Introduction
Historical monuments testify to human history and the heritage conservation is entrusted to the attention and care of the scientific community in its disciplinary entirety. As summarized in the more recent state-of-the-art contributions [
1,
2,
3,
4,
5,
6] and in others specifically cited throughout the text, stone monuments are subject to physical, chemical and biological degradation which, in addition to disfiguring their surface walls, gradually affect their load-bearing structure, compromising their stability. The process of deterioration of monumental assets, inevitable and progressive, begins immediately after their implementation and the speed of interacting phenomena depends on both natural and anthropogenic factors. Thus, timing and impact modes are specifically linked to the characteristics of the monuments (location, orientation, mineralogical and structural properties) and how they are affected by microclimate (temperature, humidity, solar radiation, wind regime, precipitations), air pollution
, presence of specific flora and fauna that are settling the built spaces of the heritage site under consideration.
Among the environmental parameters, wind and solar radiation influence surface reactivity and morphology inducing erosion and fragments detachment, while water (as rainfall/infiltration of various origins) causes dissolution, migration of dissolved salts and re-crystallization and may alter the matrix structure causing expansion, stress and fractures favourable sites to biological life. Furthermore, it is reported that climate changes increase the level of humidity, therefore, the growth of microorganisms and soiling through the better adhesion of pollutants to wetter surfaces; to properly understand these interactions and their effects on stones, short and long-term observations are recommended to extrapolate deterioration trends and plan preventive interventions based on environmental factors and constitutive characteristics that identify the given monument.
In this context, we continue our investigation on historical monuments made of calcarenite stones carried within the national project SCN_00520, aimed at the protection and safeguard of natural and cultural heritage [
7], aims now conveyed into the Tech4You (PNRR) project integrated with sustainable actions to promote inclusive, safe and resilient smart cities/communities (
see Acknowledgements).
As reported in previous work [7 and references cited therein], Calcarenites are sedimentary rocks widespread in the Mediterranean area, used for centuries as a construction material thanks to their conjugation as ‘soft rocks’ of sufficient mechanical strength and good workability. The Gravina’ calcarenite played a crucial role in the construction of the historic Sassi district in Matera: initially favoring the settlements of cave-dwelling civilizations was then extracted and utilized for the urban expansion of this magnificent heritage, UNESCO site since 1993. However, calcarenite as a carbonate rock of high porosity always faces challenges related to its weathering susceptibility. In order to preserve the historical fabric of the Sassi of Matera (Basilicata Region, Southern Italy) conservation interventions focused on structural reinforcement, surface treatments for protection from atmospheric agents and monitoring systems to assess the condition of the assets have been carried out within the framework of the research projects mentioned above, some experimental parts still in progress.
In this work the monument in calcarenite referred to is a private historical building (an old farmhouse dating back to the 18th century) located between the archaeological park of Lavello, a small town in Basilicata (southern Italy) and the industrial area surrounding the incinerator ‘Fenice’. The territorial maps were previously reported [
8,
9] with the objectives of experiments designed for long-term research, including a doctoral project hosted at the University of Basilicata (Potenza, Italy) and carried out in cooperation with CICRP (
Centre interrégional de Conservation et Restauration du Patrimoine, Marseille, France) (
see Acknowledgments), whose final aim was to retrieve the degradation pathways of calcarenite stones by temporally controlling the effects of biotic and abiotic interactions of any kind, in the given external conditions and for long-time exposures. The proposed methodology envisaged the comparative diagnostics of the old building in an advanced state of degradation and of a new block of calcarenite stones, built for this purpose in the immediate vicinity. Monitoring the new building over time, taking the old building as the ultimate reference, it was intended to verify whether it was possible to obtain adequate information on the ‘incipit’ of the whole degradation process and to predict its evolution.
The complete monitoring required multi-aspect analysis to identify the prevalent chemical, physical and biological factors that trigger the calcarenite 'degradation and how their mutual interactions evolve, possibly converging towards a sort of stabilization over long times. In particular, the results published from the thesis work [
8,
9] were derived from XPS and several other experiments, performed in combination during the first year(s) of temporal monitoring, summarized below in small dotted sections:
•The influence of some climatic parameters (humidity, rainfall, temperature, light irradiation, wind intensity), pollution (SO2, CO, NO2, Ozone and heavy metals) and biological colonization were investigated. Micro-climate parameters and air quality were monitored by the Agency for the Environmental Protection of Basilicata Region (ARPAB).
•Chemical analysis, surface analysis and biological analysis were carried out by University of Basilicata while capillarity test, sound speedy test and petro-graphic analysis by using optic microscopy and scanning electronic microscopy (SEM), were carried out by CICRP (Centre Interrégional de Conservation et Restauration du Patrimoine) in Marseille (France) during the doctorate stages, agreed for the project cooperation.
•XRD analysis showed that sample stones are composed of calcite containing a low magnesium content, minor constituents as kaolinite, hillite, chlorite, smectite and halloysite, gibbsite and goethite finely disseminated, quartz and feldspar present as individual grains. In the structure were found grains and lithic fragments of limestone and benthic foraminifera (nummulite), gastropods, echinoderms that confirmed their sedimentary origin [
10]. Chemicals parameters (pH, EC, OC, etc.) were registered together with water retention favoured by the high porosity of the rocks inducing the biocolonization as hereafter explained.
•The biological analysis gave information about the microorganism biodiversity and on the sequence of their attachments aimed at the colonization of calcarenite. Algae were found at the beginning, during the first 6 months of outdoor exposition, then lichens formed by symbiotic association with fungi. Cyanobacteria were not found but Bacillus bacteria were identified as the second phototrophic colonizers, appearing later in the sequence.
The first two years of the project were thus entirely dedicated to the multi-characterization of the new building through recurrent planned sampling of its walls for the multifaceted analyses reported above [
8,
9].
Afterwards, the diagnostics were less recurrent, the new building was left uncontrolled only subject to sporadic diagnoses, under conditions reflecting those of the old farm in the century, taken as the final reference of degradation.
In particular, the XPS monitoring of the four walls of the new building, performed every three months for the first year, was extended, although with much more sporadic sampling, to almost five years, in order to better evaluate the comparison of the investigated buildings. The acquired spectra of the collected samples were gradually curve-fitted and processed by the team of researchers who followed one another over the years, ensuring the completion of the data set.
Thus, using XPS as a surface-specific technique, this work focuses on the rationalization of the compositional variability of calcarenite surfaces as a function of outdoor exposition for the planned monitoring period. XPS analysis was continued maintaining over the five years the analytical procedure for the collection and conservation of samples and for the acquisition of spectra, recalling where appropriate the results initially obtained with other techniques and biological essays [
8,
9].
The derived XPS dataset proved to be quite consistent in estimating the degradation trend of the calcarenite walls over time, under the conditions studied. The progressively recorded compositional variations of the new building were shown to converge towards the surface composition of the old building, as will be demonstrated in the Results section.
