Submitted:
05 May 2025
Posted:
06 May 2025
You are already at the latest version
Abstract
Keywords:
1. Summary
1.1. Autism Spectrum Disorders (ASD)
1.2. Disorders Gut-Brain Interaction (DGBIs)
1.3. Rome Criteria
1.4. Functional International Digestive Epidemiological Research Survey – FINDERS
2. Data Description
2.1. Informed Consent for Parents and/or Caregivers of Children with ASD
2.2. Target Population
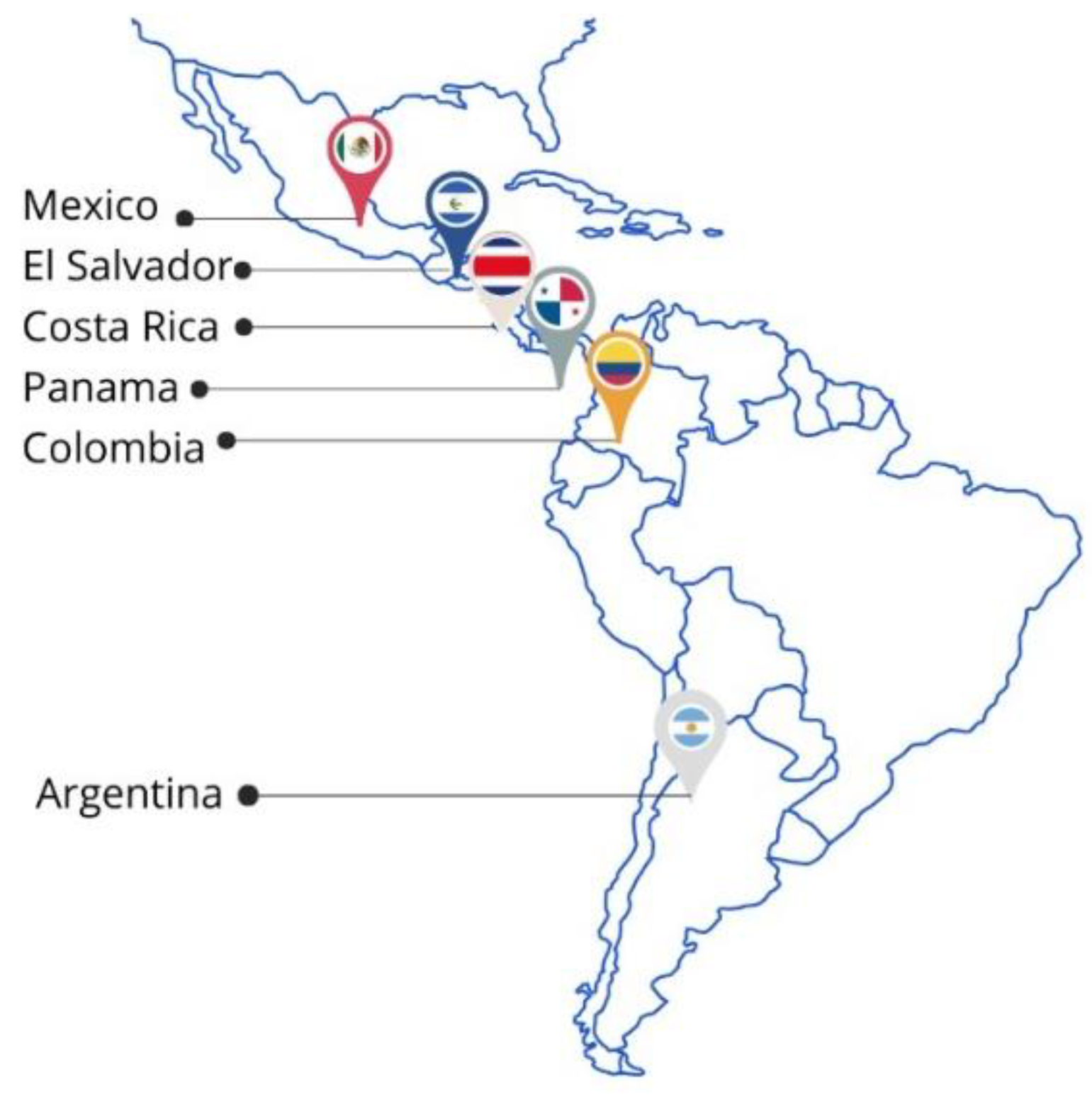
2.3. Description of the Countries Included
2.4. Demographic Variables
2.5. Clinical Variables
2.6. Nutritional Variables
2.7. Family Variables
2.8. Questionnaire for Pediatric Gastrointestinal Symptoms Rome IV (QPGS-IV)
2.9. Questionnaire Interpretation
3. Methods
3.1. Sample Size
3.2. Data Collection
3.3. Data Handling
3.4. Identifying and Handling Biases
3.5. Strengths and Weaknesses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DGBIs | Disorders of Gut-Brain interaction |
| ASD | Autism Spectrum Disorders |
| FINDERS | Functional International Digestive Epidemiological Research Survey |
| QPGS-IV | Questionnaire for Pediatric Gastrointestinal Symptoms Rome IV |
| DSM-V | Diagnostic and statistical manual of mental disorders |
| BMI | Body Mass Index |
| HA | Height for Age |
| OR | Odds Ratio |
| 95% CI | 95% Confidential Interval |
References
- Koppen, I.J.; Nurko, S.; Saps, M.; Di Lorenzo, C.; Benninga, M.C. The pediatric Rome IV criteria: what’s new?. Expert Rev Gastroenterol Hepatol 2017, 11, pp. 193–201. doi: 10.1080/17474124.2017.1282820. [CrossRef]
