Submitted:
22 April 2025
Posted:
23 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
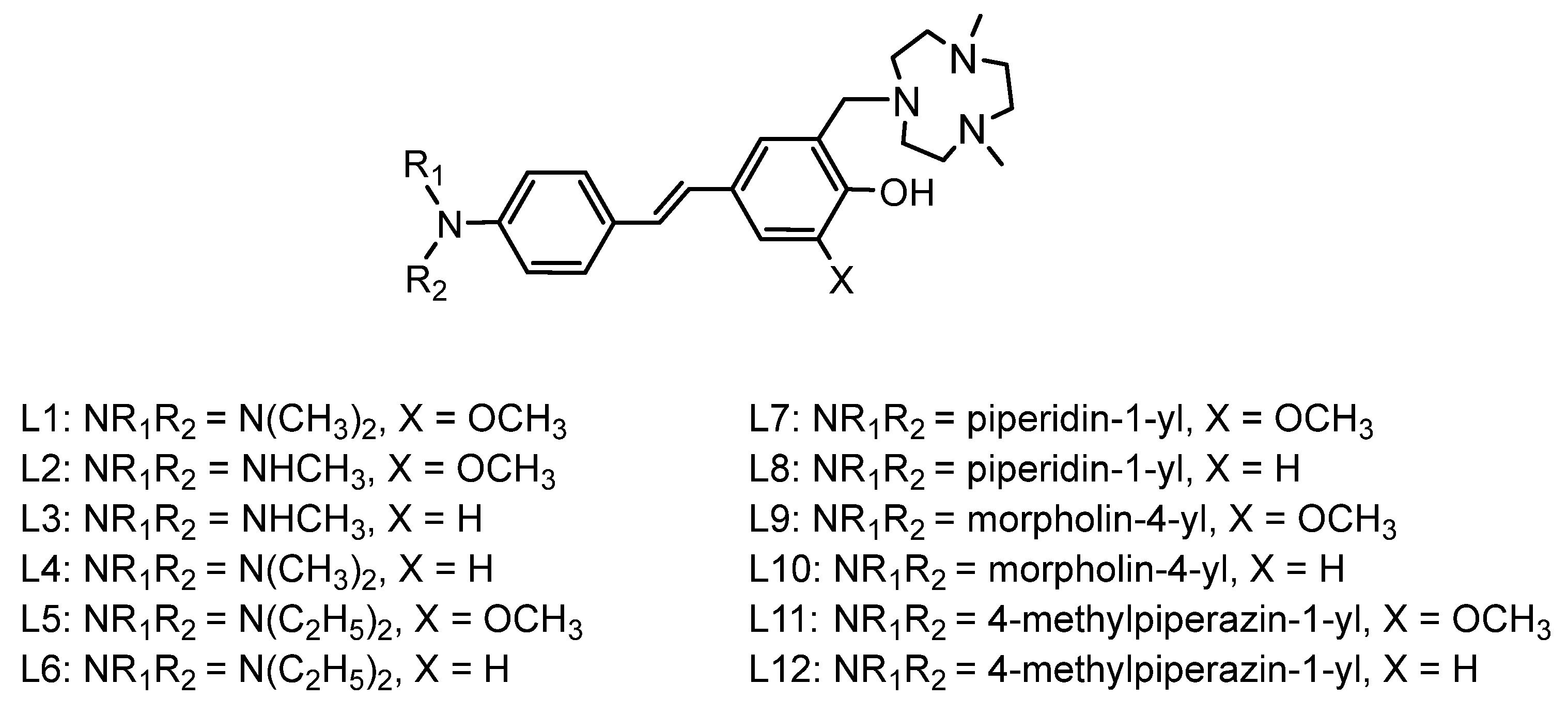
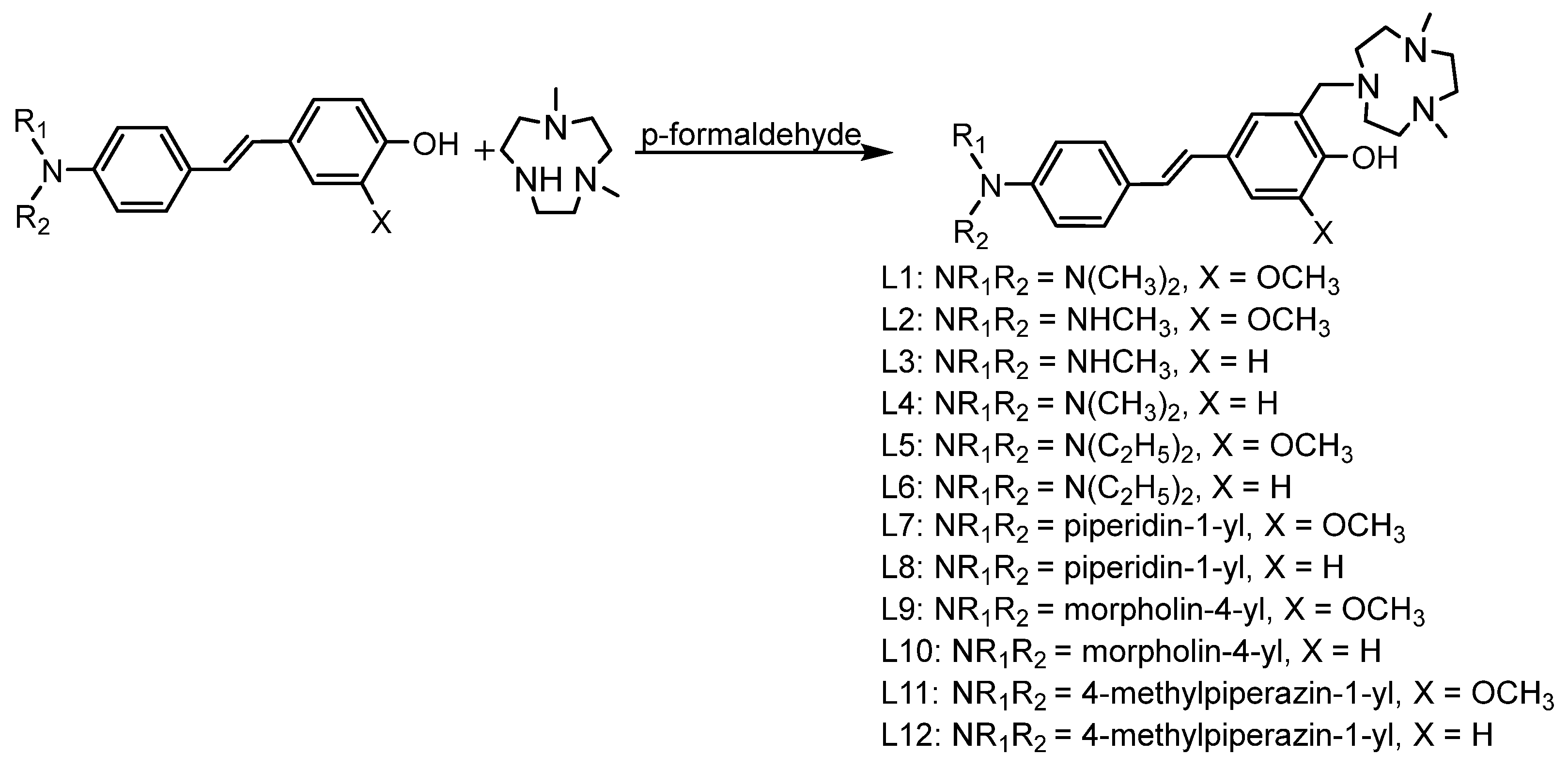
2.1. Design and Synthesis of Compounds
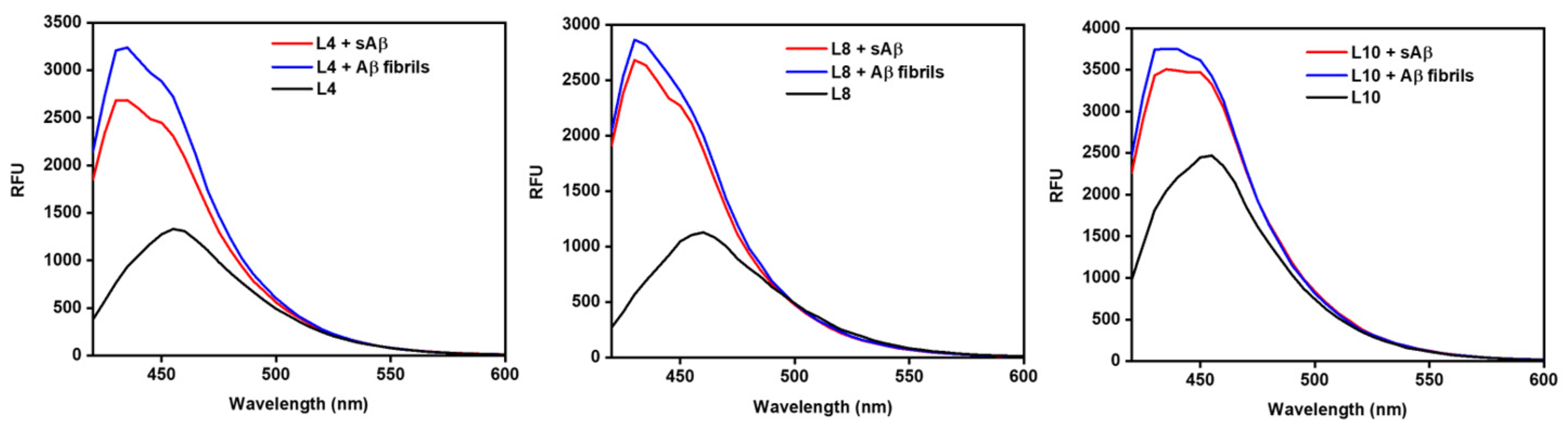
2.2. Fluorescence Turn-on Effect of Compounds with Aβ Species
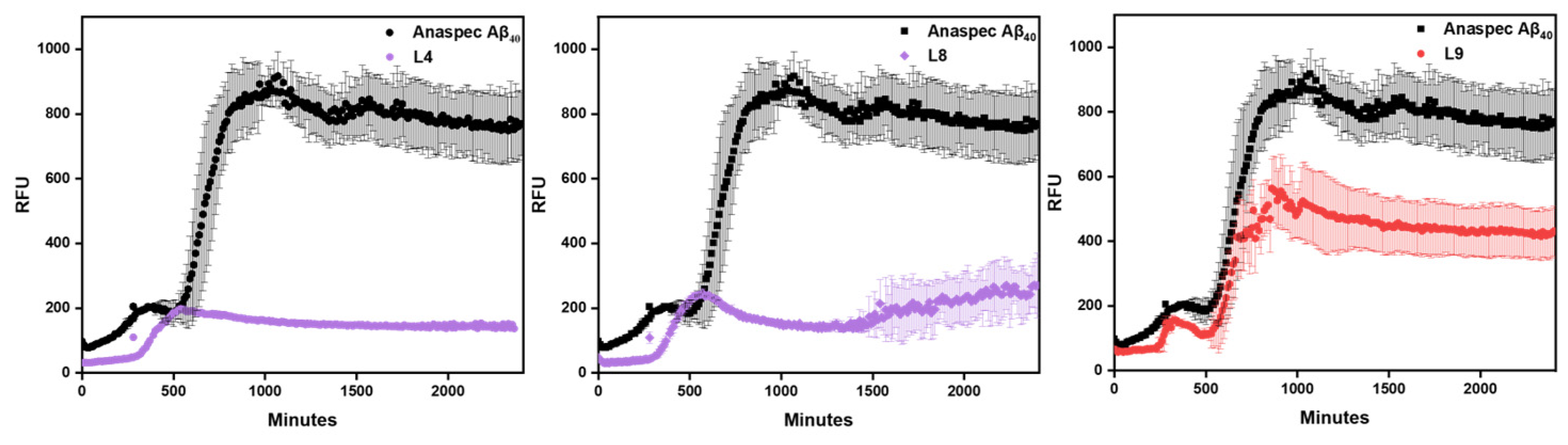
2.3. Aβ Inhibition Assays
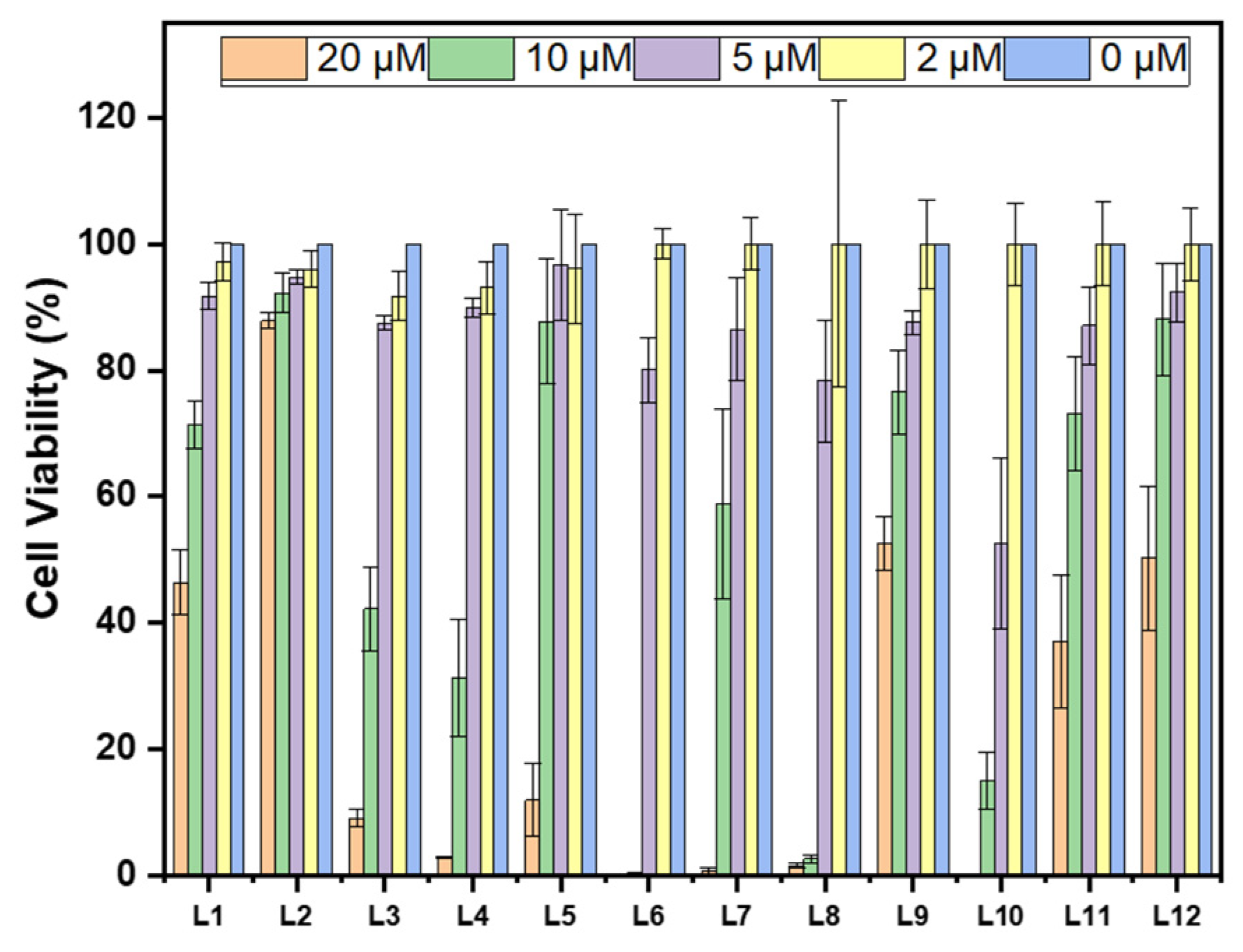
2.4. Cytotoxicity of Compounds in N2a Cells
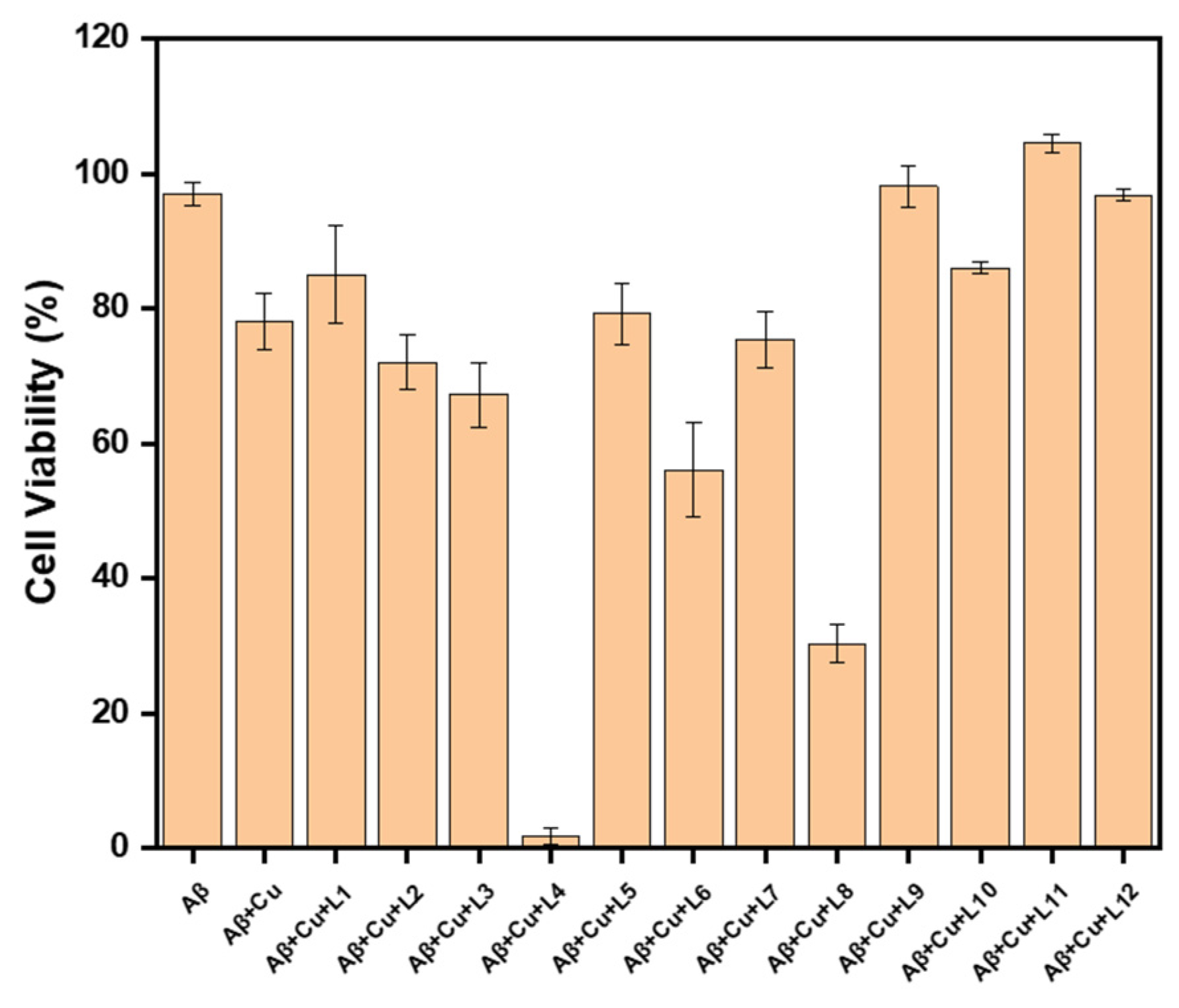
2.5. Effect of Inhibitors on Aβ42 Neurotoxicity in N2a Cells
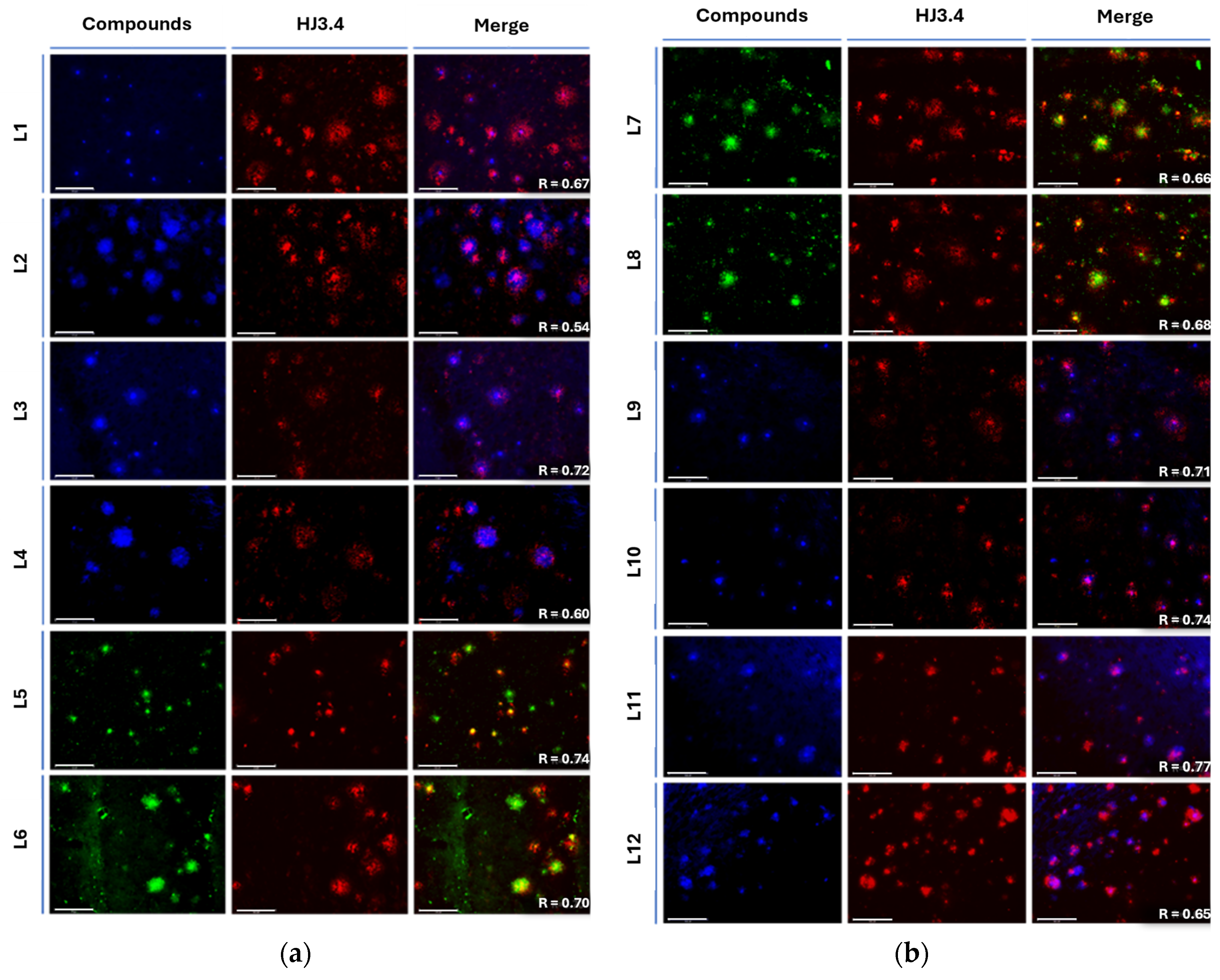
2.6. Fluorescence Imaging of Amyloid Plaques in 5xFAD Mice Brain Sections
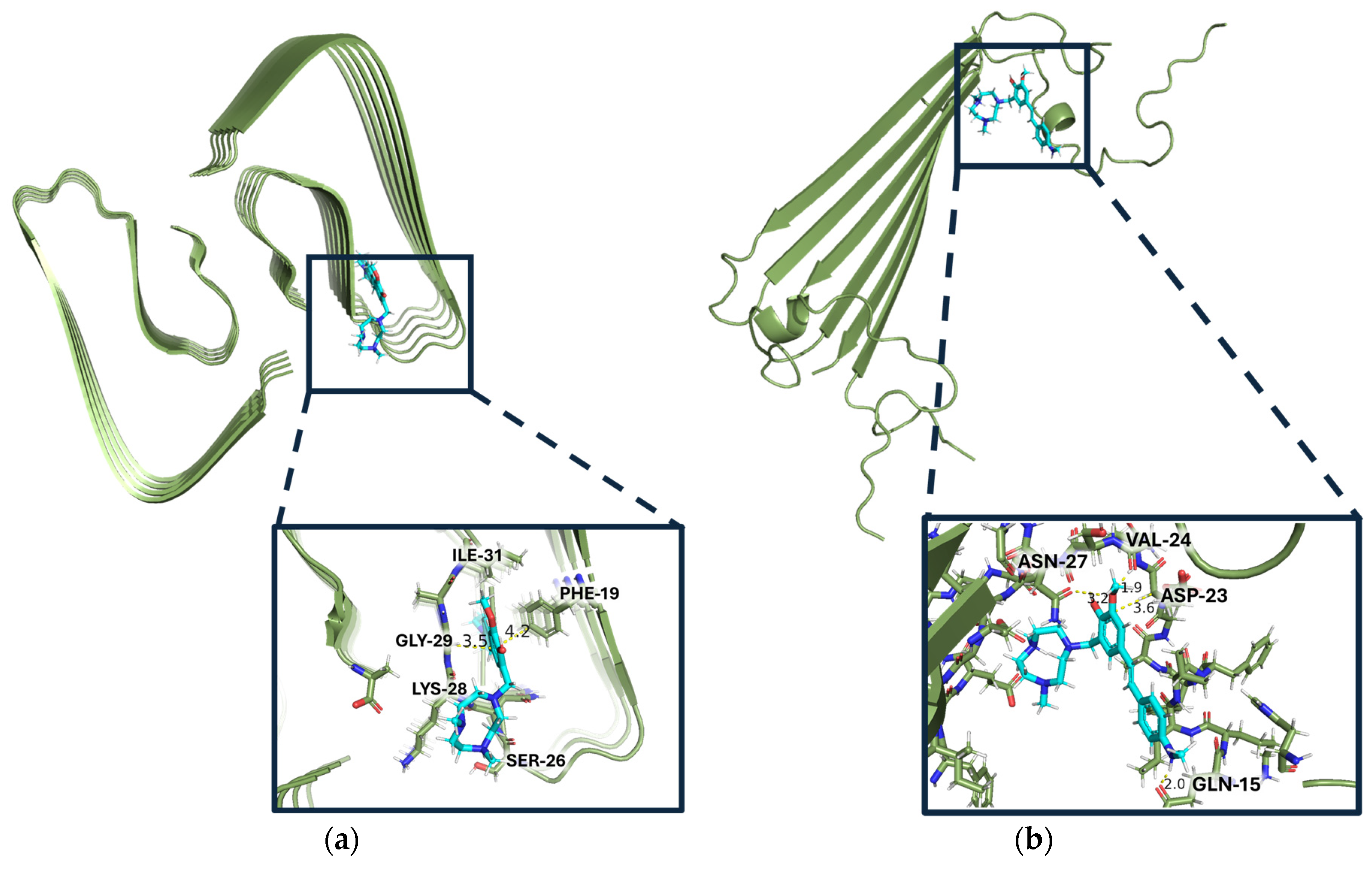
2.7. Docking Studies of Compounds on Aβ
3. Discussion
3.1. Interpretation of Docking Results
3.2. Application of Amyloid Inhibitors
4. Materials and Methods
4.1. General Experimental Details
4.2. Synthetic Details
4.2.1. Synthesis of Precursors: 1a, 1b, 2a, 2b, 3a, 3b, 4a, and 4b
4.2.1. Synthesis of Compounds L1-L12
4.3. Amyloid β Peptide Experiments
4.3.1. Fluorescence Turn-on and Cell Studies
4.3.2. Inhibition Assay
4.4. Fluorescence Spectra Measurements
4.5. Alamar Blue Assay
4.5.1. Cytotoxicity Studies
4.5.2. N2a Cell Rescue Studies
4.6. Molecular Docking
4.7. Histological Staining of 5xFAD Mice Brain Sections
