Submitted:
06 May 2025
Posted:
08 May 2025
You are already at the latest version
Abstract
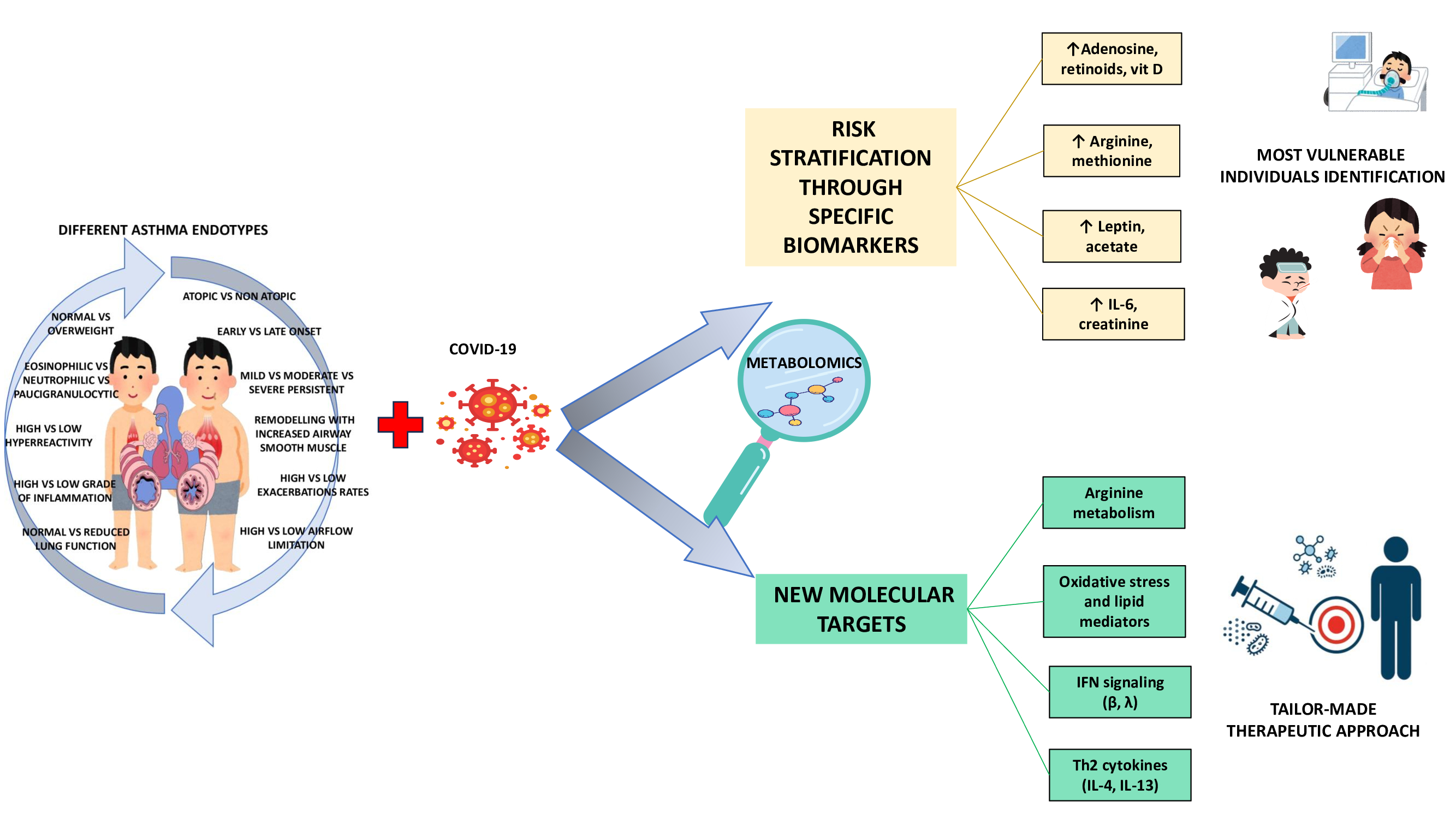
Keywords:
1. Introduction
2. Pediatric Asthma Endotypes
3. Metabolomics Endotyping of Asthmatic Children
4. Pediatric SARS-CoV-2
5. Pediatric Asthma and SARS-CoV2
6. Shared Inflammatory and Metabolic Networks Between Asthma and Pediatric SARS-CoV-2
7. From Asthma Endotypes to SARS-CoV-2 Risk Stratification: Integrative Analysis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Jiehao, C.; Jin, X.; Daojiong, L.; Zhi, Y.; Lei, X.; Zhenghai, Q.; Yuehua, Z.; Hua, Z.; Ran, J.; Pengcheng, L.; et al. A Case Series of Children With 2019 Novel Coronavirus Infection: Clinical and Epidemiological Features. Clin. Infect. Dis. 2020, 71, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Busse, WW.; Bacharier, L.B.; Kattan, M.; O’Connor, G.T.; Wood, R.A.; Visness, C.M.; Durham, S.R.; Larson, D.; Esnault, S.; et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J. Allergy Clin. Immunol. 2020, 146, 203–206e3. [Google Scholar] [CrossRef]
- Holley, AB. Medscape: Does asthma lead to worse COVID-19 outcomes? [Updated 2020]. [Accessed , 2021]. https://www.medscape. 4 July.
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Zachariah, P.; Johnson, C.L.; Halabi, K.C.; et al. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr. 2020, 174. [Google Scholar] [CrossRef]
- Willis, Z.I.; Oliveira, C.R.; Abzug, M.J.; Anosike, B.I.; Ardura, M.I.; Bio, L.L.; Boguniewicz, J.; Chiotos, K.; Downes, K.; Grapentine, S.P.; et al. Guidance for prevention and management of COVID-19 in children and adolescents: A consensus statement from the Pediatric Infectious Diseases Society Pediatric COVID-19 Therapies Taskforce. J. Pediatr. Infect. Dis. Soc. 2024, 13, 159–185. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Mathioudakis, A.G.; Custovic, A.; Deschildre, A.; Phipatanakul, W.; Wong, G.; Xepapadaki, P.; Abou-Taam, R.; Agache, I.; Castro-Rodriguez, J.A.; et al. Childhood asthma outcomes during the COVID-19 pandemic: Findings from the PeARL multi-national cohort. Allergy. 2021, 76, 1765–1775. [Google Scholar] [CrossRef]
- Beken, B.; Ozturk, G.K.; Aygun, F.D.; et al. Asthma and allergic diseases are not risk factors for hospitalization in children with coronavirus disease 2019. Ann Allergy Asthma Immunol.