The same data set obtained from the detailed analysis with the curve-fitting program NewGoogly was then employed to perform Principal Component Analysis (PCA) in the attempt to further investigate the deterioration trend, as recently reported in the study of the factors affecting the vulnerability of heritage churches, using PCA as a clustering tool [
11].
Results and Discussion
In this ‘core’ section we will try to best show the trend of degradation for the calcarenite rocks under study by summarizing the numerous XPS data collected over the five-year period, elaborated via our well-established curve-fitting procedure [
14,
15], and comment on their exploration and visualization with PCA analysis using the CAT software [
18].
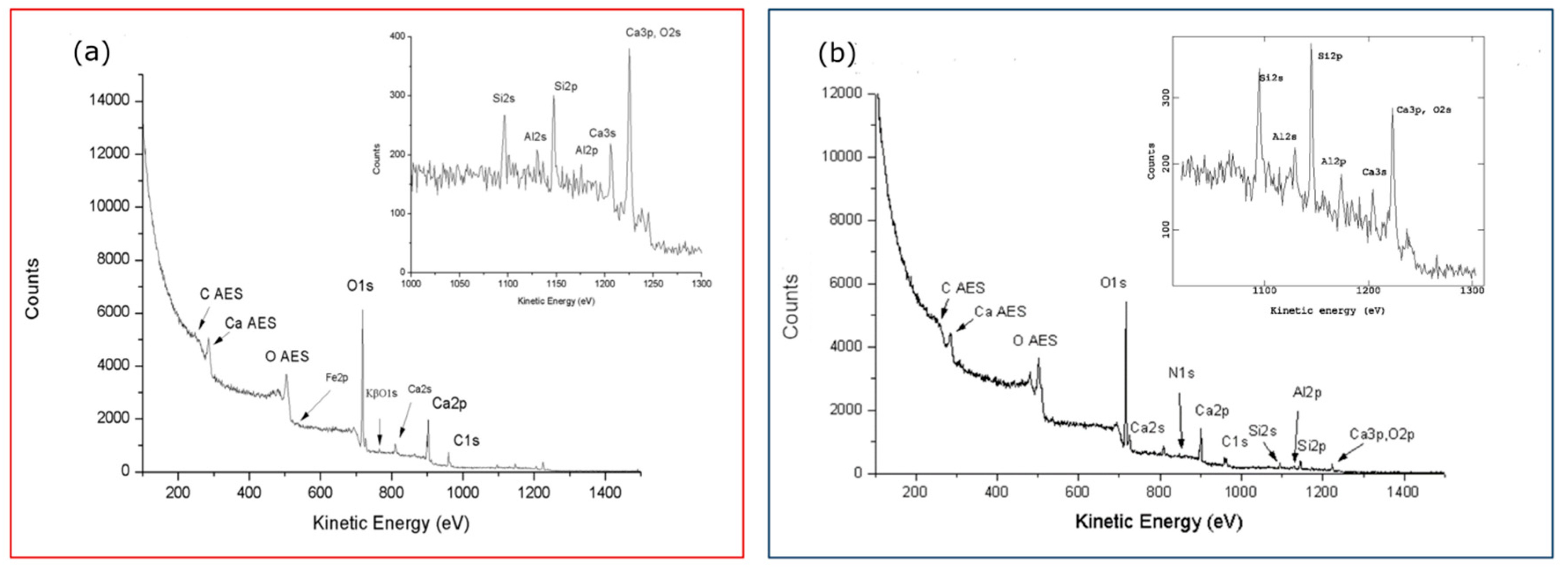
Figure 3.
Wide spectra comparison of blank calcarenites, as reference samples for the new a) and old b) building, referred to ‘0’ time exposition and to a long exposed/ renewed surface, respectively.
Figure 3.
Wide spectra comparison of blank calcarenites, as reference samples for the new a) and old b) building, referred to ‘0’ time exposition and to a long exposed/ renewed surface, respectively.
First of all, it is interesting to note, aided by the peaks labelling in both wide spectra of Figs3, the same main elements composing the two samples with iron in traces in the ‘true’ reference 3a replaced by nitrogen in reference 3b of the old farm. It will be also confirmed in the next sections the ubiquity of organic particulate/functionalized carbons on calcarenitic surfaces, revealed by XPS at the nanometric depth. Such organic matter, gradually contributing to the surface layers, increasingly affects the relative intensity of the main carbonate and oxides/silicate components of the rock, as can also be seen in the insets of both spectra.
Effectively, the peaks labelling in the wide spectrum of Fig.3a better reflects the elemental composition of the calcarenite stones [
7,
10] being used to build the new calcarenite block ‘just installed’, therefore, with “relatively clean" surfaces. The main regions, best representative of each detected element and its chemical states, first acquired at higher resolution and then resolved into the component peaks using ‘NewGoogly’ [
14,
15], are reported in
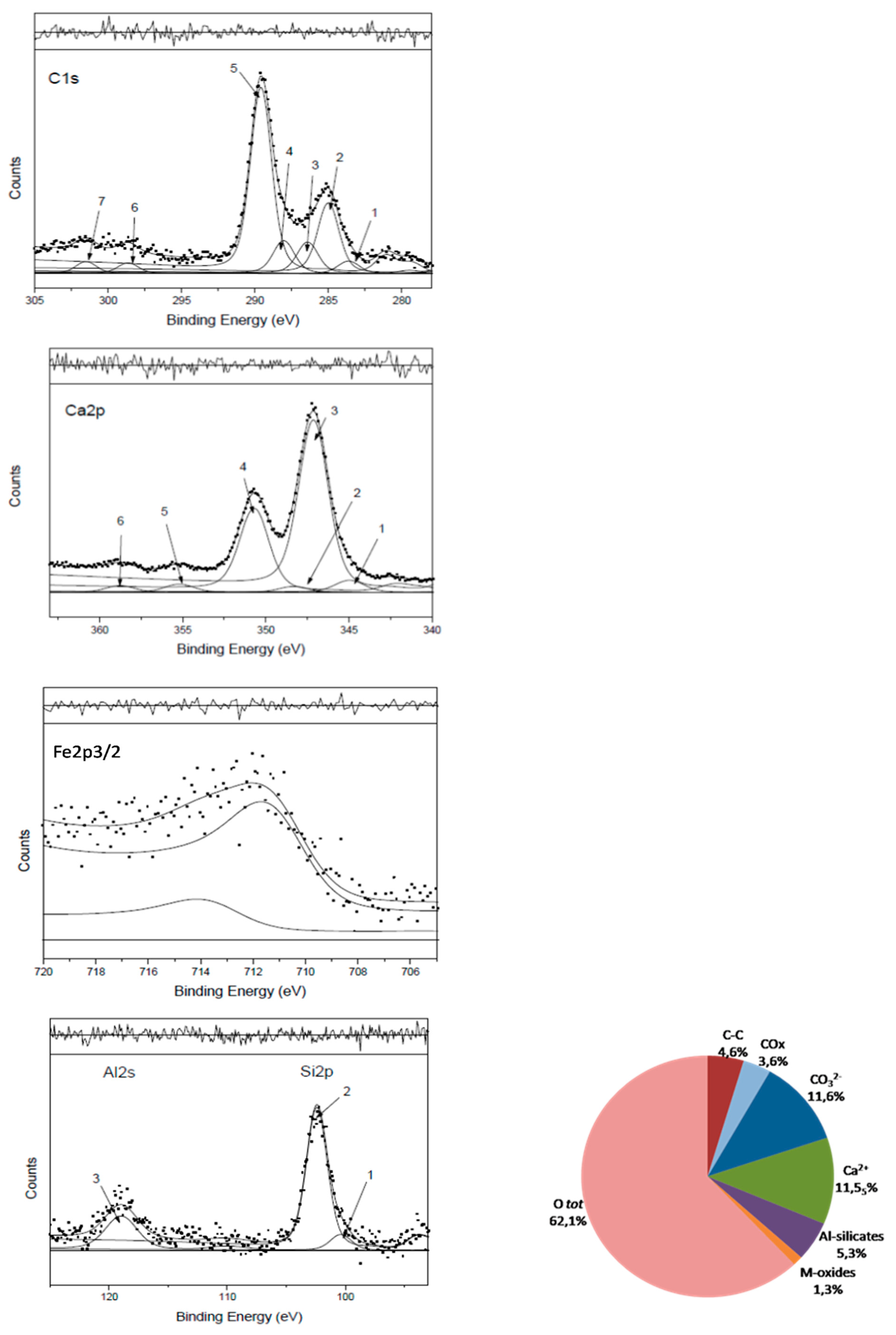
Figure 4 and the correspondent curve-fitting results listed in
Table 1 for deriving the percentage of chemical groups all detailed in the Legend and shown in the pie chart at the bottom right of
Figure 4.