4.8. Log D Measurement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | Amyloid beta |
| CR | Congo Red |
| EAG | Electron accepting group |
| EDG | Electron donating group |
| NMR | Nuclear magnetic resonance |
| sAβ | Soluble amyloid beta |
| tacn | 1,4,7-triazacyclononane |
| ThT | Thioflavin T |
| TICT | Twisted Intramolecular Charge Transfer |
References
- Liu, P. P.; Xie, Y.; Meng, X. Y.; Kang, J. S., History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target Ther. 2019, 4, 29. [CrossRef]
- Ono, K.; Condron, M. M.; Teplow, D. B., Structure–neurotoxicity relationships of amyloid β-protein oligomers. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 14745. [CrossRef]
- Tew, D. J.; Bottomley, S. P.; Smith, D. P.; Ciccotosto, G. D.; Babon, J.; Hinds, M. G.; Masters, C. L.; Cappai, R.; Barnham, K. J., Stabilization of Neurotoxic Soluble β-Sheet-Rich Conformations of the Alzheimer’s Disease Amyloid-β Peptide. Biophys. J. 2008, 94, 2752. [CrossRef]
- Jin, L.; Wu, W.-H.; Li, Q.-Y.; Zhao, Y.-F.; Li, Y.-M., Copper inducing Aβ42 rather than Aβ40 nanoscale oligomer formation is the key process for Aβ neurotoxicity. Nanoscale 2011, 3, 4746. [CrossRef]
- Yang, J.; Zeng, F.; Li, X.; Ran, C.; Xu, Y.; Li, Y., Highly specific detection of Aβ oligomers in early Alzheimer’s disease by a near-infrared fluorescent probe with a “V-shaped” spatial conformation. Chem. Commun. 2020, 56, 583. [CrossRef]