- Zheng, X.Y.; Xu, Y.J.; Guan, W.J.; Lin, L.F. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch Virol, 8: 163(4).
- Gaietto, K.; Bergum, N.; Rosser, F.; Snyder, O.; Acevedo-Torres, N.; DiCicco, L.A.; Butler, G.; Rauenswinter, S.; Iagnemma, J.; Wolfson, D.; Han, Y.Y.; Kazmerski, T.M.; Forno, E. Odds of COVID-19-Associated Asthma Exacerbations in Children Higher During Omicron Wave. Pediatr Pulmonol. 3179. [Google Scholar]
- Chan, K.; Luu, T.; Vogrin, S.; Bryant, P.A.; Babl, F.E.; Danchin, M.; South, M. Asthma and Susceptibility to COVID-19 in Australian Children During Alpha, Delta and Omicron Waves of the COVID-19 Pandemic. J Asthma Allergy. 1139. [Google Scholar]
- Zhou, Y.; Hou, Y.; Shen, J.; et al. A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19. PLoS Biol. 3000. [Google Scholar]
- Rayner, D.G.; Wang, E.; Su, C.; et al. Risk factors for long COVID in children and adolescents: a systematic review and meta-analysis. World J Pediatr.
- Chang, T.H.; Chen, Y.C.; Chen, W.Y.; et al. Weight Gain Associated with COVID-19 Lockdown in Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients.
- Li, C.X.; Gao, J.; Zhang, Z.; et al. Multiomics integration-based molecular characterizations of COVID-19. Brief Bioinform.
- Tyler, S.R.; Bunyavanich, S. Leveraging -omics for asthma endotyping. J Allergy Clin Immunol.
- Urbani, F.; Cometa, M.; Martelli, C.; Santoli, F.; Rana, R.; Ursitti, A.; Bonato, M.; Baraldo, S.; Contoli, M.; Papi, A. Update on virus-induced asthma exacerbations. Expert Rev Clin Immunol. 2023, 19(10), 1259–1272. [Google Scholar] [CrossRef]
- Roland, D.; Teo, K.W.; Bandi, S.; Lo, D.; Gaillard, E.A. COVID-19 is not a driver of clinically significant viral wheeze and asthma. Arch Dis Child. 2021, 106(4), e22. [Google Scholar] [CrossRef]
- Fainardi, V.; Esposito, S.; Chetta, A.; Pisi, G. Asthma phenotypes and endotypes in childhood. Minerva Med. 2022, 113(1), 94–105. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Cheng, M.L.; Chiang, M.H.; Wang, C.J.; Tsai, M.H.; Lin, G. Integrated metabolic and microbial analysis reveals host-microbial interactions in IgE-mediated childhood asthma. Sci Rep. 2340. [Google Scholar]
- Ferraro, V.A.; Zanconato, S.; Carraro, S. Metabolomics Applied to Pediatric Asthma: What Have We Learnt in the Past 10 Years? Children (Basel). 1452. [Google Scholar]
- Turi, K.N.; Romick-Rosendale, L.; Ryckman, K.K.; Hartert, T.V. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. J Allergy Clin Immunol, 1191. [Google Scholar]
- Barh, D.; Aljabali, A.A.; Tambuwala, M.M.; Tiwari, S.; Serrano-Aroca, Á.; Alzahrani, K.J. Predicting COVID-19-Comorbidity Pathway Crosstalk-Based Targets and Drugs: Towards Personalized COVID-19 Management. Biomedicines.
- Conrad, L.A.; Cabana, M.D.; Rastogi, D. Defining pediatric asthma: phenotypes to endotypes and beyond. Pediatr Res. 2021, 90(1), 45–51. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.; Ghezzi, M.; Kantar, A.; Song, W.J.; Bush, A.; Peroni, D.; D’Auria, E. Pediatric obesity and severe asthma: Targeting pathways driving inflammation. Pharmacol Res. 2023, 188, 106658. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Salam, M.T.; Alderete, T.L.; Habre, R.; Bastain, T.M.; Berhane, K.; Gilliland, F.D. Effects of Childhood Asthma on the Development of Obesity among School-aged Children. Am J Respir Crit Care Med. 2017, 195(9), 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Fainardi, V.; Passadore, L.; Labate, M.; Pisi, G.; Esposito, S. An Overview of the Obese-Asthma Phenotype in Children. Int J Environ Res Public Health. 2022, 19(2), 636. [Google Scholar] [CrossRef]
- Makrinioti, H.; Zhu, Z.; Camargo, C.A.J.; Fainardi, V.; Hasegawa, K.; Bush, A.; Saglani, S. Application of Metabolomics in Obesity-Related Childhood Asthma Subtyping: A Narrative Scoping Review. Metabolites. 2023, 13(3), 328. [Google Scholar] [CrossRef]
- Carraro, S.; Giordano, G.; Reniero, F.; Carpi, D.; Stocchero, M.; Sterk, P.J.; Baraldi, E. Asthma severity in childhood and metabolomic profiling of breath condensate. Allergy. 2013, 68(1), 110–7. [Google Scholar] [CrossRef]
- Sinha, A.; Desiraju, K.; Aggarwal, K.; Kutum, R.; Roy, S.; Lodha, R.; Kabra, S.K.; Ghosh, B.; Sethi, T.; Agrawal, A. Exhaled breath condensate metabolome clusters for endotype discovery in asthma. J Transl Med. 2017, 15(1), 262. [Google Scholar] [CrossRef]
- Cottrill, K.A.; Stephenson, S.T.; Mohammad, A.F.; Kim, S.O.; McCarty, N.A.; Kamaleswaran, R.; Fitzpatrick, A.M. Chandler JD. Exacerbation-prone pediatric asthma is associated with arginine, lysine, and methionine pathway alterations. J Allergy Clin Immunol.