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: advances in understanding and management. Lancet 2020, 396, pp. 1664–1674. doi: 10.1016/S0140-6736(20)32115-2. [CrossRef]
- Dargenio, V.N.; Dargenio, C.; Castellaneta, S.; De Giacomo, A.; Laguardia, M.; Schettini, F.; et al. Intestinal barrier dysfunction and microbiota-gut-brain axis: Possible implications in the pathogenesis and treatment of autism spectrum disorder. Nutrients 2023, 15, pp. 1620. doi: 10.3390/nu15071620. [CrossRef]
- Buie, T.; Campbell, D.B.; Fuchs, G.J.; Furuta, G.T.; Levy, J.; Vandewater, J.; et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDS: a consensus report. Pediatrics 2010, 125, pp. S1–S18. doi: 10.1542/peds.2009-1878C. [CrossRef]
- Velasco-Benítez, C.A.; Rojas-Cerón, C.A.; Ortiz-Rivera, C.J.; Velasco-Suárez, D.A.; Juvinao-Quintero, M.A.; Zubiri, C.; et al. Disorder of Gut-Brain Interaction in Schoolchildren and Adolescents with Developmental Disabilities. Preprint 2025. doi: 10.20944/preprints202504.0161.v1. [CrossRef]
- Hyman, S.L.; Levy, S.E.; Myers, S.M. Identification, Evaluation, and Management of Children With Autism Spectrum Disorder. Pediatrics 2020, 145, pp. e20193447. doi: 10.1542/peds.2019-3447. [CrossRef]
- American Psychiatric Association; DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5™. 5th ed.; American Psychiatric Publishing, Inc: Washington, DC, 2013.