- Matsumura, K.; Ono, M.; Kitada, A.; Watanabe, H.; Yoshimura, M.; Iikuni, S.; Kimura, H.; Okamoto, Y.; Ihara, M.; Saji, H., Structure–Activity Relationship Study of Heterocyclic Phenylethenyl and Pyridinylethenyl Derivatives as Tau-Imaging Agents That Selectively Detect Neurofibrillary Tangles in Alzheimer’s Disease Brains. J. Med. Chem. 2015, 58, 7241. [CrossRef]
- Leuzy, A.; Chiotis, K.; Lemoine, L.; Gillberg, P.-G.; Almkvist, O.; Rodriguez-Vieitez, E.; Nordberg, A., Tau PET imaging in neurodegenerative tauopathies—still a challenge. Mol. Psychiatry 2019, 24, 1112. [CrossRef]
- Vadukul, D. M.; Maina, M.; Franklin, H.; Nardecchia, A.; Serpell, L. C.; Marshall, K. E., Internalisation and toxicity of amyloid-beta 1-42 are influenced by its conformation and assembly state rather than size. FEBS Lett. 2020, 594, 3490. [CrossRef]
- Tolar, M.; Hey, J.; Power, A.; Abushakra, S., Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer’s Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression. Int. J. Mol. Sci. 2021, 22, 6355. [CrossRef]
- Fu, Z.; Yang, J.; Wei, Y.; Li, J., Effects of piceatannol and pterostilbene against β-amyloid-induced apoptosis on the PI3K/Akt/Bad signaling pathway in PC12 cells. Food & Function 2016, 7, 1014. [CrossRef]
