1. Introduction
The hyperintense vessels on FLAIR identical to diffuse leptomeningeal enhancement ( such as
Ivy sign and
Spaghetti sign )on post-contrast imaging[
1]. On MR fluid-attenuated inversion recovery (FLAIR) images, it has been described as a continuous linear high signal intensity in the leptomeningeal region along the cortical sulci and subarachnoid spaces.[
2]. On FLAIR, it is frequently observed along the cortical sulci on the cerebral surface, and it shows a strong correlation with a decrease in cerebral vascular reserve (CVR) [
3,
4]. As a result, recent research has explored the potential of MR FLAIR images as a monitoring tool following superficial temporal artery (STA)-middle cerebral artery (MCA) anastomosis in patients with symptomatic steno-occlusive disorders and Moyamoya disease.[
4]
Leptomeningeal high signal intensity on unenhanced FLAIR imaging, known as the ivy sign, is thought to reflect reduced cerebral perfusion, expanded pial blood vessels, and sluggish blood flow through the developed leptomeningeal collateral circulation. A significantly developed and widespread pial arterial network was observed over the cortical surface following the performance of a superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis. The findings led to the conclusion that the leptomeningeal enhancement indicates the presence of fine neovascularization across the cerebral cortex. Collateral circulation through leptomeningeal anastomosis plays a crucial role during certain stages of moyamoya disease. Since this collateral flow is thought to be very slow due to its retrograde direction through the leptomeningeal anastomosis, the engorged pial network is likely to show enhancement after the administration of contrast agents. HV is characterized by focal, tubular, or serpentine hyperintensities, typically observed in the subarachnoid space, contrasting with the relative hypo intensity of cerebrospinal fluid (CSF). HV suggests slow retrograde blood flow through leptomeningeal collaterals and is associated with abnormal brain hemodynamics.
Conventional perfusion imaging modalities such as dynamic susceptibility contrast MRI and SPECT yield valuable hemodynamic data but also present notable practical limitations. These techniques typically require intravenous contrast agents or radioactive tracers (often with vasodilatory challenges like acetazolamide), which expose patients to radiation, entail potential side effects, and contribute to significant discomfort. Moreover, perfusion studies tend to be time-consuming and expensive, with lengthy acquisition protocols that make frequent serial monitoring impractical. In contrast, FLAIR MRI offers a non-invasive, efficient alternative for follow-up assessment of cerebral hemodynamics, especially with its ability to visualize the “ivy sign.” This sequence can be completed in minutes without the need for contrast injection or radiation, allowing safe and convenient repeat examinations during longitudinal follow-up. The ivy sign appears on FLAIR as serpentine leptomeningeal hyperintensities resembling ivy along the cortical surface, a finding that reflects slow flow in engorged pial collaterals developed in response to chronic hypoperfusion. While FLAIR cannot directly quantify cerebral blood flow or cerebrovascular reserve, the presence and extent of an ivy sign serve as useful indirect markers of reduced perfusion and compensatory leptomeningeal collateralization in affected territories. Indeed, a pronounced ivy sign has been shown to correlate with diminished CVR in Moyamoya disease, underscoring its value as a practical tool for non-invasive hemodynamic evaluation. In this way, FLAIR-based assessment (despite its qualitative nature) provides an accessible means to monitor cerebrovascular patients over time, complementing the more quantitative but invasive perfusion imaging techniques in routine clinical practice.
