Submitted:
24 April 2025
Posted:
25 April 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Study Population
OCT Image Acquisition and Analysis
Categorization of Patients
Statistical Analysis
Results
Baseline Characteristics of CAC
Plaque Vulnerability and CPB
Plaque Vulnerability and Calcification Patterns
Inflammation Index and Calcification
Risk Factors for the Presence of Calcification Deposits and CPB
Discussion
Study Limitations
Conclusions
Supplementary Materials
Funding
Data Availability Statement
Ethics Approval
Declaration of Competing Interest
Authorship Contribution Statement
Abbreviations
| CAC | Coronary artery calcification; |
| TCFA | Thin cap fibroatheromas; |
| OCT | Optical coherence tomography; |
| CPB | calcified plaque burden; |
| PCI | percutaneous coronary intervention; |
| UAP | unstable angina pectoris; |
| NSTEMI | non-ST-segment elevation myocardial infarction; |
| STEMI | ST-segment elevation myocardial infarction; |
| AMI | acute myocardial infarction |
| hs-CRP | high-sensitivity C-reactive protein; |
| LDLC | low-density lipoprotein cholesterol; |
| HDLC | high-density lipoprotein cholesterol; |
| NHR | neutrophil/HDL ratio; |
| MLA | minimal lumen area; |
| FCT | fibrous cap thickness; |
| ACS | acute coronary syndrome |
References
- Onnis, C, Virmani, R, Kawai, K, et al. Coronary Artery Calcification: Current Concepts and Clinical Implications. Circulation 2024, 149, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Razavi, AC, Agatston, AS, Shaw, LJ, et al. Evolving Role of Calcium Density in Coronary Artery Calcium Scoring and Atherosclerotic Cardiovascular Disease Risk. JACC Cardiovasc Imaging 2022, 15, 1648–1662. [Google Scholar] [CrossRef] [PubMed]
- Osborne-Grinter, M, Kwiecinski, J, Doris, M, et al. Association of coronary artery calcium score with qualitatively and quantitatively assessed adverse plaque on coronary CT angiography in the SCOT-HEART trial. Eur Heart J Cardiovasc Imaging 2022, 23, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Mori, H, Torii, S, Kutyna, M, et al. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC Cardiovasc Imaging 2018, 11, 11–142. [Google Scholar]
- Vancheri, F, Longo, G, Vancheri, S, et al., Coronary Artery Microcalcification: Imaging and Clinical Implications, Diagnostics (Basel), 2019;9.
- Nakahara, T, Dweck, MR, Narula, N, et al., Coronary Artery Calcification: From Mechanism to Molecular Imaging, JACC Cardiovasc Imaging, 2017;10:582-593.
- Onea, HL, Olinic, M, Lazar, FL, et al., A Review Paper on Optical Coherence Tomography Evaluation of Coronary Calcification Pattern: Is It Relevant Today?, J Cardiovasc Dev Dis, 2024;11.
- Fujimoto, D, Usui, E, Vergallo, R, et al. Relationship Between Coronary Artery Calcium Score and Vulnerability of Culprit Plaque Assessed by OCT in Patients With Established Coronary Artery Disease. Circ Cardiovasc Imaging 2025, 18, e017099. [Google Scholar]
- Fujimoto, D, Kinoshita, D, Suzuki, K, et al. Relationship Between Calcified Plaque Burden, Vascular Inflammation, and Plaque Vulnerability in Patients With Coronary Atherosclerosis. JACC Cardiovasc Imaging 2024, 17, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z, Yu, L, Zhang, Y, et al., Coronary artery calcification and plaque stability: an optical coherence tomography study, Heliyon, 2023;9:e23191.
- Araki, M, Park, SJ, Dauerman, HL, et al., Optical coherence tomography in coronary atherosclerosis assessment and intervention, Nat Rev Cardiol, 2022;19:684-703.
- Milzi, A, Burgmaier, M, Burgmaier, K, et al., Type 2 diabetes mellitus is associated with a lower fibrous cap thickness but has no impact on calcification morphology: an intracoronary optical coherence tomography study, Cardiovasc Diabetol, 2017;16:152.
- Tearney, GJ, OCT imaging of macrophages: a bright spot in the study of inflammation in human atherosclerosis, JACC Cardiovasc Imaging, 2015;8:73-75.
- Shi, X, Gao, J, Lv, Q, et al., Calcification in Atherosclerotic Plaque Vulnerability: Friend or Foe?, Front Physiol, 2020;11:56.
- Gallone, G, Bellettini, M, Gatti, M, et al., Coronary Plaque Characteristics Associated With Major Adverse Cardiovascular Events in Atherosclerotic Patients and Lesions: A Systematic Review and Meta-Analysis, JACC Cardiovasc Imaging, 2023;16:1584-1604.
- Sakaguchi, M, Hasegawa, T, Ehara, S, et al., New insights into spotty calcification and plaque rupture in acute coronary syndrome: an optical coherence tomography study, Heart Vessels, 2016;31:1915-1922.
- Ehara, S, Kobayashi, Y, Yoshiyama, M, et al., Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study, Circulation, 2004;110:3424-3429.
