5. Towards Supraspinal Structures
It must be emphasized that, rostral to the spinal cord, nociceptive signals are fed into a cascade of structures characterized by two important properties. First, these structures receive inputs from other than nociceptive ones, the ascending nociceptive limb not being a mono-modal labelled line. Second, they exert more than nociceptive functions. Hence, they are multiple-input multiple-output and multi-functional nodes. This has already become clear in the DH.
In humans, a diverse array of CNS structures reacts to painful stimuli. In human brain imaging, acute experimental pain most commonly activates spinal and brainstem structures, the THAL, primary somatosensory (S1) and secondary somatosensory cortex (S2), anterior cingulate cortex (ACC), insular cortex (IC; or briefly insula), prefrontal cortex (PFC), nucleus accumbens (NAc) in the basal ganglia (BG), and AMY. S1 and S2 activations contribute to the sensory-discriminative dimension of pain. The ACC, PFC, IC, NAc, and amygdala (AMY) have been implicated in the affective component of pain (Bushnell et al. 2013; Doan et al. 2015; Henderson and Keay 2018). Signals associated with the pain experience also reach the CNS via the blood stream, by inflammatory mediators that ultimately cause the sickness response of fever, general muscle and joint ache, anorexia and lethargy (Bartfai 2001; Sandkühler 2009).
A meta-analysis of many human neuroimaging studies showed a core of areas exhibiting a largely bilateral pattern of pain-related activation in the THAL, S2, IC, and mid-cingulate cortex (MCC). These regions were activated regardless of stimulation technique, location of induction, and participant sex (Xu et al. 2020). Another meta-analysis showed that experimental pain stimuli activated S1, S2, IC, ACC, PFC, and THAL. Discrimination of pain intensity activated a ventrally directed pathway from the IC to the PFC, while discrimination of the spatial pain aspects involved a dorsally directed pathway from the posterior parietal cortex (PPC) to the dorso-lateral PFC (dlPFC) (Ong et al. 2019). Individual studies showed more diverse patterns. For example, noxious cold exposure activates the THAL, putamen and right anterior insular cortex (aIC), while innocuous cold exposure activates the posterior IC (pIC), medial orbito-frontal cortex (mOFC) and PPC (King and Carnahan 2019). Heat-evoked acute pain activates both somatic-specific areas such as the ventro-lateral THAL, the S2 and dorsal pIC, as well as regions related to affect and mood, such as the aIC, the dorsal anterior cingulate cortex (dACC) and the medial THAL (Kuner and Kuner 2021). The medial PFC (mPFC) mediates anti-nociceptive effects via its connections with other cortical areas and via its input to the peri-aqueductal gray (PAG) for modulation of pain (Ong et al. 2019).
Neuroimaging in (mostly anesthetized) animals shows commonalities with (awake) humans. Upon hindpaw thermal stimulation in rats, activations have been seen in the medial and lateral posterior THAL nuclei, S1, IC, cingulate cortex (CC), retro-splenial cortex (RSC), pretectal area as well as in the descending pain-modulatory centers, such as the diencephalic hypothalamus (HYP) and the midbrain PAG (Da Silva and Seminowicz 2019; Kuner and Kuner 2021).
5.1. Spinal Projection Neurons with Multiple Supraspinal Targets
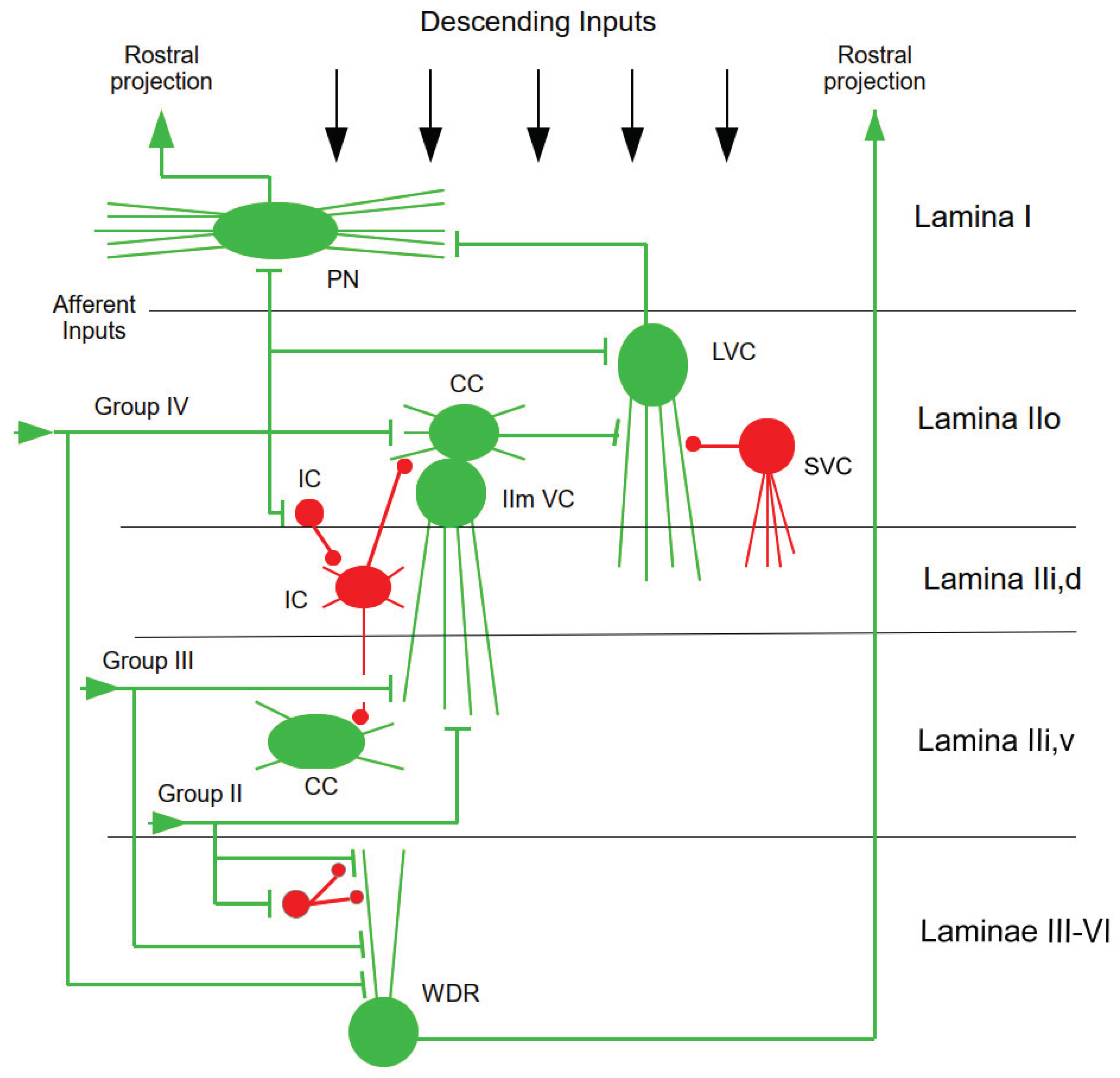
Ascending Nociceptive Tracts (Figure 2). Nociceptive group III and group IV afferents contact a minority of `projection´ neurons in the spinal DH or spV. Projection neurons convey nociceptive signals via multiple parallel pathways to multiple supraspinal targets in the brainstem, diencephalon, THAL and thence to the cerebral cortex. Ascending tracts include the spino-THAL tract (STTr), spino-cervico- THAL pathway, spino-hypothalamic pathway, spino-parabrachio-amygdaloid pathway, spino-reticular tracts, spino-mesencephalic tract, spino-limbic tracts, and postsynaptic dorsal column pathway (Bushnell et al. 2013; Coghill 2020; Dostrovsky 2000; Kuner and Kuner 2021; Poisbeau et al. 2018).
Properties of Projection Neurons. The somata of projection neurons are located in the superficial DH (lamina I), deep DH (laminae V, VI), VH (lamina VII) of primates, and the central gray (lamina X). At spinal level, projection neurons produce axon collaterals that are widely distributed within and between spinal segments, whose functions are hardly known, however (Browne et al. 2020). Many projection neurons in lamina I are nociceptive-specific (NS), with inputs from nociceptive afferents only. Lamina V contains wide-dynamic-range (WDR) neurons with inputs from nociceptive and non-nociceptive sensory afferents and appear to be able to encode noxious stimulus intensity (Braz et al. 2014). In the mouse, genetic methods have revealed distinct modular circuits made up of molecularly defined interneurons that process nociceptive (pain), pruritic (itch) and cutaneous mechano-sensitive (innocuous touch) stimuli. Excitatory interneurons transmit somatosensory information and inhibitory interneurons operate as gates to prevent innocuous stimuli from activating nociceptive and pruritic pathways (Koch et al. 2018). The properties of these neurons can change depending on context; for example, depolarization may transform NS neurons into WDR neurons (Berger et al. 2011; Sandkühler 2009). Neuron properties are also altered by the actions of several classes of neuromodulators and neuropeptides (Zeilhofer et al. 2012).
Spino-THAL Tract (STTr).The STTr has two parts, a lateral and an anterior part. The former originates in DH lamina I and, on its way to the THAL, sends collaterals to (i) the brainstem RF, interpeduncular area, noradrenergic (NA) cell groups A1, A5, A6, A7 (the latter three not illustrated in
Figure 2, for graphical reasons), and the adrenergic C1 cell group, A1 projecting further to the HYP; (ii) the parabrachial nucleus (PBN, projecting on to the AMY), the PAG, the HYP, the central nucleus of the AMY (CeA), and finally to the THAL [posterior portion of the ventral medial nucleus (Vmpo), ventro-posterior inferior nucleus (VPI), and ventral caudal portion of the medial dorsal nucleus (MDvc)] (Dostrovsky 2000; Kuner and Kuner 2021). The anterior STTr originates in DH laminae IV-V and, in the brainstem, sends collaterals to the sub-nucleus reticularis dorsalis (SRD) and other sites, and ends in VPI, ventral posterolateral nucleus (VPL) and central lateral nucleus (CL) (Dostrovsky 2000).
Other Ascending Nociceptive Pathways. Some ascending pathways transmit signals associated with motivational and cognitive aspects [the spino-reticular tract and spino-parabrachial tract (
Figure 2)], affectivity (the spino-mesencephalic tract and spino-parabrachial tract), motor responses, as well as neuro-endocrine and autonomic responses (the spino-hypothalamic tract). The spino-reticular tract is a multi-synaptic pathway originating from neurons mainly located in the spinal cord laminae IV–V and VII–VIII targeting areas of the medullary and pontine reticular formation, which have collaterals of the STTr (Martins and Tavares 2017). The spino-parabrachial tract is a significant site of convergence for both somatic and visceral nociceptive stimuli (Merighi 2018). Ascending nociceptive signals can also be indirectly conveyed to the THAL by the spino-mesencephalic and dorsal postsynaptic dorsal-column pathway (Almeida et al. 2004; Braz et al. 2014; Coghill 2020; Todd 2010; Yen and Lu 2013). The ascending pathways transmit nociceptive information across bilateral routes. That is, the spino-THAL projections may arise from deep DH and ventral-horn neurons with bilaleral and/or whole-body receptive fields. The projections of other spino-THAL neurons travel ipsilaterally instead of contralaterally to the cell body (Coghill 2020).
Visceral Projections. Spinal visceral afferent neurons project into the laminae I, II (outer part IIo) and V of the spinal DH over several segments, medio-lateral over the whole width of the DH and contralateral. Their activity is synaptically transmitted in laminae I, IIo and deeper laminae to viscero-somatic convergent neurons that receive additionally afferent synaptic (mostly nociceptive) input from the skin and from deep somatic tissues of the corresponding dermatomes, myotomes and sclerotomes. The second-order neurons consist of excitatory and inhibitory interneurons and tract neurons activated monosynaptically in lamina I by visceral afferent neurons and di- or polysynaptically in deeper laminae. Viscero-somatic tract neurons project through the contralateral ventro-lateral tract and presumably other tracts to the lower and upper brainstem, the HYP and via the THAL to various cortical areas. Visceral pain is presumably (together with other visceral sensations and nociceptive as well as non-nociceptive somatic body sensations) primarily represented in the posterior dorsal IC (primary interoceptive cortex). In primates, this cortex receives its spinal synaptic inputs mainly from lamina I tract neurons via the ventro-medial posterior nucleus of the THAL (Jänig 2014).
The ascending nociceptive pathways thus include many nodes, which will be treated below in some detail in terms of their multiple inputs, outputs and functions, and thus to emphasize their complexity (Figure 2).
5.2. Itch Pathways
Although pain and itch are distinct sensations, most noxious chemicals are not very specific to one sensation over the other. An important difference between these sensations is that itch is initiated by irritation of the skin, whereas pain can be elicited from almost anywhere in the body. Thus, itch may be encoded by the selective activation of specific sub-sets of neurons that are tuned to detect harmful stimuli at the surface and have specialized central connectivity that is specific to itch. Within the spinal cord, cross-modal inhibition between pain and itch may help sharpen the distinction between these sensations. Just as there are inhibitory circuits in the DH that mediate cross-inhibition between modalities, it appears that there are also excitatory connections that can be unmasked upon injury or in disease, leading to abnormally elevated pain states such as allodynia (Ross 2011).
Itch is a unique sensation that urges organisms to scratch away external threats, and scratching in turn induces an immune response that can enhance itchiness. The central pathways and circuits processing itch and pain overlap anatomically, but some neurons transmit itch signals independently of other modalities (Lay and Dong 2020). After spinal processing, itch signals are transferred via projection neurons that connect to the STTr. The next supraspinal itch-processing station is the PBN, which projects to different brain regions including the AMY, which links stress and anxiety to chronic itch.
Functional MRI in humans implicates cortical regions including the S1 and S2, the CC and PFC (Cevikbas and Lerner 2020).
In transgenic mice, one kind of polymodal nociceptors containing galanin (GAL) and one type of pruriceptors expressing neurotensin (NT) took different routes. NT-expressing pruriceptors avoided the STTr, although both ascending projections shared the spino-bulbar projections but occupied different sub-nuclei. In the somatic motor system, more neurons in the red nucleus (nucleus ruber) and primary motor cortex (M1) participated in the GAL-containing nociceptor-derived network, while more neurons in the NTS and the dorsal motor nucleus of vagus nerve (DMX) of the emotional motor system were found in the NT-expressing pruriceptor-derived network. Functional validation of differentially labeled nuclei by c-fos test and chemogenetic inhibition suggested that the red nucleus is involved in facilitating the response to noxious heat and the NTS/DMX in regulating the histamine-induced scratching (Chen et al. 2022).
Genetic deletion techniques have proposed that gastrin-releasing peptide (GRP) may be a key neurotransmitter for itch in the spinal cord. Glutamate but not GRP acts as the key neurotransmitter in the primary afferents in the transmission of itch. GRP is more likely to serve as an itch-related neuromodulator. The ACC plays a significant role in both itch and pain sensations (Chen and Zhuo 2023).
5.3. Nucleus Tractus Solitarii (NTS)
The NTS (or nucleus of the solitary tract) is a complex of sub-nuclei aligned in a vertical slice located in the dorso-medial medulla oblongata. The NTS has been divided cytoarchitectonically into various sub-nuclei, which are partly correlated with the areas of projection of peripheral afferent endings. Gustatory and somatic afferents from the oro-pharyngeal region project with a crude somatotopy within the rostral part of the NTS (rNTS) and visceral afferents from cardio-vascular, digestive, respiratory and renal systems terminate viscero-topically within its caudal part (cNTS) (Jean 1991; Holt and Rinaman 2022).
Functions. The extensive connections indicate that the NTS is a central structure for autonomic and neuro-endocrine functions as well as for integration of somatic and autonomic responses in certain behaviors. Painful stimuli can evoke dramatic responses in the cardio-vascular and respiratory systems. The NTS has a major role as a site for integrating nociceptive and cardio-respiratory afferents including and for mediating the reflex tachycardia evoked by somatic noxious stimulation. Similar noxious stimulation attenuates the cardiac component of the peripheral chemo-receptor reflex and inhibits the peripheral chemoreceptor-evoked excitatory synaptic response of some NTS neurons. Hence, by depressing homeostatic reflexes in the NTS, noxious stimulation-evoked cardio-respiratory changes can be expressed and maintained, which may be essential for the survival of the animal (Boscan et al. 2002). The NTS has extensive connections with the vestibular nuclei, both directly and via the PBN; whereby the vestibular nuclei could also receive nociceptive inputs (Saman et al. 2020).
Inputs. The NTS receives fibers from the superficial laminae (I-III) of the spinal DH terminating bi-laterally in the cNTS, and fibers from the deeper DH laminae (IV-V) terminating ipsilaterally, mostly in the lateral areas of the cNTS (Gamboa-Esteves et al. 2001). In rat lamina I neurons receiving noxious cutaneous and visceral stimuli via NK1 receptor activation project to NTS and so may be involved in coordinating nociceptive and cardio-respiratory responses (Gamboa-Esteves et al. 2004). In rats, NTS cells receive hindlimb somatosensory inputs from low- and high-threshold cutaneous mechano-receptors, respond to capsaicin delivered into the hindlimb arterial supply, lack thermal sensitivity, and respond to activation of mechano-sensitive as well as metabo-sensitive endings in skeletal muscle. Visceral sensory information is conveyed via the afferent glossopharyngeal (IX) and vagus (X) nerves (Toney and Mifflin 2000). The NTS receives cardio-pulmonary vagal inputs from small-diameter afferents responding to mechanical distension (lung stretch, group III fibers) and noxious stimuli/immune processes (lung irritants/cytokines, via group IV-fibers/nociceptors) leading to efferent vagal activity that evokes airway defensive reflexes (Zyuzin and Jendzjowsy 2022). The NTS also receives telencephalic inputs from a large array of structures (Gasparini et al. 2020; Holt 2022; Toney and Mifflin 2000). Direct projections from the cerebral cortex to the NTS have been identified (Jean 1991).
Outputs. At the level of the area postrema (AP), axon collaterals of most small NTS cells (soma <150 μm2) establish excitatory or inhibitory local micro-circuits likely to control the activity of nearby NTS cells and to transfer peripheral signals to efferent projection neurons. At least two cell types with efferent projections from the cNTS were distinguished: (i) a greater numbers of small cells, apparently forming local excitatory micro-circuits via recurrent axon collaterals, which project specifically and unidirectionally to the lateral parabrachial nucleus (lPBN); and (ii) much less numbers of cells likely to establish multiple global connections with a wide range of brain regions, including the ventro-lateral medulla (VLM), HYP, CeA, bed nucleus of the stria terminalis (BNST), spinal DH, brainstem RF, LC, PAG and peri-ventricular diencephalon (Kawai 2018). The NTS also projects to the PBN, ventro-lateral reticular formation, raphé nuclei, motor nuclei of several cranial nerves, and others, and long connections to diencephalic and telencephalic structures,spinal cord (Holt 2022).
5.4. Parabrachial Nucleus (PBN)
The PBN surrounds the superior cerebellar peduncles in the dorso-lateral pons. The PBN is a collection of cell groups that, in rodents, can be divided into more than a dozen sub-nuclei based on cytoarchitecture. The medial PBN (mPBN) comprises populations of neurons heterogeneous in size and morphology, whereas the lPBN includes several homogeneous groups, which are also characterized by differential connectivity and neurochemistry and contains numerous co-localized peptides, including CGRP, SP, NT, and dynorphin (Chiang et al. 2019).
Functions. The PBN nuclei are involved in many homeostatic functions including nociception, chemoreception and autonomic control. The lPBN is required for escape behaviors and aversive learning in response to noxious stimulation (Chiang et al. 2020).
Nociceptive Inputs. The PBN receives substantial projections from nociceptive neurons in the contralateral superficial DH, and less dense inputs from the ipsilateral superficial DH and deeper lamina (Peng et al. 2023). The PBN, particularly lPBN, is the primary supraspinal target of nociceptive, pruritic and thermal signals transmitted via the SPT from the trigeminal and spinal DHs. The STTr sends collaterals to PBN (Kuner and Kuner 2021). The PBN is reciprocally connected with CeA, BNST, and multiple HYP nuclei, including the preoptic area (POA). This hyper-excitability appeared in part to reflect a loss of recurrent inhibition from the CeA. CeA not only receives a significant projection from lPBN, but also sends a dense inhibitory reciprocal connection back to lPBN (Chiang et al. 2019). – In mice, catecholaminergic input from the cNTS caused amplification of PBN activity and their sensory afferents. Noxious mechanical and thermal stimuli activated cNTS neurons and produced prolonged NA transients in PBN. Similar NA transients could be evoked by focal electrical stimulation of cNTS, a region that contains the NA A2 cell group that projects densely on PBN. A2 neurons of the cNTS increase excitability and potentiate responses of PBN neurons to sensory inputs (Ji et al. 2023).
Outputs from the PBN are widespread and complex. Major outputs target the paraventricular and gustatory THAL, the IC, intra-limbic (IL) PFC and pre-limbic (PL) PFC, with direct projections to the CeA and BNST and, through a THAL relay, to the IC, implicating PBN in both emotional and autonomic aspects of pain. Thus, stimulation of the PBN connections with CeA and BNST drove avoidance behavior (real-time place aversion) and aversive learning Activation of efferent projections to the ventro-medial hypothalamus (vmHYP) or lateral peri-aqueductal gray (lPAG) drove escape behaviors, whereas activation of lPBN efferents to the BNST or CeA generated an aversive memory. lPBN is also related to motor functions. Thus, activation of the lPBN projections to the caudal-dorsal medullary RF facilitated motor responses evoked by noxious stimulation, particularly during inflammation. Stimulation of the projections to vmHYP and PAG evoked running and jumping. PBN projects to the paraventricular nucleus (PVN) of HYP and may play a role in neuro-endocrine-autonomic integration. lPBN projects to the PAG and to the RVM, both of which are implicated in descending pain modulation (Chiang et al. 2019, 2020). PBN projects directly to the RVM. Under physiological conditions and when exposed to acute pain stimuli, the contralateral PBN transmits signals to the RVM ON- and OFF-cells and then triggers acute hyperalgesia, while the ipsilateral PBN is involved in the RVM ON-and OFF-cells-induced modulation of persistent inflammation and chronic pain (Peng et al. 2023). lPBN neurons with strong nociceptive inputs from the DH project to the capsular part of the CeA, which is an important structure linking nociception and emotion. The lPBN to CeA synaptic transmission is enhanced in various pain models. In rats, light stimulation evoked monosynaptic excitatory postsynaptic currents (EPSCs), with very small latency fluctuations, followed by a large polysynaptic inhibitory postsynaptic current in AMY neurons. Intra-plantar formalin injection at 24 h before slice preparation significantly increased EPSC amplitude in late firing-type CeA neurons. This indicate that direct monosynaptic glutamatergic inputs from the lPBN not only excite CeA neurons but also regulate CeA network signaling through robust feed-forward inhibition, which is under plastic modulation in response to persistent inflammatory pain (Sugimura et al. 2016). – A sub-population of lPBN neurons relays nociceptive signals from the spinal cord to the substantia nigra pars reticulata (SNr). Pain decreases the activity of many ventral tegmental area (VTA) DA neurons. lPBN-targeted and nociception-recipient SNr neurons regulate VTA DA activity directly through feedforward inhibition and indirectly by inhibiting a distinct sub-population of VTA-projecting lPBN neurons, thereby reducing excitatory drive to VTA DA neurons. Correspondingly, ablation of SNr-projecting lPBN neurons suffices to reduce pain-mediated inhibition of DA release in vivo (Yang et al. 2021).
5.5. Cerebellum
In human neuroimaging, the cerebellum was consistently activated after a peripheral nociceptive stimulus, be it electrical, laser, capsaicin, or other types of nociceptive stimulation, the activation sites mainly involving the vermis (in lobules IV–V), the ipsilateral cortex (lobules IV–VI, Crus I), and the contralateral cortex (lobule VI, Crus I) (Welman et al. 2018).
Beyond a role in nociception, the cerebellum has been implicated in a number of functions, including oculomotor control, control of upright stance and locomotion, reaching and grasping and speech, timing and coordination of movement, control of motor-cortex excitability, prediction of sensory consequences of actions, error detection and correction, motor learning, classical conditioning (e.g. eyeblink conditioning), and even reward, language, and social behavior, emotional, motivational and cognitive functions (Dibaj and Windhorst 2024a). The cerebellum also has a role in pain processing and/or modulation, possibly due to its extensive connections with the PFC and brainstem regions involved in descending pain control (Adamaszek et al. 2017; Baumann et al. 2015; Ong et al. 2019; Wang et al. 2022). The cerebellum also has bi-directional connections to the HYP, which may be involved in feeding, cardio-vascular, osmotic, respiratory, micturition, immune, emotion, and other non-somatic regulation (Zhu et al. 2006).
Nociceptive Inputs. Animal studies suggested spinally projecting multi-sensory inputs from the skin, including tactile group III and nociceptive group III and IV fiber (Welman et al. 2018). For example, in cats, stimulation of cutaneous group III and IV fiber nociceptor afferents activated climbing fibers (CFs) that terminate on Purkinje cells (PCs) in the cerebellar anterior lobe ipsilateral to stimulation. Group IV afferents conveyed neural input through the postsynaptic dorsal columns as part of a proposed spino-olivo-cerebellar (SOC) pathway. In addition to CF input, group IV fiber input may also act through mossy fibers (MFs) to reach PCs. In rats, noxious colo-rectal distention activates visceral neurons in the lateral medullary RF, including several with direct projections to the cerebellar vermis. In addition, noxious visceral stimulation can modulate PC activity in the posterior cerebellar vermis. However, what pathway conveys these signals is yet unknown (Moulton et al. 2010). In rodents and cats, stimulation of cutaneous and visceral nociceptors and group III and/or group III/IV afferents can activate and modulate PC activity. At least two possible nociceptive spino-cerebellar pathways have been proposed: (i) a SOC pathway that conveys nociceptive group III and group IV fiber input to PCs in the cerebellar anterior lobe ipsilateral to stimulation, and (ii) a spino-ponto-cerebellar (SPC) pathway conveying group IV fiber input to PCs in the cerebellar vermis. In addition to sensory afferent input, the cerebellum receives input from brain areas associated with nociceptive processing, including cognition, affect, and motor function. With the cerebellum receiving both descending information from other brain areas and ascending nociceptive information from the spinal cord, the structure is ideally positioned to be influenced by, or to influence, the processing of pain (Baumann et al. 2015).
Other Connections. In toto, the cerebellum has extensive connections to multiple CNS regions: S1, premotor cortex (PM), mPFC, IC, ACC, hippocampus (HIPP), AMY, THAL, HYP, RF, red nucleus (nucleus ruber: NRu), PBN, PAG, spV, trigeminal ganglion, LC, vestibular nuclei, and spinal cord. Among these are several pain-related regions (Wang et al. 2022). Nociceptive signals also reach the cerebellum (Moulton et al. 2010; Saab and Willis 2003). The cerebellum is reciprocally connected to the PAG, an anti-nociceptive processing center (Wang et al. 2022).
Role in Nociception/Pain. The functional role of the cerebellum in pain processing remains largely unclear. One widely accepted idea is that cerebellar activity is related to the fine-tuning of the motor output when we experience pain, in order to protect it from further harm. However, a role in pain anticipation, in the inhibition of pain, and in perceiving pain induced in others has also been posited. Many questions remain open. One question is whether the cerebellum is involved in modulating the processing of the incoming nociceptive signals or in the preparation and execution of a motor response to the nociceptive signal. Another question is whether it is invvolved in producing tasks that are localization-independent, like pain inhibition and the production of warning signals, or whether it is involved in the precise localization and precise movement planning in response to the nociceptive signals. In the latter case, a more detailed processing of the pain signal would be required that would require a precise somatotopical organization (Welman et al. 2018).
5.6. The PAG-Triad Connection
Extensive interconnections between the ANS, endocrine, somatic and limbic networks orchestrate pain modulation, stress responses, behavioral arousal, emotion, homeostasis, cardio-vascular and respiratory control, and micturition and defecation reflexes. This system receives various inputs from diverse sources, including modulatory input from cholinergic (ANS), monoaminergic, and peptidergic neurons, as well as signals mediated by NO, purines, endocannabinoids, and neurosteroids. Visceral inputs regulate autonomic output through both the sympathetic and parasympathetic pre-ganglionic neurons in the forebrain arousal system, medulla and, spinal cord. The central ANS includes monoaminergic neurons in the brainstem RF and nuclei that use as neurotransmitters and neuromodulators monoamines such as dopamine (DA), adrenaline and noradrenaline (NA), 5-HT, and histamine (Delbono et al. 2022).
Brainstem structures of major importance in the present context are the following: PAG, RVM, CVLM, DA neurons in HYP, substantia nigra pars compacta (SNc) and VTA, NA LC, 5-HT neurons in the raphé nuclei (RN).
The PAG sends strong inputs to the RVM, which by intricate connections is integrated with the CVLM and DReN into a triad (Martins and Tavares 2017)..
