Submitted:
25 April 2025
Posted:
28 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
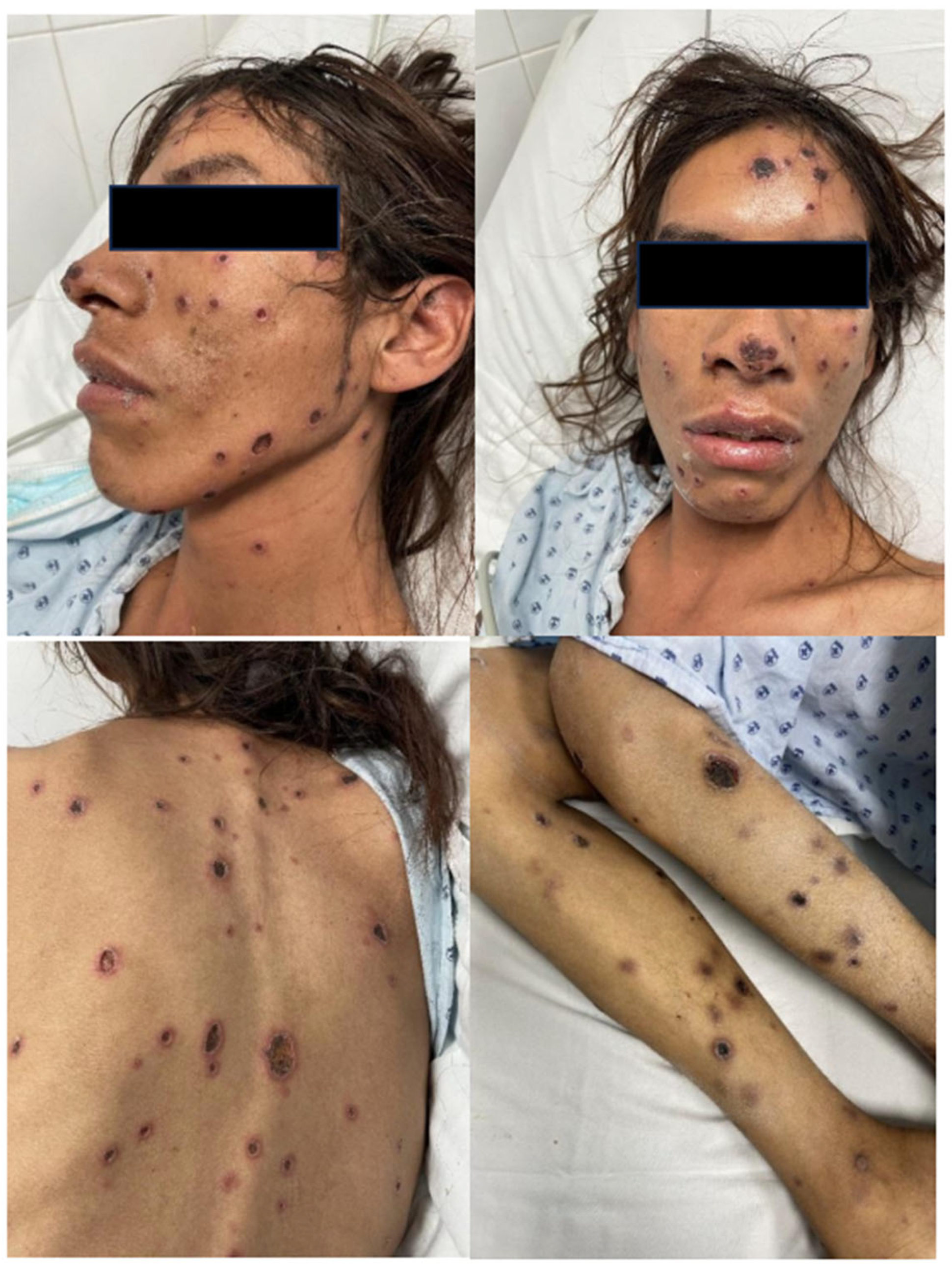
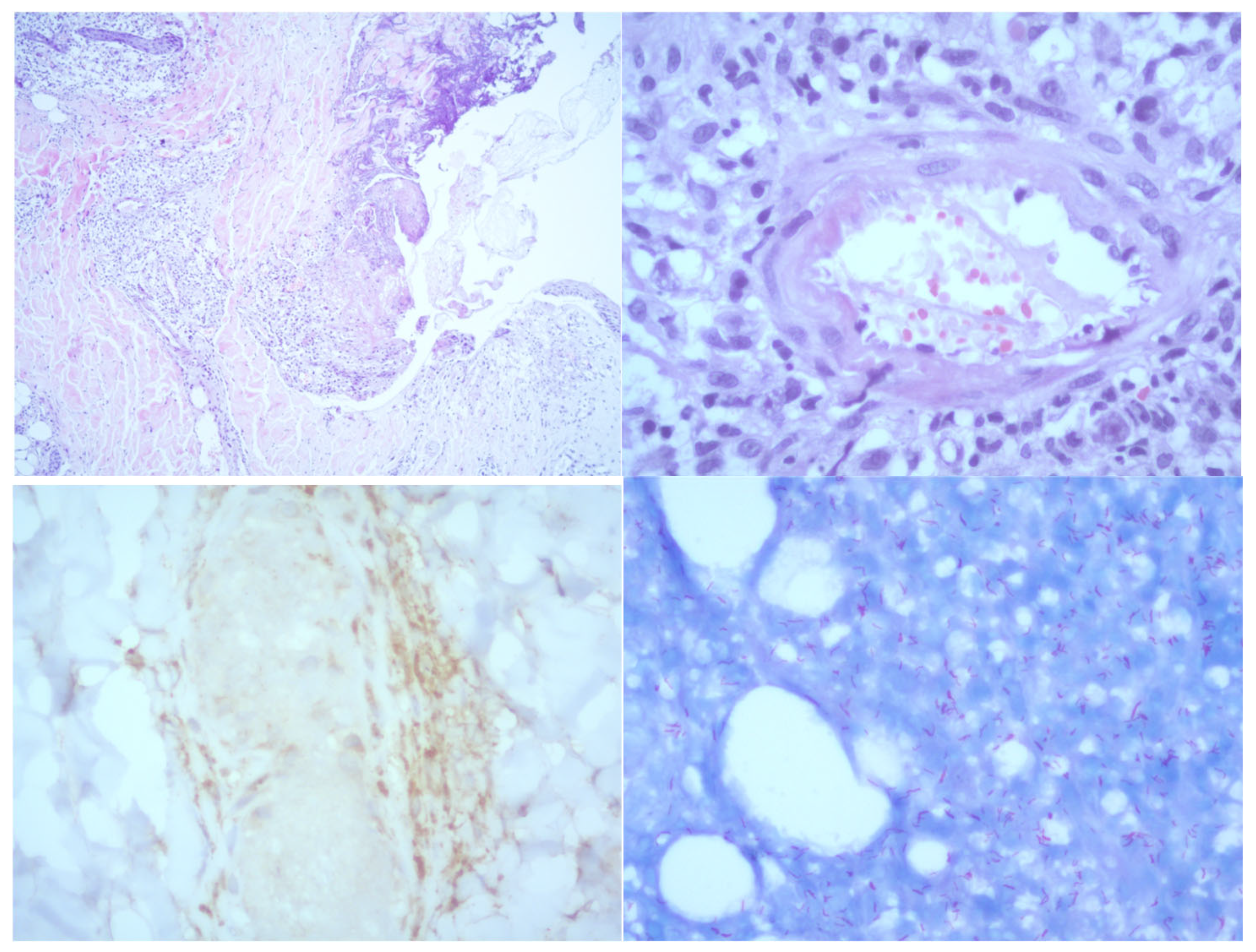
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, X.Y.; Mistry, N.A.; Thompson, E.J.; Tang, H.L.; Khanna, K.; Zhang, L. Draft Genome Sequence of New Leprosy Agent Mycobacterium lepromatosis. Genome Announc. 2015, 3. [Google Scholar] [CrossRef]
- Singh, P.; Benjak, A.; Schuenemann, V.J.; Herbig, A.; Avanzi, C.; Busso, P.; et al. Insight into the evolution and origin of leprosy bacilli from the genome sequence of Mycobacterium lepromatosis. Proceedings of the National Academy of Sciences. 2015, 112, 4459–4464. [Google Scholar] [CrossRef]
- Antunes, D.E.; Santos, D.F.; Lima, M.I.S.; Caixeta, L.P.; Correa, M.B.C.; Moraes ECdos, S.; et al. Clinical, epidemiological, and laboratory prognostic factors in patients with leprosy reactions: A 10-year retrospective cohort study. Front Med (Lausanne). 2022, 9. [Google Scholar] [CrossRef]
- Luo, Y.; Kiriya, M.; Tanigawa, K.; Kawashima, A.; Nakamura, Y.; Ishii, N.; et al. Host-Related Laboratory Parameters for Leprosy Reactions. Front Med (Lausanne). 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Góes, L.D.M.; Morais PMde Rebello, P.F.B.; Schettini, A.PM. Necrotic erythema nodosum reaction associated with histological alterations of Lucio’s phenomenon. An Bras Dermatol. 2022, 97, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Tourlaki, A.; Marzano, A.V.; Gianotti, R.; Fiallo, P.; Nunzi, E.; Alessi, E. Necrotic Erythema Nodosum Leprosum as the First Manifestation of Borderline Lepromatous Leprosy. Arch Dermatol. 2008, 144. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Singh, N.; Chatterjee, D.; Saikia, U.N.; Narang, T.; Dogra, S. Lucio phenomenon or necrotic erythema nodosum leprosum: walking the thin line. Int J Dermatol. 2020, 59. [Google Scholar] [CrossRef]