- Kinoshita, D, Suzuki, K, Usui, E, et al., High-Risk Plaques on Coronary Computed Tomography Angiography: Correlation With Optical Coherence Tomography, JACC Cardiovasc Imaging, 2024;17:382-391.
- Arnson, Y, Rozanski, A, Gransar, H, et al., Comparison of the Coronary Artery Calcium Score and Number of Calcified Coronary Plaques for Predicting Patient Mortality Risk, Am J Cardiol, 2017;120:2154-2159.
- Otsuka, F, Sakakura, K, Yahagi, K, et al., Has our understanding of calcification in human coronary atherosclerosis progressed?, Arterioscler Thromb Vasc Biol, 2014;34:724-736.
- Kolte, D, Yonetsu, T, Ye, JC, et al., Optical Coherence Tomography of Plaque Erosion: JACC Focus Seminar Part 2/3, J Am Coll Cardiol, 2021;78:1266-1274.
- Torii, S, Sato, Y, Otsuka, F, et al., Eruptive Calcified Nodules as a Potential Mechanism of Acute Coronary Thrombosis and Sudden Death, J Am Coll Cardiol, 2021;77:1599-1611.
- New, SE and Aikawa, E, Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification, Circ Res, 2011;108:1381-1391.
- Nelles, G, Abdelwahed, YS, Alyaqoob, A, et al., Spotty calcium deposits within acute coronary syndrome (ACS)-causing culprit lesions impact inflammatory vessel-wall interactions and are associated with higher cardiovascular event rates at one year follow-up: Results from the prospective translational OPTICO-ACS study program, Atherosclerosis, 2023;385:117284.
- Shaalan, WE, Cheng, H, Gewertz, B, et al., Degree of carotid plaque calcification in relation to symptomatic outcome and plaque inflammation, J Vasc Surg, 2004;40:262-269.
- Niyonzima, N, Bakke, SS, Gregersen, I, et al., Cholesterol crystals use complement to increase NLRP3 signaling pathways in coronary and carotid atherosclerosis, EBioMedicine, 2020;60:102985.
- Janoudi, A, Shamoun, FE, Kalavakunta, JK, et al., Cholesterol crystal induced arterial inflammation and destabilization of atherosclerotic plaque, Eur Heart J, 2016;37:1959-1967.