- Yuan, W.; Shang, Z.; Qiang, X.; Tan, Z.; Deng, Y., Synthesis of pterostilbene and resveratrol carbamate derivatives as potential dual cholinesterase inhibitors and neuroprotective agents. Res. Chem. Intermed. 2014, 40, 787. [CrossRef]
- Garcia, G. X.; Larsen, S. W.; Pye, C.; Galbreath, M.; Isovitsch, R.; Fradinger, E. A., The functional group on (E)-4,4′–disubstituted stilbenes influences toxicity and antioxidative activity in differentiated PC-12 cells. Bioorg. Med. Chem. Lett. 2013, 23, 6355. [CrossRef]
- Breitung, E. M.; Shu, C.-F.; McMahon, R. J., Thiazole and Thiophene Analogues of Donor−Acceptor Stilbenes: Molecular Hyperpolarizabilities and Structure−Property Relationships. J. Am. Chem. Soc. 2000, 122, 1154. [CrossRef]
- Gao, Y.; Li, J.; Wu, Q.; Wang, S.; Yang, S.; Li, X.; Chen, N.; Li, L.; Zhang, L., Tetrahydroxy stilbene glycoside ameliorates Alzheimer’s disease in APP/PS1 mice via glutathione peroxidase related ferroptosis. Int. Immunopharmacol. 2021, 99, 108002. [CrossRef]
- Freyssin, A.; Page, G.; Fauconneau, B.; Rioux Bilan, A., Natural stilbenes effects in animal models of Alzheimer’s disease. Neural Regeneration Research 2020, 15, 843. [CrossRef]
