Submitted:
16 April 2025
Posted:
17 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
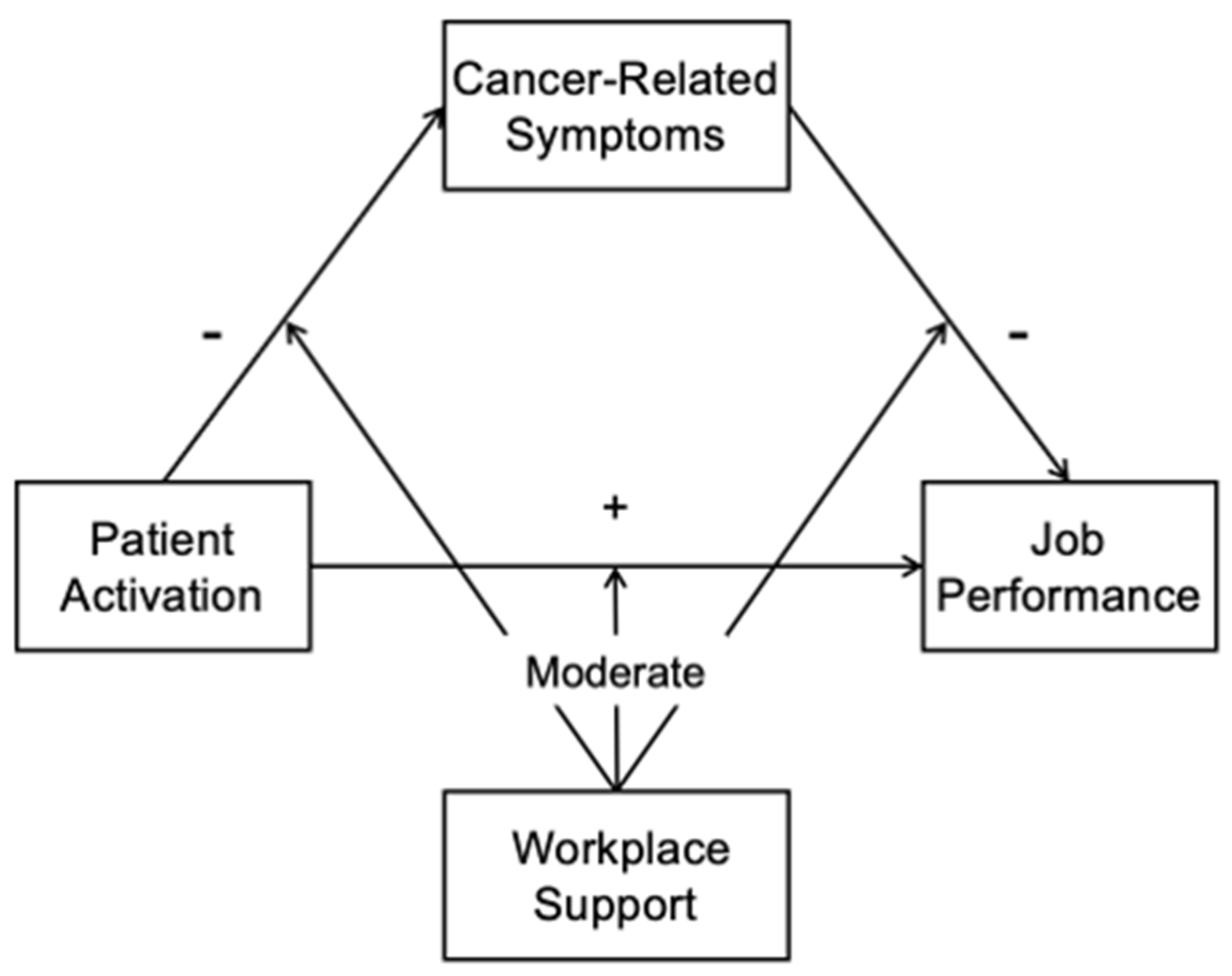
2. Materials and Methods
2.1. Study Participants and Procedures
2.2. Measures
2.2.1. Demographic and Clinical Characteristics
2.2.2. Patient Activation
2.2.3. Cancer-Related Symptoms
2.2.4. Job Performance
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGFI | Adjusted Goodness-of-Fit Index |
| CFI | Comparative Fit Index |
| CFS | Cancer Fatigue Scale |
| CMIN | Chi-Square Statistic |
| df | Degrees of Freedom |
| EORTC QLQ-C30 |
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 |
| GFI | Goodness-of-Fit Index |
| K6 | Kessler-6 |
| New BJSQ | New Brief Job Stress Questionnaire |
| PAM-13 | 13-item version of Patient Activation Measure |
| RMSEA | Root Mean Square Error of Approximation |
| SD | Standard Deviation |
| SEM | Structural Equation Modeling |
| WHO-HPQ | World Health Organization Health and Performance Questionnaire |
References
- de Boer A.G.; Taskila T.;, Ojajärvi A.; van Dijk F.J.; Verbeek J.H. Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA 2009, 301, 753–762. [CrossRef]
- Jeon S.H. The long-term effects of cancer on employment and earnings. Health Econ 2017, 26, 671–684. [CrossRef]
- Tison A.; Sagaon-Teyssier L.; Sansonetti C.; Blatier J.F.; Paraponaris A.; VICAN group. Transitions in the labor market after cancer: a comparison of self-employed workers and salaried staff. Support Care Cancer 2016, 24, 4879–4886. [CrossRef]
- Torp S.; Paraponaris A.; Van Hoof E.; Lindbohm M.L.; Tamminga S.J.; Alleaume C.; Campenhout N.V.; Sharp L.; de Boer A.G. Work-related outcomes in self-employed cancer survivors: a European multi-country study. J Occup Rehabil 2019, 29, 361–374. [CrossRef]
- Bradley C.J.; Brown K.L.; Haan M.; Glasgow R.E.; Newman L.S.; Rabin B.; Ritzwoller D.P.; Tenney L. Cancer survivorship and employment: intersection of oral agents, changing workforce dynamics, and employers’ perspectives. J Natl Cancer Inst 2018, 110, 1292–1299. [CrossRef]
- Desiron H.A.; Crutzen R.; Godderis L.; Van Hoof E.; de Rijk A. Bridging health care and the workplace: formulation of a return-to-work intervention for breast cancer patients using an intervention mapping approach. J Occup Rehabil 2016, 26, 350–365. [CrossRef]
- Todd B.L.; Feuerstein E.L.; Feuerstein M. When breast cancer survivors report cognitive problems at work. Int J Psychiatry Med 2011, 42, 279–294. [CrossRef]
- Calvio L.; Peugeot M.; Bruns G.L.; Todd B.L.; Feuerstein M. Measures of cognitive function and work in occupationally active breast cancer survivors. J Occup Environ Med 2010, 52, 219–227. [CrossRef]