5.6.1. Peri-Aqueductal Gray (PAG)
The midbrain PAG is a cell-dense region surrounding the midbrain aqueduct. It shows a high degree of anatomical and functional organization, which takes the form of longitudinal columns of afferent inputs, output neurons and intrinsic interneurons (Bandler and Shipley 1994; Koutsikou et al. 2017).
Inputs. Ascending inputs come from the STTr (Kuner and Kuner 2021; Figure 2). The ventro-lateral PAG (vlPAG) receives inputs from regions that are targets of the ascending nociceptive fibers, including the PBN and spinal cord. The lPAG receives direct inputs from the DH and spV organized in a roughly somatotopic map, with orofacial afferents terminating rostrally and afferents from the legs terminating caudally (Mills et al. 2021). – The cortical projections to the PAG originate from the PFC, in particular, from the mPFC (BAs 25, 32), the dorso-medial convexity of the medial wall (BA 9) extending into the ACC (BA 24), and the posterior orbito-frontal/anterior IC (BA 13, 12) (Ong et al. 2019). In the cat, significant sources of cortical PAG projections are the somatosensory cortex, frontal cortex, IC, and CC. Tract paths could be defined between the PFC, AMY, THAL, HYP and RVM bilaterally, and the PAG. Functional magnetic resonance imaging (fMRI) has shown that the vlPAG is functionally connected to brain regions associated with descending pain modulation including the ACC, upper pons/medulla (Bouchet and Ingram 2020; Ong et al. 2019; Ossipov et al. 2014; Vázquez-León et al. 2021; Yetnikoff et al. 2014). – The PFC-AMY-dorsal PAG-pathway may mediate fear-conditioned analgesia, i.e., a reduction in pain response upon re-exposure to a context, previously paired with an aversive stimulus. The mPFC-PAG projection plays a role in modulation of autonomic responses to pain. In addition, the PAG receives inputs from the BNST, the midbrain retro-rubral field (RRF) and VTA. – Particularly important inputs reach the PAG indirectly from the PFC. fMRI revealed that the vlPAG is functionally connected to brain regions associated with descending pain modulation including the ACC, upper pons/medulla, whereas the lPAG and dorso-lateral PAG (dlPAG) are connected with brain regions implicated in executive functions, such as the PFC, striatum, and HIPP. In vivo tracing of neuronal connections using probabilistic tractography seeded in the right dlPFC and left rostral ACC (rACC) showed that stronger placebo analgesic responses are associated with increased mean fractional anisotropy values in white matter tracts connecting the PFC with the PAG. Tensor imaging showed that tract paths could be defined between the PFC, AMY, THAL, HYP, PAG and RVM bilaterally. The PFC-AMY-dPAG pathway may mediate fear-conditioned analgesia, i.e., a reduction in pain response upon re-exposure to a context, previously paired with an aversive stimulus. The mPFC-PAG projection also plays a role in modulation of autonomic responses to pain (Ong et al. 2019).
Outputs. The PAG sends descending outputs to the pons, cerebellum, medulla oblongata (Figure 3) and spinal cord (Yetnikoff et al. 2014). The vlPAG projects to the mPFC, lateral septum, BNST, HIPP, magnocellular basal forebrain (BFB), caudate putamen, NAc, AMY (CeA), to the substantia nigra (SN), lateral habenula (lHb), VTA and the RVM (Bouchet and Ingram 2020; Yetnikoff et al. 2014). The pain modulation results from descending, excitatory and inhibitory, onnections from the PAG to the RVL, NRM and other brainstem structures which in turn send glutamatergic, GABAergic, 5-HT and enkephalin (ENK) projections to the spinal cord (Lamotte et al. 2021; Lau and Vaughan 2014). Via the RVM, the PAG exerts both anti- and pro-nociceptive influences on nociceptive signal tramission in the DH or spV. The endogenous pain-modulation structures are strongly interconnected, e.g., LC and SRD, which also send direct projections to the DH and spV modulate nociception (Mills et al. 2021). Under normal conditions, PAG output neurons to the RVM are inhibited by GABA. Removal of this inhibition resulted in activation of the descending pain modulatory circuit and analgesia. The assumption is that this disinhibition promotes excitatory neurotransmission from the PAG to RVM. Opioid-triggered analgesia was mediated by projections from the vlPAG to RVM, while non-opioid-triggered analgesia was elicited by projections of the lPAG and dlPAG to RVM (Peng et al. 2023). However, opioid receptors are expressed on both GABAergic and glutamatergic terminals in the PAG and both cell populations project to RVM. Thus, PAG to RVM connections are more complicated than simply eliciting disinhibition of excitatory descending projections and probably reflect the existence of parallel circuits that contribute to the bi-directional control of pain mediated by the RVM (Bouchet and Ingram 2020). The PAG has indirect routes to DH via the LC and NRM (Willis and Westlund 1997).
Functions. The PAG plays a crucial role in conveying the modulatory influences from higher brain regions involved in aspects of pain responses such as cognition and emotion, including the PFC, IC and the AMY. The PAG collects the modulatory influences from these areas and uses the RVM as a relay to indirectly target the spinal cord (Martins and Tavares 2017). The PAG columns serve various functions. Stimulation of the vlPAG produces opioid-mediated analgesia, as well as freezing and quiescent behaviors, whereas stimulation of the lateral column and more dorsal columns produce escape behaviors such as jumping and flight responses (Bouchet and Ingram 2020; Mills et al. 2021). Overall, PAG functions are related to pain modulation, anxiety, panic, unconditioned, conditioned, as well as learned behaviors such as fear, vocalization, food intake, and sexual behavior, and the integration of autonomic responses. PAG neurons integrate negative phenomena such as anxiety, stress, and pain with the autonomic, neuro-endocrine, and immune systems to facilitate responses to threat (Vázquez-León et al. 2021). The PAG plays a coordinative role in the management of threat, arousal, anxiety, fear, and defence systems for survival by organizing changes in sensory processing including anti-nociception, ANS activity and motor behavior, e.g., `fight or flight´ or `freezing´ (Koutsikou et al. 2017; Roelofs 2017). Different classes of threatening or nociceptive stimuli trigger distinct co-ordinated patterns of musculo-skeletal, autonomic and anti-nociceptive adjustments by selectively targeting specific PAG columnar circuits (Bandler and Shipley 1994).
Inescapable and Escapable Pain. Deep pain evokes passive emotional coping that includes quiescence and vaso-depression. Inescapable, persistent pain generated by nociceptive inputs from deep structures drive neurons in the vlPAG that co-ordinate passive emotional coping. By contrast, brief escapable cutaneous pain evokes an active emotional coping: the fight-or-flight response, which activates the dlPAG that co-ordinate active coping strategies. Hence, it has been suggested that it is the behavioral significance of the nociceptive input, rather than its organ of origin per se, that determines the characteristics of the affective response. Differential representations of escapable and inescapable pain in the PAG may extend to distinct representations of `first pain´ and `second pain´, as indicated by the columnar distribution of neurons activated by inputs from group III (Aδ) and IV (C) nociceptive afferents (Lumb 2002).
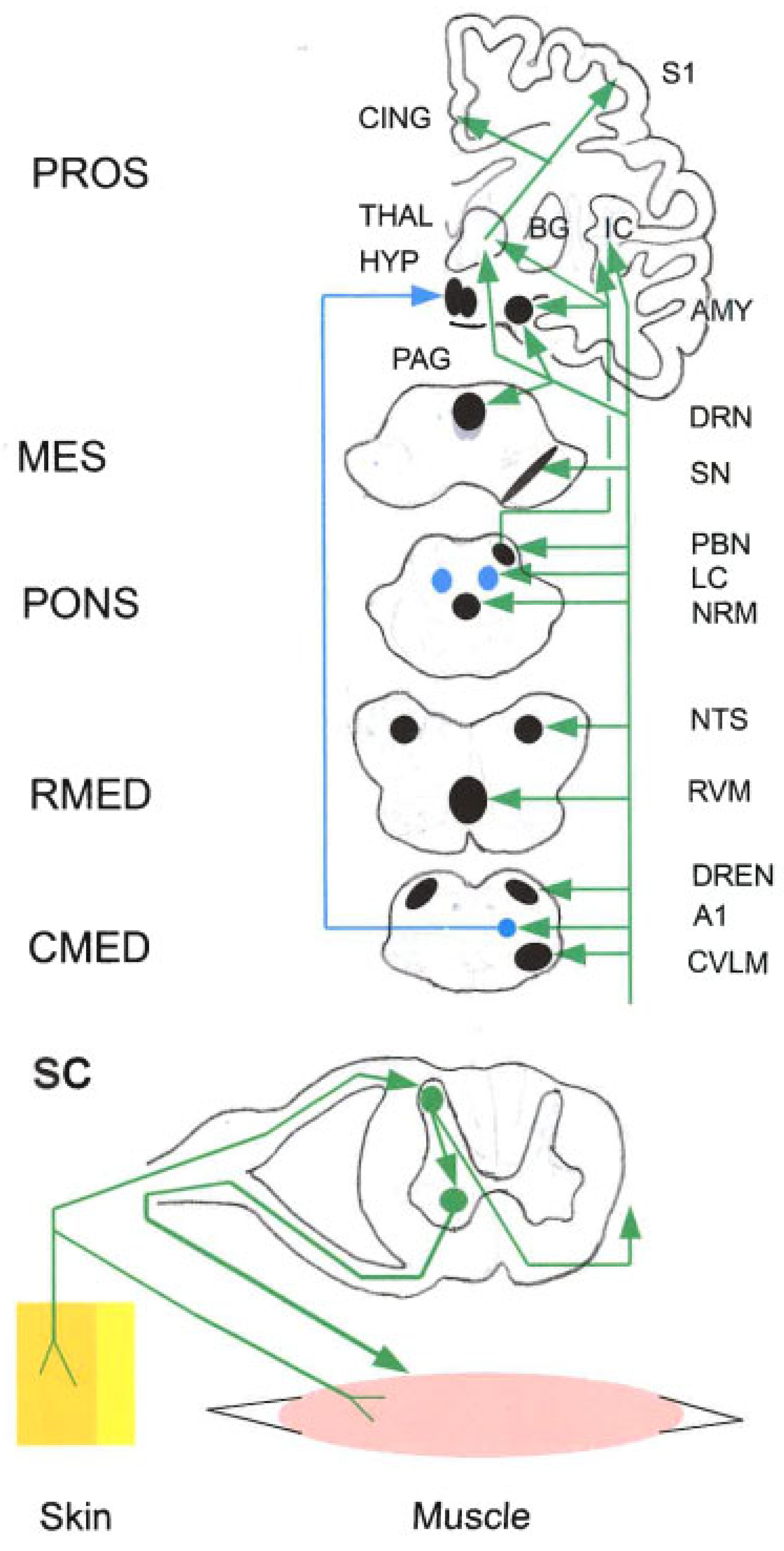
Figure 3.
Simplified and rarefied scheme of the approximate locations of some nuclei and brain structures and of connections involved in the descending control of nociceptive transmission in the spinal DH. The sections are not scaled. Some structures (e.g., raphé nuclei) distribute quite far rostro-caudally and may occur in two cross-sections, which is not shown for graphical reasons. Connections often are composed of parallel fiber systems, but are here lumped and represented by arrowed lines. Connections may be excitatory (green lines) or inhibitory (red lines). For example and importantly, the connections from RVM to DH are both facilitory and inhibitory. Other arrowed lines, e.g., from HYP to DH, comprise DA and OXT influences. Therefore, and for graphical reasons, most lines are indifferently black. Abbreviations: A1: NA A1 cell group; AMY: amygdala; BG: basal ganglia; CING: cingulate cortex; CMED: caudal medulla; CVLM: caudal ventro-lateral medulla; dlPFC: dorso-lateral prefrontal cortex; DH: dorsal horn; DReN: dorsal reticular nucleus; DRN: dorsal raphé nucleus; HYP: hypothalamus; IC: insular cortex; LC: locus coeruleus; MES: mesencephalon; mPFC: medial prefrontal cortex; NRM: nucleus raphé magnus; NTS: nucleus tractus solitarii; PAG: peri-aqueductal gray; PBN: parabrachial nucleus; PROS: prosencephalon; RMED: rostral medulla; RVM: rostral ventro-medial medulla; SN: substantia nigra; THAL: thalamus; vlPFC: ventro-lateral prefrontal cortex (Data from papers cited in the text).
Figure 3.
Simplified and rarefied scheme of the approximate locations of some nuclei and brain structures and of connections involved in the descending control of nociceptive transmission in the spinal DH. The sections are not scaled. Some structures (e.g., raphé nuclei) distribute quite far rostro-caudally and may occur in two cross-sections, which is not shown for graphical reasons. Connections often are composed of parallel fiber systems, but are here lumped and represented by arrowed lines. Connections may be excitatory (green lines) or inhibitory (red lines). For example and importantly, the connections from RVM to DH are both facilitory and inhibitory. Other arrowed lines, e.g., from HYP to DH, comprise DA and OXT influences. Therefore, and for graphical reasons, most lines are indifferently black. Abbreviations: A1: NA A1 cell group; AMY: amygdala; BG: basal ganglia; CING: cingulate cortex; CMED: caudal medulla; CVLM: caudal ventro-lateral medulla; dlPFC: dorso-lateral prefrontal cortex; DH: dorsal horn; DReN: dorsal reticular nucleus; DRN: dorsal raphé nucleus; HYP: hypothalamus; IC: insular cortex; LC: locus coeruleus; MES: mesencephalon; mPFC: medial prefrontal cortex; NRM: nucleus raphé magnus; NTS: nucleus tractus solitarii; PAG: peri-aqueductal gray; PBN: parabrachial nucleus; PROS: prosencephalon; RMED: rostral medulla; RVM: rostral ventro-medial medulla; SN: substantia nigra; THAL: thalamus; vlPFC: ventro-lateral prefrontal cortex (Data from papers cited in the text).
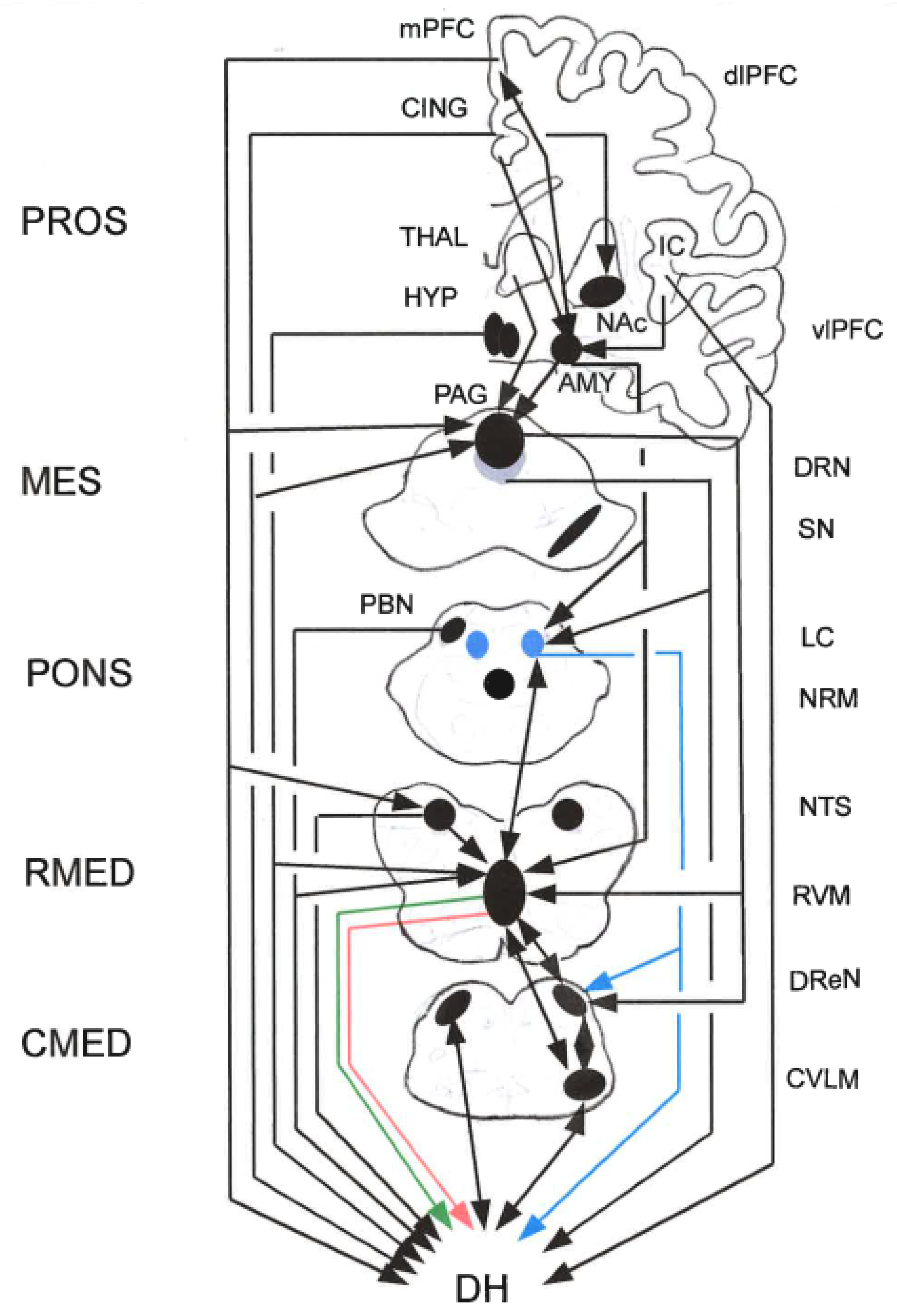
5.6.2. Rostral Ventro-Medial Medulla (RVM)
The RVM mainly consists of the midline NRM, the nucleus reticularis giganto-cellularis-pars alpha, and the nucleus paragiganto-cellularis lateralis, as well as GABAergic and glycinergic cell populations, all of which project diffusely to the spV and to superficial and deep DH layers (Heinricher et al. 2009; Mills et al. 2021; Ossipov et al. 2014; Peng et al. 2023). The RVM contains three populations of neurons, ON-cells, OFF-cells, and NEUTRAL cells that show different responses to noxious stimuli and are recruited by higher structures to enhance or inhibit pain (Heinricher et al. 1987, 2009; Peng et al. 2023).
Inputs. Nociceptive information is transmitted to the RVM through specific groups of spinal ascending neurons. The RVM also receives nociceptive-related inputs from a dense projection from the PAG, from the PBN, THAL, HYP (directly and indirectly), and the AMY, and further inputs from a variety of other cortical and sub-cortical areas as well as from the NA LC (Bouchet and Ingram 2020; Ossipov et al. 2014; Peng et al. 2023). The output from the CeA targets the RVM and PAG, which are crucial for mediating behavioral coping responses in the face of threat (Kuner and Kuner 2021). When experiencing noxious heat stimuli or continuous neuropathic pain, not only is the contralateral RVM but also the ipsilateral/median RVM activity increased, as indicated in a fMRI study of human supraspinal structures (Peng et al. 2023). – The AMY has direct and indirect projections to the RVM, and thereby influences the descending pain modulatory system. After micro-injection of morphine into different sites of the AMY, several findings suggest that its analgesic effects were mainly attributed to the direct projections from the AMY to the RVM. Infusing morphine into the BLA increased OFF-cell activity, modestly decreased ON-cell activity, strongly attenuated the OFF-cell pause, and increased TFL (Peng et al. 2023).
In humans, fMRI studies showed that somatic and visceral noxious stimulation led to activation of brainstem regions including the PAG and RVM, and at the primary nociceptive synapse, at either the spV or DH, during noxious muscle and cutaneous inputs. There was an association between the activation in, or signal coupling between, these regions and the intensity of pain reported during acute noxious inputs (Mills et al. 2021). fMRI also showed that when experiencing noxious heat stimuli or continuous neuropathic pain, not only the contralateral RVM but also the ipsilateral/median RVM activity was increased. In animals, extracellular recordings showed changes in RVM neuronal responses to nociceptive stimulation. Pro-nociceptive ON-cells increased activity immediately before facilitation of the withdrawal response. Anti-nociceptive OFF-cells decreased their relatively high spontaneous activity up to a pause, which triggered the nociceptive response. NEUTRAL cells did not respond to painful stimuli (Heinricher et al. 2009; Peng et al. 2023).
Outputs. RVM neurons project diffusely to the spV and, via ventro-lateral funiculus and spinal dorso-lateral funiculus separately, to DH laminae important in nociceptive processing, including superficial and deep DH layers that receive nociceptor primary afferents (Heinricher et al. 2009; Mills et al. 2021; Peng et al. 2023). It has been suggested that RVM has a distinctive role as the `main gatekeeper of descending pain modulation´, bi-directionally facilitating or inhibiting spinal nociceptive transmisison (Peng et al. 2023). ON-cell firing increased and OFF-cell firing decreasesd while NEUTRAL cells showed no responses to nociceptive stimuli (Heinricher et al. 1987).
PAG-RVM Connections. PAG neurons project extensively to RVM neurons, which in turn project to the spinal cord, and two-thirds of these reticulo-spinal neurons, appear to be GABAergic (contain GAD67 immuno-reactivity). The majority of PAG fibers that contact RVM reticulo-spinal GAD67-immuno-reactive neurons also contained GAD67 immuno-reactivity. Thus, there is an inhibitory projection from PAG to inhibitory RVM reticulo-spinal neurons. There were also PAG projections to the RVM, though, that did not contain GAD67 immuno-reactivity. Similar to the pattern above, both GAD67- and non-GAD67-immuno-reactive PAG neurons projected to RVM ON-, OFF-, and NEUTRAL cells in the RVM. These inputs included a GAD67-immuno-reactive projection to GAD67-immuno-reactive ON-cells and non-GAD67 projections to GAD67-immuno-reactive OFF-cells. This pattern is consistent with PAG neurons producing anti-nociception by direct excitation of RVM OFF-cells and inhibition of ON-cells (Morgan et al. 2008).
5.6.3. Caudal Ventro-Lateral Medulla (CVLM)
In several species including rat, mouse, cat, monkeys and man, the CVLM is located in the ventro-lateral quadrant of the caudal-most aspect of the medulla oblongata (Figure 3). The ascending projections from the spinal cord to the CVLM are anatomically segregated, with the lateral CVLM (CVLMlat) receiving mainly afferents from the superficial DH, namely from nociceptively responsive neurons located in lamina I. An important proportion of VLMlat-projecting neurons is located at lamina II, which appears to be a special feature of this spino-fugal pathway. Circuits capable of conveying CVLM-elicited anti-nociception include a direct reciprocal CVLM-spinal loop (Figure 3). Additionally, the CVLM is also bi-directionally connected with both the RVM and DReN (below), building a cooperative `triad´ (Martins and Tavares 2017).
Nociceptive Inputs. In pentobarbitone-anesthetized control and monoarthritic rats, electrophysiological recordings were used to characterize neuronal responses to noxious pinch, heat, cold and colo-rectal distension. CVLM neurons gave excitatory, inhibitory or no response to noxious test stimulation. Response patterns for part of the neurons varied with sub-modality of test stimulation; e.g., a cell with an excitatory response to heat could give no or an inhibitory response to cold (Pinto-Ribeiro et al. 2011). Visceral and somatic types of pain exhibit crucial differences not only in the experience, but also in their peripheral and central processing. In urethane-anesthetized adult male Wistar rats, responses of CVLM neurons were investigated to visceral (colo-rectal distension, CRD) and somatic (squeezing of the tail) noxious stimulations. The CVLM of healthy control rats, along with harboring of cells excited by both stimulations (23.7%), contained neurons that were activated by either visceral (31.9%) or somatic noxious stimuli (44.4%) (Lyubashina et al. 2019). In anesthetised rats, following both direct muscle stimulation and L5 ventral-root stimulation, fatigue-related c-fos gene expression was most prominent in the DH of the ipsilateral L2-L5 segments and within the ipsilateral NTS, the CVLM and RVL, and the intermediate reticular nucleus, and contralaterally (Maisky et al. 2002). Note the muscle fatigue activates group III/IV afferents.
Other Inputs. The CVLMlat receives inputs from the somatosensory and motor cortices, the IL PFC, IC, and limbic cortices, the CeA, lateral (lHYP), posterior HYP (pHYP), PVN, PAG, red nucleus, PBN, NRM, NTS, lateral reticular nucleus (LRN), dorsal and ventral medullary RF, and the lateral cerebellar nucleus (Cobos et al. 2003).
Outputs. The CVLMlat projects to the PAG, red nucleus or lateral cerebellar nucleus (Cobos et al. 2003). The projections to spinal DH laminae involved in nociceptive transmission originate exclusively in the CVLMlat. The CVLMlat is integrated in a disynaptic pathway involving spinally projecting pontine NA A5 neurons, which appears to convey α2-adreno-receptor-mediated analgesia produced from the VLM. The descending CVLMlat-spinal pathway targets lamina I, IV–V and X. Terminal boutons from lamina I neurons on CVLMlat neurons, that project to the spinal cord, suggest that the ascending nociceptive input from the spinal cord directly activates CVLMlat neurons. Electrophysiological mapping of the VLM has shown that it contains inhibitory neurons (OFF-like neurons) along with excitatory cells (ON-like cells) which indicates that the descending modulation from the VLM may include facilitatory modulation, along with the inhibitory effects. Neurons in the CVLMlat and in DH lamina I are reciprocally connected by a closed loop that is likely to mediate feedback control of supraspinal nociceptive transmission (Martins and Tavares 2017; Tavares and Lima 2002).
The CVLMlat is also activated in response to increases in blood pressure. Increases in blood pressure are a feature of the `fight or flight”´ response. Altogether, the VLM is an integrative center which is involved in producing the adequate pain, motor and cardio-vascular responses (Martins and Tavares 2017).
5.6.4. Dorsal Reticular Nucleus (DReN)
The DReN is located in the caudal-most aspect of the medulla oblongata in several species including rat, cat, monkey and man. It is located in the most caudal, dorso-lateral portion of the medulla (Figure 3; Martins and Tavares 2017). DReN neurons are reciprocally connected with DH lamina I neurons, thus forming a reverberative nociceptive circuit.
Inputs. The DReN is targeted from fibers originating from deeper layers in the spinal cord, namely from laminae IV–V, which specifically terminate in the lateral part of the DReN, and from lamina VII which terminate at the medial part of the nucleus. DReN neurons are activated only or mainly by noxious stimulation, whose intensity is reflected in the firing rate. They have large receptive fields that often cover the whole body surface. The DReN also receives projections from the A1 and C1 cell groups (Lima and Almeida 2002; Martins and Tavares 2017).
Outputs. The DReN is reciprocally connected with sensory medullary nuclei (e.g., nucleus cuneatus and spinal trigeminal nucleus pars caudalis), and with many brainstem and diencephalic nuclei involved in anti-nociception and/or autonomic control. Anterograde tracing showed that fibers and terminal boutons labeled from the DReN were located predominately in the brainstem, although extending also to the forebrain. In the rat medulla oblongata, anterograde labeling appeared in the RVM, CVLM, NTS, orofacial motor nuclei, and inferior olive (IO). Labeling was also present in the LC, NA A5 and A7 cell groups, PBN and deep cerebellar nuclei. In the midbrain, it was located in the PAG, SN, deep mesencephalic, oculomotor and anterior pretectal nuclei. In the diencephalon, fibers and terminal boutons occurred mainly in THAL nuclei, and in the HYP arcuate (HYP ARC), PVN, lateral, posterior, peri- and paraventricular areas. Telencephalic labeling was less intense and concentrated in the septal nuclei, globus pallidus and AMY. This suggests that the DReN is possibly implicated in the modulation of: (i) the ascending nociceptive transmission involved in the motivational-affective dimension of pain; (ii) the endogenous supraspinal pain control system centered in the PAG -RVM-spinal cord circuitry; (iii) the motor reactions associated with pain (Leite-Almeida et al. 2006). The medullary DReN is also reciprocally connected with the spinal DH (Leite-Almeida et al. 2006; Martins and Tavares 2017).
DH-DReN-Cerebellum Connection. The cerebellum receives input from nociceptors, which may serve to adjust motor programmes in response to pain and injury. A significant proportion of spino-reticular DH cells projecting to the DReN respond to noxious mechanical stimuli. One of the functions of this pathway may be to provide the cerebellum with nociceptive information (Huma et al. 2015).
5.6.5. Case Report: Acute Thoracoabdominal Trauma with Severe Pain and Autonomic Dysregulation
A 30-year old male patient arrived in the Emergency Department in acute distress following blunt trauma (high-speed motor vehicle collision) to the thorax and abdomen. He was conscious but exhibited shallow breathing, diaphoresis, tachycardia (HR 135 bpm), and fluctuating blood pressure (ranging from 90/60 to 150/100 mmHg). He reported excruciating, stabbing pain rated 10/10 (visual analogue scale), radiating from the lower ribs to the epigastrium. His voice was weak, and he appeared anxious and intermittently unresponsive. Neurological findings were hyperalgesia over lower thoracic dermatomes, reflexive guarding of abdominal muscles, and panic-like behavior. CT scan showed Rib fractures (T7–T10), and hepatic laceration. Ketamine infusion, high-flow oxygen therapy, and fluid resuscitation were initiated. Psychological support was necessary to manage affective amplification of pain. Within 48 hours, pain scores dropped to 4/10, cardiovascular parameters stabilized, and the patient regained emotional composure. By day 4, he was ambulating with mild discomfort and discharged with outpatient pain and trauma follow-up.
