1. Introduction
The COVID-19 pandemic had widespread fear and uncertainty given its high transmission and serious health risks. Zoonotic diseases are infectious diseases that may majorly be classified as smallpox, monkeypox, chickenpox, and cowpox, these cause severe health problems for humans [
1]. The outbreak of monkeypox is also a concern as the covid-19 where spread may raise more anxiety associated with the fear of lockdown, isolation, and restrictions. The rise of the emergence of zoonotic diseases, mostly pox from the poxvirus [
2]. Traditional methods used are not convenient for the diagnosis of the pox virus, there is a gap between the new approaches developed. The World Health Organization has classified Mpox as a viral disease spread from animal to human or human to human. Over history, pox diseases have increasingly challenged human health. Smallpox is the deadliest in the history of pox virus which was globally eradicated in the 1980s [
3]. The monkeypox is less severe than the smallpox. The transmission and evolution of the virus should be analyzed for the crucial development of effective diagnosis and preventive measures to control the spread. The pox virus is identified by the skin rashes and the lesions over the body but it’s hard to identify the category of poxes as chickenpox, monkeypox, or cowpox [
4]. The visual representation of the monkeypox is larger, deeper, and appears all at once, the chickenpox is smaller, red itchy with fluid-filled blisters, and for the cowpox will be red papules, larger. Compared to monkeypox chickenpox is less painful, they visually look almost similar and the visual identification will be inaccurate [
5]. This underscores the need for an accurate diagnosis. This paper highlights the major health challenge by concentrating on pox detection by artificial intelligence.
To diagnose the pox disease in history they used the method to identify the pox diseases by the visual identification through eyes, differentiating that on various stages will be difficult. The diagnosis with the wrong assumptions may lead to complications. This method may confuse poxes. The medical practitioner treated the poxes through a laboratory test called the PCR polymerase chain reaction. This test is used to find and copy small amounts of DNA [
6]. This is majorly used to detect the Mpox, by many copies of their genetic material so the virus can be easily identified. PCR is an effective method for the poxvirus which detects the small traces of sample present but the major drawback of the PCR is it consumes time and is costly, which can be done by a trained lab expert. It’s hard for rural people because PCR is not always available [
7].
The pox-infected patient in the starting stage may be confused about which type of pox they have infected, with and what are medication should they consume. In the proposed work the method is used to rectify the problem faced by the infected host. The main idea of the project is to classify the pox diseases using artificial intelligence. In artificial intelligence, deep learning is the subset of machine learning which is a powerful branch that favors identifying computer patterns in large datasets, useful for medical image processing and speech recognition [
8]. These approaches consist of two major steps known as feature extraction and classification. Convolutional neural networks recognize main patterns in the data, the multi-layered neural networks can be used for extracting the features to classify the input accurately. Neural Networks are developed by the inspiration of the human brain, which processes the data through the interconnected layers, with CNNs analyzing the images. The transfer learning makes the models in CNN more effective and efficient by using pre-trained networks like VGG19, and ResNet, allowing the model to adapt quickly to newer tasks like detecting diseases. The dataset used for image processing is downloaded from the website called Kaggle. It is used to train and validate the model. The model’s efficiency will be analyzed through customized metrics including accuracy, precision, f1-score, sensitivity, and specificity. Pattern recognition enhances artificial intelligence by recognizing the trends and grouping the same data, enabling the application in facial recognition, and medical diagnostics [
9].
2. Related Works
The significant raise in artificial intelligence and the deep learning had played a major role in the disease’s prediction with fast, accurate, and assessable diagnostic tool. Recently the growth of the artificial intelligence has developed the convolution neural network and transfer learning to enhance the pox disease detection.
Oyola et al. developed a model with statistical analysis to differentiate between chickenpox and herpes zoster with edge detection, and color transformation, which displays the efficiency of computer vision [
10]. Islam et al. developed a dataset MONKEYPOX SKIN IMAGE DATASET 2022 for disease detection like monkeypox, chickenpox, cowpox, measles, and healthy skin to train the AI models more efficiently [
11]. Sorayaie Azar et al. analyzed seven deep learning neural networks DNN and the denseNet201 performed with a high accuracy of 97.63%. The proposed work of this project Grad-Cam, an explainable AI technique that highlights the skin lesions influencing predictions, increasing model efficiency [
12]. Nayak et al. developed a deep learning model with the pre-trained network ResNet and SqueezeNet to distinguish between poxes like monkeys from measles, chickenpox, and cowpox. The model achieved an F1-score of 92.55%. The research paper focuses on distinguishing between visually the same infections, to classify them with great accuracy optimization techniques were used [
13]. Eliwa et al. implemented a deep learning model for the disease classification of monkeypox using Grey Wolf Optimizer (GWO) in CNN achieving an accuracy of 95.3% [
14]. Bala et al. designed a CNN model for pox disease detection using deep learning DenseNet-201 with an augmented and original dataset achieving accuracy of 93.19% and 98.91% [
15]. Labcharoenwongs et al. innovated an Efficient net of deep learning techniques for the classification of monkeypox lesions on skin with an accuracy of .95 for pox and .97 for rash stage classification [
16].
