Submitted:
12 May 2025
Posted:
13 May 2025
You are already at the latest version
Abstract
Keywords:
Main
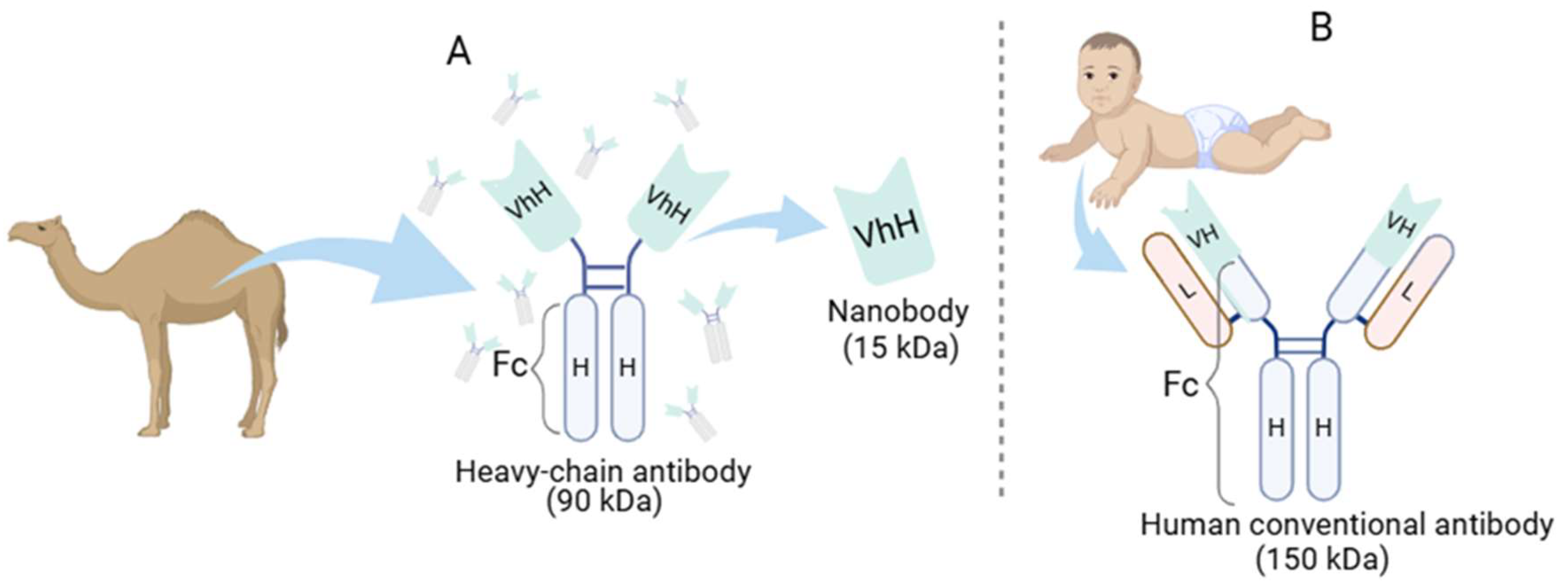
How Are Nanobodies Structured, and What Are Their Properties?
