Submitted:
15 April 2025
Posted:
16 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Case Report
- Visual acuity: Significant bilateral decrease in BCVA.
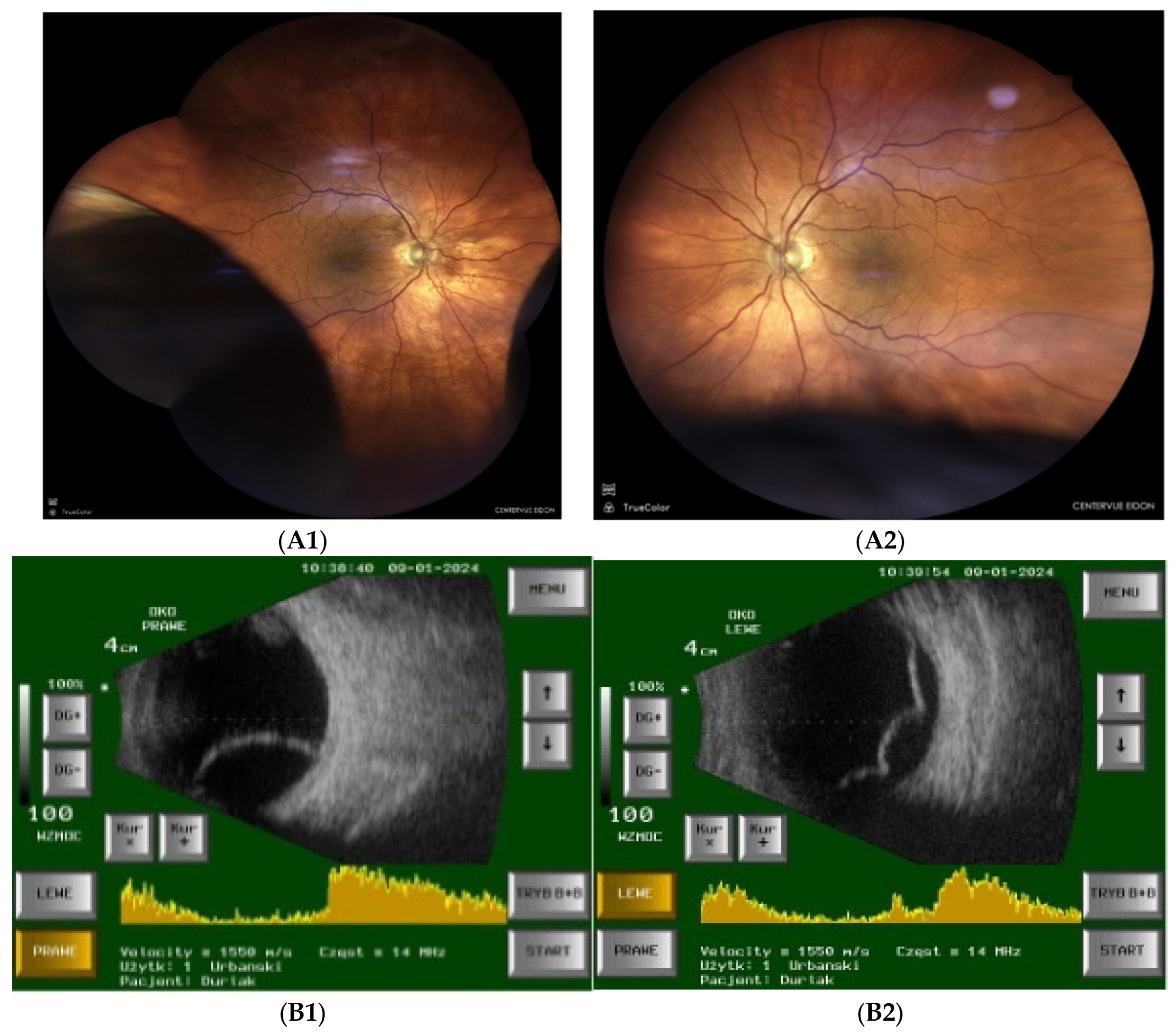
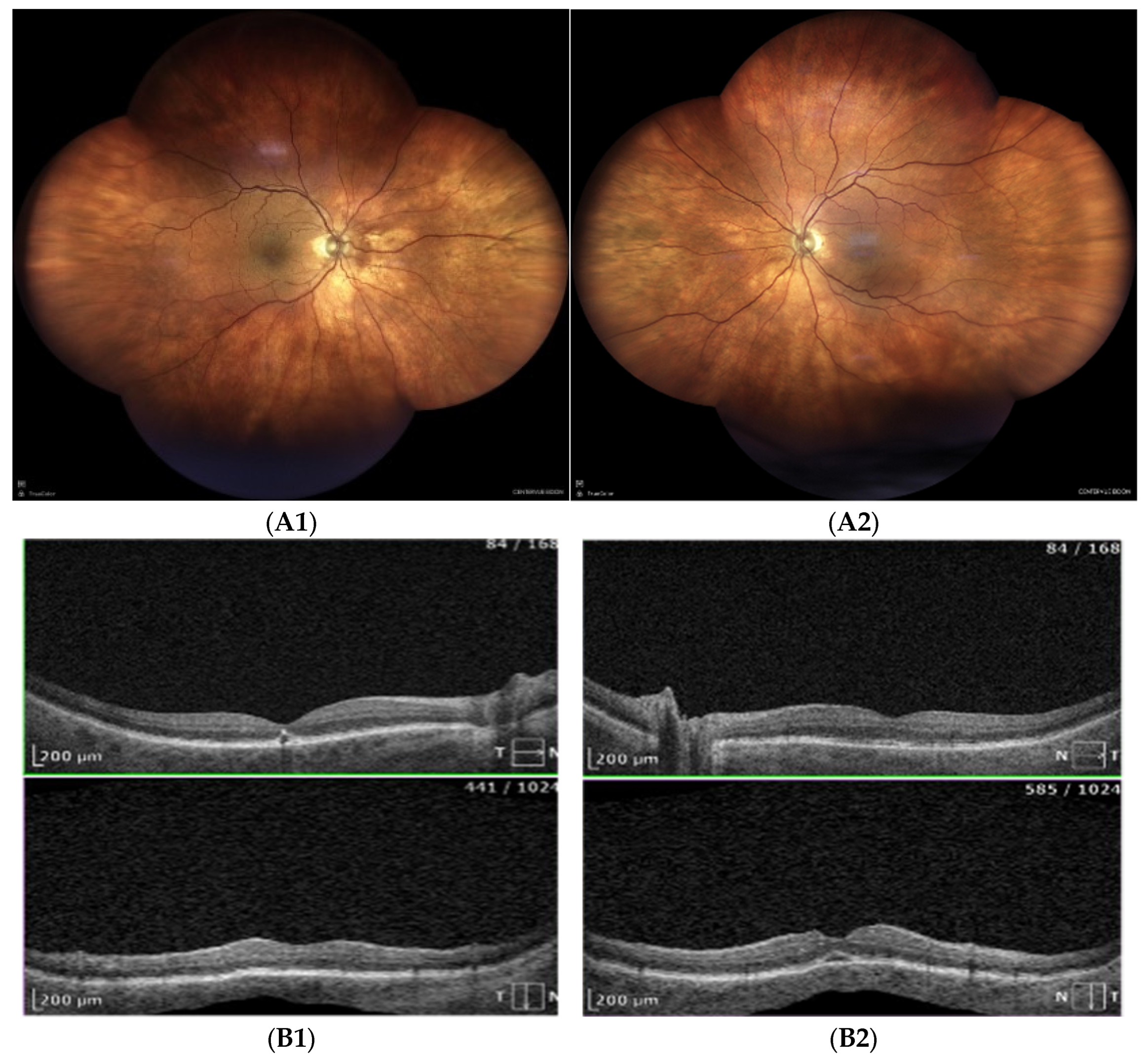
- Fundoscopy and ultrasonography: Bilateral inferotemporal choroidal detachment (Figure 2A,B).
