Submitted:
23 April 2025
Posted:
24 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. I/R Damage and the Regulation by Oxidative Preconditioning Through Low-Dose Medical Ozone
2.1. Mechanism of Action and the Ozone Effect
2.2. Heart Protection Against I/R Damage Through Ozone-Mediated Mitochondrial Biogenesis
Can Medical Ozone Induce Mitochondrial Fission and Increase the Mitochondrial Density Through Preventive Intraperitoneal Ozone Administration?
2.3. Protection of Mitochondria in the Heart Muscle Against I/R Damage
3. Protection Against Brain I/R Injury
3.1. Cell Model: Protection of Neuronal Cells
3.2. Cerebral I/R Injury Animal Model
4. Conclusion and Future Perspective
References
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J; Gao, F; Wang, S.; Tan, R.; Yuan, J. Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 2024, 9, 12. [CrossRef]
- Léon, O.S.; Menéndez, S.; Merino, N.; Castillo, R.; Sam, S.; Pérez, L.; Cruz, E.; Bocci, V. Ozone oxidative preconditioning: A protection against cellular damage by free radicals. Mediat. Inflamm. 1998, 7, 289–294. [CrossRef]
- Ajamieh, H.; Merino, N.; Candelario-Jalil, E.; Menendez, S.; Martinez-Sanchez, G.; Re, L.; Giuliani, A.; Leon, O.S. Similar protective effect of ischemic and ozone oxidative preconditioning in liver/ischemia/reperfusion injury. Pharmacological Research. 2002, 45, 333-339. [CrossRef]
- Ajamieh, H.; Menendez, S.; Martınez-Sanchez, G.; Candelario-Jalil, E.; Re, L.; Giuliani, A.; Leon Fernandez. O. Effects of ozone oxidative preconditioning on nitric oxide generation and cellular redox balance in a rat model of hepatic ischaemia–reperfusion. Liver International. 2004, 24, 55–62. [CrossRef]
- Ajamieh, H.; Berlanga, J.; Merino, N.; Martinez Sanchez, G.; Carmona, A.; Menendez Cepero, S.; Giuliani, A.; Re, L.; Leon, O.S. Role of protein synthesis in the protection conferred by ozone-oxidative-preconditioning in hepatic ischaemia/reperfusion. Transpl. Int. 2005, 18, 604–612. [CrossRef]
- Calunga, J.; Zamora, Z.; Borrego, A.; Del Rıo, S.; Barber, E.; Menendez, S.; Hernandez, F.; Montero, T.; Taboada, D. Ozone Therapy on Rats Submitted to Subtotal Nephrectomy: Role of Antioxidant System. Mediators of Inflammation. 2005, 4, 221–227. [CrossRef]
- Zamora, Z.; Borrego, A.; Lopez, O., Delgado, T.; Gonzalez, R.; Menedez, S.; Hernandez, F.; Schulz, S. Effects of ozone oxidative preconditioning on TNF-alpha release and intracellular antioxidant-prooxidant balance in mice during endotoxic shock. Mediators Inflamm., 2005, Feb 24, 16-22. [CrossRef]
- Santana-Rodríguez, N.; Llontop, P.; Clavo, B.; Fiuza-Perez, M.; Zerecero, K.; Ayub, A.; Alshehri, K.; Yordi, N.; Re, L.; Raad, W.; Fernandez-Perez, L.; García-Herrera, R.; Huang, C.; Bhora, F. FACS. Ozone Therapy Protects Against Rejection in a Lung Transplantation Model: A NewTreatment? Ann Thorac Srg. 2017, 104, 258-464. https://www.annalsthoracicsurgery.org/article/S0003-4975(17)30360-0/fulltext.