- Kim S.Y.; Kissane D.W.; Richardson G.; Senior J.; Morgan J.; Gregory P.; Birks S.; Ooi C.; Lipton L.; Antill Y.; Vereker M.; Michael N.; Bobevski I. The role of depression and other psychological factors in work ability among breast cancer survivors in Australia. Psychooncology 2022, 31, 167–175. [CrossRef]
- Dahl A.A.; Fosså S.D.; Lie H.C.; Loge J.H.; Reinertsen K.V.; Ruud E.; Kiserud C.E. Employment status and work ability in long-term young adult cancer survivors. J Adolesc Young Adult Oncol 2019, 8, 304–311. [CrossRef]
- Dorland H.F.; Abma F.I.; Van Zon S.K.R.; Stewart R.E.; Amick B.C.; Ranchor A.V.; Roelen C.A.M.; Bültmann U. Fatigue and depressive symptoms improve but remain negatively related to work functioning over 18 months after return to work in cancer patients. J Cancer Surviv 2018, 12, 371–378. [CrossRef]
- Von Ah D.; Storey S.; Crouch A. Relationship between self-reported cognitive function and work-related outcomes in breast cancer survivors. J Cancer Surviv 2018, 12, 246–255. [CrossRef]
- van Muijen P.; Duijts S.F.A.; Bonefaas-Groenewoud K.; van der Beek A.J.; Anema J.R. Predictors of fatigue and work ability in cancer survivors. Occup Med 2017, 67, 703–711. [CrossRef]
- Hydeman J.A.; Uwazurike O.C.; Adeyemi E.I.; Beaupin L.K.. Survivorship needs of adolescent and young adult cancer survivors: a concept mapping analysis. J Cancer Surviv 2019, 13, 34–42. [CrossRef]
- Stone D.S.; Ganz P.A.; Pavlish C.; Robbins W.A. Young adult cancer survivors and work: a systematic review. J Cancer Surviv 2017, 11, 765–81. [CrossRef]
- Braun I.; Friedrich M.; Morgenstern L.; Sender A.; Geue K.; Mehnert-Theuerkauf A.; Leuteritz K. Changes, challenges and support in work, education and finances of adolescent and young adult (AYA) cancer survivors: a qualitative study. Eur J Oncol Nurs 2023, 64, 102329. [CrossRef]
- Act for eliminating discrimination against persons with disabilities. Available online: https://www.japaneselawtranslation.go.jp/ja/laws/view/3052 (accessed on 25 May 2024).
- Act to Facilitate the Employment of Persons with Disabilities. Available online: https://www.japaneselawtranslation.go.jp/ja/laws/view/3845 (accessed on 25 May 2024).
- Ishida Y.; Hayashi M.; Inoue F.; Ozawa M. Recent employment trend of childhood cancer survivors in Japan: a cross-sectional survey. Int J Clin Oncol 2014, 19, 973-981. [CrossRef]
- Jigyojyo ni okeru chiryo to shigoto no ryoritsu shien no tame no gaidorain [Guidelines for supporting the balance of treatment and work in the workplace]. Available online: https://chiryoutoshigoto.mhlw.go.jp/dl/download/guideline.pdf (accessed on 10 Jun 2021).