Campana et al. developed a transfer learning model with a self-screening mobile tool for quick earlier detection in remote areas [
17]. Ali et al. introduced racially diverse skin lesion datasets, improving AI fairness and removing systematic errors in models’ classification. Epidemiology, Evolution, and Transmission Studies, While AI advancements have focused on image-based classification, epidemiological studies have explored the spread and transmission of monkeypox [
18]. Alakunle et al. have written a review about monkeypox, which concentrates on a moto of one health approach for humans, animals, and the environment. to control outbreaks happening around public health worldwide [
19]. Khattak et al. Strengthening the risk of monkeypox virus turns into a weapon used in bioterrorism of MPXV, claiming for lab tests beside Artificial Intelligence developed earlier detection tools. Transfer Learning and pre-trained model trained with high accuracy for earlier pox detection [
20]. Almufareh et al. trained the transfer learning for monkeypox detection, replacing the traditional methods like laboratory tests, and polymerase chain reactions. They developed earlier easy detection of poxes without depending on any special lab facilities [
21].
Demir et al. introduced a multi-layered deep learning framework on the MNPDenseNet. They used techniques like nested patch division and SVM-based feature extraction. Majorly focused on the engineering in improving deep learning detection of the pox model with an accuracy of 91.87% [
22]. Pal et al. classified the pox, monkeypox, chickenpox, and smallpox. They explored the multiclass classification using some pre-trained networks, VGG19, ResNet50, and Autoencoders. The role of AI in public health disease prevention and outbreak management will be explained with an accuracy of 96.56% [
23]. Singhal et al. investigated ring vaccination strategies and underscored the AI-powered epidemiological modelling for monkeypox outbreaks [
24]. Thakur et al. examined the transmission of the pox virus by analyzing the patterns, which supported the integration of AI with public health surveillance to track mutation trends in emerging orthodox viral marks [
25]. Torky et al. introduced DenseNet-121, a deep learning architecture developed for the detection of Mpox, strengthening the value of deep learning for the earlier detection of disease diagnostics. The model of Dense Net achieved 93% accuracy [
26].
3. Materials and Methods
The methodology we propose will be discussed in this section and shown in
Figure 1.
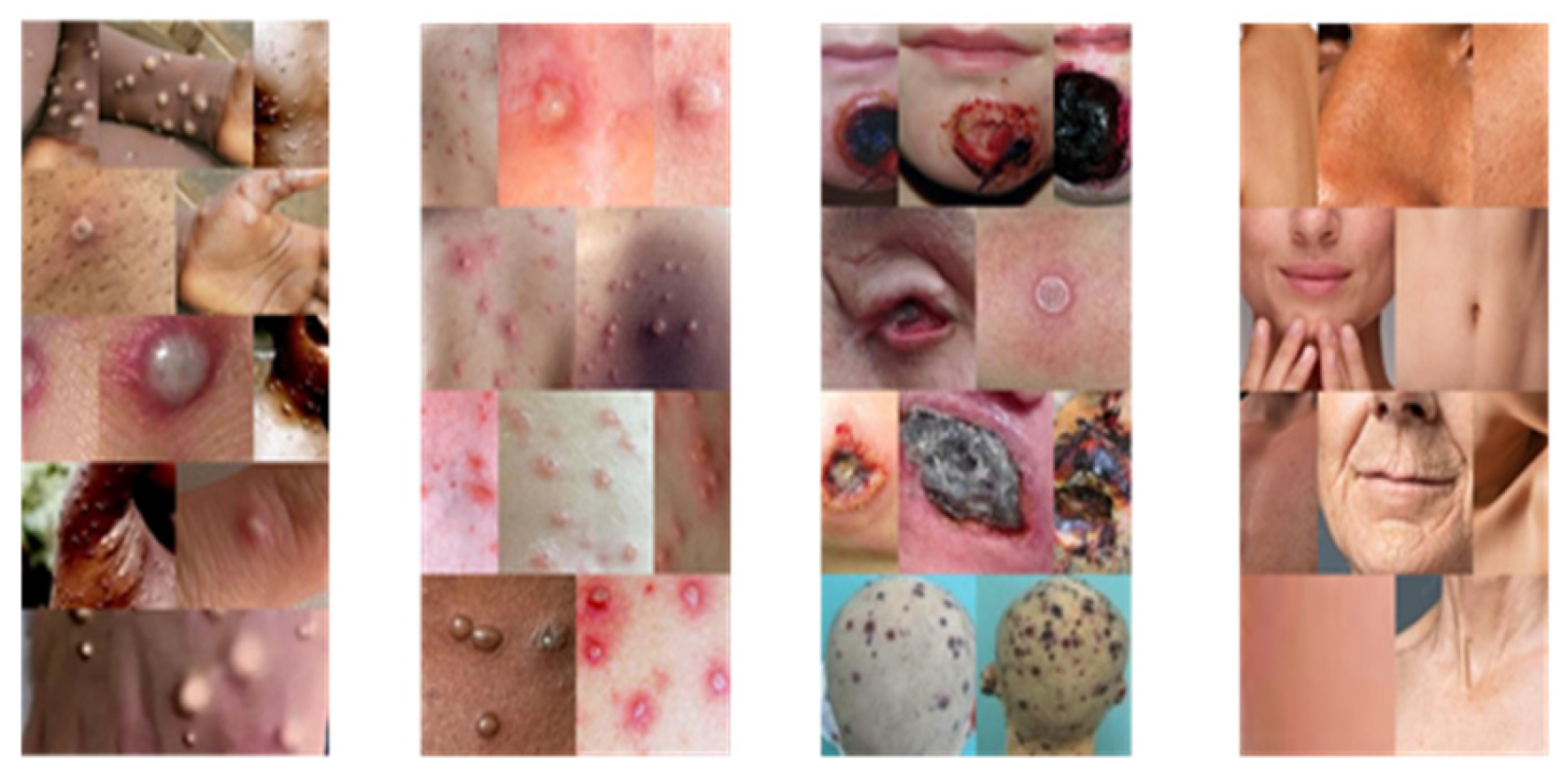
To develop an image processing model the initial criterion is the dataset, which is collected from the Kaggle website. The dataset contains the poxes like monkeypox, chickenpox, cowpox, and healthy. The images are resized in the 224x224 the height and weight of the pixel size. The RGB is the 3 color channels red, green, and Blue. Some of the dataset images are shown in
Figure 2.