- Qin, Z, Cao, M, Xi, X, et al., Cholesterol crystals in non-culprit plaques of STEMI patients: A 3-vessel OCT study, Int J Cardiol, 2022;364:162-168.
- Xue, C, Chen, Q, Bian, L, et al., The relationships between cholesterol crystals, NLRP3 inflammasome, and coronary atherosclerotic plaque vulnerability in acute coronary syndrome: An optical coherence tomography study, Front Cardiovasc Med, 2022;9:905363.
- Dai, J, Tian, J, Hou, J, et al., Association between cholesterol crystals and culprit lesion vulnerability in patients with acute coronary syndrome: An optical coherence tomography study, Atherosclerosis, 2016;247:111-117.
- Kataoka, Y, Puri, R, Hammadah, M, et al., Spotty calcification and plaque vulnerability in vivo: frequency-domain optical coherence tomography analysis, Cardiovasc Diagn Ther, 2014;4:460-469.
- Puchner, SB, Mayrhofer, T, Park, J, et al. Differences in the association of total versus local coronary artery calcium with acute coronary syndrome and culprit lesions in patients with acute chest pain: The coronary calcium paradox. Atherosclerosis 2018, 274, 251–257. [Google Scholar] [CrossRef] [PubMed]
| No Calcification (N=106) | Low CPB (N=144) |
Intermediate CPB (N=146) |
High CPB (N=144) |
p | |
|---|---|---|---|---|---|
| Age | 57.4±12.4a,d | 59.7±10.5b | 61.6±9.83c,d | 65.5±9.89a,b,c | <0.001 |
| male | 75 (71%) | 110 (76%) | 110 (75%) | 108 (75%) | 0.771 |
| Prior MI | 8 (8%) | 12 (8%) | 12 (8%) | 24 (17%) | 0.038 |
| Prior PCI | 6 (6%)a | 16 (11%) | 16 (11%) | 31 (22%)a | 0.001 |
| Hypertension | 61 (58%) | 80 (56%) | 93 (64%) | 97 (67%) | 0.157 |
| Diabetes mellitus | 28 (26%) | 40 (28%) | 42 (29%) | 53 (37%) | 0.236 |
| Clinical Diagnosis | |||||
| SAP | 11 (10%) | 10 (7%) | 9 (6%) | 4 (3%) | <0.001 |
| UAP | 54 (51%)a | 83 (58%)b | 91 (62%)c | 116 (81%)a,b,c | |
