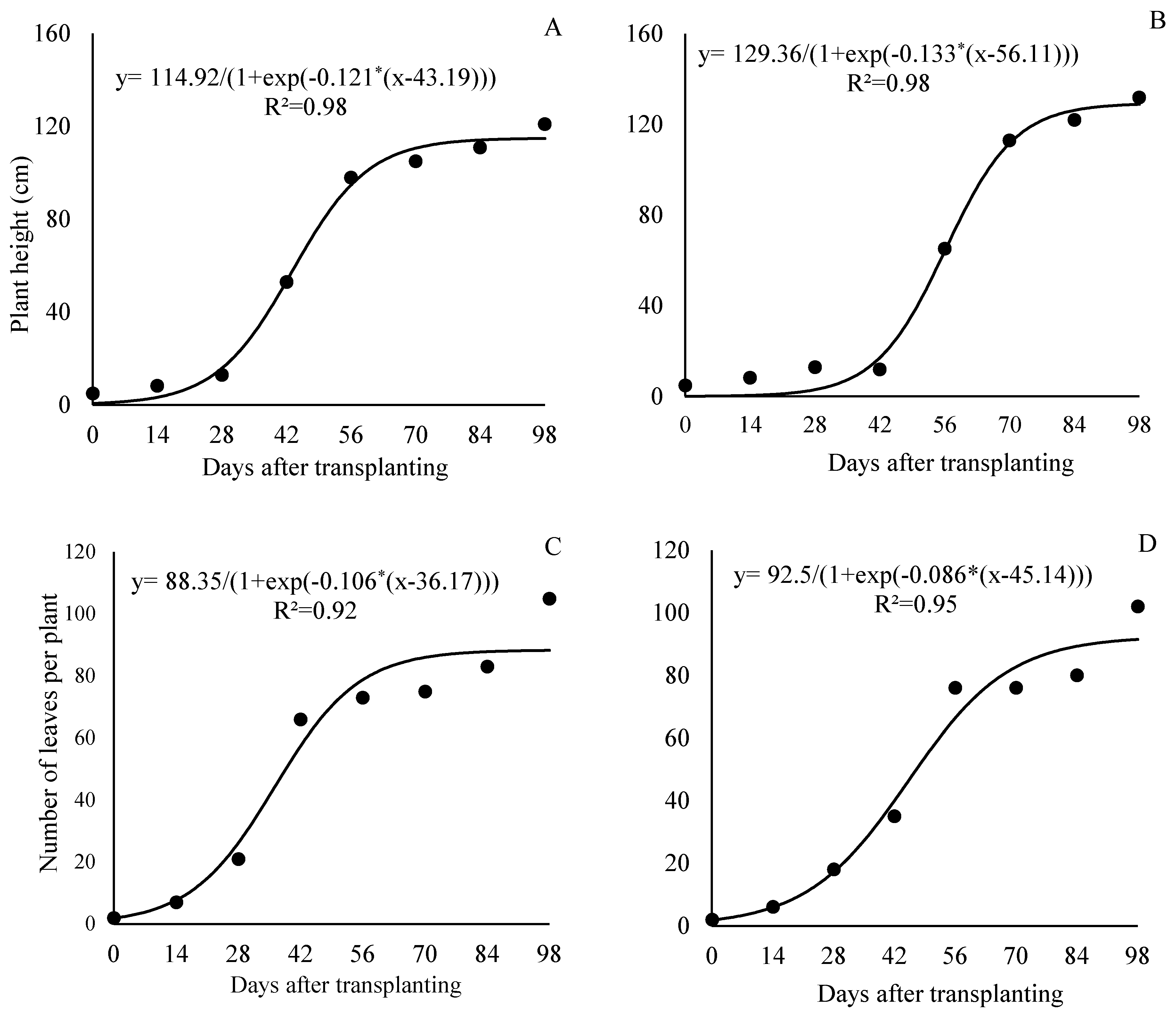
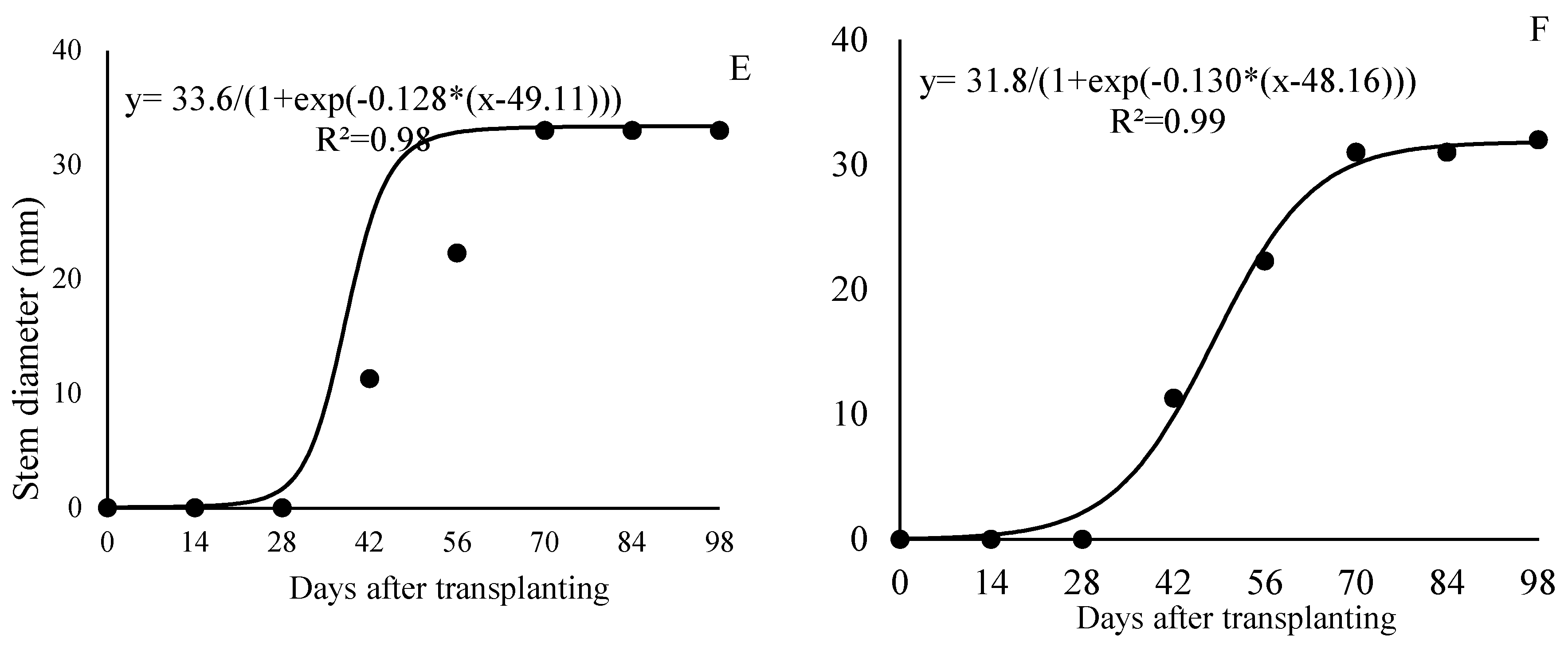
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
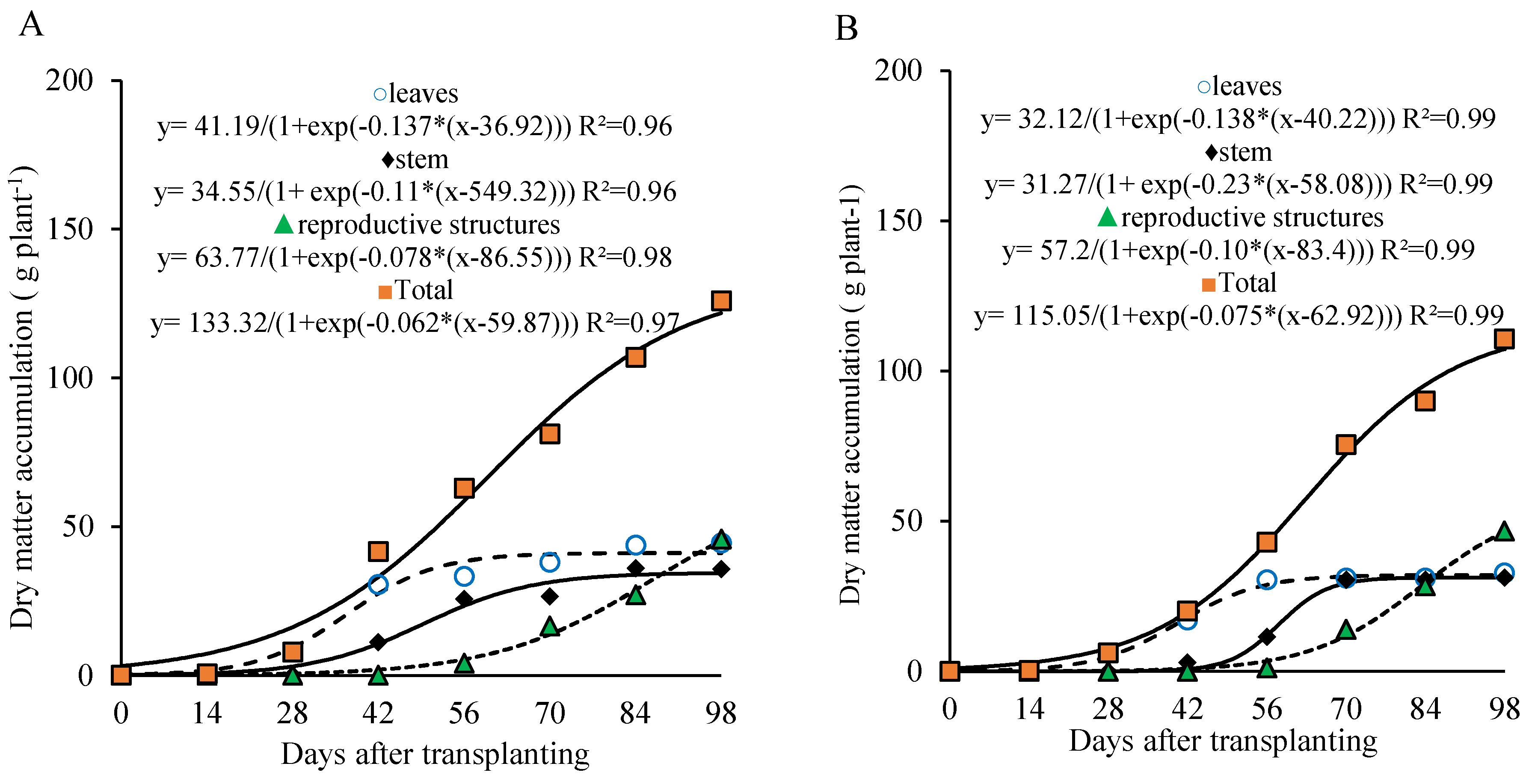
3.2. Dry Matter Accumulation
Total dry matter accumulation in lettuce plants grown for seed production was continuous throughout the crop cycle and could be divided into two distinct stages: the first from transplanting to 28 days after transplanting (DAT), and the second from 29 DAT to seed harvest (98 DAT), for both phosphorus doses 800 kg ha
−1 of P
2O
5 (
Figure 2A) and 320 kg ha
−1 of P
2O
5 (
Figure 2B).
The first stage (0 to 28 DAT) was characterized by low dry matter accumulation, with an average of 5.27 g plant−1 under the 320 kg ha−1 P2O5 treatment, representing 4.8% of the total accumulated by the end of the cycle (109.8 g plant−1); and 13.05 g plant−1 under the 800 kg ha−1 P2O5 treatment, corresponding to 10.8% of the final accumulated total of 120.8 g plant−1 at 98 DAT.
Figure 2.
Dry matter accumulation in the leaves, stem, reproductive organs, and total of lettuce plants under organic fertilization management and phosphorus doses of 800 (A) and 320 (B) kg ha−1 of P2O5.
Figure 2.
Dry matter accumulation in the leaves, stem, reproductive organs, and total of lettuce plants under organic fertilization management and phosphorus doses of 800 (A) and 320 (B) kg ha−1 of P2O5.
In the leaves and stem, under the dose of 800 kg ha−1 of P2O5, dry matter accumulation followed three stages, the first stage occurred from 0 to 28 DAT, the second from 29 to 56 DAT, and the third from 57 to 98 DAT. In the first stage, the plants accumulated 9.37 and 3.02 g plant−1 of dry matter in the leaves and stem, which represented 22.7% and 8.8% of the total dry matter accumulated by 98 DAT, respectively. During the second stage, dry matter accumulation