- Chandrashekar, L.; Kumari, R.; Thappa, D.; Badhe, B.; Ranugha, P. Is it lucio phenomenon or necrotic erythema nodosum leprosum? Indian J Dermatol. 2013, 58, 160. [Google Scholar] [CrossRef]
- Fogagnolo, L.; Souza EMde Cintra, M.L.; Velho, P.E.N.F. Vasculonecrotic reactions in leprosy. Brazilian Journal of Infectious Diseases. 2007, 11, 378–382. [Google Scholar] [CrossRef]
- Benard, G.; Sakai-Valente, N.Y.; Bianconcini Trindade, T.r.i.n.d.a.d.e. MA. Concomitant Lucio Phenomenon and Erythema Nodosum in a Leprosy Patient: Clues for Their Distinct Pathogeneses. Am J Dermatopathol. 2009, 31, 288–292. [Google Scholar] [CrossRef]
- Barreto Spandonari, C.; Villagra, D.J.; Flor, L.; Agüero Zaputovich, F.; Di Martino, B.; Aldama, A. Lucio´s phenomenon. About a case. Anales de la Facultad de Ciencias Médicas (Asunción). 2022, 55, 88–91. [Google Scholar] [CrossRef]
- Frade, M.A.C.; Coltro, P.S.; Filho, F.B.; Horácio, G.S.; Neto, A.A.; da Silva, V.Z.; et al. Lucio’s phenomenon: A systematic literature review of definition, clinical features, histopathogenesis and management. Indian J Dermatol Venereol Leprol. 2021, 88, 464. [Google Scholar] [CrossRef] [PubMed]
- Velarde-Félix, J.S.; Alvarado-Villa, G.; Vera-Cabrera, L. “Lucio’s Phenomenon” Associated with Mycobacterium lepromatosis. Am J Trop Med Hyg. 2016, 94, 483–484. [Google Scholar] [CrossRef]
- Ang, P.; Tay, Y.K.; Ng, S.K.; Seow, C.S. Fatal Lucio’s phenomenon in 2 patients with previously undiagnosed leprosy. J Am Acad Dermatol. 2003, 48, 958–961. [Google Scholar] [CrossRef]