- Clavo, B.; Catalá, L.; Pérez, J.; Rodríguez, V.; Robaina, F. Ozone Therapy on Cerebral Blood Flow: A Preliminary Report. Evid Based Complement Alternat Med. 2004, 1, 315-319. [CrossRef]
- Zhu, F.; Ding, S.; Liu, Y.; Wang, X.; Wu, Z. Ozone-mediated cerebral protection: Unraveling the mechanism through ferroptosis and the NRF2/SLC7A11/GPX4 signaling pathway. Journal of Chemical Neuroanatomy. 2024, 136, 102387. [CrossRef]
- Wang, L.; Chen, Z.; Liu, Y.; Du, Y.; Liu, X. Ozone oxidative postconditioning inhibits oxidative stress and apoptosis in renal ischemia and reperfusion injury through inhibition of MAPK signaling pathway. Drug Des. Devel Ther. 2018, 12, 1293–1301. [CrossRef]
- Wang, P.; Cui, Y.; Ren, Q., Yan, B.; Zhao, Y.; Yu, P.; Gao, G.; Shi, H.; Chang, S.; Chang, Y.; Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 2021, ,12, 447. [CrossRef]
- Barakat, S.; Saleh, N.; Thabet, S.; El Missiri, A.; Badawy, M. Ischämie/Reperfusions-Modell am Herzen nach oxidativer Konditionierung durch Ozon. [Ozone Oxidative Preconditioning in Ischemia/Reperfusion Model on the Myocardium]. Ozone-Textbook, Basics-Prevention-Therapy. Ecomed Publisher Landsberg, Germany. 2006, (Viebahn-Hänsler, R.; Knoch, H.G., Eds.).
- Meng, W.; Xu, Y.; Lic, D.; Zhua, E.; Deng, L.; Liu, Z.; Zhang, G.; Liu, H. Ozone protects rat heart against ischemia-reperfusion injury: A role for oxidative preconditioning in attenuating mitochondrial injury. Biomedicine & Pharmacotherapy. 2017, 88,1090-1097. [CrossRef]
- Cai, H.; Xi T.; Zheng, L.; Huang, L.; Peng, Y.; Liao, R.; Zhu, Y. Ozone alleviates ischemia/reperfusion injury by inhibiting mitochondrion-mediated apoptosis pathway in SH-SY5Y cells. Cell Biol Int. 2020, 44, 975–984. [CrossRef]
- Wang, R.; Liu, F.; Huang, P.; Zhang.; He, J.; Pang, X.; Zhang, D.; Guan,Y. Ozone preconditioning protects rabbit heart against global ischemia-reperfusion injury in vitro by up-regulating HIF-1. Biomedicine & Pharmacotherapy. 2022, 150, 113033. https://www.sciencedirect.com/science/article/pii/S075333222200422X?via%3Dihub.
- Ding, S.; Duanmu, X.; Xu, L.; Zhu, L.; Zhouquan, Wu, Z . Ozone pretreatment alleviates ischemia–reperfusion injury-induced myocardial ferroptosis by activating the Nrf2/Slc7a11/Gpx4. Biomedicine & Pharmacotherapy. 2023,165, 115185. [CrossRef]
- Zhu, F.; Ding, S .; Liu, Y.; Wang, X.; Wu, Z. Ozone-mediated cerebral protection: Unraveling the mechanism through ferroptosis and the NRF2/SLC7A11/GPX4 signaling pathway. Journal of Chemical Neuroanatomy. 2024, 136, 102387. [CrossRef]
- Dash, U.; Nayak, V.; Navani, S.; Samal, R.; Agrawal, P.; Singh, A.; Majhi, S.; Mogaref, D.G.; Duttaroy, A.; Jena, A.B. Understanding the molecular bridges between the drugs and immune cell. Pharmacology & Therapeutics. 2025, 267,108805.
- Viebahn-Haensler, R.; León Fernández, O. Ozone in Medicine. The Low-Dose Ozone Concept. The Redox-Bioregulatory Effect as Prominent Biochemical Mechanism and the Role of Glutathione. Ozone Sci. Eng. 2024, 46, 267–279. [CrossRef]
- Sagai, M.; Bocci, V. Mechanisms of Action Involved in Ozone Therapy: Is Healing Induced via a Mild Oxidative Stress? Medical Gas Research. 2011, 1, 29. [CrossRef]
- Togi, M.; Nagashima, S.; Kitay, Y.; Muromoto, R.; Kashiwakura, J.; Miura, T.; Matsuda, T. Implication of NF-kB Activation on Ozone-Induced HO-1 Activation. BPB Reports 2021, 4, 59-63. doi.org/10.1248/bpbreports.4.2_59.