5.7. Hypothalamus (HYP)
The HYP is a diencephalic structure in the BFB, consisting of several nuclei (Takayanagi and Onaka 2021). The HYP comprises thousands of distinct cell types that form redundant yet functionally discrete circuits (Fong et al. 2023).
Functions. The HYP is involved in multiple functions serving homeostasis, which is defined as the maintenance of the internal environment that includes physiological variables such as heart rate, blood pressure, body temperature and blood sugar concentration within a certain narrow ranges. Specific functions include stress responses, control of arousal, regulation of sleep/wake cycles, regulation of body temperature and metabolism, feeding behavior, and reproductive behavior etc. (Takayanagi and Onaka 2021).
Nociceptive Inputs. The STTr sends collaterals to HYP, also indirectly via the noradrenergic A1 cell group (Figure 2; Kuner and Kuner 2021). The HYP receives converging nociceptive and visceral inputs from the spinal DHs and trigeminal nuclei (Benarroch 2006; Jänig 2014), and direct and indirect (via PBN and NA A1 cells) nociceptive inputs from the STTr (Kuner and Kuner 2021). In mice, formalin injection induced significantly increased expression of fos in the PVN, among which OXT-containing neurons are one neuronal phenotype. Under inflammatory pain, neurons in the lPBN may play essential roles in transmitting noxious information to the PVN (Ren et al. 2024).
Other Inputs. The different HYP nuclei receive and emit differentiated inputs and outputs. Largely, in addition to nociceptive inputs, the HYP receives direct or indirect inputs from somatic and visceral sensory receptors of different kinds, as well as from the HIPP formation, gyrus cinguli, piriform cortex, OFC, mammilary body, septum, AMY, THAL, from retinal, olfactory and auditory fibers, from the brainstem RF, PAG, raphé nuclei, LC, and NTS (Brodal 1981).
Outputs. In part, the efferent HYP projections are reciprocal to the afferent inputs. Among `ascending´ connections, the mamillary tract to the anterior THAL nucleus is the most massive. Other efferents target the septum, HIPP, pulvinar, AMY, vlPAG, pretectal area, superior colliculi, midbrain RF, raphé nuclei, LC, NTS, dorsal motor nucleus of the vagus, pre-ganglionic visceral nuclei, inter-medio-lateral cell column (IML) of the spinal cord (Brodal 1981). There is a descending HYP-DH DA system. In the adult albino rat, cells in the PVN project to autonomic centers in the brainstem or in the spinal cord of the adult albino rat. Both OXT- and AVP-stained cells in the PVN project to the spinal cord and (or) to the dorsal vagal complex (Sawchenko and Swanson 1982).
Hypothalamic Dopamine (DA) Cell Cluster. The dorsal posterior HYP contains a DA cluster called A11 cell group. These neurons, approximately 300 in rats and 130 in mice, project to the neocortex which might be related to changes in the perception of ascending sensory information; 5-HT dorsal raphé nucleus (DRN), promoting cardio-vascular and sympathetic activity. They also send descending projections as the source of spinal DA. The terminals are most concentrated in the superficial sensory-related DH and inter-medio-lateral nucleus. The loss of A11 neurons causes a disinhibition of sensory inputs and favors the occurrence of abnormal visceral or muscular sensations. The spinal cord of rats, cats, monkeys, and humans express DA receptors D1, D2, and D3. DA and D2 agonists can depress the monosynaptic reflex amplitude, dependent on D3 receptors, since this effect was absent in D3 knockout mice. Hence, A11 modulatory neurons could hypothetically inhibit spinal somatosensory and sympathetic autonomic circuits (Klein et al. 2019).
5.8. Midbrain Dopamine (DA) Neurons
The midbrain DA complex comprises the SNc, VTA and RRF, which contain the A9, A10 and A8 groups of nigro-striatal, meso-limbic and meso-cortical DA neurons, respectively. Additionally, there are dorsal-caudal A10dc and rostro-ventral A10 extensions into the vlPAG and supra-mammillary nucleus, respectively. Where they intermingle, NA A8 cells are morphologically indistinguishable from cells in A9 or A10 cells, as are A9 and A10 neurons at any imaginary boundary between the VTA and SNc. Still, A8, A9 and A10 are structurally and functionally differentiated, as also reflected in the relatively distinct, albeit broadly overlapping, topographies and functions of their ascending projections. The A8–10 nomenclature refers explicitly to DA neurons, whereas the VTA, SNc, RRF are brainstem structures that also contain locally and distantly projecting neurons that utilize as transmitters, either co-expressed with DA or separately, GABA, glutamate, cholecystokinin (CCK) and NT and possibly as yet unknown compounds (Yetnikoff et al. 2014). In the VTA, the different kinds of neurons interact via intrinsic connections and have differentiated external inputs and outputs (Morales and Margolis 2017).
Functions. DA is a neurotransmitter, synthesized in both the CNS and the periphery. DA receptors are widely expressed in the body and function in both the PNS and the CNS. DA is not simply an excitatory or inhibitory neurotransmitter, since it can bind to different G protein-coupled receptors (GPCRs). The DA system plays important roles in neuromodulation, such as arousal, attention, motivation, affect, feeding, olfaction, hormone regulation, maternal and reproductive behaviors, sleep regulation, spatial memory function, motivation, reward, cognitive function, and influences the immune, cardio-vascular, gastro-intestinal, and renal systems, as well as movement and motor control. Regarding its physiological role (Klein et al. 2019).
5.8.1. General Inputs
In rodents and primates, SNc and VTA receive a large array of differentially distributed inputs from, among others, telencephalon, diencephalon, mesencephalon, pons, medulla, and cerebellum, the connections varying in strength. Inputs to the RRF resemble those to the VTA (Kelly and Fudge 2018; Morales and Margolis 2017; Yetnikoff et al. 2014). For example, VTA DA neurons receive glutamatergic inputs from the mPFC, BNST, lHb, pedunculo-pontine tegmentum, latero-dorsal tegmental nucleus (LDT), PAG, DRN, GABAergic inputs from the ventral pallidum, lHYP, rostro-medial meso-pontine tegmental nucleus (for further inputs to glutamatergic and GABAergic cells see Morales and Margolis 2017).
5.8.2. Nocicpeptive Inputs
Midbrain DA neurons change their excitability upon noxious stimulation or relief from pain-like states. DA cells are preferentially activated by appetitive versus aversive stimuli. By an acute aversive stimulus (foot pinch), DA neurons in the VTA were uniformly inhibited and a non-DA neuronal population was excited. In rodents, DA neurons, particularly in the dorsal VTA, were inhibited by noxious foot shocks while in ventral VTA DA neurons, foot-shocks induced phasic excitation (Mitsi and Zachariou 2016). The meso-limbic DA system indirectly receives somatosensory inputs, including nociceptive inputs, from the lHYP mediated by the lHb. The lHYP-lHb pathway is necessary for nociceptive modulation of the DA system (Dai et al. 2022; Ogawa and Watabe-Uchida 2018). Furthermore, nociceptive signals from the spinal cord are relayed by a sub-population of lPBN neurons to the SNr. – In anesthetized rats, many DA neurons exhibited a short-latency response to noxious stimuli, which appears to be mediated by the nociceptive-recipient PBN. During the application of noxious foot shock, simultaneous extracellular recordings showed that the PBN neurons exhibited a short-latency, short-duration excitation to foot shock, while DA neurons exhibited a short-latency but slightly later inhibition, suggesting that the PBN is an important source of short-latency nociceptive input to the DA neurons (Coizet et al. 2010). Furthermore, nociceptive signals from the spinal cord were relayed by a sub-population of lPBN neurons to the SNr. SNr-projecting lPBN neurons are activated by noxious stimuli, and silencing them blocks pain responses in two different models of pain (Yang et al. 2021). In adult anesthetized female albino rats, extracellular recordings were obtained from neurons in the VTA, the SN, including the zona compacta (SNc) and the zona reticulata (SNr), and the midbrain RF. Based on electrophysiological characteristics, the neurons were divided into two types. Type I neurons, with relatively long spike durations and slow discharge rates, were confined to the VTA and SNc. Type II neurons, with shorter spike durations and faster discharge rates, were observed in the SNr and RF as well as the VTA and SNc. Aversive foot pinch (FP) and tail pinch (TP) elicited locomotion, sniffing and gnawing responses, and stimulation of the vaginal cervix (VC) eliciting lordosis responses, vocalization and immobility. For approximately two-thirds of the neurons, the effects of the three peripheral stimuli were similar, they were either activated or suppressed. This is consistent with the view that VTA and SN neurons integrate a number of central and peripheral inputs (Maeda and Mogenson 1982). In the anesthetized rats, a majority of 194 extracellularly recorded presumed DA neurons (78%) were inhibited by intensive electrical stimulation performed at the tail (PNS) and 15% were excited. Single-shock stimulation of the lHb inhibited 89% of the tested DAergic neurons, most of which (83.8%) were also inhibited by PNS. lHb stimulation increased PNS-induced inhibition of DA neurons and electrical destruction of ipsilateral lHb depressed their nociceptive responses. This may suggest that DAergic neurons the lHb shares a step in nociceptive projection to the SN (Gao et al. 1990). Using simultaneous extracellular single-unit recordings in the SN pars compacta and in the lHb of rats, of 45 pairs of neurons responding to peripheral nociceptive stimulation, 41 pairs of nigral DA neurons were inhibited by peripheral nociceptive stimulation, while lHb neurons were excited. In 14 pairs, when sweeps were triggered randomly by spontaneous spikes from lHb neurons, the spontaneous firing rate of the DA neurons during the first 250 ms after the sweep was much lower than rates after this time period. These cross-correlations between the spontaneous activities of these two nuclei suggest that the excitation of lHb neurons induced by peripheral nociceptive stimulation might be directly responsible for inhibition of nigral DA neurons (Gao et al. 1996).
5.8.3. Outputs
There are four main ascending pathways: nigro-striatal, meso-limbic, meso-cortical, and tubero-infundibular. The midbrain DA complex gives rise to a meso-limbic pathway to the limbic forebrain and orbito-frontal cortex (OFC) and a nigro-striatal pathway to the BG striatum. Generally, nearly every telencephalic region receiving DA innervation has a medial aspect innervated by the VTA and a lateral aspect innervated by the SNc, the two separated by a broad district in which VTA and SNc projections overlap. Still, SNc projects mainly to the ventro-medial caudate-putamen and to a lesser extent to cortical structures, AMY and the subthalamic nucleus (STN) (Morales and Margolis 2017; Yetnikoff et al. 2014). Furthermore, DA efferents target in particular the mPFC (Vander Weele et al. 2019). Spinally descending DA fibers originate from the HYP (Li et al. 2019; Lindvall et al. 1983; Puopolo 2019).
In humans, midbrain DA neurons from the VTA project to the PFC via the meso-cortical pathway and to the NAc via the meso-limbic pathway. These pathways constitute the meso-cortico-limbic system, which plays a role in reward and motivation. The VTA region also gives rise to DA projections to the AMY, HIPP, CC, and olfactory bulb. The meso-limbic DA system has been implicated in positive reward and appetite-motivated behaviors. However, aversive stimuli and stress may also lead to DA release by this same system, which might correspond to a generalized behavioral arousal involving seeking safety. In animal models, micro-injections of DA into the NAc increased locomotor activity, exploratory behaviors, conditioned approach responses, and anticipatory sexual behaviors. When a GABAA receptor antagonist was injected into the VTA, locomotion was increased. This phenomenon occurred because GABAergic neurons inhibited DA neurons and the antagonist blocked this inhibition. Therefore, enhanced DA function in meso-limbic system increased behavioral activity, while lesions of this system could eliminate exploratory and appetitive behaviors (Klein et al. 2019).
In rats anesthetised with halothane, 226 spontaneously active neurons were recorded from the SN. 112 neurons (50%) were nociceptive. Approximately equal proportions of nociceptive and non-nociceptive SN neurons projected to the THAL. The majority of nigro-striatal neurons were non-nociceptive (Pay and Barasi 1982). In the rat, immediately after foot-shock termination, extracellular DA concentrations were increased in the NAc shell but remained unaltered in the NAc core. Such activation, especially in the ventral striatum and NAc, also occurred after the application of acute noxious (thermal) stimulus. In rodents, voltammetry showed changes in NAc DA release upon termination of a noxious stimulus (tail-pinch). DA release in the NAc was promoted by noxious tail stimulation and local VTA micro-injection of capsaicin. On the other hand, non-DA neurons in the VTA of anesthetized rats were excited by aversive stimuli, including pain. Moreover, fMRI in both humans and rodents showed that the offset of a noxious stimulus increased the activation of the meso-limbic DA system. Micro-dialysis supported the hypothesis that pain alleviation is modulated by changes in DA concentrations in the NAc (Mitsi and Zachariou 2016).
5.9. Locus Coeruleus (LC) and Other Cell Groups
The brainstem contains a number of catecholamine nuclei and cell groups. Through action on α₁- and α₂-adrenoceptors, NA is involved in intrinsic control of pain (Pertovaara 2013).
5.9.1. Locus Coeruleus (LC)
The locus coeruleus (actually: caeruleus: celestial blue) is a cluster of relatively large neurons containing NA, located bilaterally in the brainstem just under the cerebellum and lateral to the fourth ventricle (Poe et al. 2020). Like other neuromodulatory structures, the LC contains an exceedingly small number of cells, yet projects to much of the brain. The human LC is estimated to contain approximately 30,000 neurons that provide NA to a substantial fraction of the brain´s 100 billion neurons. The LC is therefore well positioned to modulate a wide range of functions, including homeostasis, sensory processing, motor behavior, and cognition (Bari et al. 2019). Virtually all neurons within rodent and primate LC contain NA as the primary transmitter. However, multiple peptides co-localize within LC neurons, including vasopressin (AVP), STT, neuropeptide Y (NPY), ENK, NT, corticotropin-releasing hormone (CRH) and GAL. NA neurons project broadly throughout the neuraxis, from the spinal cord to the neocortex (Aston-Jones and Waterhouse 2016).
Inputs. Almost all areas of the neocortex project to the LC, with strong glutamatergic projections from the PFC and CRH projections from the AMY (Bari et al. 2019). Besides by noxious stimuli (below), the LC-NA neurons in behaving rats to monkeys respond to (even mild) non-noxious environmental stimuli of many modalities, including auditory, visual, proprioceptive and somatosensory stimuli, depending on the present vigilance state of the animal (Aston-Jones and Bloom 1981). All LC neurons receive inputs related to autonomic arousal, but distinct sub-populations can encode specific cognitive processes, presumably through more specific inputs from the forebrain areas. LC neurons receive inputs from many regions of the brainstem and forebrain, including the PFC, BNST, CeA, HYP PVN, NTS, nucleus paragigantocellularis (PG), and the nucleus prepositus hypoglossi (NPH) (Ross and van Bockstaele 2020). The PFC is a major source of input to LC, linking circuits involved in higher cognitive and affective processes to the LC efferent system (Berridge and Waterhouse 2003).
Nociceptive Inputs. The STTr projects to the THAL, but on its way, it sends collaterals to the brainstem RF, NA cell groups A1. A5-A7, that projects further to the HYP and many other structures (Kuner and Kuner 2019; Woulfe et al. 1990; below)). Noxious peripheral stimuli increase the activity in LC. In anesthetized rats, LC neurons were potently activated by foot shock, this effect being indirect because attenuated or blocked by pharmacologic blockade of the PG (Chiang and Aston-Jones 1993). In awake mice, upon exposure to acute nociceptive stimulation consisting of a pinch and application of heat (55 °C) to the tail, both stimuli resulted in rapid and transient (<15 s) increases in the activity of LC NA neurons while control stimuli did not induce any changes (Moriya et al. 2019).
Outputs. LC (A6 group) neurons send diffuse, but regionally specific, projections throughout the neuraxis where they release NA as a neurotransmitter and neuromodulator (Berridge and Waterhouse 2003; Foote et al. 1983; Valentino and van Bockstaele 2015). LC provides the sole source of NA to the neocortex and HIPP, but less strongly innervates the BG (Berridge and Waterhouse 2003). NA cells in groups A5, A7 in the ventro-caudal LC portion and the sub-coeruleus area (together with rostral C1 adrenergic neurons) belong to the `descending catecholaminergic system´, projecting to the spinal cord including the DH and sympathetic pre-ganglionic neurons in the thoracic segments. These neurons represent the efferent arm of the central sympathetic system that influences the sympathetic outflow (Kvetnansky et al. 2009). In part, LC projections are segregated into distinct output channels and have the potential for differential release and actions of NA on its projection targets, thereby enabling differentiated modulation of diverse behaviors and cognitive functions. There are also projections to motor areas, such as the motor cortex, BG, cerebellum and inferior olive (IO) (Poe et al. 2020).
Descending Pain Modulation. The LC is involved in the descending modulation of pain, mainly through direct spinal cord projections and effects on RVM activity via NAergic projections. Ventrally located LC neurons were labeled after injecting pseudorabies virus (PRV) into the RVM. Moreover, stimulation of LC NA neurons increased the release of NA and increased 1-adrenoceptor concentrations of α1-adrenoceptors (NAα1R) in NRM, leading to analgesia. By contrast, LC-NRM projecting NA neurons possibly induced hyperalgesia by activating NRM NAα1R. During opioid withdrawal, administration of the NAα1R antagonist prazosin in NRM significantly decreased the activities of LC NA projections and suppressed hyperalgesia. RVM ON-cells received LC NA inputs and contained NAα1R, which contributed to hyperalgesia during opioid withdrawal, while inhibition of NAα2R-expressing ON-cells by clonidine suppressed DAMGO-mediated analgesia instead of modulating hyperalgesia during opioid withdrawal. OFF-cells also received dense LC NA input, and mainly expressed NAα1R and some co-expressed NAα2R. Overall, this suggests that LC-RVM projections trigger not only anti-nociceptive but also pro-nociceptive effects by modulating RVM ON- and OFF-cells (Peng et al. 2023).
5.9.2. A and C Cell Groups
Ascending projections from spinal lamina I run through and terminate in brainstem regions that contain adrenergic/NA cells. In the Cynomolgus monkey, the lamina I projections were anterogradely labeled and adrenaline/NA-containing neurons were labeled immuno-cytochemically. The terminations of the lamina I ascending projections through the medulla and pons strongly overlapped with the locations of adrenaline/NA cells in the entire rostro-caudal VLM extent (A1 caudally, C1 rostrally): the NTS and the dorso-medial medullary RF (A2 caudally, C2 rostrally); the ventro-lateral pons (A5); the LC (A6); and the sub-coerulear region, the Kölliker-Fuse nucleus, and the medial and lateral PBN (A7). Close appositions between lamina I terminal varicosities and adrenaline/NA dendrites and somata occurred, particularly in the A1, A5 and the A7 cell groups on the contralateral side. The afferent input relayed by these lamina I projections could provide information about pain, temperature, and metabolic state. Nociceptive lamina I input to adrenaline/NA cell regions with projections back to the spinal cord could form a feedback loop for control of spinal sensory, autonomic and motor activity (Westlund and Craig 1996).
Brainstem NA neuron receive dissimilar afferents via various circuits to coordinate organismal responses to internal and environmental challenges, including pain. A1, A5, A6, and A7 receive nociceptive inputs from the lateral STTr (Dostrovsky 2000; Kuner and Kuner 2021) and show extensive descending projections to the spinal cord. In rats treated with formalin into the hindpaw 30 minutes after sub-cutaneous morphine injection, NA A5 and A7 cell groups contained significantly increased fos in response to intra-plantar formalin injection (an acute noxious input) (Bajic and Commons 2010).
Brainstem NA neuron project to local segmental (brainstem), cephalic (telencephalon and diencephalon), and caudal (spinal cord anterior, lateral, and DH) regions of the CNS, to the LC (A6), the primary source of NA in the brain, and to spinal pre-ganglionic neurons, which synapse onto post-ganglionic neurons, and thence to NA neurons located at the paravertebral sympathetic ganglia, supporting an anatomical hierarchy that regulates skeletal muscle innervation, neuromuscular transmission, and muscle trophism. Together with the parasympathetic nervous system, this NA neuron network accounts for the integrated organismal response to physiological or pathological challenges, including pain. Increased sympathetic activity is associated with better cognitive performance in individuals over 65 year leading to hypothesize interactions between the ANS and higher-level brain functions in neurological and neuropsychiatric disorders (Delbono et al. 2022)
A1 Group. The rat A1 NA cell group lies in the CVLM from upper cervical spinal cord levels to the level of the area postrema. In anesthetized rats, noxious thermal stimulation of the hindleg caused c-fos activation in the HYP PVN, in the adeno-hypophysis, and in other brain nuclei, including the cathecholaminergic cell groups of the caudal medulla. The NA A1 cells receive nociceptive inputs from the lateral STTr (Dostrovsky 2000) and relay it to the HYP PVN (Figure 2) and other structures (not shown for graphical reasons) (Kuner and Kuner 2021). Consequently, noxious stimulation caused corticosterone plasma release (Pan et al. 1999). Moreover, presumed efferent target sites include the NTS, RVM, dorsal PBN, Kölliker-Fuse nucleus, PAG, HYP dorso-medial nucleus (DMH) and lateral nucleus (lHYP), PVN, peri-fornical region, zona incerta, supraoptic nucleus (SON), BNST, and organum vasculosum of the lamina terminalis (Woulfe et al. 1990).
A5 Group. These cells receive a wide range of inputs. Importantly, these include connections from neurons of spinal lamina I (Dostrovsky 2000; Kuner and Kuner 2021), and from the ventral region of the medulla, more specifically from the CVLM. Both lamina I and the CVLM region play a predominant role in the integration of nociceptive responses. Connections have also been described from the trigeminal afferents from the nasal cavity (Rocha et al. 2024). The A5 group projects segmentally to A6 [locus coeruleus (LC), actually: caeruleus: celestial blue], with a possible role in cognition, and spinally, to target pre-ganglionic ACh neurons in the IML. The synapse between A5 pre-ganglionic and post-ganglionic NA neurons located at the paravertebral sympathetic ganglia support a role in skeletal muscle regulation. Skeletal muscle post-ganglionic sympathetic neuron projections have been related to neuromuscular organization, transmission, and skeletal muscle mass maintenance with development and ageing. The A5 group is supposed to contribute to the regulation of nociceptive messages at the spinal cord level (Kwiat et al. 1993).
A6 Group. The A6 cluster (LC) projects to the cerebellum, midbrain, globus pallidum, and various HYP areas. It also projects to the spinal DH to regulate pain perception and plays a role in sensory-motor behavior (Delbono et al. 2022).
A7 Group. A7 cell terminals are closely related to the ACh MNs in the VH and may influence motor output through NA binding to NA receptors expressed by the VH neurons (Delbono et al. 2022). Electrical lHYP stimulation produced anti-nociception, which was partially blocked by intra-thecal α-adrenergic antagonists. SP-immuno-reactive neurons in the lHYP project near the NA A7 cell group, which effects anti-nociception in the DH. However, while some A7 cells inhibit nociception through the action of α2-adrenoceptors in the spinal DH, other A7 cells increase nociception through the action of α1-adrenoceptors in the spinal DH (Holden and Naleway 2001).
5.10. Raphé Nuclei
The raphé nuclei are a collection of functionally and anatomically diverse cell groups that span the brainstem and contain the majority of the 5-TH-producing neurons in the CNS (Brodal 1981). From caudal to rostral, the raphé nuclei are, in the medulla oblongata: nucleus raphé obscurus, NRM, nucleus raphé pallidus; in the pons: nucleus raphé pontis, nucleus centralis inferior; in the midbrain: nucleus centralis superior, median raphé nucleus, DRN, caudal linear nucleus (Nieuwenhuys et al. 1978).
Inputs. Noxious peripheral stimuli cause activity changes in neurons of the RVM, which contains the NRM. For example, in anesthetized rats, 63 raphé-spinal units in NRM were classified into 5-HT and non-5-HT units. Except one, all units showed either excitatory (n = 39) or inhibitory (n = 23) responses to noxious stepwise heating of the tail with hot water at 52o C. The threshold temperature was around 44oC. The excitatory or inhibitory responses of raphé-spinal units correlated well with those elicited by noxious pinching, but did not correlate with the different transmitter populations (5-HT or non-5-HT) of the NRM units. In addition, in parallel to LC neurons, 5-HT neurons are activated by noxious peripheral stimuli in awake mice. Exposure to acute nociceptive stimulation consisting of a pinch and application of heat (55 °C) to the tail resulted in rapid and transient (<15 s) increases in the activity of RVM/NRM 5-HT neurons while control stimuli did not induce any changes (Moriya et al. 2019).
Many upper brain areas control the 5-HT neurons in the NRM. 5-HT neurons in the RVM receive direct excitatory inputs from the S1. Other regions control the 5-HT, including the orbital cortex, CC, medial and lateral POA, several areas of the HYP and the habenula (Hb). Both NRM and DReN are the targets of regulation via projections from several nuclei in the brainstem and the midbrain, including several catecholaminergic and cholinergic (ACh) cell groups (Cortes-Altamirano et al. 2018; Kuner and Kuner 2021).
Outputs. In general, the raphé nuclei can be divided into neuron groups with primarily ascending and primarily descending projections. The ascending raphé nuclei (primarily the DRN, which also projects to the spinal cord, though, and the median raphé nucleus) contain 5-HT neurons that project to the majority of the brain, including the PFC, CC, parietal and occipital cortical regions, HIPP, THAL, cerebellum, and midbrain, where they regulate a variety of behaviors related to stress, mood, anxiety (Brodal 1981; Myers et al. 2017). The descending nuclei, including the DRN, NRM and raphé pallidus, project to the spinal cord and regulate autonomic and motor functions and control transmission of nociceptive signals in the DH. The NRM in the RVM sends dense 5-HT fibers to the spinal DH, building both inhibitory and facilitatory pathways (Kuner and Kuner 2021; Zhang et al. 2024). In the cat, more than 80% of the raphé neurons that project to the spinal cord are 5-HT neurons in each of the nuclei. More than 85% of the descending raphé-spinal neurons in the two caudal nuclei, nucleus raphé pallidus and nucleus raphé obscurus, are 5-HT, whereas 75% of the raphé-spinal neurons in the NRM contain 5-HT (Bowker et al. 1987).
5.11. Basal Ganglia (BG)
In higher mammals, the BG consist of bilateral sub-cortical nuclei in the basement of the brain. There are three broad domains: the dorso-lateral, dorso-medial and ventral BG (Groenewegen 2003; Humphries and Prescott 2010; Tewari et al. 2016). The dorso-lateral BG contains the striatum (consisting of nucleus caudatus and putamen), the STN, globus pallidus externus (GPe), globus pallidus internus (GPi) and SNr. The STN is integrated into the network of BG nuclei (Nelson and Kreitzer 2014). The NAc is the ventro-medial input station, with sub-territories including a core and a shell (Harris and Peng 2020).
Functions. The BG have been implicated in processes as diverse as feedforward motor planning, organization of rapidly alternating or sequential motor acts, predicting future events, reward, reinforcement, habit formation, procedural motor learning, retention and recall of well-learned motor skill, working memory, attention, emotional, motivational, associative and cognitive processes (Haber 2016; Herrero et al. 2002; Nelson and Kreitzer 2014; Roth and Ding 2024). The BG including the STN and SNr are also involved in pain-related responses and modulation (Jia et al. 2022). It has been suggested that the BG may be implicated in the (i) sensory-discriminative dimension of pain, (ii) affective dimension of pain, (iii) cognitive dimension of pain, (iv) modulation of nociceptive information and (v) sensory gating of nociceptive information to higher motor areas (Chudler and Dong 1995).
Nociceptive Inputs. The BG process non-noxious and noxious somatosensory information. Some BG neurons encode stimulus intensity, but do not appear to encode stimulus location since they have large receptive fields. Many BG neurons responsive to somatosensory stimulation are activated exclusively or differentially by noxious stimulation (Chudler and Dong 1995). – The putamen is one of the major BG input sites and is frequently activated during pain. Nine individuals with lesions involving the putamen underwent both psychophysical and functional imaging assessment of perceived pain and pain-related brain activation. These individuals exhibited intact tactile thresholds, but reduced heat-pain sensitivity and widespread reductions in pain-related cortical activity in comparison with 14 age-matched healthy subjects. This indicates that the putamen may contribute importantly to the shaping of an individual´s subjective sensory experience by utilizing internal cognitive information to influence activity of large areas of the cerebral cortex (Starr et al. 2011).
BG Outputs. The major BG outputs emerge from the GPi and SNr and are inhibitory. Their outputs project to the brainstem and spinal cord and the THAL, which exchanges excitatory connections with the cerebral cortex (Brodal 1981; DeLong and Wichmann 2007; Wichmann and DeLong 2016). The dorsal striatum is connected to the descending pain modulatory system and in particular to the RVM through the DReN (Boccella et al. 2020). The NAc projects to areas within the diencephalon and pallidal complex, such as the BNST, nucleus medio-dorsalis THAL, the globus pallidus and the sub-pallidal region, lHb nucleus, lHYP and SN. The core projects to areas such as the dorso-lateral region of the ventral pallidum, whereas the shell projects to the rostral-caudal regions of the lHYP, and ventro-medial region of the ventral pallidum (Harris and Peng 2020).