- Hirota, T.; King, B.H. Autism Spectrum Disorder: A Review. JAMA 2023, 329, pp. 157–168. doi: 10.1001/jama.2022.23661. [CrossRef]
- Alonso-Bermejo, C.; Barrio, J.; Fernández, B.; García-Ochoa, E.; Santos, A.; Herreros, M.; et al. Functional gastrointestinal disorders frequency by Rome IV criteria. An Pediatr (Engl Ed) 2022, 96, pp. 441–447. doi: 10.1016/j.anpede.2021.05.013. [CrossRef]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Childhood Functional Gastrointestinal Disorders: Child/Adolescent. Gastroenterology 2016, 150, pp. 1456–1468. doi: 10.1053/j.gastro.2005.08.063. [CrossRef]
- Velasco-Benítez, C.A.; Collazos-Saa, L.I.; García-Perdomo, H.A. A systematic review and meta-analysis in schoolchildren and adolescents with functional gastrointestinal disorders according to Rome IV Criteria. Arq Gastroenterol 2022, 59, pp. 304–313. doi: 10.1590/S0004-2803.202202000-53. [CrossRef]
- Bonilla, S.; Saps, M. Los acontecimientos de la vida temprana predisponen a la aparición de trastornos gastrointestinales funcionales infantiles. Rev Gastroenterol Mex 2013, 78, pp. 82–91.
- Drossman, D.A.; Chang, L.; Kellow, J.; Chey, W.D.; Tack, J.; Whitehead, W.E., eds. Rome IV Diagnostic Questionnaires and Tables for Investigators and Clinicians. 1st ed.; Rome Foundation: Raleigh, NC, 2016: pp. 192-221 doi: 10.1053/j.gastro.2016.02.014. [CrossRef]
- Nurko, S.; Di Lorenzo, C., eds. Rome IV Pediatric Functional Gastrointestinal Disorders – Disorders of Gut-Brain Interaction. 1st ed.; Rome Foundation: Raleigh, NC, 2016.
- Hreinsson, J.P.; Törnblom, H.; Tack, J.; Drossman, D.A.; Whitehead, W.E.; Bangdiwala, S. Factor Analysis of the Rome IV Criteria for Major Disorders of Gut-Brain Interaction (DGBI) Globally and Across Geographical, Sex, and Age Groups. Gastroenterology 2023, 164, pp. 1211–1222. doi: 10.1053/j.gastro.2023.02.033. [CrossRef]
- Velasco-Benítez, C. Trastornos Digestivos Funcionales En Pediatría. 1st ed.; Grupo Distribuna: Bogotá, Colombia, 2022. pp. 4–5.
- Instituto Geográfico Nacional de Argentina. Introducción a la Geografía de la República Argentina. Buenos Aires, 2023.
- Agencia Nacional de Discapacidad. Personas con certificado único de discapacidad con condiciones de salud vinculadas a trastornos del espectro autista. Buenos Aires: Agencia Nacional de Discapacidad, 2023.
- Oficina de Información Diplomática del Ministerio de Asuntos Exteriores, Unión Europea y Cooperación. Ficha país: Panamá, 2024.
- Instituto Panameño de Habilitación Especial. Uniendo esfuerzos para concienciar sobre el Autismo. Panamá, 2024.
- Oficina de Información Diplomática del Ministerio de Asuntos Exteriores, Unión Europea y Cooperación. Ficha país: Costa Rica, 2025.
- Alvarado, J.; Contreras, L.; Cruz, C. Experiencias familiares, estrategias de afrontamiento y salud de madres y padres de niñez con autismo. ECA 2021, 76, pp. 89–111. doi: 10.51378/eca.v76i764.4576. [CrossRef]
- Oficina de Información Diplomática del Ministerio de Asuntos Exteriores, Unión Europea y Cooperación. Ficha país: México, 2025.
- Fundación Teletón México. Panorama del autismo en México y el mundo, 2024.
- Oficina de Información Diplomática del Ministerio de Asuntos Exteriores, Unión Europea y Cooperación. Ficha país: El Salvador, 2024.
- Oficina de Información Diplomática del Ministerio de Asuntos Exteriores, Unión Europea y Cooperación. Ficha país: Colombia, 2024.
- Rojas, K.J. En Colombia no hay estadísticas oficiales acerca del autismo. Bogotá: Edición Médica, 2021.
- World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. Adopted by the 29th WMA General Assembly, Tokyo, Japan, October 1975.