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J.; Mitochondrial dysfunction: mechanisms and advances in Therapy. Sig Transduct Target Ther. 2024, 9, 124. [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta. 2017, 1863, 1066–1077. [CrossRef]
- Viebahn-Haensler, R.; León Fernández, O.S. Mitochondrial Dysfunction, Its Oxidative Stress-Induced Pathologies and Redox Bioregulation through Low-Dose Medical Ozone: A Systematic Review. Molecules. 2024, 29, 2738. [CrossRef]
- Ambrosio, G.; Tritto, I. Reperfusion injury: Experimental evidence and clinical implications. Am Heart J. 1999, 138, 69-75. [CrossRef]
- Jun, L.; Knight, E.; Broderick, T.L.; Al-Nakkash, L.; Tobin, B.; Geetha, T.; Babu, J.R. Moderate-Intensity Exercise Enhances Mitochondrial Biogenesis Markers in the Skeletal Muscle of a Mouse Model Affected by Diet-Induced Obesity. Nutrients. 2024, 16, 1836. [CrossRef]
- Galié, M.; Covi, V.; Tabaracci, G.; Malatesta, M. The Role of Nrf2 in the Antioxidant Cellular Response to Medical Ozone Exposure. Int. J. Mol. Sci. 2019, 16, 4009. [CrossRef]
- .