5.11.1. Striatum
The striatum, NAc and STN form the major input stations of the BG. Inputs derive from the entire cerebro-cortical mantle, and various THAL nuclei (the centro-median (CM) nucleus and parafascicular (Pf) nucleus). Cerebral inputs to the striatum arise from neurons whose axons remain in the telencephalon or from pyramidal tract neurons (PTNs) that project to the brainstem/spinal cord and give off collaterals to more than one BG region (Haber 2016; Herrero et al. 2002; Nelson and Kreitzer 2014; Roth and Ding 2024).
Roles of Striatum in Pain. The BG process non-noxious and noxious somatosensory information. Some BG neurons encode stimulus intensity, but do not appear to encode stimulus location since they have large receptive fields. A large proportion of somatosensory neurons within the neostriatum and globus pallidus receive nociceptive information (Chudler et al. 1993), but the electrical or chemical stimulation of the striatum inhibits pain. The pain inhibition passes through the activation of the descending pain modulatory system and involves the RVM. The point of convergence between the dorsal striatum and the descending pain modulatory system lies in the medullary DReN, projecting in turn to the spinal DH. The dorsal striatum is characterized by a large concentration of opiates and cannabinoids (Boccella et al. 2020).
5.11.2. Nucleus Accumbens (NAc)
Neuroimaging studies have implicated the NAc in acute and chronic pain responses and as a source of analgesia (Harris and Peng 2020; Thompson and Neugebauer 2019), as well as in motivational and emotional processing, pleasure, addiction and reward, beyond simply reflecting the rewarding experience of pain offset. The NAc shell may evaluate impending pain and utilize spatial information from the HIPP for appetitive learning, and the core activates with expectation of relief of an aversive stimulus and signals the reward value of pain cessation, as well as utilizes information from the baso-lateral amygdala (BLA) for appetitive learning. The NAc is involved in reward pathways and integration of cortical and affective information in order to assign motivation and value for the selection of appropriate behavioral responses to outside stimuli (Thompson and Neugebauer 2019).
Inputs. The NAc receives signals from pain-related neural structures, including the PFC, ACC, HIPP, subiculum, AMY, ventral pallidum, Hb, THAL, lHYP, VTA, SN, PAG, raphé nuclei and LC. Some connections are bi-directional, such as those to the BG, ventral pallidum, AMY, THAL, HYP, VTA and SN. Afferents to the NAc core include the peri-rhinal cortices, dorsal PL PFC, the SN, and the ACC. Afferents to the shell originate from the ventro-medial prefrontal cortex (vmPFC), the BLA, and the lHYP. Both core and shell receive afferent DA input from the VTA. The NAc responds heavily to painful stimuli, due to its high density of μ-opioid receptors and the activation of several different neurotransmitter systems in the NAc, such as opioids, DA, CGRP, GABA, glutamate, and SP, each of which have been shown to elicit analgesic effects. In both preclinical and clinical models, deep brain stimulation of the NAc has elicited successful analgesia. The multi-functional NAc is important in motivational behavior, including the motivation for avoiding pain (Harris and Peng 2020). In freely moving rats, NAc activity decreased with thermal and electrical noxious stimuli (Thompson and Neugebauer 2019). – The paraventricular thalamic nucleus (PVT) sends putative glutamatergic inputsto the NAc (PVT→NAc), which neural pathway is implicated in pain sensation and non-opioid analgesia. PVT→NAc neuronal activity increased in response to acute thermal/mechanical stimuli and persistent inflammatory pain. Direct optogenetic activation of these PVT neurons or their terminals in the NAc induced pain-like behaviors. Conversely, inhibition of PVT→NAc neurons or their NAc terminals exhibited a potent analgesic effect in both naïve and pathological pain mice, which could not be prevented by pretreatment of naloxone. The PVT→NAc circuit bi-directionally modulated pain behaviors. DA receptor 3 is a candidate target for pain modulation and non-opioid analgesic development (Zhang et al. 2023).
Interaction between Ascending and Descending Pain Control. In humans, employing thermal stimulation to the left radial forearm and a new MRI acquisition protocol, revealed BOLD responses in the ipsilateral DH of segment C6 and corresponding neuronal responses in typical pain-processing brain regions. Correlations showed the functional connectivity between the spinal C6-DH and the THAL, primary somatosensory cortex (S1), bilateral IC, bilateral striatum, and structures of the descending pain-modulatory system such as the HYP, PAG, and AMY. The individual strength of the spinal-PAG coupling predicted individual pain ratings (Sprenger et al. 2015).
5.11.3. Subthalamic Nucleus (STN)
Besides the striatum, the STN is an input station of the BG.
Inputs. The STN receives major inputs from the frontal cortex, THAL, GPe, LC, NRD, SNc and pedunculo-pontine nucleus (PPN) (Brodal 1981; Hamani et al. 2004). Nociception is altered following STN lesions. Nociceptive STN responses are abnormal in a rat model of PD, suggesting that they depend on the integrity of the nigro-striatal DA system. STN neurons exhibit complex responses to noxious stimuli (Pautrat et al. 2018). STN neurons can detect nociceptive stimuli, encode their intensity and generate windup-like plasticity, like WDR neurons in the spinal DH. In the 6-OHDA rodent model of PD, these effects were impaired during DA depletion, as the intensity response was altered in both spinal and subthalamic neurons. In rats, deep brain stimulation in the STN ameliorated 6-OHDA-induced allodynia, and this effect is mediated by descending brainstem projections leading to normalization of nociceptive integration in DH neurons (Charles et al. 2025).
Outputs. The STN sends major projections to the striatum, GPe and GPi, entopeduncular nucleus (in rodents), SNr, THAL, which projects back to the cerebral cortex, and brainstem (Brodal 1981; Grillner and Robertson 2016; Haber 2016; Hamani et al. 2004; Hammond et al. 1983). It also projects to the cerebellum via pontine nuclei (Bostan and Strick 2018).
5.12. Amygdala (AMY)
The AMY is an almond-shaped, heterogeneous nuclear complex embedded deep within the rostral pole of the cerebral temporal lobe (Neugebauer et al. 2020). It is comprised of different nuclei; the lateral amygdala (LA), BLA and CeA nuclei and in between, the intercalated cells (ITC). Altogether, it has nine sub-nuclei (Vogt 2019).
Functions. The AMY has an important role in the processing of pain, emotions and attaching emotional valence to memories and other experiences, in fear conditioning and affect (Vogt 2019). The lateral capsular region of the CeA is crucial for the negative emotional aspects of pain and is also termed as the `nociceptive amygdala´. The BLA is involved in generating the fear response following exposure to a variety of potentially hazardous stimuli and is paramount for memory consolidation of cued fear. By conveying information to the CeA, it helps form responses to avoid injury or pain, which are then executed by brainstem centers (Kuner and Kuner 2021). Thus, the AMY is an important center for the emotional-affective dimension of pain and for pain modulation (Neugebauer 2015).
Inputs. The CeA receives direct and indirect nociceptive inputs from the STTr and from the PBN through the spino-parabrachio-amygdaloid pathway (Allen et al. 2021; Kuner and Kuner 2021). The AMY also receives inputs from other sensory systems, including somatosensory, taste, auditory, and visual (Vogt 2019). The CeA additionally receives more highly processed cortical (mPFC, ACC, IC and rostral HIPP) and THAL inputs (polymodal sensory information) via the LA and BLA. PBN input is integrated with polymodal sensory information from the BLA to generate AMY-mediated pain responses (Allen et al. 2021; Thompson and Neugebauer 2019; Vogt 2019).
Outputs. The CeA forms the major output nucleus of the AMY and provides information back to brainstem areas that control the expression of innate behaviors. The lateral capsular region of the CeA is crucial for the negative emotional aspects of pain and is termed the `nociceptive amygdala´ (Kuner and Kuner 2021). The medial division of the AMY (MeA) and CRH-positive neurons in the lateral CeA project to other limbic structures, including the PFC, the BFB, the NAc, the septal nucleus, THAL, HYP, and brainstem regions involved in behavioral expression, such as PAG and PBN, ANS centers, that project to the spinal cord and regulate autonomic outflow. The LA and BLA also project to areas in the OFC, IC, and to ACC and less to anterior mid-cingulate cortex (aMCC) (Vogt 2019). Especially, BLA projections to the mPFC are involved in pain-related cognitive dysfunction and in fear conditioning, and preferentially target cortico-PAG projection neurons in layer V of the infra-limbic (IL) cortex and cortico-AMY projection neurons in layer II of the PL cortex (Allen et al. 2021; Thompson and Neugebauer 2019). The BLA sends dense glutamatergic projections to the PFC, the CA1 of the ventral hippocampus (vHIPP), and the NAc (Kuner and Kuner 2021). Amygdaloid terminals also appeared to contact catecholaminergic cells in several brainstem regions. The most heavily innervated catecholaminergic cells were the A9 (lateral) and A8 DA cell groups and the C2/A2 adrenergic/NA cell groups in the NTS. A moderate innervation by AMY terminals occurred on rostral LC NA cells (A6 rostral) and adrenergic cells of the RVM (C1). The NA A5 cell group, the main body of LC, the A7 cell group, and subcoeruleus were sparsely innervated (Wallace et al. 1992).
5.13. Habenula (Hb)
The Hb is a phylogenetically old small bilateral epi- THAL structure in the posterior-medial aspect of the dorsal THAL. The Hb can be divided into two major areas, the lHb and medial habenula (mHb), which can be further subdivided, resulting in six main subregions (Antunes et al. 2022).
Functions. The lHb is supposed to play a role in regulating negatively motivated behavior. Specifically, the Hb has been implicated in a large variety of acute and chronic processes, such as pain and analgesia, olfaction, ingestion, mating, endocrine functions, circadian rhythms, reward processing, social behavior, behavioral flexibility, decision-making, cognitive flexibility, contextual memory, as well as in the neurobiology of several psychiatric disorders and neuropsychiatric symptoms, e.g., anxiety, major depressive disorder (MDD), bipolar disorder, autism, schizophrenia, anti-reward or aversion, defensive behavior, agitation/aggressive behavior, substance use disorder, eating disorders, and sleep disturbances (Baker et al. 2022; Dai et al. 2022; Gouveia and Ibrahim 2022; Gouveia et al. 2022; Hu et al. 2020; Metzger et al. 2017).
Inputs. The Hb receives diverse inputs that conyey information about the sensory world and the animal´s internal state (Fore et al. 2018). The lHb receives inhibitory inputs from the mPFC, lateral septum, entopeduncular nucleus, diagonal band of Broca, BG (globus pallidus, ventral pallidum), HYP [lateral preoptic area (lPOA), BNST], supra-chiasmatic nucleus (SCN), and pineal gland (reciprocal). The mHb receives mostly excitatory inputs from the BFB (septum, diagonal band of Broca), pineal gland, and NAc (Baker and Mizumori 2017; Boulos et al. 2017; Gouveia and Ibrahim 2022; Gouveia et al. 2022; Hikosaka 2010; Roy and Parhar 2022). In anesthetized rats, about two thirds of the neurons in the lHb responded to peripheral noxious stimuli. The firing pattern of lHb cells was either excitatory (75%) or inhibitory (24%), and it was also related to the intensity of the stimulus, and the receptive field were large and bilateral. Most of these cells did not respond to non-noxious stimuli (Boadas-Vaello et al. 2017). In male rats, extracellular single-unit recordings and head-mounted micro-endoscopic Ca2+ imaging revealed that nociceptive stimulation by tail-pinch excited lHYP and lHb neurons. This suggests that noxious stimulation recruits the lHYP-lHb pathway to inhibit the meso-limbic DA system. The lHYP-lHb pathway is critical for nociception-induced modulation of meso-limbic DA release (Lee et al. 2022).
Outputs.The lHb projects to the ventro-lateral septum, THAL, HYP, and PBN, dorsal raphé, median raphé, SNc, VTA, rostro-medial tegmental nucleus (rmTN), PAG, and LC. In addition, the mHb sends projections to the commissural septum and lHb (Baker and Mizumori 2017; Boulos et al. 2017; Gouveia and Ibrahim 2022; Hikosaka 2010; Hu et al. 2020; Metzger et al. 2017; Roy and Parhar 2022).
In rats, the effects were assessed of intra-habenular injection of morphine on acute trigeminal pain as well as the involvement of DRN opioid and 5HT3 receptors on the anti-nociceptive activity of intra-Hb morphine to explore the possibility of existence of descending anti-nociceptive relay between the Hb and DRN. The numbers of eye wiping response elicited by applying a drop of NaCl (5 M) solution on the corneal surface were taken as an index of acute trigeminal nociception. Intra-habenular micro-injection of morphine at higher doses produced anti-nociception. Pretreatment of the DRN with ondansetron but not naltrexone prevented intra-habenular morphine induced anti-nociception. Intra-habenular injection of lidocaine reduced the corneal pain response. Hence, this suggests that the activation of the habenular μ-opioid receptor by micro-injection of morphine or inhibition of habenular neurons by micro-injection of lidocaine produced an analgesic effect in the acute trigeminal model of pain in rats. The analgesic effect of intra-habenular morphine was blocked by intra-DRN injection of 5-HT3 antagonist (Khalilzadeh and Vafaei Saiah 2017).
5.14. Thalamus (THAL)
”The thalamus receives and processes all nociceptive information that is destined to reach the cortex“ (Dostrovsky 2000).
The THAL forms the larger dorsal sub-division of the diencephalon located medially to the capsula interna and nucleus caudatus. It is a large mass of gray matter made up of a multitude of nuclei, through which sensory information is relayed and processed on its way to the cerebral cortex; the THAL also transmits and processes information from the BG and cerebellum (Brodal 1981; Dostrovsky 2000).
Functions. The THAL has roles in pain processing, the control of awareness, sleep and wakefulness, arousal and attention, reward, emotion regulation, motor control, and cognition. THAL functional abnormalities are thought to contribute to the dysregulation of sensory processing, circadian rhythms, levels of alertness, and consciousness (Kuner and Kuner 2021; Yoshii 2021; Zhou et al. 2021).
Nociceptive Input. The main spinally ascending pathways conveying nociceptive information are the STTr and the trigemino-THAL tracts. The lateral STTr originates primarily in lamina I of the spinal and medullary DH, and the anterior STTr from cells in deeper layers. The STTr terminates in the venral posterior nucleus (VP), VPI, VMpo, ventral lateral nucleus (VL), CL, parafascicular nucleus (Pf), and the MDvc (Dostrovsky 2000). Ascending nociceptive signals can also be indirectly conveyed to the THAL by the spino-reticular, spino-mesencephalic and medio-lemnical pathways (Todd 2010; Yen and Lu 2013).
Outputs. The different THAL portions targeted by the STTr contain different nuclei, which project on to different cortical areas. Thus, the VP projects to S1, the VPI to S2, the VMpo to the insula and area 3a, and the MDvc to the ACC (Dostrovsky 2000). The THAL also transmits and processes information from the BG and cerebellum (Bostan and Strick 2018; Wichmann and DeLong 2016).
Cortico-THAL-cortical Loops. The THAL is not merely a relay for sensory information to the cortex, but also receives cortical inputs that outnumber peripheral inputs. These projections typically originate in primary sensory cortices (S1) and project via two types of synaptic connections to higher-order THAL nuclei. Conversely, the relay cells of the higher-order nuclei project back to S1 and S2 as well as M1. ”The contributions of such corticothalamocortical loops to pain processing remain unknown, yet these loops are in a key position to compute differences between an initial representation generated in the S1 and the ongoing stream of sensory inputs that reach the thalamus.” Moreover, the THAL contributes to modulating cortical activity in perceptual decision-making, executive control, and attention (Kuner and Kuner 2021).
5.15. Cerebral Cortex
Structure. The cerebral cortex has a very complicated internal network structure. Cortical networks are composed of glutamatergic (Glu) excitatory pyramidal cells (projection neurons) and a minority of local GABAergic inhibitory interneurons that gate signal flow and sculpt network dynamics. GABA interneurons are highly heterogeneous, building functional classes based on their morphological, electrophysiological, and molecular features, as well as connectivity and in vivo patterns of activity (Tremblay et al. 2016).
Acute Skin and Muscle Pain. The human cerebral processing of noxious input from skin and muscle was compared by using positron emission tomography (PET). During each of eight scans, 11 normal subjects rated the intensity of stimuli delivered to the non-dominant (left) forearm on a scale ranging from 0 to 100 with 70 as pain threshold. Cutaneous pain was produced with a high-energy CO2 laser stimulator. Muscle pain was elicited with high-intensity intra-muscular electrical stimulation. The mean ratings of perceived intensity for innocuous and noxious stimulation were 32.6 +/- 4.5 (SE) and 78.4 +/- 1.7 for cutaneous stimulation and 15.4 +/- 4.2 and 73.5 +/- 1.4 for intra-muscular stimulation. The pain intensity ratings and the differences between noxious and innocuous ratings were similar for cutaneous and intra-muscular stimuli (P > 0.05). Significant increases in regional cerebral blood flow (rCBF) to both noxious cutaneous and intra-muscular stimulation occurred in the contralateral secondary somatosensory cortex (S2) and inferior parietal lobule (IPL) [Brodmann area (BA) 40]. Comparable levels of rCBF increase occurred in the contralateral aIC, THAL, and ipsilateral cerebellum. Noxious cutaneous stimulation caused significant activation in the contralateral lateral (BA 10/46) and ipsilateral PM (BA 4/6). Noxious intra-muscular stimulation evoked rCBF increases in the contralateral ACC (BA 24) and sub-significant responses in the contralateral primary sensorimotor cortex (M1/S1) and lenticular nucleus. These activated cerebral structures may represent those recruited early in nociceptive processing because both forms of stimuli were near pain threshold. Correlation analyses showed a negative relationship between changes in rCBF for THAL and M1/S1 for cutaneous stimulation, and positive relationships between THAL and aIC for both stimulus modalities. The similar cerebral activation patterns suggest that the perceived differences between acute skin and muscle pain are mediated by differences in the intensity and temporo-spatial pattern of neuronal activity within similar sets of forebrain structures (Svensson et al. 1997).
5.15.1. Functional Differentiation of the Cerebral Cortex
The multiple dimensions of pain are processed differentially in a distributed brain network (Chen 2018; Legrain et al. 2010). A simple model suggests that: (i) S1 and S2 represent the sensory-discriminative pain aspect (“when, where, how strong”). (ii) The posterior IC seems to contribute to nociceptive processing, cognitive aspects of pain, and the generation of the pain percept. (iii) The medial THAL with several nuclei whose relay cells receive inputs from the STTr projects mainly to motor areas of the CC, but also to ACC and PFC, these projections mediating motor aspects, attentional orienting, affective, motivational and emotional component of the pain percept (Kuner and Kuner 2021).
Brain Imaging. Multiple, highly interconnected cerebro-cortical regions receive parallel inputs from the THAL. In humans (and animals), brain imaging of different sorts has led to a widespread consensus that acute pain results from the integrated function of a `brain network for acute pain´ that has been proposed to contain the following main components: THAL, S1, S2, IC, ACC, PFC (Apkarian et al. 2005). Other regions without direct spinal nociceptive input (the aIC, frontal operculum, precuneus and dlPFC) are activated next, and the PPC and peri-genual CC are activated last (Kummer et al. 2020; Kuner asd Kuner 2021; Ong et al. 2019).
In human intra-cranial electroencephalogram (EEG) recordings showed activation of the pIC, operculum, MCC, and AMY before conscious activity; activation of the PFC, aIC, and PPC during time-frames coinciding with conscious voluntary reactions; followed by activation of the HIPP, peri-genual, and peri-splenial cingulate cortices (CCs) well after conscious perception occurred. In human fMRI, the discrimination intensity activated a ventrally directed pathway, extending bilaterally from the IC to the PFC, while discrimination of the spatial aspects of pain involved a dorsally directed pathway from the PPC to the dlPFC. Both of these tasks activate similar regions of the ACC (Ong et al. 2019).
The precise activation patterns of cortical areas depend on the type of pain, and de-activation of other areas may occur with activation (Neugebauer et al. 2009). S1, ACC and PFC, for example BA 10 (Peng et al. 2018), are activated more frequently and more robustly in individuals who are highly sensitive to pain than in insensitive individuals (Coghill et al. 2003). Mere anticipation of pain may also activate the pain matrix, whereas this activation and the subsequent pain may be reduced by the feeling to be able to control the intensity of an impending nociceptive stimulus (Brodal 2017).
However, due to the various experimental approaches and recording methods used, the imaging results have been interpreted differently. For example, supraspinal regions were activated in heat-evoked acute pain, delineated from pain-unrelated aversion, and included both somatic-specific areas such as the ventro-lateral THAL, the S2 and the dorsal pIC as well as regions related to affect and mood, such as the aIC, dACC, and the medial THAL (Wager et al. 2013). Hence, largely, neocortical regions with nociceptive inputs include the somatosensory, IC, CC and PFC, which respectively are involved in the processing of sensory, emotional, and cognitive aspects of pain. In humans, brain activity patterns and regions underlying the affective dimension of pain, i.e., its unpleasantness, are still debated, however. Some researchers have suggested that key elements of the limbic brain, i.e., the HIPP, AMY, ventral striatum, and the mPFC, which govern emotion, expectation, and salience, need to be recruited (Baliki and Apkarian 2015), which was not confirmed by others. Nonetheless, it is broadly agreed that the limbic network plays a key role in emotional modulation of pain and is significant in the transition to several forms of chronic pain (Kuner and Kuner 2021).
Lesions of specific brain regions do not completely eliminate pain perception. For example, lesions of S1 disrupt the ability to perceive light touch, but not the ability to perceive pain. This ability is also preserved with lesions to S2, IC, ACC and PFC, as well as to regions providing sub-cortical input to the PFC (Coghill 2020).
Sensory Discrimination and Intensity Scaling of Pain. Within the pain network, S1 has been proposed to be devoted to more general sensory discrimination and intensity scaling as for touch and proprioception. Many human brain regions are activated in a fashion related to noxious stimulus intensity and perceived pain intensity. These regions include bilateral portions of the THAL, contralateral S1, bilateral S2, bilateral pIC, bilateral aIC, bilateral ACC, and bilateral portions of the putamen. Thus, intensity-related information is widely distributed in regions ipsilateral to stimulation, as well as regions like the ACC that are typically associated with affective processing rather than sensory-discriminative processing (Coghill 2020).
Subjective Recognition of Pain. The opercular/IC region, including S2 and pIC of primates, is supposed to be the earliest truly pain-encoding region. The opercular/IC region is thought responsible for the subjective recognition of pain, encoding of pain intensity, learning and memory of pain experiences (Chen 2018).
Unpleasantness and Motivational Aspects are represented in IC, CC and sub-cortical structures, e.g., the AMY and NAc of the BG (Becerra et al. 2013; Bushnell et al. 2013; Chen 2018; Craig 2003; Neugebauer et al. 2009).
Evaluation and Integration. At a high integrative level, BA 10 might contribute to evaluate and integrate sensory-discriminative pain information, affective-motivational aspects, pain modulation, stress, anxiety and fear response, and pain memory (Peng et al. 2018).
Saliency Network. It has been proposed that the above cortical network is not specifically related to pain representation but more generally is involved in detecting, orienting attention toward and reacting to salient events of several sensory modalities. Thus, the ACC and IC are included in the so-called `saliency network´ which is activated by any event catching attention and raising arousal (Brodal 2017; Legrain et al. 2010; Zhou et al. 2021).
First Pain and Second Pain. Generalized cortical reaction to several sensory modalities is consistent with the spatio-temporal cortical representations of first pain and second pain, as assessed by magnetoencephalography. First pain evoked by brief cutaneous laser pulses is associated particularly with transient activation of contralateral S1, whereas second pain is related to longer-lasting activation of the ipsilateral ACC, and either of the pain sensations activates S2 bilaterally. These distinct distributions suggest different functions. The first pain may signal threat and provide precise sensory information about the noxious nature and location of the stimulus for an appropriate fast motor response such as a fast withdrawal reflex, while the second pain has a strong affective component, attracts attention and elicits behavioral responses to limit further injury and optimize recovery (Ploner et al. 2002).
5.15.2. Somatosensory Cortex
As noted above, the lateral THAL projects to S1 and S2 where the sensory-discriminative pain aspect is represented. The posterior THAL projects to the posterior IC where pain perception and intensity are represented, and the medial THAL projects to the CC and PFC where affective, motivational and motor aspects are represented (Kuner and Kuner 2021).
5.15.3. Prefrontal Cortex (PFC)
The so-called PFC covers large expanses of the frontal lobe and is situated rostral to Brodmann area (BA) 6, including that on the orbital surface, and is particularly well developed in primates, especially man. It is a heterogeneous brain region composed of multiple structures and exhibits species-specific differences between rodents, primates, and humans in connectivity, cytoarchitecture, electrophysiological properties, protein expression, and responses following damage (Ong et al. 2019; Thompson and Neugebauer 2019).
Division. The term PFC and its partitions are not used unaminously, due to the vast expansion that the PFC underwent during evolution from rodents to non-human primates and humans. The PFC can be divided into the mPFC on the medial surface of the hemisphere, vmPFC, the ventro-lateral PFC (vlPFC), the dlPFC, the OFC facing the eye orbits, and the caudal PFC (Maletic and Raison 2009; Ong et al. 2019; Toledo and Carson 2022).
Functions. The PFC is important for pain processing (Ong et al. 2019). In line with its complex composition, the PFC has been assigned various functions, differentiated according to area. The PFC underlies executive functions such as planning, problem solving, and social control. It is able to represent information not currently in the environment, and this representational information is used to intelligently guide thought, actions, and emotions, including the inhibition of inappropriate thoughts, distractions, actions, and feelings. Functions include cognitive (executive) control over the organization of thought, memory and action in accordance with internal goals, maintenance of task sets and the encoding, representation and storage of knowledge about the consequences of behaviors in complex situations, attention and decision-making processes, the processing of intrinsic and extrinsic sensory information, and behavior, memory, social cognition, and assigning salience to self-related information processing (Arnsten 2009; Ong et al. 2018: Ploski and Aidya 2021, Toledo and Carson 2022).
Inputs. The mPFC processes ascending nociceptive inputs and is involved in both sensory and affective aspects of pain (Kummer et al. 2020). The PFC receives signals from a wide spectrum of structures. The PL and IL mPFC receive inputs from regions including the THAL, BLA, HIPP, and contra-lateral mPFC. A portion of BLA inputs terminate on GABAergic interneurons, allowing for feedforward inhibition of mPFC output through modulation of mPFC projection neurons (Thompson and Neugebauer 2019). There is a general, topographic organization whereby the dlPFC receives sensory inputs about the external world (i.e., auditory and visual inputs), while the vmPFC receives information about our internal world (somatosensory, olfactory, taste, pain), thus creating a topography of circuits mediating cognitive to emotional control. Excitatory afferents to the PFC arise from several regions including limbic areas related to emotion such as the HIPP, AMY and HYP (Brockway and Crowley 2020).
Outputs. The PFC sends glutamatergic projections to multiple brain regions responsible for regulating emotional behaviors (some of them reciprocal), including the BNST, striatum, AMY and PAG. It has reciprocal connections with the medio-dorsal nucleus of the THAL (MD) (Brockway and Crowley 2020). Projections from both PL and IL target the anterior piriform cortex, olfactory forebrain areas, entorhinal cortex (EC), orbito-medial cortex, IC, the medial THAL nuclei, the midbrain VTA, and medial regions of the PAG (Kummer et al. 2020). The PL mPFC predominately targets the BLA, whereas the IL cortex projects to BLA and other AMY divisions including the LA, intercalated cell mass of the AMY (ITC), and possibly to the lateral central nucleus of the AMY (CeL). The ITC sends GABAergic projections to central AMY (CeA) projection neurons, allowing for feedforward inhibitory control of AMY output by the mPFC (Thompson and Neugebauer 2019).
Pain Processing. In human fMRI, the discrimination of pain intensity activates a ventrally-directed pathway extending bilaterally from the IC to the PFC. Spatial discrimination of pain is represented by a dorsally directed activation of the PPC and right dlPFC. The PFC is also involved in empathy for pain. Bilateral aIC, rostral ACC, brainstem, and cerebellum were activated when subjects received pain and also by a signal that a loved one is experiencing pain. Empathy is reflected in increased activation of the aIC and ACC as well as the mPFC and temporal pole when sharing other´s social suffering. Responses of vmPFC to somatosensory representations of others´ pain are present even in patients with CIP. In contrast to non-nociceptive stimuli, nociceptive stimuli enhanced gamma-band oscillations, and these oscillations are a feature of nociceptive signaling in the mPFC and the IC (Ong et al. 2019). In humans, fMRI activity in the ACC and the NAc appears to reflect affective and motivational aspects of pain. In both humans and rats, the onset (aversion) and offset (reward) of a noxious heat stimulus to a dorsal part of a limb showed different responses. Pain onset resulted in negative activity change in the NAc and pain offset produced positive activity change in the ACC and NAc (Becerra et al. 2013).