was more pronounced, with the plants accumulating 38.4 and 23.3 g plant-1 in the leaves and stem, accounting for approximately 70% and 60% of the total accumulation, respectively. In the third stage, plants accumulated 2.9 and 10.9 g plant-1 of dry matter (7% and 31.7% of the total accumulated by 98 DAT), respectively.
Under the dose of 320 kg ha−1 of P2O5, dry matter accumulation in leaves and stem also followed three stages, with the first stage occurring from 0 to 28 DAT (leaves) and 0 to 42 DAT (stem), the second from 28 to 56 DAT (leaves) and 43 to 70 DAT (stem), and the third from 57 to 98 DAT (leaves) and 71 to 98 DAT (stem). In the first stage, the plants accumulated 5.0 and 0.8 g plant−1 in the leaves and stem (15.65% and 2.42% of the total accumulated by 98 DAT), respectively. In the second stage, the plants accumulated approximately 23.8 and 28.7 g plant−1 in the leaves and stem, representing 74% and 91% of the total accumulated, respectively.
At the harvest point for fresh consumption, i.e., at 35 DAT, plants had accumulated 11.1 and 24.9 g plant−1 of dry matter, corresponding to 10.1% and 20.6% of the total dry matter accumulated at the doses of 320 and 800 kg ha−1 of P2O5, respectively.
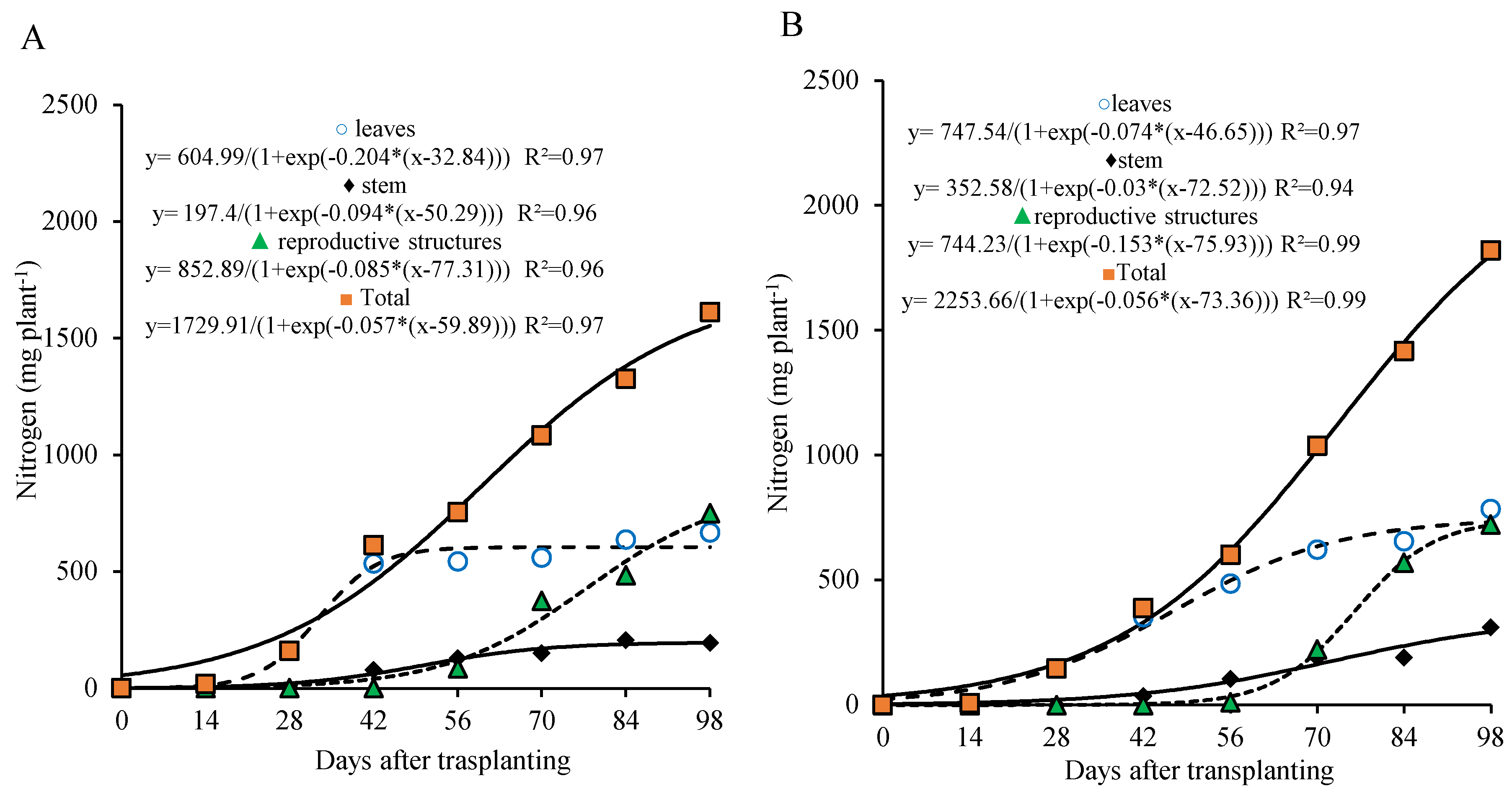
3.3. Macronutrients Accumulation
At the dose of 800 kg ha
−1 of P
2O
5, nitrogen accumulation in the leaves occurred sharply during the first 42 days after transplanting (DAT) and then stabilized. The stem showed a more gradual nitrogen accumulation compared to the leaves during the first 42 DAT, followed by a more pronounced increase after 42 DAT, coinciding with stem elongation due to the formation of the reproductive stalk. In the reproductive part, nitrogen accumulation was more intense and almost linear from 56 DAT onward, at the time of floral bud emergence (
Figure 4A).