- Carvajal-Oliveros, A.; Uriostegui-Arcos, M.; Zurita, M.; Melchy-Perez, E.; Narváez-Padilla, V.; Reynaud, E. The BE (2)-M17 cell line has a better dopaminergic phenotype than the traditionally used for Parkinson´s research SH-SY5Y, which is mostly serotonergic. IBRO Neurosci Rep. 2022, 13, 543-551. [CrossRef]
- Viebahn-Haensler, R.; León Fernández, O.S.; Fahmy, Z. Ozone in medicine: The low-dose ozone concept. Guidelines and treatment strategies. Ozone Sci. Eng. 2012, 34, 408–424. [CrossRef]
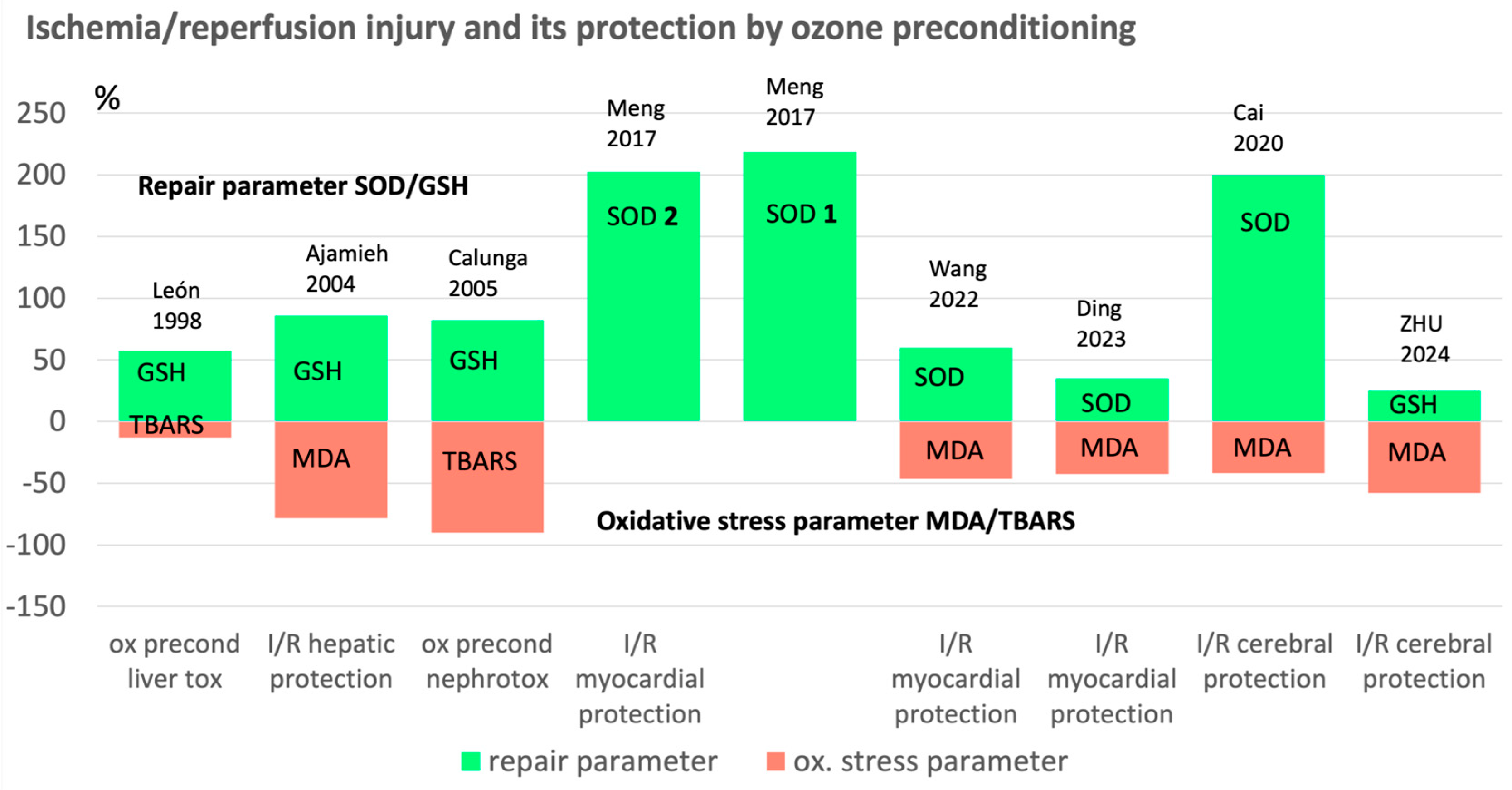

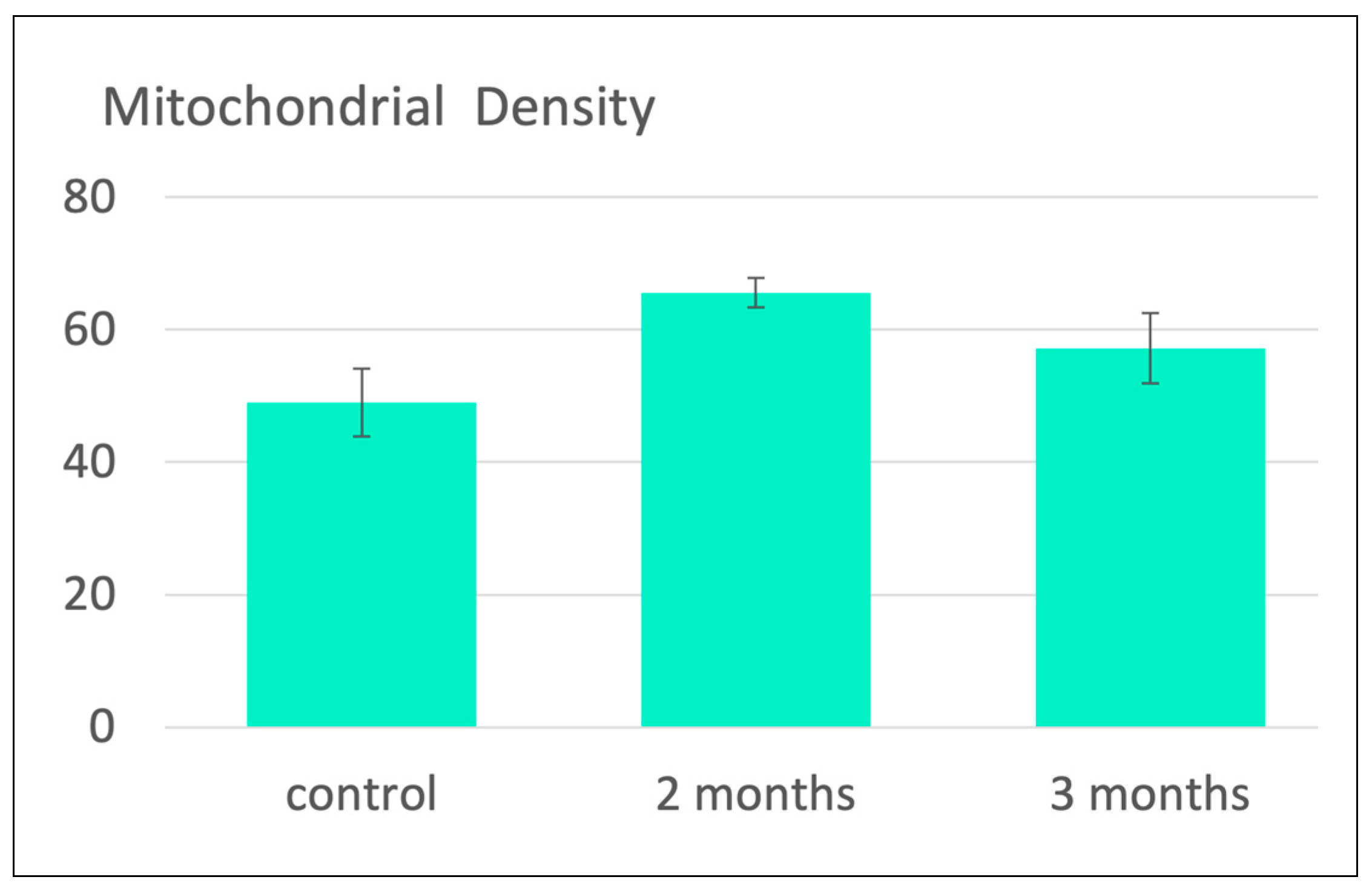
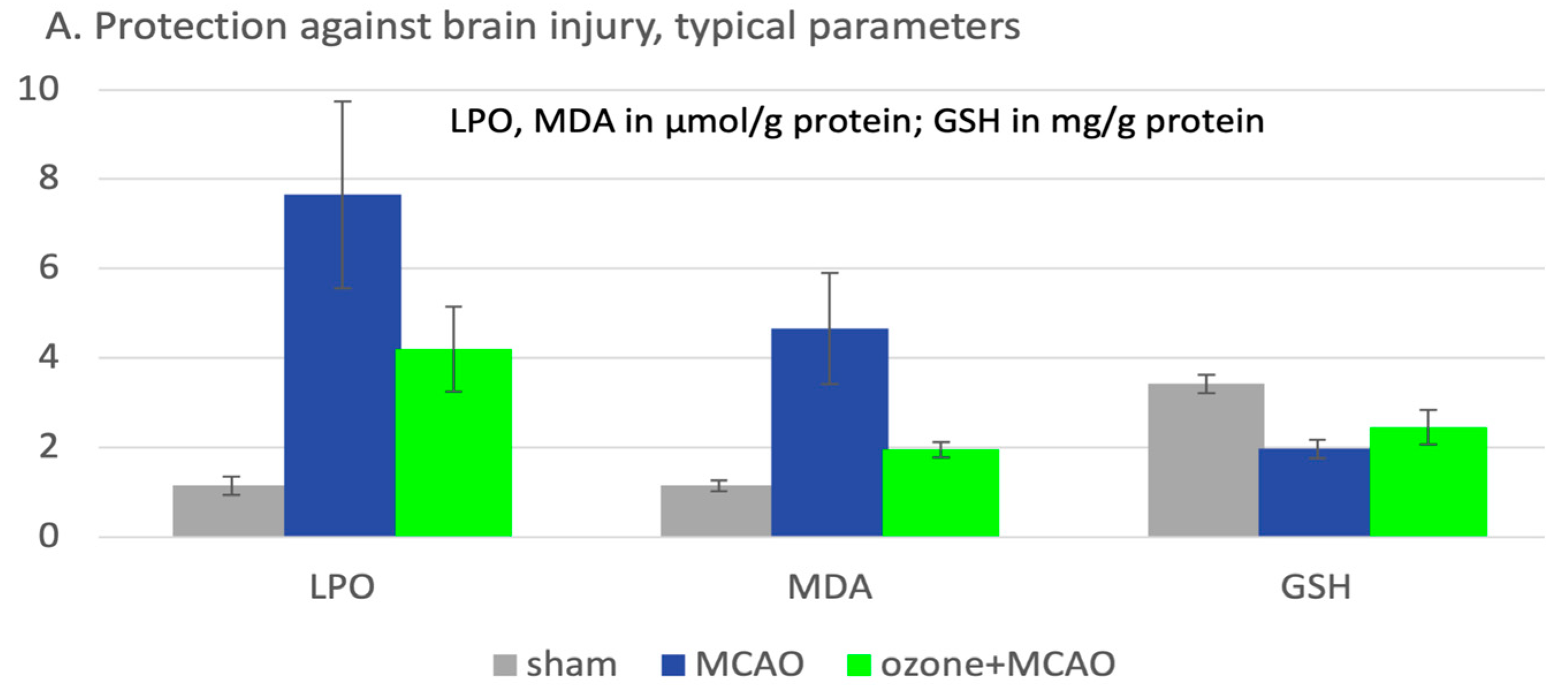
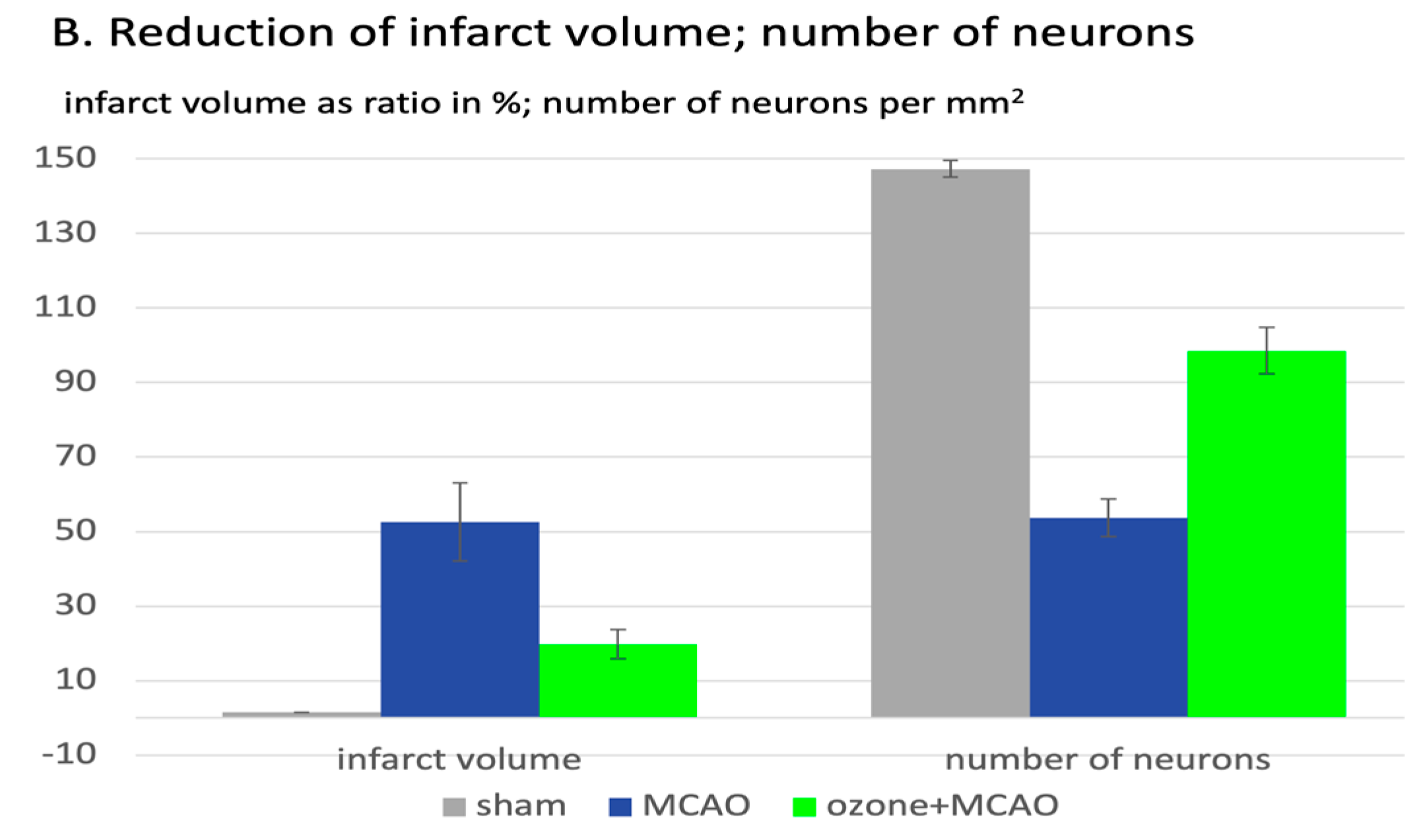
| Subject, type of study | Procedure | Antioxidants / oxidative stress parameters relevant for ozone effect | References |
|---|---|---|---|
| Preclinical trial in rats. Ozone oxidative preconditioning: A protection against cellular damage by free radicals. |
6 animal groups: adult female Sprague-Dawley rats, 220–250 g. 10 animals per group.4 control groups Toxicity-producing ROS: by CCl4 solution; ozone preconditioning: 15 O3 treatments as rectal insufflation (1mg/kg), 4,5-5 mL, 50 µg/mL. |
Only a few redox parameters, relevant to ozone effect here discussed: SOD in u/g; GSH in mmol/g: TBARS in nmol/g protein. | León et al. 1998 [2]. |