- Fitzpatrick, A.M.; Park, Y.; Brown, L.A.; Jones, D.P. Children with severe asthma have unique oxidative stress-associated metabolomic profiles. J Allergy Clin Immunol.
- Papamichael,M.M.;Katsardis,C.;Tsoukalas,D.;Itsiopoulos,C.;Erbas,B.Plasma lipid biomarkers in relation to BMI, lung function, and airway inflammation in pediatric asthma. Metabolomics.
- Kelly, R.S.; Virkud, Y.; Giorgio, R.; Celedón, J.C.; Weiss, S.T.; Lasky-Su, J. Metabolomic profiling of lung function in Costa-Rican children with asthma. Biochim Biophys Acta Mol Basis Dis. 1590. [Google Scholar]
- Kelly, R.S.; Mendez, K.M.; Huang, M.; Hobbs, B.D.; Clish, C.B.; Gerszten, R.; Cho, M.H.; Wheelock, C.E.; McGeachie, M.J.; Chu, S.H.; et al. Metabo-Endotypes of Asthma Reveal Differences in Lung Function: Discovery and Validation in Two TOPMed Cohorts. Am J Respir Crit Care Med.
- Childhood Asthma Management Program Research, Group; Szefler, S.; Weiss, S.; Tonascia, J.; Adkinson, N.F.; Bender, B.; Cherniack, R.; Donithan, M.; Kelly, H.W.; Reisman, J. Childhood Asthma Management Program Research Group; Szefler, S.; Weiss, S.; Tonascia, J.; Adkinson, N.F.; Bender, B.; Cherniack, R.; Donithan, M.; Kelly, H.W.; Reisman, J.; et al.Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 1054. [Google Scholar]
- Fitzpatrick, A.M.; Mutic, A.D.; Mohammad, A.F.; Stephenson, S.T.; Grunwell, J.R. Obesity Is Associated with Sustained Symptomatology and Unique Inflammatory Features in Children with Asthma. J Allergy Clin Immunol Pract.
- Tobias, T.A.M.; Wood, L.G.; Rastogi, D. Carotenoids, fatty acids and disease burden in obese minority adolescents with asthma. Clin Exp Allergy.
- Thompson, D.; Wood, L.G.; Williams, E.J.; McLoughlin, R.F.; Rastogi, D. Endotyping pediatric obesity-related asthma: Contribution of anthropometrics, metabolism, nutrients, and CD4+ lymphocytes to pulmonary function. J Allergy Clin Immunol.
- Qu, H.Q.; Glessner, J.; Qu, J.; Gilhool, S.; Mentch, F.; Campbell, I.; Sleiman, P.; Connolly, J.J.; Hakonarson, H. IHCC consortium. Metabolomic profiling of samples from pediatric patients with asthma unveils deficient nutrients in African Americans. iScience. 1046. [Google Scholar]
- Chiu, C.Y.; Chou, H.C.; Chang, L.C.; Fan, W.L.; Dinh, M.C.V.; Kuo, Y.L.; Chung, W.H.; Lai, H.C.; Hsieh, W.P.; Su, S.C. Integration of metagenomics-metabolomics reveals specific signatures and functions of airway microbiota in mite-sensitized childhood asthma. Allergy. 2020, 75(11), 2846–2857. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.; Dedrick, S.; Momeni, B.; Kelly, R.S.; Zeiger, R.S.; O’Connor, G.T.; Sandel, M.T.; Bacharier, L.B.; Beigelman, A.; Laranjo, N.; Gold, D.R.; Lasky-Su, J.; Litonjua, A.A.; Liu, Y.Y.; Weiss, S.T. Association of the gut microbiome and metabolome with wheeze frequency in childhood asthma. J Allergy Clin Immunol. 2022, 150(2), 325–336. [Google Scholar] [CrossRef]
- Gomez-Llorente, M.A.; Martínez-Cañavate, A.; Chueca, N.; Rico, M.C.; Romero, R.; Anguita-Ruiz, A.; Aguilera, C.M.; Gil-Campos, M.; Mesa, M.D.; Khakimov, B.; et al. Approach Reveals New Signatures in Obese Allergic Asthmatic Children. Biomedicines.
- Park, Y.H.; Fitzpatrick, A.M.; Medriano, C.A.; Jones, DP. High-resolution metabolomics to identify urine biomarkers in corticosteroid-resistant asthmatic children. J Allergy Clin Immunol. 1518. [Google Scholar]
- Yonker, L.M.; Neilan, A.M.; Bartsch, Y.; et al. Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical Presentation, Infectivity, and Immune Responses. J Pediatr.
- Zeng, Z.Q.; Chen, D.H.; Tan, W.P.; et al. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: a study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur J Clin Microbiol Infect Dis.
- Dong, Y.; Mo, X.; Hu, Y.; et al. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020. [Google Scholar]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med.
- de Souza, T.H.; Nadal, J.A.; Nogueira, R.J.N.; Pereira, R.M.; Brandão, M.B. Clinical manifestations of children with COVID-19: A systematic review. Pediatr Pulmonol. 1892. [Google Scholar]
- Rabha, A.C.; Oliveira Junior, F.I.; Oliveira, T.A.; Cesar, R.G.; Fongaro, G.; Mariano, R.F.; Camargo, C.N.; Fernandes, F.R.; Wandalsen, G.F. Clinical manifestations of children and adolescents with COVID-19: Report of the first 115 cases from Sabará Hospital Infantil. Rev Paul Pediatr. 2020. [Google Scholar]
- Rajapakse, N.; Dixit, D. Human and novel coronavirus infections in children: a review. Paediatr Int Child Health.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T. ; Erichsen, S; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell.
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 1735. [Google Scholar]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol.
- Gui, M.; Song, W.; Zhou, H.; et al. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res.
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med.