| STEMI | 22 (21%)a | 32 (22%)b | 23 (16%) | 11 (8%)a,b | |
| NSTEMI | 19 (18%) | 19 (13%) | 23 (16%) | 13 (9%) | |
| WBC (109/L) | 7.39±2.53 | 7.22±2.33 | 7.36±2.27 | 6.74±1.98 | 0.067 |
| neutrophils | 4.91±2.23 | 4.76±2.09 | 4.91±2.12 | 4.32±1.67 | 0.046 |
| lymphocytes | 1.83±0.67 | 1.85±0.55 | 1.83±0.67 | 1.80±0.65 | 0.951 |
| monocytes | 0.471±0.174 | 0.433±0.160 | 0.446±0.191 | 0.433±0.148 | 0.263 |
| platelets (109/L) | 228±61 | 228±63 | 223±52 | 225±66 | 0.878 |
| hemoglobin (g/L) | 139±17 | 140±13 | 140±15 | 137±16 | 0.303 |
| hs-CRP (mg/l) | 2.10 (0.98-5.05)a | 1.31 (0.60-3.91) | 1.49 (0.50-4.20) | 0.99 (0.50-2.73)a | 0.007 |
| FBG (mmol/l) | 5.40 (4.93-6.36) | 5.54 (4.99-6.57) | 5.59 (5.01-6.70) | 5.55 (5.03-6.59) | 0.647 |
| HbA1c | 6.00 (5.70-6.80) | 6.10 (5.70-7.20) | 6.10 (5.78-7.30) | 6.37 (5.65-7.30) | 0.464 |
| TC (mmol/l) | 4.17±0.99 | 4.25±1.17 | 4.17±1.08 | 3.93±1.06 | 0.080 |
| TG (mmol/l) | 1.54 (1.18-2.20) | 1.49 (1.10-2.14) | 1.56 (1.13-2.06) | 1.31 (1.06-2.08) | 0.086 |
| LDLC (mmol/l) | 2.66±0.91 | 2.71±0.95 | 2.65±0.93 | 2.48±1.00 | 0.187 |
| HDLC (mmol/l) | 1.03±0.24 | 1.01±0.22 | 1.04±0.24 | 1.05±0.22 | 0.541 |
| NHR | 5.07±2.67a | 4.99±2.62b | 4.99±2.38c | 4.31±1.93a,b,c | 0.029 |
| MHR | 0.457 (0.326-0.577) | 0.411 (0.309-0.556) | 0.406 (0.304-0.566) | 0.399 (0.309-0.530) | 0.285 |
| LDL/HDL | 2.67±0.96 | 2.79±1.07 | 2.67±1.07 | 2.45±1.10 | 0.051 |
| TGs | 1.52±0.65 | 1.58±0.72 | 1.55±0.63 | 1.39±0.61 | 0.065 |
| eGFR (ml/min1.73m2) | 90.2±17.8 | 90.0±14.3 | 89.0±14.6 | 86.6±15.4 | 0.197 |
| Uric acid (umol/L) | 338 (277-405) | 325 (273-393) | 319 (268-369) | 317 (272-396) | 0.336 |
| D-dimer (μg/ml) | 0.23 (0.19-0.31) | 0.24 (0.19-0.34) | 0.24 (0.19-0.37) | 0.26 (0.22-0.39) | 0.076 |
| BNP (pg/ml) | 16.5 (0-71.3) | 19 (0-57) | 13 (0-65) | 21 (0-51) | 0.788 |