- Gao, D.; Hao, J.-p.; Li, B.-y.; Zheng, C.-c.; Miao, B.-b.; Zhang, L.; Li, Y.-l.; Li, L.; Li, X.-j.; Zhang, L., Tetrahydroxy stilbene glycoside ameliorates neuroinflammation for Alzheimer’s disease via cGAS-STING. Eur. J. Pharmacol. 2023, 953, 175809. [CrossRef]
- Firdoos, S.; Dai, R.; Tahir, R. A.; Khan, Z. Y.; Li, H.; Zhang, J.; Ni, J.; Quan, Z.; Qing, H., In silico identification of novel stilbenes analogs for potential multi-targeted drugs against Alzheimer’s disease. J. Mol. Mod. 2023, 29, 209. [CrossRef]
- Yu, Z.; Guo, W.; Patel, S.; Cho, H.-J.; Sun, L.; Mirica, L. M., Amphiphilic stilbene derivatives attenuate the neurotoxicity of soluble Aβ42 oligomers by controlling their interactions with cell membranes. Chem. Sci. 2022, 13, 12818. [CrossRef]
- Hilt, S.; Liu, R.; Maezawa, I.; Rojalin, T.; Aung, H. H.; Budamagunta, M.; Slez, R.; Gong, Q.; Carney, R. P.; Voss, J. C., Novel Stilbene-Nitroxyl Hybrid Compounds Display Discrete Modulation of Amyloid Beta Toxicity and Structure. Front. Chem. 2022, 10. [CrossRef]
- Yu, Z.; Moshood, Y.; Wozniak, M. K.; Patel, S.; Terpstra, K.; Llano, D. A.; Dobrucki, L. W.; Mirica, L. M., Amphiphilic Molecules Exhibiting Zwitterionic Excited-State Intramolecular Proton Transfer and Near-Infrared Emission for the Detection of Amyloid β Aggregates in Alzheimer’s Disease. Chem. Eur. J. 2023, 29, e202302408. [CrossRef]