- Holingue, C.; Newill, C.; Lee, L.C.; Pasricha, P.J.; Fallin, M.D. Gastrointestinal symptoms in autism spectrum disorder: a review of the literature on ascertainment and prevalence. Autism Res 2018, 11, pp. 24–36. doi: 10.1002/aur.1854. [CrossRef]
| Variable Name | Field Name | Value | Definition | Variable Type |
|---|---|---|---|---|
| Current date | current_date | day/month/year | Current date from which the questionnaire is answered | Quantitative – Interval |
| Subject identification | subject_ID | Initials of the country/number of patient | Subject identification number | Qualitative – Ordinal |
| Date of Birth | birth_date | day/month/year | Chronological data indicating the day, month, and year the participant was born | Quantitative – Interval |
| Age | age_years | 4–18 years | Age in completed years at the time of enrollment in the study. Calculated by subtracting the date of birth from the current date | Quantitative – Ratio |
| Age Group | age_group | 0 = Preschool 1 = School-age 2 = Adolescent |
Classification by age group: preschool (4–8 years old), school-age (9–12 years old), and adolescent (13–18 years old) | Qualitative – Ordinal categorical |
| Sex | sex | 0 = Female 1 = Male |
Biological characteristics that distinguish participants based on reproductive and genetic attributes | Qualitative – Nominal categorical |
| Race | race | 0 = Mixed race 1 = Indigenous 2 = White 3 = Afrodescendant |
Socio-cultural category used to classify people based on physical characteristics such as skin color, facial features, or hair type | Qualitative – Nominal categorical |
| Country of Origin | country_1 | 0 = Argentina 1 = Panama 2 = Costa Rica 3 = Mexico 4 = El Salvador 5 = Colombia |
Geographic, cultural, or ethnic origin of the participant | Qualitative – Nominal categorical |
| Type of Educational Institution | type_school | 0 = Public 1 = Private |
Classification according to the nature of the educational institution attended by participants: public or private | Qualitative – Nominal categorical |
| Variable Name | Field Name | Value | Definition | Variable Type |
|---|---|---|---|---|
| Cesarean Birth Method | c_section | 0 = No 1 = Yes |
Surgical technique in which the baby is delivered from the mother's uterus through an incision in the abdominal wall and uterus | Qualitative – Nominal categorical |
| Prematurity | premature | 0 = No 1 = Yes |
Birth occurring before 37 weeks of gestation | Qualitative – Nominal categorical |
| Type of Medical Consultation | consultation_type | 0 = Public 1 = Private |
Classification of the medical care service received by the patient, according to the sector of the providing institution | Qualitative – Nominal categorical |
| Comorbidities | comorbidities | 0 = No 1 = Yes |
Presence of one or more additional diseases or medical conditions that coexist with the participant's main condition | Qualitative – Nominal categorical |
| Medication Use | medications | 0 = No 1 = Yes |
Intake, administration, or use of one or more drugs by the participant, either occasionally, continuously, or under medical indication | Qualitative – Nominal categorical |
| Hospitalizations | hospitalizations | 0 = No 1 = Yes |
Admission of the participant to a healthcare center or hospital for 24 hours or more to receive specialized medical care | Qualitative – Nominal categorical |
| Level of Autism According to DSM-V | autism_level | 0 = Level 1 1 = Level 2 2 = Level 3 3 = Don’t know |
Degree of ASD severity, assessing the level of support required in social communication and restricted, repetitive behaviors | Qualitative – Nominal categorical |
| Variable Name | Field Name | Value | Definition | Variable Type |
|---|---|---|---|---|
| Date of the weight and height | date_weight_height | day/month/year | Date on which it was weighed and stemmed | Quantitative – Interval |