- Yao, Y.; Wang, H.; Liu, Z. Expression of ACE2 in airways: Implication for COVID-19 risk and disease management in patients with chronic inflammatory respiratory diseases. Clin Exp Allergy. 1313. [Google Scholar]
- Bunyavanich, S. ; Do. A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA. 2427. [Google Scholar]
- Zhang, H.P.; Sun, Y.L.; Wang, Y.F.; Yazici, D.; Azkur, D.; Ogulur, I.; Azkur, A.K.; Yang, Z.W.; Chen, X.X.; Zhang, A.Z.; et al. Recent developments in the immunopathology of COVID-19. Allergy.
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 1233. [Google Scholar]
- Felsenstein, S.; Herbert, J.A.; McNamara, P.S.; Hedrich, C.M. COVID-19: Immunology and treatment options. Clin Immunol. 1084. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 1022. [Google Scholar]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 1022. [Google Scholar]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 1022. [Google Scholar]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat Immunol. 2022; 23(1):40-49.
- Flores-Vega, V.R.; Monroy-Muñoz, I.E.; Galicia-Velasco, M.; Villalobos, G.; Gonzalez-Bonilla, C.R.; Rosas-Salazar, C. SARS-CoV-2: Evolution and emergence of new viral variants. Viruses.
- Soriano-Arandes, A. COVID-19 clinical manifestations in children: What is the impact of the different SARS-CoV-2 variants and do we need to distinguish between different respiratory viruses? Acta Paediatr.
- Wurm, J.; Uka, A.; Bernet, V.; Fuchs, E.; Lemaitre, E.; Dehaes, S.; Hug, M.I.; Dolci, M.; Mosimann, L.; Berger, C.; Rimensberger, P.; L’Huillier, A.G.; Posfay-Barbe, K.M.; Kaiser, L.; Eckerle, I.; Bornhauser, M.; Buettcher, M. The changing clinical presentation of COVID-19 in children during the course of the pandemic. Acta Paediatr. 2024; 113(4):771–777.
- Pokorska-Śpiewak, M.; Talarek, E.; Pawłowska, M.; Mania, A.; Mazur-Melewska, K.; Służewski, W.; Nowak, I.; Czajka, H.; Kuchar, E. The influence of SARS-CoV-2 variants B.1.1.7 and B.1.617.2 on a different clinical course and severity of COVID-19 in children hospitalized in 2021 compared with 2020. Pediatr Infect Dis J. 2023; 42(7):584–589.
- Gentile, A.; Juárez, M.D.V.; Romero Bollon, L.; Viegas, M.; Paniagua, A.; Caballero, M.T.; Trotta, F.; Ávila, M.M.; Mistchenko, A.; Aguirre, D.; Galloni, C.; Moragas, A.; Santoro, J.; Ruvinsky, S.; Sánchez, A.; Gómez, R.; Neyro, S.; Stambulian, D.; Tenembaum, S.N. Comparison of epidemiologic and clinical COVID-19 profiles in children in Argentina, during circulation of original and variant (alpha, gamma and lambda) strains. Pediatr Infect Dis J. 2023; 42(2):136–142.
- Abraham, D.R.; Butters, C.; Yunis, N.A.; du Plessis, N.M.; Oliver, C.; van Rooyen, E.; van der Zalm, M.M.; van Toorn, R.; Rabie, H.; Dramowski, A. The impact of SARS-CoV-2 variants on the clinical phenotype and severity of multisystem inflammatory syndrome in children in South Africa. Pediatr Infect Dis J.
- Putri, N.D.; Laksanawati, I.S.; Husada, D.; Laksono, B.; Supriyatno, B.; Setyanto, D.B.; Dewi, N.M.A.R.; Rismawati, D.; Utomo, T.; Kurniati, N.; Maghfiroh, A.A.; Sadarjoen, S.S. A systematic review of post COVID-19 condition in children and adolescents: Gap in evidence from low-and middle-income countries and the impact of SARS-CoV-2 variants. PLoS One, e: 20(3), 0315. [Google Scholar]
- Calcaterra, V.; Verduci, E.; Vandoni, M.; Rossi, V.; Di Profio, E.; Carnevale Pellino, V. Telehealth: A Useful Tool for the Management of Nutrition and Exercise Programs in Pediatric Obesity in the COVID-19 Era. Nutrients.
- Amezquita, M.V. El impacto de COVID-19 en la obesidad pediátrica. Andes Pediatr. 2021;92(4).
- Zeng, F.; Huang, Y.; Guo, Y.; Yin, M.; Chen, X.; Xiao, L. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int J Infect Dis. 2020. [Google Scholar]
- Aparicio, C.; Willis, Z.I.; Nakamura, M.M.; Wolf, J.; Little, C.; Maron, G.M. Risk Factors for Pediatric Critical COVID-19: A Systematic Review and Meta-Analysis. J Pediatric Infect Dis Soc.
- Harwood, R.; Yan, H.; Talawila Da Camara, N.; Smith, C.; Ward, J.; Tudur-Smith, C. Which children and young people are at higher risk of severe disease and death after hospitalisation with SARS-CoV-2 infection in children and young people: A systematic review and individual patient meta-analysis. EClinicalMedicine, 1012. [Google Scholar]
- Browne, N.T.; Snethen, J.A.; Greenberg, C.S.; Frenn, M.; Kilanowski, J.F. ; Gance-Cleveland. When Pandemics Collide: The Impact of COVID-19 on Childhood Obesity. J Pediatr Nurs.
- Al Agha, A.E.; Alharbi, R.S.; Almohammad, O.A.; Yousef, S.Y.; Sulimani, A.E.; Alaama, R.A. Impact of COVID-19 lockdown on glycemic control in children and adolescents. Saudi Med J.
- Marigliano, M.; Maffeis, C. Glycemic control of children and adolescents with type 1 diabetes improved after COVID-19 lockdown in Italy. Acta Diabetol.
- Anderson, L.N.; Yoshida-Montezuma, Y.; Dewart, N.; Jalil, E.; Khattar, J.; De Rubeis, V. Obesity and weight change during the COVID-19 pandemic in children and adults: A systematic review and meta-analysis. Obes Rev, 1355. [Google Scholar]
- Pietrobelli, A.; Pecoraro, L.; Ferruzzi, A.; Heo, M.; Faith, M.; Zoller, T. Effects of COVID-19 Lockdown on Lifestyle Behaviors in Children with Obesity Living in Verona, Italy: A Longitudinal Study. Obesity.