The peak assignments reported in
Table 1 were qualitatively validated by combining binding energy (BE) indications retrievable from the XPS database, literature, laboratory curve fitting results of standard compounds, and quantitatively by the cross check of normalized peak areas used to perform partial and total mass balances, considered satisfactory if in the limits of XPS accuracy of +/- 10% [
12,
13]. The groups percentage summarized in the pie chart of
Figure 4 satisfy these premises and indicate the right area ratios for the carbonate components (carbon: calcium: oxygen ≈ 1:1:3) as for reference CaCO
3, while the sum of oxygenated contributions associated to CaO+Fe
2O
3 and Al
2O
3+SiO
2, respectively representing M-oxides and Al-silicates, using the stoichiometric coefficients of each single compound, required the addition of almost 20% of hydration water/hydroxyls, not specifically assigned, to match the total O1s area within the above-mentioned uncertainty in the area ratio.
H2O/hydroxyls
Table 1 Peaks resolved by curve-fitting the detailed regions of Figs.4 (0R sample), identified and quantified as explained in the Figure caption. The chemical groups associated in the pie chart of Fig.4f, are the following:
•Ca2+ and CO3—of the Carbonate strutture, both reported to visualize their ratio (1:1 in reference CaCO3) (Ca2p peaks 2+4+5 and C1s peaks 5+6+7)
•C-C carbide/graphitic and polyciclic carbons (C1s peak 1) + Aliphatic/aromatic (C1s peak 2): IS • COx oxygen functionalized carbons (C1s peaks 3+4) to which CN groups can be added if nitrogen is present in aminic and/or amidic forms
•Mixed oxides*, in this case: CaO (Ca2p peaks 1+3) and Fe2O3 (Fe2p3/2 peak) •Al-silicates: Al2O3 (Al2s peak) + SiO2 (Si2p peak 2)
•The oxygen percentage, arbitrarily resolved in three components (see related text) is reported in the pie chart as O1s total area.
*Mixed oxides in reference calcarenitic rocks normally comprise Ca/Mg/K/Fe oxides not always contemporarily revealed in the surface layers. Other elements if present are reported as ‘extra’ in captions
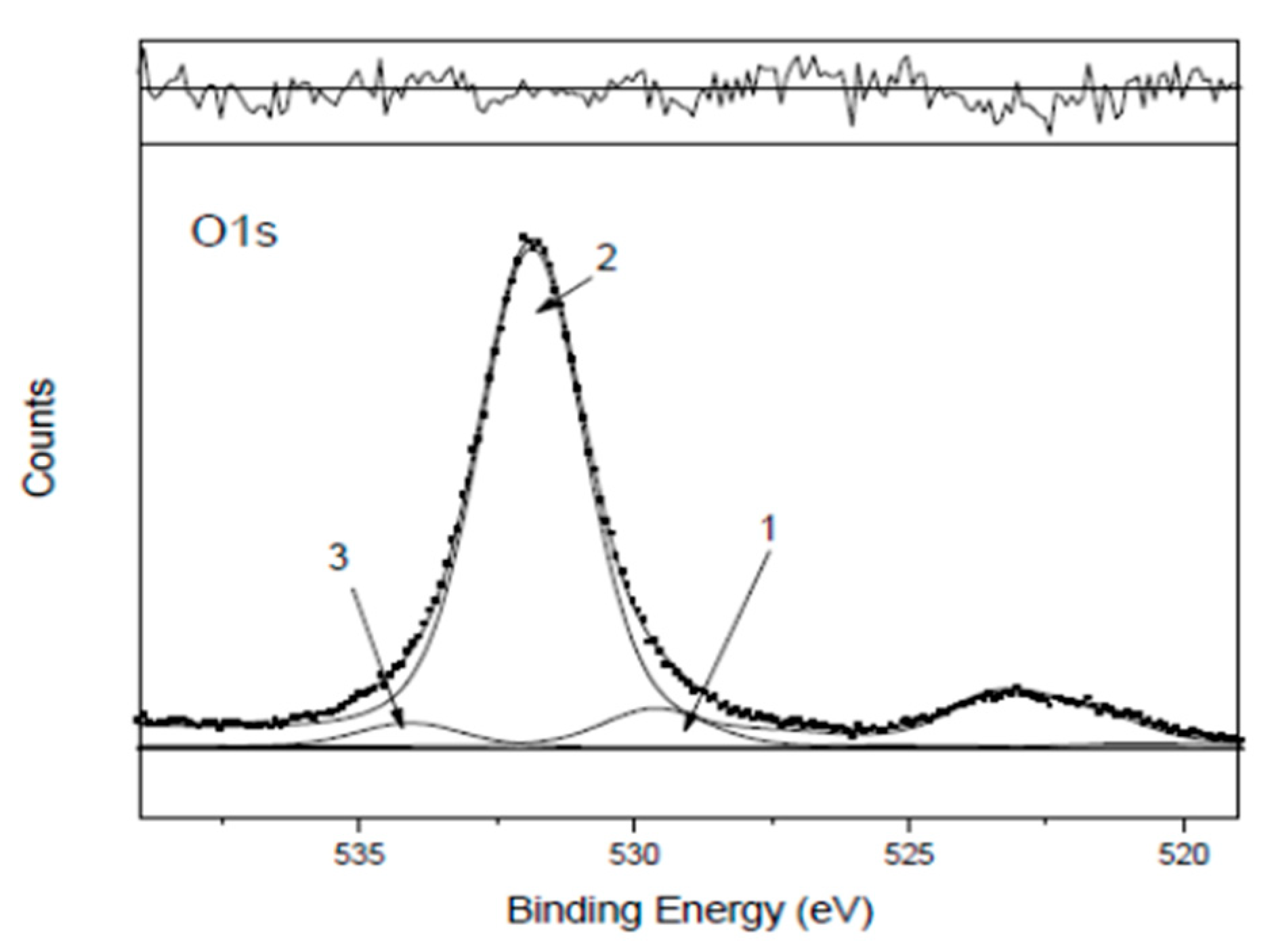
As already found in previous works [7 and references cited therein], it is often difficult when dealing with real samples to resolve the O1s region into the component peaks if many oxygenated groups contribute to it and their chemical states are too close in terms of binding energy, BE, for the given instrumental resolution, using achromatic X-ray sources. In this case, the three curve-fitted oxygen peaks, although belonging to differentiated classes of oxygenated compounds, characterizing the reference calcarenite 0R [
7,
10], actually do not result to be of the right intensity to individually correspond to the oxygenated compounds defined in the assignments and identified in the corresponding detailed regions, listed in
Table 1. Therefore, also in this work, the only way to successfully perform the oxygen ‘mass balance’ is to refer all the oxygenated compounds to the total O1s area, taking into account their stoichiometry.