| LVEF (%) | 60 (55-61) | 60 (56-62) | 60 (56-62) | 60 (57-62) | 0.189 |
| culprit vessel | |||||
| LAD | 61 (58%)a | 101 (70%) | 112 (77%)a | 109 (76%) | 0.033 |
| RCA | 33 (31%)a | 31 (21%) | 23 (16%)a | 26 (18%) | |
| LCX | 12 (11%) | 11 (8%) | 11 (8%) | 7 (5%) | |
| LM | 0 (0%) | 1 (1%) | 0 (0%) | 2 (1%) | |
| multivessel disease | |||||
| one vessel | 55 (52%)a | 51 (36%)b | 35 (24%)a | 19 (13%)a,b | <0.001 |
| two vessels | 25 (24%) | 51 (35%) | 46 (31%) | 46 (32%) | |
| three vessels | 26 (24%)a | 42 (29%)b | 65 (45%)a,b | 79 (55%)a,b | |
| Medications | |||||
| Statins | 35 (33%) | 47 (33%) | 52 (36%) | 58 (40%) | 0.525 |
| β-blockers | 41 (39%) | 50 (35%) | 52 (36%) | 53 (37%) | 0.928 |
| ACEI/ARB | 21 (20%) | 36 (25%) | 40 (27%) | 37 (26%) | 0.571 |
| CCB | 26 (25%) | 38 (26%) | 39 (27%) | 41 (29%) | 0.920 |
| aspirin | 91 (86%) | 122 (85%) | 127 (87%) | 119 (83%) | 0.765 |
| No Calcification (N=106) | Microcalcification (N=9) |
spotty calcification (N=230) | Macrocalcification (N=195) |
p | |
|---|---|---|---|---|---|
| Age | 57.4±12.4a,c | 55.6±10.6c | 60.7±10.2a,b,c | 64.3±10.1a,b,c | <0.001 |
| male | 75 (71%) | 7 (78%) | 179 (78%) | 142 (73%) | 0.481 |
| Prior MI | 8 (8%) | 0 (0%) | 21 (9%) | 27 (14%) | 0.182 |
| Prior PCI | 6 (6%)a | 2 (22%) | 28 (12%) | 33 (17%)a | 0.035 |
| Hypertension | 61 (58%) | 4 (44%) | 136 (59%) | 130 (67%) | 0.210 |
| Diabetes mellitus | 28 (26%) | 1 (11%) | 66 (29%) | 68 (35%) | 0.208 |
| Clinical Diagnosis | |||||
| SAP | 11 (10%) | 0 (0%) | 14 (6%) | 9 (5%) | 0.006 |
| UAP | 54 (51%)a | 5 (56%) | 143 (62%) | 142 (73%)b | |
| STEMI | 22 (21%) | 4 (44%)a | 42 (18%) | 20 (10%)a | |
| NSTEMI | 19 (18%) | 0 (0%) | 31 (14%) | 24 (12%) | |
| WBC (109/L) | 7.39±2.53 | 7.39±3.86 | 7.19±2.19 | 6.99±2.15 | 0.523 |
| neutrophils | 4.91±2.23 | 5.01±3.33 | 4.72±2.01 | 4.58±1.87 | 0.556 |
| lymphocytes | 1.83±0.67 | 1.76±0.65 | 1.86±0.61 | 1.79±0.64 | 0.716 |