5.15.4. Cingulate Cortex (CC)
The CC is a long gyrus (gyrus cinguli) on the medial hemispheric side, riding on and around the corpus callosum. In primates, the CC has three major regions: an agranular ACC; a dysgranular MCC; and a granular posterior cingulate cortex (PCC)/RSC. The regions are additionally divided into sub-regions or areas: ACC has sub-genual and posterior parts (sACC and pACC, respectively); MCC has anterior and posterior parts (aMCC and pMCC, respectively); PCC has dorsal and ventral parts (dPCC and vPCC, respectively). Each of these divisions has a different cytoarchitecture, sets of connections and functions. All regions are related to emotion and motivation, and memory insofar as they are connected to the HIPP (Oane et al. 2023; Vogt 2005, 2016, 2019).
5.15.4.1. Anterior Cingulate Cortex (ACC)
Inputs/Outputs. Nociceptive inputs to the ACC are mediated via the pathway through the midline and intra-laminar THAL nuclei (including the paraventricular nucleus of the THAL: PVT), which also project to other limbic cortices, robustly to the AMY, and to the PAG. In humans, both pACC and MCC were activated when noxious heat was applied to the back of the hand when controlled for innocuous heating to the same skin, while there were fewer activation sites in pACC and almost none in sACC or dPCC. Imaging studies also showed coding for the intensity of noxious stimulation in pACC and MCC. Nociceptive visceral responses showed a preference for pACC and to a lesser extent aMCC. In rabbits, neurons in ACC did not signal the location of the noxious stimulus on the body surface because stimulation anywhere on their body can evoke a discharge. These neurons respond mainly to noxious stimuli including pressure and temperatures above 46°C. Largely, these responses reflect those of their input THAL neurons that have large and bilateral receptive fields (Vogt 2016, 2019). Besides their local mPFC connectivity, ACC cells synapse onto neurons in the neighboring MCC and RSC. Pyramidal neurons located in deeper layers project to the HYP, AMY, PAG, the contralateral hemisphere or other supraspinal areas as well as directly to the spinal DH (Kummer et al. 2020).
In rodents, the ACC also receives inputs from S1, IC, HIPP CA1 region and subiculum, BFB nuclei, BLA, VTA, PAG, NRD, and LC. While the PL PFC receives inputs mainly from anterior forebrain regions, the IL PFC is preferentially targeted by projections from anterior HYP, medial and lateral septum, substantia innominata, as well as the dorso-lateral tegmentum. Cholinergic (ACh) inputs to the mPFC arise from the BFB and are associated with attentional tasks and cognitive performance, from the diagonal band of Broca, and from the medial septum, which might contribute to the cognitive and emotional impairments induced by pain (Kummer et al. 2020).
5.15.4.2. Mid-Cingulate Cortex (MCC)
Inputs/Outputs. The aMCC is activated by nociceptive information from the midline, medio-dorsal and intra-laminar THAL nuclei, which mediate nocifensive behaviors and evoke fear. Painful laser stimulation evoked cortical potentials simultaneously in MCC and in the S2/IC region. Neuronal responses to noxious stimuli have been recorded in the MCC in both monkeys and humans. Hence, regions of MCC receive nociceptive information from the STTr system. In cebus monkeys, STTr inputs reached multiple contralateral cortical areas, the major targets being several areas in the cingulate sulcus, IC and S2, such that the human equivalents of the three cingulate motor areas (CMAs) correspond to sites of pain-related activation. STTr inputs to the cingulate sulcus is directed solely to the CMAs. Thus, there is substantial overlap between an ascending afferent pathway involved in pain processing and the CMAs that have substantial connections with the M1, dorsal premotor cortex (PMd) and ventral premotor cortex (PMv), as well as to the spinal cord. STTr inputs to CMAs may thus be involved in sensory-motor integration, which conveys information about the state of segmental interneurons involved in motor mechanisms such as spinal reflexes, posture and locomotion (Dum et al. 2016).
5.15.4.3. Posterior Cingulate Cortex (PCC)
Several imaging studies have reported activity changes in the PCC during pain and proposed its speculative involvement to modulate the conscious experience of pain according to elements from the context and awareness of the self and others (Peyron et al. 2019). In an fMRI study of human volunteers, a thermal painful stimulus applied to the right hand activated areas in the PCC, S2, and IC. The PCC activity was in a region that, in the monkey, receives nociceptive inputs from posterior medial and lateral THAL nuclei that in turn are targets for STTr terminations (Gelnar et al. 1999). After noxious stimulation, nociceptive stimulus discrimination had peak latency at 172 milliseconds in dPCC. Both pMCC and dPCC showed short-latency, nociceptive responses using cranial surface EEG recording. Furthermore, evoked potentials were used to show that painful and non-painful electrical stimulation of muscle activated the caudal CMA and dPCC. Lastly, simple finger movements evoked movement-associated activity (recorded using magnetoencephalography) at sites in the caudal CMA and dPCC. This suggests that pMCC and dPCC are involved in orienting the body to sensory stimuli including nociceptive ones. The extent to which pain activation of pMCC and dPCC requires emotion depends upon the role of both regions in emotion (Vogt 2016).
Functions. The ACC is supposed to sustain functions such as pain-related attention, arousal, pain modulations, engagement of endogenous pain control system, and motor withdrawal reflex (Peyron et al. 2019). It has been suggested that nociceptive information in the ACC is combined with motivational (reward-related) and affective information received from other areas of the brain, such as the mPFC, OFC, IC and BLA. Additionally, the ACC interacts with pain circuitry in the PAG, which accounts for the activation of the ACC and PAG in the presence of noxious stimuli (Arnsten et al. 2023; Thompson and Neugebauer 2019; Yang and Chang 2019). The ACC would then generate affective and motivational pain responses through its projections to the AMY, NAc, and mPFC (Yang and Chang 2019). Caudal parts of the aMCC are separately activated by innocuous and noxious activity and might be related to a third site for cognitive processing rather than nociception and intensity ratings as such. The dorsal peri-genual ACC projects to the motor nucleus of the VIIth cranial nerve, generating affectively modulated facial expressions and vocalizations (Vogt 2016).
5.15.5. Insular Cortex (IC)
The IC or island of Reil is a region of the cerebral cortex located in the center of the cerebral hemisphere, within the lateral fissure (Sylvian fissure) and covered by parts of the frontal, temporal and parietal cortices (Nagai et al. 2007). The IC is parcellated into various areas, which, in different species from rodents to primates, show roughly graded differences in macro- and micro-anatomy, cyto-architectonics, connectivity and function, The IC can grossly be divided into the rostral agranular IC, the middle dysgranular IC, and the caudal granular IC (Evrard 2019; Labrakakis 2023; Livneh and Andermann 2021; Vogt 2019).
Inputs. The IC is nested in a widespread network of connections. The IC receives direct or indirect sensory inputs (including nociceptive, thermoreceptive, gustatory, olfactory, auditory, vestibular, and visceral signals) from different organs and systems: mouth, tongue, esophagus, stomach, intestines, liver, pancreas, blood, kidneys, heart, lungs, urinary bladder, and genitals. The different sensory projections are differentially distributed in the different IC sub-division (Vogt 2019). In the macaque, the posterior dorsal fundus of the IC responds to innocuous and noxious thermal stimuli as well as to noxious mechanic pinch stimuli, with a somatotopic representation of the foot, hand, and face from posterior to anterior (Evrard 2019). The dorsal visual system has direct anatomical projections to the aIC (Lu et al. 2016).
Outputs. The IC projects to multifarious structures. The aIC is connected to the vlPFC and OFC, while the pIC has connections to the S1 and S2 (Ong et al. 2019). The pIC also projects to the brainstem vestibular nuclei and is strongly interconnected with the primary vestibular cortex (parieto-insular vestibular cortex) and with other cortical vestibular processing sites (Evrard 2019). Stimulation of the IC causes changes in blood pressure, heart rate, and respiration. Projections descending from IC neurons can have potent effects on viscero-motor functions involving control of smooth muscles and glands, regulation of gastric motility, heart rate, breathing, and salivation (Livneh and Andermann 2021).
The IC sends extensive connections to the main brainstem areas of descending pain control. These include the PAG and NRM, as well as an extensive connection to the PBN. There are less extensive but consistent IC axonal inputs to different NA nuclei, the LC, subcoereuleus, and the A5 cell group. These connections suggest an important role of the IC in pain processing through descending pathways (Liang and Labrakakis 2024). In rats, retrograde and anterograde tract tracing showed that the granular and dysgranular parts of IC send direct descending projections to the trigeminal caudal sub-nucleus (spVc), especially the superficial laminae (I/II) (Wang et al. 2015).
A sub-area of the agranular aIC projects strongly to the anterior part of the lHYP, the ventro-lateral part of the BLA, and the PAG, the LHa and BLA projecting further to the PAG, all three regions having crucial roles in autonomic and emotional behavioral responses. The same sub-area of the aIC also projects to the SN, VTA, and median raphé nucleus. In macaques, micro-stimulation of the aIC produced both physiological changes with effects on cardio-vascular and respiratory functions and behavioral effects reminiscent of disgust facial expression (Evrard 2019).
Functions. The IC plays an important role in pain processing (Labrakakis 2023). Pain intensity is presumed to occur in the IC and/or the secondary somatosensory (S2) area (Peyron et al. 2019). Human functional imaging led to the view of the aIC as an association cortex that computes sensory predictions, while mid-posterior IC integrates these predictions with incoming visceral and gustatory inputs to compute prediction errors in terms of positive/negative valence or appetitive/aversive sensations. For example, the activity in more anterior agranular regions of human IC more strongly reflects anticipation of expected pain, whereas activity in posterior granular regions more strongly reflects the actual intensity of painful stimuli (Livneh and Andermann 2021). The IC has been supposed to participate in both sensory-discriminative and affective-motivational aspects of pain. Due to abundant connections with other brain areas, the IC likely serves as an interface where cross-modal shaping of pain occurs. In chronic pain, however, this mode of emotional awareness and the modulation of pain are disrupted (Lu et al. 2016). Moreover, the IC is involved in processing taste, smell, auditory, visual and vestibular signals, in viscero-sensory, viscero-motor and interoceptive functions, attention, emotions, verbal and motor information (Kuner and Kuner 2021; Nagai et al. 2007; Ong et al. 2019).
Descending Pain Modulation. In healthy subjects, a conditioned pain modulation paradigm to study descending pain modulation showed that weaker pain inhibition was related to increased connectivity between the IC and AMY (Labrakakis 2023).
Laterality of IC. Brain laterality seems to be important, with the right aIC responding to arousing stimuli and autonomic sensations, such as pain, while the left aIC is activated by sensory salient and emotional feelings (Paulus and Stein 2006; Sliz and Hayley 2012).
5.15.6. Hippocampus (HIPP)
The HIPP is an evolutionarily old and complicated structure with the shape of a seahorse located in the medial temporal lobe of the brain. The HIPP formation is part of the limbic system and includes the dentate gyrus (DG), C1-C4 sectors, pro-subiculum, subiculum, and pre-subiculum. It has been proposed that the HIPP is composed of an anterior, middle and posterior division (Vogt 2019).
Input/Output. The HIPP connec ts extensively with other brain regions. Its major input and output structure is the EC, which receives additonal inputs from the peri-rhinal and para-HIPP cortices from cortical and sensory sources (Garcia and Buffalo 2020; Lavenex and Amaral 2000; Witter et al. 2017). Thus, the HIPP receives indirect input from multiple sensory modalities, including olfaction, somatosensation, audition and vision, as well as inputs from the PFC, e.g., information about goals, task rules, and contexts), septum (cholinergic), anterior THAL, and HYP. The vHIPP receives limbic input from the BLA and indirect input from the mPFC. The HIPP activity is modulated by the ACh, DA, NA and 5-HT systems (Brodal 1981; Garcia and Buffalo 2020; Thompson and Neugebauer 2019; Witter 2009). The HIPP participates in both the processing and modulation of nociceptive signals. Nociceptive signals reach the HIPP indirectly via the STTr and PBN. Septo-HIPP neurons receive direct input from the spinal cord and respond to intense thermal stimuli. Neurons in the CA1 region and the DG react ro painful stimuli. Injection of lidocaine directly into the DG produces analgesia. A HIPP lesion can alter the perception of noxious stimuli and partially alleviate pain. The HIPP plays a role in integrating sensory, autonomic and affective information, and by way of complex networks modulates spinal nociceptive processing via activation of descending monoaminergic fibers. The HIPP may be involved in the development of chronic pain (Fasick et al. 2015).
7. Descending Pain Control
“A Long Way Down”
(Nick Hornby 2005)
As if the anatomical architecture of the nociceptive and acute pain systems were not complex enough, a huge variety of additional modulators adds to it. Pain can be such a desolate experience that the CNS may be expected to have evolved counter-measures to reign it in. This is the case, although the opposite is also true. The coumter-measures open new dimensions. However, they are anything but simple (Nguyen et al. 2023; Willis and Westlund 1997). Descending control arises from several supraspinal sites, most with their upstream influencers, and give rise to parallel pathways to the DH, sometimes with receprocal connections.
A selected number of structural pathways involved in descending pain modulation from the highest cerebro-cortical levels to the spinal DH are schematically shown in Figure 3.
We will follow a long way down. This is also justified by the presumption that probably the most significant pain modulation is exerted by systems descending from forebrain sources (Gamal-Eltrabily et al. 2021), and since the spinal DH and spV host the first synapse in the ascending pathways from peripheral nociceptors to the cerebral cortex, they offer a powerful target for regulation of nociceptive transmission. Since acute and chronic pain symptoms involve a sensory dimension, an immediate affective dimension, and sometimes a secondary affective dimension, termed pain-related suffering, pain modulation and neuroplasticity occur all along the nociceptive pathways and involve many different mechanisms (Boadas-Vaello et al. 2017; Heinricher et al. 2009; Kuner and Kuner 2021). Consequently, after the initial activation of nociceptors, the final experience of pain is the result of complex interactions between the DH neuronal circuits engaged to transduce and transmit the pain signals and the modulatory actions from higher brain centers (Puopolo 2019). Unfortunately, these modulatory actions are ambivalent because they may suppress or enhance pain sensation.
Descending Facilitation vs. Inhibition. The descending pain modulatory system operates as an endogenous control of pain, being recruited following painful stimuli perception alongside many other brain regions, including the primary and secondary somatosensory cortices (S1, S2), ACC, PFC, IC, AMY, NAc, VTA and PAG. The first structures encode sensorial aspects of pain, including location, duration, and intensity, which are presumed to be processed by the S1, S2 and IC. The latter encode emotional and contextual aspects of pain, such as suffering and contextual avoidance, and are processed by the PFC, ACC, NAc, AMY, and VTA (Pagliusi and Gomes 2023). One of the important projections to the RVM arises from the PAG. When stimulated by endogenously or exogenously (e.g., through chemogenetics), PAG-RVM projections exert analgesia by facilitating the descending inhibition of pain. Inversely, projections from the ACC to the RVM enhance the descending facilitation of pain. The over-activation of ACC-RVM projections is thought to be involved in pain chronification (Pagliusi and Gomes 2023).
The descending facilitatory system includes the ACC, AMY, HYP, PAG, RVM, and NTS. In the physiological state, the activation of this system improves the response ability to nociceptive stimuli by lowering pain threshold. The descending inhibitory system is centered around the PAG, which receives afferent projections from the above upstream cortical and sub-cortical brain regions, and sends efferent projections to the RVM and thence to spinal cord neurons. It is composed of the cerebral cortex sending a descending inhibitory pathway containing the arcuate hypothalamic nucleus (HYP ARC) pain modulation system, and the descending inhibitory pain modulation system of the limbic system via the NAc, Hb, PAG, RVM to the spinal cord/(spV (Cui et al. 2023).
7.1. Cerebral Cortex
Cerebro-cortical areas involved in the modulation of nociception include the PFC, ACC, ventro-lateral orbito-frontal cortex (vlOFC), IC, motor cortex, and somatosensory cortices. The modulatory effects are mediated by cortico-cortical or cortico-subcortical interactions, by direct cortico-spinal projections, or by intermediate activation of brainstem structures, i.e., PAG, LC, NRM and RVM (Gamal-Eltrabily et al. 2021).
Expectation. Expectations and associative learning processes are important psychological determinants of placebo effects, but yet their underlying brain mechanisms are little understood, in particular with regard to the brain systems underlying placebo effects on pain, autonomic, and immune responses. The vmPFC, IC, AMY, HYP, and PAG emerge as central brain structures underlying placebo effects. The vmPFC appears to be a core element of a network that represents structured relationships among concepts, providing a substrate for expectations and a conception of the situation – the self in context – that is crucial for placebo effects. Such situational representations enable multi-dimensional predictions that are combined with incoming sensory information to construct percepts and shape motivated behavior. They influence experience and physiology via descending pathways to physiological effector systems, including the spinal cord and other peripheral organs (Geuter et al. 2017).
Negative Experience. Nocebo hyperalgesia means increased pain sensitivity resulting from negative experiences. Various methods have been used to unravel the neurobiology and have yielded inconsistent results. Structural and functional neuroimaging demonstrated that nocebo hyperalgesia amplified pain signals in the spinal cord and brain regions involved in sensory and cognitive-affective processing including the PFC, IC, AMY, and HIPP (Thomaidou et al. 2021).
7.1.1. Somatosensory Cortices
Direct Cortico-spinal Pain Modulation. While many pro-nociceptive and anti-nociceptive effects are mediated by brainstem structures, there are also direct cortico-spinal modulatory effects. For example, in mice, a sub-set of cortico-spinal neurons (CSN) in the S1/S2 somatosensory cortices and travelling in the cortico-spinal tract (CST) exerted anti-nociceptive effects mediated by direct innervation of the DH. Either reduction in somatosensory CSN activity or transection of the CST selectively impaired behavioral responses to light touch without altering responses to noxious stimuli. Moreover, such CSN manipulation greatly attenuated tactile allodynia in a model of peripheral neuropathic pain. Tactile stimulation activated somatosensory CSNs, and their cortico-spinal projections facilitated light-touch-evoked activity of CCK interneurons in the deep DH. This touch-driven feed-forward spinal-cortical-spinal sensitization loop is important for the recruitment of spinal nociceptive neurons under tactile allodynia (Liu et al. 2018).
Primary Somatosensory Cortex (S1) efferents can modulate signals from mechanical and cooling stimuli but not heat stimuli. Presumably, therefore, the responsiveness to particular somatosensory stimuli occurs in a modality specific fashion. In a mouse model, inhibition of output from the S2 increased the sensitivity to mechanical and heat stimuli, but not cooling stimuli. S2 projections to the secondary motor cortex (M2) governed mechanical and heat sensitivity without affecting motor performance or anxiety (Taub et al. 2024).
Secondary Somatosensory Cortex (S2). The trigeminal spinal subnucleus caudalis (spVc), also known as the `medullary DH´, receives orofacial somatosensory inputs, particularly nociceptive inputs, from the trigeminal nerve. Higher brain regions, including the cerebral cortex, LC, and DRN, send axons to the spVc. Among these descending projections, cortico-trigeminal projections from the somatosensory cortex (S2) to the spVc play a suppressive role in nociceptive information processing, whereas a facilitative role of the IC in nociceptive information processing at the spVC level (Kobayashi et al. 2024).
In rats, the distribution of S2 neurons projecting to the trigeminal principal nucleus (Vp) or oral subnucleus (Vo) of the trigeminal sensory nuclear complex (TSNC) were studied after injections of a retrograde tracer into five regions in the Vp/Vo, which were responsive to stimulation of trigeminal nerves innervating the orofacial tissues. A large number of labeled neurons were found with a somatotopic arrangement in the dorsal areas of S2 (orofacial S2 area). The projections to the TSNC showed somatotopic arrangements. This suggests that the orofacial S2 projects selectively to certain rostro-caudal levels of the contralateral TSNC, and the projections may allow the orofacial S2 to accurately modulate orofacial somatosensory transmission to higher brain centers including the orofacial S2 itself (Haque et al. 2012).
7.1.2. Primary Motor Cortex (M1)
Motor cortex stimulation provided pain relief by motor cortex plasticity and activating descending inhibitory pain control systems (Ye et al. 2014). The primary motor cortex (M1) is involved in the control of voluntary movements, but also in the modulation of pain. There is a connection from the M1 to the NAc reward circuitry through a M1 layer 6-medio-dorsal THAL pathway, which in neuropathic pain specifically suppresses negative emotional valence and associated coping behaviors. By contrast, layer 5 M1 neurons connect with specific cell populations in zona incerta and PAG to suppress sensory hypersensitivity without altering pain affect. Thus, the M1 uses different, layer-specific pathways to dampen sensory and aversive-emotional components of neuropathic pain (Gan et al. 2022).
In the adult male mouse, neurons involved in the efferent control of the left gastrocnemius muscle were identified following visualization of retrograde tracing and were detected in spinal cord, PAG and motor cortex. It has thus been hypothezized that MC4R signaling in the motor cortex-PAG-spinal cord pathway may participate in the modulation of the melanocortin-sympathetic signaling and contribute to the descending modulation of nociceptive transmission (Ye et al. 2014).altering pain affect. Thus, the M1 uses different, layer-specific pathways to dampen sensory and aversive-emotional components of neuropathic pain (Gan et al. 2022).
In the adult male mouse, neurons involved in the efferent control of the left gastrocnemius muscle were identified following visualization of retrograde tracing and were detected in spinal cord, PAG and motor cortex. It has thus been hypothezied that MC4R signaling in the motor cortex-PAG-spinal cord pathway may participate in the modulation of the melanocortin-sympathetic signaling and contribute to the descending modulation of nociceptive transmission (Ye et al. 2014).
7.1.3. Prefrontal Cortex (PFC)
Anticipation and anxiety of pain that enhance pain experience, activate brain regions including the PFC, EC, aIC, AMY, ventral brainstem areas and PAG (Neugebauer et al. 2009; Tracey and Mantyh 2007).
7.1.3.1. Medial Prefrontal Cortex (mPFC)
The mPFC is composed of granular cortical areas (medial BA 9 and 10) and agranular regions (BA 24, 25, and 32) which encompass the anterior cingulate cortex (ACC, BA 24), infra-limbic cortex (BA 25), and the pre-limbic cortex (BA 32) (Ong et al. 2019). The mPFC has emerged as a critical region for top-down cognitive control over emotion-driven behaviors via processes including fear conditioning and extinction. The mPFC, comprising, in rats, the ACC, PL PFC and IL PFC, receives ascending nociceptive inputs, but also exerts top-down control of pain sensation. Human fMRI studies have shown activation of mPFC during the perception of acute pain stimuli and functional de-activation in chronic pain patients. Neurons in the mPFC responded to noxious stimuli, and electrical stimulation of mPFC inhibited nociceptive responses (Kummer et al. 2020).
The mPFC could serve dual, opposing roles in pain: (i) It mediates anti-nociceptive effects, due to its connections with other cortical areas, and as the main source of cortical afferents to the PAG for modulation of pain. Indeed, activation of the mPFC and ACC is related to increased activity of the PAG. (ii) It could induce pain chronification via its cortico-striatal projection, possibly depending on the level of DA receptor activation (or lack of) in the reward pathway from the VTA to NAc. There is a role for the PFC during placebo analgesia, and in establishing links between pain and anxiety, depression, and loss of cognition. The mPFC is also involved in modulation of pain catastrophizing, reduction of pain-induced sympathetic activity, and decrease in facial expressions of pain (Ong et al. 2019).
mPFC-DH Projection. In rats, there are direct projections from the dorsal peduncular cortex (DP) in the mPFC to the trigeminal brainstem sensory nuclear complex and other lower brainstem areas. mPfC neurons projecting directly to the medullary DH (trigeminal sub-nucleus caudalis [Vc]) and trigeminal sub-nucleus oralis (Vo) are known to receive direct projections from the IC. Injections of a retrograde tracer into the rostro-dorso-medial part of laminae I/II of Vc (rdm-I/II-Vc) labeled many neurons bilaterally (with an ipsilateral predominance) in the rostro-caudal middle level of DP (mid-DP) and not in other mPfC areas. Anterograde tracer injections into the mid-DP labeled many axons and terminals bilaterally (with an ipsilateral predominance) in the rdm-I/II-Vc, PAG and NTS, and ipsilaterally in the PBN and trigeminal mesencephalic nucleus. Many tracer-labeled axons and terminals from the mid-DP also occurred ipsilaterally in the caudal-most level of the granular and dysgranular IC (GI/DI). Retrograde injections into the caudal-most GI/DI labeled many axons and terminals ipsilaterally in the mid-DP. The projections from the mid-DP to the rdm-I/II-Vc and other brainstem nuclei suggest that mid-DP neurons may regulate intra-oral and peri-oral sensory processing (including nociceptive processing) of rdm-I/II-Vc neurons directly or indirectly through the brainstem nuclei (Akhter et al. 2014).
dmPFC-vlPAG Projection. The dorso-medial prefrontal cortex (dmPFC) is an important cortical area for nociceptive modulation. Lesions in the dmPFC induced an algesic and anxious state. An excitatory descending pathway runs from the dmPFC to the vlPAG. In a mouse model of chronic pain, activation of the dmPFC-to-vlPAG pathway by optogenetic manipulation produced analgesic and anti-anxiety effects. Chemogenetic activation of inhibitory neurons in the dmPFC produced an algesic and anxious state under both normal and chronic pain conditions. Antagonists of the GABAAR or mGluR1 applied to the dmPFC produced analgesic and anti-anxiety effects. This suggests that the dmPFC-vlPAG pathway might participate in the maintenance of pain thresholds and anti-anxiety behaviors under normal conditions, while silencing or suppressing the dmPFC-vlPAG pathway might be involved in the initial stages and maintenance of chronic pain and the emergence of anxiety-like behaviors (Yin et al. 2020).
7.1.3.2. Ventro-Medial Prefrontal Cortex (vmPFC)
Expectations and associative learning processes are important psychological determinants of placebo effects, but yet their underlying brain mechanisms are little understood, in particular with regard to the brain systems underlying placebo effects on pain, autonomic, and immune responses. The vmPFC, IC, AMY, HYP, and PAG emerge as central brain structures underlying placebo effects. The vmPFC appears to be a core element of a network that represents structured relationships among concepts, providing a substrate for expectations and a conception of the situation – the self in context – that is crucial for placebo effects. Such situational representations enable multi-dimensional predictions that are combined with incoming sensory information to construct percepts and shape motivated behavior. They influence experience and physiology via descending pathways to physiological effector systems, including the spinal cord and other peripheral organs (Geuter et al. 2017).
vmPFC-PAG Connectivity during Conditioned Pain Modulation. Pain interacts with the ANS, possibly involving descending pain modulatory mechanisms. The PAG is involved both in descending pain modulation and ANS. Humans underwent conditioned pain modulation (CPM), in which they rated painful pressure stimuli applied to their thumbnail, either alone or with a painful contralateral cold stimulation. Heart-rate variability (HRV) was simultaneously recorded. Normalized low-frequency HRV (LF-HRVnu) and the CPM score were negatively correlated. Subjects with higher LF-HRVnu during pain reported reductions in pain during CPM. PAG-vmPFC and PAG-RVM functional connectivity correlated negatively with the CPM. PAG-vmPFC functional connectivity mediated the strength of the LF-HRVnu-CPM association. CPM response magnitude was also negatively correlated with vmPFC GM volume (Makovac et al. 2021).
7.1.3.3. Dorso-Lateral Prefrontal Cortex (dlPFC)
Petrides 2005
The dlPFC is composed of the lateral part of BA 9 and all of BA 46 (Ong et al. 2019). The dlPFC is large and functionally heterogeneous. Compared to other primates, the dlPFC is substantially expanded in humans, suggesting a role in complex cognitive processes. It is involved in cognitive processes, such as attention, value encoding, working memory, creativity, decision-making, and emotional regulation. It is generally associated with maintenance and regulation of top-down modulation, and driving appropriate behavioral responses. Neuroimaging showed that the dlPFC was often activated in response to nociceptive stimuli in healthy subjects, and exhibited abnormally increased function in chronic pain populations. Non-invasive brain stimulation of the dlPFC exerted acute pain modulation and some effectiveness as a treatment for certain chronic pain conditions. The dlPFC is also involved in placebo modulation of pain by integrating incoming nociceptive signals with the expectation of pain – an important feature of placebo analgesia. Evidence supports a role for the dlPFC in the suppression of pain and maintenance of pain inhibition. Uncontrollable pain resulted in increased activation of pain-related areas including the THAL and IC, but bilateral dlPFC had increased negative connectivity strength during controllable pain to both the THAL and right aIC. Thus, the dlPFC suppressed IC and THAL activity and reduced pain sensitization associated with uncontrollable pain. The dlPFC is involved in cognitive control over pain. Cognitive control can reduce pain and has in part been attributed to a brain network comprising prefrontal regions including dlPFC, vlPFC and OFC, the aIC, ACC, and brainstem regions, such as the PAG and the RVM. Activation of part of this network, including the dlPFC, ACC, and cerebellum, has been implicated in mediating the analgesic effects of spinal cord stimulation in chronic back pain patients. In sum, these studies suggest that the dlPFC acts as an interface between cognitive processing and pain regulation (Seminowicz and Moayedi 2017). As mentioned above, while the discrimination of pain intensity, a non-spatial aspect of pain, activates a ventrally directed pathway from the IC to the PFC, a dorsally directed activation of the PPC and right dlPFC occurs during spatial discrimination of pain. The dlPFC is connected to the mPFC by short association fibers (Ong et al. 2019).