- Yang, S.; Guo, B.; Ao, L.; Yang, C.; Zhang, L.; Zhou, J.; Jia, P. Obesity and activity patterns before and during COVID-19 lockdown among youths in China. Clin Obes.
- Cipolla, C.; Curatola, A.; Ferretti, S.; Giugno, G.; Condemi, C.; Delogu, A.B. Eating habits and lifestyle in children with obesity during the COVID-19 lockdown: a survey in an Italian center. Acta Biomed.
- Di Riso, D.; Bertini, S.; Spaggiari, S.; Olivieri, F.; Zaffani, S.; Comerlati, L. Short-Term Effects of COVID-19 Lockdown in Italian Children and Adolescents with Type 1 Diabetes Mellitus: The Role of Separation Anxiety. Int J Environ Res Public Health.
- Moreno, J.P.; Johnston, C.A.; Woehler, D. Changes in weight over the school year and summer vacation: results of a 5-year longitudinal study. J Sch Health.
- Kim, E.S.; Kwon, Y.; Choe, Y.H.; Kim, M.J. COVID-19-related school closing aggravate obesity and glucose intolerance in pediatric patients with obesity. Sci R.
- Nassar, M.F.; Allam, M.F.; Shata, M.O. Effect of COVID-19 Lockdown on Young Egyptian Soccer Players. Glob Pediatr Health.
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science. 6490. [Google Scholar]
- Piątkowska-Chmiel, I.; Krawiec, P.; Ziętara, K.J.; Pawłowski, P.; Samardakiewicz, M.; Pac-Kożuchowska, E. The Impact of Chronic Stress Related to COVID-19 on Eating Behaviors and the Risk of Obesity in Children and Adolescents. Nutrients.
- Golebski, K.; Kabesch, M.; Melén, E.; Potočnik, U.; van Drunen, C.M.; Reinarts, S. Childhood asthma in the new omics era: challenges and perspectives. Curr Opin Allergy Clin Immunol.
- www. ginasthma.org. 07 February.
- Chatziparasidis, G.; Kantar, A. COVID-19 in Children with Asthma. Lung. 2021, 199(1), 7–12. [Google Scholar] [CrossRef]
- Kloepfer, K.M.; Olenec, J.P.; Lee, W.M.; Liu, G.; Vrtis, R.F.; Roberg, K.A.; Evans, M.D.; Gangnon, R.E.; Lemanske, R.F.J.; Gern, J.E. Increased H1N1 infection rate in children with asthma. Am J Respir Crit Care Med. 2012, 185(12), 1275–9. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.R.; Strong, K.; Cameron, A.; Walton, R.P.; Jackson, D.J.; Johnston, S.L. Viral infections in allergy and immunology: How allergic inflammation influences viral infections and illness. J Allergy Clin Immunol. 2017, 140(4), 909–920. [Google Scholar] [CrossRef]
- Bateman, E.D.; Bousquet, J.; Busse, W.W.; Clark, T.J.; Gul, N.; Gibbs, M.; Pedersen, S. ; GOAL Steering Committee and Investigators. Stability of asthma control with regular treatment: an analysis of the Gaining Optimal Asthma controL (GOAL) study. Allergy.
- Garg, S.; Kim, L.; Whitaker, M.; O’Halloran, A.; Cummings, C.; Holstein, R.; Prill, M.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020, 69(15), 458–464. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Forno, E. Asthma and COVID-19 in children: A systematic review and call for data. Pediatr Pulmonol. 2020, 55(9), 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, X.; Wan, X.G.; Wang, M.L.; Qiu, Z.H.; Chen, J.L.; Shi, M.H.; Zhang, S.Y.; Xia, Y. L. Pediatric asthma control during the COVID-19 pandemic: A systematic review and meta-analysis. Pediatr Pulmonol. 2022, 57(1), 20–25. [Google Scholar] [CrossRef]
- Moreno-Sánchez, E.; Castillo-Viera, E.; Vélez-Moreno, E.; Gago-Valiente, F.J. Facts and Challenges about Asthma and COVID-19 among the Paediatric Population: A Systematic Literature Review. Medicina (Kaunas). 2021, 57(12), 1306. [Google Scholar] [CrossRef]
- Kimura, H.; Francisco, D.; Conway, M.; Martinez, F.D.; Vercelli, D.; Polverino, F.; Billheimer, D.; Kraft, M. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol.
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A. S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 1016. [Google Scholar]
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin Rev Allergy Immunol. 2020, 64(1), 90–107. [Google Scholar] [CrossRef]
- Radzikowska, U.; Ding, M.; Tan, G.; Zhakparov, D.; Peng, Y.; Wawrzyniak, P.; Wang, M.; Li, S.; Morita, H.; Altunbulakli, C.; et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020, 75(11), 2829–2845. [Google Scholar] [CrossRef]
- Sajuthi, S.P.; DeFord, P.; Li, Y.; Jackson, N.D.; Montgomery, M.T.; Everman, J.L.; Rios, C. L.; Pruesse, E.; Nolin, J.D.; Plender, E. G.; et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun. 2020, 11(1), 5139. [Google Scholar] [CrossRef]
- Lipworth, B.; Chan, R.; RuiWen, K.C. Type 2 Asthma Inflammation and COVID-19: A Double Edged Sword. J Allergy Clin Immunol Pract. 2021, 9(3), 1163–1165. [Google Scholar] [CrossRef]
- Muñoz, X.; Pilia, F.; Ojanguren, I.; Romero-Mesones, C.; Cruz, M.J. Is asthma a risk factor for COVID-19? Are phenotypes important? ERJ Open Res. 0021. [Google Scholar]
- Ferastraoaru, D.; Hudes, G.; Jerschow, E.; Jariwala, S.; Karagic, M.; de Vos, G.; Rosenstreich, D.; Ramesh, M. Eosinophilia in Asthma Patients Is Protective Against Severe COVID-19 Illness. J Allergy Clin Immunol Pract. 1152. [Google Scholar]
- Yamaya, M.; Nishimura, H.; Deng, X.; Sugawara, M.; Watanabe, O.; Nomura, K.; Shimotai, Y.; Momma, H.; Ichinose, M.; Kawase, T.l. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020, 58(3), 155–168. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; Sajuthi, S.; Deford, P.; Christenson, S.; Rios, C.L.; Montgomery, M.T.; Woodruff, P. G.; Mauger, D.T.; Erzurum, S.C.; Johansson, M.W.; et al. COVID-19-related Genes in Sputum Cells in Asthma. Am J Respir Crit Care Med. 2020, 202(1), 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.M. A network approach predicts NFKBIA and BIRC3 as pathogenic genes in childhood asthma. Genet Mol Res.
