Submitted:
23 April 2025
Posted:
23 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Diatomaceous Earths
2.3. Study insects
2.4. Bioassay
2.5. F1 productivity
2.6. Statistical analysis
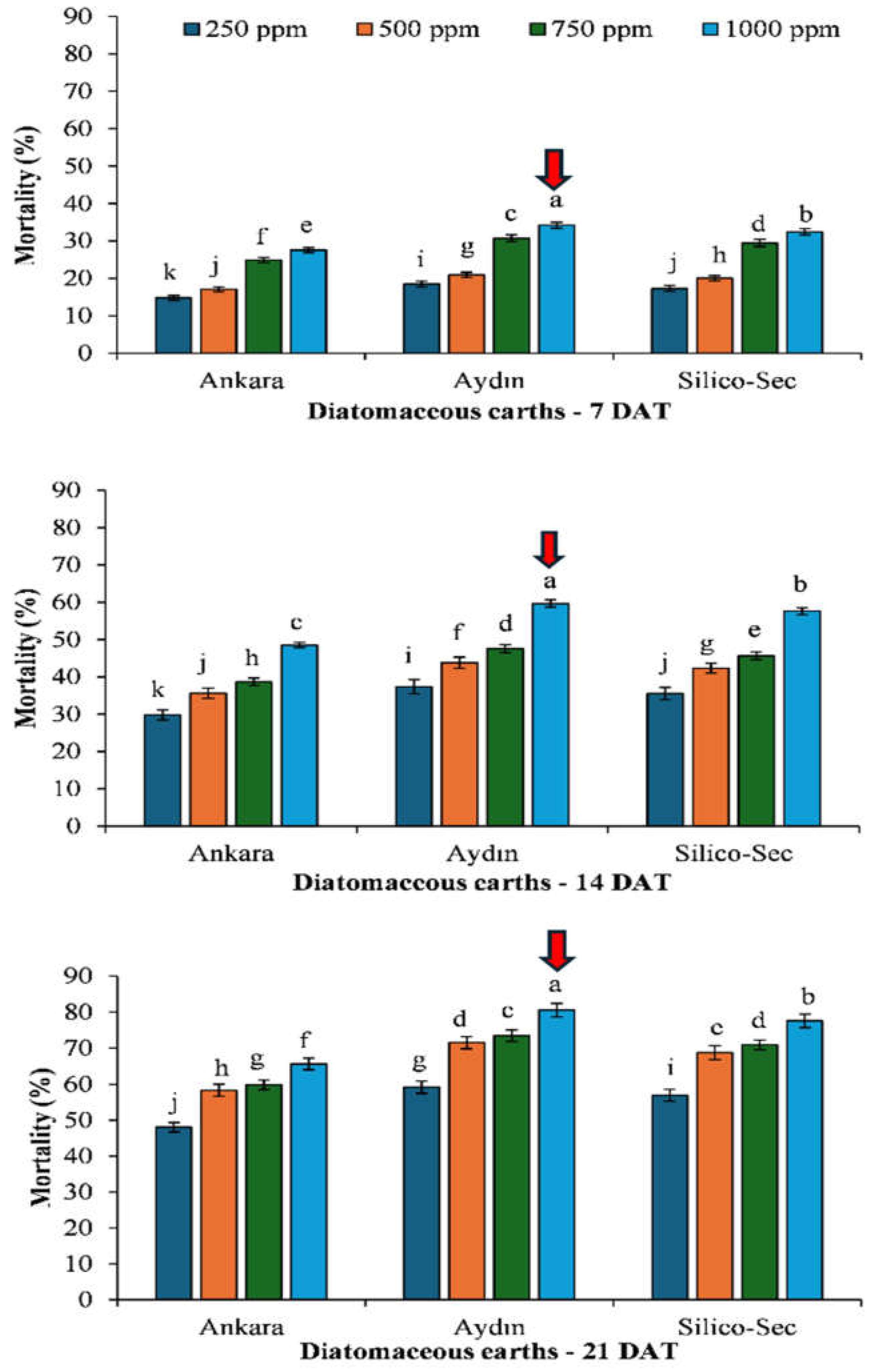
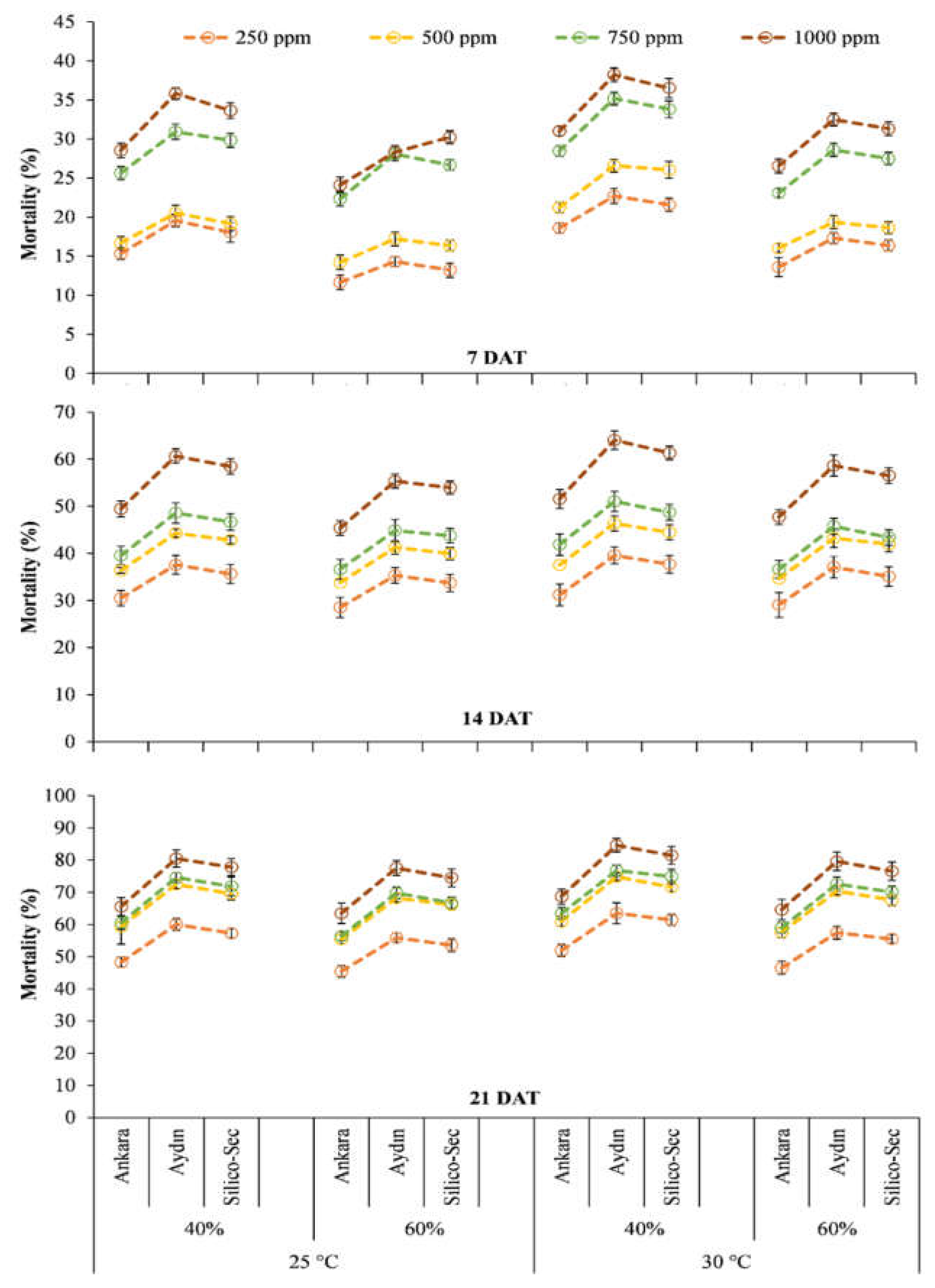
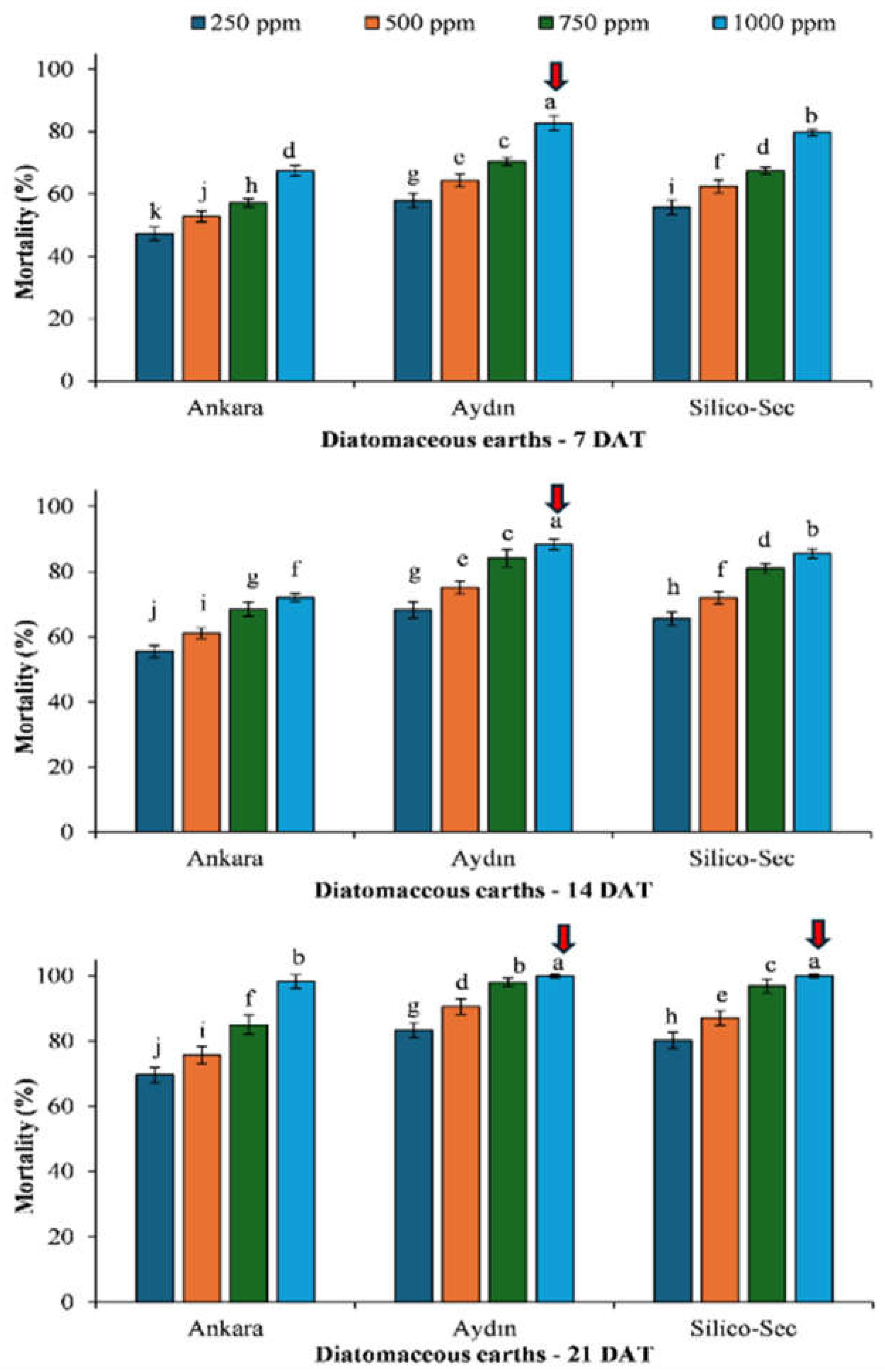
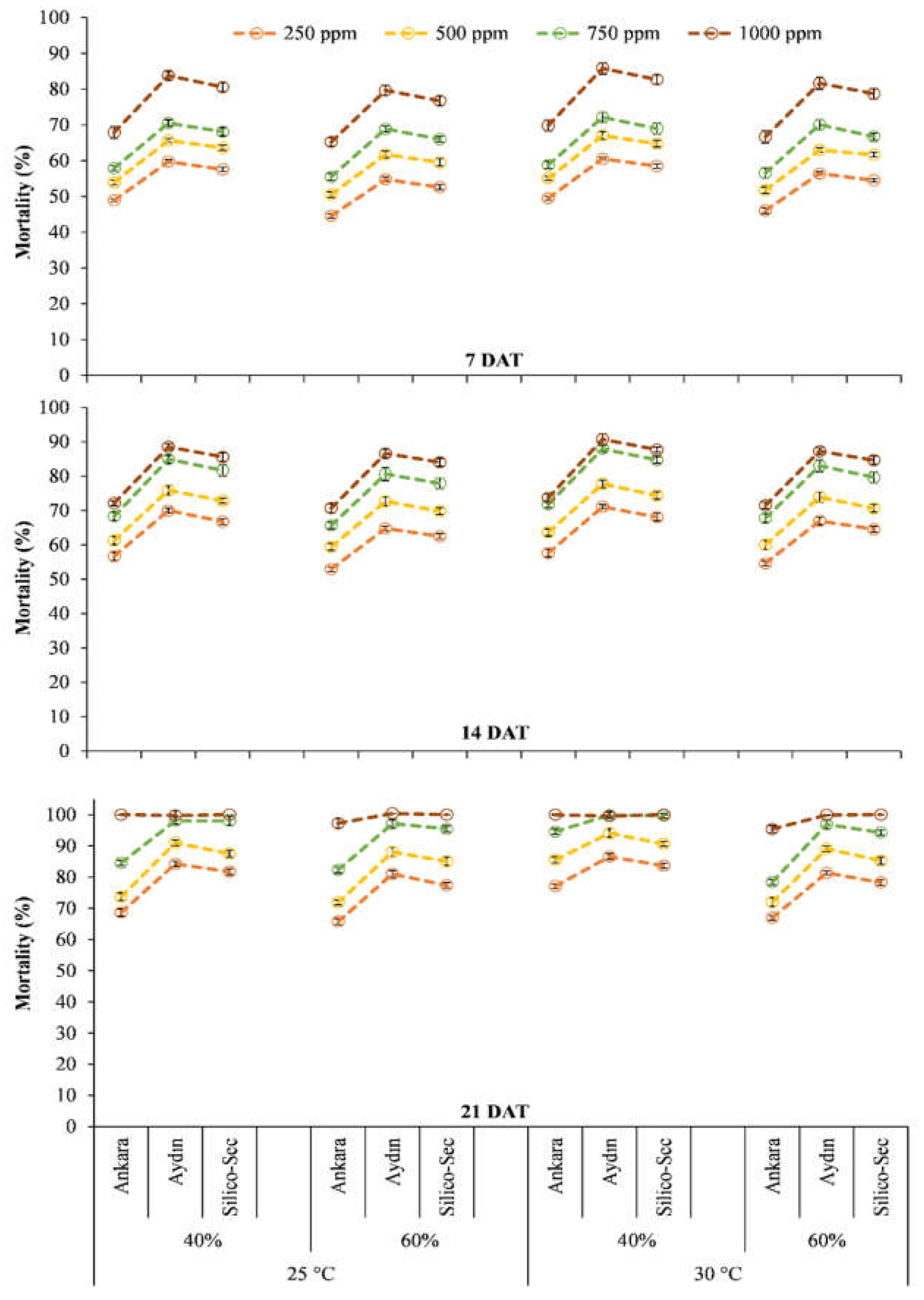
3. Results
4. Discussion
5. Conclusions
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DE | Diatomaceous earth |
| DAT | Days after treatment |
References
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops That Feed the World 10. Past Successes and Future Challenges to the Role Played by Wheat in Global Food Security. Food Secur 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.-J. Global Trends in Wheat Production, Consumption and Trade. In Wheat Improvement; 2022.
- Ibba, M.I.; Gupta, O.P.; Govindan, V.; Johnson, A.A.T.; Brinch-Pedersen, H.; Nikolic, M.; Taleon, V. Editorial: Wheat Biofortification to Alleviate Global Malnutrition. Front Nutr 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.W.; Sarwar, G.; Farooqi, M.A.; Jamil, M. Extent and Pattern of Damage in Wheat Caused by Three Different Species of Storage Insect Pests. Int J Trop Insect Sci 2021, 41, 593–599. [Google Scholar] [CrossRef]