Pain is strongly modulated by expectations and beliefs. Across species, sub-regions of vmPFC are implicated in a variety of functions germane to pain, predictions, and learning. Human fMRI studies show that vmPFC activity tracks expectations about pain and mediates expectancy effects on pain-related activity in other brain regions. Prior lesion studies suggest that vmPFC may instead play a more general role in generating affective responses to painful stimuli. To test whether vmPFC is required to generate affective responses to pain or is more specifically involved in expectancy-based pain modulation, responses to heat stimuli were studied in five adults with bilateral surgical lesions of vmPFC and twenty healthy adults without brain damage. All participants underwent a quantitative sensory testing procedure followed by a pain expectancy task in which cues predicting either low or high pain were followed by intermittent medium intensity heat stimuli. Compared to adults without brain damage, individuals with vmPFC lesions reported larger differences in expected pain based on predictive cues and failed to update expectations following the covert introduction of unexpected medium temperature stimuli. Consistent with observed expectancy differences, subjective pain unpleasantness ratings in the vmPFC lesion group were more strongly modulated by cue during thermal stimulation. No group differences occurred in overall pain sensitivity, nor in relationships between pain and autonomic arousal, suggesting that vmPFC damage specifically enhances the effect of expectations on pain processing, likely driven by impaired integration of new sensory feedback to update expectations about pain. These results provide essential new data regarding the specific functional contribution of vmPFC to pain modulation (Motzkin et al. 2023).
7.1.3.4. Ventro-Lateral Prefrontal Cortex (vlPFC)
Petrides 2005
The down-regulation of pain through beliefs is commonly envisaged as a form of emotion regulation. The analgesic effect co-occurs with reduced anxiety and increased activity in the vlPFC, which is an important region of emotion regulation. The neural basis of the analgesic and anxiolytic effect of two types of threat modulation were compared: a `behavioral control´ paradigm, which involved the ability to terminate a noxious stimulus, and a `safety signaling´ paradigm, which involved visual cues that signaled the threat (or absence of threat) that a subsequent noxious stimulus might be of unusually high intensity. Analgesia was paralleled by vlPFC activity during behavioral control. Safety signaling engaged elements of the descending pain control system, including the rACC that showed increased functional connectivity with the PAG and vlPFC. In contrast, anxiety reduction scaled with dlPFC activation during behavioral control but had no distinct neural signature during safety signaling. This suggests that analgesic and anxiolytic effects are exerted in distinct neural mechanisms and differ between distinct stress- and pain-modulatory approaches (Wiech et al. 2014).
vlPFC and Social Pain. The right vlPFC (rvlPFC) has been implicated in mitigating social pain, a multi-faceted emotional response triggered by inter-personal rejection or criticism. Transcranial magnetic stimulation (TMS) integrated with fMRI recordings were used during a social-pain task on eighty participants who underwent either active TMS targeting the rvlPFC or control stimulation at the vertex. TMS-induced rvlPFC facilitation significantly reduced self-reported social pain, confirming the causal role of the rvlPFC in social pain relief. Functional connectivity analyses demonstrated enhanced interactions between the rvlPFC and the dlPFC, pointing to collaborative engagement of prefrontal regions in emotion regulation. Significantly, negative social feedback led to negative social attitudes, whereas rvlPFC activation countered this detrimental effect (Li et al. 2024).
7.1.3.5. OFC-THAL-PAG Connection
The nucleus submedius (Sm) in the medial THAL, the vlOFC, and the PAG are supposed to constitute a pain-modulatory pathway, activation of which leads to activation of the PAG-brainstem descending inhibitory system and depression of the nociceptive inputs in the spinal cord and trigeminal nucleus. There are indications that the Sm-vlOFC-PAG pathway plays an important role in the analgesia induced by electroacupuncture stimulation of the acupuncture point (acupoint) for exciting small-diameter group III and group IV afferents (Tang et al. 2009).
7.1.4. Cingulate Cortex (CC)
Anterior Cingulate Cortex (ACC). A top-down cortico-spinal descending pathway from the ACC to the spinal DH could directly regulate nociceptive transmission. Pyramidal cells in the deep layers of the ACC send direct descending projecting terminals to the DH of the spinal cord (lamina I-III). (Chen et al. 2014; Wang et al. 2015). The peri-genual ACC sub-division has been suggested to participate in top-down control of pain, including the placebo effects known to be opioid mediated, mainly (but not exclusively) through the connection between the orbitofrontal/sub-genual ACC and the PAG (Peyron et al. 2019). Descending facilitatory pain modulation is an important mechanism underlying the induction and maintenance of neuropathic and inflammatory pain. The ACC widely connects with relevant regions of the descending modulation system. Group IV (C)-fiber-evoked field potentials in the spinal DH were produced by electrical stimulation of the sciatic nerve at an intensity high enough to excite group IV fibers, and paw-withdrawal latencies (PWLs) to noxious heating were recorded. High-frequency tetanic electrical stimulation of the ACC both unilaterally enhanced the group IV fiber-evoked field potentials in the spinal DH and bilaterally shortened PWLs, indicating a facilitation of spinal nociception. Similar effects occurred after micro-injection of NMDA or homocysteic acid into the ACC. After electrolytical lesion of the DRN, ACC-induced facilitation of spinal nociception was blocked. This indicates that (i) activation of the ACC may facilitate spinal nociception; (ii) NMDA receptors in the ACC may be involved in descending facilitation; and (iii) the DRN plays a crucial role in mediating ACC-induced facilitation of spinal nociception (Zhang et al. 2005).
Direct Cortico-spinal Pain Modulation. While many pro-nociceptive and anti-nociceptive effects are mediated by brainstem structures (below), there are also direct cortico-spinal modulatory effects. For example, in mice, part of the CST originating in the S1/S2 somatosensory cortices exerted anti-nociceptive effects mediated by direct innervation of the DH. Tactile stimulation activated CST neurons, and their cortico-spinal projections facilitated light touch-evoked activity of CCK interneurons in the deep DH. This represents a touch-driven spinal-cortico-spinal sensitization loop. In fact, reduction in CST activity or transection of the CST selectively reduced behavioral responses to light touch without altering responses to noxious stimuli, but greatly attenuates tactile allodynia in a peripheral neuropathic pain model (Liu et al. 2018).
7.1.5. Insular Cortex (IC)
The IC is one of the brain areas that are consistently activated during pain. Anterograde tracing revealed extensive IC connections to the main brainstem areas of descending pain control. These included the PAG and NRM. There was also an extensive IC connection to the PBN. There were less extensive but consistent IC axonal inputs to different NA nuclei, the LC, subcoereuleus, and the A5 cell group. These connections suggest an important role of the IC in pain processing through descending pathways (Liang and Labrakakis 2024). The dorsal visual system has direct anatomical projections to the aIC. In rats, retrograde and anterograde tract tracing showed that the granular and dysgranular parts of IC send direct descending projections to the trigeminal caudal sub-nucleus (spVc), especially the superficial laminae (I/II) (Wang et al. 2015).
The rostral agranular insular cortex (RAIC) is a relevant structure in nociception. Recruitment of GABAergic activity in RAIC promotes the disinhibition of the LC, which in turn inhibits (by NA action) the peripheral nociceptive input at the spinal level. At the cortical level, OXT can modulate the GABAergic transmission. Consequently, an interaction modulating nociception could exist between OXT and GABA at RAIC. In male Wistar rats, the effect of OXT micro-injection into RAIC was tested during an inflammatory (by sub-cutaneous peripheral injection of formalin) nociceptive input. OXT micro-injection produced a diminution of (i) flinches induced by formalin and (ii) spontaneous firing of spinal WDR cells. The above anti-nociceptive effect was abolished by micro-injection (at RAIC) of the following: (i) the OXT receptor (OXTR) antagonist L-368899, or by (ii) the GABAA receptor blocker) bicuculline, suggesting a GABAergic activation induced by OXTR. Since intra-thecal injection of a α2A-adrenoceptor antagonist partially reversed the OXT effect, a descending NA anti-nociception is suggested. Further, injection of L-368899 per se induced a pro-nociceptive behavioral effect, suggesting a tonic endogenous OXT release during inflammatory nociceptive input. There is a bilateral projection from the HYP PVN to RAIC. Some of the PVN-projecting cells are OXT and destinate GABAergic and OXTR-expressing cells inside RAIC. Aside from the direct anatomic link between PVN and RAIC, this provided evidence about the role of OXT mechanisms modulating the pain process at the RAIC level (Gamal-Eltrabily et al. 2020).
Projections from the rat IC to lower brainstem areas are possibly involved in orofacial pain processing. IC neurons projecting directly to the trigeminal caudal subnucleus (Vc, medullary dorsal horn) and oral subnucleus (Vo) receive orofacial nociceptive inputs. Retrograde tracing from the medial and lateral part of laminae I/II of Vc, many IC neurons were labeled bilaterally. Tracer injections into the Vo labelled many neurons bilaterally. Injections of an anterograde tracer into the rostral granular IC (GI) and dysgranular IC (DI) labelled, many axons and terminals occurred bilaterally with a contralateral predominance in the medial part of laminae I/II of Vc, dorso-medial Vo, juxta-trigeminal region, RVM, and NTS and with an ipsilateral predominance in the PBN, Kölliker-Fuse nucleus (KF) and trigeminal mesencephalic nucleus. This suggest that orofacial nociceptive processing of Vc and Vo neurons may be regulated by GI/DI directly or indirectly through brainstem nuclei such as lPAG, PBN, KF and RVM (Sato et al. 2013).
aIC and lPFC. The rostral aIC and the lateral PFC (lPFC) seem to act as a pain-control system that alters emotional and behavioral responses to various types of painful stimuli (Tracey and Mantyh 2007). Pain relief has a rewarding effect engaging activation of midbrain DA neurons, release of DA in the NAc, and opioid signaling in the ACC (Harris and Peng 2020; Kuner and Kuner 2021; Mitsi and Zachariou 2016; Navratilova et al. 2015).
7.2. Basal Ganglia (BG)
The NAc plays an important role as a source of analgesia (Harris and Peng 2020; Thompson and Neugebauer 2019), as well as in motivational and emotional processing, pleasure, addiction and reward, beyond simply the rewarding experience of pain offset. The NAc shell may evaluate impending pain and utilize spatial information from the HIPP for appetitive learning, and the core activates in anticipation of relief from an aversive stimulus and signals the reward value of pain cessation (Thompson and Neugebauer 2019). The NAc provides pain-induced analgesia, in part through its projection to the RVM (Doan et al. 2015).
7.2.1. Striatum
Electrical and chemical stimulation of the striatum produced inhibition of the nociceptive jaw-opening reflex. This analgesic action of the striatum was mediated by activation of its DA D2 receptors and transmitted through the indirect pathways of the BG and the medullary DReN to the sensorial nuclei of the trigeminal nerve. Its mechanism of action was by inhibition of the nociceptive response of the second-order neurons of the nucleus caudalis of the V par (Barceló et al. 2012).
In urethane-anesthetized rats, the jaw-opening reflex was produced by supra-threshold stimulation of the tooth pulp and measured as electromyographic response in the digastric muscle, with simultaneous recording of noxious responses in single-unit neurons of the spVc. The micro-injection of glutamate into striatal JOR inhibitory sites significantly decreased the evoked response mediated by group III (Aδ) and group IV (C) fibers in 92% of nociceptive spVc neurons. This suggests that the striatum could be involved in the modulation of nociceptive inputs and confirms the role of the BG in the processing of nociceptive information (Belforte and Pazo 2005).
7.2.2. Nucleus Accumbens (NAc)
The NAc responds heavily to painful stimuli, due to its high density of μ-opioid receptors and the activation of several different neurotransmitter systems in the NAc, such as opioids, DA, CGRP, GABA, glutamate, and SP, each of which elicit analgesic effects. In both preclinical and clinical models, deep brain stimulation of the NAc has elicited successful analgesia. The multi-functional NAc is important in motivational behavior, including the motivation for avoiding pain (Harris and Peng 2020).
Opioid Link Mediating Anti-nociception. In the NAc, an opioid link exists that mediates anti-nociception produced by a novel ascending pain modulation pathway. For example, noxious stimulation induces hetero-segmental anti-nociception that is mediated by both μ- and δ-opioid receptors in NAc. However, spinal intra-thecal administration of the μ-receptor agonist DAMGO also induced hetero-segmental anti-nociception. In the rat, the intra-NAc opioid receptors was identified that mediate the antinociceptive effects of spinally administered DAMGO and to determine the effect of NAc efferent activity on nociception. Intra-NAc administration of either a μ-opioid receptor antagonist or a δ-opioid receptor antagonist blocked the anti-nociceptive effect of spinally administered DAMGO on the jaw-opening reflex. Injection of lidocaine attenuated the jaw-opening reflex, suggesting that the output of NAc is pro-nociceptive. Intra-NAc injection of kainate enhanced the jaw-opening reflex. Thus, it is possible to modulate activity in NAc to bi-directionally attenuate or enhance nociception, suggesting a potential role for NAc in setting nociceptive sensitivity (Gear and Levine 2011).
Effects of Music and Sleep on NAc.The NAc has been implicated in sleep, reward, and pain modulation. It was determined whether NAc function at the onset and offset of a noxious thermal stimulus would be enhanced by rewarding music, and whether that effect is reversed by experimental sleep disruption. Healthy subjects underwent fMRI scans on two separate days after both uninterrupted sleep and experimental sleep disruption. During fMRI scans, participants received noxious stimulation while listening to individualized rewarding or neutral music. Behavioral results revealed that rewarding music significantly reduced pain intensity compared with neutral music, and disrupted sleep was associated with decreased pain intensity in the context of listening to music. Sleep disruption attenuated NAc activation at pain onset and during tonic pain. Rewarding music altered NAc connectivity with nodes of the cortico-striatal circuits during pain onset. Sleep disruption increased reward-related connectivity between the NAc and the MCC at pain onset. Hence, experimental sleep disruption modulates NAc function during the onset of pain in a manner that may be conditional on the presence of competing reward-related stimuli (Seminowicz et al. 2019).
7.2.3. Subthalamic Nucleus (STN)
The STN is the only glutamatergic nucleus in the BG and modulates motor, limbic, and cognitive functions. In humans and rodents, the STN may also play a significant role in pain perception and modulation. STN neurons have fast spontaneous firing and readily respond to painful stimuli. In mice, optogenetic activation of STN neurons decreased pain thresholds. In Parkinsonian mice, optogenetic inhibition of hyperactive STN neurons ameliorated hyperalgesia and central sensitization. There is a pathway, consisting of GABAergic neurons in the SNr (SNrGABA) and glutamatergic neurons in the STN (STNGlu) and the lateral parabrachial nucleus (lPBNGlu) that modulates acute and persistent pain states in both male and female mice. In such pain states, the activity of STN neurons was enhanced. This enhancement was accompanied by hypoactivity in SNrGABA neurons and strengthening of the STN-lPBN glutamatergic projection. Reversing the dysfunction in the SNrGABA-STNGlu-lPBNGlu pathway attenuated activity of lPBNGlu neurons and mitigated pain-like behaviors. Therefore, the SNrGABA-STNGlu-lPBNGlu pathway regulates pathological pain and is a potential target for pain management (Jia et al. 2022).
7.3. Amygdala (AMY)
The AMY is an important center for the emotional-affective dimension of pain and for pain modulation (Neugebauer 2015). Concerning pain modulation, it appears to be asymmetrical. The right CeA has a strong pro-nociceptive function across pain models. The left CeA has often been characterized as having no effect on pain modulation, a dampened pro-nociceptive function, or most recently an anti-nociceptive function (Allen et al. 2021).
CeA output targets the PAG and the RVM, which are critical for mediating behavioral coping responses in the face of threat (Kuner and Kuner 2021). The analgesic effects of micro-injection of morphine into different sites of the AMY suggest that they were mainly attributed to the direct projections from the AMY to the RVM. Infusing morphine into the BLA increased OFF-cell activity, modestly decreased ON-cell activity, and increased tail-flick latency (TFL). Applying morphine into the cortical and medial nuclei exerted smaller effects on ON- and OFF-cells than infusion directly into BLA, and only the increased TFL to a small degree. Introducing morphine into the central and dorso-lateral nuclei failed to modulate activities of the RVM ON- and OFF-cells and the TFL (Peng et al. 2023).
7.4. Thalamus (THAL)
The Sm of the medial THAL is supposed to be involved in the modulation of nociception. The vlOFC, NSm and PAG establish a pain-modulatory pathway, activation of which activates the PAG descending inhibitory system and depression of the nociceptive inputs in the spinal DH and spV (Tang et al. 2009).
The THAL medio-dorsal (MD) and ventro-medial (VM) nuclei have been hypothesized to serve as `promoters´ in initiating descending facilitation and inhibition, respectively, of spinal nociceptive transmission, with specific spatio-temporal characteristics. According to this hypothesis, the MD would initiate descending facilitation via the CC, CST or dlPAG to the DH superficial layers. By contrast, the VM would initiate descending inhibition via the IC and vlPAG to the deep DH layers. The engagement of these THAL nuclei in descending modulation of nociception is supposed to be `silent´ or inactive during the physiological state as well as in conditions of insufficient noxious stimulation. Once sufficient activity from noxious or innocuous group IV sensory afferents arises, the MD and VM nuclei exhibit different effects: facilitation and inhibition, respectively, upon noxious mechanical and heat-evoked nociception (You et al. 2022).
7.5. Habenula (Hb)
The Hb could play a role in descending pain control.
In rats, the effects were assessed of intra-habenular injection of morphine on acute trigeminal pain as well as the involvement of DRN opioid and 5HT3 receptors on the anti-nociceptive activity of intra-Hb morphine to explore the possibility of existence of descending anti-nociceptive relay between the Hb and DRN. The numbers of eye wiping response elicited by applying a drop of NaCl (5 M) solution on the corneal surface were taken as an index of acute trigeminal nociception. Intra-habenular micro-injection of morphine at higher doses produced anti-nociception. Pretreatment of the DRN with ondansetron but not naltrexone prevented intra-habenular morphine induced anti-nociception. Intra-Hb injection of lidocaine reduced the corneal pain response. This suggests that the activation of the Hb μ-opioid receptor by micro-injection of morphine or inhibition of Hb neurons by micro-injection of lidocaine produced an analgesic effect in the acute trigeminal model of pain in rats. The analgesic effect of intra-Hb morphine was blocked by intra-DRN injection of 5-HT3 antagonist (Khalilzadeh and Vafaei Saiah 2017).
7.6. Hypothalamus (HYP)
Due to its heterogeneous complexity, the HYP emanates various strings by which it contributes to pain modulation, mostly via intermediate stages or finally in the DH. At least three pathways are direct to the DH. One is an OXT path. Another is a VAP path. The third is a DA path to the DH. Indirect HYP-DH connections include the following.
As mentioned above, electrical lHYP stimulation produces anti-nociception probably mediated by brainstem NA A7 cells. SP-immuno-reactive neurons in the lHYP project near the NA A7 cell group, which effects anti-nociception in the DH (Holden and Naleway 2001).
HYP-RVM Connections participate in pain modulation. In female Sprague-Dawley rats, electrical stimulation of the lHYP produced anti-nociception mediated in part by spinally projecting 5-HT neurons in the ventro-medial medulla, which acted at 5-HT1A, 5-HT1B, and 5-HT3 receptors in the DH (Holden et al. 2005). While intense or highly arousing stressors suppress pain, relatively mild or chronic stress can enhance pain, causing stress-induced hyperalgesia (SIH). The physiological and neuroendocrine effects of mild stress are mediated by the DMH, which has connections with the RVM. Stress could engage both the DMH and the RVM to produce hyperalgesia. In awake animals, direct pharmacological activation of the DMH increased the sensitivity to mechanical stimulation, confirming that the DMH can mediate behavioral hyperalgesia. A behavioral model of mild stress also produced mechanical hyperalgesia, which was blocked by inactivation of either the DMH or the RVM. The DMH has a role in stress, but may also be engaged in a number of chronic or abnormal pain states (Wagner et al. 2013).
7.6.1. Neurons Releasing Corticotropin-Releasing Hormone (CRH)
CRH is an initiating hormone of the hypothalamic-pituitary-adrenal (HPA) axis. Moreover, secreted CRH travels to other CNS sites and affects its stress-associated function including pain modulation. It elicits strong anti-nociceptive effects at the peripheral, spinal, and brain levels (Kuner and Kuner 2021). CRH is also expressed in nociceptors and their neighboring components, giving rise to hypotheses for possible pain modulations at this level (Zheng et al. 2020).
7.6.2. Neurons Releasing Oxytocin (OXT) and Vasopressin (AVP)
Much evidence indicates that HYP-derived neuropeptides, OXT, AVP, and ORX A and B, inhibit nociceptive transmission in the rat spinal DH (Kumamoto 2019).
OXT and AVP are closely related pituitary nona-peptides and are synthesized in overlapping regions of the HYP, primarily in morphologically and electrophysiologically distinct magnocellular and parvocellular neurons located in the HYP PVN and SON (evolution: Jurek and Neumann 2018). However, they are not only released into the blood from nerve endings in the posterior pituitary but also, independently, from dendrites in the PVN and SON (Bali et al. 2014; Hökfelt et al. 2018; Lefevre et al. 2021). Both magnocellular and parvocellular AVP and OXT neurons project to extra-HYP sites including the AMY, and AVP neurons occur in the MeA of rodents but not primates (Neugebauer et al. 2020). OXT is secreted and circulated in the systemic vasculature following transport to and release from the posterior pituitary gland. In contrast, neurosecretory cells in the parvocellular part of the PVN synthesize OXT and transport it via axons to remote parts of the CNS, suggesting the existence of intricate OXT circuits (Kuner and Kuner 2021; Takayanagi and Onaka 2021). Receptors for both OXT and AVP are localized in areas of the nervous system that regulate social, emotional, and adaptive behaviors.
Both magnocellular and parvocellular OXT and AVP neurons project to extra-hypothalamic sites including the AMY, and AVP neurons are found in the medial AMY of rodents but not primates. Evidence suggests mutually opposing functions of OXT and AVP in the CeA through GABA interneurons. Both AVP and OXT exert anti-nociceptive effects in tail-flick, hot-plate and formalin tests after systemic, peripheral, intra-thecal or intra-cerebro-ventricular (ICV) administration. Micro-injection of AVP into the CeA suppressed an electromyographic nociceptive jaw-opening reflex evoked by electrical stimulation. Stereotaxic administration of AVP into the CeA had no effect on mechano-sensitivity (nocifensive reflexes) but increased emotional responses (vocalizations to noxious stimuli) and anxiety-like behavior (elevated plus maze) (Neugebauer et al. 2020).
7.6.2.1. Neurons Releasing Oxytocin (OXT)
Inputs. In the mouse, inputs to PVN OXT neurons derive from the CC, IC, OFC, subfornical organ, Hb, HYP ARC nucleus, dorso-medial HYP area, pHYP nucleus, zona incerta (ZI), lateral lemniscal nucleus, medial POA (mPOA), posterior intra-laminar THAL, medial raphé nucleus, NRM, and PPN (Lefevre et al. 2021). OXT contributes to descending pain control (below), but it remain unclear how ascending nociceptive signals would impact HYP OXT/AVP neurons (Zheng et al. 2021).
Outputs. The parvocellular OXY neurons project to brain areas such as BNST, medial pre-optic areas, vmHYP, NAc, NTS, dorsal nucleus of the vagus nerve, area postrema (Bali et al. 2014). In the mouse, PVN OXT neurons project to the posterior pituitary gland, olfactory bulb, accessory olfactory nucleus, cerebral cortex, vHIPP, VTA, LC (Lefevre et al. 2021). In Wistar rats, a sub-set of approximately 30 parvocellular OXT neurons sends collaterals to magnocellular OXT neurons and longer-range projections to neurons of spinal deep layers. Evoked OXT release from these OXT neurons suppressed nociception and promoted analgesia in an animal model of inflammatory pain (Eliava et al. 2016).
Descending Pain Processing. A neuronal pathway from the HYP PVN to the spinal cord and trigeminal nucleus caudalis (spVc) has been described (Condés-Lara et al. 2024). OXT is involved in the pain-inhibitory descending pathway and generally assumed to exert analgesic effects (Kawasaki et al. 2024). Electrical stimuli applied to the HYP PVN reduced the nociceptive neuronal activity, i.e., neuronal discharge associated with activation of group III (Aδ) and group IV (C) fibers, the spinal DH wide dynamic range (WDR) cells and nociceptive behavior (Godínez-Chaparro et al. 2016). Optogenetic activation of these specific OXT neurons dampened nociception and inflammatory pain, both via direct suppression of nociceptive processing in the spinal cord and via release of OXT in the bloodstream (Kuner and Kuner 2021; Takayanagi and Onaka 2021). Direct PVN-DH projections could contribute to this action. HYP PVN projections to the spinal DH are related to anti-nociception. OXT, AVP and ENK could be released in the DH. There is anatomical evidence of interactions between OXT, AVP, ENK, and dynorphin (DYN) along the PVN projections to the spinal DH at L3 level. A high percentage of co-localizations between two of the peptides (OXT-AVP, OXT-DYN, AVP-ENK, and DYN-ENK) were present along the PVN, as well as in the DH at L3 level. This showed that different anti-nociceptive peptides may interact with each other to evoke PVN anti-nociceptive effects as part of the endogenous system of nociceptive modulation (Gamal-Eltrabily et al. 2018). OXT infusions into the CeA (which receives inputs from HYP OXT) produced analgesia. HYP descending anti-nociception appears to occcur predominantly in OXTR-positive WDR neurons in DH lamina V, but not in superficial laminae. Overall, HYP OXT neurons appear to constitute a separate descending inhibitory pain control pathway (Zheng et al 2021).
OXT HYP-DH Projection. OXT transmission blocks nociception at the peripheral, spinal, and supraspinal levels through the OXT receptor (OXTR). There is a direct neuronal pathway from the HYP PVN to the spinal cord and spVc. The trigemino-cervical complex (TCC), an area spanning the spVc, C1, and C2 regions, plays a role in some pain disorders associated with cranio-facial structures (e.g., migraine). In male Wistar rats, in vivo electrophysiological recordings of TCC wide-dynamic range (WDR) cells sensitive to stimulation of the peri-orbital or meningeal region were performed. PVN electrical stimulation diminished the neuronal firing evoked by peri-orbital or meningeal electrical stimulation. This inhibition was reversed by OXTR antagonists administered locally. Accordingly, there existed neuronal projections from the PVN to the WDR cells (Condés-Lara et al. 2024).
HYP-spVc Projection.The spVc/C1 region or medullary DH (MDH) plays an important role modulating the nociceptive input arriving from cranio-facial structures. OXT could play a role in modulating the nociceptive input at the MDH level. Using an electrophysiological and pharmacological approach, the effect of OXT on the nociceptive signaling in the MDH and the receptor involved were determined. In anesthetized rats, electrophysiological unitary recordings were made from second-order neurons at the MDH region responding to peripheral nociceptive-evoked responses of the first branch (V1; ophthalmic) of the trigeminal nerve. Under this condition, dose-response curves were constructed analyzing the effect of local spinal OXT on MDH nociceptive neuronal firing. The role of OXT receptors (OXTRs) or AVP V1A receptors (V1ARs) were determined. OXT dose-dependently inhibited the peripheral-evoked activity in nociceptive MDH neurotransmission. This inhibition was associated with a blockade of neuronal activity of group III (Aδ) and group IV (C) fibers. Since this anti-nociception was abolished by pretreatment (in the MDH) with the potent and selective OXTR antagonist and remained unaffected after the V1AR antagonist, the role of OXTR is implied. Hence, OXT inhibited the peripheral-evoked neuronal activity at MDH, through OXTR activation (García-Boll et al. 2018).
7.6.2.2. Neurons Releasing Vasopressin (AVP)
The nonapeptide AVP is mainly synthesized in the HYP PVN and SON and secreted from the posterior pituitary gland to the systemic circulation. AVP neurons are also found in the lateral septum, HIPP, AMY, and other brain structures. The most sensitive stimulus for the secretion of AVP is serum osmolality, although the secretion of AVP is also affected by changes in blood volume and blood pressure (Mavani et al. 2015). AVP plays multifarious non-pressor and non-antidiuretic roles in pain, infection and inflammation, regulation of the HPA axis, cognition, social behavior, bone and lipid metabolism, cellular proliferation, body fluid balance, blood pressure regulation, sodium homeostasis, hypertension, metabolic syndrome, diabetes, and kidney function. AVP has an agonistic activity on the OXTRs. Conversely, OXT can act on AVPR1A receptors (Cid-Jofré et al. 2021; Koshimizu and Tsujimoto 2009). AVP activates three AVP receptors, which are located in the brain and in the periphery: AVPR1A, AVPR1B, and AVPR2. AVPR1a receptors are present in the brain, vascular smooth muscle cells, adrenal cortex, pancreas, adipose tissue, hepatocytes, osteoblasts and osteoclasts (Cid-Jofré et al. 2021). AVPR1B receptors are mainly present in anterior pituitary, adrenal medulla, pancreatic islet cells of Langerhans, and white adipose tissue. AVPR2 receptors occur in the kidney, alveolar epithelial cells, osteoblasts and osteoclasts (Mavani et al. 2015).