At the dose of 320 kg ha
−1 of P
2O
5, nitrogen accumulation in the leaves and stem increased throughout the cycle, although it was less intense after 70 DAT and did not reach stabilization as observed with the higher dose and in the dry matter data (
Figure 2). In the reproductive part, accumulation became more pronounced after 70 DAT (
Figure 4B).
Figure 4.
Nitrogen accumulation in different parts of lettuce plants grown for seed production under organic fertilization with 800 (A) and 320 (B) kg ha−1 of P2O5.
Figure 4.
Nitrogen accumulation in different parts of lettuce plants grown for seed production under organic fertilization with 800 (A) and 320 (B) kg ha−1 of P2O5.
Nitrogen accumulation in the plant (total) followed a continuous growth pattern up to 98 days after transplanting (DAT), fitting a sigmoidal curve. This accumulation could be divided into two distinct stages for both phosphorus doses, similarly to the dry matter accumulation pattern. The first stage occurred from 0 to 28 DAT, during which nitrogen accumulation reached 198.5 and 171.7 mg per plant—representing approximately 13% and 9.8% of the total at the end of the cycle—for the 800 and 320 kg ha−1 doses, respectively. The daily accumulation rate during this phase averaged 7.0 and 6.1 mg per plant per day.
From 29 to 98 DAT, nitrogen uptake intensified considerably. During this second stage, plants accumulated 1329.2 and 1571.5 mg per plant—equivalent to 87.0% and 90.2% of the total nitrogen content—for the 800 and 320 kg ha
−1 doses, respectively. The average daily accumulation rates in this phase were 19.0 and 22.45 mg per plant per day (
Figure 4A,B).
At the end of the cycle, total nitrogen accumulation for the 800 kg ha
−1 dose reached 1527.7 mg per plant, with 40% allocated to the leaves, 13% to the stem, and 48% to the reproductive structures. A similar distribution was observed under the 320 kg ha
−1 dose, with total nitrogen accumulation of 1743.17 mg per plant, 42% in the leaves, 17% in the stem, and 41% in the reproductive part (
Figure 4A,B).
The overall nitrogen accumulation pattern was similar to that of total dry matter accumulation, with limited uptake before 28 DAT and most of the nitrogen being absorbed between 29 and 98 DAT under both phosphorus treatments (
Figure 2A,B).
Interestingly, even though the reproductive structures represented only 37% (
Figure 2A) and 34% (
Figure 2B) of total dry mass at 98 DAT for the 800 and 320 kg ha
−1 doses, respectively, they accounted for a significant proportion of total nitrogen accumulation, 48% and 41%, respectively.
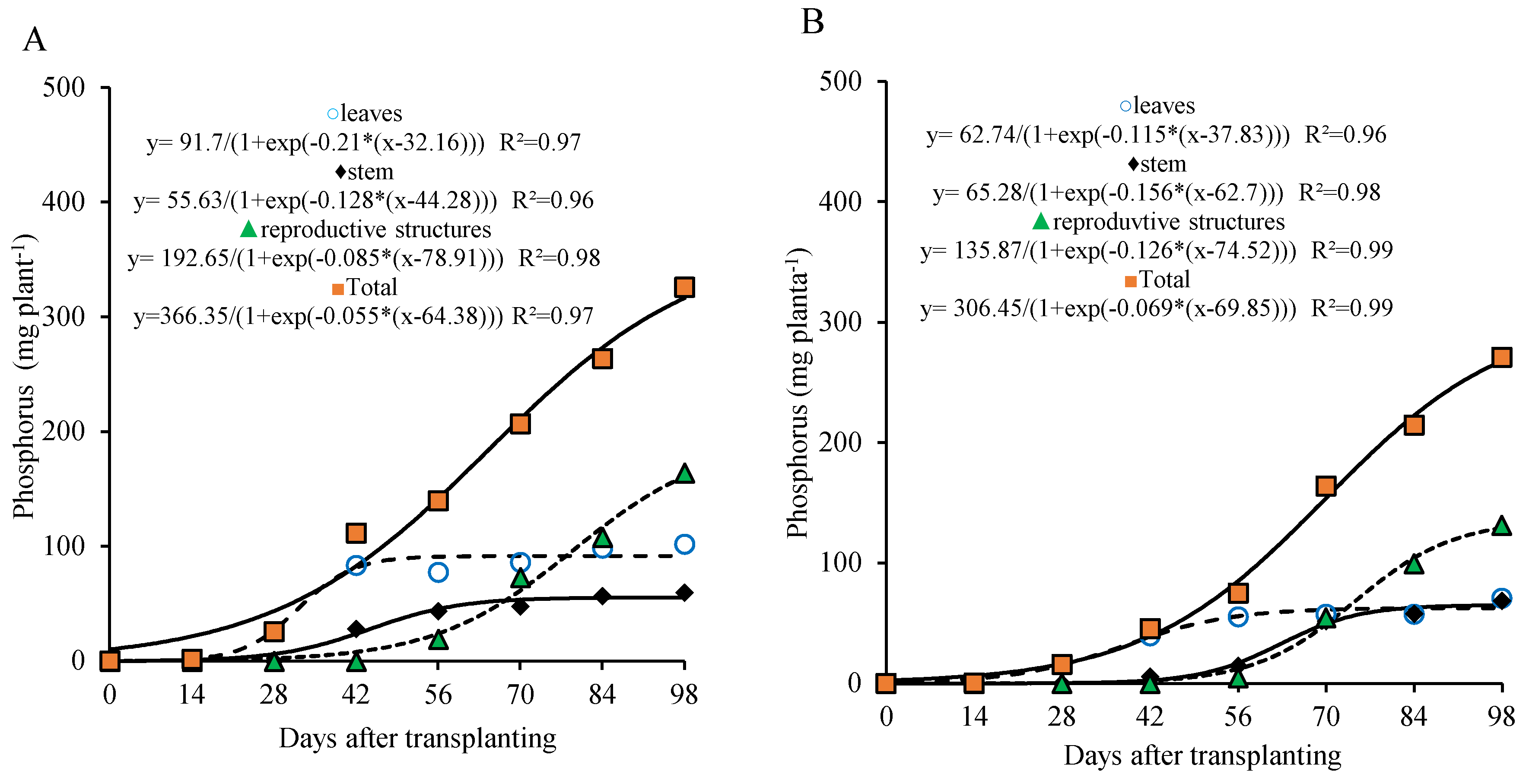
Phosphorus accumulation exhibited a pattern similar to that of nitrogen in the different parts of the plant, with faster accumulation in the leaves and a more gradual increase in the stem during the first weeks after transplanting. This accumulation tended to stabilize from 42 and 56 days after transplanting (DAT), respectively, and became more intense in the reproductive structures from 70 DAT onward (
Figure 5A,B).