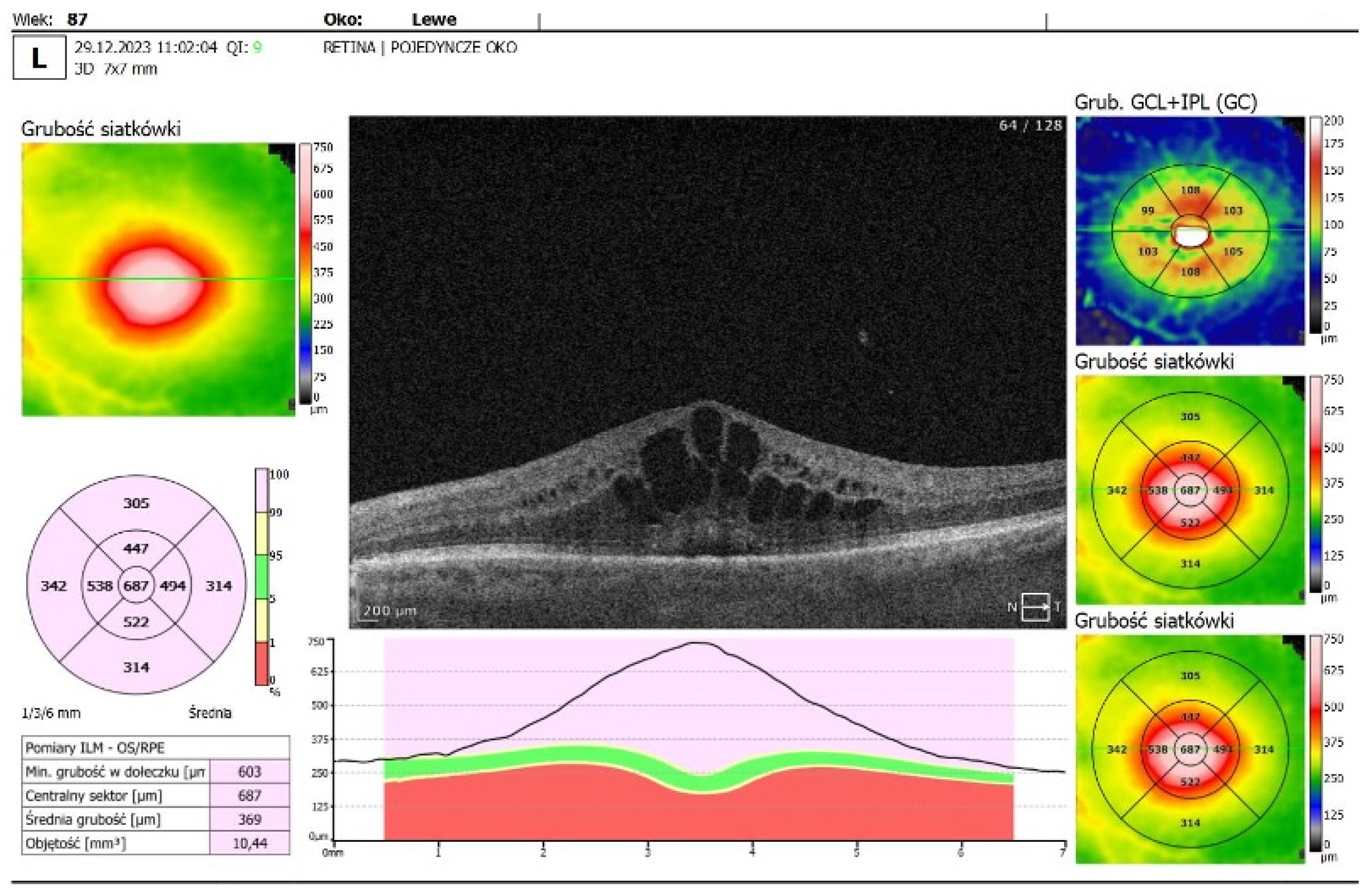
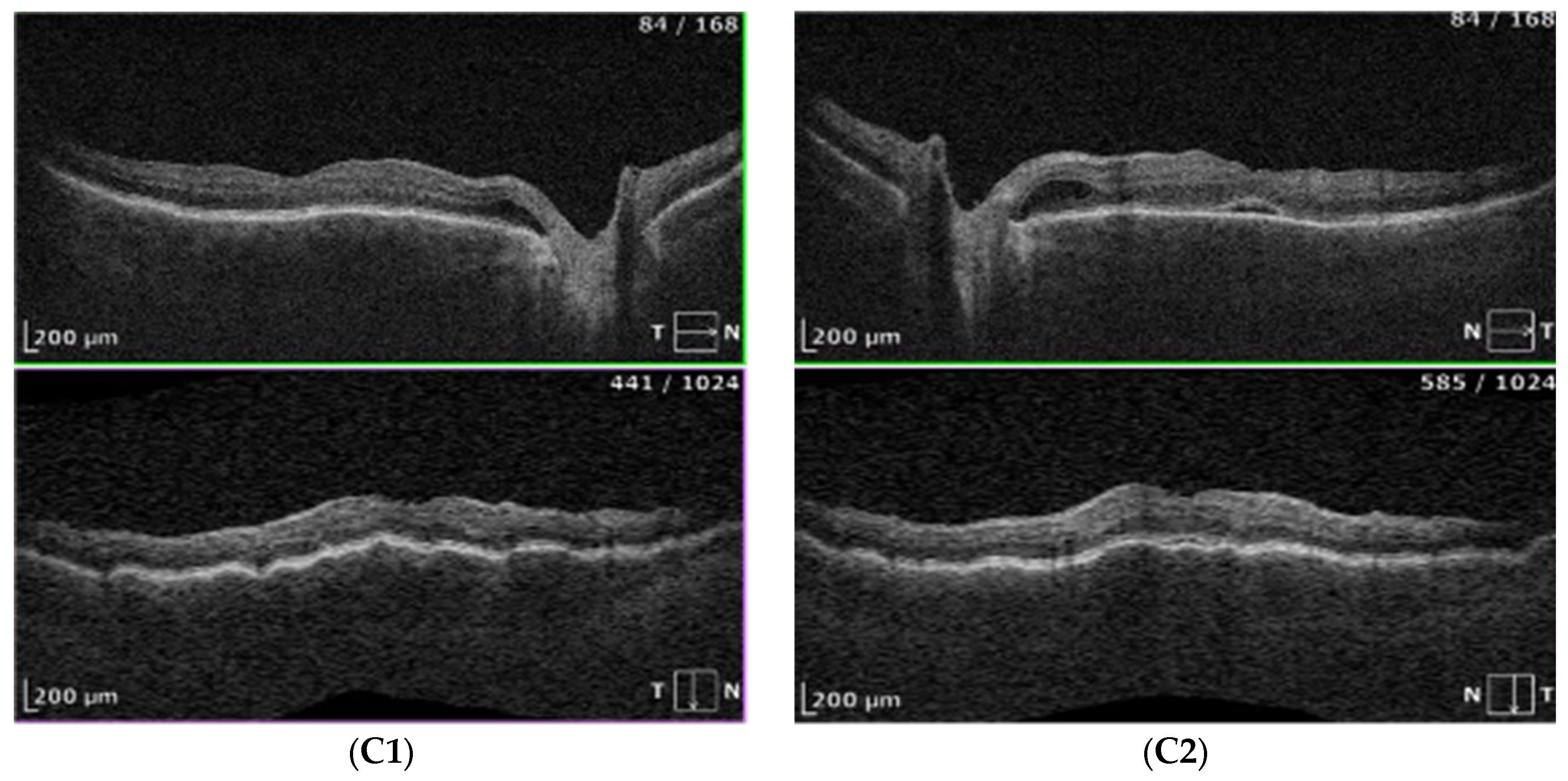
- OCT imaging: Complete resolution of macular edema in the left eye; bilateral choroidal folds and subretinal fluid (SRF) were present (Figure 2C).
