1. Introduction
Despite the unprecedented rise in global life expectancy, healthspan—the proportion of life spent in good functional health—has not kept pace (Crane et al., 2022; WHO, 2025). This disparity between longer survival and earlier onset of functional decline underscores a key paradox of modern aging: while we are living longer, we are not necessarily living better. Increasingly, evidence suggests that this gap may be rooted not in isolated disease processes, but in a systemic failure of the body’s adaptive capacity to meet the cumulative demands of modern stress exposure (McEwen, 2022; Monzel et al., 2023).
Central to this emerging perspective is the concept of stress adaptation as a metabolically governed trajectory, in which the ability to resolve, rather than merely respond to, stress determines long-term resilience. Classical models, such as Selye’s General Adaptation Syndrome, framed stress as a linear sequence of alarm, resistance, and exhaustion (Selye, 1950). Contemporary refinements—such as the concepts of allostasis and the integrated stress response—emphasize the dynamic, resource-dependent nature of this process (Costa-Mattioli & Walter, 2020; McEwen & Wingfield, 2003). These models converge on a common insight: chronic or unresolved stress imposes a persistent bioenergetic cost that progressively undermines system integrity, accelerating biological aging and predisposing to chronic disease (Bobba-Alves et al., 2023; Ryan & Ryznar, 2022).
This burden is further magnified by the exposome—the totality of exposures an individual accumulates across the lifespan, spanning both external and internal domains (Rappaport et al., 2014; Vermeulen et al., 2020). External exposures include pollutants, pathogens, dietary factors, psychosocial stressors, and circadian disruption, while internal exposures arise from endogenous processes such as inflammation, oxidative stress, metabolic byproducts, and microbiome activity. These factors interact continuously with the body, shaping health trajectories over time. The exposome captures not only the environment we live in but also how our biology responds to and is shaped by these influences—emphasizing the dynamic interplay between context and physiology that governs resilience and vulnerability. Even in well-nourished individuals living under relatively low external stress, biological systems are never free from the cumulative pressure of adaptation. Chronic interaction with the internal and external exposome imposes ongoing bioenergetic demands that, over time, contribute to functional aging—even in the absence of overt malnutrition or pathology.
We do not inhabit a clean or neutral world; rather, our systems are constantly navigating a complex and evolving landscape of challenges that intersect with genetic, epigenetic, and metabolic susceptibilities. In this context, equilibrium is not a fixed state (homeostasis), but a dynamic process (homeodynamics) requiring ongoing adaptation and resource reallocation to preserve function (Demirovic & Rattan, 2013; Rattan, 2020). When adaptive capacity is strained—whether through depletion, inefficiency, or chronic overload—this resilience erodes, giving rise to adaptive failure phenotypes that often precede or underlie clinical disease and contribute to the progressive acceleration of biological aging (Kivimäki et al., 2023; Wu et al., 2024).
Within this framework, Exposure-Related Malnutrition (ERM) is proposed as a mechanistically distinct, early-stage phenotype of stress maladaptation, marking the tipping point between successful resolution and unresolved, energy-intensive adaptation. Unlike classical malnutrition, which is typically characterized by overt nutritional deficits, weight loss, or insufficient intake, ERM reflects a state of functional undernourishment, where the ongoing energetic demands of unresolved stress deplete available resources, forcing a trade-off that deprioritizes essential housekeeping functions—including cellular maintenance, repair, and long-term regenerative processes. This may occur despite seemingly adequate—or even excessive—caloric intake (Cederholm & Bosaeus, 2024).
The foundational logic of ERM draws on Bruce Ames’ nutrient triage theory, which proposed that during periods of limited micronutrient availability, the body preferentially allocates resources to short-term survival functions at the expense of long-term health maintenance (Ames, 2006). ERM extends this concept to the broader bioenergetic landscape, highlighting how chronic stress can drive substrate misallocation not only in micronutrient pathways but across major energy-requiring systems.