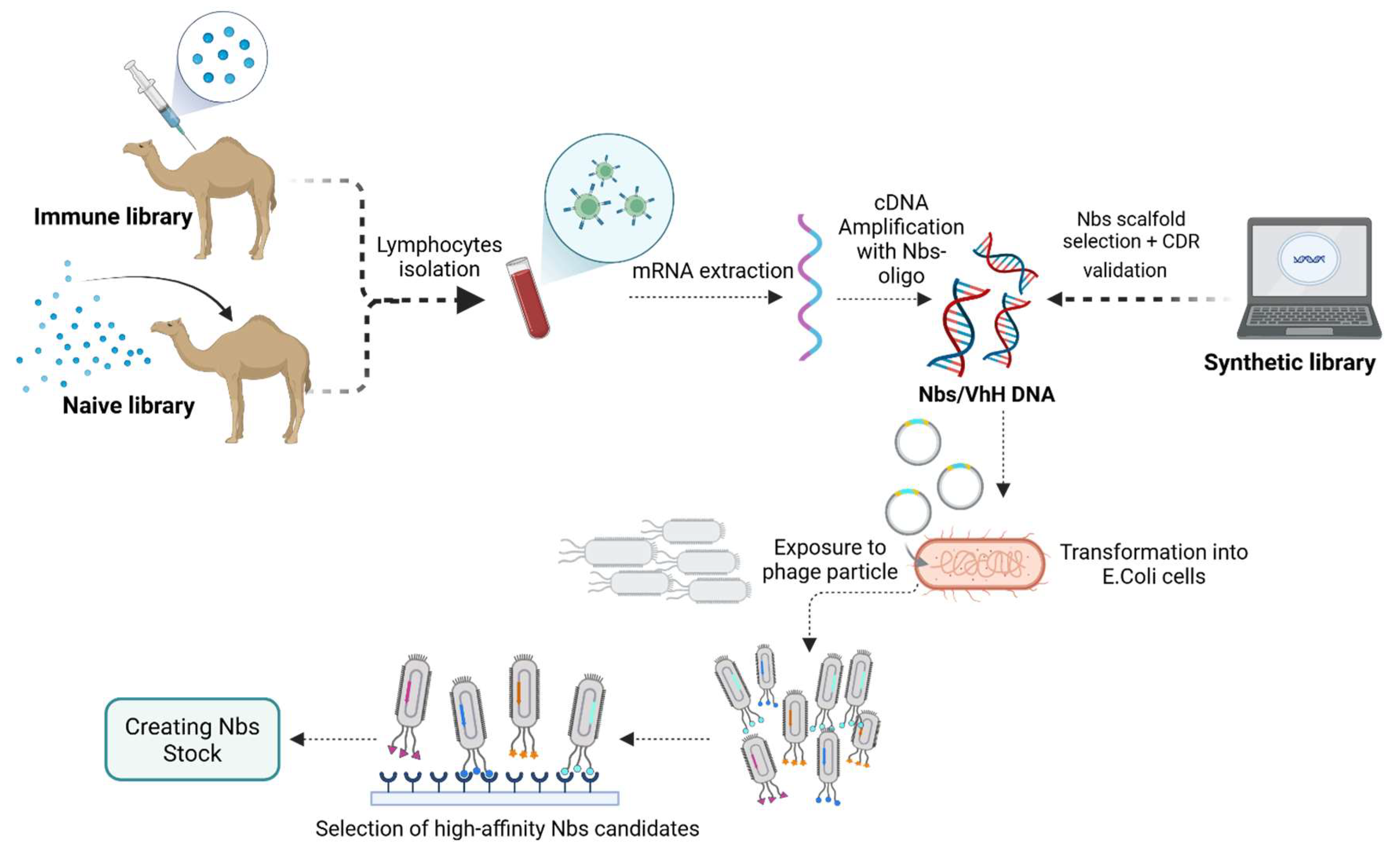
Overview of Nanobody Generation
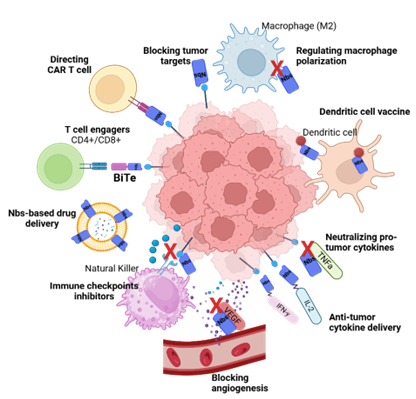
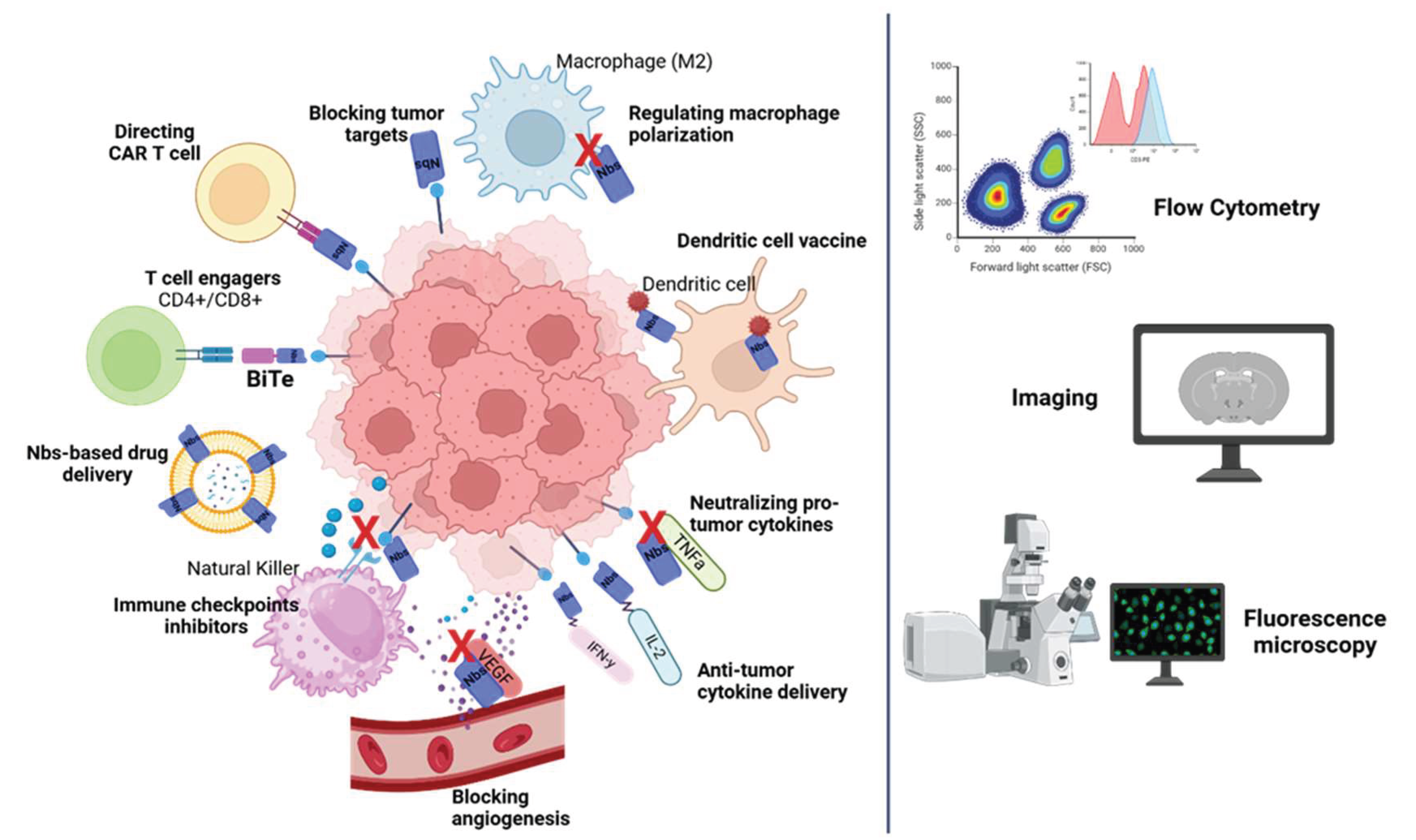
Applications: Nanobody-Based Strategies for Cancer
Why Nanobody-Based Cancer Therapies?