- Ali, S.; Hirschfeld, A.F.; Mayer, M.L.; Fortuno, E.S.; Corbett, N.; Kaplan, M. Functional genetic variation in NFKBIA and susceptibility to childhood asthma, bronchiolitis, and bronchopulmonary dysplasia. J Immunol, 3949. [Google Scholar]
- Suren Garg, S.; Kushwaha, K.; Dubey, R.; Gupta, J. Association between obesity, inflammation and insulin resistance: Insights into signaling pathways and therapeutic interventions. Diabetes Res Clin Pract, 1106. [Google Scholar]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev Med Virol.
- Bantulà, M.; Roca-Ferrer, J.; Arismendi, E.; Picado, C. Asthma and Obesity: Two Diseases on the Rise and Bridged by Inflammation. J Clin Med.
- Marks KJ, Whitaker M, Anglin O, et al. Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 States, 21–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):271–278. 20 July.
- Zhou R, To KK, Wong YC, et al. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2022;55(1):26–41.e6.
- Gómez-Ochoa SA, Franco OH, Rojas LZ, Franco OH, Rojas LZ, Wyssmann BM, Gea-Horta T, Raguindin PF, Roa-Díaz ZM, Echeverría LE. COVID-19 in healthcare workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2021;190(1):161–175.
- Goldstein E, Lipsitch M, Cevik M. On the effect of age on the transmission of SARS-CoV-2 in households, schools, and the community. J Infect Dis. 2021;223(3):362–369.
- Ssentongo P, Ssentongo AE, Hehnly C, Ba DM, Ericson JE, Jordan AR, Nunez JJ. Clinical profiles of COVID-19 in children and young adults by machine learning clustering of multiple comorbidities: a retrospective cohort study. PLoS One. 2022;17(6):e0267795.
- Mahdavinia M, Codispoti CD, Khan T, Kreiner M, Fathi M, Bassiri H, et al. Asthma and COVID-19: Unveiling outcome disparities and long-term sequelae. Front Allergy. 2023;4:1215921.
- Samuel, C.E. Interferon at the crossroads of SARS-CoV-2 infection and COVID-19 disease. J Biol Chem. 2023; 299(8):104960.
- U.S. Centers for Disease Control and Prevention. Obesity is a risk factor for decrease in lung function after COVID-19 infection in asthmatic children. MMWR Morb Mortal Wkly Rep. 2022;71(17):573–578.
- Bauer RN, Vijeyakumaran M, Zhou L, Story R, Dillon P, Park JJ, et al. Asthma and COVID-19: a dangerous liaison? J Allergy Clin Immunol. 2021;148(1):31–40.
- Izquierdo JL, Rodríguez JM, Albert P, Soriano JB. Severe asthma in the era of COVID-19: A narrative review. Respir Med. 2021;180:106347.
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110(7), 2208–2211. [Google Scholar] [CrossRef]
- Ghirardo, V.; Ciprandi, G.; Ricci, G.; Marseglia, G.L. Paucigranulocytic Asthma: Clinical Features and Therapeutic Perspectives. J Pers Med. 2022, 12(5), 850. [Google Scholar]
- Altman, M.C.; Gill, M.A.; Whalen, E.; Babineau, D.C.; Shao, B.; Liu, A.H.; et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat Immunol. 2019, 20(6), 637–651. [Google Scholar] [CrossRef]
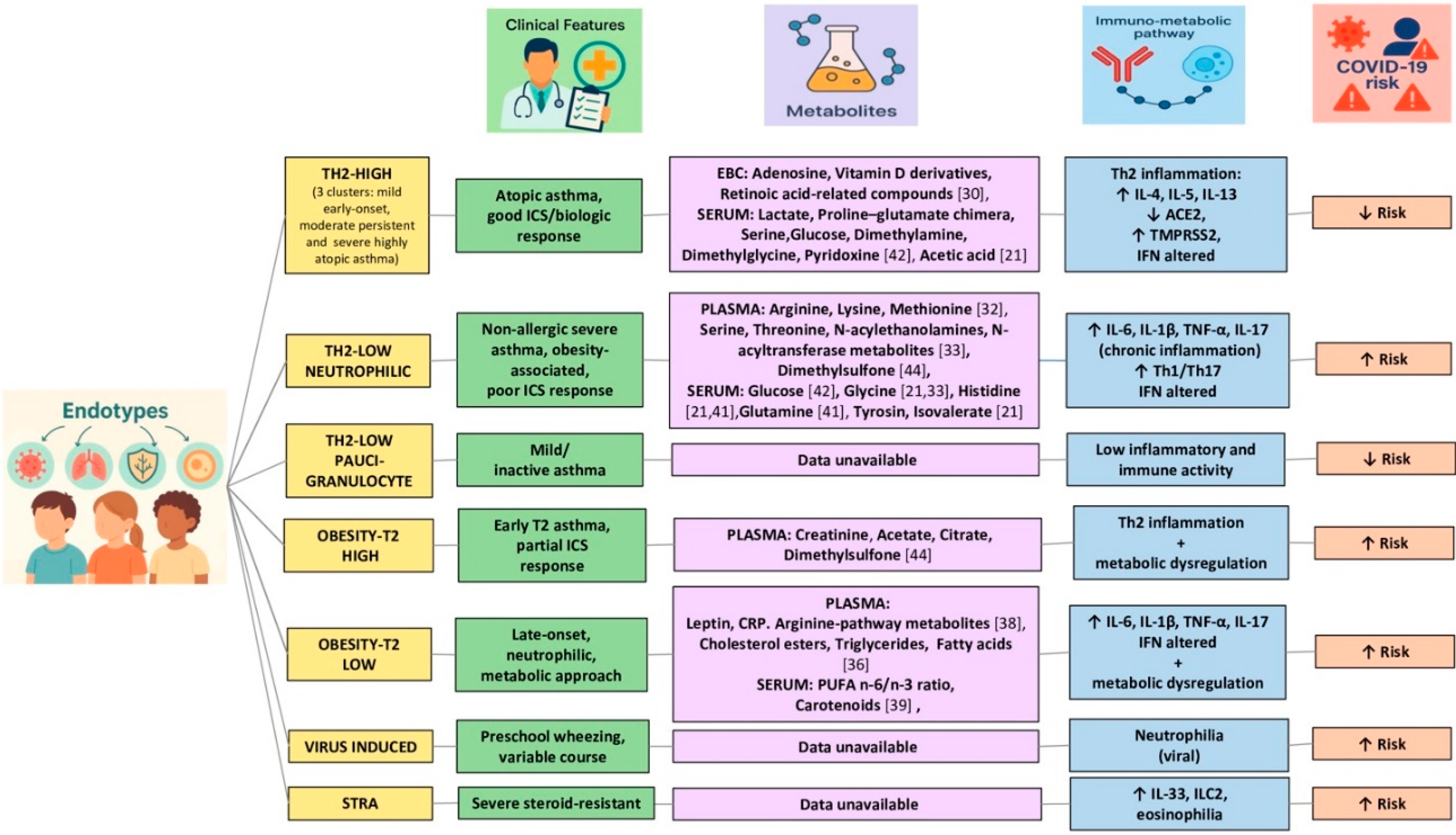
| Endotype | Immunological Features | Clinical Phenotype | Treatment Response |
|---|---|---|---|
| Th2-high | ↑ IL-4, IL-5, IL-13, eosinophilia, ↑ IgE,↓ IFN-I/III | Cluster 1: mild early-onset atopic asthma (low exacerbations, preserved lung function)Cluster 2: moderate persistent atopic asthma (↓ lung function, severe hyperreactivity)Cluster 3: severe highly atopic asthma (significant symptoms, intensive therapy required, impaired lung function) | Good response to ICS and biologics |
| Th2-lowneutrophilic | ↑ Th17, IL-17, IL-12, TNF-α,neutrophilia,↓ IFN-I/III, ↑ IFN-γ,Th1/Th17 activation | Mild early-onset non-atopic asthma (normal lung function)Severe persistent non-allergic asthma (poor control, obesity-associated, high exacerbations) | Poor response to ICSrequires specialist evaluation |
| Th2-low paucigranulocytic | Absence of eosinophils or neutrophils,low systemic inflammation | Mild or inactive asthma,airway remodeling and bronchial hyperreactivity,persistent airflow limitation | Variable |
| Obesity-related (T2-high) | Eosinophilic inflammation,high BMI, predominant Th2 profile,possible ↓ IFN-I/III | Early-onset asthma with T2 inflammation | Partial, nutritional intervention may beneficial |
| Obesity-related (T2-low) | Neutrophilic inflammation,↑ IL-6, IL-1β, ↑ IL-17, ↑ IFN-γ, ↓ IFN-I/III, Th1/Th17 activation | Late-onset asthma with systemic inflammation | Poor, targeted metabolic interventions required |