To design the neural network transfer learning is one of the methods which is used to train the model. The collected dataset is loaded by the image datastore function, through the folder path, consisting of Train and valid folders. Load the CNN pre-trained networks, to evaluate the accuracy of the model to be developed.
We have majorly 15 networks, load the any net freeze the initial layer and replace the final layers. To freeze the initial layers, the main thing is to prevent from updating during training the dataset. The final layer will be replaced by unlocking the layers replace with the new fully connected to achieve the new task for the given dataset. The dataset will be split into valid and trained for 70 % and 30%.
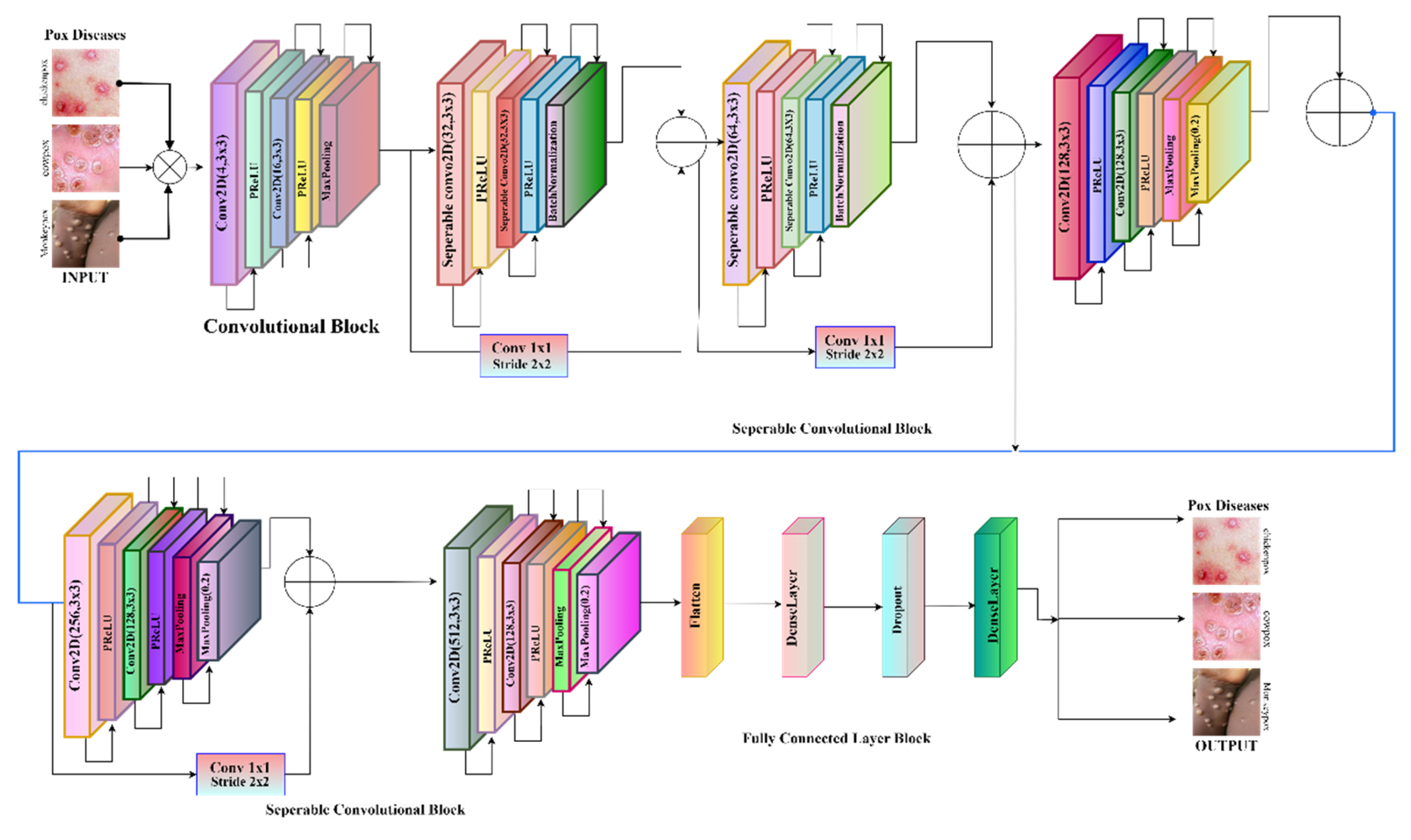
To train the transfer learning model will be run through the deep network designer. For 15 pretrained models, we got mostly accuracies between the 80-90%. The highest accuracy was for the Xception net with an accuracy of 95.76%. Proposed Xception Net is shown in
Figure 3. The Xception net is also known as the extreme inception net, the improved architecture of the inception by replacing traditional convolution with depth-wise separable convolution can reduce the computation and improve efficiency.