- Hammond A.; O’Brien R.; Woodbridge S.; Bradshaw L.; Prior Y.; Radford K.; Culley J.; Whitham D.; Pulikottil-Jacob R. Job retention vocational rehabilitation for employed people with inflammatory arthritis (WORK-IA): a feasibility randomized controlled trial. BMC Musculoskelet Disord 2017, 18, 315. [CrossRef]
- van Vilsteren M.; Boot C.R.; Twisk J.W.; van Schaardenburg D.; Steenbeek R.; Voskuyl A.E.; Anema J.R. Effectiveness of an integrated care intervention on supervisor support and work functioning of workers with rheumatoid arthritis. Disabil Rehabil 2017, 39, 354–362. [CrossRef]
- Tamminga S.J.; Verbeek J.H.; Bos M.M.; Fons G.; Kitzen J.J.E.M.; Plaisier P.W.; Frings-Dresen M.H.W.; de Boer A.G. Effectiveness of a hospital-based work support intervention for female cancer patients - a multi-centre randomised controlled trial. PLoS One 2013, 8, e63271. [CrossRef]
- Rabin C. Cancer-related self-disclosure in the workplace/school by adolescent and young adult cancer survivors. J Adolesc Young Adult Oncol 2020, 9, 528–533. [CrossRef]
- Stergiou-Kita M.; Pritlove C.; van Eerd D.; Holness L.D.; Kirsh B.; Duncan A.; Jones J. The provision of workplace accommodations following cancer: survivor, provider, and employer perspectives. J Cancer Surviv 2016, 10, 489–504. [CrossRef]
- Stergiou-Kita M.; Pritlove C.; Kirsh B. The "Big C"-stigma, cancer, and workplace discrimination. J Cancer Surviv 2016, 10, 1035–1050. [CrossRef]
- Fujisawa D.; Umezawa S.; Fujimori M.; Miyashita M. Prevalence and associated factors of perceived cancer-related stigma in Japanese cancer survivors. Jpn J Clin Oncol 2020, 50, 1325–1329. [CrossRef]
- Takahashi M.; Tsuchiya M.; Horio Y.; Funazaki H.; Aogi K.; Miyauchi K.; Arai Y. Job resignation after cancer diagnosis among working survivors in Japan: timing, reasons and change of information needs over time. Jpn J Clin Oncol 2018, 48, 43–51. [CrossRef]
- Soejima T.; Kamibeppu K. Are cancer survivors well-performing workers? A systematic review. Asia Pac J Clin Oncol 2016, 12, e383–97. [CrossRef]
- Hibbard J.H.; Mahoney E.R.; Stockard J.; Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res 2005, 40, 1918–1930. [CrossRef]
- Hibbard J.H.; Mahoney E.; Sonet E. Does patient activation level affect the cancer patient journey? Patient Educ Couns 2017, 100, 1276–1279. [CrossRef]
- Rijken M.; Heijmans M.; Jansen D.; Rademakers J. Developments in patient activation of people with chronic illness and the impact of changes in self-reported health: results of a nationwide longitudinal study in The Netherlands. Patient Educ Couns 2014, 97, 383–390. [CrossRef]