- Rizwan, M.; Atta, B.; Ali, M.Y.; Ashraf, I.; Arshad, M.; Tahir, M.; Rizwan, M.; Sabir, A.M.; Shehzadi, N.; Khalid, U. Bin; et al. The Comparison of Interstitial Relative Humidity and Temperatures of Hermetic and Polypropylene Bag for Wheat Grain Storage under Different Agro-Climatic Conditions of Rice-Wheat Ecosystem of Pakistan: Effect on Seed Quality and Protection against Insect Pests. J Stored Prod Res 2022, 96, 101936. [Google Scholar] [CrossRef]
- Kalpna; Hajam, Y.A.; Kumar, R. Management of Stored Grain Pest with Special Reference to Callosobruchus Maculatus, a Major Pest of Cowpea: A Review. Heliyon 2022, 8, e08703. [CrossRef]
- Wicochea-Rodríguez, J.D.; Rigou, P.; Lullien-Pellerin, V.; Chalier, P. A New Green Insecticide for Stored Wheat Grains: Efficiency against Rhyzopertha Dominica and Risk Assessment. J Cereal Sci 2021, 101, 103312. [Google Scholar] [CrossRef]
- Hubert, J.; Stejskal, V.; Athanassiou, C.G.; Throne, J.E. Health Hazards Associated with Arthropod Infestation of Stored Products. Annu Rev Entomol 2018, 63. [Google Scholar] [CrossRef] [PubMed]