Tumor Targets
Nanobodies as Modulators of Checkpoint Inhibitors
Dendritic Cell Vaccine Based on Nanobody
Nanobodies Engineering for CAR-T Technology
Nanobody-Based Drug Delivery
Nanobody-Drug Conjugates
Nanobodies-Based Viral Vectors
T Cell Engagers
Limitations of Nanobody-Based Therapy
Conclusions
Author contributions
Acknowledgements
Competing interests
Additional information
References
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Portilla, P.A.; Cancino-Villeda, L.; Coronado-Aceves, E.W.; Espitia-Pinzón, C. Nanoanticuerpos: desarrollo biotecnológico y aplicaciones. TIP 2021, 24. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Ding, C.; Zheng, D.; Ma, X.; Yi, L.; Tong, X.; Wu, C.; Xue, C.; Yu, Y.; Zhou, Q. Nanobody Conjugates for Targeted Cancer Therapy and Imaging. Technol. Cancer Res. Treat. 2021, 20. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Molina-Zapata, A.; Pose, A.G.; Moreno, E. Structural Insights into the Design of Synthetic Nanobody Libraries. Molecules 2022, 27, 2198. [Google Scholar] [CrossRef]
- Alexander, E.; Leong, K.W. Discovery of nanobodies: a comprehensive review of their applications and potential over the past five years. J. Nanobiotechnology 2024, 22, 1–36. [Google Scholar] [CrossRef]
- Liu, W.; Song, H.; Chen, Q.; Yu, J.; Xian, M.; Nian, R.; Feng, D. Recent advances in the selection and identification of antigen-specific nanobodies. Mol. Immunol. 2018, 96, 37–47. [Google Scholar] [CrossRef]
- Duggan, S. Caplacizumab: First Global Approval. Drugs 2018, 78, 1639–1642. [Google Scholar] [CrossRef]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2015, 57, 27–33. [Google Scholar] [CrossRef]
- Roovers, R.C.; Laeremans, T.; Huang, L.; De Taeye, S.; Verkleij, A.J.; Revets, H.; de Haard, H.J.; Henegouwen, P.M.P.v.B.E. Efficient inhibition of EGFR signalling and of tumour growth by antagonistic anti-EGFR Nanobodies. Cancer Immunol. Immunother. 2006, 56, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Rossotti, M.A.; Henry, K.A.; van Faassen, H.; Tanha, J.; Callaghan, D.; Hussack, G.; Arbabi-Ghahroudi, M.; MacKenzie, C.R. Camelid single-domain antibodies raised by DNA immunization are potent inhibitors of EGFR signaling. Biochem. J. 2019, 476, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, S.; Varese, M.; Sánchez-Navarro, M.; Vincke, C.; Teixidó, M.; García, J.; Muyldermans, S.; Giralt, E. Blocking EGFR Activation with Anti-EGF Nanobodies via Two Distinct Molecular Recognition Mechanisms. Angew. Chem. Int. Ed. Engl. 2018, 57, 13843–13847. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Xiao, Z.; Li, W.; Dimitrov, D.S.; Chen, W. Human Domain Antibodies to Conserved Epitopes on HER2 Potently Inhibit Growth of HER2-Overexpressing Human Breast Cancer Cells In Vitro. Antibodies 2019, 8, 25. [Google Scholar] [CrossRef]
- Araste, F.; Ebrahimizadeh, W.; Rasooli, I.; Rajabibazl, M.; Gargari, S.L.M. A novel VHH nanobody against the active site (the CA domain) of tumor-associated, carbonic anhydrase isoform IX and its usefulness for cancer diagnosis. Biotechnol. Lett. 2013, 36, 21–28. [Google Scholar] [CrossRef]
- Sadeghnezhad, G.; Romão, E.; Bernedo-Navarro, R.; Massa, S.; Khajeh, K.; Muyldermans, S.; Hassania, S. Identification of New DR5 Agonistic Nanobodies and Generation of Multivalent Nanobody Constructs for Cancer Treatment. Int. J. Mol. Sci. 2019, 20, 4818. [Google Scholar] [CrossRef]
- Godar, M.; Morello, V.; Sadi, A.; Hultberg, A.; De Jonge, N.; Basilico, C.; Hanssens, V.; Saunders, M.; Lambrecht, B.N.; El Khattabi, M.; et al. Dual anti-idiotypic purification of a novel, native-format biparatopic anti-MET antibody with improved in vitro and in vivo efficacy. Sci. Rep. 2016, 6, 31621. [Google Scholar] [CrossRef]
- Vosjan, M.J.; Vercammen, J.; Kolkman, J.A.; Walsum, M.S.-V.; Revets, H.; van Dongen, G.A. Nanobodies Targeting the Hepatocyte Growth Factor: Potential New Drugs for Molecular Cancer Therapy. Mol. Cancer Ther. 2012, 11, 1017–1025. [Google Scholar] [CrossRef]
- Sánchez-Martín, D.; Martínez-Torrecuadrada, J.; Teesalu, T.; Sugahara, K.N.; Alvarez-Cienfuegos, A.; Ximénez-Embún, P.; Fernández-Periáñez, R.; Martín, M.T.; Molina-Privado, I.; Ruppen-Cañás, I.; et al. Proteasome activator complex PA28 identified as an accessible target in prostate cancer by in vivo selection of human antibodies. Proc. Natl. Acad. Sci. 2013, 110, 13791–13796. [Google Scholar] [CrossRef]
- Schoonaert, L.; Rué, L.; Roucourt, B.; Timmers, M.; Little, S.; Chávez-Gutiérrez, L.; Dewilde, M.; Joyce, P.; Curnock, A.; Weber, P.; et al. Identification and characterization of Nanobodies targeting the EphA4 receptor. J. Biol. Chem. 2017, 292, 11452–11465. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Murad, Y.M.; Chang, C.-C.; Yang, M.-C.; Baral, T.N.; Cowan, A.; Tseng, S.-H.; Wong, A.; MacKenzie, R.; Shieh, D.-B.; et al. Single domain antibody against carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) inhibits proliferation, migration, invasion and angiogenesis of pancreatic cancer cells. Eur. J. Cancer 2012, 50, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Samec, N.; Jovcevska, I.; Stojan, J.; Zottel, A.; Liovic, M.; Myers, M.P.; Muyldermans, S.; Šribar, J.; Križaj, I.; Komel, R. Glioblastoma-specific anti-TUFM nanobody for in-vitro immunoimaging and cancer stem cell targeting. Oncotarget 2018, 9, 17282–17299. [Google Scholar] [CrossRef]
- Wang, D.; Hu, X.; Liu, C.; Jia, Y.; Bai, Y.; Cai, C.; Wang, J.; Bai, L.; Yang, R.; Lin, C.; et al. Protein C receptor is a therapeutic stem cell target in a distinct group of breast cancers. Cell Res. 2019, 29, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; et al. Screening and antitumor effect of an anti-CTLA-4 nanobody. Oncol. Rep. 39, 511–518 (2018).