| monocytes | 0.471±0.174 | 0.424±0.155 | 0.436±0.170 | 0.440±0.165 | 0.315 |
| platelets (109/L) | 228±61 | 249±132 | 224±55 | 224±62 | 0.655 |
| hemoglobin (g/L) | 139±17 | 141±12 | 139±14 | 139±15 | 0.900 |
| hs-CRP (mg/l) | 2.10 (0.98-5.05)a | 1.20 (0.41-2.72) | 1.25 (0.65-4.94) | 1.00 (0.48-3.12)a | 0.004 |
| FBG (mmol/l) | 5.40 (4.93-6.36) | 6.02 (4.69-7.21) | 5.54 (4.94-6.57) | 5.60 (5.04-6.65) | 0.644 |
| HbA1c | 6.00 (5.70-6.80) | 6.05 (5.75-6.58) | 6.10 (5.60-7.10) | 6.30 (5.70-7.40) | 0.324 |
| TC (mmol/l) | 4.17±0.99 | 4.04±1.03 | 4.25±1.11 | 3.96±1.09 | 0.053 |
| TG (mmol/l) | 1.54 (1.18-2.20) | 1.43 (1.26-2.66) | 1.52 (1.08-2.15) | 1.45 (1.09-2.10) | 0.527 |
| LDLC (mmol/l) | 2.66±0.91 | 2.48±0.53 | 2.73±0.94 | 2.48±1.00 | 0.054 |
| HDLC (mmol/l) | 1.03±0.24 | 0.94±0.19 | 1.03±0.23 | 1.03±0.22 | 0.656 |
| NHR | 5.07±2.67 | 5.65±4.04 | 4.80±2.33 | 4.68±2.27 | 0.405 |
| MHR | 0.457 (0.326-0.577) | 0.488 (0.279-0.632) | 0.405 (0.314-0.537) | 0.400 (0.304-0.558) | 0.273 |
| LDL/HDL | 2.67±0.96 | 2.75±0.71 | 2.76±1.08 | 2.48±1.09 | 0.060 |
| TGs | 1.52±0.65 | 1.69±0.77 | 1.53±0.67 | 1.47±0.64 | 0.610 |
| eGFR (ml/min1.73m2) | 90.2±17.8 | 91.7±16.4 | 89.4±14.1 | 87.2±15.5 | 0.312 |
| Uric acid (umol/L) | 338 (277-405) | 350 (278-365) | 318 (272-390) | 323 (271-384) | 0.610 |
| D-dimer (μg/ml) | 0.23 (0.19-0.31) | 0.22 (0.18-0.32) | 0.23 (0.19-0.32) | 0.25 (0.21-0.39) | 0.058 |
| BNP (pg/ml) | 16.5 (0-71.3) | 10 (0-23) | 19 (0-56) | 21 (0-53) | 0.765 |
| LVEF (%) | 60 (55-61) | 60 (58-63) | 60 (57-62) | 60 (57-62) | 0.206 |
| culprit vessel | |||||
| LAD | 61 (58%)a | 6 (67%) | 166 (72%)a | 150 (77%)a | 0.027 |
| RCA | 33 (31%)a | 3 (33%) | 41 (18%)a | 36 (19%) | |
| LCX | 12 (11%) | 0 (0%) | 21 (9%) | 8 (4%) | |
| LM | 0 (0%) | 0 (0%) | 2 (1%) | 1 (1%) | |
| multivessel disease | |||||
| one vessel | 55 Ca | 3(33%) | 73 (32%)a,b | 29 (15%)a,b | <0.001 |