| Preclinical trial in rats. Similar protective effect of ischemic and ozone oxidative preconditioning in liver/ischemia/reperfusion injury. |
Adult male Wistar rats (250–300 g). n=32, hepatic ischaemia I/R (n = 8): 90 min hepatic ischaemia 90 min reperfusion (n=8). ozone preconditioning: 15 O3 treatments as rectal insufflation (n=8) (1mg/kg), 5–5.5 mL, 50 μg/mL. |
MDA+4-hydroxynonenal in mmol/g; total SH-groups in mol/mg protein. | Ajamieh et al. 2002 [3]. |
| Preclinical trial in rats. Effects of ozone oxidative preconditioning on nitric oxide generation and cellular redox balance in a rat model of hepatic ischemia/reperfusion. |
60 adult male Wistar rats (250–300 g), 10 per group. hepatic ischemia I/R 90 min ischemia, 90 min reperfusion; ozone preconditioning: 15 O3 treatments as rectal insufflation (1mg/kg), 5–5.5 mL, 50 μg/mL. | GSH in μg/g tissue, MDA+4 hydroxynonenal in mmol/g. |
Ajamieh et al. 2004 [4]. |
| Preclinical trial in rats. Role of protein synthesis in protection conferred by ozone-oxidative-preconditioning in hepatic ischemia/reperfusion. |
Adult male Wistar rats (10 per group, 250–275 g) hepatic ischemia 90 min ischemia, ozone preconditioning: 15 O3 treatments as rectal insufflation (1mg/kg), 5–5.5 mL, 50 μg/mL. | Mn-SOD in U/g tissue (SOD 2 mitochondrial SOD); Cu/Zn-SOD in U/g tissue (SOD 1 cytosol), MDA+4 hydroxynonenal in mmol/g. | Ajamieh et al. 2005 [5]. |