Initial XPS processing of calcarenite degradation.
The curve fitting procedure adopted for the reference sample (0R) was repeated for all acquired XP- Spectra, following the sampling interval of 3, 6, 9, 12, 21 and 52 months from the North, East, South and West walls of the externally exposed calcarenite cube, as shown in Materials and Methods.
As found by the XPS characterization of sample 0R, the composition of calcarenite surfaces is already of a certain complexity in the starting/unexposed reference and, from the visual comparison of the two wide spectra in Fig. 3, it is expected to evolve over time. Indeed, the coexistence of single stoichiometric compounds identified by curve-fitting the detailed regions of Fig. 4, is certainly a simplification of the interconnected chemical bonds representing the composition of "real" surfaces. However, the grouping classification, mainly consisting of mixed compounds, listed in the legend of
Table 1 and reported in the pie chart, proves very useful to verify the counterions balance so as to estimate the neutrality of the surfaces composition. In this way, also a good assessment of their compositional variation over time can be performed, which is necessary to extrapolate the trend of calcarenite degradation from comparative XPS characterizations.
The first processing of the XPS temporal monitoring is shown in the supplementary figures S1a-d represented by equidistant analyses, three months-spaced sampling, of the cube walls, cardinally oriented, in the first nine months of outside exposure.
The XPS results related to that period were previously commented together with others instrumental techniques [
8,
9] also taking into account online data provided by the control unit of the regional agency ARPAB on the local weather and emissions from the nearby incinerator ‘Fenice’ on the winds direction and strength (retrievable by the wind rose graphic) and combined microscopy and biological analysis. In these supplementary figures, the XPS results were re-proposed using the grouped components, equally shown in the pie-chart of Fig.4 for the reference 0R samples, as the appropriate ‘indicators’of the calcarenite decay versus time.
As evident in S1a-d supplements, the new building in the nine months of exposure to the outside since its installation (July 2009) show compositional variation of its wall surfaces, the changes of the main chemical groups in their relative intensity certainly linked to the seasonal characteristics of the year (temperature, rainfall, winds etc.):
-in the first 3 months, traces of other elements are seen deposited on the West, South and East walls, consisting of sulphur, phosphorus and chlorine anions, all mass balanced by sodium counter cation, most likely coming from the incinerator fumes and transported by the winds blowing from the North.
-after 3 months it can be noted that the contribution of carbons, C-C, COx/CN, other than carbonates, has ‘on average’ increased compared to the reference. This can be attributed both to the deposition of carbonaceous particles possibly contributed by the combustion process of the ‘Fenice’ incinerator (PTS) and/or to the presence of micro-organisms colonies, already localized and developed in the subsurface/inner areas, as attested by slight chromatic changes of surfaces and coloured stains along some parts, porous and with interstices, of the cube walls and corroborated by SEM images (see next sections)
-the excess of carbon carbonate compared to calcium carbonate, clearly visible in all S1a-d graphs, could be attributed to the formation of calcium bicarbonate Ca(HCO
3)
2, the unbalance attesting the first form of degradation of calcite, the predominant component of calcarenite. In fact, when the calcarenite comes into contact with humidity and acid rainwater, the calcite hydrates and dissolves, forming carbonate complexes such as bicarbonate ions [
19,
20]. Depending on outdoor conditions the main carbonate structure of calcarenite can be differently hydrated thus leading to different H
2CO
3--/CO3
-- mixture differently balanced by calcium while ‘mixed oxides’ may include flowing ions sharing the hydroxyls of the phyllosilicate structure and the oxygens of funcionalized carbons and other combinations to be verified in the presence of oxalic acid and other metabolites similarly due to biological activities [
6,
7,
21,
22]
- hydration and de-hydration process could be followed by the oscillation percentage of oxygen for each wall during the nine months. The oscillations could be the result of meteorological conditions favoring/disfavoring the synergistic actions of abiotic and biotic pollutants locally interacting with the calcarenite surfaces. The calcarenite cube, from the moment of its installation, has certainly experienced hot but also rainy periods, as reported on the portal of the Lavello weather station (
https://www.3bmeteo.com/meteo/lavello/storico). The high temperature and the abundant rainfall represent the fundamental conditions for condensation phenomena to occur, which constitute an extremely efficient transport mechanism for atmospheric pollutants. In fact, while the rain immediately removes the products resulting from the attack of the original material, the condensation water, not normally being sufficient to flow on the surface, evaporates leaving behind reaction products that can give rise to further destructive processes, eventually with disintegration of the surface area itself [
22,
23]
Obviously XPS observations of the cubic calcarenite after only 9 months of outdoor exposition do not allow to identify clearly the prevalent factors and/or their combined actions responsible of its decay. It was already possible, however, to notice that while the first quarterly sampling showed some variation in the composition of the cube walls due to the wind blowing from the north, carrying additional elements (extra in the legends) mainly in EWS directions, later on the XPS differentiation was no longer so evident and gradually the effects of the environmental interactions on the four walls could be considered averagely comparable.
It was therefore decided to graphically represent the degradation trend of cubic calcarenite, expressed by the percentage intensity versus time of the component groups, averaged over the four walls, with associated standard deviation. In the supplementary "average" plots, S2 figures, two options are presented: in S2a the percentage of oxygen is retained to control also the variation of the average degree of hydration (i.e. the hydration de-hydration oscillations observed for the single walls) while in S2b the oxygen is omitted to enlarge the scale and therefore better estimate the behaviour of the component groups, the total area of the O1s region being implicitly considered as the stoichiometric sum of all oxygenated compounds. The comparison of the two S2 plots shows that indeed the trend in the nine-month interval is mainly characterized by the variations of the component groups, to be thus confirmed as the main indicators of degradation, therefore, the S2b option was chosen to complete the plot of calcarenite decay over time to also simplify the processing and visualization of the larger data set, without considering the total oxygen percentage as an additional variable.