2. Materials and Methods
2.1. Patients
From JAN 2015 to FEB 2020, among 49 patients, 17 were treated with moyamoya disease and 32 with symptomatic steno-occlusive disorder underwent STA-MCA anastomosis (mean age, 51.3 years; range, 23-64 years), All patients underwent STA-MCA anastomosis on preoperative and postoperative MR FLAIR images. The criteria for performing STA-MCA bypass surgery include a combination of clinical symptoms and advanced imaging findings. For surgery to be considered, patients must exhibit significant cerebral artery stenosis or occlusion, confirmed through angiography or high-resolution MR imaging. Additionally, evidence of impaired cerebral perfusion, as seen in diminished cerebrovascular reserve on imaging techniques like SPECT or perfusion MRI, is essential. Recurrent neurological symptoms that do not respond to medical therapy, such as TIAs or strokes, are indicators for surgical intervention. Favorable anatomical features, including the availability of superficial temporal artery and recipient vessels (middle cerebral artery), are also considered. Contraindications for STA-MCA bypass surgery include several conditions where the risks of surgery outweigh the potential benefits. First, patients with severe systemic diseases, such as advanced cardiovascular, pulmonary, or renal conditions, or those of advanced age with poor recovery potential, may not be suitable for surgery due to high perioperative risks. Second, patients with extensive atherosclerosis is affecting multiple vessels or other major arteries outside the MCA/ACA territories may not benefit from the procedure, as the underlying vascular pathology may compromise the success of the surgery. Third, active infections, particularly those in the head or neck, are contraindications due to the increased risk of surgical site infection and complications during recovery. Severe brain atrophy, particularly in areas of the brain with irreversible damage, reduces the likelihood of a favorable outcome, as the improvement in blood flow may not result in significant functional recovery. Furthermore, patients who are unlikely to comply with postoperative care, including the use of antiplatelet therapy or regular follow-up appointments, may face increased risks of graft failure and recurrent ischemic events. Lastly, pregnancy may be considered a relative contraindication due to the potential risks associated with anesthesia and radiation exposure from preoperative imaging studies, such as SPECT. In summary, STA-MCA bypass surgery should be performed on patients who meet specific clinical, anatomical, and imaging criteria. Careful evaluation of both the indications and contraindication is essential to ensure the selection of appropriate candidates, thereby optimizing the chances of a successful outcome and minimizing the risks associated with the procedure.
2.2. Imaging Studies
Preoperative and postoperative imaging, including MRI, CT angiography, and SPECT, were performed on all patients. The MRI scans included perfusion and FLAIR sequences. A radiologist with board certification reviewed the images and assigned scores for the ivy sign.
The cerebral hemisphere’s cortico-subcortical region was divided into four areas:
The study focused on the anterior cerebral artery (ACA), the anterior portion of the middle cerebral artery (ant-MCA), the posterior portion of the middle cerebral artery (post-MCA), and the posterior cerebral artery (PCA). The central sulcus served as a reference point to differentiate between the ant-MCA and post-MCA regions. Preoperative and postoperative FLAIR images were compared using a three-level scoring system based on the presence of the ivy sign: Grade 0 (negative; no visible ivy sign), Grade 1 (minimal; the ivy sign appears faint or unclear with mild high signal intensity), and Grade 2 (positive; the ivy sign is clearly defined with linear and punctate high signal intensity).[
4]
SPECT results were displayed using a 20-level color scale, ranging from black to white. Scores were given on a scale of 0 to 100, with each color level corresponding to 5 points. This scoring system was feasible due to the precise definition of the 20 color levels in the SPECT images, which were confirmed by a radiologist. Adequate cerebral perfusion was represented by red hues with an intensity range of 60 to 80. We evaluated cerebral vascular reserve (CVR) both before and after acetazolamide administration. For this study, we utilized the post-acetazolamide CVR data, which is strongly correlated with a higher risk of stroke recurrence in patients with symptomatic cerebral infarction. We examined the connection between the Ivy sign on MR FLAIR images and the CVR on SPECT images by comparing pre- and postoperative results after performing STA-MCA anastomosis.
The radiological characteristics that distinguish normal hyper vascular intensity from pathological hypervascularity on MRI include vascular distribution patterns, signal changes after contrast administration, blood flow velocity and dynamic patterns, and temporal changes. Pathological hypervascularity typically shows irregular and asymmetric vascular distribution, strong contrast enhancement, and rapid blood flow, while normal hypervascularity usually presents with a symmetric and regular distribution. Leptomeningeal high-signal intensity observed on unenhanced FLAIR imaging, known as the ivy sign, is thought to reflect reduced cerebral perfusion, enlarged pial vasculature, and slow flow through developed leptomeningeal collaterals. Following superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis, a significantly expanded and diffuse pial arterial network was seen over the cortical surface. This led to the conclusion that leptomeningeal enhancement signifies fine neovascularization on the cerebral cortex.
Collateral circulation through leptomeningeal anastomosis plays a crucial role at certain stages of Moyamoya disease. Since this collateral flow is expected to be slow due to its retrograde direction through the leptomeningeal anastomosis, it is plausible that such a dilated pial network would exhibit enhancement after contrast media injection. Hyperintensities (HV) are described as focal, tubular, or serpentine structures, typically appearing in the subarachnoid space, contrasting with the relative hypo intensity of cerebrospinal fluid (CSF). HV signifies slow retrograde flow within leptomeningeal collaterals and is associated with abnormal brain hemodynamics.