- Potter, C. The Biology and Distribution of Rhizopertha dominica (Fab.). Transactions of the Royal Entomological Society of London 2009, 83, 449–482. [Google Scholar] [CrossRef]
- Edde, P.A. A Review of the Biology and Control of Rhyzopertha Dominica (F.) the Lesser Grain Borer. J Stored Prod Res 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Toews, M.D.; Cuperus, G.W.; Phillips, T.W. Susceptibility of Eight U.S. Wheat Cultivars to Infestation by Rhyzopertha Dominica (Coleoptera: Bostrichidae). Environ Entomol 2000, 29, 250–255. [Google Scholar] [CrossRef]
- Mason, L.; McDonough, M. Biology, Behavior, and Ecology of Stored Grain and Legume Insects. Stored Product Protection 2011. [Google Scholar]
- Boniecki, P.; Koszela, K.; Świerczyński, K.; Skwarcz, J.; Zaborowicz, M.; Przybył, J. Neural Visual Detection of Grain Weevil (Sitophilus Granarius l.). Agriculture (Switzerland) 2020, 10. [Google Scholar] [CrossRef]
- Boniecki, P.; Piekarska-Boniecka, H.; Świerczyński, K.; Koszela, K.; Zaborowicz, M.; Przybył, J. Detection of the Granary Weevil Based on X-Ray Images of Damaged Wheat Kernels. J Stored Prod Res 2014, 56, 38–42. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Steenberg, T. Insecticidal Effect of Beauveria Bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreaes) in Combination with Three Diatomaceous Earth Formulations against Sitophilus Granarius (L.) (Coleoptera: Curculionidae). Biological Control 2007, 40, 411–416. [Google Scholar] [CrossRef]
- Kumar, H.; Vijay, V.K.; Subbarao, P.M.V.; Chandra, R. Studies on the Application of Bio-Carbon Dioxide as Controlled Atmosphere on Pest Management in Wheat Grain Storage. J Stored Prod Res 2022, 95, 101911. [Google Scholar] [CrossRef]
- Huang, F.; Subramanyam, B. Management of Five Stored-Product Insects in Wheat with Pirimiphos-Methyl and Pirimiphos-Methyl plus Synergized Pyrethrins. Pest Manag Sci 2005, 61, 356–362. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Reed, C.; Kenkel, P. Management of Stored Wheat Insect Pests in the USA. Integrated Pest Management Reviews 1999, 4, 127–143. [Google Scholar] [CrossRef]
- Morrison, W.; Bruce, A.; Wilkins, R.; Albin, C.; Arthur, F. Sanitation Improves Stored Product Insect Pest Management. Insects 2019, 10, 77. [Google Scholar] [CrossRef]