- Aldama, A.; Wattiez, V.; Mendoza, G. Fenómeno de Lucio. Comunicación de 14 casos. Piel. 2018, 33, 81–85. [Google Scholar] [CrossRef]
- Curi, P.F.; Villaroel, J.S.; Migliore, N.; Albertengo, A.; Aquino, M.L.; Ceccato, F.; et al. Lucio’s phenomenon: report of five cases. Clin Rheumatol. 2016, 35, 1397–1401. [Google Scholar] [CrossRef]
- Yang, H.M.; Liu, S.W.; Li, Y.F.; Wang, Y.C. Lucio’s phenomenon. Journal of Microbiology, Immunology and Infection. 2023, 56, 647–648. [Google Scholar] [CrossRef]
- Dilipbhai Bodar, P.; Kailashbhai Patel, J.; Subramonia Pillai, D.; Vipul, V.o.r.a.R. Lucio phenomenon; A case report. Indian J Dermatol Venereol Leprol. 2023, 0, 1. [Google Scholar] [CrossRef]
- Han, X.Y.; Seo, Y.H.; Sizer, K.C.; Schoberle, T.; May, G.S.; Spencer, J.S.; et al. A New Mycobacterium Species Causing Diffuse Lepromatous Leprosy. Am J Clin Pathol. 2008, 130, 856–864. [Google Scholar] [CrossRef]
- Han, X.Y.; Quintanilla, M. Diffuse Lepromatous Leprosy Due to Mycobacterium lepromatosis in Quintana Roo, Mexico. J Clin Microbiol. 2015, 53, 3695–3698. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, V.D. Mycobacterium lepromatosis Lepromatous Leprosy in US Citizen Who Traveled to Disease-Endemic Areas. Emerg Infect Dis. 2019, 25, 389–390. [Google Scholar] [CrossRef]
- Collin, S.M.; Lima, A.; Heringer, S.; Sanders, V.; Pessotti, H.A.; Deps, P. Systematic Review of Hansen Disease Attributed to Mycobacterium lepromatosis. Emerg Infect Dis. 2023, 29. [Google Scholar] [CrossRef] [PubMed]
- Lucio, R.; Alvarado, I.; Francis, A. Opúsculo sobre el mal de San Làzaro, ó, Elefanciasis de los Griegos / escrito por los professores de medicina y cirugía Rafael Lucio e Ygnacio Alvarado.