- Cho, H.-J.; Huynh, T. T.; Rogers, B. E.; Mirica, L. M., Design of a multivalent bifunctional chelator for diagnostic 64Cu PET imaging in Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 30928. [CrossRef]
- Cui, M.; Ono, M.; Watanabe, H.; Kimura, H.; Liu, B.; Saji, H., Smart Near-Infrared Fluorescence Probes with Donor–Acceptor Structure for in Vivo Detection of β-Amyloid Deposits. J. Am. Chem. Soc. 2014, 136, 3388. [CrossRef]
- Sun, L.; Cho, H.-J.; Sen, S.; Arango, A. S.; Bandara, N.; Huang, Y.; Huynh, T. T.; Rogers, B. E.; Tajkhorshid, E.; Mirica, L. M., Amphiphilic Distyrylbenzene Derivatives as Potential Therapeutic and Imaging Agents for the Soluble Amyloid-β Oligomers in Alzheimer’s Disease. J. Am. Chem. Soc. 2021, 143, 10462. [CrossRef]
- Kung, H. F.; Choi, S. R.; Qu, W.; Zhang, W.; Skovronsky, D., 18F Stilbenes and Styrylpyridines for PET Imaging of Aβ Plaques in Alzheimer’s Disease: A Miniperspective. J. Med. Chem. 2010, 53, 933. [CrossRef]
- Martí-Centelles, R.; Falomir, E.; Murga, J.; Carda, M.; Marco, J. A., Inhibitory effect of cytotoxic stilbenes related to resveratrol on the expression of the VEGF, hTERT and c-Myc genes. Eur. J. Med. Chem. 2015, 103, 488. [CrossRef]
- Kung, H., F.; Kung, M.-P.; Zhuang, Z.-P. Stilbene derivatives and their use for binding and imaging amyloid plaques. International patent no. 05854410.7, 2005.
