1. Introduction
With the increasing prevalence of low-dose computed tomography (LDCT) for lung cancer screening, the detection of pulmonary nodules has significantly risen [
1]. Among these, deep pulmonary nodules represent a unique challenge in video-assisted thoracoscopic surgeries (VATS) due to their small size, ground-glass matrix appearance, and deep location relative to the pleural surface [
2]. Accurate localization of these nodules prior to surgery is essential to ensure successful resection while minimizing the need for open thoracotomy, which is associated with increased morbidity [
3]. Moreover, this challenge is further compounded for nodules situated deeply in the central thorax, where conventional localization methods often prove inadequate.
Several techniques have been developed for preoperative localization of pulmonary nodules. These include CT-guided hook-wire placement, microcoil localization, and injection of dyes, barium, or radiotracer [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16]. While hook-wire techniques are widely adopted, they are associated with patient discomfort and a risk of dislodgement . They also have “blind areas” in deep thoracic locations, such as the mediastinum vicinity, areas near the interlobar fissures, and scapula-shadowed regions [
3,
17]. Microcoil placement has emerged as a safer alternative for these scenarios, but its reliance on intraoperative fluoroscopy adds complexity and radiation exposure. Disadvantages such as coil migration and air embolism have also been reported [
3,
6,
7]. Dye injection techniques are simpler and cost-effective but have historically been limited by dye diffusion issues, particularly with methylene blue [
3,
8,
9,
13].
Patent blue dye (PBD) offers a potential solution to these challenges. Its precise marking capabilities, even in deep-seated nodules, and its long staining effect make it a promising tool for localization [
12,
18]. However, achieving successful localization for deeply situated nodules requires careful consideration of factors such as needle depth, which may be associated with complications like pneumothorax and pulmonary hemorrhage [
19]. These complications are likely related to the extent of lung parenchyma penetration, emphasizing the need for strategic planning of the needle pathway. Additionally, Tsai et al. highlight the importance of combining superficial marking and deep marking for successful localization of deeply situated pulmonary nodules [
20]. Despite these advancements, the utility of patent blue injection specifically for deep thoracic nodules remains under-researched.
To address this gap, we categorized four scenarios based on anatomical locations, applied corresponding needle approach paths, and injected the dye from the nodule to the subpleural area. This retrospective study aims to evaluate the efficacy and safety of these methods.