| Weight | weight | Weight in kilograms | Measurement of the participant’s body mass, commonly expressed in kilograms (kg) | Quantitative – Continuous |
| Height | height | Height in centimeters | Measurement of the participant’s stature from feet to head, usually expressed in centimeters (cm) | Quantitative – Continuous |
| Body Mass Index (BMI) diagnosis | dx_bmi | 0= Normal 1= Obesity 2= Overweight risk 3= Overweight 4= malnutrition 5= Severe malnutrition |
BMI-for-height interpretation according to WHO charts for children aged 4 to 18 years: Obesity: > +2 SD; Overweight: +1 to +2 SD; Normal: +1 to -2 SD; malnutrition: < -2 SD; Severe malnutrition: < -3 SD | Qualitative – Nominal categorical |
| Height-for-Age diagnosis | dx_ha | 0 = Normal 1= Short height 2 = Severe short height 3 = Tall height |
Height-for-age interpretation based on WHO growth charts for children aged 4 to 18 years: Normal: -2 to +3 SD; Short stature: -2 to -3 SD; Severe short stature: < -3 SD; Tall stature: > +3 SD | Qualitative – Nominal categorical |
| Variable Name | Field Name | Value | Definition | Variable Type |
|---|---|---|---|---|
| Only Child | only_child | 0 = No 1 = Yes |
Participant who has no siblings | Qualitative – Nominal categorical |
| First-born | first_born | 0 = No 1 = Yes |
Participant who is the first-born among their siblings | Qualitative – Nominal categorical |
| Separated/Divorced Parents | separated_divorced_parents | 0 = No 1 = Yes |
Situation in which the participant does not live with either parent | Qualitative – Nominal categorical |
| Family History of Illness | family_history_illness | 0 = No 1 = Yes |
Presence of one or more diseases or medical conditions in the participant’s family | Qualitative – Nominal categorical |
| Family History of Autism | family_autism | 0 = No 1 = Yes |
Presence of an autism diagnosis in a family member of the participant | Qualitative – Nominal categorical |
| Section | Explanation | Cronbach's Alpha | Interpretation |
|---|---|---|---|
|
Section A Pain and discomfort above the belly button |
This section of the questionnaire explores gastrointestinal symptoms located above the umbilical region, specifically functional dyspepsia and its subtypes: postprandial dyspepsia and epigastric dyspepsia; some questions for diagnosing irritable bowel syndrome and functional abdominal pain not otherwise specified | 0.7331 | High |
|
Section B Pain and discomfort in, around, or below the belly button |
This section assesses gastrointestinal symptoms located around or below the umbilical region. In particular, it helps establish the diagnosis of irritable bowel syndrome, abdominal migraine, and functional abdominal pain not otherwise specified | 0.470 | Moderate |
|
Section C Bowel movements (“poop”, “number 2”) |
This section evaluates the child's bowel movement patterns to diagnose defecation disorders such as functional constipation and non-retentive fecal incontinence | 0.6535 | High |
|
Section D Nausea and vomiting |
This section explores symptoms associated with functional nausea and vomiting disorders, including cyclic vomiting syndrome, nausea, functional vomiting, and adolescent rumination syndrome | 0.6110 | High |
|
Section E Other symptoms |
Inquires about symptoms related to aerophagia | 0.6367 | High |
| H1. Functional Nausea and Vomiting Disorders |
| H1a. Cyclic Vomiting Syndrome |
| (D8) Two or more episodes of repeated vomiting in the past 6 months, AND (D8b) Presence of nausea (“Yes”), AND (D8c) Vomit free intervals is “Several weeks” or longer. |
| H1b. Functional Nausea and Functional Vomiting |
|
H1b1. Functional Nausea (D1) Nausea “2 times a week” or more in the past 2 months, AND (D2) Nausea for 2 months or longer, AND (D3) Nausea not usually related to meal (“No”), AND (D4) No vomiting during nausea episode (“No”), AND (D4a) If comorbid pain is present during nause episode, nausea is more bothersome than pain. H1b2. Functional Vomiting (D5) Vomiting on average one or more times per week, AND (D6) Vomiting for 2 months or longer, AND (D7) Vomiting is not self-induced (“Never” or “Once in a while”), AND Child does not meet criteria for rumination. |