- Rozan, C.; Cornillon, A.; Pétiard, C.; Chartier, M.; Behar, G.; Boix, C.; Kerfelec, B.; Robert, B.; Pèlegrin, A.; Chames, P.; et al. Single-Domain Antibody–Based and Linker-Free Bispecific Antibodies Targeting FcγRIII Induce Potent Antitumor Activity without Recruiting Regulatory T Cells. Mol. Cancer Ther. 2013, 12, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; et al. A GPC3-targeting bispecific antibody, GPC3-S-Fab, with potent cytotoxicity. J. Vis. Exp. 12, 57588 (2018).
- Li, Y.; Zhou, C.; Li, J.; Liu, J.; Lin, L.; Li, L.; Cao, D.; Li, Q.; Wang, Z. Single domain based bispecific antibody, Muc1-Bi-1, and its humanized form, Muc1-Bi-2, induce potent cancer cell killing in muc1 positive tumor cells. PLOS ONE 2018, 13, e0191024. [Google Scholar] [CrossRef]
- Homayouni, V.; Ganjalikhani-Hakemi, M.; Rezaei, A.; Khanahmad, H.; Behdani, M.; Lomedasht, F.K. Preparation and characterization of a novel nanobody against T-cell immunoglobulin and mucin-3 (TIM-3). 2016, 19, 1201–1208.
- Ma, L.; Zhu, M.; Gai, J.; Li, G.; Chang, Q.; Qiao, P.; Cao, L.; Chen, W.; Zhang, S.; Wan, Y. Preclinical development of a novel CD47 nanobody with less toxicity and enhanced anti-cancer therapeutic potential. J. Nanobiotechnology 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Farajpour, Z.; Rahbarizadeh, F.; Kazemi, B.; Ahmadvand, D. A nanobody directed to a functional epitope on VEGF, as a novel strategy for cancer treatment. Biochem. Biophys. Res. Commun. 2014, 446, 132–136. [Google Scholar] [CrossRef]
- Khodabakhsh, F.; Norouzian, D.; Vaziri, B.; Ahangari Cohan, R.; Sardari, S.; Mahboudi, F.; Behdani, M.; Mansouri, K.; Mehdizadeh, A. Development of a novel nano-sized anti-VEGFA nanobody with enhanced physicochemical and pharmacokinetic properties. Artif. Cells, Nanomed. Biotechnol. 2018, 46, 1402–1414. [Google Scholar] [CrossRef]
- Behdani, M.; Zeinali, S.; Khanahmad, H.; Karimipour, M.; Asadzadeh, N.; Azadmanesh, K.; Khabiri, A.; Schoonooghe, S.; Anbouhi, M.H.; Hassanzadeh-Ghassabeh, G.; et al. Generation and characterization of a functional Nanobody against the vascular endothelial growth factor receptor-2; angiogenesis cell receptor. Mol. Immunol. 2012, 50, 35–41. [Google Scholar] [CrossRef]
- Baharlou, R.; Tajik, N.; Behdani, M.; Shokrgozar, M.A.; Tavana, V.; Kazemi-Lomedasht, F.; Faraji, F.; Habibi-Anbouhi, M. An antibody fragment against human delta-like ligand-4 for inhibition of cell proliferation and neovascularization. Immunopharmacol. Immunotoxicol. 2018, 40, 368–374. [Google Scholar] [CrossRef]
- Khatibi, A.S.; Roodbari, N.H.; Majidzade-A, K.; Yaghmaei, P.; Farahmand, L. In Vivo Tumor-Suppressing and Anti-Angiogenic Activities of a Recombinant Anti-CD3ε Nanobody in Breast Cancer Mice Model. Immunotherapy 2019, 11, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Goyvaerts, C.; Dingemans, J.; De Groeve, K.; Heirman, C.; Van Gulck, E.; Vanham, G.; De Baetselier, P.; Thielemans, K.; Raes, G.; Breckpot, K. Targeting of Human Antigen-Presenting Cell Subsets. J. Virol. 2013, 87, 11304–11308. [Google Scholar] [CrossRef] [PubMed]
- Goyvaerts, C.; De Vlaeminck, Y.; Escors, D.; Lienenklaus, S.; Keyaerts, M.; Raes, G.; Breckpot, K. Antigen-presenting cell-targeted lentiviral vectors do not support the development of productive T-cell effector responses: implications for in vivo targeted vaccine delivery. Gene Ther. 2017, 24, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.J.; Dougan, M.; Jailkhani, N.; Ingram, J.; Fang, T.; Kummer, L.; Momin, N.; Pishesha, N.; Rickelt, S.; Hynes, R.O.; et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc. Natl. Acad. Sci. 2019, 116, 7624–7631. [Google Scholar] [CrossRef]
- An, N.; Hou, Y.N.; Zhang, Q.X.; Li, T.; Zhang, Q.L.; Fang, C.; Chen, H.; Lee, H.C.; Zhao, Y.J.; Du, X. Anti-Multiple Myeloma Activity of Nanobody-Based Anti-CD38 Chimeric Antigen Receptor T Cells. Mol. Pharm. 2018, 15, 4577–4588. [Google Scholar] [CrossRef]
- Taheri, F.H.; Hassani, M.; Sharifzadeh, Z.; Behdani, M.; Arashkia, A.; Abolhassani, M. T cell engineered with a novel nanobody-based chimeric antigen receptor against VEGFR2 as a candidate for tumor immunotherapy. IUBMB Life 2019, 71, 1259–1267. [Google Scholar] [CrossRef]
