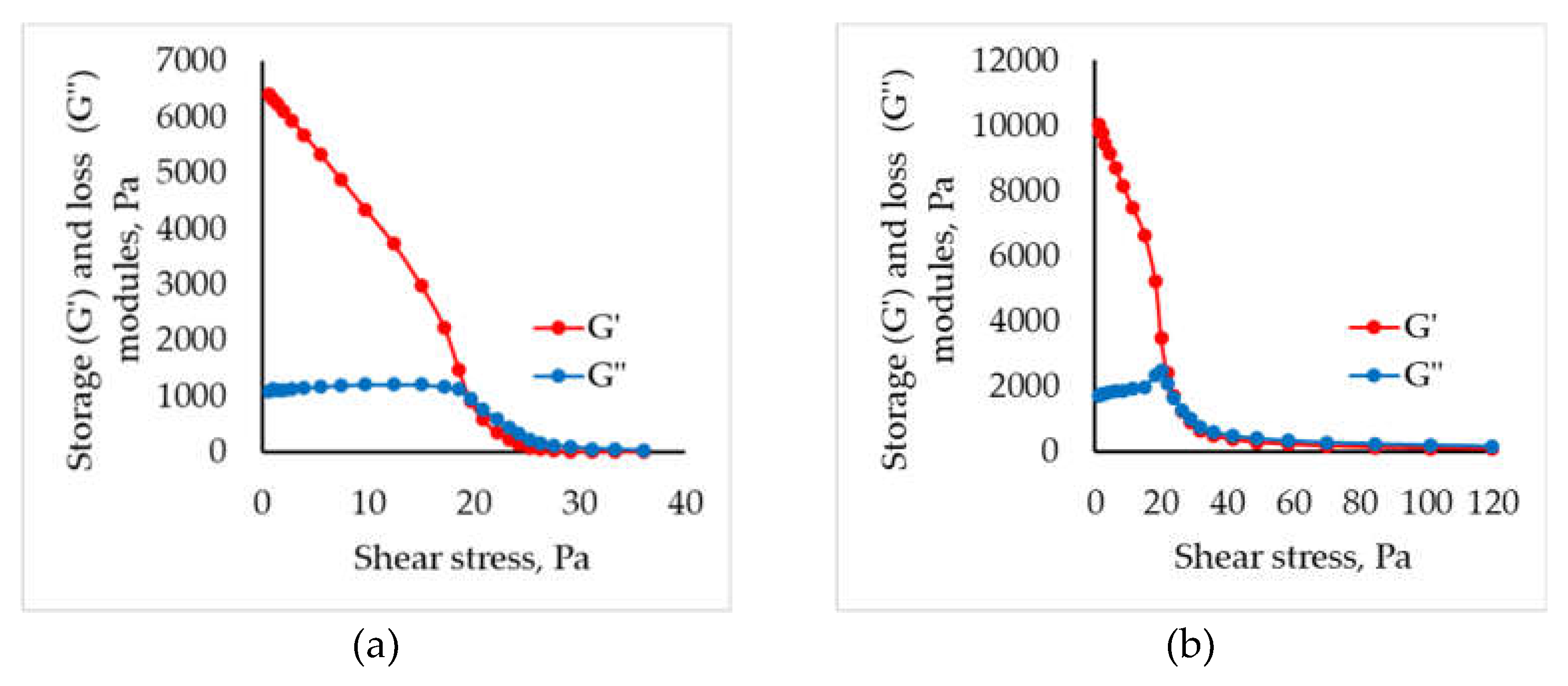
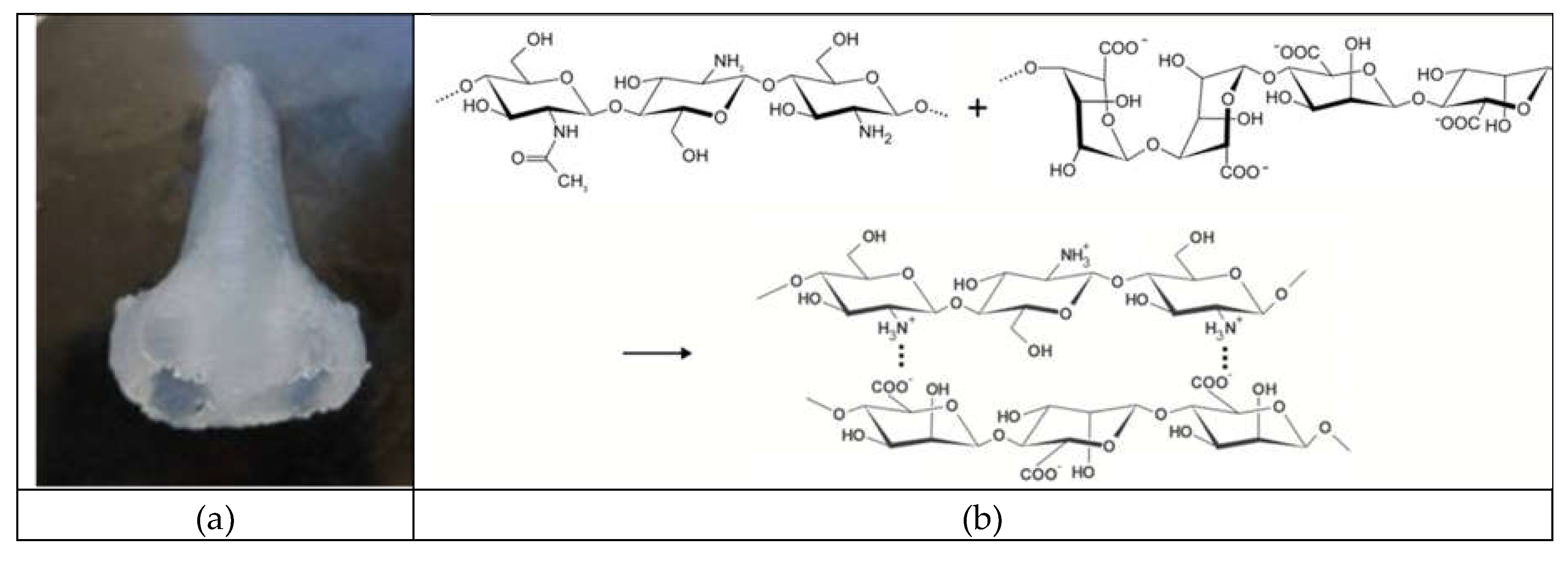
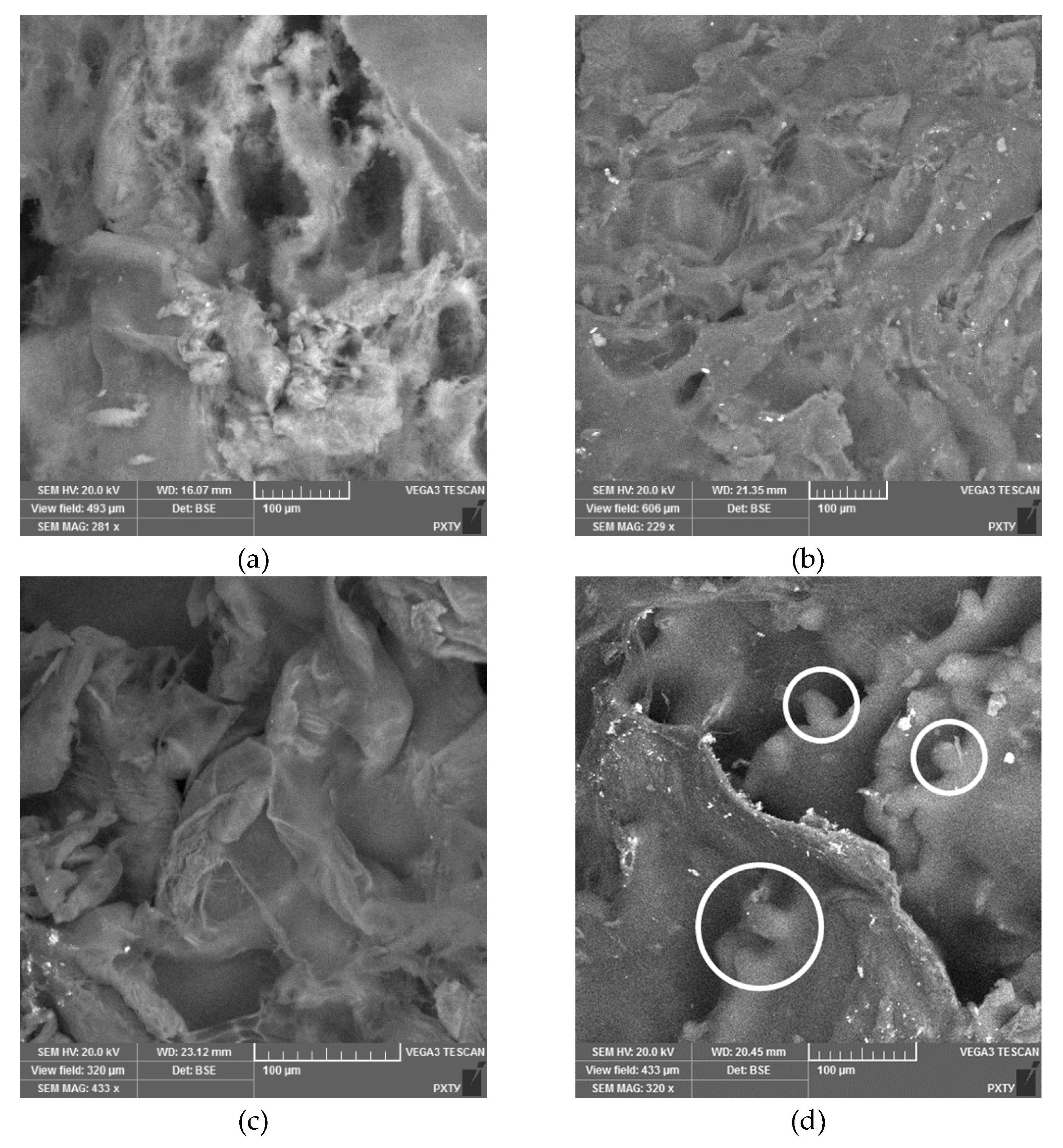
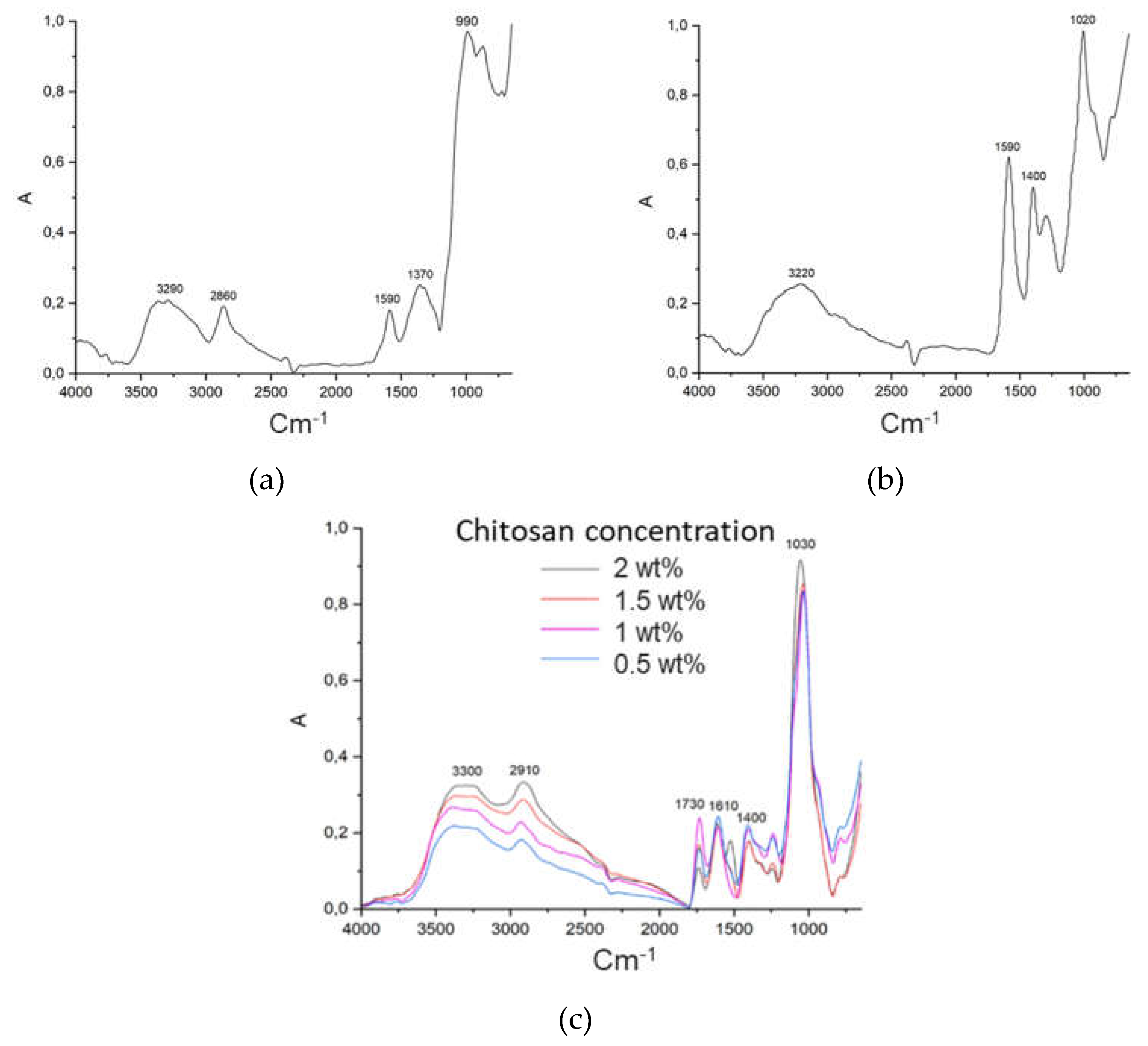
| Concentration of chitosan, wt.% | Viscosity, Pa ⋅ s | |
| Alginate | Partly cross-linked alginate | |
| 0,0 | 0,8 | 1032,4 |