While conceptually closer to Disease-Related Malnutrition (DRM)— in which illness-driven metabolic demands exceed nutritional supply—ERM may arise earlier, during a phase when adaptive systems are still actively compensating, albeit at escalating energetic cost (Muscaritoli et al., 2023). Unlike DRM, which typically presents with recognizable clinical and biomarker signs of classical malnutrition, ERM can remain silent, occurring in individuals who appear outwardly well-nourished. It reflects a subtle, progressive decline in resilience that precedes overt malnutrition, organ dysfunction, or clinical disease.
ERM reflects a state of energy and substrate rationing, in which declining availability, coupled with persistent adaptive demands, drives systematic misallocation of resources across neuroendocrine, immune, muscular, and mitochondrial systems. These covert trade-offs suppress anabolism, immune surveillance, and cellular turnover—while sustaining low-grade inflammation, mitochondrial dysfunction, fatigue, and early functional decline (Paulussen et al., 2021). They reflect a physiological state in which the body has responded and adapted but failed to resolve the stressor—resulting in unfinished resolution or maladaptation. Critically, these shifts represent adaptive strategies that become progressively unsustainable, ultimately eroding resilience and predisposing to chronic dysfunction. When identified early, they may be reversible, offering a vital window for intervention before irreversible dysfunction or structural disease emerges (Picard et al., 2018; Shaulson et al., 2024).
The purpose of this review is to synthesize current evidence across biological systems to propose a bioenergetic model of stress adaptation in aging. We introduce ERM as a unifying early-stage phenotype marked by declining energy availability, substrate misallocation, and failure to resolve prolonged adaptation. We trace how these unresolved adaptive processes give rise to maladaptive consequences across multiple levels of biological organization—including mitochondrial dysfunction, impaired cellular turnover, organ-level decline, and neuroendocrine disruption—positioning ERM as a critical contributor to the molecular basis of aging. This model reframes aging not as a passive or predetermined process, but as an energetically governed trajectory—shaped by cumulative stress exposure and potentially modifiable through early detection and targeted intervention.