- Hassani, M.; Taheri, F.H.; Sharifzadeh, Z.; Arashkia, A.; Hadjati, J.; van Weerden, W.M.; Modarressi, M.H.; Abolhassani, M. Construction of a chimeric antigen receptor bearing a nanobody against prostate a specific membrane antigen in prostate cancer. J. Cell. Biochem. 2019, 120, 10787–10795. [Google Scholar] [CrossRef]
- Hassani, M.; Taheri, F.H.; Sharifzadeh, Z.; Arashkia, A.; Hadjati, J.; van Weerden, W.M.; Abdoli, S.; Modarressi, M.H.; Abolhassani, M. Engineered Jurkat Cells for Targeting Prostate-Specific Membrane Antigen on Prostate Cancer Cells by Nanobody-Based Chimeric Antigen Receptor. Iran. Biomed. J. 2019, 24, 81–88. [Google Scholar] [CrossRef]
- De Munter, S.; et al. Nanobody-based dual-specific CARs. Int. J. Mol. Sci. 19, 403 (2018).
- Xie, Y.J.; Dougan, M.; Ingram, J.R.; Pishesha, N.; Fang, T.; Momin, N.; Ploegh, H.L. Improved Antitumor Efficacy of Chimeric Antigen Receptor T Cells that Secrete Single-Domain Antibody Fragments. Cancer Immunol. Res. 2020, 8, 518–529. [Google Scholar] [CrossRef]
- Dougan, M.; Ingram, J.R.; Jeong, H.-J.; Mosaheb, M.M.; Bruck, P.T.; Ali, L.; Pishesha, N.; Blomberg, O.; Tyler, P.M.; Servos, M.M.; et al. Targeting Cytokine Therapy to the Pancreatic Tumor Microenvironment Using PD-L1–Specific VHHs. Cancer Immunol. Res. 2018, 6, 389–401. [Google Scholar] [CrossRef]
- Sadeghian-Rizi, T.; Behdani, M.; Khanahmad, H.; Sadeghi, H.M.; Jahanian-Najafabadi, A. Generation and Characterization of a Functional Nanobody Against Inflammatory Chemokine CXCL10, as a Novel Strategy for the Treatment of Multiple Sclerosis. CNS Neurol. Disord. - Drug Targets 2019, 18, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Li, R.; Li, Z.; Cho, J.H.; Guzman, J.S.; Kamm, R.D.; Ploegh, H.L. Remodeling of the Tumor Microenvironment by a Chemokine/Anti-PD-L1 Nanobody Fusion Protein. Mol. Pharm. 2019, 16, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Eichhoff, A.M.; Börner, K.; Albrecht, B.; Schäfer, W.; Baum, N.; Haag, F.; Körbelin, J.; Trepel, M.; Braren, I.; Grimm, D.; et al. Nanobody-Enhanced Targeting of AAV Gene Therapy Vectors. Mol. Ther. - Methods Clin. Dev. 2019, 15, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Ahani, R.; Roohvand, F.; Cohan, R.A.; Etemadzadeh, M.H.; Mohajel, N.; Behdani, M.; Shahosseini, Z.; Madani, N.; Azadmanesh, K. Sindbis Virus-Pseudotyped Lentiviral Vectors Carrying VEGFR2-Specific Nanobody for Potential Transductional Targeting of Tumor Vasculature. Mol. Biotechnol. 2016, 58, 738–747. [Google Scholar] [CrossRef]
- Shoae-Hassani, A.; Mortazavi-Tabatabaei, S.A.; Sharif, S.; Madadi, S.; Rezaei-Khaligh, H.; Verdi, J. Recombinant λ Bacteriophage Displaying Nanobody towards Third Domain of HER-2 Epitope Inhibits Proliferation of Breast Carcinoma SKBR-3 Cell Line. Arch. Immunol. et Ther. Exp. 2012, 61, 75–83. [Google Scholar] [CrossRef]
- Xing, J.; Lin, L.; Li, J.; Liu, J.; Zhou, C.; Pan, H.; Shu, R.; Dong, B.; Cao, D.; Li, Q.; et al. BiHC, a T-Cell–Engaging Bispecific Recombinant Antibody, Has Potent Cytotoxic Activity Against Her2 Tumor Cells. Transl. Oncol. 2017, 10, 780–785. [Google Scholar] [CrossRef]
- Li, L.; He, P.; Zhou, C.; Jing, L.; Dong, B.; Chen, S.; Zhang, N.; Liu, Y.; Miao, J.; Wang, Z.; et al. A Novel Bispecific Antibody, S-Fab, Induces Potent Cancer Cell Killing. J. Immunother. 2015, 38, 350–356. [Google Scholar] [CrossRef]
- Harwood, S.L.; Alvarez-Cienfuegos, A.; Nuñez-Prado, N.; Compte, M.; Hernández-Pérez, S.; Merino, N.; Bonet, J.; Navarro, R.; Henegouwen, P.M.P.V.B.E.; Lykkemark, S.; et al. ATTACK, a novel bispecific T cell-recruiting antibody with trivalent EGFR binding and monovalent CD3 binding for cancer immunotherapy. OncoImmunology 2017, 7, e1377874–e1377874. [Google Scholar] [CrossRef]
- van der Linden, R.; Frenken, L.; de Geus, B.; Harmsen, M.; Ruuls, R.; Stok, W.; de Ron, L.; Wilson, S.; Davis, P.; Verrips, C. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim. et Biophys. Acta (BBA) - Protein Struct. Mol. Enzym. 1999, 1431, 37–46. [Google Scholar] [CrossRef]
- Chen, Y. & Duong Van Hoa, F. Peptidisc-assisted hydrophobic clustering toward the production of multimeric and multispecific nanobody proteins. Biochemistry 64, 655–665 (2025).