- Greene J.; Hibbard J.H. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med 2012, 27, 520–526. [CrossRef]
- van Maarschalkerweerd P.; Rademakers J.; Rijken M. Cancer survivors’ activation to self-management and its relationship with participation in paid work and work-related problems. Psychooncology 2017, 26, 1881–1887. [CrossRef]
- Hallgren E.; Ayers B.L.; Moore R.; Purvis R.S.; McElfish P.A.; Maraboyina S.; Bryant-Smith G. Facilitators and barriers to employment for rural women cancer survivors. J Cancer Surviv 2023, 17, 1338-1346. [CrossRef]
- Kollerup A.; Ladenburg J.; Heinesen E.; Kolodziejczyk C. The importance of workplace accommodation for cancer survivors - The role of flexible work schedules and psychological help in returning to work. Econ Hum Biol 2021, 43, 101057. [CrossRef]
- Alleaume C.; Paraponaris A.; Bendiane M.K.; Peretti-Watel P.; Bouhnik A.D. The positive effect of workplace accommodations on the continued employment of cancer survivors five years after diagnosis. Support Care Cancer 2020, 28, 4435–4443. [CrossRef]
- Bochimoto H.; Ishimaru T.; Nakano A.; Hasegawa K.; Kimura E.; Tajima S.; Yoshikawa T.; Nemoto H. Association between workplace social support and use of health-promoting wearable devices: a prospective cohort study of Japanese employees. J UOEH 2023, 45, 95–103. [CrossRef]
- Inoue A.; Kawakami N.; Shimomitsu T.; Tsutsumi A.; Haratani A.; Yoshikawa T.; Shimazu A.; Odagiri Y. Development of a short questionnaire to measure an extended set of job demands, job resources, and positive health outcomes: the new brief job stress questionnaire. Industrial Health 2014, 52, 175–189. [CrossRef]
- Fujita E.; Kuno E.; Kato D.; Kokochi M.; Uehara K.; Hirayasu Y. Seishin no Kenko Kanri heno Sekkyokusei Hyoka Syakudo Nihongoban no Kaihatsu [Development and validation of the Japanese version of the patient activation measure 13 for mental health]. Seishin Igaku 2010, 52, 765–772. [CrossRef]
- Okuyama T.; Akechi T.; Kugaya A.; Okamura H.; Shima Y.; Maruguchi M.; Hosaka T.; Uchitomi Y. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage 2000, 19, 5–14. [CrossRef]
- Furukawa T.A.; Kawakami N.; Saitoh M.; Ono Y.; Nakane Y.; Nakamura Y.; Tachimori H.; Iwata N.; Uda H.; Nakane H.; Watanabe M.; Naganuma Y.; Hata Y.; Kobayashi M.; Miyake Y.; Takeshima T.; Kikkawa T. The performance of the Japanese version of the K6 and K10 in the World Mental Health Survey Japan. Int J Methods Psychiatr Res 2008, 17, 152–158. [CrossRef]