The subsequent and final phases of the project specifically concerning the temporal monitoring of calcarenite by XPS were then considered in the following order.
●XPS analysis of the old farm: sampling of ‘selected zones’ differently degraded and coloured
As already specified in Materials and methods section, the samples were taken on the North-East walls of the old building following the sampling sequences reported in Figs.2 and selecting the four specific zones, different in their appearance, therein indicated.
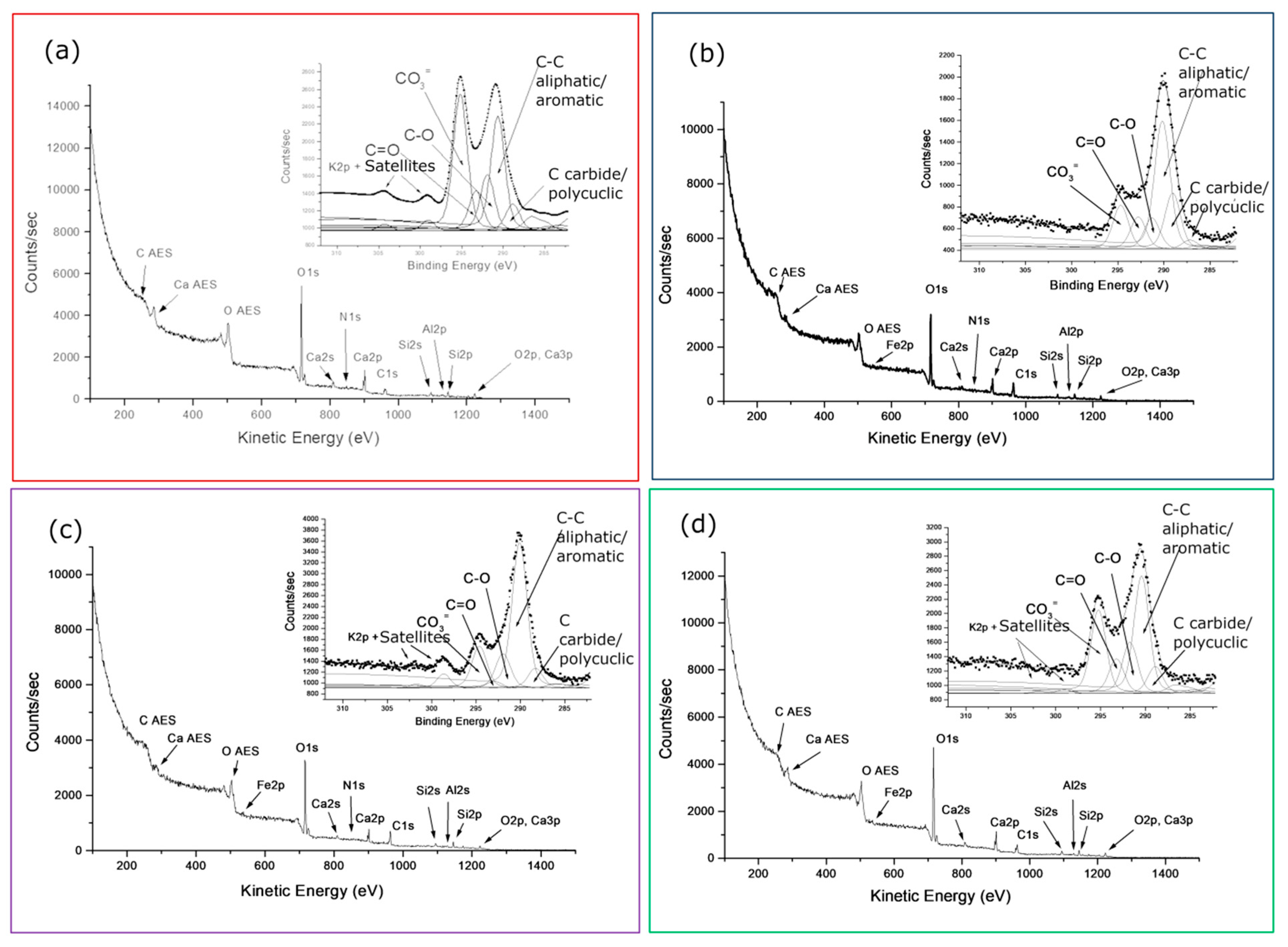
The wide spectra of the four coloured samples with associated peaks labelling and the curve-fitted C1s regions inserted are compared in
Figures 5. The overall percentage composition derived by curve-fitting, without the total oxygen percentage, reported in
Table 2 using the same grouped components retrieved for the reference 0R sample, being no extra elements detected by XPS.
Table 2 shows the curve-fitting results of the old building samples 1v-4v, in percentage form according to the S2b option, and those correspondent to sample 0R of the new building added for comparison. We can now better judge the differences of the two references, 0R and a-1v, for the new and old building, respectively, qualitatively predicted by comparing their wide spectra in Figs.3. We can also extend the comparison to the remaining samples 2v-4v, all together representative of the old farm building and derive some anticipations on the evolution of the surfaces composition due to their very different aging time.
Observing the four spectra in Figs. 5a-d with the related grouped components listed in
Table 2, the surface increase of the carbonaceous components, including nitrogen- functionalized carbons, the inverse intensity of the carbonate and silicate components, compared to the reference sample of the new building, seem to be the prevalent indications of long- weathered calcarenite, common to samples 1v-4v of the old building,
independently of their very different appearance.
Buildings in calcarenite are continuously exposed to various environmental interactions generated by wind, rain, sun or humidity with prevailing effects due to the specific location [
23,
24]. Among these, organic contaminants deposited on their surfaces are suitable to create ideal conditions for biological colonization. A variety of microorganisms can coexist in structured biofilms strongly adherent/anchored to these calcareous surfaces with molecular mechanisms involving the Ca
2+ ions of the carbonate substrate whose weathering modifications contribute mainly to the observed calcarenite degradation, while the (initially minor) silicate constituents are less affected by biotic impact and chemical dissolution [
25,
26,
27].
Taking into account the above considerations, the reason for the colorations that visually differentiate the samples in Figs. 5 could then be traced back to biological processes occurring at greater depths than the outer surfaces, probably originally colonized, therefore visible to direct observation and detectable by the complementary techniques in use during the research project [
8,
9].
All these aspects that emerged from the monitoring of the old farmhouse in direct comparison with the 0R sample (from unexposed cubic calcarenite) will be reconsidered with the completion of the temporal monitoring of the new building, illustrated in the next paragraph. The final phase was dedicated to the processing of the entire data set obtained in the hope of deriving useful information on the degradation processes of calcarenitic stones: their onset and their temporal evolution and finally their possible stabilization/convergence over time.