- Alexander, E.; Leong, K.W. Discovery of nanobodies: a comprehensive review of their applications and potential over the past five years. J. Nanobiotechnology 2024, 22, 1–36. [Google Scholar] [CrossRef]
- Papadopoulos, K. P.; et al. Unexpected hepatotoxicity in a phase I study of TAS266, a novel tetravalent agonistic nanobody® targeting the DR5 receptor. Cancer Chemother. Pharmacol. 75, 887–895 (2015).
- Albert, S.; Arndt, C.; Feldmann, A.; Bergmann, R.; Bachmann, D.; Koristka, S.; Ludwig, F.; Ziller-Walter, P.; Kegler, A.; Gärtner, S.; et al. A novel nanobody-based target module for retargeting of T lymphocytes to EGFR-expressing cancer cells via the modular UniCAR platform. OncoImmunology 2017, 6, e1287246. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, R. C. G.; et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells. Oncoimmunology 7, e1375641 (2017).
- Liu, B.; Wu, P.; Sha, H.; Qian, H.; Wang, Q.; Yang, Y.; Yang, M.; Bian, X.; Cheng, L. Anti-EGFR-iRGD recombinant protein conjugated silk fibroin nanoparticles for enhanced tumor targeting and antitumor efficiency. OncoTargets Ther. 2016, 9, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Van De Water, J. A. J. M.; et al. Therapeutic stem cells expressing variants of EGFR-specific nanobodies have antitumor effects. Proc. Natl Acad. Sci. USA 109, 16642–16647 (2012).
- Karges, J.; Jakubaszek, M.; Mari, C.; Zarschler, K.; Goud, B.; Stephan, H.; Gasser, G. Synthesis and Characterization of an Epidermal Growth Factor Receptor-Selective RuII Polypyridyl–Nanobody Conjugate as a Photosensitizer for Photodynamic Therapy. ChemBioChem 2019, 21, 531–542. [Google Scholar] [CrossRef]
- Fang, Y.; Yao, G.; Zhong, W.; Duan, W.; Zhou, Z.; Wen, X.; Chen, Y.; Fang, J.; Wang, Y.; Jiang, W.; et al. Abstract 2857: DR30303, a SMART-VHHBody powered anti-CLDN18.2 VHH-Fc with enhanced ADCC activity for the treatment of gastric and pancreatic cancers. Cancer Res. 2022, 82, 2857–2857. [Google Scholar] [CrossRef]
- Yamamoto, N.; Koyama, T.; Shimizu, T.; Todaka, A.; Kawakami, T.; Erzen, D.; Sarashina, A.; Li, B.; Hou, J.; Yamazaki, K. Phase I study of the VEGF/Ang-2 inhibitor BI 836880 alone or combined with the anti-programmed cell death protein-1 antibody ezabenlimab in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2023, 91, 469–480. [Google Scholar] [CrossRef]
- Shimizu, T.; Nakajima, T.E.; Yamamoto, N.; Yonemori, K.; Koyama, T.; Kondo, S.; Sunakawa, Y.; Izawa, N.; Horie, Y.; Xiang, S.; et al. Phase I study of envafolimab (KN035), a novel subcutaneous single-domain anti-PD-L1 monoclonal antibody, in Japanese patients with advanced solid tumors. Investig. New Drugs 2022, 40, 1021–1031. [Google Scholar] [CrossRef]
- Li, J.; Deng, Y.; Zhang, W.; Zhou, A.-P.; Guo, W.; Yang, J.; Yuan, Y.; Zhu, L.; Qin, S.; Xiang, S.; et al. Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors. J. Hematol. Oncol. 2021, 14, 1–10. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Harb, W.; Peer, C.J.; Hua, Q.; Xu, S.; Lu, H.; Lu, N.; He, Y.; Xu, T.; Dong, R.; et al. First-in-Human Phase I Study of Envafolimab, a Novel Subcutaneous Single-Domain Anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors. Oncol. 2021, 26, e1514–e1525. [Google Scholar] [CrossRef]
- D’huyvetter, M.; De Vos, J.; Caveliers, V.; Vaneycken, I.; Heemskerk, J.; Duhoux, F.P.; Fontaine, C.; Vanhoeij, M.; Windhorst, A.D.; van der Aa, F.; et al. Phase I Trial of 131I-GMIB-Anti-HER2-VHH1, a New Promising Candidate for HER2-Targeted Radionuclide Therapy in Breast Cancer Patients. J. Nucl. Med. 2020, 62, 1097–1105. [Google Scholar] [CrossRef]