- Kobayashi K.; Takeda F.; Teramukai S.; Gotoh I.; Sakai H.; Yoneda S.; Noguchi Y.; Ogasawara H.; Yoshida K. A cross-validation of the European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30) for Japanese with lung cancer. Eur J Cancer 1998, 34, 810–815. [CrossRef]
- Mayo S.J.; Lustberg M.; Dhillon H.M.; Nakamura Z.M.; Allen D.H.; Von Ah D.; Janelsins C.M.; Chan A.; Olson K.; Tan C.J.; Toh Y.L.; Oh J.; Grech L.; Cheung Y.T.; Subbiah I.M.; Petranovic D.; D’Olimpio J.; Gobbo M.; Koeppen S.; Loprinzi C.L.; Pang L.; Shinde S.; Ntukidem O.; Peters K.B. Cancer-related cognitive impairment in patients with non-central nervous system malignancies: an overview for oncology providers from the MASCC Neurological Complications Study Group. Support Care Cancer 2021, 29, 2821–2840. [CrossRef]
- Suzuki T.; Miyaki K.; Song Y.; Tsutsumi A.; Kawakami N.; Shimazu A.; Takahashi M.; Inoue A.; Kurioka S. Relationship between sickness presenteeism (WHO-HPQ) with depression and sickness absence due to mental disease in a cohort of Japanese workers. J Affect Disord 2015, 180, 14–20. [CrossRef]
- Schermelleh-Engel K.; Moosbrugger H.; Müller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Methods of Psychological Research Online 2003, 8, 23–74. [CrossRef]
- Endo M.; Muto G.; Imai Y.; Mitsui K.; Nishimura K, Hayashi K. Predictors of post-cancer diagnosis resignation among Japanese cancer survivors. J Cancer Surviv 2020, 14, 106–113. [CrossRef]
- Fowles J.B.; Terry P.; Xi M.; Hibbard J.; Bloom C.T.; Harvey L. Measuring self-management of patients’ and employees’ health: further validation of the Patient Activation Measure (PAM) based on its relation to employee characteristics. Patient Educ Couns 2009, 77, 116–122. [CrossRef]
- Cousins R.; MacKay C.J.; Clarke S.D.; Kelly C.; Kelly P.J.; McCaig R.H. ‘Management Standards’ work-related stress in the UK: practical development. Work Stress 2004, 18, 113–136. [CrossRef]
- Hakanen J.J.; Lindbohm M.L. Work engagement among breast cancer survivors and the referents: the importance of optimism and social resources at work. J Cancer Surviv 2008, 2, 283–295. [CrossRef]
- Yang T.; Ma T.; Liu P.; Liu Y.; Chen Q.; Guo Y: Zhang S.; Deng J. Perceived social support and presenteeism among healthcare workers in China: the mediating role of organizational commitment. Environ Health Prev Med 2019, 24, 55. [CrossRef]
- Baeriswyl S,; Krause A.; Elfering A. Berset M. How workload and coworker support relate to emotional exhaustion: The mediating role of sickness presenteeism. Int J Stress Manag 2017, 24, 52–73. [CrossRef]
- Overview of the Basic Plan to Promote Cancer Control Programs. Available online: https://www.mhlw.go.jp/english/wp/wp-hw3/dl/2-078.pdf (accessed on 5 Dec 2022).