| two vessels | 25 (24%) | 4 (44%) | 72 (31%) | 67 (34%) | |
| three vessels | 26 (24%)a | 2 (22%) | 85 (37%)b | 99 (51%)a,b | |
| Medications | |||||
| Statins | 35 (33%) | 3 (33%) | 74 (32%) | 80 (41%) | 0.260 |
| β-blockers | 41 (39%) | 4 (44%) | 81 (35%) | 70 (36%) | 0.885 |
| ACEI/ARB | 21 (20%) | 2 (22%) | 60 (26%) | 51 (26%) | 0.606 |
| CCB | 26 (25%) | 2 (22%) | 61 (27%) | 55 (28%) | 0.902 |
| Aspirin | 91 (86%) | 9 (100%) | 192 (84%) | 1671 (86%) | 0.546 |
| OCT | No Calcification (N=106) | Low CPB (N=144) |
Intermediate CPB (N=146) |
High CPB (N=144) |
p |
|---|---|---|---|---|---|
| MLA | 2.07 (1.45-2.89)a | 1.93 (1.37-2.72)b | 1.95 (1.31-2.62) | 1.69 (1.31-2.31)a,b | 0.002 |
| FCT(um) | 126 (81-188) | 113 (71-171) | 99 (61-168) | 116 (88-160) | 0.069 |
| Max lipid arc ° | 273 (240-360)a | 290 (245-360)b | 311 (256-360) | 360 (280-360)a,b | <0.001 |
| Lipid length | 28.56±14.4a,b | 30.5±12.9c,d | 33.8±12.9b,c | 34.8±11.5a,d | <0.001 |
| Lipid index | 5768±3351a,b | 6201±2986c,d | 7034±3038b,c | 7449±2735a,d | <0.001 |
| Total plaque index | 5773±3343a,b | 6421±3007c,d | 7675±3109a,c,e | 9215±2939b,d,e | <0.001 |
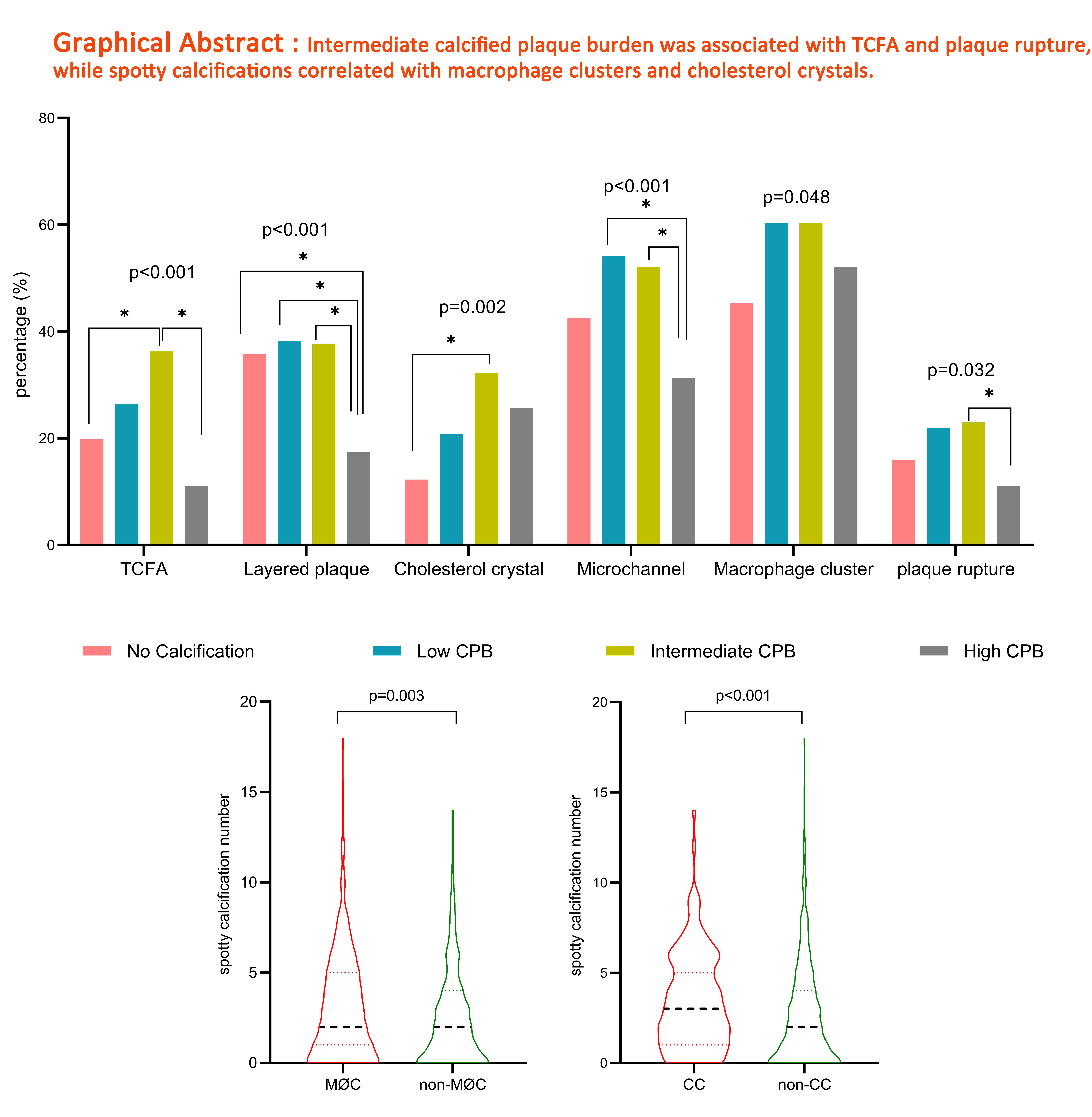
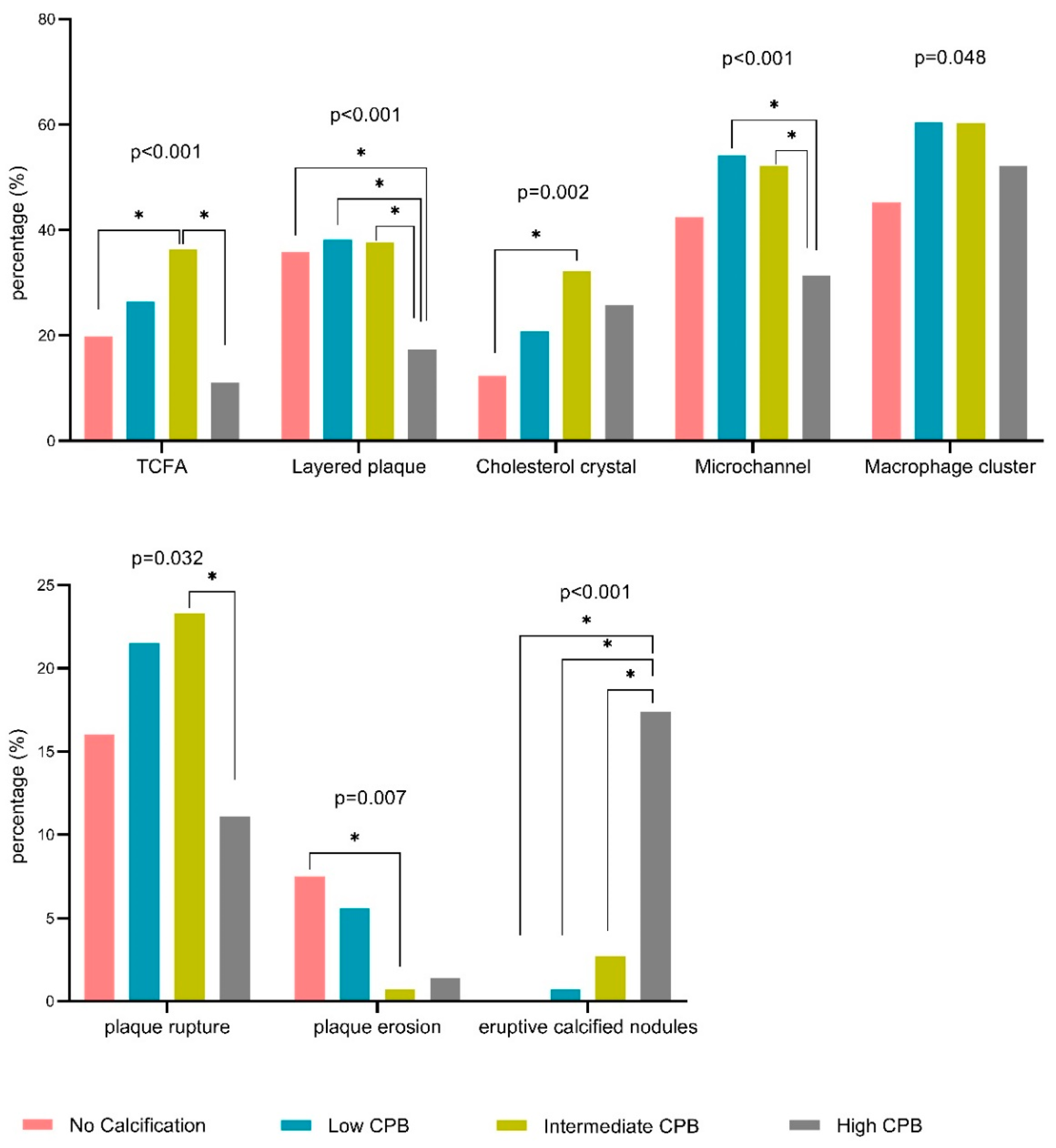
| TCFA | 21 (20%)a | 38 (26%)b | 53 (36%)a,c | 16 (11%)b,c | <0.001 |
| Layered plaque | 38 (36%)a | 55 (38%)b | 55 (38%)c | 25 (17%)a,b,c | <0.001 |
| Cholesterol crystal | 13 (12%)a | 30 (21%) | 47 (32%)a | 37 (26%) | 0.002 |
| Microchannel | 45 (43%) | 78 (54%)a | 76 (52%)b | 45 (31%)a,b | <0.001 |
| Macrophage cluster | 48 (45%) | 87 (60%) | 88 (60%) | 75 (52%) | 0.048 |
| Plaque rupture | 17 (16%) | 31 (22%) | 34 (23%)a | 16 (11%)a | 0.032 |
| Plaque erosion | 8 (8%)a | 8 (6%) | 1 (1%)a | 2 (1%) | 0.007 |
| Eruptive calcified nodules | 0 (0%)a | 1 (1%)b | 4 (3%)c | 25 (17%)a,b,c | <0.001 |