●Surface monitoring of the new calcaranite block up to 52 months and comparison with the old construction: graphical trend of averaged XPS data and PCA analysis
The data matrix in
Table 3, in the percentage format of
Table 2, represents the entire collection of XPS results, obtained through periodic monitoring of the four walls of the new cubic construction. It begins with the unexposed 0R adding the four walls results up to 52 months of outdoor exposition and, according to the project objective, the results of the four samples (1v-4v) from the old farmhouse for the ultimate comparison. Moreover, for each sample/sampling time the binding energies (BEs) of Ca
2+ and CO
3— carbonate components, the main structural costituent of calcarenite, are included as additional variables for PCA analysis to verify their variation in relation to weathering modifications of the carbonate structure.
•At% composition resulting from the curve-fitted spectra of the four walls of the cubic building at the reported sampling time, with the exception of the sampling after six months performed only on two walls, N/E, and the four NE samplings of the old building (1v-4v). •The last two columns with the (corrected) BEs for Ca2+ and CO3= peaks of the carbonates at each sampling were included as additional variables for PCA analysis to verify BE chemical shifts Vs reference calcarenite 0R as a function of time.
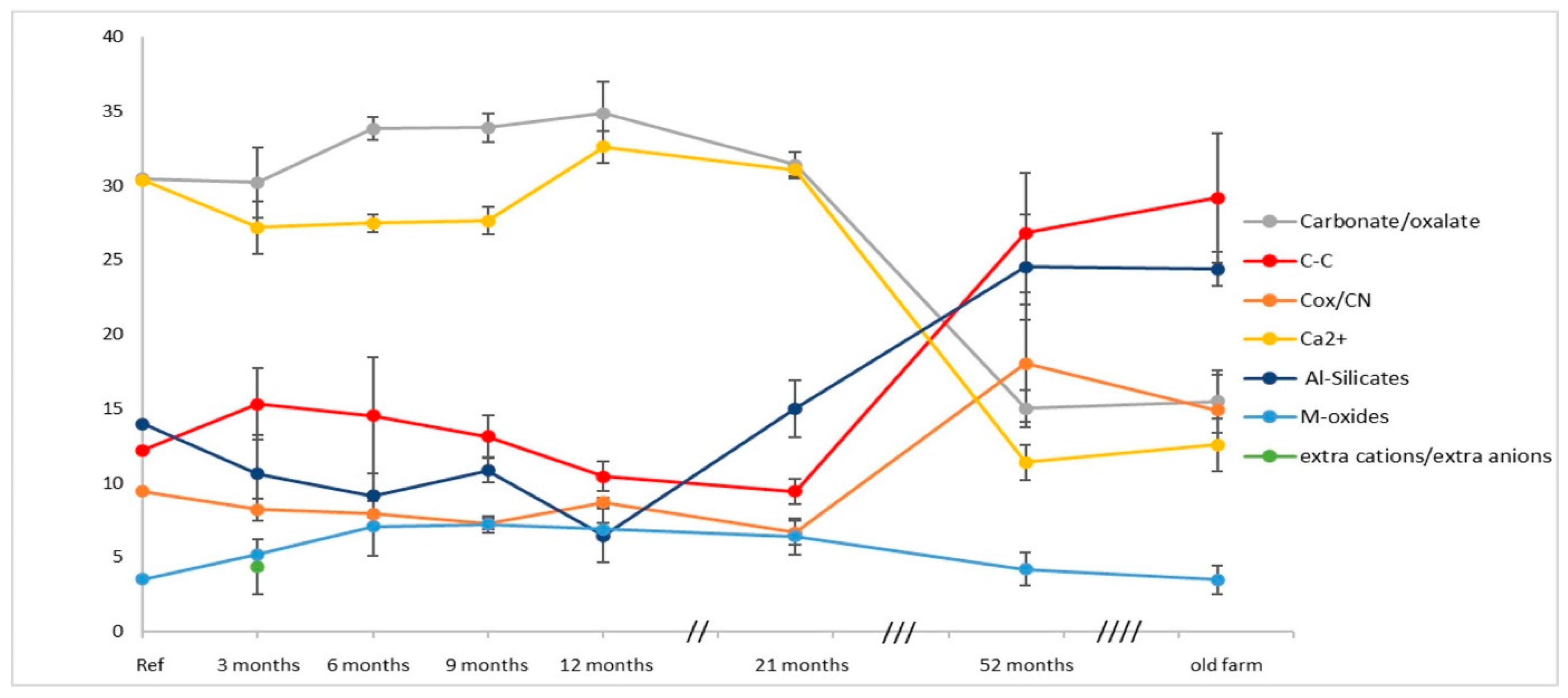
Figure 6 shows the completed S2b plot using, of all the curve-fitted data reported in
Table 3, the average of the same chemical groups (XPS indicators of degradation, listed in the legend) present on the four walls of the new building at each sampling time and, similarly, the average of the four samples collected from the walls of the old building (old farm). As anticipated, the decay trend based on the S2b option seems more justified with increasing time, in particular for the new building given its small size. Therefore, the contributions averaged on all oriented faces are more appropriate and the balance of their hydration/dehydration percentage observed in the nine-months interval, better justifies the omission of the total oxygen area as a ‘decay indicator’ being implicitly accounted for by the total contribution of oxygenated compounds, verifiable a priori during the curve-fitting procedure.
The sampling variability across the four walls, shown by the "error bar" of each decay indicator in
Figure 6, is represented by the standard deviation associated with the mean value of the four collected samples. Instead, as said and shown in the graphs S1 for single samplings, the uncertainty associated with the reference sample 0R is to be considered equal to +/-10% [
12,
13] therefore doubled for the case of only two samples collected at 6 months.
The compositional changes already observed after three months are progressing, maintaining the trend over the course of the first year of exposure, with the variability ranges reflected in the magnitude of the standard deviations associated with the means. The percentage variations are clearly linked to the different contribution of the individual walls, evident in graphs S1a-d classified by cardinal orientation. In Fig. 6, the influence of meteorological fluctuations and washing away of the wall surfaces by precipitation with consequent removal of the crystallized salts present on the surfaces ('extra cations/anions' in the graphs) and other reasonable differences in composition of the sampling zones, are all included in the variability associated with the 'averaged' indicators of samples collected from different walls/areas of the cubic building.
After the first year, sampling was no longer scheduled every three months, however, at the end of the year, at the 12 months sampling, the imbalance of the carbonate components tended to the reduction then reached with the sampling at 21 months. Subsequently, the percentage inversion of carbonates and Al-silicates was already predictable in the graphic trend, fully achieved then at the 52 months sampling, clearly represented by a progressive decrease in calcium and carbonate carbon and by a strong increase in silicates and aliphatic/aromatic and functionalized carbons.
Interestingly, in the absence of intermediate sampling, it can be inferred that exposure time prolonged over two years leads at some stage to a sort of stabilization of the degraded calcarenite, at least regarding the similarity of the surface composition for both buildings, as evident in the last part of the graph in Fig.6. Indeed, the ‘same’ component groups, of nearly the ‘same’ percentage distribution, within the indicated errors bar, characterize the averaged samples collected from the new building (52months sampling) and old (1v-4v sampling) farmhouse.