- Yahaya N.A.; Abdullah K.L.; Ramoo V.; Zainal N.Z.; Wong L.P.; Danaee M. Effects of Self-Care Education Intervention Program (SCEIP) on activation level, psychological distress, and treatment-related information. Healthcare (Basel) 2022, 10, 1572. [CrossRef]
- Knoerl R.; Lee D.; Yang J.; Bridges C.; Kanzawa-Lee G.; Smith L.G,; Smith L.E.M. Examining the impact of a web-based intervention to promote patient activation in chemotherapy-induced peripheral neuropathy assessment and management. J Cancer Educ 2018, 33, 1027–1035. [CrossRef]
- National Cancer Center Japan: Cancer statistics in Japan 2021. Available online: https://ganjoho.jp/public/qa_links/report/statistics/2021_en.html (Accessed on 25 Nov 2021).
- Education at glance 2021: OECD indicators. Available online: https://www.oecd-ilibrary.org/sites/1426642c-en/index.html?itemId=/content/component/1426642c-en (Accessed on 5 Dec 2022).
- Lerner D., Amick B.C. 3rd.; Rogers W.H.; Malspeis S.; Bungay K.; Cynn D. The work limitations questionnaire. Med Care 2001, 39, 72–85. [CrossRef]
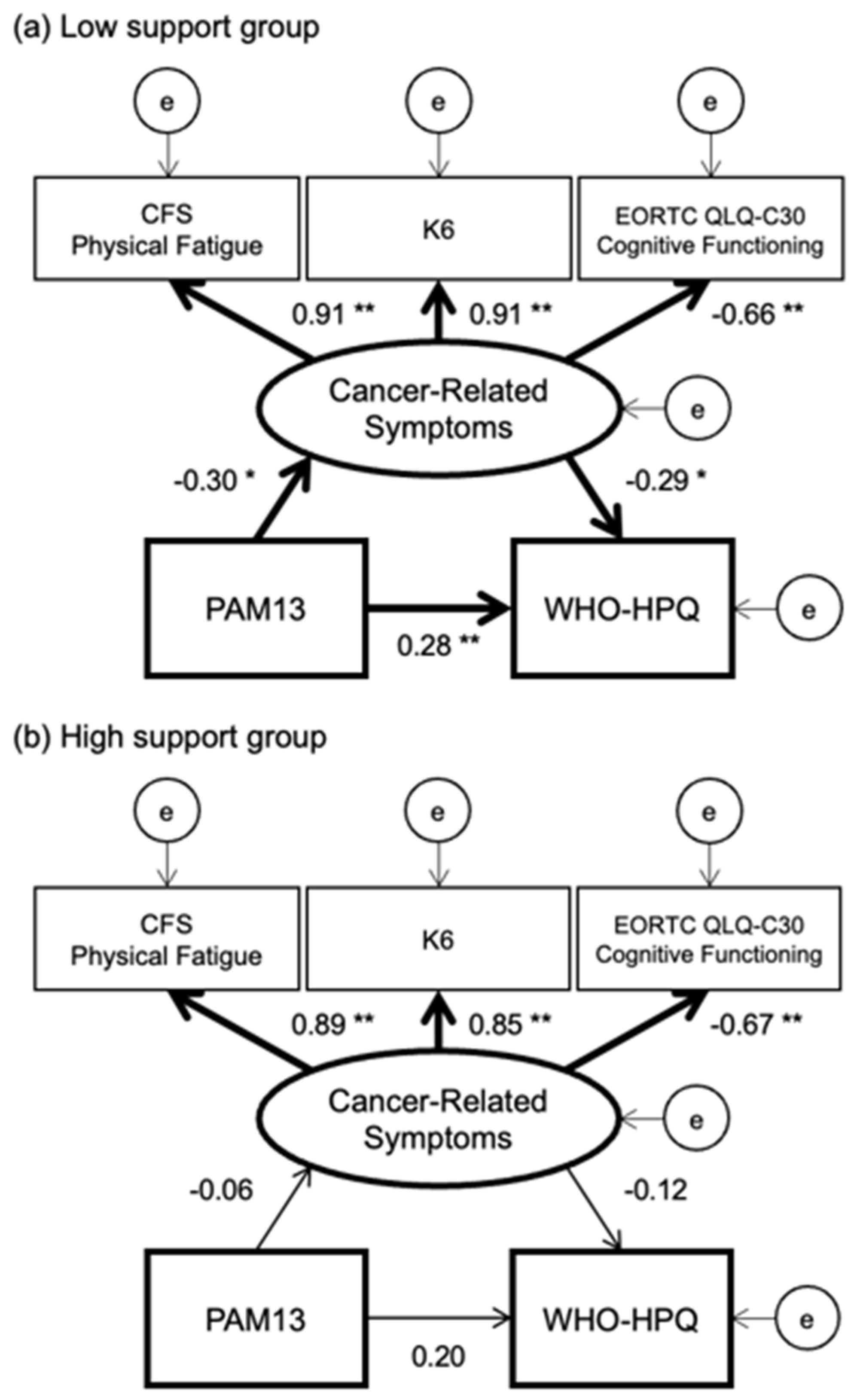
| N = 202 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Low Support Group (n = 97) | High Support Group (n = 105) | WHO-HPQ | ||||||
| n | % | n | % | n | % | p a | η2 b | p b | |
| Age | 0.07 | 0.14 | 0.29 | ||||||
| 20–24 years | 7 | 3 | 2 | 2 | 5 | 5 | |||
| 25–29 years | 27 | 13 | 19 | 20 | 8 | 8 | |||
| 30–34 years | 61 | 30 | 28 | 29 | 33 | 31 | |||
| 35–39 years | 107 | 53 | 48 | 49 | 59 | 56 | |||
| Gender | 0.06 | 0.06 | 0.40 | ||||||
| Male | 45 | 22 | 16 | 16 | 29 | 28 | |||
| Female | 157 | 78 | 81 | 84 | 76 | 72 | |||