| Superficial microcalficialtions (n) | NA | 0.076±0.292 | 0.158±0.560 | 0.118±0.450 | 0.455 |
| Deep microcalficialtions (n) | NA | 0.167±0.443 | 0.158±0.435 | 0.174±0.448 | 0.926 |
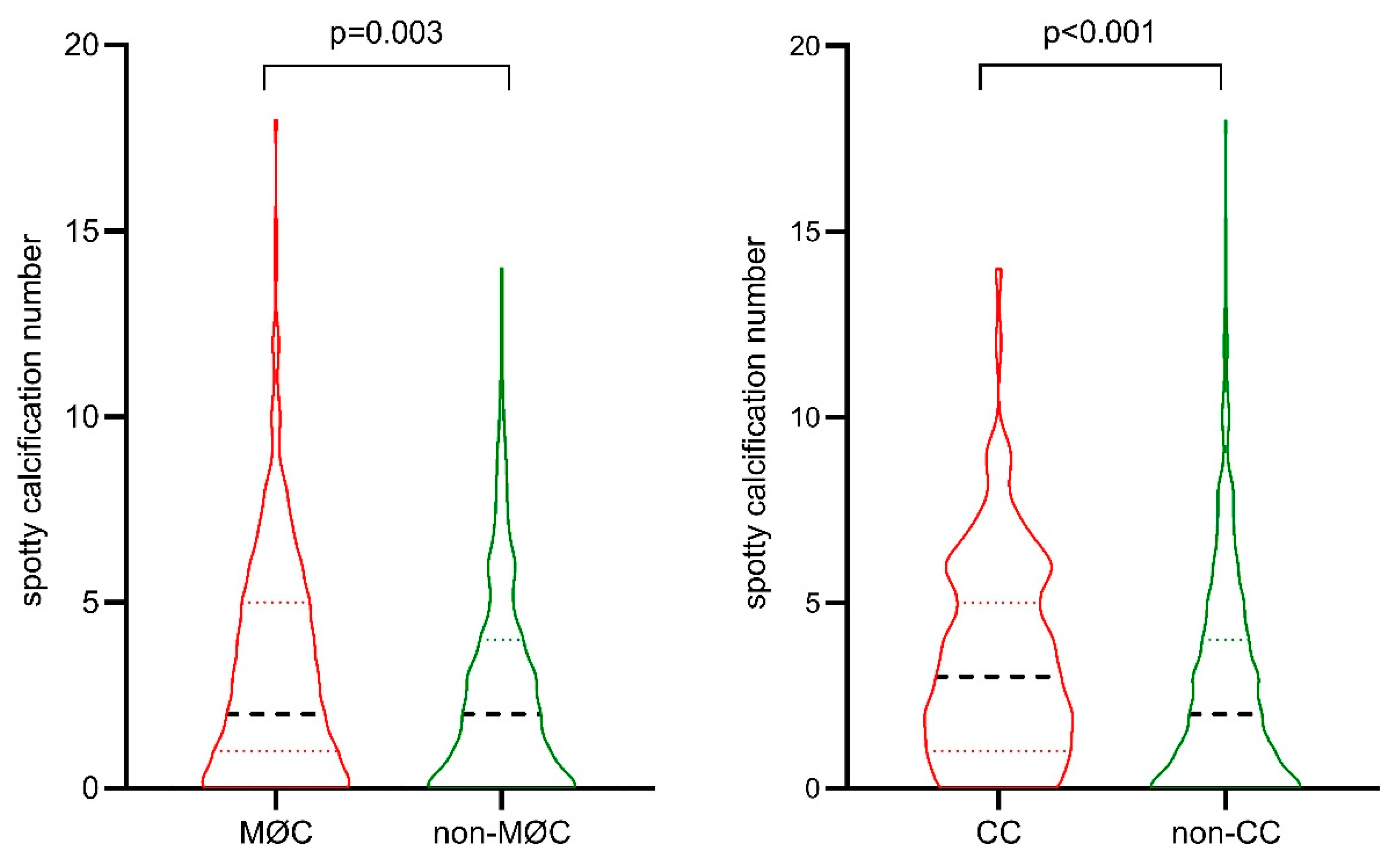
| Superficial spotty calcifications (n) | NA | 0.347±0.651a,b | 1.01±1.25a,c | 2.26±2.17b,c | <0.001 |
| Deep spotty calcifications (n) | NA | 1.17±1.01a,b | 2.55±1.88a | 3.10±2.53b | <0.001 |
| Superficial macrocalcifications (n) | NA | 0.028±0.165a,b | 0.377±0.656a,c | 2.27±1.90b,c | <0.001 |
| Deep macrocalcifications (n) | NA | 0.056±0.230a,b | 0.178±0.450a | 0.299±0.739b | <0.001 |
| OCT | No Calcification (N=106) | Microcalcification (N=9) |
spotty calcification (N=230) | Macrocalcification (N=195) |
p |
|---|---|---|---|---|---|
| MLA | 2.07 (1.45-2.89) | 2.31 (1.92-3.51) | 1.96 (1.39-2.81) | 1.79 (1.31-2.46) | 0.010 |
| FCT(um) | 126 (81-188) | 86 (60-205) | 112 (64-171) | 110 (71-155) | 0.372 |
| Max lipid arc ° | 273 (240-360)a | 280 (225-327) | 292 (250-360)b | 320 (280-360)a,b | <0.001 |
| Lipid length | 28.6±14.4a,b | 21.1±11.7c,d | 32.8±12.7a,c | 33.9±12.2b,d | <0.001 |
| Lipid index | 5768±3351a,b | 4132±2521c,d | 6740±2946a,c | 7205±2931b,d | <0.001 |
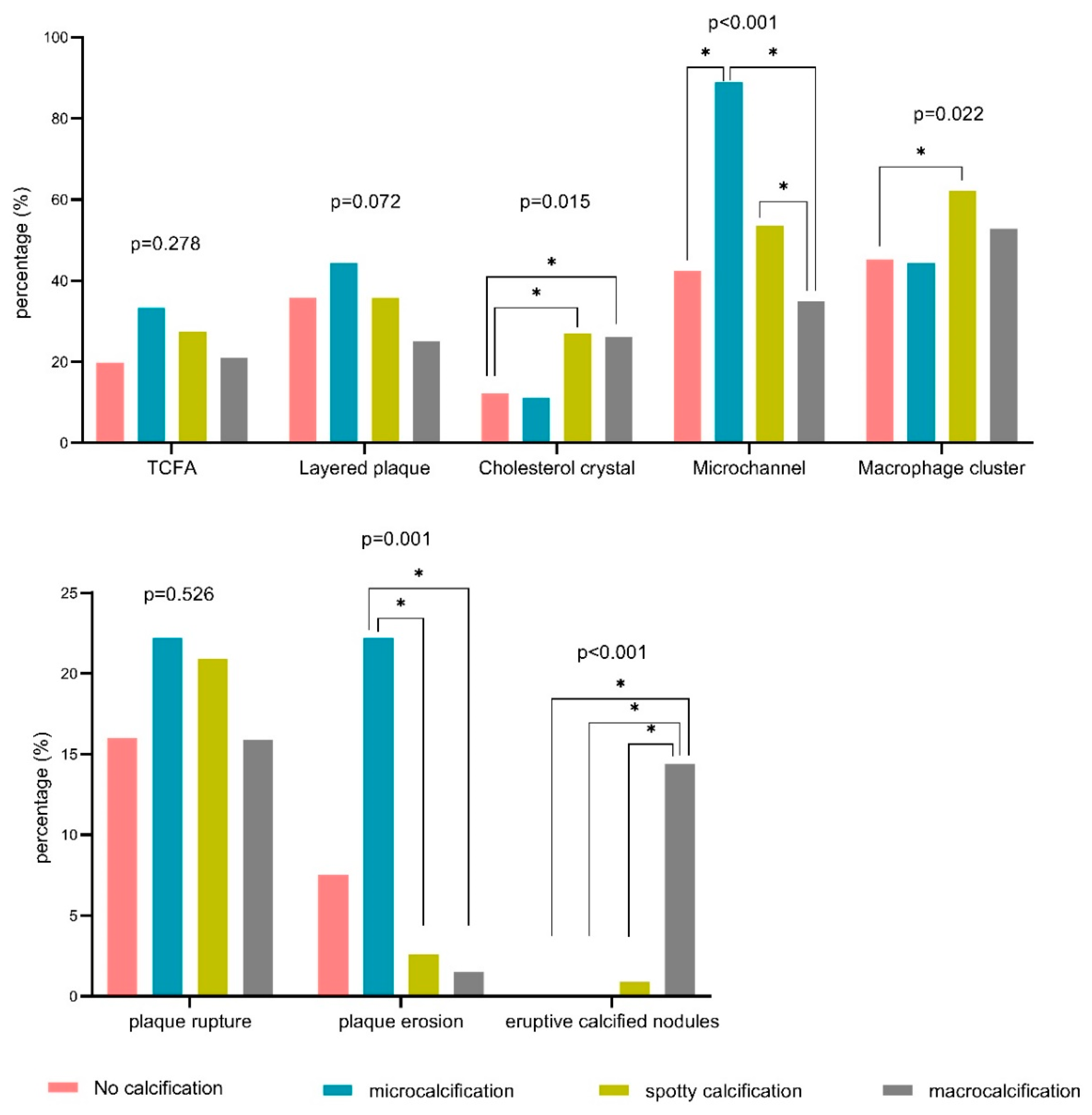
| TCFA | 21 (20%) | 3 (33%) | 63 (27%) | 41 (21%) | 0.278 |
| Layered plaque | 38 (36%)a | 4 (44%)b | 82 (36%)c | 49 (25%)a,b,c | 0.072 |
| Cholesterol crystal | 13 (12%)a,b | 1 (11%) | 62 (27%)a | 51 (26%)b | 0.015 |
| Microchannel | 45 (43%)a | 8 (89%)a,c | 123 (54%)b | 68 (35%)b,c | <0.001 |
| Macrophage cluster | 48 (45%)a | 4 (44%) | 143 (62%)a | 103 (53%) | 0.022 |
| Plaque rupture | 17 (16%) | 2 (22%) | 48 (21%)a | 31 (16%)a | 0.526 |
| Plaque erosion | 8 (8%)a | 2 (22%)b,c | 6 (3%)c | 3 (2%)a,b | 0.001 |
| Eruptive calcified nodules | 0 (0%)a | 0 (0%) | 2 (1%)b | 28 (14%)a,b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