The trend shown in Fig.6 highlights decay phenomena that can be justified by the continuous dissociation and dissolution of the carbonate matrix and by the concomitant increase of aliphatic/aromatic carbons, due to the deposition of incombustible carbon particles, and of functionalized carbons probably accentuated by the presence of lichens and algae as shown in the SEM (scanning electron microscopy) images acquired on the samples collected after 12 months of exposure from the cube walls [
8,
9]. Some images with included EDS microanalysis are reproduced in Supplementary Figures S3 together with the list of identified bacteria of the genus Bacillus, available from the (unpublished) PhD thesis. The presence of lichens in the subsurface zones supports the hypothesis according to which the calcarenite rocks, in addition to the evident degradation of their carbonate matrix due to atmospheric agents and acidity of rainwater, undergoes an increase in porosity augmented by freeze-thaw cycles [
22,
23]. As experimentally verified with timed tests on the capillarity of calcarenite stones [8,9 and PhD report], the expansion caused by the internal pressure exerted by frozen water promotes bio-colonization. The colonizing microorganisms tending to settle inside the pores and interstices of the stone material finally induce the reduction of the interstitial porosity, as shown in Figures S3.
The evolving stages of colonization were testified by the biological analysis anticipated in the Introduction, carried out on samples taken from the walls of the cubic building at the end of two years exposure and, for comparison, also from the walls of the farmhouse. The same microorganisms were found, using the analytical techniques mentioned above for both buildings, and similarly distributed on the surfaces (bacteria belonging to the Bacillus family, listed in S3) and beneath surfaces (lichens/fungi listed in reference 8,9) on inner faces more protected from environmental threats together with mono- and di-hydrated calcium oxalates detected by XRD (PhD report), derived from the dissolution of carbonates by oxalic acid, one of the biometabolic products.
Thus the degradation trend of the new building walls outlined in Fig. 6, in practice, fully accounts for the bioactivities of the ‘same’ colonizers in determining the perfect convergence towards the walls state of the old building, left exposed to the outside in similar conditions for over a century. The average XPS analyses of the four samples treated in Figs. 5 and
Table 2, so different in appearance as widely specified, align perfectly with those of the new building at the last sampling (after 52 months), within the limit of the total variability due to sampling, to the calculation of curve-fitting, as well as to the orientation of the walls and to the characteristics of the samples themselves, the latter expected with greater specificity given the dimensions of the old building and therefore of sampling areas with probable different exposure even along the same wall.
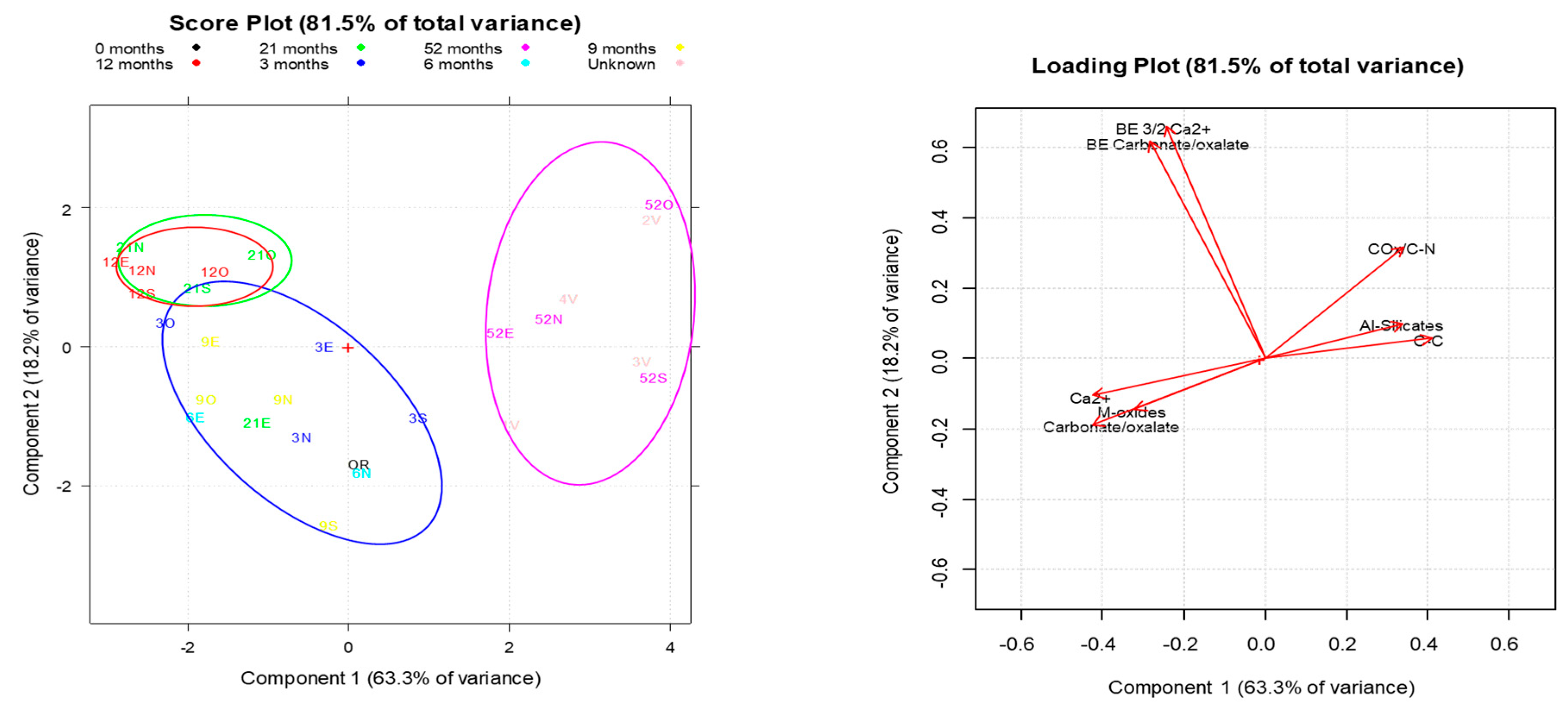
Principal Component Analysis (PCA) was finally applied to the entire XPS dataset of
Table 3, using the CAT software available online [
18] with the aim of evaluating the information content of the variables while reducing their number, eliminating the redundant ones. The Matrix includes 22 surface samples of calcarenite collected at the given time intervals, the blank calcarenite 0R, 4 samples of the old building (1v-4v) and 8 variables, i.e. the percentage of the chemical groups listed aside the XPS graphs of Fig. 6 plus Ca
2+/CO
3= binding energies (BEs) known to be sensitive to the calcium carbonate structural changes [
19,
20]. The graphical output of the CAT software is shown in
Figure 7a, b where the combined display of the two graphs, scores and loading plots, helps the interpretation of the data, hence to rationalize the degradation processes under consideration.