2. Conceptual Model:
“Respond → Adapt → Resolve: A Bioenergetic Trajectory of Stress and Aging”
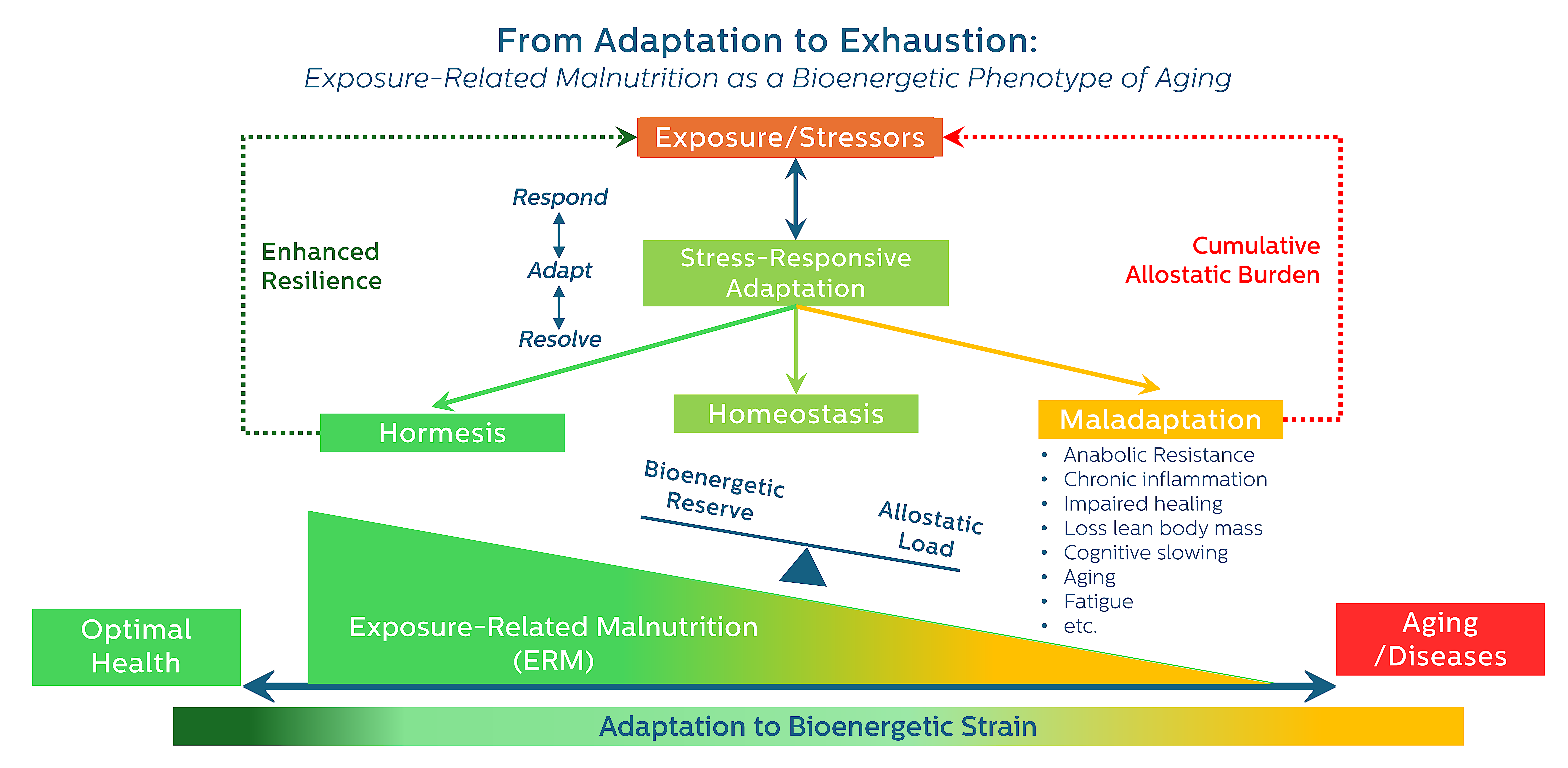
To move beyond fragmented views of aging, we propose a unifying trajectory—Respond → Adapt → Resolve— that conceptualizes aging as the cumulative outcome of unresolved physiological adaptation. Rather than emphasizing acute stress responses or isolated damage pathways, this model centers on the critical role of energy and substrate availability in determining whether adaptation leads to resolution or maladaptation. When recovery is incomplete, bioenergetic costs accumulate, triggering covert trade-offs that progressively degrade resilience (Harrell et al., 2016). This framework highlights a critical inflection point—failure to resolve—as a key determinant of biological aging and a precursor to early dysfunction, exemplified by transitional states such as ERM.
Structured around three metabolically demanding phases, the Respond → Adapt → Resolve model emphasizes that it is not the presence of stress alone, but the effectiveness of resolution that determines long-term outcomes. In the Respond phase, acute stress activates emergency systems, mobilizing energy and suppressing non-essential functions to ensure immediate survival. The Adapt phase involves sustained physiological adjustments, including neuroendocrine, immune, organ-level, cellular, and mitochondrial reprogramming to maintain function under constraint. The Resolve phase governs recovery and restoration, requiring sufficient energy and coordination to reverse adaptive changes and restore homeodynamic balance. When resolution fails—due to depleted bioenergetic reserves or chronic exposure—maladaptive consequences ensue, including persistent inflammation, mitochondrial dysfunction, and accelerated biological decline (Bobba-Alves et al., 2023; Paulussen et al., 2021).