- Beckett, S.J. Insect and Mite Control by Manipulating Temperature and Moisture before and during Chemical-Free Storage. J Stored Prod Res 2011, 47, 284–292. [Google Scholar] [CrossRef]
- Zeni, V.; Baliota, G. V.; Benelli, G.; Canale, A.; Athanassiou, C.G. Diatomaceous Earth for Arthropod Pest Control: Back to the Future. Molecules 2021, 26, 7487. [Google Scholar] [CrossRef]
- Shah, M.A.; Khan, A.A. Use of Diatomaceous Earth for the Management of Stored-Product Pests. Int J Pest Manag 2014, 60, 100–113. [Google Scholar] [CrossRef]
- Losic, D.; Korunic, Z. CHAPTER 10: Diatomaceous Earth, A Natural Insecticide for Stored Grain Protection: Recent Progress and Perspectives. In RSC Nanoscience and Nanotechnology; 2018; Vol. 2018-January.
- Afridi, I.A.K.; Parveen, Z.; Masud, S.Z. Stability of Organophosphate and Pyrethroid Pesticides on Wheat in Storage. J Stored Prod Res 2001, 37, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Arthur, F.H. Aerosols and Contact Insecticides as Alternatives to Methyl Bromide in Flour Mills, Food Production Facilities, and Food Warehouses. J Pest Sci (2004) 2012, 85.
