Submitted:
06 May 2025
Posted:
07 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
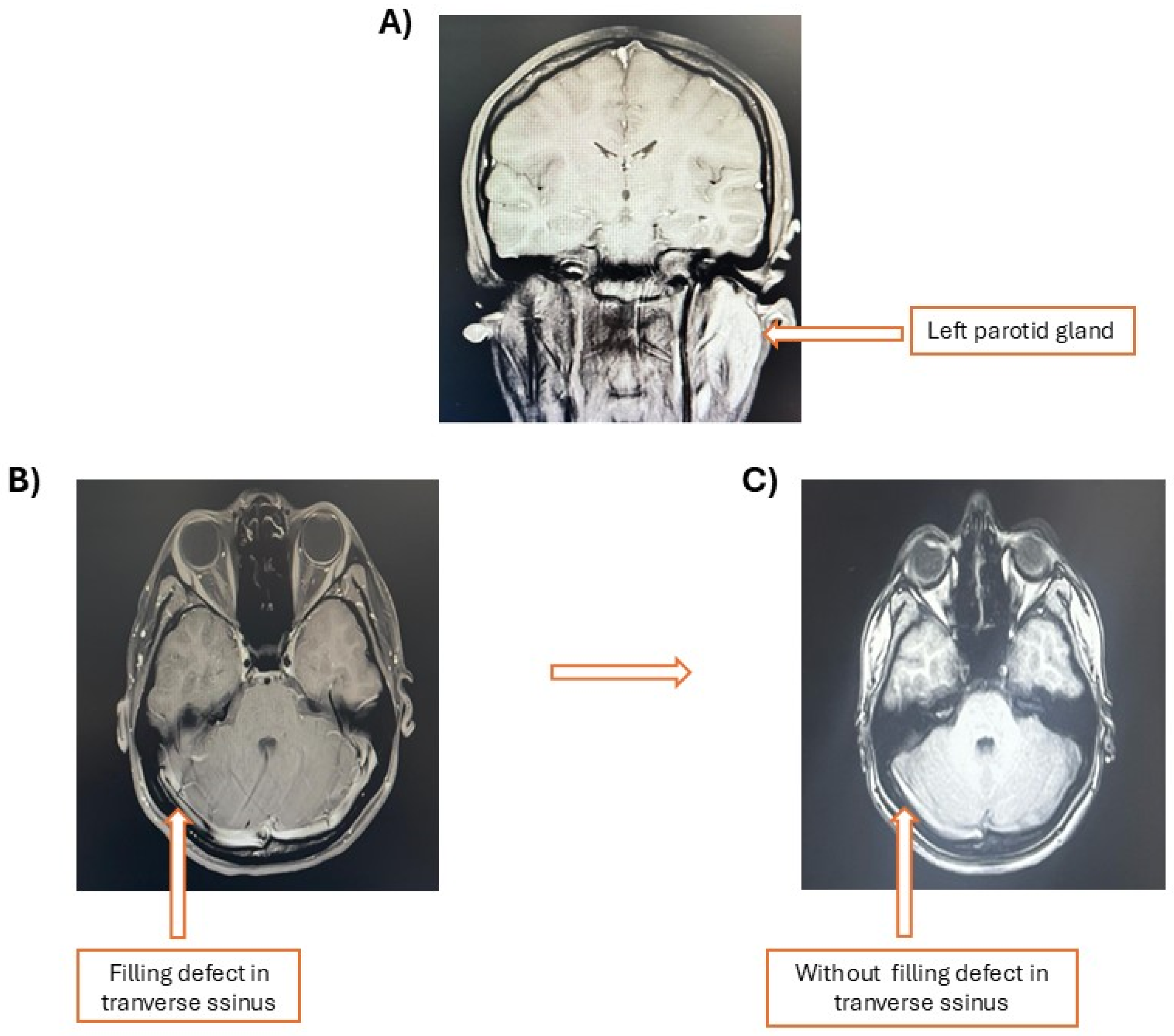
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MTHFR | methylenetetrahydrofolate reductases |
| PS | protein S |
| PC | protein C |
| FVL | Factor V Leiden |
| C4BP | complement protein 4 |
| APC | activated PC |
| TFPI | tissue factor pathway inhibitor |
| Al222Val | alanine to valine |
| BDNF | brain derived neurotrophic factor |
| CSF | cerebrospinal fluid |
| EEG | electroencephalogram |
| MRI | magnetic resonance imaging |
| ATP | Adenosin triphosphate |
References
- Méndez-López, M.; Salazar-Sánchez, L.; Porras, P. J. Trombofilia Primaria: Mejorando el Diagnóstico Basado en Evidencia. Rev. Costarric. cardiol. 2013, 15(2), 25–30. [Google Scholar]
- Castro Quismondo, N.; Rodríguez Rodríguez, M.; Zafra Torres, D.; Martínez-López, J. Trombofilia y trombosis. Medicine. 2020, 13(22), 1259–66. [Google Scholar] [CrossRef]
- Gales, A.; Masingue, M.; Millecamps, S.; Giraudier, S.; Grosliere, L.; Adam, C.; et al. Adolescence/adult onset MTHFR deficiency may manifest as isolated and treatable distinct neuro-psychiatric syndromes. Orphanet. J. Rare. Dis. 2018, 13(29). [Google Scholar] [CrossRef] [PubMed]
- Majumder, R.; Nguyen, T. Protein S: function, regulation, and clinical perspectives. Curr. Opin. Hematol. 2021, 28(5), 339–44. [Google Scholar] [CrossRef] [PubMed]
- Herrera, P.M.; Vélez Van Meerbeke, A.; Bonnot, O. Trastornos psiquiátricos secundarios a enfermedades neurometabólicas. Rev. Colomb. Psiquiatr. 2018, 47(4), 244–51. [Google Scholar] [CrossRef] [PubMed]
- El-Hadidy, M.A.; Abdeen, H.M.; Abd El-Aziz, S.M.; Al-Harrass, M. MTHFR Gene Polymorphism and Age of Onset of Schizophrenia and Bipolar Disorder. Biomed. Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, A.; Mcclellan, J. Practitioner Review: Psychosis in children and adolescents. J. Child. Psychol. Psychiatry. 2023, 64, 980–8. [Google Scholar] [CrossRef] [PubMed]
- Hoirisch-Clapauch, S.; Nardi, A.E.; Gris, J.C.; Brenner, B. Coagulation and Mental Disorders. Rambam. Maimonides. Med. J. 2014, 5(4), e0036. [Google Scholar] [CrossRef] [PubMed]
- Pujol, N.; Mané, A.; Bergé, D.; Mezquida, G.; Amoretti, S.; Pérez, L.; et al. Influence of BDNF and MTHFR polymorphisms on hippocampal volume in first-episode psychosis. Schizophr. Res. 2020, 223, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.P.; Cullen, K.R.; Deyoung, C.G. Klimes-Dougan B. The genetics of early-onset bipolar disorder: A systematic review. J. Affect. Disord. 2015, 184, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Qian, Z.Z.; Gong, F.F.; Lu, S.S.; Feng, F.; Wu, Y.L.; et al. Methylenetetrahydrofolate reductase (MTHFR) polymorphism susceptibility to schizophrenia and bipolar disorder: an updated meta-analysis. J. Neural. Transm. 2015, 122(2), 307–20. [Google Scholar] [CrossRef] [PubMed]