| Virus-induced | Neutrophilia (during viral infection), immature IFN-I/III responses in children, impaired antiviral defense | Preschool episodic wheeze | Variable, does not always progress to asthma |
| STRA | ↑ IL-33, ILC2, persistent eosinophilia, sustained Th2 profile, immune dysregulation, ↓ IFN-I/III | Severe,corticosteroid-resistant asthma | Biologics needed, intensive management, poor ICS response due to steroid resistance |
| Samples | Patients | Technique | Key Findings | Clinical Relevance |
|---|---|---|---|---|
| EBC[30] | 42 asthmatic children (8– 17 years): 31 with non severe and 11 with severe asthma. | LC-MS | EBC metabolomic profiling separated severe, non-severe asthma and controlsKey metabolites: retinoic acid, adenosine and vitamin D derivatives | Breathomics profiling may serve as a non-invasive tool to distinguish asthma severity phenotypes and support individualized therapeutic strategies in children |
| EBC[31] | 89 asthmatic children and 20 controls | NMR+ML | 3 distinct clusters outlined in the asthmatic group, with significant clinical and chemical differences (eosinophils, exacerbations and family history) | Global breathomics profiling aided by ML may facilitate endotype discovery in pediatric asthma through non-invasive spectral clustering |
| PLASMA[32] | 215 asthmatic subjects, including 41 with exacerbative asthma | UHPLC-MS | 32 unique cohort-independent metabolites distinguished exacerbation-prone from non-prone asthmatic childrenThe arginine, lysine and methionine pathways were the most affected | Specific plasma metabolites enable identification of exacerbation-prone asthma in children, even under high-dose ICS therapy, supporting a role for metabolomics in early risk stratification |
| PLASMA[33] | 22 children with mild-to-moderate asthma (8 normal weight, 7 overweight and 7 obese) and 35 with severe refractory asthma (15 normal weight, 9 overweight and 11 obese)(9-17 years) | LC-MS | Distinct plasma metabolomic profiles in severe vs. mild-to-moderate asthmaPathways involved included glycine/serine/threonine metabolism and N-acylethanolamine and N-acyltransferase signalling both associated with oxidative stress | Oxidative stress-related metabolic alterations may underlie corticosteroid insensitivity in severe pediatric asthma and represent potential therapeutic targets |
| PLASMA [34] | 64 asthmatic subjects (5-12 years) with mild asthma phenotype(35 normal, 18 overweightand 8 obese) | GC-MS | Linoleic, oleic, erucic, cis-11-eicosenoic and arachidic acids significantly associated with poorer asthma control and lung function (FEV1, FVC, FEV1/FVC, PEF, FEF25-75% e FeNO)in overweight/obese childrenNo associations for arachidonic, α-linolenic, EPA and DHA | Fatty acid profiling could guide personalized nutritional interventions to improve asthma control and therapeutic outcomes in children |
| PLASMA [35] | 380 asmathic children | LC-MS | Specific metabolites correlated with three clinical features associated with disease severity:-AHR correlated with 91 of the 574 metabolites-%FEV1/FVC ratio pre- and post-bronchodilator with 102 and 155 respectively)Key metabolites: thiamine, creatinine, fatty acids (oleic, myristic), carnitine and gammalinolenic acid | Asthma severity metabolome associated with different degrees of asthma severity in children, reflecting measurable biological alterations at the systemic levelPotential of metabolomics to refine asthma phenotyping and guide personalized severity assessment |
| PLASMA [36] | 1.165 asthmatic subjects(aged 6 and 14 years) from 2 different cohorts | LC-MS+SNF and spectral clustering | Detection of 5 metaboendotypes with significant phenotypic differences, including pre and post bronchodilator FEV1/FVCKey metabolites: cholesterol esters, triglycerides and fatty acids | Existence of reproducible, clinically meaningful metabo-endotypes in asthma, supporting metabolomics as a tool for precision medicine |
| PLASMA [38] | Asthmatics children 6-17 years (257 lean, 99 overweight, 138 obese) | UPLC-TSQ MS | Specific markers of systemic inflammation identified in obese children (↑leptin, CRP and certain amino acid metabolites associated with glutathione synthesis and oxidative stress)↓ concentrations of arginine-related metabolites in uncontrolled obese asthma patients than in obese controlled asthma at 12 months | Persistent asthma symptoms and systemic metabolic-inflammation in obesity-related asthmaSupports amino acid-based biomarker identification for stratified management |