2.3. Statistical Analysis
Statistical analysis was conducted using the Student’s t-test to examine the correlation between the presence of an ivy sign on MR FLAIR and cerebral vascular reserve (CVR) on SPECT. The difference was considered significant if the p-value was less than 0.05 (p<0.05).
3. Results
Forty-nine patients were diagnosed with moyamoya disease and symptomatic steno-occlusive disease on angiography, and they underwent STA-MCA anastomosis. As a result, the sum of CVR in all patients on post-contrast SPECT images was 3035 preoperatively and 3480 postoperatively (p < 0.05), and the surgery was successful in all patients. Among these patients, MR FLAIR and SPECT images were checked after the anastomosis (
Figure 1).
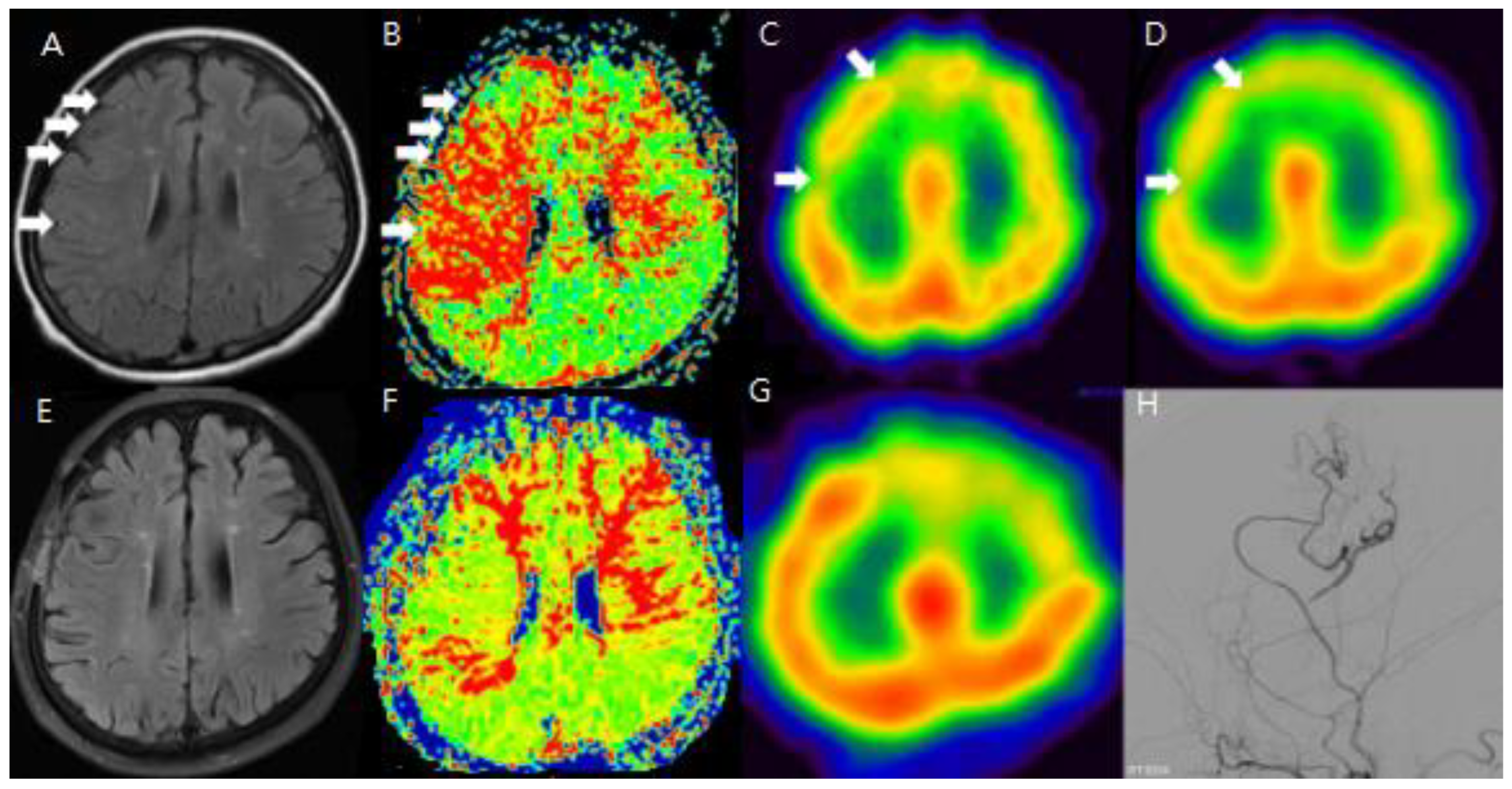
To improve the analysis of preoperative and postoperative outcomes, the cerebral hemisphere was segmented into four regions: the anterior cerebral artery (ACA), the anterior part of the middle cerebral artery (ant-MCA), the posterior part of the middle cerebral artery (post-MCA), and the posterior cerebral artery (PCA). Changes in the sum of cerebrovascular reserve (CVR) were compared between preoperative and postoperative conditions for each of these four regions. The main objective of this study was to demonstrate whether the ivy sign could serve as another indicator of hemodynamic changes, making the analysis of preoperative and postoperative HV (hyperintense vessels) the most important focus of this report.