- Peerbooms, O.L.; van Os, J.; Drukker, M.; Kenis, G.; Hoogveld, L.; Group MiP.; et al. Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: evidence for a common genetic vulnerability? Brain. Behav. Immun. 2011, 25(8), 1530–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Lee, S.Y.; Chen, S.L.; Chang, Y.H.; Chen, P.S.; Huang, S.Y.; et al. A potential interaction between COMT and MTHFR genetic variants in Han Chinese patients with bipolar II disorder. Sci. Rep. 2015, 5(1), 8813. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zheng, J.L.; Sun, M.L.; Lai, H.Y.; Wang, B.J.; Yao, J.; et al. Association between MTHFR (677C>T and 1298A>C) polymorphisms and psychiatric disorder: A meta-analysis. PLoS One. 2022, 17(7), e0271170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Yang, L.P.; Gai, C.; Cheng, C.C.; Guo, Z.Y.; Sun, H.M.; et al. Association between variants of MTHFR genes and psychiatric disorders: A meta-analysis. Front. Psychiatry. 2022, 13, 976428. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Li, Y.; Zhang, Z.; Sun, Z.; He, Y.; Li, R. Methylenetetrahydrofolate reductase and psychiatric diseases. Translational. Psychiatry. 2018, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naeem, A.; Fritts, A.; Cummins, M.; Kayes, C.; Fang, W. Discovery of Methylenetetrahydrofolate Reductase (MTHFR) Deficiency in Individuals With Common Psychiatric Comorbidities: A Retrospective Case Review. Cureus. 2024, 16(4), e58122. [Google Scholar] [CrossRef] [PubMed]
| Study | Outcome | Reference Value |
|---|---|---|
| Toxo IgG | < 5 UI/mL | Non-reactive |
| Toxo IgM | 0.02 l/mL | Non-reactive |
| Rubella IgM | 0.16 IC | Non-reactive |
| Cytomegalovirus IgG | 2.42 IC | Reactive >1.1 |
| Cytomegalovirus IgM | 0.2 IC | Non-reactive |
| Herpes I and II IgG | 0.63 IC | Non-reactive |
| Study | Outcome | Reference Value |
|---|---|---|
| Factor II mutation 20210 genotype | Not detected | Not detected |
| Mutation factor V (Leiden mutation) | Not detected | Not detected |
| MTHFR variant P.ALA22VAL | Detected | Not detected |
| C3 complement | 119.620 | 90-180 |
| C4 complement | 26.835 | 10-40 |
| ANTI-SSB Antibodies (LA) | 3.3 | <20 |
| Anti-NATIVE DNA antibodies (IFI) | Negative | Negative |
| Antineutrophil cytoplasmic antibodies (ANCA P) | Negative | Negative |
| Perinuclear neutrophil antibodies | Negative | Negative |
| Myeloperoxidase | Negative | Negative |
| Anti SSA antibodies (RO) | 4.9 | < 20 |
| Anti-beta 2 glycoprotein IGG antibodies | 6.4 | < 20 |
| Anti-beta 2 glycoprotein IGM antibodies | 2.1 | < 20 |
| Lupus anticoagulant | 1.14 | < 1.16 |
| Anticardiolipin IGG antibodies | 4.0 | < 20 |
| Anticardiolipin antibodies IGM | 2.1 | < 20 |
| Coagulation protein C | 77.0 | 65-130 |
| Coagulation protein S | 35.0 | 54.0-103.0 |
| Coagulation factor XII | 65.9 | 36.0-159.0 |
| Prothrombin time | 11.8 sec | 11-13 sec |
| International normalized ratio (INR) | 1 | 0.8-1.2 |
| Activated partial thromboplastin time | 23.2 sec | 25-45 sec |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