2. Materials and Methods
This study was approved by the Institutional Review Board for human research, with a waiver of informed consent. During the period between November 2015 and May 2023, 50 consecutive patients who underwent CT-guided localization with the injection of PBD were enrolled, all of whom had pulmonary nodules located deep within the central thorax. The definition of "deep pulmonary nodules of the thorax" and their categorization according to the nodule localization relative to the thoracic organs and the visceral pleura, which required different needle approach pathways, were as follows:
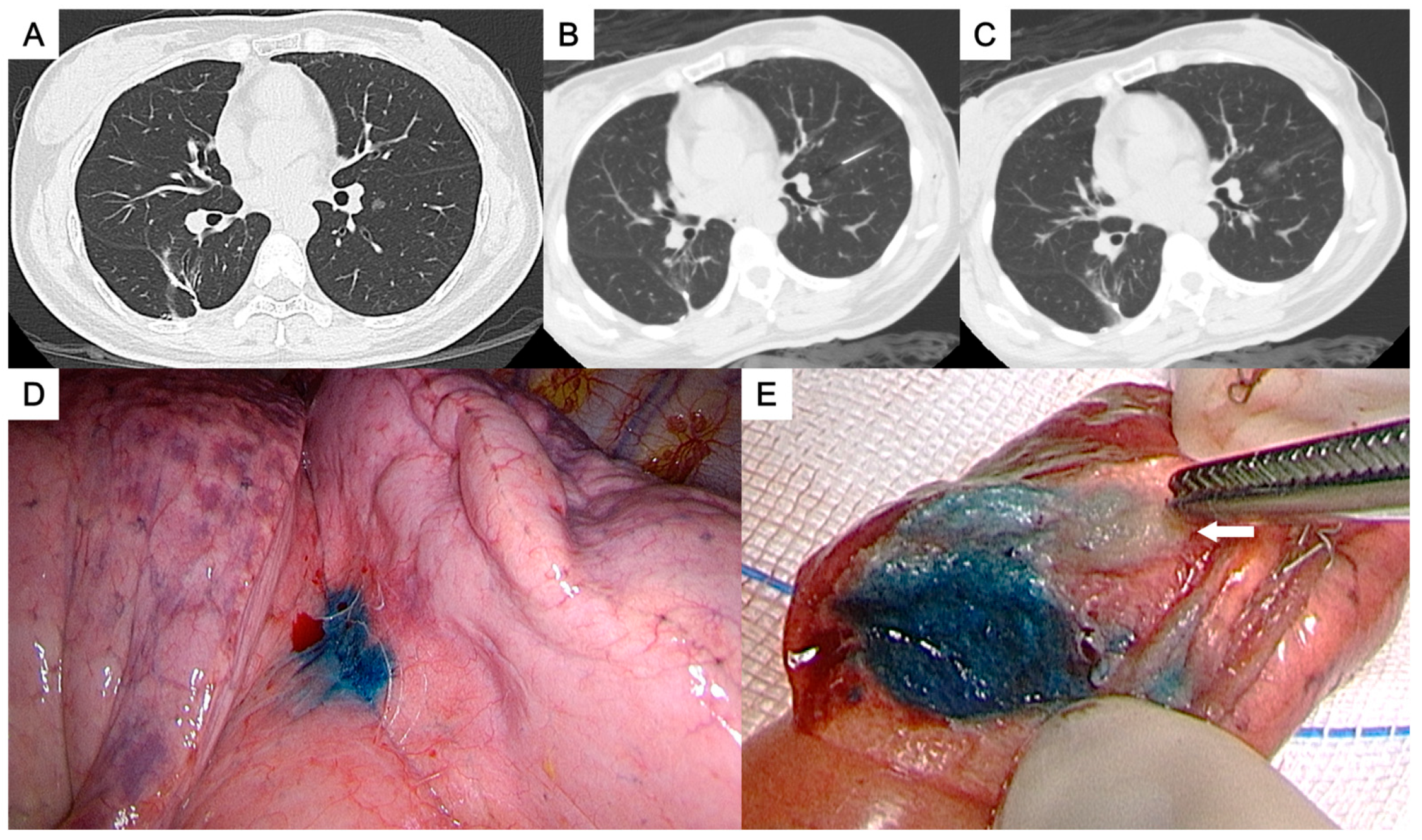
Perifissural nodules: nodules close to the interlobar fissure (
Figure 1 and
Figure 2) ;
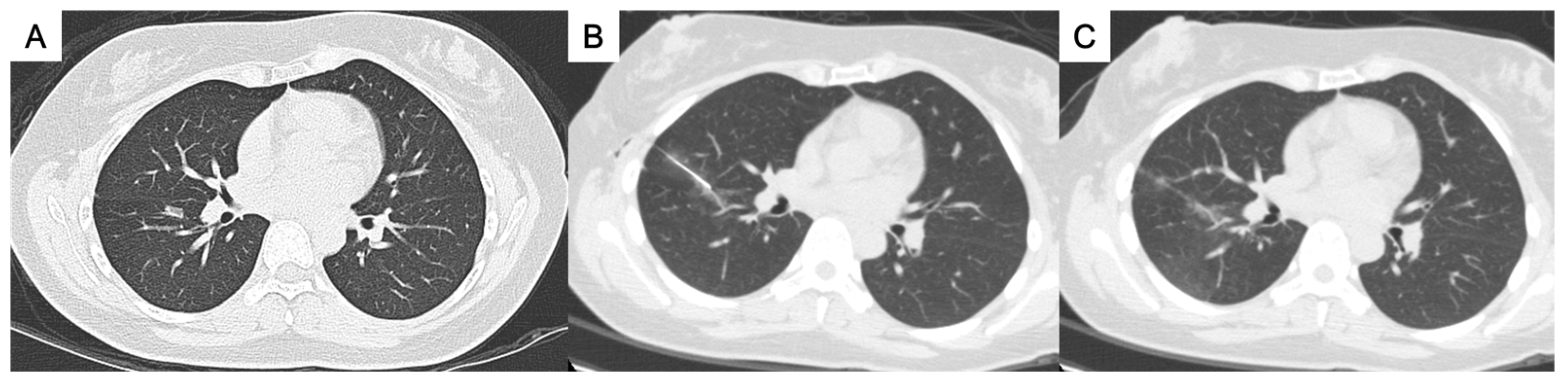
Paravertebral nodules: nodules located in the paravertebral region (
Figure 3) ;
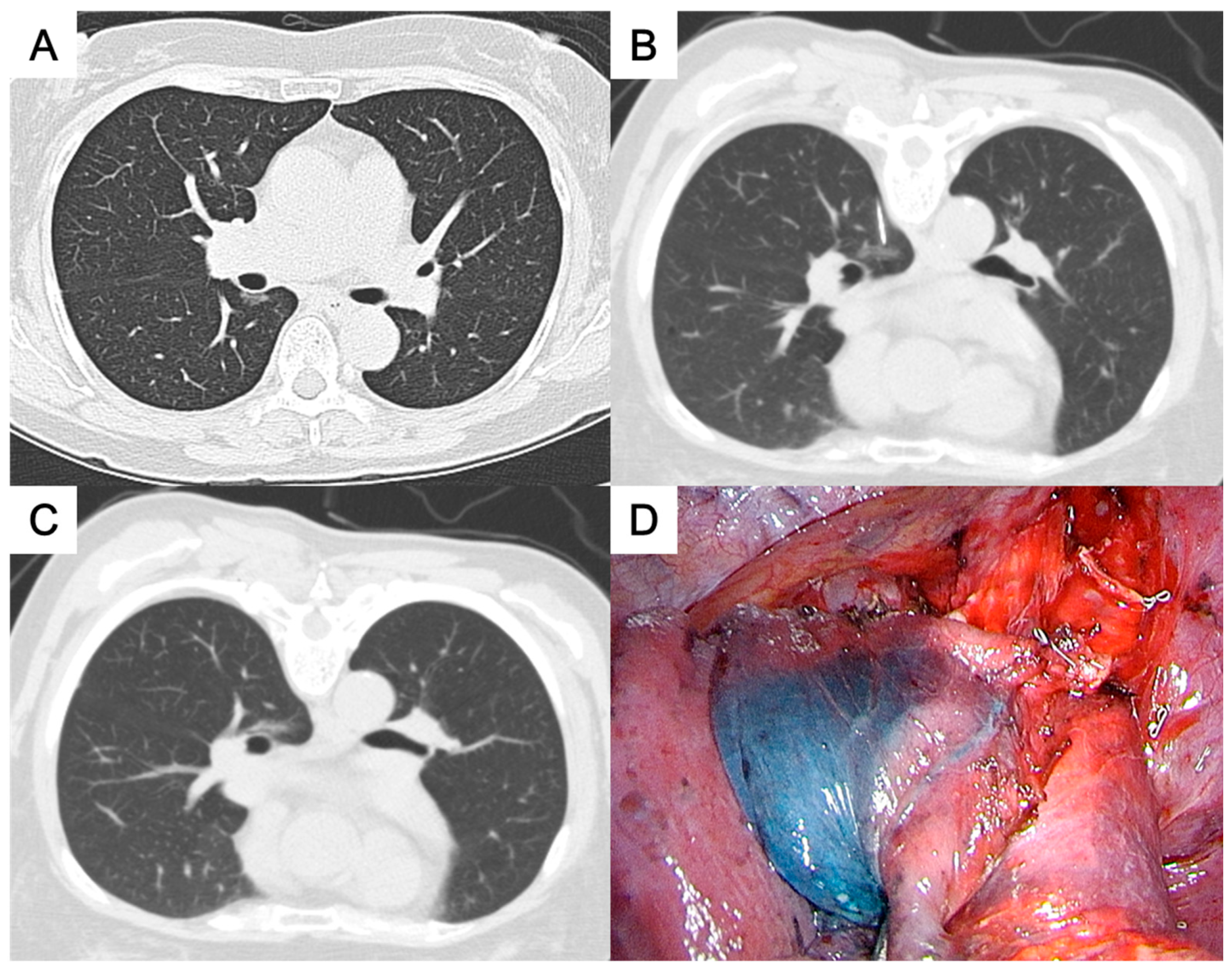
Paramediastinal nodules: nodules located close to the mediastinum (
Figure 4) ;
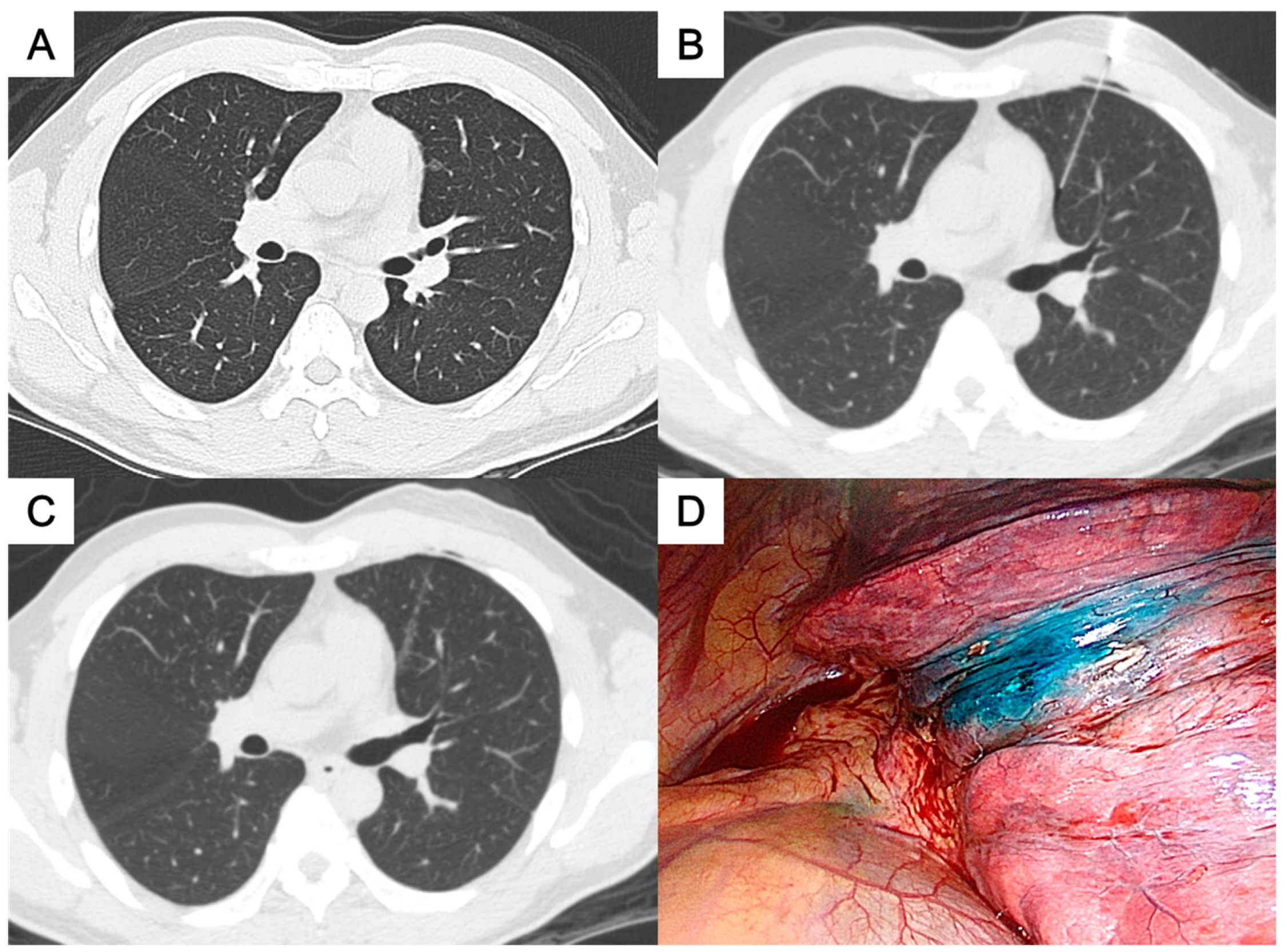
Deep parenchymal nodules: nodules situated in the center of a pulmonary lobe distant from any visceral pleura (
Figure 5).