| 0,5 | 82,2 | 1446,0 |
| 1,0 | 318,7 | 1578,2 |
| 1,5 | 1591,3 | 1754,5 |
| 2,0 | 1800,0 | 7970,0 |
| Concentration of chitosan, wt.% | Thixotropy hysteresis loops | |
| Alginate | Partly cross-linked alginate | |
| 0,0 | 9,5 | 2374,2 |
| 0,5 | 29,9 | 2405,8 |
| 1,0 | 40,2 | 2416,1 |
| 1,5 | 105,8 | 2887,9 |
| 2,0 | 659,3 | 2967,8 |
| Concentration of chitosan, wt.% | Shear stresses | |
| Alginate | Partly cross-linked alginate | |
| 0,0 | 0 | 77 |
| 0,5 | 0,8 | 42,8 |
| 1,0 | 1,8 | 32,3 |
| 1,5 | 19,7 | 26,3 |
| 2,0 | 52,8 | 12,2 |
| Concentration of chitosan, wt.% | ρист, g/cm3 | ρкаж, g/cm3 | Porosity, % | SBET, m2/g | VBDH, cm3/g |
| 0,5 | 1.684 | 0.090 | 95 | 238 | 1.23 |
| 1,0 | 1.853 | 0.099 | 95 | 199 | 0.59 |
| 1,5 | 1.884 | 0.076 | 96 | 153 | 0.64 |
| 2,0 | 1.982 | 0.062 | 97 | 108 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