For total phosphorus in the plant, two growth stages were also observed for the 800 kg ha
−1 P
2O
5 dose: the first between 0 and 28 DAT, and the second from 29 to 98 DAT. In the first stage, the plant accumulated approximately 12.0% of the total phosphorus (35.7 mg plant
−1), with an average of 1.3 mg plant
−1 day
−1. In the second stage, phosphorus accumulation was more pronounced, reaching 272.2 mg plant
−1 by the end of the period (about 88.4% of the total accumulated), with an average of 3.8 mg plant
−1 day
−1. At the end of the cycle, total accumulation reached 308.2 mg plant
−1, distributed among leaves, stem, and reproductive parts in proportions of 30%, 18%, and 52%, respectively (
Figure 5A).
For the 320 kg ha
−1 P
2O
5 dose, two growth stages were also observed, with the first occurring between 0 and 42 DAT and the second from 43 to 98 DAT. In the first stage, the plant accumulated about 16.9% of the total phosphorus (43.4 mg plant
−1), with an average of 1.03 mg plant
−1 day
−1. In the second stage, phosphorus accumulation became more pronounced, reaching 213.3 mg plant
−1 by the end of the period (approximately 83.1% of the total accumulated), with an average of 3.8 mg plant
−1 day
−1. At the end of the cycle, total phosphorus accumulation was 256.9 mg plant
−1, distributed among leaves, stem, and reproductive parts in proportions of 25%, 25%, and 50%, respectively (
Figure 5B).
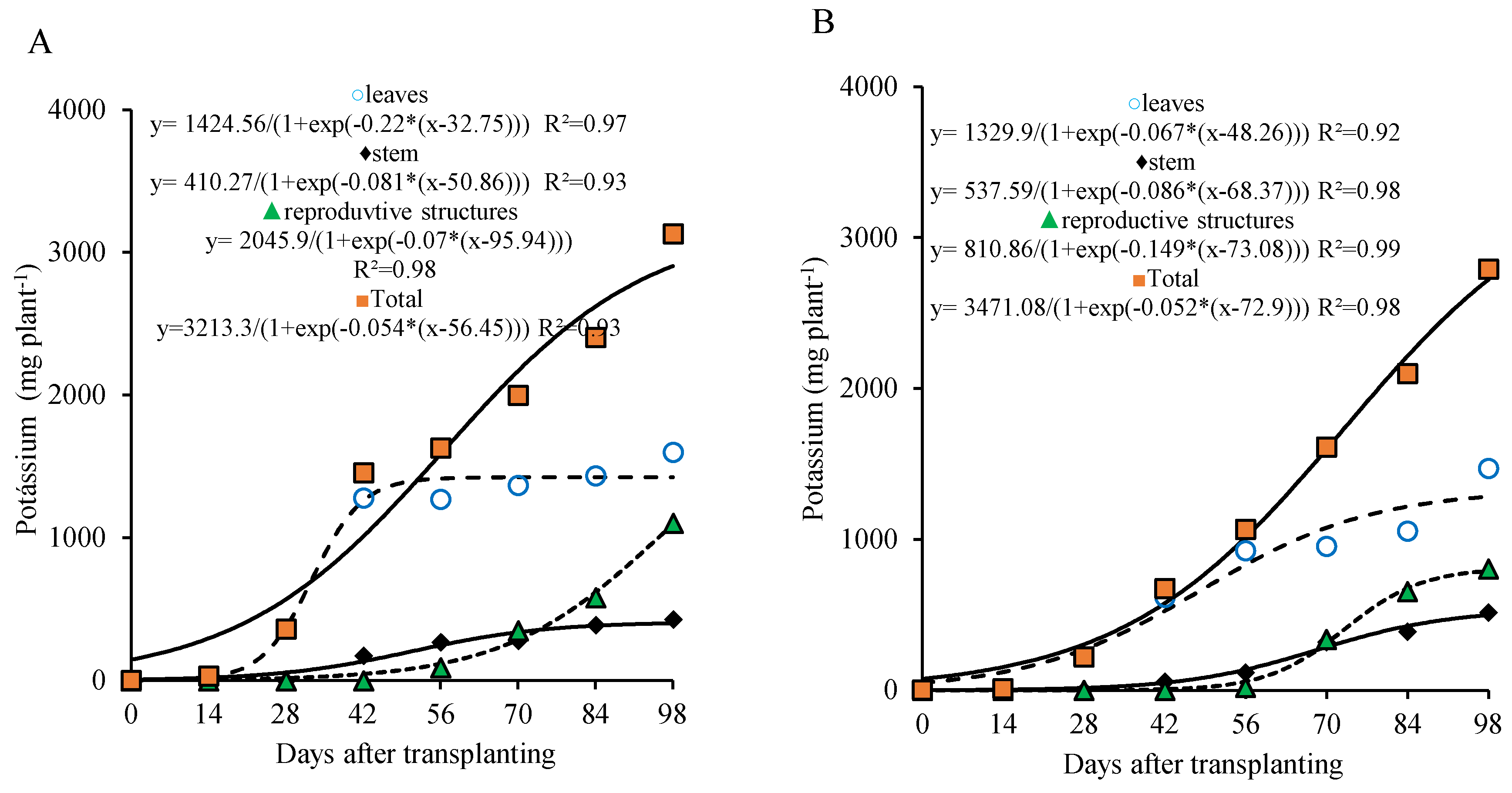
For the 800 kg ha
−1 P
2O
5 dose, potassium accumulation in the leaves was pronounced, with the highest increase occurring around 56 DAT, followed by stabilization thereafter. The stem showed a more gradual potassium accumulation, with growth leveling off after 70 DAT. On the other hand, potassium accumulation in the reproductive part began to intensify from 70 DAT onward, increasing almost steadily until the end of the cycle (
Figure 6A).
For the 320 kg ha
−1 P
2O
5 dose, the sigmoid curve shows a continuous and gradual accumulation of potassium in the leaves and stem until the end of the cycle (98 DAT), although less intense during the last two sampling dates. Potassium accumulation in the reproductive part began later, from 70 DAT, reaching a maximum of 791.54 mg plant
−1 by the end of the cycle (
Figure 6B).
The curve of total potassium accumulation in the plant shows continuous and significant increase around 98 DAT and can be divided into two stages, the first from 0 to 28 DAT and the second from 29 to 98 DAT for both doses studied. In the first stage, the plant accumulated 443.8 and 289.3 mg plant−1 (about 15.2% and 11.2% of the total accumulated by the end of the cycle), with averages of 15.8 and 10.3 mg plant−1 day−1 at 800 and 320 kg ha−1 of P2O5, respectively. In the second stage, an accumulation of 2478.8 and 2284.9 mg plant−1 was recorded (84.8% and 88.8% of the total accumulated by the end of the cycle), with averages of 38.2 and 32.6 mg plant−1 day−1, respectively.
Figure 6.
Potassium accumulation in different parts of lettuce plants grown for seed production under organic fertilization with 800 (A) and 320 (B) kg ha−1 of P2O5.
Figure 6.
Potassium accumulation in different parts of lettuce plants grown for seed production under organic fertilization with 800 (A) and 320 (B) kg ha−1 of P2O5.