The criteria for preoperative localization were as follows: (1) nodules measuring less than 3 cm in diameter, (2) nodules not in direct contact with the visceral pleura, and (3) nodules deemed challenging for intraoperative identification by the surgeons. Surgical intervention was indicated for nodules with increasing size or a growing solid part. Additionally, some patients with stable subsolid nodules (solid part <5 mm) opted for surgery due to concerns about malignancy. For each patient, clinical parameters were recorded, including age, sex, smoking status, body mass index, and the type of surgical procedure. CT findings for each nodule were analyzed, including (1) nodule size, (2) pulmonary lobe involvement, and (3) attenuation. Several technical aspects of the localization procedure were also recorded, such as the (1) nodule localization category, (2) nodule depth (the distance between the nodule and the nearest pleura), (3) the needle pathway length (from the skin insertion to the tip of the needle), (4) the distance between the nodule and the dye, and (5) the procedure duration. The time interval between localization and surgery, along with any post-localization complications, was recorded. Complications included pneumothorax (asymptomatic or symptomatic requiring preoperative intervention), parenchymal hemorrhage, hemothorax, hemoptysis, and allergic reactions to PBD. All CT scans were independently reviewed by two chest radiologists (H.H.H. and K.H.K., with 32 and 17 years of experience). Discrepancies were resolved through discussion.