However, HV was not observed throughout all regions of the hemisphere. Therefore, we selected MR FLAIR image slices that showed typical HV and compared these slices with the corresponding SPECT images to assess CVR.
To evaluate the usefulness of the ivy sign for follow-up, changes in the ivy sign on MR FLAIR images were compared with changes in CVR on SPECT images before and after STA-MCA anastomosis.
Minimal and positive HV were observed in 36 out of 49 patients (74%). Preoperative and postoperative MR FLAIR images were obtained at an average of 46.7 days (range: 8–99 days) and 196.6 days (range: 33–368 days) after surgery, respectively. A decrease in baseline cerebral blood flow (CBF) was noted in 92% of lesions, and 83% of areas with positive or minimal HV showed a decrease in CVR. In Moyamoya disease, positive HV and Ivy scores were highest in the post-MCA region, while in steno-occlusive disease, the Spaghetti score was highest in the ant-MCA region.
After STA-MCA bypass surgery, 21 out of the 49 patients showed disappearance of positive or minimal HV, and 11 patients showed a reduction in HV. In the Moyamoya disease group, HV improved as follows: from 10 to 5 in the ACA region, from 17 to 5 in the ant-MCA region, and from 24 to 11 in the post-MCA region. In the symptomatic steno-occlusive disease group, HV improved as follows: from 12 to 7 in the ACA region, from 23 to 10 in the ant-MCA region, from 7 to 4 in the post-MCA region, and from 3 to 2 in the PCA region. These results showed statistically significant correlation (p<0.05) when compared with CVR scores on SPECT images taken before and after surgery.