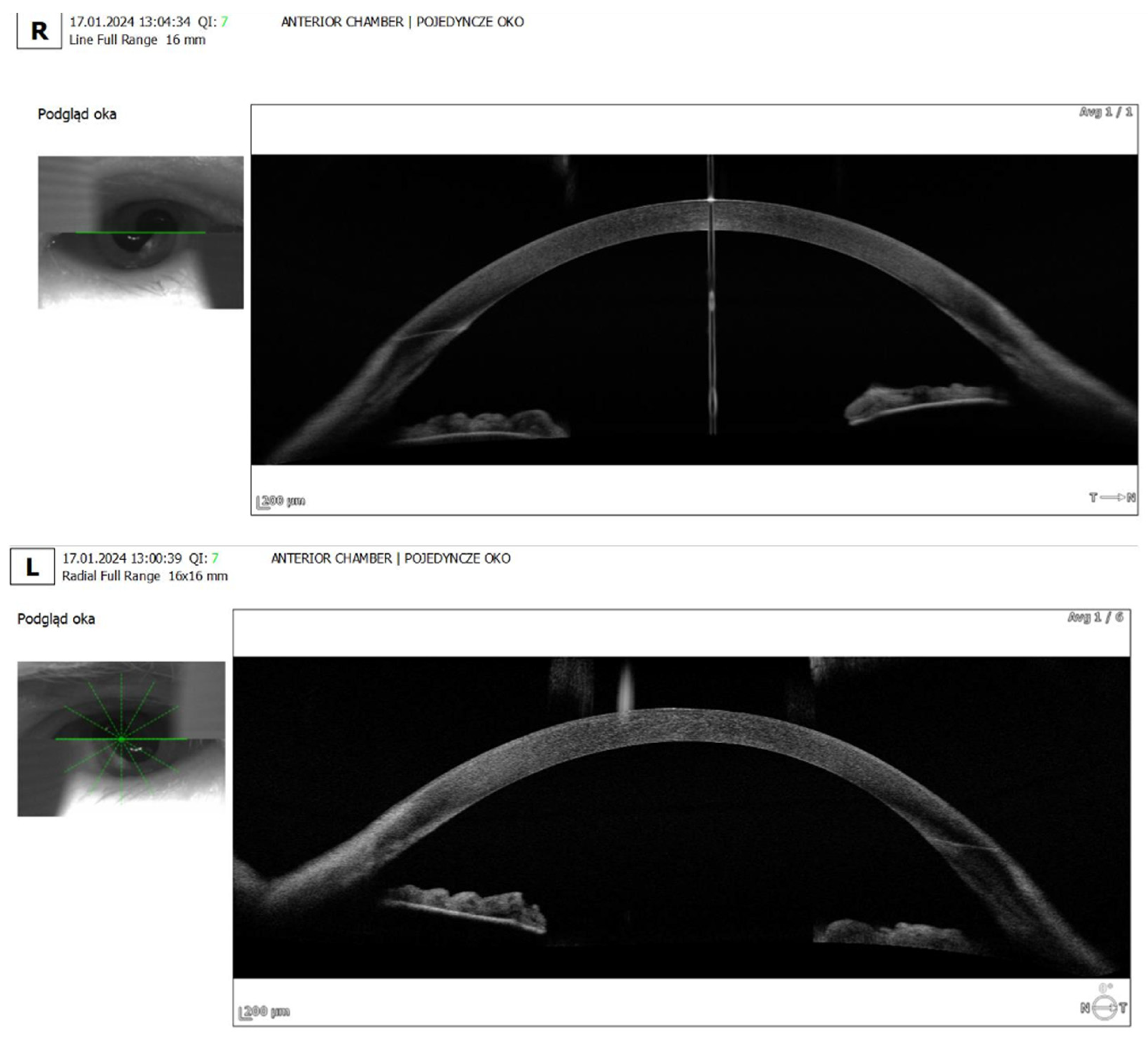
- Anterior segment OCT: Revealed iris plateau configuration, a possible contributing factor to the choroidal effusion (Figure 3).
3. Discussion
3.1. Proposed Pathophysiological Mechanisms of Acetazolamide-Induced Choroidal Effusion
- Increased Choroidal Vascular Permeability and Endothelial Dysfunction
- 2.
- Alteration in Hydrostatic and Osmotic Pressure
- 3.
- Direct Effect on the Ciliary Body and Choroidal Blood Flow
- 4.
- Idiosyncratic Drug Reaction and Immune-Mediated Response
4. Review of Previous Literature and Comparisons
- There was a case report in which a 76-year-old patient developed bilateral angle-closure glaucoma and extensive choroidal detachment following oral acetazolamide administration after routine cataract surgery. The condition improved rapidly upon discontinuation of acetazolamide and initiation of high-dose intravenous steroid therapy. This case highlights the importance of early steroid intervention in CAI-induced choroidal effusion [13].