2.1. CT-Guided PBD Localization Procedure
One experienced chest radiologist (K.H.K.) conducted all the localization procedures. Detailed information regarding the main parameter settings for the CT device [64-detector row scanner (Brilliance; Philips Medical Systems, Cleveland, OH, USA)] and the CT-guided PBD (patent blue V 2.5%; Guerbet, Aulnay-sous-Bois, France) injection had been described in our previous study [
18]. The needle approach way for each categories of deep thoracic nodules were as following:
For the perifissural nodules (
Figure 1), we introduced the needle by the shortest way from the chest wall to the nodule and injected the PBD in the subpleural area. There is an alternative transfissural approach (
Figure 2) to avoid pulmonary vessels, scapula, and ribs, with the dye retention in the subpleural area.
For the paravertebral nodules (
Figure 3), we introduced the needle by paraspinal approach from posterior chest wall with the tip placed within 1 cm to the nodule. PBD was then injected while withdraw the needle, leaving the dye retention in the needle pathway till the subpleural area.
For the paramediastinal nodules (
Figure 4), we approached the nodule by the shortest way from the chest wall to the nodule, with the needle tip placed within 1 cm and relative medial side to the nodule. We then injected the PBD with the dye retention in the subpleural area.
For the deep parenchymal nodules (
Figure 5), the needle pathway was made by the shortest way from the chest wall to the nodule, while avoiding bony structures. After confirming the needle tip placed within 1 cm to the nodule, the PBD was injected while needle retraction until the subpleural area. The dye was then deposit in the needle pathway.
Following localization, patients were sent back to the ward while awaiting transfer to the operating room. The procedure duration was measured from the first to the last CT scan.
2.2. Video-Assisted Thoracoscopic Surgery (VATS ) After Localization
VATS was performed on the same day or the following day after CT-guided preoperative localization, depending on the surgeon’s schedule and operating room availability. Successful localization was defined as the presence of a dyed area on the visceral pleura, facilitating wedge resection with complete nodule removal and negative pathological margins. The resected specimens were sent for frozen section analysis. Limited wedge resection or segmentectomy was performed for benign lesions, particularly in patients with limited cardiopulmonary reserve or for nodules smaller than 2 cm with a predominant ground-glass matrix appearance [
21]. In cases of malignancy, anatomic resection and mediastinal lymph node dissection were performed. Surgical approaches were adjusted based on the surgeon's judgment and the intraoperative circumstances. Technical failure was defined as the lack of visible dye marking on the visceral pleura.
2.3. Statistical Analysis
Categorical variables are reported as numbers and percentages, while numerical variables are presented as means or medians with ranges. The chi-square test was used for categorical variables, and the Mann–Whitney test was applied for non-normally distributed continuous variables. Multivariate logistic regression analysis was performed to identify risk factors for post-localization pneumothorax. A p-value <0.05 was considered statistically significant. All analyses were conducted using SPSS software (version 21.0; SPSS, Chicago, IL, USA).
3. Results
3.1. Clinical and Radiological Features
The characteristics of the 50 enrolled patients with 50 indeterminate pulmonary nodules are summarized in
Table 1. The patients were predominantly middle aged (mean=57.4 years) female (n=39; 78.0%) non-smokers (n=39; 78.0%) with normal range BMI (mean=23). The mean size of the nodules was 10.3 mm (range, 4.7–21.0), and most were pure ground-glass appearance (n=40; 80.0%).
3.2. Localization Parameters, Complications, and Surgical Results
The localization parameters, associated complications, and the surgical results are shown in
Table 2. The nodules could be categorized as (1) perifissural nodules (n=13; 26.0%), (2) paravertebral nodules (n=20, 40.0%), (3) paramediastinal nodules (n=4; 8.0%), and (4) deep parenchymal nodules (n=13, 26.0%). The mean distance between the nodule and the nearest pleural surface was 16.1 mm (range, 0.1–52.2 mm). The mean needle pathway from the chest wall was 7.7 cm (range, 4.5–11.7 cm). Among the 13 perifissural nodules, 11 were approached using the transfissural path, while the remaining 2 were localized via an alternative path to avoid pulmonary vessels. The median of the distance between the nodule and the dye, measured by the CT scan after dye injection, was 0.6 mm (mean, 1.6 mm; range: 0–13.5 mm). The mean procedure time was 16 minutes (range, 8–26). The median of time interval from dye injection to the operation was 139 minutes (mean, 270 ; range, 57–2641). A longer time interval was due to changed operation schedule. All 50 dyes could be identified on the pleural surface by thoracoscopy and were resected mostly by wedge resection (n=25, 50.0%).