Architecture of Xception Network is shown in
Figure 4. It has 71 layers making it deeper than standard CNN models. The operation of the Xception net contains three major steps the entry, middle, and exit flow. The entry flow of the convolution layer is the initial feature extraction using the 2d convolution layers. The depth-wise separable convolution replaces standard convolution to process spatial and channel-wise information separately. The middle flow has 36 convolutional layers structured into 14 blocks, enhancing deep feature extraction for repeated depth-wise separable convolutions.it is focused on complex pattern recognition. The exit flow, the final convolution where the refinement of extracted feature.
4. Results and Analysis
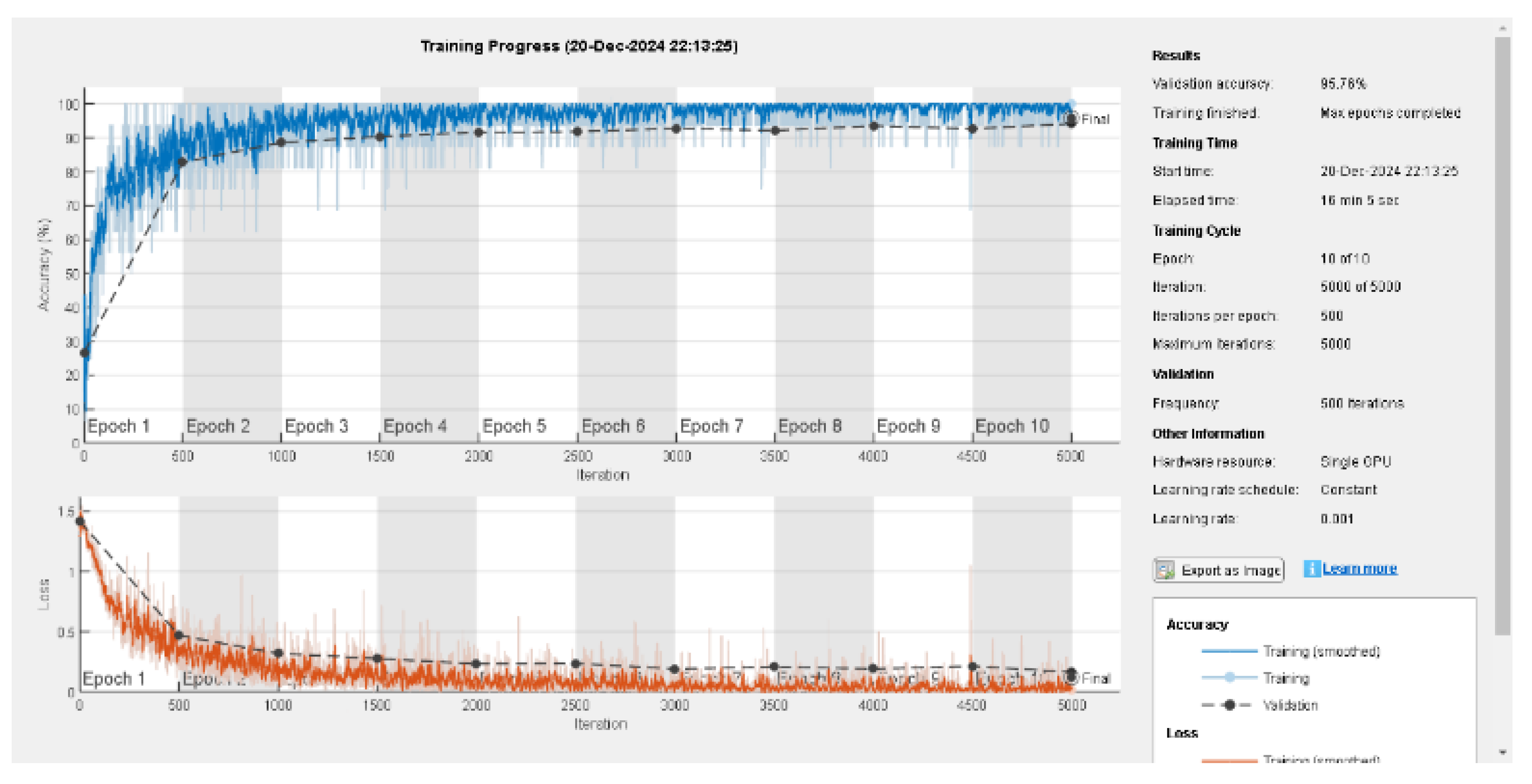
In the section the result assigned for the Xception will be explained and the evaluation matrix with the graphical representation. The training progress of the Xception network for pox disease classification is depicted in the graph shown below. The accuracy curve demonstrates a steady increase, with the training accuracy approaching 100% and the validation accuracy stabilizing at 95.76%, indicating strong generalization.
Figure 5.
Training Progress of Proposed Net.
Figure 5.
Training Progress of Proposed Net.
4.1. Experimental Manager and Evaluation Metrics
The primary details of the Evaluation metrics are essential for assessing the performance of a deep learning model. They help determine how well the model is classifying images and detecting patterns. In pox disease classification, these metrics ensure the model makes accurate and reliable predictions. One of the evaluation metrics is the confusion metric, which is used to find the accuracy, precision, recall, score, sensitivity, and specificity with the visualization of the model’s performance.