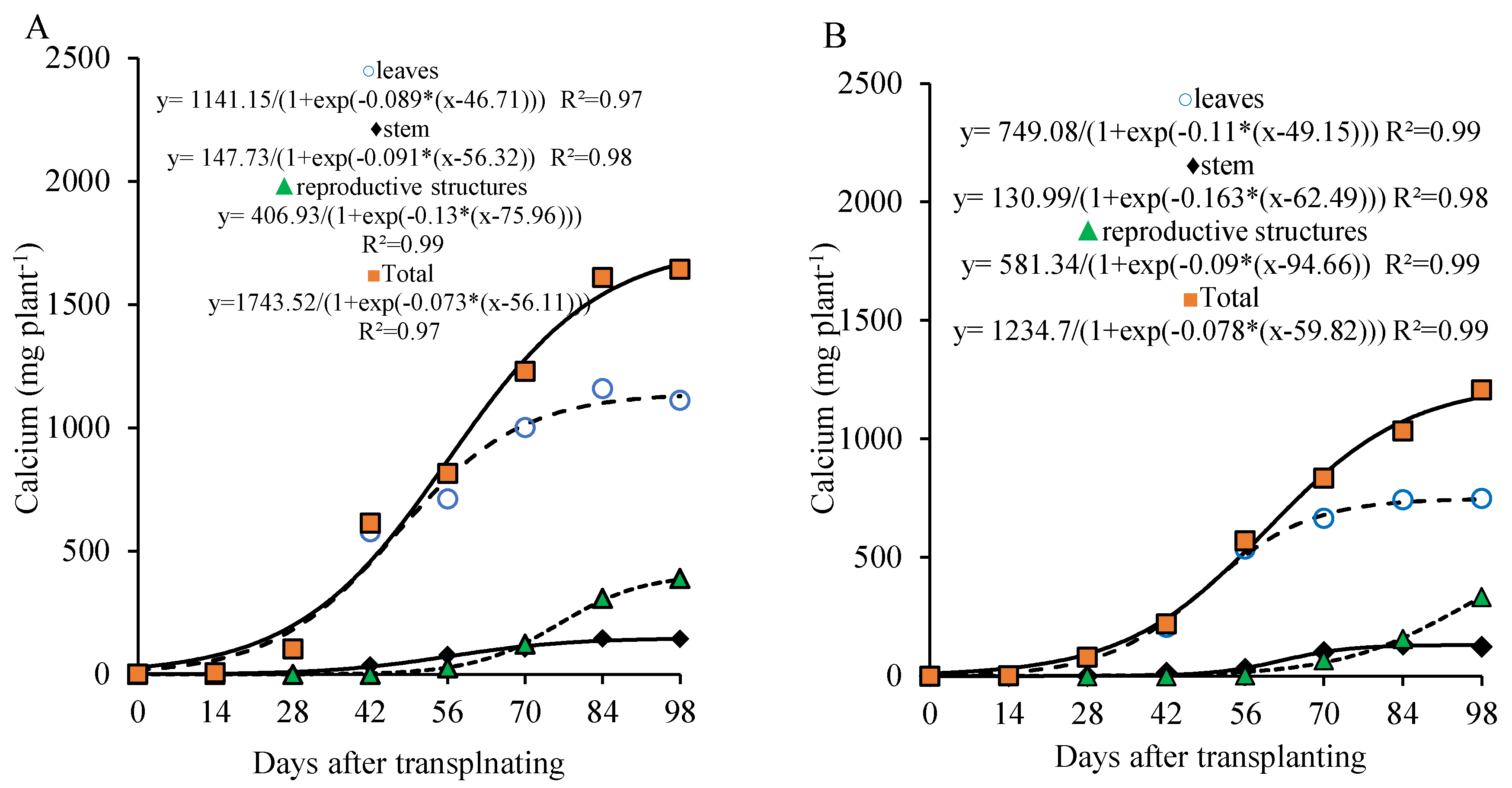
Calcium showed a marked accumulation in the leaves up to 56 DAT and a gradual increase in the stem throughout the lettuce growth cycle. In the reproductive part, calcium accumulation became more pronounced from 70 DAT onward, with 66.6% (800 kg ha
−1 of P
2O
5) and 83.05% (320 kg ha
−1 of P
2O
5) of the total calcium in these parts accumulating after this period (
Figure 7A,B).
The total calcium accumulation curve shows a continuous increase up to 98 DAT, with a more marked rate between 28 and 70 DAT, during which the plant accumulated about 64.1% of the total calcium (1,063.9 and 776.7 mg plant-1), with an average of 25.3 and 18.5 mg plant−1 day−1 at the doses of 800 and 320 kg ha−1 of P2O5, respectively. After 70 DAT, the plant accumulated approximately 24.2% and 30.7% of the total calcium, and the average daily accumulation dropped to 14.4 and 13.3 mg plant−1 day−1 until the end of the cycle, for the doses of 800 and 320 kg ha−1 of P2O5, respectively.
At 98 DAT, under the 800 kg ha−1 P2O5 dose, the total calcium accumulation was 1,658.4 mg plant−1, with approximately 77.0% found in the vegetative part and 23.0% in the reproductive part. Under the 320 kg ha−1 P2O5 dose, total calcium accumulation was 1,210.2 mg plant−1, with 72.4% in the vegetative part and 27.6% in the reproductive part.
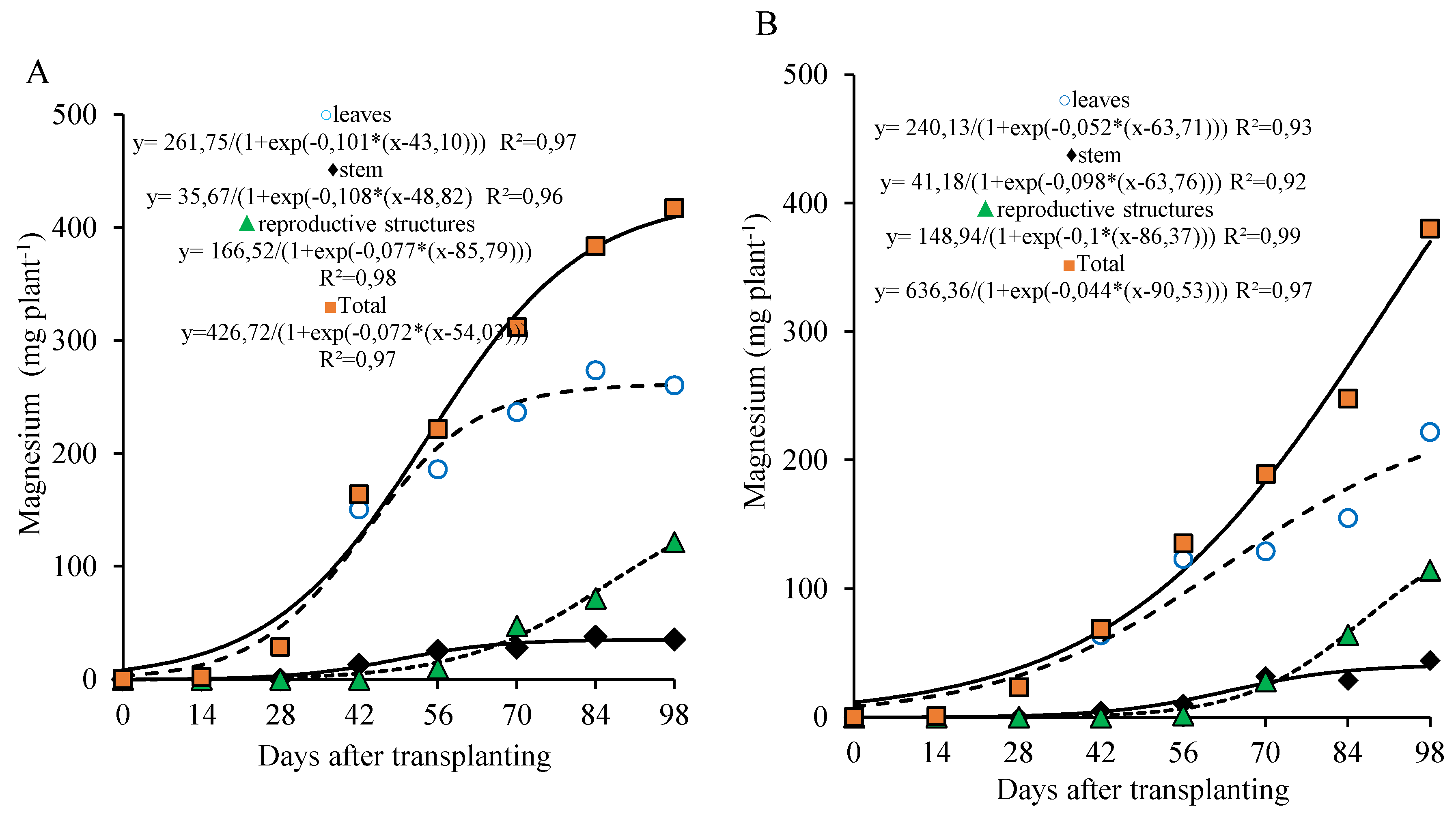
The magnesium accumulation curve for the 800 kg ha
−1 P
2O
5 dose in the leaves and stem followed a pattern similar to that of calcium, with more pronounced values in the leaves until 56 DAT and a gradual accumulation in the stem, which reached its maximum at the end of the cycle. In the reproductive part, magnesium accumulation became more intense after 70 DAT, with approximately 68.2% of the total magnesium in these organs being accumulated after this period. Total magnesium accumulation was slow from transplanting to 28 DAT, with an average of 1.86 mg plant
−1 day
−1. From 29 to 98 DAT, the average accumulation rose to 5.14 mg plant
−1 day
−1, during which about 87.5% of the total magnesium was accumulated. At 98 DAT, total magnesium accumulation reached 416 mg plant
−1, with 63% in the leaves, 9% in the stem, and 29% in the reproductive part (
Figure 8A).