- Ramlau, R.; Kowalski, D.; Szczylik, C.; Szczęsna, A.; Wiatr, E.; Demas, S.; Chao, H.; Roszkowski-Sliz, K. P2.06-006 Phase I/II Dose Escalation Study of L-DOS47 as a Monotherapy in Non-Squamous Non-Small Cell Lung Cancer Patients. J. Thorac. Oncol. 2017, 12, S1071–S1072. [Google Scholar] [CrossRef]
- Piha-Paul, S.; Simon, G.; Belani, C.P.; Chao, H.; Gaspar, K.; Lee, B.; Dowlati, A. A Phase 1, Open-Label, Dose-Escalation Study of L-DOS47 in Combination With Pemetrexed Plus Carboplatin in Patients With Stage IV Recurrent or Metastatic Nonsquamous NSCLC. JTO Clin. Res. Rep. 2022, 3, 100408. [Google Scholar] [CrossRef] [PubMed]
- Kater, A.P.; Van De Donk, N.W.; Rodríguez-Otero, P.; Mateos, M.V.; Bosch, F.; Tucci, A.; Ghia, P.; Adang, A.E.; Parren, P.W.H.; Tuinhof, I.; et al. Lava-051, a Novel Bispecific Gamma-Delta T-Cell Engager (Gammabody), in Relapsed/Refractory MM and CLL: Pharmacodynamic and Early Clinical Data. Blood 2022, 140, 4608–4609. [Google Scholar] [CrossRef]
- Mehra, N.; et al. Early dose escalation of LAVA-1207, a novel bispecific gamma-delta T-cell engager (Gammabody), in patients with metastatic castration-resistant prostate cancer (mCRPC). American Society of Clinical Oncology ( 2023. [CrossRef]
- Martin, T.; et al. Updated results from CARTITUDE-1: phase Ib/II study of ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T cell therapy, in patients with relapsed/refractory multiple myeloma. Blood 138, 549 (2021).
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Zhao, W. H.; et al. Four-year follow-up of LCAR-B38M in relapsed or refractory multiple myeloma: a phase I, single-arm, open-label, multicenter study in China (LEGEND-2). J. Hematol. Oncol. 15, 86 (2022).
- van de Donk, N.; et al. B07: Safety and efficacy of ciltacabtagene autoleucel, a chimeric antigen receptor T-cell therapy directed against B-cell maturation antigen in patients with multiple myeloma and early relapse after initial therapy: CARTITUDE-2 results. HemaSphere 6, 9–10 (2022).
- Einsele, H.; et al. P08: CARTITUDE-2 UPDATE: Ciltacabtagene autoleucel, a B-cell maturation antigen–directed chimeric antigen receptor T-cell therapy, in lenalidomide-refractory patients with progressive multiple myeloma after 1–3 prior lines of therapy. HemaSphere 6, 15 (2022).
- Cohen, Y. C.; et al. Efficacy and safety of ciltacabtagene autoleucel (ciltacel), a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T-cell therapy, in lenalidomide-refractory patients with progressive multiple myeloma after 1–3 prior lines of therapy: Updated results from CARTITUDE-2. Blood 138, 3866 (2021).
- Agha, M.E.; van de Donk, N.W.; Cohen, A.D.; Cohen, Y.C.; Anguille, S.; Kerre, T.; Roeloffzen, W.; Schecter, J.M.; De Braganca, K.C.; Varsos, H.; et al. S185: CARTITUDE-2 COHORT B: UPDATED CLINICAL DATA AND BIOLOGICAL CORRELATIVE ANALYSES OF CILTACABTAGENE AUTOLEUCEL IN PATIENTS WITH MULTIPLE MYELOMA AND EARLY RELAPSE AFTER INITIAL THERAPY. HemaSphere 2022, 6, 86–87. [Google Scholar] [CrossRef]
- Brussel, U. Z. Quantification of 68-GaNOTA-Anti-HER2 VHH1 Uptake in Metastasis of Breast Carcinoma Patients and Assessment of Repeatability (VUBAR)—Pilot Study. ClinicalTrials.gov Identifier: NCT03924466 (2024). Available from: https://clinicaltrials.gov/study/NCT03924466?a=3.
- Alexion Pharmaceuticals, I. A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel, Multicenter Study to Evaluate the Safety and Efficacy of ALXN1720 in Adults With Generalized Myasthenia Gravis. ClinicalTrials.gov Identifier: NCT05556096.