A total variance higher than 80% was explained by PCA, with the two first components PC1 and PC2 able to explain the compositional variation of ‘calcarenite’ under environmental exposure and extract the main indicators of degradation.
Three main clusters were observed in the score plot, distributed along PC1 (63.3% of the explained variance). Samples collected from the new building after 52 months of exposure and from the old building,1v-4v, were located on the same side of the plot thus suggesting the same Surface composition of long weathered samples. On the opposite side of the score plot, samples collected within 3 and 21 months were present. In detail, two main clusters were observed in this section, along PC2 (18.2% of the explained variance): the first, widely distributed, includes 0R and the four-wall samples collected in the first nine-month time interval; the second, actually consisting of two overlapping clusters each made of samples collected from the cubic walls, after 12 (red cluster) and 21 (green cluster) months, very closely distributed.
From the Loading Plot view, the variables mostly contributing to the first principal component PC1 were detected, i.e. (%Al-silicates, %C-C, %, COx/CN) and (%Ca2+, %CO32- /C2O42-, %M-oxides) which were inferred to be negatively correlated with each other. Thus, by increasing the outdoor exposure, an increased content of aliphatic/aromatic and functionalized Carbons, of Al and Si (grouped as Al-silicates in Fig. 6) could be noticed, while decreasing the content of carbonates and M(ixed)-oxides.
The PCA plots of Figs.7 overall agree with the XPS plot of Fig.6 and support the interpretation given on the decay trend of calcarenite with time. Moreover, another agreement concerns the BEs behavior of Ca
2+ and CO
3=, the carbonate constituents, considered as additional variables for PCA analysis, mostly contributing to the PC2 component. Viewing the score and loading plots together, it can be seen that their BEs increase with outdoor exposure in the first year. In fact, even considering the variability range that goes beyond the energy step (+/-0.1eV) only reported in
Table 3, the BEs therein listed record the concomitant increase for the two constituents, up to their maximum values, 348.0 eV(Ca
2+) and 290.2 eV(CO
3=), right in the time interval of the two clusters and the subsequent decrease, although not always to the same extent, for the samples collected from the cubic walls aged 52 months and old farmhouse.
The excursions in the binding energies of the carbonate constituents and in their ratio are clearly conditioned by the temporal impact of the abiotic and biotic agents on the calcarenitic walls, monitored in this work by their XPS characterization, therefore, they could be regarded as an integrative part of the set of degradation trend indicators reported in Fig.6, as hereafter summarized.
To start with, the weathering susceptibility of carbonates to dissolve and form Ca(HCO3)2 is mainly responsible of the recurrent Ca2+ < CO3= ratio, most visible in our graphical percentages up to nine months of outdoor exposure. In subitaneous succession, the reported attachment of autotrophe microorganisms takes place and the calcarenite colonization proceeds with the cooperation of different heterotrophe lithotypes mutually interacting for the construction of a protective biofilm, as indirectly testified by the increase of carbonaceous components in the Surface layers and directly by SEM, XRD and biological analysis, already mentioned.
Among the metabolic routes of the biocolonizing comunities are those responsible of dissolution by Ca
2+ complexation and those of reprecipitation, the reprecipitate CaCO
3 clearly structural different from the pristine calcite thus presumably of different BEs [
21,
25,
26,
27,
28]. From the graphical trend of
Figure 6 and data matrix of Table3 the time required for the adhering biofilm to consolidate onto calcarenite and then structurally evolve for the living of cohabitant microrganisms apparently ranges around two years or less, judging from the BE values of the samples collected at twelve and twenty-one months, further supported by the better equivalence of Ca
2+:CO
3= percentage of the last, likely due to the reprecipitation of calcium carbonate, contributing in the outermost layers.
Considering all the points paid attention to so far, the main features that characterize long-exposure calcarenitic surfaces and that most capture attention in
Figure 6 are the following:
● Carbonaceous C-C added to functionalized carbons (COx/CN) are the prevalent components of the surface layers closely followed by Al-silicate groups, in second place even with the inclusion of M-oxides, placed at the lowest percentage, if considered as ‘intricate’ parts of the silicate framework [
29].
● Contrary to the bulk composition of calcarenite and differently from the surface composition 0R, in the highly degraded surfaces the carbonates are the minor components confirming the negligible degradation of the silicates which become the major components, most probably ensuring with their solidity the surface adhesion required for the formation of structured biofilm (with trapped pollutants) for the protection of the living microorganisms [
25,
26,
27].
● To the non-equivalent intensity of carbonate constituents, visible again on long-exposed surfaces, may contribute carboxylic anions, belonging to extracellular polymeric substances (EPS) produced by microorganisms and binding Ca
2+ [
21,
28]. Unfortunately, the intrinsic resolution of conventional XPS often prevents to properly resolve detailed spectra into energetically close components, as reported for the curve-fitted O1s region. Even in the case of curve-fitted Ca2p and C1s regions, for the peaks generally assigned to ‘calcium carbonate’ constituents, the co-presence of unresolved components can be reflected/estimated only by the Ca
2+/CO
3= ratio and the chemical shift (∆BE) of the peaks maximum, both dependent on their relative intensity.
As a summary dissertation of this work, it can however be stated that, even with all the mentioned approximations, the XPS results provided by the curve-fitting procedure [
15] (home-made Googly software), confirmed by PCA using the CAT software available online [
18], seem consistently significant. The evident compositional similarity, achieved in a relatively short time, of the new weathered building with the old farmhouse clearly indicates that the degradation of the calcarenitic surfaces evolves towards a sort of stabilization, creating favorable conditions for the sequential settlement of the same microorganisms community, as revealed by biological analysis.
If the right protection mechanisms (EPS, pigments, internal shelters) are then activated for the survival of the biocolonization against external attacks, all the activities of the cohabiting microorganisms necessary for their life and for the consolidation of the surface layers, in the form of a protective structured biofilm, reach a dynamic equilibrium that can persist for an indefinite time.
The importance of biofilms that develop on external monuments and their dual role [
30], protective on external surfaces and erosive when they protrude internally, is still a matter of debate, as it was a decade ago, when the experiments reported in this work were conducted. Attempts have been made to identify antagonistic bacterial/fungal microorganisms and to extract their metabolic products (toxins) to be used for the removal of biofilms by natural cleaning (bioremediation) in place of traditional chemical products [7, 21 and SCN publications cited therein].
Bioremediation has proved effective in some cases but not in others when the removal of the biofilm induced a greater reactivity of the renovated surfaces with impediments to consolidation actions and therefore a timely regression to the structural degradation of the monumental walls. The numerous case studies have shown the importance of appropriate diagnostics to evaluate case by case the relative importance between the protection and the destruction of calcarenitic stones by microorganisms, in order to plan the most appropriate interventions.
We believe the results here reported represent a useful piece of information to be of support in the nowadays research context, recently reviewed for the use of multitechniques and multidisciplinary approaches with the support of new technnological advancements [
30] under the UNI 111882:2006 Standard Protocol and ICOMOS’s guideline recommendations.