At the 320 kg ha
−1 P
2O
5 dose, the trend of total magnesium accumulation was continuous and nearly linear, showing a low initial accumulation, with an average of 1.2 mg plant
−1 day
−1 until 28 DAT, increasing to 4.6 mg plant
−1 day
−1 between 29 and 98 DAT. During this second period, 90.5% of the total accumulated magnesium was recorded. At 98 DAT, the total magnesium accumulation was 358.8 mg plant
−1, with 57% in the leaves, 11% in the stem, and 32% in the reproductive part (
Figure 8B).
Figure 8.
Magnesium accumulation in different parts of lettuce plants grown for seed production under organic fertilization with 800 (A) and 320 (B) kg ha−1 of P2O5.
Figure 8.
Magnesium accumulation in different parts of lettuce plants grown for seed production under organic fertilization with 800 (A) and 320 (B) kg ha−1 of P2O5.
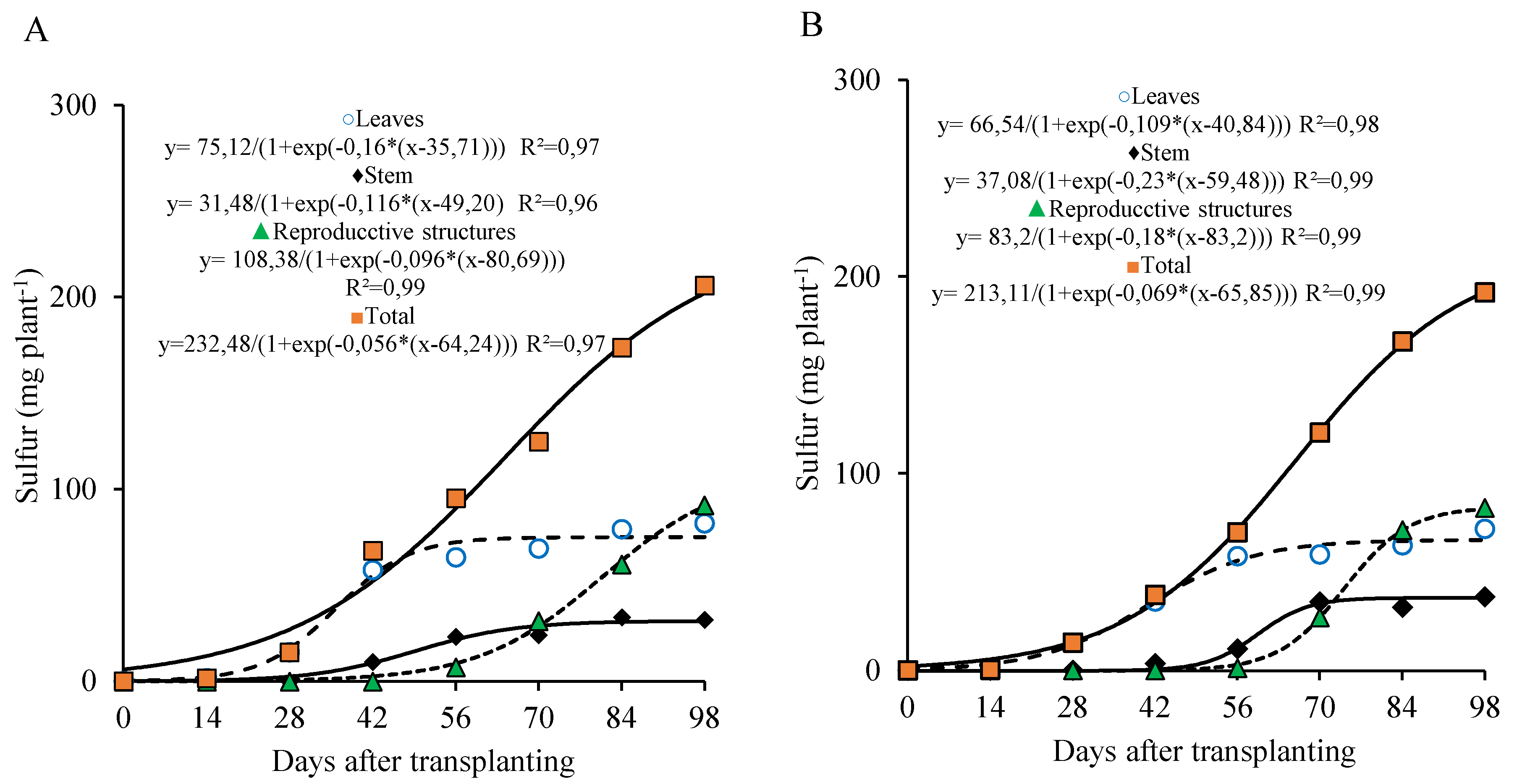
At the 800 kg ha
−1 P
2O
5 dose, sulfur accumulation in the leaves was more prominent from 0 to 56 DAT, with approximately 73.7% of the maximum accumulated in this organ occurring during this period (55.4 mg plant-1). In the stem, sulfur accumulation tended to stabilize after 70 DAT, with a slower rate from 70 to 98 DAT, reaching a maximum of 31.4 mg plant
−1. In the reproductive part, accumulation became more marked after 70 DAT, with about 69.3% of the total sulfur in this organ being accumulated during that period, ending the cycle with 91.0 mg plant
−1 (
Figure 9A).
On the other hand, under the 320 kg ha
−1 P
2O
5 dose, sulfur accumulation in the leaves was more evident between 28 and 70 DAT, with 76.7% of the maximum accumulated during this interval (50.7 mg plant
−1). In the stem, sulfur accumulation also tended to stabilize after 70 DAT, with a slower accumulation until 98 DAT, when a maximum of 37.0 mg plant-1 was observed. In the reproductive part, accumulation became more expressive after 70 DAT, with approximately 67.4% of the sulfur being accumulated in this period, ending the cycle with 82.0 mg plant
−1 (
Figure 9B).
Figure 9.
Sulfur accumulation in different parts of lettuce plants grown for seed production under organic fertilization with 800 (A) and 320 (B) kg ha−1 of P2O5.
Figure 9.
Sulfur accumulation in different parts of lettuce plants grown for seed production under organic fertilization with 800 (A) and 320 (B) kg ha−1 of P2O5.
Similar to nitrogen, the total sulfur accumulation curve followed the dry matter accumulation pattern, with the highest accumulation occurring from 28 to 98 DAT. During this period, 177.5 and 172.4 mg plant-1 of sulfur were accumulated (representing 89.8% and 93.2% of the total sulfur accumulated by the end of the cycle), with an average of 2.53 and 2.46 mg plant
−1 day
−1 under the 800 and 320 kg ha
−1 P
2O
5 doses, respectively. As observed for the other macronutrients, the sulfur accumulation curve showed a continuous upward trend until 98 DAT, with total accumulations of 197.6 and 185.5 mg plant
−1 recorded at the end of the cycle. These values were distributed among the plant organs as follows: 38% and 36% in the leaves, 16% and 20% in the stem, and 46% and 44% in the reproductive parts, for the 800 and 320 kg ha
−1 P
2O
5 doses, respectively (
Figure 9A,B).