For the localization related complications, asymptomatic pneumothorax developed in 24 patients (48.0%), and focal parenchymal hemorrhage was identified in 4 patients (8.0%). Multivariate logistic regression analysis identified no specific risk factors for procedure-related pneumothorax (
Table 3).
3.3. Pathological Reports
The majority of nodules (n=48, 96.0%) were malignancies, including invasive adenocarcinoma, minimally invasive adenocarcinoma (MIA), adenocarcinoma in situ (AIS), Squamous cell carcinoma, and atypical adenomatous hyperplasia (AAH) (
Table 4). All surgical margins were negative for tumor involvement.
4. Discussion
This retrospective study aimed to assess the efficacy and safety of CT-guided patent blue dye injection for localizing deep pulmonary nodules in the thorax. Our focus was on developing effective needle path planning to ensure accurate marking on the visceral pleura while minimizing complications, such as pneumothorax and pulmonary hemorrhage, which may be linked to excess lung parenchyma penetration. By categorizing nodules into four anatomical scenarios—(1) perifissural, (2) paravertebral, (3) paramediastinal, and (4) deep parenchymal—and adopting tailored needle approaches, the results showed a high success rate (100%), a relatively short procedure time (mean: 16 minutes), and minimal complications.
Regarding marking deep central thoracic locations, two core objectives must be achieved: creating a visible marking on the nearest visceral pleura and accurately localizing the nodule within the parenchyma to guide surgeons in obtaining an adequate surgical margin while preserving functional lung tissue. For perifissural nodules, dye injection in the subpleural area near the nodule, with or without transfissural puncture, provides a precise stained area on the fissure. For paravertebral nodules, a paraspinal approach allows the shortest path to the nodule in the prone position, with dye injected along the needle tract to create a clear marking on the posterior visceral pleura. Similarly, for paramediastinal nodules, dye injection into the subpleural area closest to the mediastinum provides precise marking relative to the mediastinal-side visceral pleura. For deep parenchymal nodules, dye is injected along the needle path to create a blue tract extending to the subpleural area, enabling visible marking on the nearest visceral pleura while indicating the lesion’s location within the parenchyma. In all cases, the needle tip was placed within 1.0 cm of the nodule, ensuring effective dye diffusion to the lesion site, resulting in a close distance between the nodule and the dye (median: 0.6 mm; mean: 1.6 mm; Range: 0–13.5 mm). The results demonstrate the reliability of this core concept, aligning with prior results on deep parenchymal nodules by Tsai et al.[
20].
In terms of complications, pneumothorax and parenchymal hemorrhage are the most common. In our study, pneumothorax occurred in 48% of patients, all of which were asymptomatic and required no further intervention. This rate is slightly higher than that reported by Lin et al., who observed pneumothorax in 29.4% of patients undergoing CT-guided patent blue dye (PBD) localization [
12], and Tsai et al., who reported a pneumothorax rate of 37.0% specifically in cases involving deep pulmonary parenchymal nodules [
20]. This discrepancy may be attributable to our focus on deep thoracic nodules, which required a deeper needle pathway (mean 7.7 cm; range 4.5–11.7 cm) and included transfissural punctures in 22% of cases. Parenchymal hemorrhage was noted in 8% of our cases, all of which were focal and self-limiting. Notably, none of our patients experienced severe complications, such as hemothorax, air embolism, or allergic reactions to PBD. These findings are consistent with those of Lin et al. and other studies[
12,
20], suggesting that PBD is a safe option for localization. Our results indicate that with careful planning and technique, PBD localization can be performed with a favorable safety profile, even in challenging cases involving deep thoracic nodules.
When comparing localization techniques for deep thoracic nodules, the CT-guided patent blue dye (PBD) injection method demonstrates several practical advantages over hook-wire, microcoil, and dual localization methods. Hook-wire localization, while effective for peripheral nodules, is often associated with dislodgement, patient discomfort, and limited utility in challenging areas such as the mediastinum or interlobar fissures [
17]. Microcoil localization provides a reliable depth marker but requires intraoperative fluoroscopy, introducing additional procedural complexity and radiation exposure. Dual localization techniques, combining microcoils and PBD [
22], offer both surface and depth marking but involve longer procedural times (mean: 39 minutes), which may not be feasible in all settings. The patent blue dye injection, combined with a tailored needle approach strategy, offers a simple and straightforward protocol. While our approach does not provide specific markers for deep safe margins as some dual localization techniques do, this study indicates that it provides sufficient information on nodule location and depth for surgeons to achieve complete resection.