- Aulicky, R.; Stejskal, V. Efficacy and Limitations of Phosphine “Spot-Fumigation” against Five Coleoptera Species of Stored Product Pests in Wheat in a Grain Store - Short Note. Plant Protection Science 2015, 51, 33–38. [Google Scholar] [CrossRef]
- Bonjour, E.L.; Opit, G.P.; Hardin, J.; Jones, C.L.; Payton, M.E.; Beeby, R.L. Efficacy of Ozone Fumigation Against the Major Grain Pests in Stored Wheat. J Econ Entomol 2011, 104, 308–316. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Economou, L.P.; Dimizas, C.B.; Vayias, B.J.; Tomanović, S.; Milutinović, M. Persistence and Efficacy of Three Diatomaceous Earth Formulations Against <I>Sitophilus Oryzae</I> (Coleoptera: Curculionidae) on Wheat and Barley. J Econ Entomol 2005, 98, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Korunic, Z. Evaluation of Two New Diatomaceous Earth Formulations, Enhanced with Abamectin and Bitterbarkomycin, against Four Stored-Grain Beetle Species. J Stored Prod Res 2007, 43, 468–473. [Google Scholar] [CrossRef]
- Korunic, Z. Diatomaceous Earths: Natural Insecticides. Pesticidi i fitomedicina 2013, 28, 77–95. [Google Scholar] [CrossRef]
- Subramanyam, B.; Roesli, R. Inert Dusts. Alternatives to pesticides in stored-product IPM 2000, 321–380.
- Fields, P.; Korunic, Z. The Effect of Grain Moisture Content and Temperature on the Efficacy of Diatomaceous Earths from Different Geographical Locations against Stored-Product Beetles. J Stored Prod Res 2000, 36, 1–13. [Google Scholar] [CrossRef]
- Nikpay, A. Diatomaceous Earths as Alternatives to Chemical Insecticides in Stored Grain. Insect Sci 2006, 13. [Google Scholar] [CrossRef]
- Ebeling, W. Sorptive Dusts for Pest Control. Annu Rev Entomol 1971, 16, 123–158. [Google Scholar] [CrossRef]
- Sousa, A.H.; Faroni, L.R.A.; Andrade, G.S.; Freitas, R.S.; Pimentel, M.A.G. Bioactivity of Diatomaceous Earth to Sitophilus Zeamais (Coleoptera: Curculionidae) in Different Application Conditions. Revista Brasileira de Engenharia Agrícola e Ambiental 2013, 17, 982–986. [Google Scholar] [CrossRef]
- Arthur, F.H. Immediate and Delayed Mortality of Oryzaephilus Surinamensis (L.) Exposed on Wheat Treated with Diatomaceous Earth: Effects of Temperature, Relative Humidity, and Exposure Interval. J Stored Prod Res 2000, 37, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ciniviz, G.; Mu, C. Effectiveness of Some Native Diatomaceous Earth against Maize Weevil, Sitophilus Zeamais (Coleoptera: Curculionidae), under Controlled Conditions. Agriculture and Forestry 2020, 66. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Arthur, F.H. Bacterial Insecticides and Inert Materials. Recent advances in stored product protection 2018, 83–98. [Google Scholar]
- Şen, R.; Işekber, A.A.; Bozkurt, H.; Sağlam, Ö. Effect of Temperature on Insecticidal Efficiency of Local Diatomaceous Earth against Stored-Grain Insects. Turkish Journal of Entomology 2019, 441–450. [Google Scholar] [CrossRef]
- Alkan, M.; Erturk, S.; Firat, T.A.; Ciftci, E. Study of Insecticidal and Behavioral Effects and Some Characteristic of Native Diatomaceous Earth against the Bean Weevil, Acanthoscelides Obtectus (Coleoptera: Chrysomelidae). Fresenius Environ Bull 2019, 28, 2916–2922. [Google Scholar]
- Kavallieratos, N.G.; Athanassiou, C.G.; Vayias, B.J.; Maistrou, S.N. Influence of Temperature on Susceptibility of Tribolium Confusum (Coleoptera: Tenebrionidae) Populations to Three Modified Diatomaceous Earth Formulations. Florida Entomologist 2007, 90, 616–625. [Google Scholar] [CrossRef]
- Arthur, F.H. Toxicity of Diatomaceous Earth to Red Flour Beetles and Confused Flour Beetles (Coleoptera: Tenebrionidae): Effects of Temperature and Relative Humidity. J Econ Entomol 2000, 93, 526–532. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Vayias, B.J.; Dimizas, C.B.; Kavallieratos, N.G.; Papagregoriou, A.S.; Buchelos, C.Th. Insecticidal Efficacy of Diatomaceous Earth against Sitophilus Oryzae (L.) (Coleoptera: Curculionidae) and Tribolium Confusum Du Val (Coleoptera: Tenebrionidae) on Stored Wheat: Influence of Dose Rate, Temperature and Exposure Interval. J Stored Prod Res 2005, 41, 47–55. [Google Scholar] [CrossRef]