| Preclinical trial in rats. Ozone Therapy on Rats Submitted to Subtotal Nephrectomy: Role of Antioxidant System. |
30 female Wistar rats (180–200 g), 10 per group: 15 treatments 2.5−2.6mL, conc. 50 μg/mL rectal insufflation 1x/day, partial nephrectomy. | GSH (nmol/mg protein) TBARS in nmol/g protein. |
Calunga et al. 2005, [6]. |
| Preclinical trial in rats. Ischemia/reperfusion animal model on rat myocard after ozone oxidative preconditioning. [Ischämie/Reperfusions-Modell am Herzen nach oxidativer Konditionierung durch Ozon]. |
30 male albino rats (100-150 g), 3 groups, each with n=10, preventive ozone i.p. 2x per week for 2 months or for 3 months, conc.: 4 μg /mL; 28 μg per 100 g rat (400 μg per 100 mL blood), followed by by 30 min ischemia and 30 min reperfusion. | SOD, MDA, Mitochondrial biogenesis. |
Barakat et al. 2006 [13]. |
| Preclinical trial in rats. Ozone Therapy Protects Against Rejection in a Lung Transplantation Model: A New Treatment? |
Male Sprague-Dawley rats, n=36, rectal O3 daily for 2 weeks prior to lung transplantation (20-50 μg per animal) and 50 μg/dose 3x/week up to 3 months. |