| Educational Level | 0.89 | 0.19 | 0.11 | ||||||
| Junior-High School | 4 | 2 | 2 | 2 | 2 | 2 | |||
| High School | 43 | 21 | 20 | 21 | 23 | 22 | |||
| College/Vocational School | 42 | 21 | 18 | 19 | 24 | 23 | |||
| University/Graduate School | 110 | 54 | 56 | 58 | 54 | 51 | |||
| Others | 3 | 1 | 1 | 1 | 2 | 2 | |||
| Type of Employment | 0.01 | 0.07 | 0.66 | ||||||
| Permanent | 135 | 67 | 55 | 57 | 80 | 76 | |||
| Temporary | 58 | 29 | 37 | 38 | 21 | 20 | |||
| Self-Employed | 9 | 4 | 5 | 5 | 4 | 4 | |||
| Type of Occupation | 0.80 | 0.18 | 0.37 | ||||||
| Technical | 38 | 19 | 17 | 18 | 21 | 20 | |||
| Managerial | 17 | 8 | 8 | 8 | 9 | 9 | |||
| Clerical | 76 | 38 | 35 | 36 | 41 | 39 | |||
| Sales | 20 | 10 | 9 | 9 | 11 | 10 | |||
| Production | 16 | 8 | 7 | 7 | 9 | 9 | |||
| Services | 25 | 12 | 14 | 14 | 11 | 10 | |||
| Others | 10 | 5 | 7 | 7 | 3 | 3 | |||
| Cancer Sites | 0.04 | 0.24 | 0.07 | ||||||
| Gastric Cancer | 16 | 8 | 6 | 6 | 10 | 10 | |||
| Colorectal Cancer | 16 | 8 | 8 | 8 | 8 | 8 | |||
| Breast Cancer | 18 | 9 | 11 | 11 | 7 | 7 | |||
| Female Genital Cancer | 85 | 42 | 50 | 52 | 35 | 33 | |||
| Thyroid Cancer | 17 | 8 | 7 | 7 | 10 | 10 | |||
| Lymphoma/Leukemia | 15 | 7 | 4 | 4 | 11 | 10 | |||
| Others | 35 | 17 | 11 | 11 | 24 | 23 | |||
| Period from Diagnosis | 0.02 | 0.11 | 0.27 | ||||||
| < 1 year | 32 | 16 | 15 | 15 | 17 | 16 | |||
| 1– < 5 years | 98 | 49 | 38 | 39 | 60 | 57 | |||
| ≥ 5 years | 72 | 36 | 44 | 45 | 28 | 27 | |||
| Treatment c | |||||||||
| Surgery | 122 | 60 | 54 | 56 | 68 | 65 | 0.19 | 0.00 | 0.97 |
| Chemotherapy | 50 | 25 | 22 | 23 | 28 | 27 | 0.51 | 0.02 | 0.76 |