In the experimental manager setup, learning rates of [0.01, 0.001, 0.0001] were tested, representing high, medium, and low learning rates. The batch sizes used were [128, 256, 512], categorized as large batch sizes, and the number of epochs varied across [5, 10, 15, 20]. According to the analysis of the experimental manager setup, the training accuracy and training loss indicates that a learning rate of 0.01 results in minimal loss and high accuracy compared to learning rates of 0.001 and 0.0001. A total of 36 trials were conducted for image classification using sweeping parameters, with 12 trials for each learning rate. When using a fixed learning rate of 0.01, three batch sizes [128, 256, 512] and four epoch values [5, 10, 15, 20] were analyzed. Customized confusion metrics, including accuracy, precision, recall, F1-score, sensitivity, and specificity, were used for evaluating the experimental setup.
4.2. Confusion Matrix
A confusion metric is a tool used to evaluate the performance of a classification model by comparing predict labels with actual labels. It is a square metric, typically for binary or multiclass classification problems, where rows represent the actual class and columns predicted classes. Confusion matrix of validation data is shown in
Figure 6.
There are four key components:
True positives—correctly predicted positive cases
True negatives- correctly predicted negative cases
False positives—negative cases incorrectly predicted as positives (Type 1 error)
False negatives- positive cases incorrectly predicted as negative (Type 2 error)
4.2.1. Accuracy
Accuracy measures the proportion of correctly predicted instances out of the total instances.
4.2.2. Precision
Precision calculates how many of the predicted positive instances were correct.
4.2.3. Recall & Sensitivity
Recall, also known as Sensitivity or True Positive Rate, measures how well a model correctly identifies actual positive cases.
4.2.4. F1-Score
F1-Score is the harmonic mean of precision and recall, balancing both metrics.
4.2.5. Specificity
Specificity measures the proportion of actual negatives correctly identified.
4.3. Analysis of Model Performance Using a Fixed Learning Rate@0.01
4.3.1. Batch 128
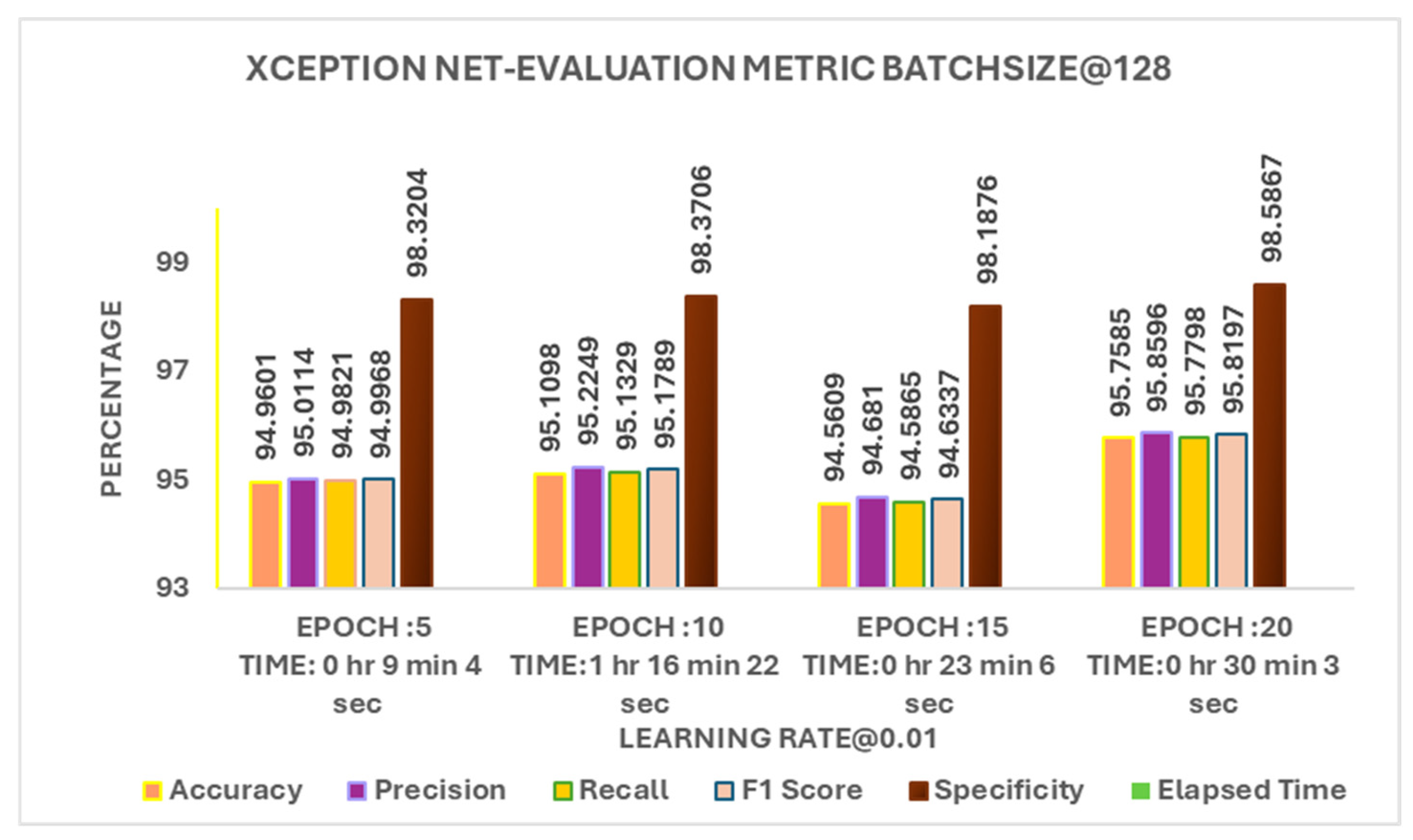
With a learning rate of 0.01 and a batch size of 128, the model trained for different epochs shown in
Figure 7. 5 epochs had the shortest training time (9 minutes) with 94% accuracy. 10 and 15 epochs achieved around 94-95% accuracy in about 20 minutes. 20 epochs gave the highest accuracy but took 30 minutes to train. This shows a trade-off more epochs improve accuracy, but they also increase training time. 10 epochs seem to be a good balance between accuracy and efficiency.