| H1c. Adolescent Rumination Syndrome |
| (D9) Food comes back up “Several times a week” or “Every day”, AND (D9a) Episodes occur shortly after eating (“Yes”). AND (D9b) Episodes do not occur during sleep (“No”), AND (D9c) Episodes are not accompanied by nausea or vomiting (“No”). |
| H1d. Aerophagia |
| [(E1) Belching “Several times a week” or “Every day”, OR (E2) Flatus “Several times a week” or “Every day”] AND (E3) Abdominal distension “Several times a week” or “Every day”, AND (E4) Swallowing air “Several times a week” or “Every day”. |
| H2. Abdominal Pain Disorders |
| H2a. Functional Dyspepsia |
| Functional dyspepsia is diagnosed if child qualifies for postprandial distress syndrome or epigastric pain syndrome or both. H2a1. Postprandial Distress Syndrome [(A3) Fullness “4 days a month” or more often, OR (A4) Satiation “4 days a month” or more often], AND (A7) Duration of upper abdominal pain or discomfort is “2 months” or longer. H2a2. Epigastric Pain Syndrome [(A1) Upper abdominal pain “4 days a month” or more often, OR (A2) heartburn “4 days a month” or more often], AND (A7) Duration of upper abdominal pain or discomfort is “2 months” or longer, AND (A8) Not related to a bowel movement: “Never” or “Once in a while”, AND (A9-12) Not associated with change in stool form or frequency: “Never” or “Once in a while”. |
| H2b. Irritable Bowel Syndrome |
| (B1 or A1) Abdominal pain “4 days a month” or more often, AND (B3 or A7) Abdominal pain is “2 months” or longer, AND (B2a) Not exclusively associated with eating (“No”), AND (B2b) For girls, not exclusively associated with menses (“No” or “No applicable”), AND [At least one (A8-A12) OR (B4-B8) Bowel symptoms “Sometimes” or more often], AND (B9) For those who use laxatives (B9 is “Yes”), question (B9a) Elimination of symptoms with laxatives must be answered “Never”, “Once in a while”, or “Sometimes” (i.e., NOT answered “Most of the time” or “Always”). |
| H2c. Abdominal Migraine |
| (B10) Severe pain causing restriction in daily activities (“Yes”), AND (B10a) Pain lasts 1 hour or more, AND (B10b) In the past 6 months, 2 or more episodes of severe pain, AND [(B10c) Two or more of the following during pain episodes:
|
| H2d. Functional Abdominal Pain-NOS |
| Lower abdominal location (B1 OR A1) Abdominal pain “4 days a month” or more often, AND (B3 OR A7) Abdominal pain is “2 months” or longer, AND (B2a) Pain is not exclusively associated with eating (“No”), AND (B2b) In girls, pains is not exclusively associated with menses (“No” or Not” applicable”), AND Does not meet criteria for the other functional gastrointestinal disorders associated with abdominal pain (e.g., functional dyspepsia, irritable bowel syndrome, abdominal migraine). |
| H3. Functional Defecation Disorders |
| H3a. Functional constipation |
| Two or more of the following: (C1) Two or fewer stools per week, OR [Either (C2) hard (“type 2”) or very hard stools (“type 1”) OR (C3) Painful stool] OR (C4) Passage of very large stools, OR (C5) Stool retention “1 time a week” or more often, OR (C6) History of large fecal mass in rectum, OR (C7) Soiling “Once a week” or more often. If child meets criteria for irritable bowel syndrome, pain should improve with laxative use (B9a= “Most of the time” or Always”). |
| H3b. Non-Retentive Fecal Incontinence |
| (C7) Soiling “1 time a week” or more often, AND (C7a) Amount of stool is small or large (not just a stain), AND (C7b) Soiling for 1 month or longer, AND (C5) No evidence of fecal retention (C5=Never), AND Does not meet criteria for functional constipation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