| Nanobodies vs. Conventional Antibody | ||
| Structural simplicity | Single-domain structure | Heterotetrameric structure |
| Size | ~15 kDa | ~150 kDa |
| CDR | 3 CDRs (Longer CDR1 and CD3) | 3 CDRs |
| Stability | High. Generally, they are functional at high temperatures and different pH levels | Less stable under extreme temperatures and pH conditions |
| Solubility | High. They are rich in hydrophilic regions, preventing aggregation | Solubility is more variable and lower in some cases due to hydrophobic regions. Such regions increase the risk of aggregation |
| Affinity | High | High |
| Immunogenicity | Low | Higher than nanobodies, can induce immune responses |
| Antigenic Diversity | Both non-planar and planar epitopes | Mainly planar epitopes |
| Efficient Tissue Penetration | High | Low, due to larger size |
| Cost of Production | Low | High |
| Examples | Reference | |
| Targeting Modules (UniCAR) | Nanobody-based targeting modules that effectively retarget UniCAR T cells to induce EGFR+ tumor lysis. | [59] |
| γδ T Cell Activator | Nanobody as part of BiTE, targeting the EGFR and Vγ9Vδ2 T cells receptor stimulated T-cell mediated cytotoxicity against EGFR+ tumor cells in vivo. | [60] |
| Tumor Penetrating Peptides | Nanobodies conjugated to penetrating peptides to improve specificity and penetration. Anti-EGFR nanobodies fused to these peptides have demonstrated antitumor activity in vivo | [61] |
| Nanobody-Secreting Stem Cells | Therapeutic stem cells that secrete either anti-EGFR nanobodies conjugated to tumor necrosis factor-related apoptosis | [62] |
| Nanobodies in Photodynamic Therapy | Nanobody-photosensitizer conjugates demonstrated targeted phototoxicity in vitro and in vivo. Anti-EGFR nanobodies conjugated to a novel RuII polypyridyl complex reported EGFR-specific targeting | [63] |
| Clinical Trial ID/Phase | Title | Results | Reference |
| NCT05639153/ Phase I | A Trial to Evaluate Safety, Tolerability, Pharmacokinetics and Preliminary Efficacy of DR30303 in Patients with Advanced Solid Tumors | Completion date-30/04/2024 | [64] |
| NCT03972150/ Phase I | A Study to Find the Best Dose of BI 836880 Alone and in Combination with BI 754091 in Japanese Patients with Different Types of Advanced Cancer | The maximum tolerated dose was not reached. BI 836880 alone and in combination with ezabenlimab had a manageable safety profile with preliminary clinical activity in Japanese patients with advanced solid tumors | [65] |
| NCT03248843/ Phase I | A Study of PD-L1 Antibody KN035 in Japanese Subjects with Locally Advanced or Metastatic Solid Tumors | Well-tolerated with efficacy. Pharmacokinetics data and preliminary anti-tumor response support dose regimens | [66] |
| NCT03667170/ Phase I | KN035 in Subjects with Advanced Solid Tumors | Completion date-15/12/2025 | [67] |
| NCT02827968/ Phase I | Phase 1 Study of Anti-PD-L1 Monoclonal Antibody KN035 to Treat Locally Advanced or Metastatic Solid Tumors | Favorable safety and pharmacokinetic profile, with promising preliminary antitumor activity in patients with advanced solid tumors | [68] |
| NCT02683083/ Phase I | [131I]-SGMIB Anti-HER2 VHH1 in Patients with HER2+ Breast Cancer | No drug-related adverse events with 131I-GMIB-anti-HER2-VHH1, primarily eliminated through the kidneys, stability in circulation, exhibited specific uptake in metastatic lesions in advanced breast cancer patients | [69] |
| NCT02340208/ Phase I/II | A Phase I/II Open-Label, Non-Randomized Dose Escalation Study of Immunoconjugate L-DOS47 | One dose-limiting toxicity (spinal pain) observed, no complete or partial responses were seen, 32 patients achieved stable disease after two treatment cycles, one patient in cohort 9 remained on treatment for 10 cycles without disease progression | [70] |
| NCT02309892/ Phase I | A Phase I, Open Label, Dose Escalation Study of Immunoconjugate L-DOS47 in Combination with Pemetrexed/Carboplatin in Patients with Stage IV (TNM M1a and M1b) Recurrent or Metastatic NSCLC Lung Cancer | L-DOS47 combined with standard pemetrexed, and carboplatin chemotherapy is well tolerated in patients with recurrent or metastatic nonsquamous NSCLC | [71] |
| NCT04887259/ Phase I/IIa | Trial of LAVA-051 in Patients with Relapsed/Refractory CLL, MM, or AML | Completion – 30/12/2024 | [72] |
| NCT05369000/ Phase I/IIa | Trial of LAVA-1207 in Patients with Therapy Refractory Metastatic Castration Resistant Prostate Cancer | Completion—30/03/2024 | [73] |
| NCT03548207/ Phase Ib/2 | A Phase 1b-2, Open-Label Study of JNJ-68284528, A Chimeric Antigen Receptor T-Cell (CAR-T) Therapy Directed Against BCMA in Subjects with Relapsed or Refractory Multiple Myeloma | With a median follow-up of 18 months, results show significant, long-lasting responses in heavily treated multiple myeloma patients, the treatment maintained a manageable safety profile without any new safety concerns | [74,75] |
| NCT03090659/ Phase 1/2 | A Clinical Study of Legend Biotech BCMA-chimeric Antigen Receptor Technology in Treating Relapsed/Refractory (R/R) Multiple Myeloma Patients | Completion—31/12/2023 | [76] |
| NCT04133636/ Phase 2 | A Phase 2, Multicohort Open-Label Study of JNJ-68284528, A Chimeric Antigen Receptor T-Cell (CAR-T) Therapy Directed Against BCMA in Subjects with Multiple Myeloma | Completion—13/11/2028. Interim results—responses with manageable safety, responses in pts with ineffective or insufficient response to autologous stem cell transplantation | [77,78] |
| NCT03924466/ Phase II | Quantification of 68-GaNOTA-Anti-HER2 VHH1 Uptake in Metastasis of Breast Carcinoma Patients and Assessment of Repeatability (VUBAR) – Pilot Study | Completion-31/12/2024 | [79] |
| NCT05556096/ Phase III | A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel, Multicenter Study to Evaluate the Safety and Efficacy of ALXN1720 in Adults with Generalized Myasthenia Gravis | Completion-07/07/2027 | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