- Latapi, F.; Chevez-Zamora, A. The “spotted” leprosy of Lucio: an introduction to its clinical and histological study. Int J Lepr. 1948, 16, 421–423. [Google Scholar]
- Han, X.Y.; Sizer, K.C.; Velarde-Félix, J.S.; Frias-Castro, L.O.; Vargas-Ocampo, F. The leprosy agents Mycobacterium lepromatosis and Mycobacterium leprae in Mexico. Int J Dermatol. 2012, 51, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Aung, F.M.; Choon, S.E.; Werner, B. Analysis of the Leprosy Agents Mycobacterium leprae and Mycobacterium lepromatosis in Four Countries. Am J Clin Pathol. 2014, 142, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, P.; McCoy, R.C.; Lenz, S.M.; Donovan, K.; Ochoa, M.T.; et al. Isolation of Mycobacterium lepromatosis and Development of Molecular Diagnostic Assays to Distinguish Mycobacterium leprae and M. lepromatosis. Clinical Infectious Diseases. 2020, 71, e262–e269. [Google Scholar] [CrossRef]
- Trave, I.; Barabino, G.; Cavalchini, A.; Parodi, A. Long-term ulcerations caused by Mycobacterium lepromatosis. Int J Mycobacteriol. 2020, 9, 223. [Google Scholar] [CrossRef]
- Han, X.Y.; Jessurun, J. Severe Leprosy Reactions Due to Mycobacterium lepromatosis. Am J Med Sci. 2013, 345, 65–69. [Google Scholar] [CrossRef]
- Vera-Cabrera, L.; Escalante-Fuentes, W.G.; Gomez-Flores, M.; Ocampo-Candiani, J.; Busso, P.; Singh, P.; et al. Case of Diffuse Lepromatous Leprosy Associated with “Mycobacterium lepromatosis”. J Clin Microbiol. 2011, 49, 4366–4368. [Google Scholar] [CrossRef]
- Massone, C.; Talhari, C.; Ribeiro-Rodrigues, R.; Sindeaux, R.H.M.; Mira, M.T.; Talhari, S.; et al. Leprosy and HIV coinfection: a critical approach. Expert Rev Anti Infect Ther. 2011, 9, 701–710. [Google Scholar] [CrossRef]
- Ustianowski, A.P.; Lawn, S.D.; Lockwood, D.N. Interactions between HIV infection and leprosy: a paradox. Lancet Infect Dis. 2006, 6, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Deps, P.; Lucas, S.; Porro, A.M.; Maeda, S.M.; Tomimori, J.; Guidella, C.; et al. Clinical and histological features of leprosy and human immunodeficiency virus co-infection in Brazil. Clin Exp Dermatol. 2013, 38, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.A.A.; Miranda MFRde Bittencourt Mde, J.S.; Brito ACde, X.a.v.i.e.r. MB. Comparison between histopathologic features of leprosy in reaction lesions in HIV coinfected and non-coinfected patients*. An Bras Dermatol. 2015, 90, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, E.P.; Caneshi, J.R.; Nery, J.A.; Duppre, N.C.; Pereira, G.M.; Vieira, L.M.; et al. Cellular immune response to Mycobacterium leprae infection in human immunodeficiency virus-infected individuals. Infect Immun. 1995, 63, 1848–1854. [Google Scholar] [CrossRef]
- Couppié, P.; Domergue, V.; Clyti, E.; Guedj MEl Vaz, T.; Sainte-Marie, D.; et al. Increased incidence of leprosy following HAART initiation: a manifestation of the immune reconstitution disease. AIDS. 2009, 23, 1599–1600. [Google Scholar] [CrossRef]