4.3.2. Batch 256
With a learning rate of 0.01 and a batch size of 256, the model trained for different epochs shown in
Figure 8. 5 epochs had the shortest training time with 93.86% accuracy. 10 and 15 epochs improved accuracy to around 94.51% and 94.96%, showing better precision, recall, and F1-score. 20 epochs achieved the highest accuracy (95.66%) and specificity (98.55%) but required more training time. This highlights a trade-off—higher epochs improve accuracy but increase training time. 10-15 epochs seem to provide a good balance between accuracy and efficiency.
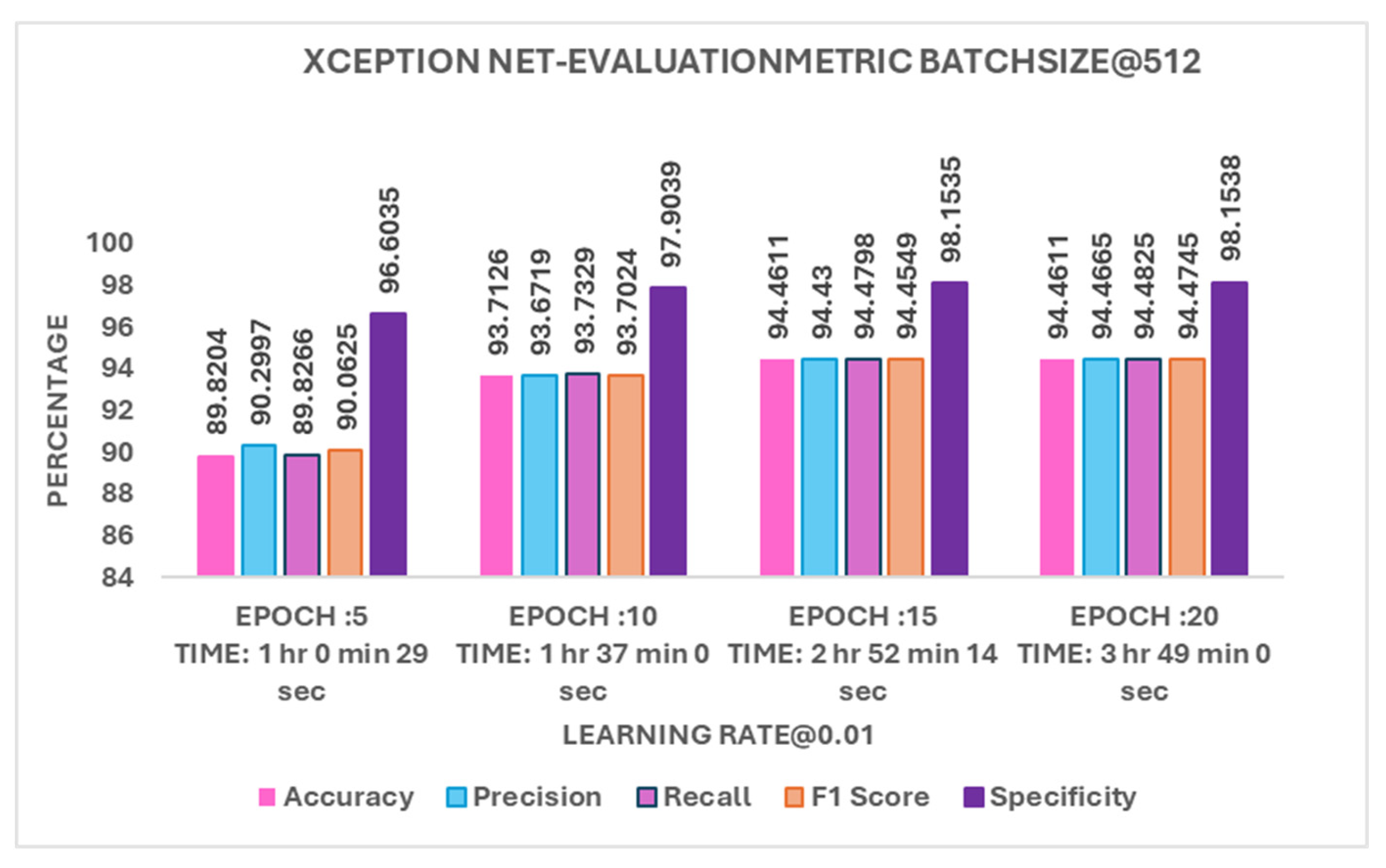
4.3.3. Batch 512
The model performed better as the number of epochs increased with batch 512 as shown in
Figure 9. At 5, accuracy was 89.82%. With 10, it improved to 93.71%, and by 15 and 20, it reached 94.46% with little difference between them. Since training beyond 15 did not add much improvement, 10-15 provides a good balance between accuracy and training time.