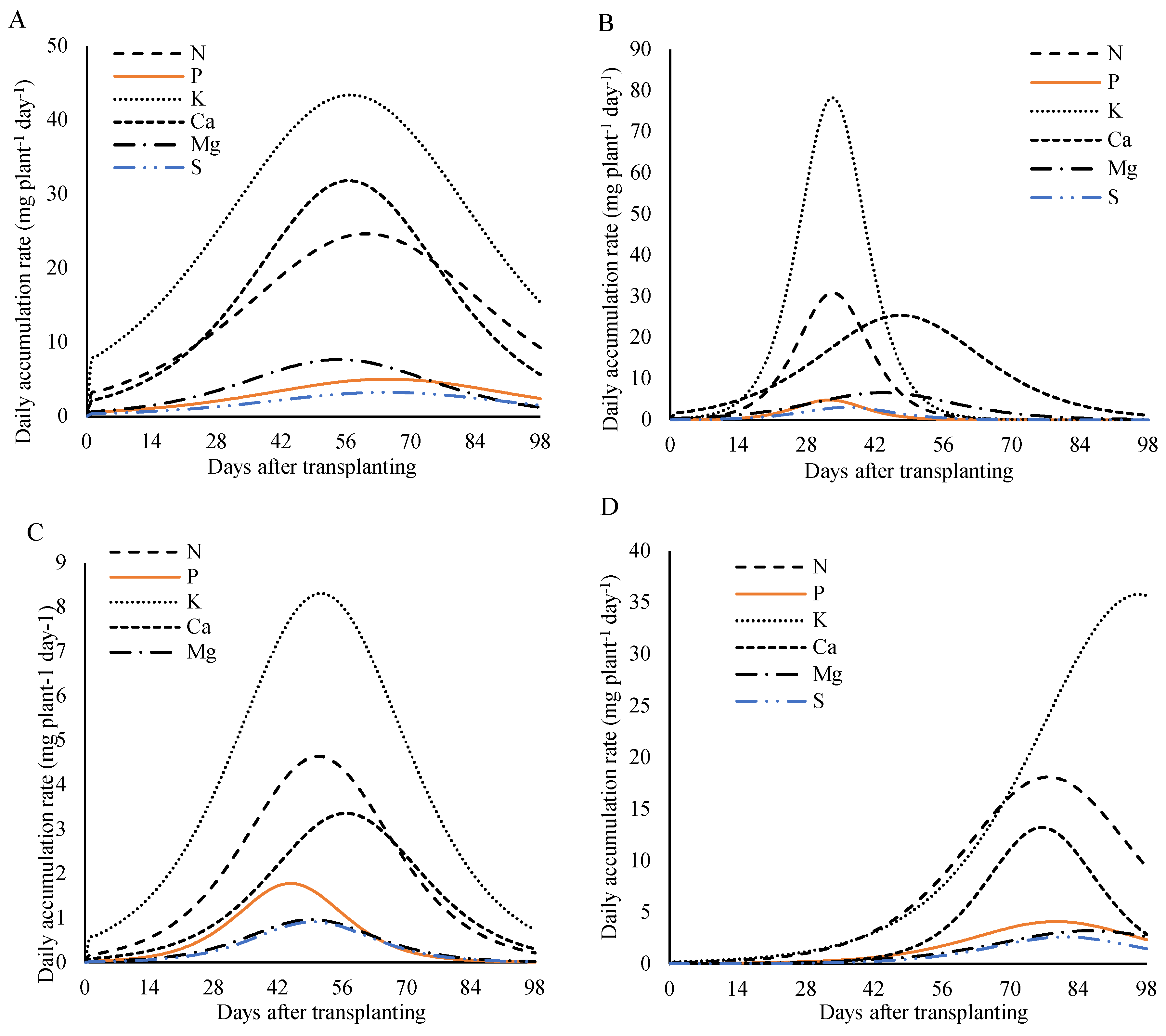
3.4. Daily Accumulation Rate
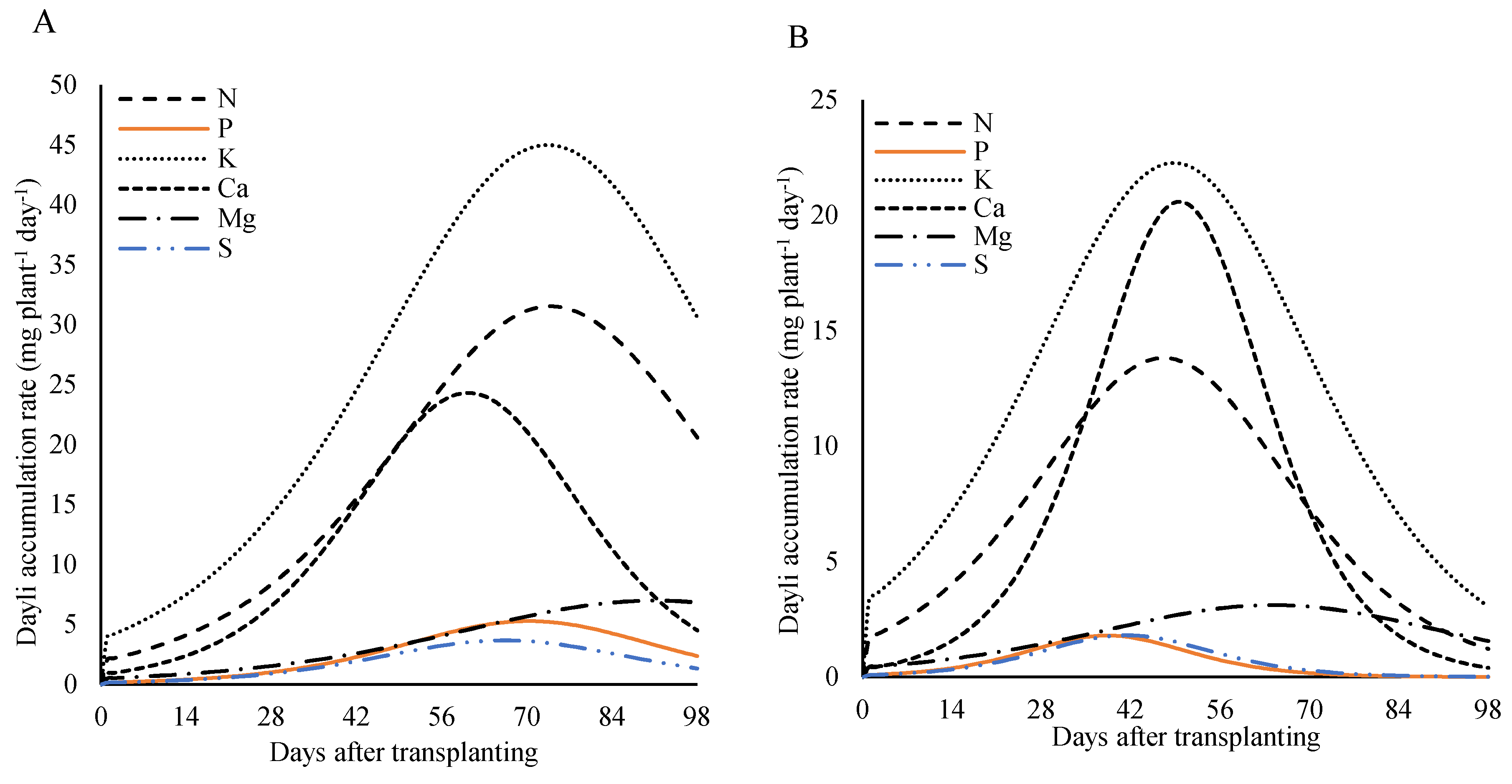
Analyzing the nutritional demand of lettuce plants over the days after transplanting (
Figure 10), under fertilization with 800 kg ha
−1 of P
2O
5, a rise in the daily accumulation rate was observed throughout the cultivation cycle, reaching a peak—known as the maximum daily accumulation rate (MDAR)—followed by a decline until the end of the cycle. This pattern was evident for all plant parts (leaves, stem, reproductive structures, and total plant) and for nearly all nutrients. However, in the reproductive parts, the daily accumulation rate of potassium continued to increase, with its maximum observed close to harvest.
For the plant as a whole, the MDAR occurred between 55 and 65 days after transplanting (DAT). The maximum daily accumulation rates were: 24.6 mg plant-1 day-1 for nitrogen at 60 DAT; 5.0 and 3.2 mg plant
−1 day
−1 for phosphorus and sulfur, respectively, at 65 DAT; 43.4 and 31.8 mg plant
−1 day
−1 for potassium and calcium at 57 DAT; and 7.7 mg plant-1 day-1 for magnesium at 55 DAT. These results highlight the periods of greatest nutritional demand throughout the plant’s development cycle (
Figure 10A).