Additionally, changes in the ivy sign on MR FLAIR images were compared with MR perfusion images. The degree of change in mean transit time (MTT) was greater in areas with positive or minimal HV compared to areas with negative HV. After STA-MCA bypass surgery, regions where HV decreased or disappeared showed improved hemodynamics, as reflected in both SPECTand MR perfusion imaging (
Figure 2).
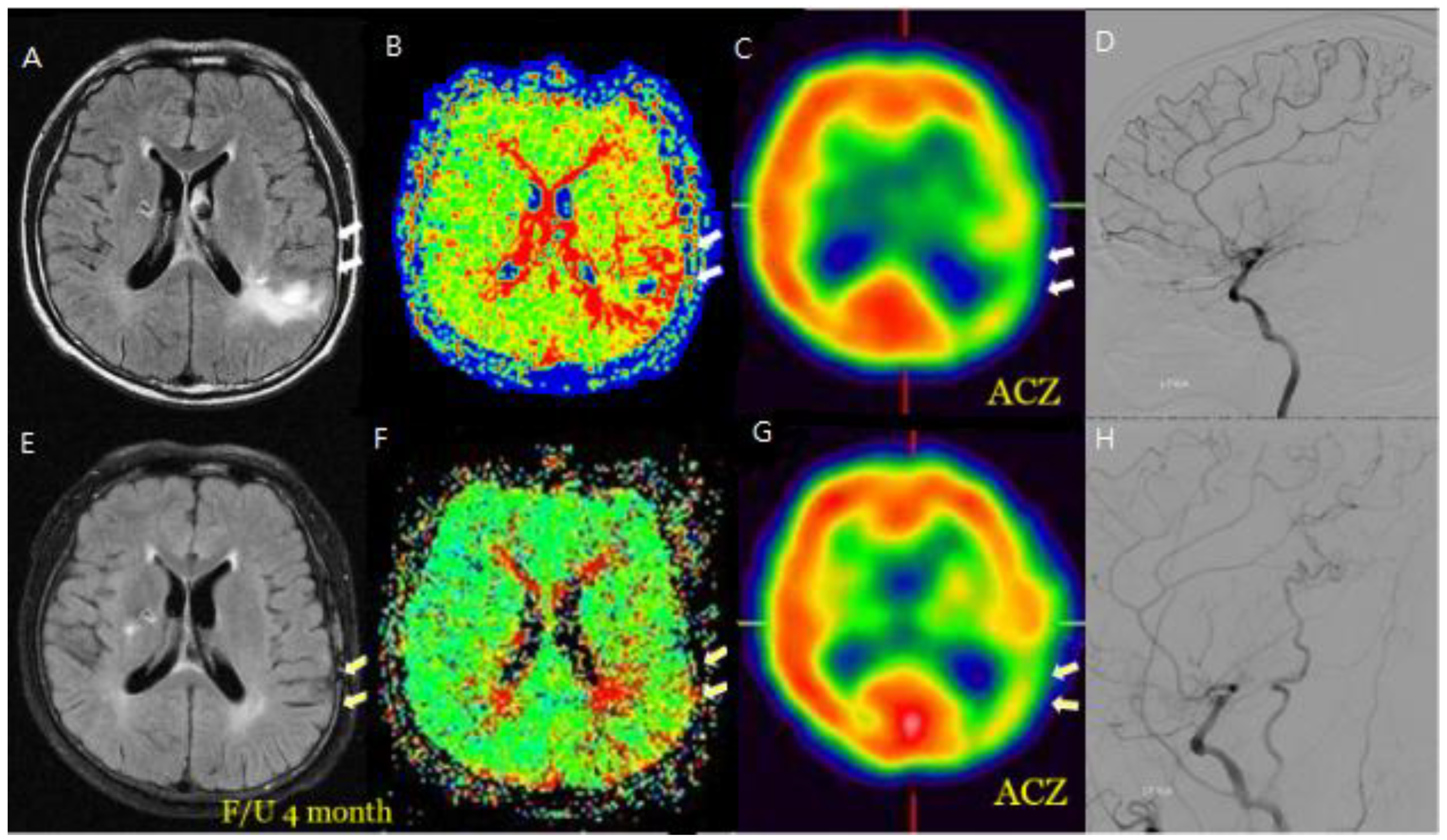
Figure 2.
M/58 symptomatic steno-occlusive patient (Rt. Hemiparesis with Lt. M1 occlusion). Preoperative MR FLIAR and Perfusion MR, SPECT (A, B, C, D) shows Hyperintense vessels indicated decreased cerebral vascular reserve and postoperative decrease or disappeared hyperintense vessels were revealed revascularization effective for decreasing HV after F/U and decrease of HV on bypass-established hemisphere associated with improved hemodynamic status (E, F, G, H).
Figure 2.
M/58 symptomatic steno-occlusive patient (Rt. Hemiparesis with Lt. M1 occlusion). Preoperative MR FLIAR and Perfusion MR, SPECT (A, B, C, D) shows Hyperintense vessels indicated decreased cerebral vascular reserve and postoperative decrease or disappeared hyperintense vessels were revealed revascularization effective for decreasing HV after F/U and decrease of HV on bypass-established hemisphere associated with improved hemodynamic status (E, F, G, H).