AVP exerts its actions through various receptors: AVPR1A, AVPR1B, AVPR2, and AVPR3. AVP receptors are expressed on CRH-producing PVN cells, and on neurons in the BNST, CeA, and particular neurons in the Edinger-Westphal nucleus (Bali et al. 2014).
Inputs to AVP neurons in PVN and SON are heterogeneous. Generally, PVN and SON releasing AVP neurons receive inputs from septal nuclei, AMY, arcuate and peri-ventricular THAL nuclei, circumventricular organs, BNST, diagonal band of Broca, NRD, lPBN, AP, lateral tegmenal area, LC, NTS, and others (Iovino et al. 2016). However, PVN AVP neurons received relatively broader and denser inputs than did SON AVP neurons (Wei et al. 2021).
Outputs. The tracts from the HYP AVP-containing neurons innervate other areas of brain and the spinal cord, enabling AVP to exert its actions locally in the brain and spinal cord. Smaller amounts are also produced outside the HYP in many tissues. Thus, apart from its endocrine effect, AVP exerts autocrine and paracrine effects (Mavani et al. 2015). Both magnocellular and parvocellular AVP and OXT neurons project to extra-HYP sites including the AMY, and AVP neurons occur in the MeA of rodents but not primates (Neugebauer et al. 2020).
7.6.3. Neurons Releasing Orexin (ORX)
ORXs are excitatory neuropeptides that include two substances derived from the same precursor (ORXA and ORX B, also called hypocretins 1 and 2, respectively), which.are primarily synthesized and secreted by the lHYP and pHYP. ORXs are not only involved in the regulation of feeding, the sleep-wake cycle, energy metabolism, but are also closely associated with various physiological functions, such as cardio-vascular control, reproduction, the modulation of pain transmission, arousal, stress regulation, fear, anxiety, reward, addiction and cognition. ORX receptors and projections are localized in wide regions like NAc, VTA, PAG, and spinal cord, which are involved in pain modulation. ORXA activates both ORXA receptors (ORX1Rs) and ORXB receptors (ORX2Rs) with approximately equal potency, while ORXB preferentially activates ORXBRs. The distribution of ORXARs and ORXBRs in the brain shows partially overlapping and partially distinct patterns (Han et al. 2020; Jacobson et al. 2022; Kang et al. 2021; Rezaee ez al. 2018; Sartori and, Singewald 2019).
Inputs. ORX neurons are activated by nociceptive mechanical and heat stimuli. Inputs regulating ORX neurons arise from ACh neurons in the BFB, GABAergic neurons in the POA, lateral septum, BNST, AMY, many HYP regions, 5-HT raphé neurons, and PAG (Grafe and Bhatnagar 2018; Han et al. 2020; Ma et al. 2018; Sartori and Singewald 2019).
Outputs. Although the number of HYP ORX neurons is limited, they project to many pain-related brain regions, where they modulate pain, including the mPFC, CC, BFB, BNST, dorsal HIPP, caudate nucleus, NAc, CeA, THAL (PVT), PVN, lHYP, histaminergic tubero-mamillary nucleus (TMN), NA LC, 5-HT NRD, DA VTA, LDT, PPN, NTS, RVM, RF, and the spinal DH (Grafe and Bhatnagar 2018; Han et al. 2020; Ma et al. 2018; Sartori and Singewald 2019). Some of these regions contain only ORX1Rs and others only ORX2Rs (Han et al. 2020).
In mouse, rat, and human spinal cord, long descending axonal projections containing ORX innervate all levels of the spinal cord from cervical to sacral segments. High densities of axonal innervation are found in regions of the spinal cord related to modulation of sensation and pain, notably in the marginal zone (lamina 1). Innervation of the intermedio-lateral column and lamina 10 as well as strong innervation of the caudal region of the sacral cord suggest that ORX may participate in the regulation of both the sympathetic and parasympathetic parts of the ANS. In cultured rat spinal cord neurons, digital-imaging with fura-2 detected a rise in intracellular Ca2+ in response to ORX, indicating that spinal cord neurons express ORX-responsive receptors. A greater number of cervical cord neurons responded to ORX than OXT (Van den Pol 1999).
In male Wistar rats, the effects were investigated of intra-thecal administration of an ORX-1 receptor antagonist in spinal anti-nociception induced by intra-LH administration of carbachol (cholinergic receptor agonist) in both early and late phases of pain-related behaviors. Pain-related behaviors (pain scores) were evaluated using the formalin test during 5-min block intervals for a 60-min period Intra-LH injection of carbachol attenuated the formalin-induced biphasic pain responses, and intra-thecal administration of the ORX-1 receptor antagonist dose-dependently decreased lHYP stimulation-induced anti-nociceptive responses during both phases. The anti-nociceptive role of ORX system in the formalin test through a neural pathway from the LH to the spinal cord provides evidence that ORXs can induce pain relief (Rezaee et al. 2018).
7.6.4. Neurons Releasing Hypothalamic Dopamine (DA) Neurons
DA is a multi-functional endogenous catecholamine found in many brain regions. DA acts via five G-protein-coupled receptors, two of which activate (D1, D5) and three of which (D2, D3, D4) reduce cAMP. The midbrain DA complex comprises the SNc, VTA and RRF, which contain the A9, A10 and A8 groups of nigro-striatal, meso-limbic and meso-cortical DA neurons, respectively. The VTA is a complex group of DA neurons that are primarily regulated through glutamatergic innervation, resulting in both short- and long-term changes in DA activity (Kelly and Fudge 2018).
The HYP hosts some DA A11 cell groups that are involved in descending pathways. The A11 nucleus projects to both the brainstem and spinal cord, and their terminals are in the spinal DH and around the preganglionic sympathetic neurons in the thoraco-lumbar spinal cord. Available data favor the participation of the spinal DA system in pain modulation and autonomic and motor responses. The excitation the DA A11 neurons inhibited orofacial neuropathic pain (Li et al. 2019; Lindvall et al. 1983; Puopolo 2019).
Electrical stimulation of HYP DA A11 cells induced outward current and suppressed the frequency and amplitude of EPSCs. Hence, this HYP DA descending pathway has an anti-nociceptive effect via D2-like receptors on substantia-gelatinosa neurons (Taniguchi et al. 2011).
Inputs. In rodents, HYP A11 DA neurons receive inputs from cortical areas, including the CC, IL and BNST, which are involved in the affective and emotional aspects of pain and the behavioral responses to aversive or threatening stimuli, as well as from midbrain and brainstem nuclei involved in pain modulation, such as the PAG and the PBN. A11 DA neurons are predominantly driven by sensory inputs, responding to tactile and visual sensory modalities (Puopolo 2019).
Outputs. Descending fibers from the A11 cell group terminate in both the spinal DH and VH and form axo-dendritic synapses or terminate sparsely. DA can exert both anti-nociceptive and pro-nociceptive effects, with activation of D2-like receptors mediating the anti-nociceptive effects, and activation of D1-like receptors mediating the pro-nociceptive effects (Puopolo 2019). Electrical stimulation of HYP DA A11 cells, which project to the spinal cord, induced outward current and suppressed the frequency and amplitude of EPSCs. Hence, this HYP DA descending pathway has an anti-nociceptive effect via D2-like receptors on substantia-gelatinosa neurons (Taniguchi et al. 2011).
Actions. An example of DA actions on spinal nociceptive reflexes is the following. In high spinal cats, noxious radiant heat induced reflex facilitation, with an early component being mediated by group III fibers and a late component by group IV fibers. After injection of L-DOPA, the onset of reflex facilitation induced by noxious radiant heat was delayed by 4 to 10 s, i.e. the early component was blocked, while the late component persisted. Presumably, therefore, DA preferentially blocks the transmission in nociceptive reflex pathways from group III fibers (Schomburg et al. 2011b).
7.7. Midbrain Neurons Releasing Dopamine (DA)
Among the brainstem structures that are involved in endogenous pain modulation is the midbrain SN plus VTA that produce DA.
There are four major DA pathways emerging from the brainstem are: (i) The meso-limbic pathway from VTA to NAc; (ii) The meso-cortical pathway from VTA to PFC; (iii) The nigro-striatal pathway from the SNc to the caudate nucleus; and (iv) The tubero-infundibular pathway from tuberal HYP to pituitary gland. Four minor pathways are from VTA to AMY, HIPP, CC, and olfactory bulb (Li et al. 2019).
Pain Modulation. The analgesic effects of DA receptors, particularly D1 and D2 receptors, are different in different regions of the CNS, including the striatum, NAc, PAG and spinal cord. These regions express a high density of DA receptors and thus are well suited for pain modulation by DA. D2-like receptors may exert a higher analgesic potency, but D1-like receptors act in different manners across several mechanisms in the mentioned regions. In the spinal cord and striatum, anti-nociception of DA is mainly mediated by D2-like receptors, while in the NAc and PAG, both D1- and D2-like receptors are involved as analgesic targets. D2-like receptor agonists can act as adjuvants of μ-opioid receptor agonists to potentiate analgesic effects and provide a better approach to pain relief (Wang et al. 2021).
7.8. Brainstem Noradrenergic (NA) Cell Groups
7.8.1. Locus Coeruleus (LC)
Other brainstem regions are able to significantly modulate incoming peripheral nociceptive signals. One source are brainstem NA neurons. The LC and A5 NA cell groups project to the DReN. The increased activation of NA LC and A5 neurons in traumatic neuropathic models leads to increased release of NA into DReN, which was proposed to enhance descending facilitation of nociceptive transmission from that medullary area (Tavares et al. 2021). The LC release of NA can have both inhibitory and facilitating effects on neurotransmission throughout the CNS via the activation of α1 and α2 adrenoreceptors (Mills et al. 2021).
Functional LC Heterogeneity. LC neurons appear to be a functionally heterogeneous. In part, LC projections are segregated into distinct output channels and have the potential for differential release and actions of NA on its projection targets, thereby enabling differentiated modulation of diverse behaviors and cognitive functions (Kuner and Kuner 2021; Waterhouse et al. 2022).
Ascending Nordrenergic Pain Modulation. Brainstem NA neurons are widely distributed and influence nociceptive responses in supraspinal structures, including the PAG, RVM, mesencephalic LRN, HYP, THAL, AMY, striatum of the BG, and the cerebral cortex. The effects can be facilitatory or inhibitory, depending on the site of release and sub-type of adrenoceptor, the duration of the pain and pathophysiological conditions (De Felice and Ossipov 2016; Pertovaara 2006, 2013).
Descending Outputs. Descending NA projections to the spinal DH and spV arise from groups A5, A6 and A7 (Kölliker-Fuse) pontine NA nuclei, and these regions communicate with the RVM and PAG (Ossipov et al. 2014). Descending projections target the spinal DH and mainly, but not exclusively, inhibit nociceptive transmission. For spinally projecting neurons, an anti-nociceptive role has been suggested for cells in the ventral LC and a pro-nociceptive role for NA neurons of the dorsal LC (Kuner and Kuner 2021; Waterhouse et al. 2022). The LC NA system has a dual role in descending pain control. First, it exerts anti-nociception due to its direct inhibitory action on the spinal cord. Second, the LC NA system may induce pro-nociception by directly acting on brainstem pain modulatory circuits, namely, at the LC and medullary DReN (Tavares et al. 2021; Figure 3).
7.8.2. Noradrenergic (NA) A Cell Groups
NA A5-A7 Cell Groups. A5/A7 NA and B2 5-HT cell groups form descending pathways and project to the DH. Acute nociceptive stimuli rapidly increased the activities of A5/A7 NA or B2 5-HT neurons but the non-noxious stimuli did not (Moriya et al. 2022). The pontine NA cell groups, A5, A6 (LC), and A7, provide the only NA innervation of the spinal cord. The A6 group (LC) provides the densest innervation at all spinal levels, and includes all parts of the spinal gray matter, but it is particularly dense in the DH. The A7 group provides the next most dense innervation, again including all parts of the spinal cord, but it is denser in the VH. The A5 group supplies only sparse innervation to the DH and VH and to the cervical and lumbo-sacral levels, but provides the densest innervation to the thoracic intermedio-lateral cell column, and in particular to the sympathetic preganglionic neurons. The observed pattern of spinal projections may suggest that the LC might have the greatest effect on somatosensory transmission, the A7 group on motor function, and the A5 group on sympathetic function (Bruinstroop et al. 2012). NA A5-A7 groups projecting to the DH possibly exert pain transmission control (Dostrosky 2000; Gamal-Eltrabily et al. 2018; Kwiat and Basbaum 1992; Pertovaara 2006, 2013). In the rat, the pontine NA A5 cells, which project to the DH, are reciprocally connected with the CVLM in the rat (Tavares et al. 1997). The PAG projects to A7 and LC, which connect reciprocally with RVM (De Felice and Ossipov 2016).
A5/A7 NA and B2 5-HT cell groups form descending pathways and project to the DH. Acute nociceptive stimuli rapidly increased the activities of A5/A7 NA or B2 5-HT neurons but the non-noxious stimuli did not (Moriya et al. 2022).
A7 Cell Group. Electrical lHYP stimulation produced anti-nociception partially blocked by intra-thecal α-adrenergic antagonists. SP-immuno-reactive neurons in the lHYP project near the NA A7 cell group, which effects anti-nociception in the DH. The cholinergic agonist carbachol, micro-injected into the lHYP, significantly increased response latencies of tail-flick and nociceptive foot-withdrawal latencies. However, two sequential doses of yohimbine blocked lHYP-induced anti-nociception on both tests. In contrast, two sequential doses of WB4101 increased nociceptive responses on both the tail-flick and foot-withdrawal tests. This suggests that neurons in the lHYP activate spinally projecting methionine enkephalin neurons, as well as two populations of A7 NA neurons that exert a bi-directional effect on nociception. One of these populations increases nociception through the action of α1-adrenoceptors and the other inhibits nociception through the action of α2-adrenoceptors in the spinal DH (Holden and Naleway 2001).
7.9. Peri-Aqueductal Gray (PAG)
The PAG plays an important role in conveying the modulatory influences from higher brain regions involved in aspects of pain responses such as cognition and emotion, including the PFC and IC, and the AMY. The PAG collects the modulatory influences from these areas and uses the RVM as a relay to indirectly target the spinal cord (Martins and Tavares 2017). Thus, the PAG and RVM take a central position, complemented by further systems. In general, activation of all vlPAG neurons by delivery of glutamate or any agonist of ionotropic glutamate receptors results in an elevation of sensory thresholds and/or reduced responses to noxious input. By contrast, injection of GABA and GABA agonists into the vlPAG enhances behavioral response to noxious input, suggesting that pharmacological manipulations that increase PAG excitation facilitate anti-nociceptive behaviors (Nguyen et al. 2023).
Inputs. The PFC-AMY-dorsal PAG-pathway may mediate fear-conditioned analgesia, i.e., a reduction in pain response upon re-exposure to a context, previously paired with an aversive stimulus. The mPFC-PAG projection also plays a role in modulation of autonomic responses to pain. In addition, the PAG receives inputs from the BNST, the midbrain RRF and VTA. fMRI has shown that the vlPAG is functionally connected to brain regions associated with descending pain modulation including the ACC, upper pons/medulla (Bouchet and Ingram 2020; Ong et al. 2019; Ossipov et al. 2014; Vázquez-León et al. 2021; Yetnikoff et al. 2014). As mentioned above, there appears to an important PFC-AMY-dPAG pathway, which may mediate fear-conditioned analgesia, i.e., a reduction in pain response upon re-exposure to a context, previously paired with an aversive stimulus. The mPFC-PAG projection also plays a role in modulation of autonomic responses to pain (Ong et al. 2019).
Outputs.The PAG and RVM are the twin core of descending pain control. The pain modulation results from descending onnections from the PAG to the RVL, NRM and other brainstem structures which send glutamatergic, GABAergic, 5-HT and ENK projections to the spinal cord (Lamotte et al. 2021). Via the RVM, the PAG exerts both anti- and pro-nociceptive influences on nociceptive signal tramission in the DH or spV. The endogenous pain-modulation structures are strongly interconnected, e.g., LC and SRD, which also send direct projections to the DH and spV modulate nociception (Mills et al. 2021). Under normal conditions, PAG output neurons to the RVM are inhibited by GABA. Removal of this inhibition resulted in activation of the descending pain modulatory circuit and analgesia. The assumption is that this disinhibition promotes excitatory neurotransmission from the PAG to RVM. Opioid-triggered analgesia was mediated by projections from the vlPAG to RVM, while non-opioid-triggered analgesia was elicited by projections of the lPAG and dlPAG to RVM (Peng et al. 2023). However, opioid receptors are expressed on both GABAergic and glutamatergic terminals in the PAG and both cell populations project to RVM. Thus, PAG to RVM connections are more complicated than simply eliciting disinhibition of excitatory descending projections and probably reflect the existence of parallel circuits that contribute to the bi-directional control of pain mediated by the RVM (Bouchet and Ingram 2020). The PAG has indirect routes to DH via the LC and NRM (Willis and Westlund 1997).
Pain Modulation. The PAG exerts its pain-modulatory effects in part via descending NA pathways, which originate in cell groups A5-A7, A5 and A6 in the LC and pontine A7. The PAG projects to A7 and LC, which connect reciprocally with RVM. LC also receives inputs from RVM, AMY, THAL nuclei and IC (De Felice and Ossipov 2016). LC NA neurons are important for supraspinal opioid anti-nociception, which is primarily driven by excitatory output from the vlPAG to the LC. The suppression of opioid-sensitive inhibitory input from the RVM disinhibits LC neurons to drive spinal NA anti-nociception (Lubejko et al. 2024). In the vlPAG, ORX release from incoming HYP projections may contribute to stress-induced analgesia (SIA) (Brodal 2017; Butler and Finn 2009; Kuner and Kuner 2021).
Selectivity. PAG control of spinal DH responses is highly selective for noxious inputs. Selective suppression of nociception would allow an organism to respond in an appropriate manner to a life-threatening situation without the distraction or counter-productive motor responses that might be evoked by noxious input. There are also clear differences in the descending control of group IV fiber- versus group III fiber-mediated spinal nociception from the PAG. Group IV fiber-evoked activity is powerfully suppressed, whereas III-fiber nociception is unaffected or even enhanced. Such differential control is of considerable behavioral significance given the central role of the PAG in coordinating survival strategies (Heinricher et al. 2009).
7.10. Nuclei in the Reticular Formation (RF)
The RF contains areas that are involved in bi-directional pain control. Among them are specific neuronal populations involved in anti-nociceptive or pro-nociceptive behavioral responses, namely the RVM, VLM, and DReN.
Inputs/Outputs. The PAG especially, but also the RVM, VLM, and DReN receive important direct and indirect inputs from limbic forebrain areas, including the mPFC, ACC, AMY, DMH (Heinricher et al. 2009). The mentioned structures target the spinal DH. The RVM receives nociceptive signals via a direct connection from the PBN, these signals also being conveyed to the RVM pain-modulating neurons under basal conditions. Additional inputs from PBN have the capacity to activate both classes of RVM pain-modulating neurons (below) (Chen et al. 2017).
Activation of the PBN projection to the PAG also produced analgesia. PBN links ascending pain-transmission pathways with descending pain-modulation pathways, including the PAG-RVM circuit that has been implicated in both analgesia (including opioid analgesia) and hyperalgesia and persistent pain states. By contrast, the lPBN output to the RVM exerts a net pro-nociceptive effect, which is most pronounced in persistent pain states (Chiang et al. 2020).
Brainstem Prodynorphin (Pdyn)-expressing LJA5 Neurons. In the mouse, there is a population of inhibitory prodynorphin-expressing neurons named LJA5 (located in lateral pons, juxta A5). LJA5 neurons receive inputs from sensory and stress areas such as S1 and IC, POA, PVN, DMH and lHYP, PAG, and lPBN. The projections of the Pdyn-expressing LJA5 neurons had terminal fields, which were densest in the lPAG and vlPAG, lPBN, caudal pressor area, and lamina I of the spV and all levels of the spinal cord. LJA5 neurons send the only known inhibitory descending projection specifically to spinal lamina I. This pattern of inputs and outputs suggests that LJA5 neurons are well positioned to be activated by sensation and stress, and in turn, inhibit pain and itch (Agostinelli et al. 2021).
7.10.1. Rostral Ventro-Medial Medulla (RVM)
The RVM comprises the midline located NRM and the adjacent reticular formation at the level of the facial nucleus and ventral to the gigantocellular reticular nucleus. The ascending projections from the spinal cord to the RVM are very scarce. By contrast, the descending projection from the RVM to the spinal cord is strong and targets almost all spinal segments, reaching mainly the DH laminae I–V, but also lamina X (Martins and Tavares 2017; Pagliusi and Gomes 2023).
Connections. The RVM has connections with most pain-related structures, providing a mechanism through which cortical and sub-cortical structures influence pain perception and processing of nociceptive inputs at the DH level. The RVM connectivity underlies the placebo analgesia, which shows the relevance of the RVM in pain control, as it can impact both sensorial-discriminative and affective-motivational aspects of pain. Projections from the ACC to the RVM are supposed to enhance the descending facilitation of pain. In addition, the over-activation of ACC-RVM projections is thought to be involved in pain chronification. The RVM can be considered the output of the midline pain-modulation system. It is an important node of the descending system that functions as an endogenous pain control being recruited following painful stimuli perception alongside many other brain regions, including S1 and S2, ACC, PFC, IC, AMY, NAc, VTA, and PAG. The RVM connectivity also underlies the placebo analgesia, which can impact both sensorial-discriminative and affective-motivational aspects of pain (Pagliusi and Gomes 2023).
The RVM has been implicated as an important brain region in the switch from acute to chronic pain. Pain chronification may result from an imbalance between the facilitatory and inhibitory roles of the RVM in controlling nociceptive input. It has been proposed that the basal sensorial processing threshold is maintained by a fine balance between the descending pain facilitatory and inhibitory systems, which can be disturbed by illness, lesions, inflammation, or even exposure to psychological stressors (Pagliusi and Gomes 2023).
Inhibitory control from the PAG-RVM system preferentially suppresses nociceptive inputs mediated by group IV (C) fibers, preserving sensory-discriminative information conveyed by more rapidly conducting group A fibers. The circuitry within the RVM provides the neuronal basis for bi-directional control from the midline system, containing two populations of neurons: ON-cells and OFF-cells, which are differentially recruited by higher structures important in psychological stress, fear, and illness, to enhance or inhibit pain (Figure 3). OFF-neurons are involved in pain inhibition and their electrophysiological responses pause when the animal exhibits a nocifensive behavior. By contrast, ON-neurons facilitate nociceptive signal transmission as their electrophysiological activity increases just prior to the appearance of a nociceptive withdrawal reflex. Besides the ON- and OFF-cells, the RVM harbors NEUTRAL neurons, which exhibit an electrophysiological activity that is not related to identify animal behavior (Heinricher et al. 2009; Martins and Tavares 2017). Electrical stimulation within the RVM can produce biphasic effects. Stimulation at relatively high current intensities can inhibit DH neurons, whereas RVM stimulation at lower intensities can facilitate DH neuron. Within both the PAG and RVM, scattered cell populations have distinct nociception-modulating properties (Mills et al. 2021). Inhibitory and facilitatory RVM influences appear to involve anatomically distinct, independent spinal pathways and are mediated by different lumbar spinal receptors. Spinal nociceptive inhibition is mediated via descending projections from the RVM in the dorso-lateral funiculi, whereas nociceptive facilitation is transmitted via the ventro-lateral funiculi (Bannister and Dickenson 2016; Urban and Gebhart 1999). When stimulated endogenously or exogenously (e.g., through chemogenetics), the PAG-to-RVM projections exert analgesia by facilitating the descending pain inhibition. Conversely, projections from the ACC to the RVM enhance the descending facilitation of pain. The over-activation of ACC-to-RVM projections is considered to be involved in pain chronification (Pagliusi and Gomes 2023).
Dynamic shifts in the balance between pain inhibiting and facilitating RVM effects contribute to setting the gain of nociceptive processing as dictated by behavioral priorities, but are also likely to contribute to pathological pain states (Heinricher et al. 2009; Martins and Tavares 2017).
Cell Types. The circuitry within the RVM revealed that the neural basis for bi-directional control from the midline system is two populations of neurons, ON-cells and OFF-cells that are differentially recruited by higher structures to enhance or inhibit pain. ON- cells exhibit increased discharge during noxious stimulation, are inhibited by morphine, and have been proposed to facilitate nociceptive responses. By contrast, OFF-cells exhibit decreased discharge during noxious stimulation, are excited by morphine, and are considered to inhibit nociceptive responses. A third neuron group, NEUTRAL cells, are unaffected by noxious cutaneous stimuli or by exogenous opioids. While their role in nociception is unclear, they are generally thought to be largely 5-HT and are believed to participate in autonomic and homeostatic functions. Inhibitory control from the PAG-RVM system preferentially suppresses nociceptive inputs mediated by group IV (C)-fibers, preserving sensory-discriminative information conveyed by more rapidly conducting group III (Aδ)-fibers. Dynamic shifts in the balance between pain inhibiting and facilitating outflows from the brainstem play a role in setting the gain of nociceptive processing as dictated by behavioral priorities, but are also likely to contribute to pathological pain states (Heinricher et al. 2009; Nguyen et al. 2023; Peng et al. 2023).
RVM 5-HT Cells. 5-HT cells could inhibit or facilitate nociception due to the existence of many sub-types of 5-HT receptors with opposing effects (De Felice and Ossipov 2016). Selective activation of 5-HT cells in mice induced persistent pain sensitization (Cai et al. 2014). Hence, the facilitatory effects appear to predominate over inhibition (Bannister and Dickenson 2016).
RVM 5-HT neurons receive direct excitatory inputs from the S1. Moreover, nociceptive neurons located in spinal laminae V-VIII project back to the NRM, but not the NRD, thus establishing a spino-NRM-spinal loop for regulating the strength of nociceptive processing (Cortes-Altamirano et al. 2018; Kuner and Kuner 2021).
RVM Non-5-HT Cells. In the rat, the RVM contains three classes of non-5-HT neurons with phasic pain-modulating effects: NEUTRAL cells, OFF and ON cells, at least some of which project to the spinal cord, especially to the DH (De Felice and Ossipov 2016; Foo and Mason 2003a; Heinricher et al. 2009; Mason 2001; Porreca et al. 2002; Wu et al. 2010). In rats, about 20% of the RVM cells projecting to the spinal cord are 5-HT. The rest probably comprises GABAergic and/or glycinergic cells, which inhibit nociceptive processing in the DH (De Felice and Ossipov 2016).
NEUTRAL Cells do not react during nocifensive withdrawals or acute inflammation nor to micro-injections of opioids, eCBs, α2-NA agonists or CCK. At least some NEUTRAL cells are 5-HT (Heinricher et al. 2009). NEUTRAL cells are believed to participate in responses to prolonged inflammatory nociceptive stimulation (Wu et al. 2010). Their role in descending pain control is still unclear, but they may change their activity and be recruited as ON- or OFF-cells during pain chronification (Pagliusi and Gomes 2023).
OFF-cells are continuously active during slow-wave sleep (SWS) and only sporadically during waking. They are inhibited by noxious heat, excited by PAG activation and by analgesic doses of opioids which make them become continuously active. OFF-cells provide for the anti-nociceptive effects on nociceptive signal transmission in the spinal DH (Heinricher et al. 2009; Pagliusi and Gomes 2023).
ON-cells are active just prior to a noxious heat-evoked tail flick or withdrawal reflex, excited by noxious heat anywhere on the body surface, inhibited by PAG activation and opioids, and show bursts of activity during waking but very little activity during SWS. Injury or inflammation enhance ON-cell activity (Ossipov et al. 2014). ON cells mediate facilitatory effects on nociceptive signal transmission in the spinal DH (Heinricher et al. 2009; Pagliusi and Gomes 2023).
The evidence above implies that because ON-cells are most active during waking, and OFF-cells more active during SWS, the former facilitate alertness and awareness of a painful stimulus during the waking state, while the latter suppresses during sleep. These behaviors change during inflammation and following nerve injury. For instance, in neuropathic pain models, ON and OFF cells show novel responses to non-noxious mechanical stimuli and enhanced responses to noxious heat (Heinricher et al. 2009).
DMH stimulation induced robust activation of RVM ON-cells along with suppression of OFF-cell firing, leading to behavioral hyperalgesia, in contrast to other data showing that HYP stimulation produced analgesia that could be inhibited by systemic naloxone. Hyperalgesia induced by DMH stimulation recruited ON-cells under mild and persistent stress, a response known as SIH. However, micro-injection of lidocaine into the RVM potentiated HYP-mediated analgesia, as evidenced by the increase in the pain threshold and inhibition of the tail-flick reflex. Collectively, this confirms that the HYP projects directly to the RVM and influences the activities of ON-and OFF-cells, thus exerting bi-directional pain modulation (Peng et al. 2023).