In the stem, maximum daily accumulation rates occurred between 43 and 56 DAT. Nitrogen, magnesium, and sulfur peaked at 4.6, 1.0, and 0.9 mg plant
−1 day
−1 at 48 DAT, respectively. Phosphorus reached 1.8 mg plant
−1 day
−1 at 43 DAT, potassium reached 8.3 mg plant
−1 day
−1 at 50 DAT, and calcium reached 3.4 mg plant
−1 day
−1 at 56 DAT (
Figure 10B).
In the leaves, the highest daily accumulation rates for nitrogen, phosphorus, and potassium were 30.8, 4.8, and 78.2 mg plant
−1 day
−1, respectively, reached at 33 DAT. Calcium reached its peak later, at 25 mg plant
−1 day
−1 on 50 DAT. Magnesium peaked at 6.6 mg plant
−1 day
−1 at 44 DAT, and sulfur had the lowest peak at 3.0 mg plant
−1 day
−1 at 36 DAT (
Figure 10C).
In the reproductive structures (
Figure 10D), the maximum daily accumulation rate occurred between 69 and 87 DAT for most nutrients. The peak values were 18.1, 4.1, and 13.2 plant
−1 day
−1 for nitrogen, phosphorus, and calcium, respectively, at 77 DAT; 3.2 mg plant-1 day-1 for magnesium at 83 DAT; and 2.6 mg plant
−1 day
−1 for sulfur at 79 DAT. For potassium, demand remained continuous throughout the cycle, with the maximum value of 35.8 mg plant
−1 day
−1 reached at 97 DAT.
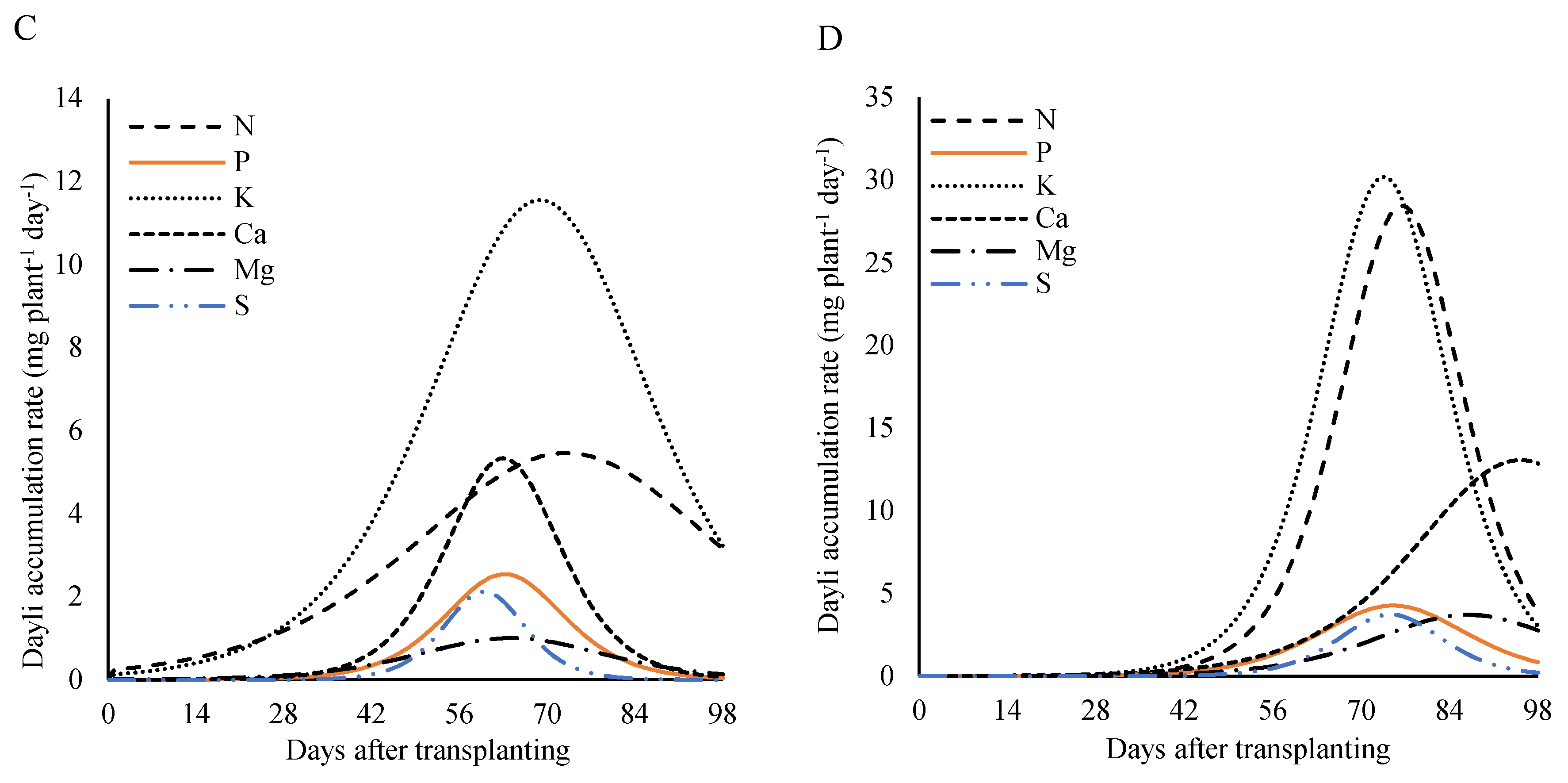
Analyzing the nutritional demand of the plants as a function of days after transplanting at the dose of 320 kg ha
−1 of P
2O
5 (
Figure 11), a similar pattern was observed for all plant parts (leaves, stem, reproductive part, and total) for almost all nutrients. On the other hand, for the reproductive part, the daily accumulation rate of calcium showed a continuous increase, with the peak occurring close to harvest (
Figure 11A).
In the stem, the maximum rate occurred between 61 and 75 DAT, with peaks of 5.5 mg plant
−1 day
−1 at 72 DAT for nitrogen, 2.5, 5.2, 1.0, and 2.1 mg plant
−1 day
−1 at 61 DAT for phosphorus, calcium, magnesium, and sulfur, respectively. For potassium, the maximum rate was 11.5 mg plant
−1 day
−1 at 67 DAT (
Figure 11B).
The maximum daily accumulation rate in the leaves occurred between 38 and 59 DAT. For nitrogen, potassium, and calcium, maximum values of 13.8, 22.7, and 20.6 mg plant
−1 day
−1 were recorded at 49 DAT. Phosphorus and sulfur reached a rate of 1.8 mg plant
−1 day
−1 at 38 DAT, while magnesium, the slowest nutrient, reached a rate of 3.1 mg plant
−1 day
−1 at 59 DAT (
Figure 11C).
Figure 10.
Daily accumulation rate of macronutrients in the whole plant (A), stem (B), leave (C), and reproductive structure (D) of lettuce plants fertilized with 800 kg ha−1 of P2O5, as a function of days after transplanting.
Figure 10.
Daily accumulation rate of macronutrients in the whole plant (A), stem (B), leave (C), and reproductive structure (D) of lettuce plants fertilized with 800 kg ha−1 of P2O5, as a function of days after transplanting.
Figure 11.
Daily accumulation rate of macronutrients in the in the whole plant (A), leaves (B), stem (C), and reproductive part (D) of lettuce plants fertilized with 320 kg ha−1 of P2O5, as a function of days after transplanting.
Figure 11.
Daily accumulation rate of macronutrients in the in the whole plant (A), leaves (B), stem (C), and reproductive part (D) of lettuce plants fertilized with 320 kg ha−1 of P2O5, as a function of days after transplanting.
In the reproductive part, the demand for nutrients was highest between 74 and 95 DAT. For nitrogen and phosphorus, the maximum rate was 28.4 and 4.3 mg plant
−1 day
−1 at 76 DAT, potassium and sulfur reached 30.2 and 3.7 mg plant
−1 day
−1 at 74 DAT, respectively, and calcium reached 13.1 mg plant
−1 day
−1 at 95 DAT (
Figure 11D).