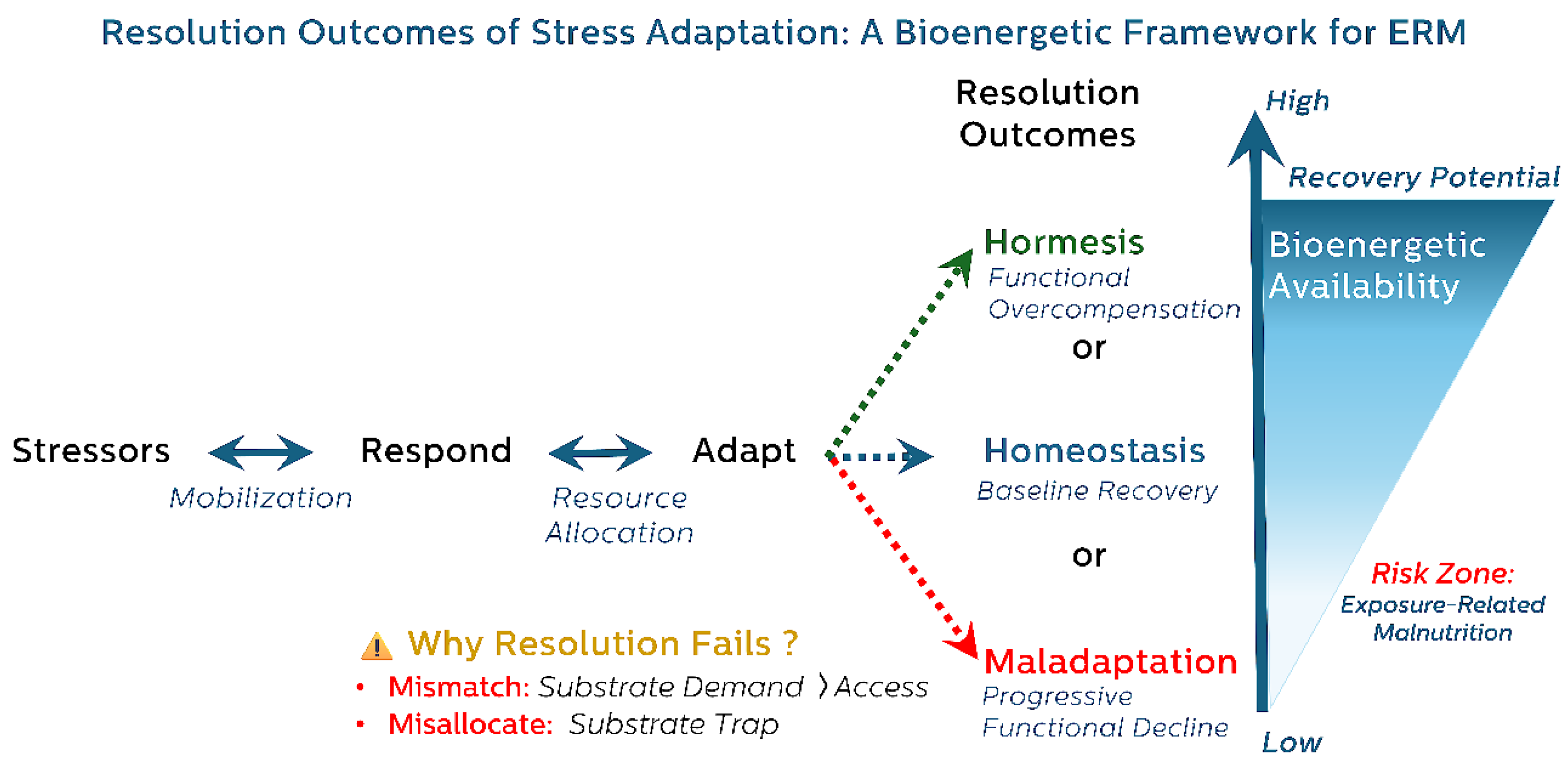
This adaptive trajectory is visually summarized in
Figure 1, which maps resolution outcomes as a function of energy and substrate availability and highlights ERM as an early phenotype of failed resolution.
Within this model, ERM is conceptualized as an early and distinct phenotype of impaired stress resolution. This covert trade-off can occur even in individuals with adequate or excessive caloric intake, rendering it undetectable by conventional assessments. Clinically, ERM may manifest as features that frequently precede the onset of frailty or overt chronic disease (Arron et al., 2024; Cabre et al., 2022; National Academies of Sciences & Medicine, 2024).