- George, A.; Vidyadharan, S. Hansen’s disease in association with immune reconstitution inflammatory syndrome. Indian Dermatol Online J. 2016, 7, 29. [Google Scholar] [CrossRef]
- Viard, J.P.; Caux, F.; Bille, E.; Lévy, A.; Charlier, C.; Lortholary, O.; et al. Unmasking Leprosy: An Unusual Immune Reconstitution Inflammatory Syndrome in a Patient Infected with Human Immunodeficiency Virus. Am J Trop Med Hyg. 2010, 83, 13–14. [Google Scholar]
- Mouchard, A.; Blaizot, R.; Graille, J.; Couppié, P.; Bertin, C. Leprosy as immune reconstitution inflammatory syndrome in patients living with HIV: Description of French Guiana’s cases over 20 years and systematic review of the literature. PLoS Negl Trop Dis. 2022, 16, e0010239. [Google Scholar] [CrossRef]
- Lockwood, D.N.J.; Lambert, S.M. Human Immunodeficiency Virus and Leprosy: An Update. Dermatol Clin. 2011, 29, 125–128. [Google Scholar] [CrossRef]
- Batista, M.D.; Porro, A.M.; Maeda, S.M.; Gomes, E.E.; Yoshioka, M.C.N.; Enokihara, M.M.S.S.; et al. Leprosy Reversal Reaction as Immune Reconstitution Inflammatory Syndrome in Patients with AIDS. Clinical Infectious Diseases. 2008, 46, e56–60. [Google Scholar] [CrossRef]
- Pires, C.A.A.; Jucá Neto, F.O.M.; de Albuquerque, N.C.; Macedo, G.M.M.; Batista Kde, N.M.; Xavier, M.B. Leprosy Reactions in Patients Coinfected with HIV: Clinical Aspects and Outcomes in Two Comparative Cohorts in the Amazon Region, Brazil. PLoS Negl Trop Dis. 2015, 9, e0003818. [Google Scholar] [CrossRef] [PubMed]
- Menezes, V.M.; Nery, J.A.C.; Sales, A.M.; Miranda, A.; Galhardo, M.C.G.; Bastos, F.I.; et al. Epidemiological and clinical patterns of 92 patients co-infected with HIV and Mycobacterium leprae from Rio de Janeiro State, Brazil. Trans R Soc Trop Med Hyg. 2014, 108, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Arakkal, G.; Damarla, S.; Chanda, G. Immune reconstitution inflammatory syndrome unmasking erythema nodosum leprosum: A rare case report. Indian J Dermatol. 2015, 60, 106. [Google Scholar] [CrossRef]
- Serrano-Coll, H.A.; Beltrán-Alzate, J.C.; Buitrago, S.M.; Cardona-Castro, N. Lepromatous leprosy and human immunodeficiency virus co-infection associated with phenomenon of Lucio versus immune reconstitution inflammatory syndrome. Infectio. 2016, 20, 272–275. [Google Scholar] [CrossRef]
- Cusini, A.; Gunthard, H.F.; Weber, R.; Huber, M.; Kamarashev, J.; Bertisch, B.; et al. Lepromatous leprosy with erythema nodosum leprosum as immune reconstitution inflammatory syndrome in an HIV-1 infected patient after initiation of antiretroviral therapy. Case Reports. 2009, 2009, bcr0520091904–bcr0520091904. [Google Scholar] [CrossRef] [PubMed]
- Bernardes Filho, F.; Pess, D.; Akabane, A.L.; Foss, N.T.; Frade, M.A.C. Lucio’s phenomenon: A life-threatening medical emergency. International Journal of Infectious Diseases. 2018, 69, 94–95. [Google Scholar] [CrossRef]
- Kumari, R.; Thappa, D.M.; Basu, D. A fatal case of Lucio phenomenon from India. Dermatol Online J. 2008, 14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