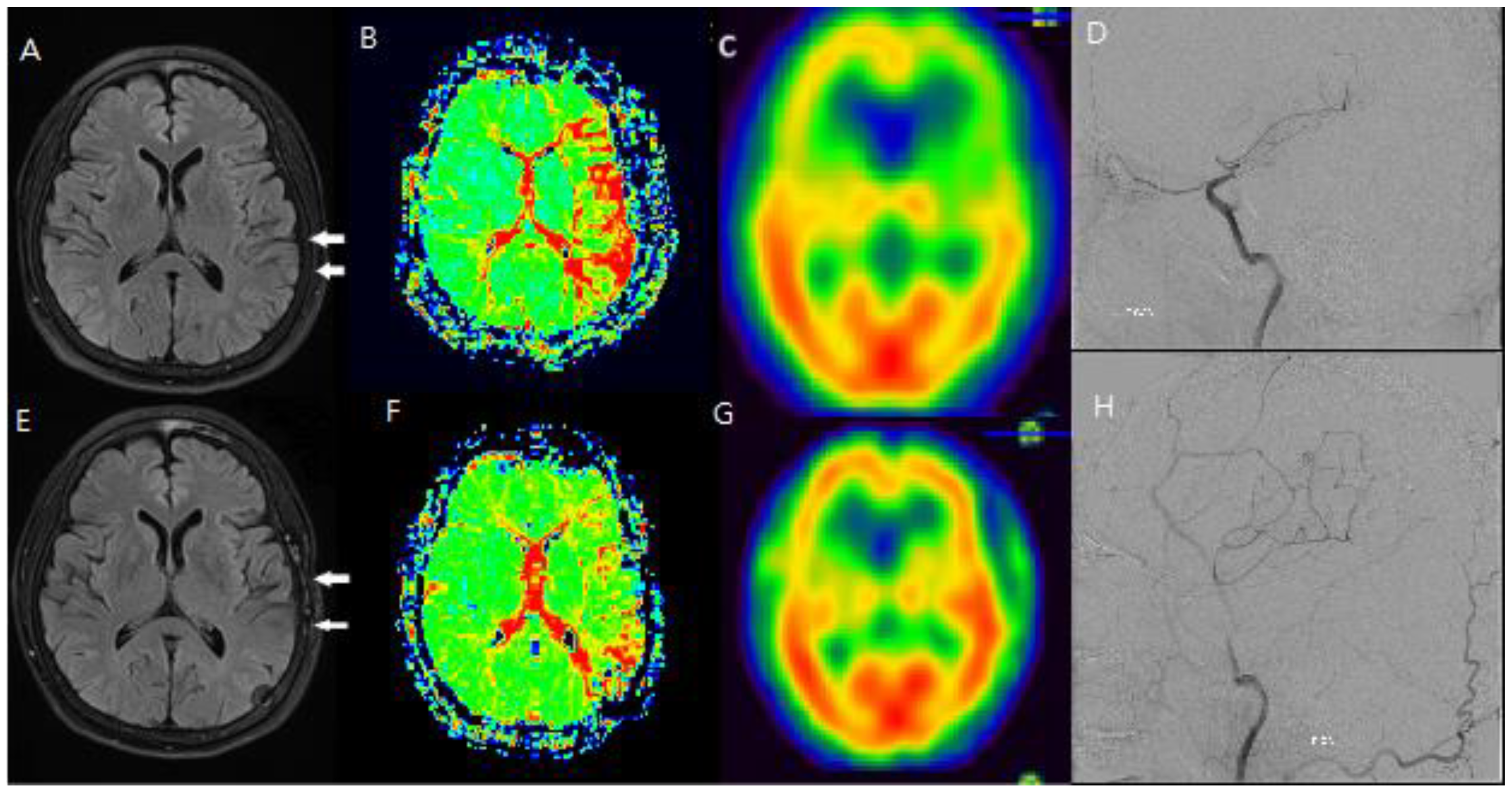
Figure 3.
F/66 Lt. distal ICA severe stenosis patient. Hyperintense vessels were observed in areas with reduced blood flow and reserve before surgery (A, B, C, D), and it was confirmed that the hyperintense vessels had disappeared on the FLAIR scan after the hemodynamic condition improved following STA-MCA bypass surgery (E, F, G, H).
Figure 3.
F/66 Lt. distal ICA severe stenosis patient. Hyperintense vessels were observed in areas with reduced blood flow and reserve before surgery (A, B, C, D), and it was confirmed that the hyperintense vessels had disappeared on the FLAIR scan after the hemodynamic condition improved following STA-MCA bypass surgery (E, F, G, H).
4. Discussion
Numerous studies have examined brain hemodynamic changes to date. Initially, these changes were measured based on CO2 reactivity, and SPECT became the standard method for assessing cerebral vascular reserve (CVR) by utilizing the metabolic effects of intravenous acetazolamide (ACZ) administration [
5,
6,
7]. Additionally, mean transit time (MTT) from MR perfusion has been valuable in evaluating hemodynamic changes in patients with Moyamoya disease and steno-occlusive disorders [
2,
8]. However, side effects have been reported with SPECT and MR perfusion, including discomfort for patients, especially with T1-weighted MRI. As a result, extra caution is needed when administering ACZ to patients with severe ischemic symptoms. Furthermore, both MR perfusion and T1-weighted MRI require contrast agents, which means patients must undergo intravenous administration while fasting, requiring several hours of fasting prior to the procedure.