This study has several limitations. It is a single-center retrospective analysis with a relatively small sample size, which may limit the generalizability of our findings. Although our method effectively localized deep thoracic nodules, its applicability to cases involving multiple nodules or nodules located outside the four anatomical scenarios warrants further investigation. Future research with larger, multi-center cohorts is needed to validate these results and explore the potential for broader applications.
5. Conclusions
The findings of this study demonstrated that the four categorized needle approach paths enabled precise and reliable PBD localization of deep thoracic pulmonary nodules for VATS surgery with minimal complications. By providing a simple and effective framework, this method may serve as a valuable reference for less experienced operators, offering practical guidance to enhance confidence and consistency in such challenging cases.
Author Contributions
K.-H.K. conceptualized, designed, and supervised the study, and critically revised the manuscript. C.-H.L. collected and analyzed the data, performed the statistical analysis, and drafted the manuscript. T.-W.H., H.-H.H., and W.-C.T. contributed to data acquisition and interpretation, and supervised the writing and editing of the manuscript. All authors have read and approved the final version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Tri-Service General Hospital (approval number: C202405198 and date of approval: 2024/12/18).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study, which was conducted using de-identified data derived from standard clinical records without any direct patient contact or intervention.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CT |
Computer Tomography |
| PBD |
Patent Blue Dye |
| VATS |
Video-Assisted Thoracoscopic Surgery |
| BMI |
Body Mass Index |
| RUL |
Right Upper Lobe |
| RML |
Right Middle Lobe |
| RLL |
Right Lower Lobe |
| LUL |
Left Upper Lobe |
| LLL |
Left Lower Lobe |
| MIA |
Minimally Invasive Adenocarcinoma |
| AIS |
Adenocarcinoma In Situ |
| AAH |
Atypical Adenomatous Hyperplasia |
References
- Krochmal, R.; Arias, S.; Yarmus, L.; Feller-Kopman, D.; Lee, H. Diagnosis and management of pulmonary nodules. Expert Rev Respir Med 2014, 8, 677-691. [CrossRef]
- Suzuki, K.; Nagai, K.; Yoshida, J.; Ohmatsu, H.; Takahashi, K.; Nishimura, M.; Nishiwaki, Y. Video-Assisted Thoracoscopic Surgery for Small Indeterminate Pulmonary Nodules. Chest 1999, 115, 563-568. [CrossRef]
- McDermott, S.; Fintelmann, F.J.; Bierhals, A.J.; Silin, D.D.; Price, M.C.; Ott, H.C.; Shepard, J.-A.O.; Mayo, J.R.; Sharma, A. Image-guided Preoperative Localization of Pulmonary Nodules for Video-assisted and Robotically Assisted Surgery. RadioGraphics 2019, 39, 1264-1279. [CrossRef]
- Ichinose, J.; Kohno, T.; Fujimori, S.; Harano, T.; Suzuki, S. Efficacy and Complications of Computed Tomography-Guided Hook Wire Localization. The Annals of Thoracic Surgery 2013, 96, 1203-1208. [CrossRef]
- Hsu, H.-H.; Shen, C.-H.; Tsai, W.-C.; Ko, K.-H.; Lee, S.-C.; Chang, H.; Huang, T.-W. Localization of nonpalpable pulmonary nodules using CT-guided needle puncture. World Journal of Surgical Oncology 2015, 13. [CrossRef]
- Lizza, N.; Eucher, P.; Haxhe, J.-P.; De Wispelaere, J.-F.; Johnson, P.M.; Delaunois, L. Thoracoscopic resection of pulmonary nodules after computed tomographic–guided coil labeling. The Annals of Thoracic Surgery 2001, 71, 986-988. [CrossRef]
- Liu, L.; Zhang, L.J.; Chen, B.; Cao, J.M.; Lu, G.M.; Yuan, L.; Li, K.; Xu, J. Novel CT-guided coil localization of peripheral pulmonary nodules prior to video-assisted thoracoscopic surgery: a pilot study. Acta Radiologica 2014, 55, 699-706. [CrossRef]