Based on the analysis, all three batch sizes showed the highest performance at epoch 20. The batch size of 128 achieved the highest accuracy of 95.76%, followed by batch size 256 with 95.66% accuracy at epoch 20. For batch size 512, both epochs 15 and 20 performed best, reaching 94.46% accuracy. This indicates that batch size 128 with 20 epochs provides the best overall performance in terms of accuracy and efficiency. We have compared other models with same dataset for the detection of pox diseases in
Table 1. Comparing these algorithms our proposed model performed better than models proposed by [
29,
30,
31]. Furthermore, we need to optimize proposed models to get better results comparing to YOLO and GAN based networks.
7. Conclusions
In this study, the solution for the pox diseases is developed through the deep learning-based model using transfer learning with the Xception network. The model trained using the dataset collected from the Kaggle website, consist of four class, as chickenpox, monkeypox, cowpox, and healthy skin. Through an experimental setup with various epochs and batches were analyzed different setups. on the Thorough analysis the batch 128 got the high evaluation of accuracy of 95.76%. The model evaluated through the customized metrics for the efficient performance precision of 95.85%, Recall 95.77%, F1-score 95.81%, sensitivity 95.77% and specificity 98.58%. The experimental results confirmed that the proposed model provides a reliable method for pox disease detection from earlier stage, with minimal false positives and false negatives. The use of transfer learning with Xception proved to be highly efficient, leveraging pretrained knowledge to enhance feature extraction and classification accuracy.
The future work will be focused on increasing the quality of the dataset and integrating the deep learning Xception network model into a real time application or website. By adding the extra feature to enhance the model performance, as connecting to the physician / clinician for the final decision making. The improvements will be done for making the model with high accuracy as more accessible for the remote area people as detect the skin lesions in the earlier stages through a mobile phone. This research highlights the potential of deep learning in dermatological disease detection, the way for AI-driven solutions in medical diagnostics.
References
- Deshmukh, R., Sawant, N., Gadgil, I., Pawar, G., & pagar, khushali. (2024). Monkey pox—A Review on Transmission, Diagnosis, treatment and current scenario. International Journal For Multidisciplinary Research, 6(4). [CrossRef]
- Anil, S., Joseph, B., Thomas, M., Sweety, V. K., Suresh, N., & Waltimo, T. (2024). Monkeypox: A Viral Zoonotic Disease of Rising Global Concern. Infectious Diseases & Immunity, 4(3), 121–131. [CrossRef]
- MacNeill AL. Comparative Pathology of Zoonotic Orthopoxviruses. Pathogens. 2022 Aug 9;11(8):892. PMID: 36015017; PMCID: PMC9412692. [CrossRef]
- Kundu, N. K., Karim, M., Kobir, S., & Farid, D. M. (2023, December). Attention Based Feature Fusion Network for Monkeypox Skin Leison Detection. In 2023 26th International Conference on Computer and Information Technology (ICCIT) (pp. 1-6). IEEE. [CrossRef]
- Oncu, E. (2024). Combining CNNs and Symptom Data for Improved Monkeypox Virus Detection. [CrossRef]
- Nayak, Tushar & Chadaga, Krishnaraj & Sampathila, Niranjana & Mayrose, Hilda & Bairy, Muralidhar & Prabhu, Srikanth & K. S., Swathi & Umakanth, Shashikiran. (2023). Detection of Monkeypox from skin lesion images using deep learning networks and explainable artificial intelligence. Applied Mathematics in Science and Engineering. 31. 2225698. [CrossRef]
- Yu, T., Rong, Z., Gu, Z., Wei, H., Wang, Y., Song, R., ... & Wang, S. (2024). Detection of monkeypox virus based on a convenient and sensitive single-step RPA-CRISPR/Cas12a strategy. RSC advances, 14(21), 14775-14783. [CrossRef]
- Aldi, F., Nozomi, I., Sentosa, R. B., & Junaidi, A. (2023). Machine Learning to Identify Monkey Pox Disease. Sinkron: jurnal dan penelitian teknik informatika, 7(3), 1335-1347.
- Ali, M. U., Khalid, M., Alshanbari, H., Zafar, A., & Lee, S. W. (2023). Enhancing Skin Lesion Detection: A Multistage Multiclass Convolutional Neural Network-Based Framework. Bioengineering, 10(12), 1430.