- Arthur, F.H. Evaluation of Methoprene Alone and in Combination with Diatomaceous Earth to Control Rhyzopertha Dominica (Coleoptera: Bostrichidae) on Stored Wheat. J Stored Prod Res 2004, 40, 485–498. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Pashalidou, F.G.; Andris, N.S.; Tomanović, Ž. Influence of Grain Type on the Insecticidal Efficacy of Two Diatomaceous Earth Formulations AgainstRhyzopertha Dominica (F) (Coleoptera: Bostrychidae). Pest Manag Sci 2005, 61, 660–666. [Google Scholar] [CrossRef]
- Vassilakos, T.N.; Athanassiou, C.G.; Kavallieratos, N.G.; Vayias, B.J. Influence of Temperature on the Insecticidal Effect of Beauveria Bassiana in Combination with Diatomaceous Earth against Rhyzopertha Dominica and Sitophilus Oryzae on Stored Wheat. Biological Control 2006, 38, 270–281. [Google Scholar] [CrossRef]
- Wakil, W.; Riasat, T.; Ashfaq, M. Residual Efficacy of Thiamethoxam, Beauveria Bassiana (Balsamo) Vuillemin, and Diatomaceous Earth Formulation against Rhyzopertha Dominica F. (Coleoptera: Bostrychidae). J Pest Sci (2004) 2012, 85, 341–350. [Google Scholar] [CrossRef]
- Vardeman, E.A.; Arthur, F.H.; Nechols, J.R.; Campbell, J.F. Efficacy of Surface Applications with Diatomaceous Earth to Control Rhyzopertha Dominica (F.) (Coleoptera: Bostrichidae) in Stored Wheat. J Stored Prod Res 2007, 43, 335–341. [Google Scholar] [CrossRef]
- Arthur, F.H. Impact of Food Source on Survival of Red Flour Beetles and Confused Flour Beetles (Coleoptera: Tenebrionidae) Exposed to Diatomaceous Earth. J Econ Entomol 2000, 93, 1347–1356. [Google Scholar] [CrossRef]
- Golnaz Shams Insecticidal Effect of Diatomaceous Earth against Callosobruchus Maculatus (F.) (Coleoptera: Bruchidae) and Sitophilus Granarius (L.) (Coleoptera: Curculionidae) under Laboratory Conditions. African Journal Of Agricultural Reseearch 2011, 6. [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D. Principles and Procedure of Statistics. A Biometrical Approach 3rd Ed. McGraw HillBookCo. Inc., New York 1997, 352–358.
- IBM SPSS Statistics for Windows. IBM Corp. Released 2012 2015.
- Busvine, J.R. A Critical Review of the Techniques for Testing Insecticides. A critical review of the techniques for testing insecticides. 2nd Edition. 1971. [Google Scholar]
- CABI Sitophilus Granarius (Grain Weevil). CABI Compendium 2022, CABI Compendium. [CrossRef]
- Wakil, W. Rhyzopertha Dominica (Lesser Grain Borer). CABI Compendium 2022, CABI Compendium. [CrossRef]
- Morrison, W.R.; Larson, N.L.; Brabec, D.; Zhang, A. Methyl Benzoate as a Putative Alternative, Environmentally Friendly Fumigant for the Control of Stored Product Insects. J Econ Entomol 2019, 112, 2458–2468. [Google Scholar] [CrossRef]
- Arthur, F.H. Immediate and Delayed Mortality of Oryzaephilus Surinamensis (L.) Exposed on Wheat Treated with Diatomaceous Earth: Effects of Temperature, Relative Humidity, and Exposure Interval. J Stored Prod Res 2000, 37, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, L.; Tran Thi Lan, H.; Brostaux, Y.; Haubruge, E. Efficacy of Diatomaceous Earth Formulations Admixed with Grain against Populations of Tribolium Castaneum. J Stored Prod Res 2005, 41, 121–130. [Google Scholar] [CrossRef]
- Vayias, B.J.; Athanassiou, C.G.; Kavallieratos, N.G.; Buchelos, C.T. Susceptibility of Different European Populations of Tribolium Confusum (Coleoptera: Tenebrionidae) to Five Diatomaceous Earth Formulations. J Econ Entomol 2006, 99, 1899–1904. [Google Scholar] [CrossRef]
- Korunic, Z. ReviewDiatomaceous Earths, a Group of Natural Insecticides. J Stored Prod Res 1998, 34, 87–97. [Google Scholar] [CrossRef]
- Dodanlı, A.; Mutlu, Ç. Biological Activity of Some Native Diatomaceous Earth Against Khapra, Trogoderma Granarium Everts (Coleoptera: Dermestidae), Larvae Under Laboratory Conditions. Uluslararası Tarım ve Yaban Hayatı Bilimleri Dergisi 2020, 44–54. [CrossRef]
- Quarles, W.; Winn, P.S. Diatomaceous Earth and Stored Product Pests. IPM practitioner 1996, 18, 1–10. [Google Scholar]
- Baliota, G. V.; Lampiri, E.; Athanassiou, C.G. Differential Effects of Abiotic Factors on the Insecticidal Efficacy of Diatomaceous Earth against Three Major Stored Product Beetle Species. Agronomy 2022, 12. [Google Scholar] [CrossRef]
- Golob, P. Current Status and Future Perspectives for Inert Dusts for Control of Stored Product Insects. J Stored Prod Res 1997, 33, 69–79. [Google Scholar] [CrossRef]
- Subramanyam, B.; Hagstrum, D.W. Alternatives to Pesticides in Stored-Product IPM; Springer Science & Business Media, 2012; ISBN 1461543533.