FLAIR imaging can provide reliable longitudinal follow-up for patients with Moyamoya disease or other cerebrovascular conditions without requiring invasive procedures, allowing for safer and more frequent monitoring of disease progression. Since FLAIR imaging is non-invasive, it is easy to perform during long-term follow-up, and it is being used for evaluation alongside clinical symptom tracking in outpatient visits
Recent studies suggest that the hypervascularity (HV) observed in MR FLAIR images offers new diagnostic value in detecting hemodynamic changes [
2,
9]. Several studies have indicated that HV on MR FLAIR images reflects the brain’s hemodynamic status in patients with Moyamoya disease and steno-occlusive disorders. We believe MR FLAIR may be more beneficial for follow-up evaluations compared to SPECT, perfusion MRI, or T1-weighted MRI, as it is less discomforting for patients with these conditions [
5,
10]. The MR FLAIR study has the advantage of being easily conducted in clinical settings. Compared to SPECT, MR perfusion, and T1-weighted MRI, MR FLAIR imaging can be completed in under ten minutes, has fewer side effects, doesn’t require fasting, and is a non-invasive method.
While the Ivy sign itself does not directly measure blood flow, it indicates the brain’s attempt to develop collateral circulation in response to reduced flow. This can serve as an indirect measure of how CVR is functioning—if there is significant collateral formation, it suggests that CVR is impaired, as the brain is relying on these vessels to compensate for the lack of normal blood flow
Although Ivy sign cannot directly replace dynamic blood flow measurements or CVR testing (such as using functional MRI or PET to directly assess perfusion, it can still provide valuable information about the state of cerebral perfusion and the compensatory mechanisms in place, especially in situations where more invasive methods are not feasible.
The correlation between the distribution of the Ivy sign and CVR reduction was consistent with the results of the SPECT study and FLAIR follow-up imaging.
The current sample has shown statistically significant results. By increasing the sample size, we can improve the reliability of the findings. We are currently in the process of collecting additional samples and plan to provide further updates in the future. Differences in surgeons can introduce various variables, including surgical outcomes. However, this study was conducted by a single experienced neurosurgeon and a single neurologist with expertise in neuroimaging, both from one center.
The Ivy sign is the visualization of leptomeningeal collateral vessels, which form in response to cerebral hypoperfusion (insufficient blood flow). These vessels develop as an attempt to compensate for the stenosis or occlusion of major arteries, such as the internal carotid artery and middle cerebral artery. The presence of Ivy sign suggests that collateral circulation has developed in response to ischemia, but if CVR is reduced, this means the brain’s ability to increase blood flow and compensate for ischemia is failing, which can lead to more significant ischemic events like stroke or TIA (transient ischemic attacks).The Ivy sign in Moyamoya disease is closely associated with CVR reduction and ischemic symptoms. It reflects the formation of collateral vessels as a compensatory mechanism for insufficient blood flow. While Ivy signs itself do not directly assess CVR, it can serve as a non-invasive indicator of reduced CVR and the ischemic burden of the disease. However, for more accurate assessment of CVR, additional dynamic blood flow measurements and functional imaging techniques are still needed to complement the findings from Ivy sign imaging. The Ivy sign is a useful non-invasive marker to assess collateral circulation and can indirectly reflect the degree of CVR reduction in MMD. By using MRI or CT angiography, the Ivy sign can be identified, and it can provide insights into the compensatory blood vessels’ development in response to ischemia. While the Ivy sign itself does not directly measure blood flow, it indicates the brain’s attempt to develop collateral circulation in response to reduced flow. This can serve as an indirect measure of how CVR is functioning—if there is significant collateral formation, it suggests that CVR is impaired, as the brain is relying on these vessels to compensate for the lack of normal blood flow. Although Ivy sign cannot directly replace dynamic blood flow measurement s or CVR testing (such as using functional MRI or PET to directly assess perfusion, it can still provide valuable information about the state of cerebral perfusion and the compensatory mechanisms in place, especially in situations where more invasive methods are not feasible. The correlation between the distribution of the Ivy sign and CVR reduction was consistent with the results of the SPECT study and FLAIR follow-up imaging.
FLAIR imaging can provide reliable longitudinal follow-up for patients with Moyamoya disease or other cerebrovascular conditions without requiring invasive procedures, allowing for safer and more frequent monitoring of disease progression. Since FLAIR imaging is non-invasive, it is easy to perform during long-term follow-up, and it is being used for evaluation alongside clinical symptom tracking in outpatient visits.