In rats, the modulatory effects of electrical and chemical (glutamate) stimulation in the RVM on spinal nociceptive transmission and a spinal nociceptive reflex were studied. Electrical stimulation at a total 86 sites in the RVM in the medial raphé nuclei (n = 54) and adjacent gigantocellular areas (n = 32) produced biphasic (facilitatory and inhibitory, n = 43) or only inhibitory (n = 43) modulation of the tail-flick reflex (TFR). At these 43 biphasic sites in the RVM, facilitation of the TFR was produced at low intensities of stimulation and inhibition was produced at greater intensities of stimulation. At 43 sites in the RVM, electrical stimulation only produced intensity-dependent inhibition of the TFR. Activation of cell bodies in the RVM by glutamate micro-injection reproduced the biphasic modulatory effects of electrical stimulation. In electrophysiological experiments, electrical stimulation at 62 sites in the RVM produced biphasic (n = 26), only inhibitory (n = 26), or only facilitatory (n = 10) modulation of responses of lumbar DH neurons to noxious cutaneous thermal (50 degrees C) or mechanical (75.9 g) stimulation. Facilitatory effects were produced at lesser intensities of stimulation and inhibitory effects were produced at greater intensities of stimulation. The spinal pathways conveying descending facilitatory and inhibitory influences were different. Descending facilitatory influences on the TFR were conveyed in ventral/ventro-lateral funiculi, whereas inhibitory influences were conveyed in dorso-lateral funiculi. This indicates that descending inhibitory and facilitatory influences can be simultaneously engaged throughout the RVM, including NRM, and that such influences are conveyed in different spinal funiculi (Zhuo and Gebhart 1997).
GABAergic RVM-DH Pathway. There appear to be more descending pain-modulating patways. In vivo opto/chemogenetic manipulations and trans-synaptic tracing of genetically identified RVM and DH neurons revealed an RVM-spinal cord-primary afferent circuit controlling pain thresholds. RVM GABAergic neurons facilitated mechanical pain by inhibiting DH ENK/GABAergic interneurons. These interneurons gated sensory inputs and controlled pain through temporally coordinated ENK- and GABA-mediated PSI of somatosensory neurons. This descending disynaptic inhibitory circuit facilitated mechanical pain, and is engaged during stress (Franҫois et al. 2017).
DMH-RVM Connection. While intense or highly arousing stressors suppress pain, relatively mild or chronic stress can enhance pain, causing stress-induced hyperalgesia (SIH). The physiological and neuroendocrine effects of mild stress are mediated by the DMH, which has connections with the RVM. Stress could engage both the DMH and the RVM to produce hyperalgesia. In awake animals, direct pharmacological activation of the DMH increased the sensitivity to mechanical stimulation, confirming that the DMH can mediate behavioral hyperalgesia. A behavioral model of mild stress also produced mechanical hyperalgesia, which was blocked by inactivation of either the DMH or the RVM. The DMH has a role in stress, but may also be engaged in a number of chronic or abnormal pain states (Wagner et al. 2013).
However, the DMH may also exert anti-nociceptive effects via endomorphin-2 and CCK containing projections. After micro-injection of Fluoro-Gold (FG) into the RVM, retrogradely labeled neurons occurred in the HYP, the majority of which were present in the lHYP and DMH. DMH stimulation directly induced robust activation of ON-cells along with suppression of OFF-cell firing, leading to behavioral hyperalgesia, in contrast to other data showing that HYP stimulation produced analgesia that could be inhibited by systemic naloxone. Hyperalgesia induced by DMH stimulation recruited ON-cells under mild and persistent stress, a response known as SIH. However, micro-injection of lidocaine into the RVM potentiated HYP-mediated analgesia, as evidenced by the increase in the pain threshold and inhibition of the tail-flick reflex (TFR). Collectively, this confirms that the HYP projects directly to the RVM and influences the activities of ON-and OFF-cells, thus exerting bi-directional pain modulation (Peng et al. 2023).
7.10.2. Caudal Ventro-Lateral Medulla (CVLM)
In addition to the PAG-RVM system, the CVLM and DReN have also been implicated in descending control of DH nociceptive processing. Pain control from the VLM is elicited from the CVLM (below), which is one important component of the endogenous pain modulatory system (Cobos et al. 2003).
CVLM Actions. The CVLM has a role as a center for the integration of nociceptive, cardio-vascular, and motor functions. It integrates `top-down´ and `bottom-up´ signals that regulate functionally defined RVM cell types, `OFF cells´ and `ON cells´ which respectively suppress of facilitate nociceptive processing (De Preter and Heinricher 2024). The CVLM modulates pain responses, autonomic functions and somatic and visceral motor activity. The CVLM exerts vaso-depression and contains the A1 NA group. The lateral-most part of the CVLM (CVLMlat) is the CVLM area responsible for pain modulation (Cobos et al. 2003).
CVLM Stimulation strongly inhibits behavioral and DH nociceptive responses, whereas lesions result in apparent disinhibition. This suggests that the CVLM exerts a tonic inhibitory control of DH nociception. Nevertheless, like the CRVM, the CVLM may exert a facilitatory influence, as neurons with features of ON- and OFF-cells have been identified in this region. Acute inflammation induced by intra-articular injection of a solution of PGE2 and bradykinin induced a strong activation both at the CVLM and the spinal cord. This suggests that at the initial phases of inflammation, descending inhibition from the CVLM fails to inhibit the strong nociceptive transmission arising from the spinal cord (Heinricher et al. 2009). Mild stimulation of the CVLM can produce profound and long-lasting analgesia. The CVLMlat, the reticular formation between the spinal trigeminal nucleus and the LRN, appears to play a major role in the anti-nociceptive effect. The projections to spinal laminae involved in nociceptive transmission originate exclusively in the CVLMlat. The CVLMlat participates in a disynaptic pathway involving spinally projecting pontine NA A5 neurons, which appears to convey α2-adrenoreceptor-mediated analgesia produced from the VLM. Neurons in the CVLMlat and in lamina I are reciprocally connected by a closed loop that is likely to mediate feedback control of supraspinal nociceptive transmission (Tavares and Lima 2002).
The descending CVLMlat-spinal pathway targets lamina I, IV–V and X. There is a reciprocal loop between lamina I and the CVLMlat. Data suggest that the arrival of nociceptive einput from the spinal cord triggers activation of CVLMlat neurons. Electrophysiological mapping of the VLM has shown that it harbors inhibitory neurons (OFF-like neurons) along with excitatory cells (ON-like cells) which indicates that the descending modulation from the VLM may include facilitatory modulation, along with the well-established inhibitory effects (Martins and Tavares 2017).
CVLM–LC–SC Pathway. In mice, there is a small group of NA neurons in the CVLM, which were robustly activated by noxious stimuli. When activated, these neurons triggered marked anti-nociception to heat without affecting behavioral responses to all other sensory modalities. They could ameliorate heat allodynia, and inhibition of CVLM neurons reduced counter-stimulus induced analgesia. Mice in which CVLM neurons were inhibited or ablated exhibited increased sensitivity to heat, suggesting that these neurons provide tonic inhibition in naïve conditions. CVLM NA neurons send projections to the LC, and.activation of NA CVLM neurons induced c-fos labeling of LC neurons. The functional coupling between the NA CVLM and LC occurred specifically in ventral LC neurons, consistent with the distribution of spinally projecting neurons in the LC. CVLM mediated anti-nociception depended on NA signaling in the spinal cord. Upon activation, these neurons produced bilateral feed-forward inhibition that attenuated nociceptive responses through a pathway involving the LC and NA in the spinal cord (Gu et al. 2023).
CVLM-A5-DH Pathway. In the rat exists a disynaptic pathway linking the CVLM to the spinal cord via the pontine NA A5 cell group. The A5 projects to the CVLM and the spinal DH. The CVLM cells act upon the A5 spinally projecting neurons, which are likely to exert a α2-adrenoreceptor-mediated inhibition on the spinal cord. The A5-CVLM pathway may be the anatomical substrate of a negative feedback circuit whereby the modulatory action of the CVLM on the spinal cord is self-inhibited through activation of the A5 (Tavares et al. 1997).
7.10.3. Dorsal Reticular Nucleus (DReN)
Adding DReN to the RVM and CVLM completes the network to a triad, the three components being reciprocally connected in a way, which allows integration of responses that are related to pain, namely cardio-vascular control and motor reactions. The triad RVM-CVLM-DReN also represents the neurobiological substrate for the emotional and cognitive modulation of pain, through pathways that involve the PAG-RVM connection. This triad may have special features to provide integrated and rapid responses in situations that are life-threatening, in particular noxious stimuli. It probably represents a phylogenetically conserved mechanism that subserves the classical `fight or flight´ response, activated by acute pain (Martins and Tavares 2017).
Inputs. The DReN receives afferent inputs from: (i) higher centers, namely the ACC, the motor, somatosensory and insular cortices, the HYP and the AMY; and (ii) several brainstem areas, namely the PAG, the LC, the A5 NA cell group along with the two components of the medullary triad (RVM and VLM) (Martins and Tavares 2017). As mentioned above, the LC NA system has a dual role in descending pain control. First, it exerts anti-nociception due to its direct inhibitory action on the spinal cord. Second, the LC NA system may induce pro-nociception by directly acting on brainstem pain modulatory circuits, namely, at the LC and medullary DReN (Tavares et al. 2021; Figure 3).
Outputs. Through its projections to the lateral ventro-medial THAL, the DReN participates in a reticulo-THAL-cortical ascending nociceptive pathway. DReN neurons project to the superficial and deep DH. The DReN neurons are reciprocally connected with spinal lamina I neurons forming a reverberative nociceptive circuit (Martins and Tavares 2017).
Lesioning the medullary DReN depresses nociceptive responses to acute and inflammatory pain, whereas stimulation produces the inverse effect. Following DReN lesioning, the decrease in formalin-induced pain behavior was accompanied by a decrease of spinal noxious-evoked c-fos neuronal activation. DReN blocking by lidocaine resulted in a decrease of the nociceptive activity of spinal DH neurons, while stimulation by glutamate had the opposite effect. A putative reciprocal disynaptic excitatory circuit linking the DReN and the DH conveys nociceptive input through the ascending branch, indicating that the DReN pain facilitating action is mediated by a reverberating spino-DReN circuit that promotes the enhancement of the response capacity of spinal neurons to noxious stimulation.The existence of a primary pro-nociceptive center in the descending pain control system supports the concept of pain modulation as a dynamic and flexible process that integrates nociceptive processing by balancing multiple excitatory and inhibitory actions as the way of adapting to the various unsteady pain determinants (Lima and Almeida 2002).
Neuromodulators. Pain facilitation from the DReN is modulated by neurotransmitters such as glutamate, opioid peptides, NA and GABA. Glutamate plays an important role in the pro-nociceptive actions of the DReN during the formalin test since the blockade of AMPA/KA, NMDA and mGlu1 glutamate receptors by the local administration of the respective antagonists significantly reduced formalin-induced pain behavior, which was accompanied by a reduction of c-Fos expression at both the superficial and deep DH laminae. NA is also involved in the mediation of pro-nociception from the DReN. The reduction of NA release in the DReN by genetic manipulation of DReN-NA afferents significantly attenuated pain behavior in the formalin test while increasing local extracellular levels of NA (Martins and Tavares 2017).
Precarious Network. The triad RVM-CVLM-DReN should be complemented by the DH, with which exist strong connections. The operation of this extended, intricate, and non-linear network is at risk of falling into instability. It must therefore be kept in a stable balance. Under unfavourable conditions, it can easily tip into a pathological condition.
7.10.4. Dorsal Raphé Nucleus (DRN)
The DRN is an important nucleus in pain modulation and has been described as the `pain inhibitory nucleus´ in the brain (Zhang et al. 2024). It is located in the ventro-medial part of the midbrain PAG (Figs. 1, 3) and has a fan-shaped structure that is symmetrically distributed around the midline. The DRN is involved in various functions, such as sleep–awake, feeding, and emotion, and analgesia. It receives diverse projections from HYP, midbrain, and pons, and projects to diverse regions such as cerebral cortex, limbic forebrain, THAL, and the midbrain. Its vast fiber connections provide a morphological basis for its pain modulating function. In ascending projections, the DRN directly modulates the responses of pain-sensitive neurons in the THAL. It can also be involved in analgesia effects induced by the hypothalamic arcuate nucleus (HYP ARC). Its descending projections, directly or via the NRM, modulate the responses caused by noxious stimulation of the spinal DH neurons. Neurophysiologic and neuropharmacologic data suggest that 5-HT neurons and ENK neurons in the DRN inhibit pain, and GABA neurons do the opposite. The activation of DRN neurons in mice prevents the establishment of chronic neuropathic pain (CNP) symptoms. Chemogenetic or optogenetic inhibition neurons in the DRN are sufficient to establish pain phenotypes, including long-lasting tactile allodynia. The DRN contains various cell types, mainly including the most abundant 5-HT neurons, as well as GABA neurons, DA neurons, glutamatergic neurons, and peptidergic neurons. The specific neuron types in the DRN involved in pain modulation are still incompletely known (Wang and Nakai 1994; Zhang et al. 2024).
7.10.5. Sub-Nucleus Reticularis Dorsalis (SRD)
Some neurons in the DH are strongly inhibited when a nociceptive stimulus is applied to any part of the body, distinct from their excitatory receptive fields. This phenomenon was termed `diffuse noxious inhibitory control´ (DNIC). DNIC influences can be triggered only by conditioning stimuli which are nociceptive, i.e., by activity of group III or group III/IV afferents and is mediated by the SRD in the dorsal medulla, which receives NA input from the LC, and is able to modulate nociceptive transmission via direct projections to the DH/spV. DNIC is a powerful analgesic mechanism that can block transmission of nociceptive information by completely inhibiting WDR neurons in the spV and deep DH. In human acute pain studies, the SRD was activated by noxious thermal stimuli, and received signals from the PAG during and after noxious thermal stimulation (Bouhassira et al. 1990; Mills et al. 2021; Villanueva and Le Bars 1995).
7.10.6. Acupuncture Against Visceral Pain
During visceral pain, acupuncture can exert analgesic effects involving brain nuclei mainly including the ACC, PVN, AMY, LC, RVM and NTS. Important connections involved are ACC-AMY, AMY-IC, claustrum-ACC, PBN-AMY, BNST-PVN, PVN-ventral lateral septum, LC-RVM. Signals generated by acupuncture can modulate the central structures and interconnected neural circuits of multiple brain regions, including the cerebral cortex, THAL, HYP and medulla oblongata. This analgesic process also involves the participation of various neurotransmitters/neuromodulators and/or receptors, such as 5-HT, ENK, and glutamate (Dou et al. 2023).
7.11. Cerebellum
The cerebellum has a role in pain processing and/or modulation, possibly due to its extensive connections with the PFC and brainstem regions involved in descending pain control (Ong et al. 2019).
In rats, electrical stimulation of the cerebellar lateral nucleus modulated the encoding of noxious stimuli in intra-laminar parafasicular neurons in the THAL. In squirrel monkeys, electrical stimulation of the intermediate portion of the anterior cerebellar lobe could raise nociceptive thresholds to tail shock. In rats, morphine micro-injection into the anterior cerebellum entailed acute analgesia, and electrical stimulation or chemical stimulation (by a non-specific glutamate receptor agonist) of the cerebellar cortex increased neural responses to a noxious visceral stimulus in and around the termination sites of nociceptive afferents in the spinal cord. Chemical stimulation of the rat cerebellar cortex also increased visceral nociceptive reflexes, whereas such stimulation of the fastigial nucleus decreased these reflexes. This may suggest that the cerebellum may engage the pain-modulating circuitry in the brainstem, including the PAG and RVM (Moulton et al. 2010).
7.12. Nucleus Tractus Solitarii (NTS)
In anesthetized rats, electrical and chemical stimulation in the NTS affected the spinal nociceptive transmission in laminae I-VI in the L3-L5 segments. The spinal neurons responded to mechanical (low and/or high intensities) and thermal stimuli (42-52 degrees C). Electrical stimulation in the NTS either ipsilateral or contralateral to the spinal unit inhibited neuronal responses to noxious thermal stimuli. Stimulation in the area ventral to the NTS produced a greater inhibition of these units than did NTS stimulation. Micro-injection of 50 nmol of glutamate into the NTS ipsilateral to the spinal unit also inhibited neuronal responses to thermal stimuli in most units. Vagal afferent stimulation had varied effects on spinal nociceptive transmission (Ren et al. 1990).
In anesthetized cats, DH neurons and lamina X neurons in the lumbar spinal cord were extracellularly recorded. The nociceptive responses of these cells evoked by peripheral nerve stimulation were significantly inhibited by stimulation of the NTS at low intensity without any noticeable cardio-vascular reaction. Stronger inhibition was induced when the stimulation site was within or in the immediate vicinity of the NTS. There was no significant difference in the efficacy of the NTS stimulation-produced inhibition of nociceptive response between DH neurons and lamina X neurons (Du and Zhou 1990).
7.13. Spinal Dorsal Horn (DH)
The DH is the location of the first synapse in pain pathways, and as such, offers a very efficient target for the regulation of nociceptive transmission by both local segmental and supraspinal mechanisms.
Anti-nociceptive and Pro-nociceptive Modulation of nociceptive DH neurons is exerted by overlapping midbrain, pontine and medullary regions, the involved anatomical connections and neurotransmitter actions being complex (De Felice and Ossipov 2016; D´Mello and Dickenson 2008; Gebhart 2004; Heinricher et al. 2009; Jennings et al. 2014; Lima and Almeida 2002; Martins and Tavares 2017; Mason 2001, 2005; Ren and Dubner 2007; Saadé and Jabbur 2008; Scholz and Woolf 2002; Tracey and Mantyh 2007; Vanegas and Schaible 2004; Wu et al. 2010; Yoshimura and Furue 2006). Important descending influences are mediated by catecholamine pathways including descending DA fibers from the HYP and SN, NA fibers from the LC, and 5-HT fibers from the RVM. Moreover, higher structures can exert modzulating effects from various structures and mechanisms.
It is likely that an imbalance between anti-nociceptive and pro-nociceptive effects contributes to chronic pain states (De Felice and Ossipov 2016; Martins and Tavares 2017). For example, while signals decending from the RVM to DH are usually anti-nociceptive, they can be the opposite. By use of in vivo opto- and hemogenetic manipulations and trans-synaptic tracing of genetically identified DH and RVM neurons to uncover an RVM-spinal cord-primary afferent circuit controlling pain thresholds, it was found that RVM GABAergic neurons facilitated mechanical pain by inhibiting DH ENK/GABAergic interneurons, which gated sensory inputs and controlled pain through temporally coordinated ENK- and GABA-mediated PSI of somatosensory neurons. This revealed a descending disynaptic inhibitory pathway that facilitates mechanical pain, is engaged during stress, and could be targeted to establish higher pain thresholds (François et al. 2017).
Feedback Loops. The final descending pathways to the spinal cord are integrated into feedback loops including sensory inputs targeting various supraspinal structures. For example, PAG cells and some neurons in the RVM and VLM receive sensory inputs, including nociceptive signals, and cells in the DReN are reciprocally connected with spinal DH (Heinricher et al. 2009).
Thus, the DH processes nociceptive signals by complex interactions between the DH neuronal circuits and modulatory actions from higher brain centers whose activity can be influenced by emotion, motivation, anxiety, and other cognitive states that can ultimately exacerbate or mitigate the overall pain experience associated with specific noxious stimuli. Descending modulatory pathways comprise, among others, NA, 5-HT, GABAergic, and DA fibers (Puopolo 2019).
Stress-induced Analgesia (SIA). The descending PAG-RVM system forms the circuitry that underlies the physiological phenomenon of SIA, which is mediated by parallel opioid and cannabinoid neurotransmitter systems in the PAG. Opioids and cannabinoids are hypothesized to activate descending analgesia through an indirect process of `GABA disinhibition´-suppression of inhibitory GABAergic inputs onto output neurons which constitute the descending analgesic pathway (Lau and Vaughan 2014).
Adrenergic Receptors. The spinal DH is an important site for the relay and modulation of nociceptive transmission by the α2-adrenergic receptors. Both the α2A- and α2C-adrenergic receptors are expressed in the DH and the DRG neurons. Capsaicin treatment in neonatal rats or resiniferatoxin treatment in adult rats removed TRPV1-expressing sensory neurons and induced a large decrease in the α2A-, but not the α2C-, adrenergic receptor immunoreactivity in the DH. This suggests that the α2A-adrenergic receptor is located primarily on the central terminals of primary afferent neurons, while the α2C subtype is located primarily on the DH neurons. Peripheral nerve injury decreased the α2A-, but not α2C-, adrenergic receptor immunoreactivity in the rat spinal cord ipsilateral to the injury side (Pan et al. 2008).
8. Clinical Syndromes
Acute pain is often associated with various clinical syndromes, typically resulting from tissue injury, inflammation, or dysfunction of the nervous system. Some important clinical syndromes related to acute pain include:
Post-operative Pain. It occurs after surgical procedures due to tissue trauma and inflammation. The treatment include the use of NSAIDs, opioids, local anesthetics, and multi-modal pain management approaches.
Acute Neuropathic Pain. It is often caused by nerve injury or irritation (e.g., herpes zoster/shingles, radiculopathy), and is characterized by burning, tingling, or electric shock-like sensations. The treatment includes the use of anticonvulsants (gabapentin, pregabalin), or anti-depressants (duloxetine for instance), as well as nerve blocks.
Acute Musculoskeletal Pain is common in conditions like fractures, sprains, muscle strains, and acute arthritis. The management includes rest, local cooling, compression, elevation, the use of NSAIDs, and physical therapy.
Acute Visceral Pain occurs in internal organs, such as acute pancreatitis, renal colic (kidney stones), myocardial infarction (heart attack), and bowel obstruction. It presents as deep, cramping, or colicky pain. The management requires targeted treatment like opioids, anti-spasmodics, or disease-specific interventions.
Headache Syndromes include tension-type headache, migraine, and cluster headache. Acute treatment may involve the use of NSAIDs, triptans, corticosteroids, or other drugs, depending on the headache type.
There is substantial evidence indicating that acute pain involves pathophysiologically significant processes in both the PNS and the CNS. These processes contribute to pain development, pain transmission, and pain processing.
Post-operative Pain. Surgical tissue trauma may lead to peripheral sensitization of nociceptors via release of inflammatory mediators (e.g., prostaglandins, bradykinin, and cytokines) that activate nociceptors (Ekman and Koman 2005; O’Neill and Lirk 2022). This lowers the activation threshold of peripheral nerve endings, resulting in hyperalgesia and allodynia. Tissue trauma leads to a release of neuropeptides like SP, which further enhance inflammation and pain signaling (D’Amico et al. 2025). Persistent afferent input from surgical wounds triggers wind-up and long-term potentiation (LTP) in the spinal DH (Smith et al. 2024; Wen et al. 2024). Important mechanisms include NMDA receptor activation and decrease of activity of spinal inhibitory interneurons, both leading to increased excitability of spinal neurons, as well as glial-cell activation (particularly microglia, and astrocytes) which release pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, further amplifying pain signals. Supraspinal processing may also change (Jiang et al. 2020). In post-operative pain, descending modulatory system involving PAG and RVM may become dysregulated, leading to prolonged pain perception. Understanding these mechanisms supports the use of multi-modal analgesia to target different pain pathways (O’Neill and Lirk 2022), including: NSAIDs (reduce peripheral inflammation), opioids (modulate pain at spinal and supraspinal levels), NMDA antagonists like ketamine (prevent central sensitization), and gabapentinoids (reduce hyperexcitability in spinal neurons). Vegetative symptoms include: Tachycardia, hypertension and sweating (diaphoresis) due to activation of the sympathetic nervous system (SNS); Nausea and Vomiting caused by activation of the area postrema (chemoreceptor trigger zone) and vagal afferents.
Acute Neuropathic Pain may also involve significant pathophysiological changes in pain-related structural areas within the PNS and the CNS. Acute neuropathic pain often arises from nerve injury or irritation, leading to abnormal excitability of primary afferent neurons. The pathophysiological mechanisms in the PNS lead to ectopic activity and peripheral sensitization (Michaelis et al. 1998). Spontaneous ectopic discharges of damaged or irritated sensory neurons lead to abnormal hyperexcitability of peripheral nerves, causing spontaneous pain (e.g., burning, tingling, or electric shock-like sensations). Up-regulation of Na+ channel (increased expression of Nav1.7, Nav1.8, and Nav1.9), and down-regulation of K+ channel (Kv7 (KCNQ) channels) contribute to neuronal hyperexcitability and sustained firing (Qian et al. 2024; Reid et al. 2022). Neurogenic inflammation by release of cytokines (TNF-α, IL-1β, IL-6), as well as by activation of immune cells (mast cells, macrophages among others) enhances nerve sensitization and pain transmission in the PNS. In the spinal cord, particularly in laminae I, II, and V of DH, NMDA receptor activation, wind-up of activity (e.g., by repeated group IV-fiber stimulation), microglial and astrocyte activation (e.g., by BDNF up-regulation), and disinhibition (loss of GABAergic and glycinergic inhibition) contribute to central sensitization (Kim et al. 2013). Dysfunction in descending pain modulation from PAG and RVM may lead to a dysbalance between pain inhibition via endogenous opioids and pain facilitation (Monhemius et al. 2001). This dysfunction entails exaggerated pain perception. Treatment strategies often target Na+ channels in the PNS (e.g., application of carbamazepine), as well as Ca2+ channels (application of gabapentinoids) and some transmitters like 5-HT and NA [Serotonin-Noradrenaline Reuptake Inhibitors (SNRIs) like duloxetine, and venlafaxine] in the CNS. Vegetative symptoms include: Hypothermia/Hyperthermia by dysregulated autonomic vasomotor function due to SNS hyperactivity or failure, respectively; hyperhidrosis or anhidrosis due to imbalance in SNS control of sweat glands.
Acute Musculoskeletal Pain arises from injury, inflammation, or mechanical stress affecting muscles, joints, tendons, ligaments, and bones. Mechanical, thermal, and chemical stimuli activate nociceptors via ion channels (Gregory et al. 2018; Savadipour et al. 2023; Wang et al. 2017) like TRPV1 (heat), ASICs (acidosis), and PIEZO2 (mechanical pressure). Comparable with acute neuropathic pain, inflammatory processes, induced by release of SP, CGRP, and other mediators (cytokines, prostaglandins and bradykinin), lower nociceptor activation thresholds, leading to peripheral sensitization (Hoegh 2022; Puntillo et al. 2021). Muscle and joint-specific pain mechanisms involve: increased lactate, acidosis, and ATP release (e.g., through muscle ischemia/hypoxia); activation of nociceptors in the synovium and joint capsule (e.g., through cartilage degradation and synovial inflammation); activation of periostal nociceptors, which contribute to severe pain in bone fractures. Similar pathophysiological processes in the spinal cord and brain as mentioned above in relation to acute neuropathic pain may also lead to central sensitization in acute musculo-skletal pain (Curatolo 2023). Treatment strategies include the application of NSAIDs, local anesthetics (e.g. lidocaine), and muscle relaxants (e.g., tizanidine, and methocarbamol) as well as physical therapy and neuromodulation techniques (e.g., transcutaneous electrical nerve stimulation – TENS). Vegetative symptoms include: Localized vasodilation and edema caused by increased vascular permeability; Pallor or flushing indicative of SNS dysregulation affecting cutaneous blood flow.
Acute Headache Syndromes often arise from activation and sensitization of the trigemino-vascular system, which consists of the trigeminal nerve, trigeminal ganglion, and meningeal blood vessels. The dura mater and large cranial blood vessels are innervated by trigeminal group III (Aδ) and group IV (C) fibers, which release CGRP, SP, and neurokinin A when activated (Edvinsson 2001). In trigeminal autonomic cephalalgias (e.g., cluster headaches, and paroxysmal hemicrania), activation of cranial autonomic pathways, particularly increased parasympathetic outflow from the sphenopalatine ganglion, lead to lacrimation, conjunctival injection, and nasal congestion (Jürgens and May 2014). In migraine, brainstem dysfunction involves hyperexcitability of the trigeminal nucleus caudalis in the medulla as well as 5-HT and NA dysregulations (Hawkins et al. 2018). Migraine aura is linked to cortical spreading depression, a wave of neuronal depolarization followed by inhibition that often affects the visual and sensory cortices. Treatment strategies of acute headache syndromes include the application of NSAIDs, triptans (e.g., sumatriptan, and rizatriptan; inhibiting 5-HT1B/1D receptors), and CGRP antagonists (e.g., rimegepant) as well as neuromodulation (e.g., TMS), and vagus-nerve stimulation – VNS), and behavioral therapies. In general and particularly in the case of headache syndromes, understanding the above mentioned vegetative symptoms and their neurophysiological mechanisms can improve targeted multimodal pain management strategies for different acute pain syndromes.