Ozone pre- and postconditioning significantly decreased the expression of all genes related to oxidative stress and chronic rejection. | Santana-Rodríguez et al. 2017 [7]. |
| Preclinical trial in rats. Ozone protects rat heart against ischemia-reperfusion injury: A role for oxidative preconditioning in attenuating mitochondrial injury. |
Adult male Sprague-Dawley rats (200–250 g) OzoneOP 2 mL ozone 100 μg/kg/day for 5 days, 30 min of cardiac ischemia followed by 2 h reperfusion. | SOD 1 and SOD 2 in u/mg protein | Meng et al. 2017 [14]. |
| Preclinical trial in cell culture. Ozone alleviates ischemia/reperfusion injury by inhibiting mitochondrion-mediated apoptosis pathway in SH-SY5Y cells. |
SH-SY5Y cells as model for neuronal function tests; ozone oxidative preconditioning via incubation with 40 μg/mL and cultured for 2, 6;12, and 24 hrs. | SOD in u/mg protein, MDA in nmol/mg protein. | Cai et al. 2020 [15]. |
| Preclinical trial in rabbits. Ozone preconditioning protects rabbit heart against global ischemia-reperfusion injury in vitro by up-regulating HIF-1. |
Adult rabbits (2.50–2.75 kg), ozone oxidative preconditioning: i.p. injections 10 mL daily for 5 days. 3 conc´s: 20; 40, 80 μg/mL followed by 20 min ischemia, 60 min reperfusion. | SOD in u/mg protein, MDA in mmol/mg protein | Wang et al. 2022 [16]. |
| Preclinical trial in cell culture and in an animal model (mice). Ozone pretreatment alleviates ischemia–reperfusion injury-induced myocardial ferroptosis by activating the Nrf2/Slc7a11/Gpx4 axis. |
1.H9c2 cardiomyocytes; 2. Male C57 mice, 7 weeks of age, 22 -24 g. n=36, 12 per group, ozone preconditioning, 25 μg/mL i.p. injections, 2 mL per day for 5 days. 30 min. ischemia, 2 hrs reperfusion. | SOD activity in % of CTL (cytotoxic T lymphocyte activity), MDA in μmol/g protein | Ding et al. 2023 [17]. |
| Preclinical trial in rats. Ozone-mediated cerebral protection: Unraveling the mechanism through ferroptosis and the NRF2/SLC7A11/GPX4 signaling pathway. |
Sprague-Dawley (SD) rats (260–300 g), middle artery occlusion for 120 min, followed by surgery and reperfusion. Ozone oxidative preconditioning, i.p. injections (intra peritoneal), 20 μg/mL with 2,5 mL/kg/d, 5 days. | GSH in mg/g protein, LPO (lipoperoxides), MDA in μmol/g protein. | Zhu et al 2024 [18]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