- Xiao, G.; Li, Y.; Qiang, X.; Xu, R.; Zheng, Y.; Cao, Z.; Luo, L.; Yang, X.; Sang, Z.; Su, F.; Deng, Y., Design, synthesis and biological evaluation of 4′-aminochalcone-rivastigmine hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2017, 25, 1030. [CrossRef]
- Lennol, M. P.; Canelles, S.; Guerra-Cantera, S.; Argente, J.; García-Segura, L. M.; de Ceballos, M. L.; Chowen, J. A.; Frago, L. M., Amyloid-β1-40 differentially stimulates proliferation, activation of oxidative stress and inflammatory responses in male and female hippocampal astrocyte cultures. Mech. Ageing Dev. 2021, 195, 111462. [CrossRef]
- Gu, L.; Guo, Z., Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305. [CrossRef]
- Sharma, A. K.; Pavlova, S. T.; Kim, J.; Kim, J.; Mirica, L. M., The effect of Cu2+ and Zn2+ on the Aβ42 peptide aggregation and cellular toxicity. Metallomics 2013, 5, 1529. [CrossRef]
- Sun, L.; Sharma, A. K.; Han, B.-H.; Mirica, L. M., Amentoflavone: A Bifunctional Metal Chelator that Controls the Formation of Neurotoxic Soluble Aβ42 Oligomers. ACS Chem. Neurosci. 2020, 11, 2741. [CrossRef]
- Cho, H.-J.; Sharma, A. K.; Zhang, Y.; Gross, M. L.; Mirica, L. M., A Multifunctional Chemical Agent as an Attenuator of Amyloid Burden and Neuroinflammation in Alzheimer’s Disease. ACS Chem. Neurosci. 2020, 11, 1471. [CrossRef]
- Liao, F.; Li, A.; Xiong, M.; Bien-Ly, N.; Jiang, H.; Zhang, Y.; Finn, M. B.; Hoyle, R.; Keyser, J.; Lefton, K. B.; Robinson, G. O.; Serrano, J. R.; Silverman, A. P.; Guo, J. L.; Getz, J.; Henne, K.; Leyns, C. E. G.; Gallardo, G.; Ulrich, J. D.; Sullivan, P. M.; Lerner, E. P.; Hudry, E.; Sweeney, Z. K.; Dennis, M. S.; Hyman, B. T.; Watts, R. J.; Holtzman, D. M., Targeting of nonlipidated, aggregated apoE with antibodies inhibits amyloid accumulation. J. Clin. Invest. 2018, 128, 2144. [CrossRef]
- Mahan, T. E.; Wang, C.; Bao, X.; Choudhury, A.; Ulrich, J. D.; Holtzman, D. M., Selective reduction of astrocyte apoE3 and apoE4 strongly reduces Aβ accumulation and plaque-related pathology in a mouse model of amyloidosis. Mol. Neurodegener. 2022, 17, 13. [CrossRef]
- Esparza, T. J.; Zhao, H.; Cirrito, J. R.; Cairns, N. J.; Bateman, R. J.; Holtzman, D. M.; Brody, D. L., Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Ann. Neurol. 2013, 73, 104. [CrossRef]
- Gremer, L.; Schölzel, D.; Schenk, C.; Reinartz, E.; Labahn, J.; Ravelli, R. B. G.; Tusche, M.; Lopez-Iglesias, C.; Hoyer, W.; Heise, H.; Willbold, D.; Schröder, G. F., Fibril structure of amyloid-β(1–42) by cryo–electron microscopy. Science 2017, 358, 116. [CrossRef]
- Zou, Y.; Qian, Z.; Chen, Y.; Qian, H.; Wei, G.; Zhang, Q., Norepinephrine Inhibits Alzheimer’s Amyloid-β Peptide Aggregation and Destabilizes Amyloid-β Protofibrils: A Molecular Dynamics Simulation Study. ACS Chem. Neurosci. 2019, 10, 1585. [CrossRef]
- Gautieri, A., The Anti-Amyloidogenic Action of Doxycycline: A Molecular Dynamics Study on the Interaction with Aβ42. Int. J. Mol. Sci. 2019, 20, 4641. [CrossRef]
- Ciudad, S.; Puig, E.; Botzanowski, T.; Meigooni, M.; Arango, A. S.; Do, J.; Mayzel, M.; Bayoumi, M.; Chaignepain, S.; Maglia, G.; Cianferani, S.; Orekhov, V.; Tajkhorshid, E.; Bardiaux, B.; Carulla, N., Aβ(1-42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage. Nat. Commun. 2020, 11, 3014. [CrossRef]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G. A.; Dahlgren, M. K.; Russell, E.; Von Bargen, C. D.; Abel, R.; Friesner, R. A.; Harder, E. D., OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291. [CrossRef]
- Friesner, R. A.; Murphy, R. B.; Repasky, M. P.; Frye, L. L.; Greenwood, J. R.; Halgren, T. A.; Sanschagrin, P. C.; Mainz, D. T., Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177. [CrossRef]
- Friesner, R. A.; Banks, J. L.; Murphy, R. B.; Halgren, T. A.; Klicic, J. J.; Mainz, D. T.; Repasky, M. P.; Knoll, E. H.; Shelley, M.; Perry, J. K.; Shaw, D. E.; Francis, P.; Shenkin, P. S., Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739. [CrossRef]
- Raju, S. K.; Sundhararajan, N.; Sekar, P.; Nagalingam, Y., Therapeutic aspects of biologically potent vanillin derivatives: A critical review. J. Drug Deliv. Therap. 2023, 13, 177.