- An echographic study was conducted evaluating the incidence of uveal effusion after cataract surgery. The findings indicated that the postoperative combination of oral acetazolamide and topical pilocarpine gel significantly increased the risk of choroidal effusion, suggesting that certain pharmacological combinations may exacerbate this condition [14].
- A case of 60-year-old male with plateau iris configuration who developed bilateral ciliochoroidal effusion syndrome after acetazolamide use was decribed. The patient presented with a myopic shift, elevated intraocular pressure, and shallow anterior chambers. Upon discontinuation of acetazolamide and the initiation of topical therapy, the choroidal effusion resolved. This case demonstrates how predisposing anatomical factors may contribute to the severity of acetazolamide-induced choroidal detachment [15]
- Liyanage et al. reported two cases of uveal effusion following acetazolamide administration, one after cataract surgery and another following prophylactic treatment for altitude sickness. In both cases, timely discontinuation of the drug led to complete resolution of symptoms, underscoring the reversibility of CAI-induced effusions with appropriate management [16]
5. Clinical Implications and Recommendations for Management
- 1.
-
Monitoring High-Risk Patients
- ○
- Elderly individuals and those with a history of choroidal detachment, angle-closure glaucoma, or uveal effusion should be carefully monitored when prescribed acetazolamide or other CAIs.
- ○
- Regular follow-up with ultrasonography and optical coherence tomography (OCT) can help in the early detection of subclinical choroidal effusion before symptomatic vision loss occurs.
- 2.
-
Avoiding Certain Drug Combinations
- ○
- The combination of acetazolamide with miotics like pilocarpine may increase the risk of uveal effusion and secondary angle closure.
- ○
- Consider alternative treatments for postoperative macular edema in patients with known risk factors.
- 3.
-
Prompt Discontinuation of CAIs in Suspected Cases
- ○
- If choroidal effusion is suspected, acetazolamide should be discontinued immediately to prevent worsening of the condition.
- ○
- Alternative anti-inflammatory therapy, including topical corticosteroids (dexamethasone) and cycloplegics (atropine), should be initiated to reduce inflammation and promote fluid resolution.
- 4.
-
Considering Steroid Therapy for Severe Cases
- ○
- In cases with significant visual impairment or extensive choroidal detachment, systemic or periocular corticosteroids may be beneficial in hastening resolution.
6. Conclusions
7. Plain Language Summary
7.1. Plain Language Summary
References
- Ying, S.; Sidoti, P.A.; Panarelli, J.F. Risk factors and management of choroidal effusions. Curr. Opin. Ophthalmol. 2022, 34, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Waheed NK, Mendonça LSM, Young LH. Choroidal Effusions and Detachments. In: Albert and Jakobiec’s Principles and Practice of Ophthalmology: Fourth Edition. 2022.
- Phylactou, M.; Matarazzo, F.; Jones, E. Indapamide-induced bilateral choroidal effusion in pseudophakic patient. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Pasquale, L.R. Causes and Treatment of Choroidal Effusion after Glaucoma Surgery. Semin. Ophthalmol. 2014, 29, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Koenigstein, D.; Neudorfer, M.; Goldenberg, D.; Habot-Wilner, Z. CHOROIDAL EFFUSION AS AN OCULAR MANIFESTATION OF IMMUNOGLOBULIN G4–RELATED DISEASE. Retin. Cases Brief Rep. 2016, 10, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Khanam Z, Krishnamoorthy S, Baskaran V, Mashruwala A. Acetazolamide –friend to foe. Indian Journal of Ophthalmology - Case Reports 2024;4(1).