5. Limitations
Although our sample size was limited (n=49), significant correlations between HV changes and hemodynamic improvements were clearly demonstrated, suggesting clinical relevance despite this limitation. Given the retrospective nature of our study, selection bias and uncontrolled confounding factors cannot be excluded, highlighting the need for prospective validation. Further large-scale, prospective studies are needed to validate the clinical utility of FLAIR imaging in longitudinal monitoring of Moyamoya disease and symptomatic steno-occlusive cerebrovascular disorders.
6. Conclusions
The presence of HV on FLAIR images was observed in areas where both cerebral perfusion and CVR were reduced. Following STA-MCA anastomosis, the HV either diminished or disappeared in the affected hemisphere. Compared to traditional diagnostic tools like SPECT or MR perfusion, HV proves to be a valuable marker for detecting hemodynamic changes in the brain before and after surgery. Our findings indicate that HV is also an effective indicator for monitoring hemodynamic changes in adult patients. Additionally, the severity of HV was more pronounced in patients with greater symptom severity. Further research is needed to determine whether the Ivy sign can also help assess improvements in hemodynamic status and evaluate the success of STA-MCA anastomosis in adult patients with Moyamoya disease and steno-occlusive disorders.
In conclusion, HV observed on FLAIR imaging correlates significantly with cerebral hemodynamics. Our findings indicate that changes in HV after STA-MCA bypass surgery reliably reflect hemodynamic improvements, suggesting its potential utility as a convenient, non-invasive imaging biomarker.
Funding
This research received no external funding.
References
- Komiyama M, Nakajima H, Nishikawa M, Yasui T, Kitano S, Sakamoto H: Leptomeningeal contrast enhancement in moyamoya: its potential role in postoperative assessment of circulation through the bypass. Neuroradiology 2001; 43:17-23. [CrossRef]
- Maeda M, Tsuchida C: „Ivy sign” on fluid-attenuated inversion-recovery images in childhood moyamoya disease. AJNR American journal of neuroradiology 1999; 20:1836-1838.
- Gauvrit JY, Leclerc X, Girot M, Cordonnier C, Sotoares G, Henon H, Pertuzon B, Michelin E, Devos D, Pruvo JP, Leys D: Fluid-attenuated inversion recovery (FLAIR) sequences for the assessment of acute stroke: inter observer and inter technique reproducibility. J Neurol 2006; 253:631-635. [CrossRef]
- Lee JK, Yoon BH, Chung SY, Park MS, Kim SM, Lee DS: The usefulness of the ivy sign on fluid-attenuated intensity recovery images in improved brain hemodynamic changes after superficial temporal artery-middle cerebral artery anastomosis in adult patients with moyamoya disease. J Korean Neurosurg Soc 2013; 54:302-308. [CrossRef]
- Settakis G, Molnar C, Kerenyi L, Kollar J, Legemate D, Csiba L, Fulesdi B: Acetazolamide as a vasodilatory stimulus in cerebrovascular diseases and in conditions affecting the cerebral vasculature. Eur J Neurol 2003; 10:609-620. [CrossRef]
- Ringelstein EB, Sievers C, Ecker S, Schneider PA, Otis SM: Noninvasive assessment of CO2-induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke 1988; 19:963-969. [CrossRef]
- Tomsick TA, Khatri P, Jovin T, Demaerschalk B, Malisch T, Demchuk A, Hill MD, Jauch E, Spilker J, Broderick JP, Committee IIE: Equipoise among recanalization strategies. Neurology 2010; 74:1069-1076. [CrossRef]
- Lee SK, Kim DI, Jeong EK, Kim SY, Kim SH, In YK, Kim DS, Choi JU: Postoperative evaluation of moyamoya disease with perfusion-weighted MR imaging: initial experience. AJNR American journal of neuroradiology 2003; 24:741-747.
- Kawashima M, Noguchi T, Takase Y, Ootsuka T, Kido N, Matsushima T: Unilateral hemispheric proliferation of ivy sign on fluid-attenuated inversion recovery images in moyamoya disease correlates highly with ipsilateral hemispheric decrease of cerebrovascular reserve. AJNR American journal of neuroradiology 2009; 30:1709-1716. [CrossRef]
- Mori N, Mugikura S, Higano S, Kaneta T, Fujimura M, Umetsu A, Murata T, Takahashi S: The leptomeningeal „ivy sign” on fluid-attenuated inversion recovery MR imaging in Moyamoya disease: a sign of decreased cerebral vascular reserve? AJNR American journal of neuroradiology 2009; 30:930-935. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).