- Lenglinger, F.X.; Schwarz, C.D.; Artmann, W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. American Journal of Roentgenology 1994, 163, 297-300. [CrossRef]
- McConnell, P.I.; Feola, G.P.; Meyers, R.L. Methylene blue–stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. Journal of Pediatric Surgery 2002, 37, 1729-1731. [CrossRef]
- Stephenson, J.A.; Mahfouz, A.; Rathinam, S.; Nakas, A.; Bajaj, A. A Simple and Safe Technique for CT Guided Lung Nodule Marking prior to Video Assisted Thoracoscopic Surgical Resection Revisited. Lung Cancer International 2015, 2015, 1-3. [CrossRef]
- Chen, J.-R.; Tseng, Y.-H.; Lin, M.-W.; Chen, H.-M.; Chen, Y.-C.; Chen, M.-C.; Lee, Y.-F.; Chen, J.-S.; Chang, Y.-C. Safety and efficacy of computed tomography-guided dye localization using patent blue V for single lung nodule for video-assisted thoracoscopic surgery: a retrospective study. Annals of Translational Medicine 2019, 7, 28-28. [CrossRef]
- Lin, M.-W.; Tseng, Y.-H.; Lee, Y.-F.; Hsieh, M.-S.; Ko, W.-C.; Chen, J.-Y.; Hsu, H.-H.; Chang, Y.-C.; Chen, J.-S. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. The Journal of Thoracic and Cardiovascular Surgery 2016, 152, 535-544.e532. [CrossRef]
- Wang, Y.-Z.; Boudreaux, J.P.; Dowling, A.; Woltering, E.A. Percutaneous localisation of pulmonary nodules prior to video-assisted thoracoscopic surgery using methylene blue and TC-99. European Journal of Cardio-Thoracic Surgery 2010, 37, 237-238. [CrossRef]
- Choi, B.G.; Kim, H.H.; Kim, B.S.; Kim, K.T.; Shinn, K.S.; Moon, S.W. Pulmonary nodules: CT-guided contrast material localization for thoracoscopic resection. Radiology 1998, 208, 399-401. [CrossRef]
- Lee, N.K.; Park, C.M.; Kang, C.H.; Jeon, Y.K.; Choo, J.Y.; Lee, H.-J.; Goo, J.M. CT-Guided Percutaneous Transthoracic Localization of Pulmonary Nodules Prior to Video-Assisted Thoracoscopic Surgery Using Barium Suspension. Korean Journal of Radiology 2012, 13, 694. [CrossRef]
- Bellomi, M.; Veronesi, G.; Trifirò, G.; Brambilla, S.; Bonello, L.; Preda, L.; Casiraghi, M.; Borri, A.; Paganelli, G.; Spaggiari, L. Computed Tomography-Guided Preoperative Radiotracer Localization of Nonpalpable Lung Nodules. The Annals of Thoracic Surgery 2010, 90, 1759-1764. [CrossRef]
- Zuo, T.; Shi, S.; Wang, L.; Shi, Z.; Dai, C.; Li, C.; Zhao, X.; Ni, Z.; Fei, K.; Chen, C. Supplement CT-Guided Microcoil Placement for Localising Ground-glass Opacity (GGO) Lesions at “Blind Areas” of the Conventional Hook-Wire Technique. Heart, Lung and Circulation 2017, 26, 696-701. [CrossRef]
- Ko, K.-H.; Huang, T.-W.; Lee, S.-C.; Chang, W.-C.; Gao, H.-W.; Hsu, H.-H. A simple and efficient method to perform preoperative pulmonary nodule localization: CT-guided patent blue dye injection. Clinical Imaging 2019, 58, 74-79. [CrossRef]
- Chiang, H.; Chen, L.-K.; Hsieh, W.-P.; Tang, Y.-X.; Lo, C.-Y. Complications during CT-Guided Lung Nodule Localization: Impact of Needle Insertion Depth and Patient Characteristics. Diagnostics 2023, 13, 1881. [CrossRef]
- Tsai, T.-M.; Chiang, X.-H.; Liao, H.-C.; Tsou, K.-C.; Lin, M.-W.; Chen, K.-C.; Hsu, H.-H.; Chen, J.-S. Computed tomography-guided dye localization for deeply situated pulmonary nodules in thoracoscopic surgery. Annals of Translational Medicine 2019, 7, 31-31. [CrossRef]
- Kato, H.; Oizumi, H.; Suzuki, J.; Hamada, A.; Watarai, H.; Nakahashi, K.; Sadahiro, M. Thoracoscopic wedge resection and segmentectomy for small-sized pulmonary nodules. Journal of Visualized Surgery 2017, 3, 66-66. [CrossRef]
- Lin, C.-W.; Ko, H.-J.; Yang, S.-M.; Chen, Y.-C.; Ko, W.-C.; Huang, H.-C.; Chen, J.-S.; Chang, Y.-C. Computed tomography-guided dual localization with microcoil and patent blue vital dye for deep-seated pulmonary nodules in thoracoscopic surgery. Journal of the Formosan Medical Association 2019, 118, 979-985. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).