| SERUM[39] | 158 adolescents (39 obese asthmatics, 39 healthy-weight asthmatics, 38 obese controls and 42 healthy-weight controls) | HPLCGC | Obese asthmatic children showed ↓ total carotenoid levels and ↑ n-6/n-3 PUFA ratioBoth markers were significantly associated with decreased FEV1 and increased insulin resistance | Diet modification may reduce asthma burden:protective role for carotenoids and n-3 PUFA in pulmonary function and metabolic regulation in obese pediatric asthma |
| SERUM[40] | 89 asthmatic children 7-11 years (49, healthy-weight, 40 obese) | Multi-omics integration with SNF (metabolomics WITH NMR, transcriptomics, epigenomics) | Anthropometric, metabolic, nutritional and immune factors contribute interdependently to the obese asthma phenotypeWHR and metabolic markers (↑ HOMA-IR, ↑ leptin, ↓ adiponectin) showed the strongest associations with reduced lung function, though none predicted symptom-based severity or control | Highlights truncal adiposity as a key driver of the asthma endotype and supports integrated metabolic–immune profiling to define pediatric obesity-related asthma |
| SERUM [41] | 602 asthmatic children, 593 controls without asthma. | NMR | ↓ levels of citrate, ketone bodies, histidine and glutamine in asthma cases compared to controlsLipid metabolites lost significance after controlling for obesity with the exception of FC% in mVLDL and SFA% | Nutrient deficiencies may contribute to the pathophysiology of asthma and represent therapeutic targets for nutritional supplementation strategies |. |
| SERUM[42] | 55 children (27 with asthma and 28 control) | NMR + shotgun metagenomics | Significant microbe–metabolite associations in asthmatic children: ↓ Prevotella sp. oral taxon 306 and ↓ DMG (dimethylglycine), ↓ dimethylamine, ↓ glucose, ↓ pyridoxine, and ↑ proline-glutamate chimera, ↑ serine, ↑ lactateSeveral control-enriched species inversely correlated with total and allergen-specific IgE levels | Integration of metagenomics and metabolomics reveals host–microbiome interactions in mite-sensitized childhood asthma, with diagnostic implications |
| SERUM[21] | 53 children, aged 3-5 years,(15 lowly sensitized non-atopic asthma, 13 highly sensitized atopic asthma, 25 healthy controls) | NMR+16S rRNA sequencing | Four metabolites (tyrosine, isovalerate, glycine, and histidine) associated with lowly sensitized asthmaAcetic acid strongly linked to highly sensitized asthma and showed a robust correlation with airway microbiota | Distinct metabolomic signatures linked to IgE sensitization profiles and microbiota–metabolite axis in the pathogenesis of childhood asthma |
| FECES[43] | 110 asthmatic subjects aged 3-5 years | UHPLC-MS/MS+16S rRNA sequencing | ↑ Veillonella and histidine metabolites (carnosine, methyl-histidine, β-alanyl-methyl-histidine) in high wheeze group↑ sphingolipids (sphinganine, sphingosine, ceramides) in ICS-treated non-responders | Gut microbiome–metabolome signatures linked to wheeze severity and ICS responseFecal histidine and sphingolipid metabolites as potential biomarkers in pediatric asthma |
| FECES/ PLASMA [44] | 46 asthmatic children, 4-13 years-old (13 normal-weight, 8 overweight, 25 obese) | NMRGC-MS+16S rRNA sequencing | ↑ Leptin ↓ plasma acetate in obese allergic asthma phenotypeChildren with worse asthma outcomes show ↑ fecal D-lactate, ↑ D/L lactate ratio, and ↑ plasma creatinine.Persistent asthma associated with ↓ plasma citrate and ↓ dimethylsulfone (DMSO₂) | Distinct metabolic alterations linked to obesity and asthma severity in childrenPlasma and fecal metabolites as potential biomarkers for endotype-specific profiling |
| URINE[45] | 30 asthmatic children 6-17 years(15 corticosteroid respondent, 15 CS-nonrespondent) | LC coupled with FTMS | Five urinary metabolites (3,6-dihydronicotinic acid, 3-methoxy-4-hydroxyphenyl(ethylene)glycol, 3,4-dihydroxyphenylalanine, γ-glutamylcysteine, cysteinylglycine) associated with corticosteroid resistance in children with severe asthmaKey pathways: tyrosine metabolism, degradation of aromatic compounds and glutathione metabolism | Urine metabolomics identifies non-invasive biomarkers of corticosteroid resistancePathway-specific profiling to guide personalized treatment in severe pediatric asthma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