- Rothwell, A.; Anderson, O. Bilateral choroidal effusions after taking acetazolamide for altitude sickness. BMJ Case Rep. 2022, 15, e246145. [Google Scholar] [CrossRef] [PubMed]
- Atisundara, S.M.; Rifada, R.M.; Umbara, S.; Gustianty, E.; Prahasta, A. ACETAZOLAMIDE INDUCED SECONDARY ANGLE CLOSURE GLAUCOMA: A RARE CASE REPORT. Ophthalmol. Indones. 2024, 49. [Google Scholar] [CrossRef]
- Yang, J.-G.; Li, J.-J.; Tian, H.; Li, Y.-H.; Gong, Y.-J.; Su, A.-L.; He, N. Uveal effusion following acute primary angle-closure: a retrospective case series. Int. J. Ophthalmol. 2017, 10, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Arrico, L.; Malagola, R.; Giannotti, R.; Pattavina, L. Acetazolamide-induced cilio-choroidal effusion after cataract surgery: unusual posterior involvement. Drug Des. Dev. Ther. 2013, 7, 33–6. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Lewis, A. Multimodal etiology of drug induced angle closure with topical glaucoma therapy. Am. J. Ophthalmol. Case Rep. 2021, 23, 101152. [Google Scholar] [CrossRef] [PubMed]
- Malagola, R.; Giannotti, R.; Pattavina, L.; Arrico, L. Acute cilio-choroidal effusion due to acetazolamide: unusual posterior involvement (OCT aspects). Eye 2013, 27, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Mancino, R.; Varesi, C.; Cerulli, A.; Aiello, F.; Nucci, C. Acute bilateral angle-closure glaucoma and choroidal effusion associated with acetazolamide administration after cataract surgery. J. Cataract. Refract. Surg. 2011, 37, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Sabti, K.; Lindley, S.K.; Mansour, M.; Discepola, M. Uveal effusion after cataract surgery: an echographic study. Ophthalmology 2001, 108, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Man, X.; Costa, R.; Ayres, B.M.; Moroi, S.E. Acetazolamide-induced bilateral ciliochoroidal effusion syndrome in plateau iris configuration. Am. J. Ophthalmol. Case Rep. 2016, 3, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Liyanage G, Bertin S, Villaret J, Paques M. Acetazolamide and uveal effusion: report of two cases. J Fr Ophtalmol 2024;47(1).
- Ashok, A.; Bhat, M.; Shetty, R. Acetazolamide-induced bilateral uveal effusion after cataract surgery. TNOA J. Ophthalmic Sci. Res. 2023, 61, 357. [Google Scholar] [CrossRef]
| Day 1 | |
| Chief Complaint (CC) | acute, painless bilateral vision deterioration |
| BCVA | BCVA RE= 0,3 cc -1,5 sph, -1,25 cyl ax 88 BCVA LE= 0,4 cc +0,25 sph, -1,0 cyl ax 107 |
| Anterior Segment | Moderately shallow anterior chambers in both eyes (confirmed on anterior segment OCT) |
| Posterior Segment Figure 2A |
choroidal effusion in inferior-temporal and inferior-nasal quadrants in both eyes |
| USG ScanB Figure 2B |
inferior-temporal and inferior-nasal choroidal detachment, more pronounce in LE |
| OCT Figure 2C |
No cystoid macular edema in the left eye; residual SRF and choroidal folds in both eyes Fluid next to the optic disc and macular choroidal folds in both eyes |
| IOP | RE 16 mmHg LE 14 mmHg |
| Treatment | 1. Immediate discontinuation of acetazolamide 2. 0,1% dexamethasone eye drops every two hours - both eyes 3. 1% Atropine eye drops twice a day - both eyes 4. Bromfenac twice a day - left eye |
| Day 8 | |
| CC | Visual acuity improvement |
| BCVA | BCVA RE= 0,5 cc 0 sph, -1,5 cyl x90, cyl ax 88 BCVA LE= 0,6 +0,5 sph, -1,0 cyl x 107 |
| Slit lamp exam and fundoscopy | AC deepen Complete resolution of choroidal effusion |
| OCT | residual submacular subretinal fluid persisted in left eye, fluid next to optic disc resolved and choroidal folds disappeared |
| IOP | OD 16 mmHg, OS 16 mmHg |
| Treatment |
|
| Day 29 | |
| CC | No complains |
| BCVA | BCVA RE= 0,7 cc 0 sph, -1,5 cyl x90, cyl ax 88 BCVA LE= 0,6 +0,5 sph, -1,0 cyl x 107 |
| Treatment | All medications discontinued |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