- Iannuzzi, C.; Liccardo, M.; Sirangelo, I., Overview of the Role of Vanillin in Neurodegenerative Diseases and Neuropathophysiological Conditions. Int. J. Mol. Sci. 2023, 24, 1817. [CrossRef]
- Kumar, N. S.; Nair, R. H.; B, P. K., Comparative analysis of anti-oxidant potential of vanillin and ferulic acid invitro. Food & Humanity 2023, 1, 1206. [CrossRef]
- Terpstra, K.; Wang, Y.; Huynh, T. T.; Bandara, N.; Cho, H.-J.; Rogers, B. E.; Mirica, L. M., Divalent 2-(4-Hydroxyphenyl)benzothiazole Bifunctional Chelators for 64Cu Positron Emission Tomography Imaging in Alzheimer’s Disease. Inorg. Chem. 2022, 61, 20326. [CrossRef]
- Dong, X.; Qiao, Q.; Qian, Z.; Wei, G., Recent computational studies of membrane interaction and disruption of human islet amyloid polypeptide: Monomers, oligomers and protofibrils. Biochim. Biophys. Acta, Biomemb. 2018, 1860, 1826. [CrossRef]
- Gao, D.; Wan, J.; Zou, Y.; Gong, Y.; Dong, X.; Xu, Z.; Tang, J.; Wei, G.; Zhang, Q., The destructive mechanism of Aβ1–42 protofibrils by norepinephrine revealed via molecular dynamics simulations. Phys. Chem. Chem. Phys. 2022, 24, 19827. [CrossRef]
- Chen, Z.-L.; Singh, P. K.; Calvano, M.; Norris, E. H.; Strickland, S., A possible mechanism for the enhanced toxicity of beta-amyloid protofibrils in Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2023, 120, e2309389120. [CrossRef]
- Vangala, V. R.; Bhogala, B. R.; Dey, A.; Desiraju, G. R.; Broder, C. K.; Smith, P. S.; Mondal, R.; Howard, J. A. K.; Wilson, C. C., Correspondence between Molecular Functionality and Crystal Structures. Supramolecular Chemistry of a Family of Homologated Aminophenols. J. Am. Chem. Soc. 2003, 125, 14495. [CrossRef]
- Jameson, L. P.; Smith, N. W.; Dzyuba, S. V., Dye-Binding Assays for Evaluation of the Effects of Small Molecule Inhibitors on Amyloid (Aβ) Self-Assembly. ACS Chem. Neurosci. 2012, 3, 807. [CrossRef]
- Terpstra, K.; Huang, Y.; Na, H.; Sun, L.; Gutierrez, C.; Yu, Z.; Mirica, L. M., 2-Phenylbenzothiazolyl iridium complexes as inhibitors and probes of amyloid β aggregation. Dalton Trans. 2024, 53, 14258. [CrossRef]
- Ran, C.; Xu, X.; Raymond, S. B.; Ferrara, B. J.; Neal, K.; Bacskai, B. J.; Medarova, Z.; Moore, A., Design, Synthesis, and Testing of Difluoroboron-Derivatized Curcumins as Near-Infrared Probes for in Vivo Detection of Amyloid-β Deposits. J. Am. Chem. Soc. 2009, 131, 15257. [CrossRef]
- Xue, C.; Lee, Y. K.; Tran, J.; Chang, D.; Guo, Z., A mix-and-click method to measure amyloid-β concentration with sub-micromolar sensitivity. R. Soc. Open Sci. 2017, 4, 170325. [CrossRef]
- Groenning, M., Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils—current status. J. Chem. Biol. 2010, 3, 1. [CrossRef]
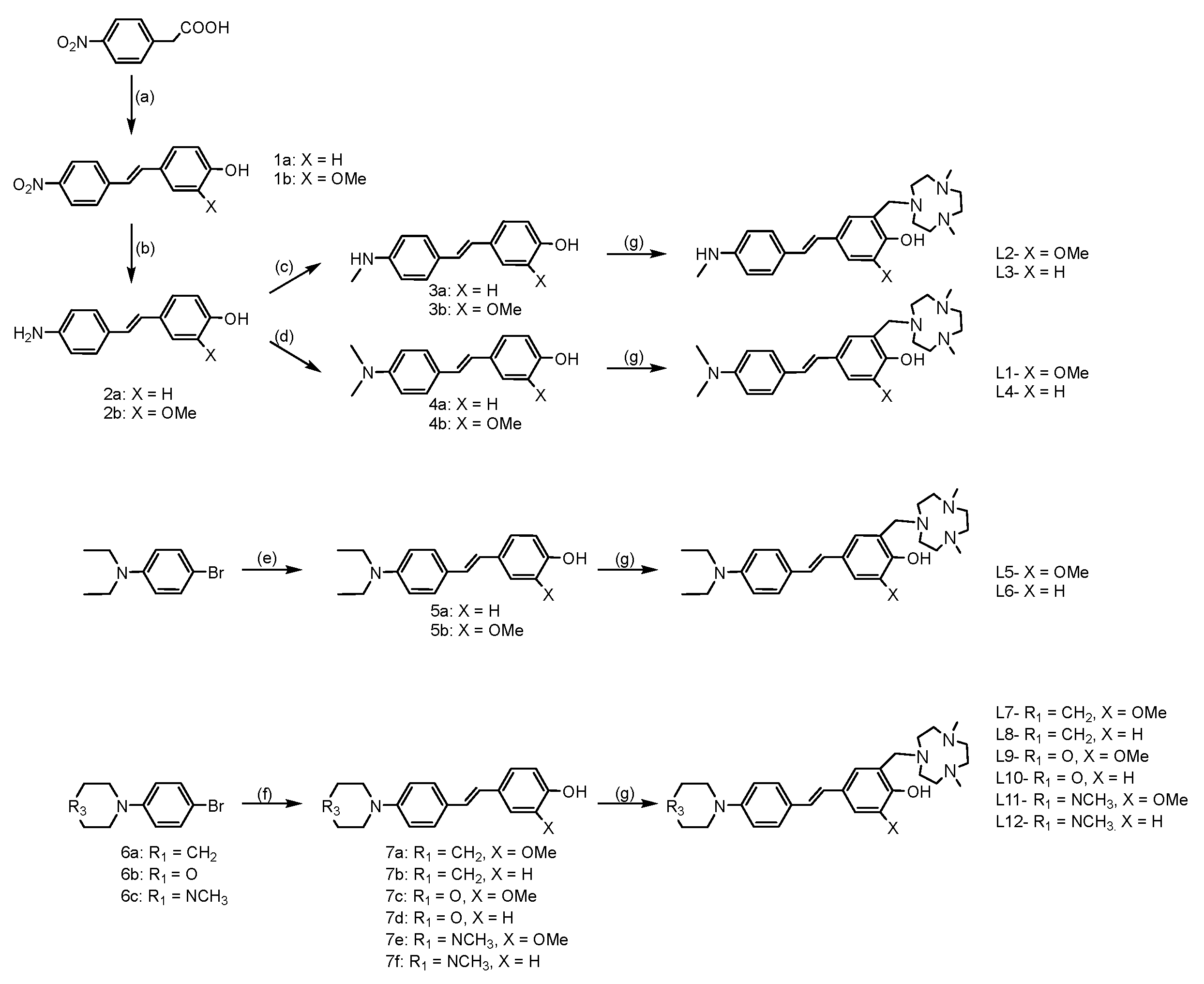
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