- Oyola, J., Arroyo, V., Ruedin, A., & Acevedo, D. (2012). Detection of chickenpox vesicles in digital images of skin lesions. In Lecture notes in computer science (pp. 583–590). [CrossRef]
- Islam, T., Hussain, M. A., Chowdhury, F. U. H., & Islam, B. M. R. (2022). A web-scraped skin image database of monkeypox, chickenpox, smallpox, cowpox, and measles. [CrossRef]
- Azar, A. S., Naemi, A., Rikan, S. B., Mohasefi, J. B., Pirnejad, H., & Wiil, U. K. (2023). Monkeypox detection using deep neural networks. BMC Infectious Diseases, 23(1). [CrossRef]
- Nayak, T., Chadaga, K., Sampathila, N., Mayrose, H., Bairy, G. M., Prabhu, S., Katta, S. S., & Umakanth, S. (2023). Detection of Monkeypox from skin lesion images using deep learning networks and explainable artificial intelligence. Applied Mathematics in Science and Engineering, 31(1). [CrossRef]
- Eliwa, E.H.I., El Koshiry, A.M., Abd El-Hafeez, T. et al. Utilizing convolutional neural networks to classify monkeypox skin lesions. Sci Rep 13, 14495 (2023). [CrossRef]
- Bala, D., Hossain, M. S., Hossain, M. A., Abdullah, M. I., Rahman, M. M., Manavalan, B., Gu, N., Islam, M. S., & Huang, Z. (2023). MonkeyNet: A robust deep convolutional neural network for monkeypox disease detection and classification. Neural Networks, 161, 757–775. [CrossRef]
- Labcharoenwongs, P., Noolek, D., & Chunhapran, O. (2024). Monkeypox lesion and rash stage classification for self-screening on mobile application using deep learning technique. Current Applied Science and Technology, e0257989. [CrossRef]
- Campana, M. G., Colussi, M., Delmastro, F., Mascetti, S., & Pagani, E. (2024). A Transfer Learning and Explainable Solution to Detect mpox from Smartphones images. Pervasive and Mobile Computing, 98, 101874. [CrossRef]
- Ali, Shams Nafisa & Ahmed, Md. Tazuddin & Jahan, Tasnim & Paul, Joydip & Sani, s.M. & Noor, Nawsabah & Asma, Anzirun & Hasan, Taufiq. (2024). A Web-based Mpox Skin Lesion Detection System Using State-of-the-art Deep Learning Models Considering Racial Diversity. Biomedical Signal Processing and Control. 98. 106742. [CrossRef]
- Alakunle E, Kolawole D, Diaz-Cánova D, Alele F, Adegboye O, Moens U, Okeke MI. A comprehensive review of monkeypox virus and mpox characteristics. Front Cell Infect Microbiol. 2024 Mar 6; 14:1360586. PMID: 38510963; PMCID: PMC10952103. [CrossRef]
- Khattak S, Rauf MA, Ali Y, Yousaf MT, Liu Z, Wu DD, Ji XY. The monkeypox diagnosis, treatments and prevention: A review. Front Cell Infect Microbiol. 2023 Feb 6;12:1088471. PMID: 36814644; PMCID: PMC9939471. [CrossRef]
- Almufareh, Maram Fahaad et al. “A Transfer Learning Approach for Clinical Detection Support of Monkeypox Skin Lesions.” Diagnostics (Basel, Switzerland) vol. 13,8 1503. 21 Apr. 2023. [CrossRef]
- Demir, F.B., Baygin, M., Tuncer, I. et al. MNPDenseNet: Automated Monkeypox Detection Using Multiple Nested Patch Division and Pretrained DenseNet201. Multimed Tools Appl 83, 75061–75083 (2024). [CrossRef]
- Pal, Madhumita et al. “Deep and Transfer Learning Approaches for Automated Early Detection of Monkeypox (Mpox) Alongside Other Similar Skin Lesions and Their Classification.” ACS omega vol. 8,35 31747-31757. 23 Aug. 2023. [CrossRef]
- Singhal, T., Kabra, S.K. & Lodha, R. Monkeypox: A Review. Indian J Pediatr 89, 955–960 (2022). [CrossRef]
- Thakur, Manish et al. “Human monkeypox: epidemiology, transmission, pathogenesis, immunology, diagnosis and therapeutics.” Molecular and cellular biochemistry vol. 478,9 (2023): 2097-2110. [CrossRef]
- Torky, M., Bakheit, A., Bakry, M., & Hassanien, A. E. (2022). Deep learning model for recognizing monkey pox based on dense net-121 algorithm. medRxiv, 2022-12.
- Yasmin, F., Hassan, M. M., Hasan, M., Zaman, S., Kaushal, C., El-Shafai, W., & Soliman, N. F. (2023). PoxNet22: A fine-tuned model for the classification of monkeypox disease using transfer learning. Ieee Access, 11, 24053-24076. [CrossRef]
- Nayak, T., Chadaga, K., Sampathila, N., Mayrose, H., Gokulkrishnan, N., Prabhu, S., & Umakanth, S. (2023). Deep learningbased detection of monkeypox virus using skin lesion images. Medicine in Novel Technology and Devices, 18, 100243. [CrossRef]
- Uysal, F. (2023). Detection of monkeypox disease from human skin images with a hybrid deep learning model. Diagnostics, 13(10), 1772. [CrossRef]
- Ali, S. N., Ahmed, M. T., Jahan, T., Paul, J., Sani, S. S., Noor, N., ... & Hasan, T. (2024). A web-based mpox skin lesion detection system using state-of-the-art deep learning models considering racial diversity. Biomedical Signal Processing and Control, 98, 106742. [CrossRef]
- Zi, S. (2022, December). Monkeypox Diagnosis with Convolutional Neural Networks Combined with Colour Space Models. In Proceedings of the 2022 4th International Conference on Robotics, Intelligent Control and Artificial Intelligence (pp. 1179-1184). [CrossRef]
- Kundu, D., Rahman, M. M., Rahman, A., Das, D., Siddiqi, U. R., Alam, M. G. R., ... & Ali, Z. (2024). Federated deep learning for monkeypox disease detection on gan-augmented dataset. IEEE Access. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).