- Vayias, B.J.; Athanassiou, C.G. Factors Affecting the Insecticidal Efficacy of the Diatomaceous Earth Formulation SilicoSec against Adults and Larvae of the Confused Flour Beetle, Tribolium Confusum DuVal (Coleoptera: Tenebrionidae). Crop Protection 2004, 23, 565–573. [Google Scholar] [CrossRef]
- Baliota, G. V.; Athanassiou, C.G. Evaluation of a Greek Diatomaceous Earth for Stored Product Insect Control and Techniques That Maximize Its Insecticidal Efficacy. Applied Sciences 2020, 10, 6441. [Google Scholar] [CrossRef]
- Ogreten, A.; Eren, S.; Kaya, C.; Mutlu, C.; Ayaz, T.; Gaafar, A.R.Z.; ul Malook, S.; Mahmoud, R.M. Insecticidal Efficacy of Native Raw and Commercial Diatomaceous Earths against Tribolium Confusum DuVal (Coleoptera: Tenebrionidae) under Different Environmental Conditions. J King Saud Univ Sci 2023, 35. [Google Scholar] [CrossRef]
- Mutlu, Ç.; ÖĞRETEN, A.; KAYA, C.; MAMAY, M. Influence of Different Grain Storage Types on Khapra Beetle, Trogoderma Granarium Everts, 1898 (Coleoptera: Dermestidae), Infestation in Southeastern Anatolia (Turkey) and Its Resistance to Malathion and Deltamethrin. Turkish Journal of Entomology 2019, 131–142. [Google Scholar] [CrossRef]
| 7 DAT | 14 DAT | 21 DAT | |||||
| Source of variation | DF | F value | P value | F value | P value | F value | P value |
| Temperature (T) | 1 | 1230.69 | < 0.0001 | 329.65 | < 0.0001 | 857.32 | < 0.0001 |
| Relative humidity (RH) | 1 | 3353.30 | < 0.0001 | 1301.84 | < 0.0001 | 2379.97 | < 0.0001 |
| Diatom dose (D) | 11 | 2157.03 | < 0.0001 | 2857.95 | < 0.0001 | 4085.85 | < 0.0001 |
| T × RH | 1 | 120.91 | < 0.0001 | 10.53 | 0.001 | 32.19 | < 0.0001 |
| T × D | 11 | 7.44 | < 0.0001 | 5.57 | < 0.0001 | 2.58 | 0.005 |
| RH × D | 11 | 3.37 | 0.000 | 10.69 | < 0.0001 | 3.86 | < 0.0001 |
| T × RH × D | 11 | 9.06 | < 0.0001 | 2.03 | 0.030 | 3.88 | < 0.0001 |
| 7 DAT | 14 DAT | 21 DAT | |||||
| Source of variation | DF | F value | P value | F value | P value | F value | P value |
| Temperature (T) | 1 | 338.79 | < 0.0001 | 591.43 | < 0.0001 | 477.12 | < 0.0001 |
| Relative humidity (RH) | 1 | 2028.46 | < 0.0001 | 2258.31 | < 0.0001 | 2940.44 | < 0.0001 |
| Diatom dose (D) | 11 | 6417.59 | < 0.0001 | 6721.19 | < 0.0001 | 5921.60 | < 0.0001 |
| T × RH | 1 | 1.90 | < 0.0001 | 19.87 | < 0.0001 | 675.12 | < 0.0001 |
| T × D | 11 | 2.17 | < 0.0001 | 5.40 | < 0.0001 | 61.56 | < 0.0001 |
| RH × D | 11 | 11.99 | < 0.0001 | 13.38 | < 0.0001 | 109.50 | < 0.0001 |
| T × RH × D | 11 | 1.15 | < 0.0001 | 3.88 | < 0.0001 | 71.60 | < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