| Radiation | 26 | 13 | 9 | 9 | 17 | 16 | 0.14 | 0.01 | 0.87 |
| Hormone Therapy | 18 | 9 | 10 | 10 | 8 | 8 | 0.50 | 0.05 | 0.44 |
| Mean | SD | Mean | SD | Mean | SD | p a | r d | p d | |
| Job Demands on New BJSQ | 2.6 | 0.7 | 2.5 | 0.7 | 2.7 | 0.7 | 0.16 | -0.08 | 0.25 |
| Workplace Support on New BJSQ |
2.5 | 0.8 | 1.9 | 0.5 | 3.2 | 0.4 | 0.02 | 0.27 | <0.01 |
| N = 202 | |||||||
|---|---|---|---|---|---|---|---|
| Total | Low Support Group (n = 97) | High Support Group (n = 105) | |||||
| Mean | SD | Mean | SD | Mean | SD | p a | |
| Patient activation score on PAM13 | 55.0 | 14.7 | 54.9 | 15.9 | 55.1 | 13.6 | 0.92 |
| CFS Physical Fatigue subscale | 9.0 | 7.4 | 9.0 | 7.3 | 9.0 | 7.5 | 0.95 |
| K6 | 6.5 | 6.4 | 6.4 | 6.2 | 6.7 | 6.6 | 0.74 |
| EORTC QLQ-C30 Cognitive Functioning subscale |
67.5 | 26.3 | 69.1 | 26.5 | 66.0 | 26.1 | 0.41 |
| WHO-HPQ | 63.9 | 17.4 | 59.4 | 18.0 | 68.1 | 15.8 | <0.01 |
| N = 202 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Models a | CMIN | Change b | p c | CMIN/df | CFI | GFI | AGFI | RMSEA | AIC |
| Model without any path constraints |
3.11 | NA | NA | 0.389 | 1.000 | 0.994 | 0.977 | 0.000 | 47.112 |
| Model with path constraints | |||||||||
| PAM13 to Cancer-Related Symptoms |
5.11 | 2.00 | 0.16 | 0.567 | 1.000 | 0.974 | 0.954 | 0.000 | 47.107 |
| PAM13 to WHO-HPQ | 3.26 | 0.15 | 0.70 | 0.362 | 1.000 | 0.978 | 0.961 | 0.000 | 45.261 |
| Cancer-Related Symptoms to WHO-HPQ |
4.55 | 1.43 | 0.23 | 0.505 | 1.000 | 0.975 | 0.956 | 0.000 | 46.545 |
| All paths | 7.02 | 3.91 | 0.27 | 0.638 | 1.000 | 0.971 | 0.954 | 0.000 | 45.201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