This Respond → Adapt → Resolve reframes aging as a dynamic, resource-sensitive trajectory in which the outcome of stress exposure is determined not solely by the magnitude of the stressor, but by the organism’s capacity to complete resolution. Divergent resolution outcomes emerge based on bioenergetic availability: when energetic and substrate resources are abundant, transient stress exposures may trigger hormetic strengthening through overcompensatory adaptive responses; when resources are merely sufficient, homeostasis may be restored without gain; whereas chronic insufficiency drives unresolved adaptation and maladaptive remodeling (Calabrese et al., 2024). ERM marks a critical threshold—a metabolically constrained state in which adaptive processes are initiated but remain energetically unsupported and unresolved. Recognizing ERM as a reversible, subclinical phenotype of maladaptation offers a vital opportunity for early intervention—shifting the clinical focus from managing downstream dysfunction to restoring upstream resolution capacity (Ryan & Ryznar, 2022).
Adaptive responses to stress follow a trajectory from Response (mobilization) to Adaptation (resource allocation), culminating in one of three resolution outcomes: Hormesis (functional overcompensation), Homeostasis (baseline recovery), or Maladaptation (progressive decline). These outcomes are contingent on the sufficiency and allocation of energy and metabolic substrates, represented by the vertical gradient on the right.
Hormesis occurs when energy is sufficient and optimally allocated, supporting repair and strengthening. Homeostasis reflects adequate but not surplus recovery. Maladaptation arises when resolution fails due to either (1) substrate mismatch (demand exceeds access) or (2) substrate misallocation (resources are diverted to short-term survival at the expense of long-term repair).
ERM is depicted as a syndrome that emerges under low energy and substrate availability—where chronic, unresolved adaptation leads to covert trade-offs and progressive loss of systemic resilience. This framework highlights the critical role of bioenergetic sufficiency in determining long-term health trajectories following stress.
3. Mechanistic Pathways Underlying ERM
3.1. From Central Command to Cellular Collapse: The Bioenergetic Logic of Maladaptive Adaptation
The failure to resolve chronic physiological stress is evident across tightly interlinked systems, beginning with central neuroendocrine regulation and extending through immune and muscular function to cellular signaling networks and mitochondrial energetics. At each level, a common pattern emerges: a metabolic phenotype characterized by substrate rationing and resource allocation under persistent bioenergetic strain. Critically, these systems operate as dynamic, feedback-regulated loop—where signals originating from mitochondria, cells, and peripheral organs ultimately shape central control. This bidirectional communication determines whether the organism adapts, recovers, or progressively declines. The ERM phenotype, therefore, reflects not isolated dysfunction but a systemic failure of resolution across the bioenergetic hierarchy.
3.2. Neuroendocrine Axis: Central Command of Substrate Allocation
The hypothalamic–pituitary–adrenal (HPA) axis serves as the central regulator of systemic energy allocation in response to stress. Activation of the HPA and sympathetic–adrenal–medullary (SAM) systems initiate the Respond phase, rapidly mobilizing glucose and fatty acids while downregulating energy-intensive processes such as reproduction, growth, and digestion (Tsigos & Chrousos, 2002). Under conditions of chronic stress, sustained activation imposes bioenergetic strain that disrupts hormonal homeostasis—flattening the diurnal cortisol rhythm and skewing the cortisol: DHEA ratio toward a catabolic profile. These changes, commonly observed with aging, reflect a shift from adaptive flexibility to maladaptive rigidity (McEwen, 2007; Yiallouris et al., 2019).
The Selfish Brain Theory posits that the brain prioritizes its access to glucose and oxygen over peripheral tissues, especially under conditions of systemic energy scarcity (Peters, 2004; Peters et al., 2022). This neurocentric substrate allocation becomes maladaptive over time, diverting resources away from essential processes such as tissue repair, immune tolerance, and muscle maintenance—hallmarks of the Adapt phase trade-offs. The Brain–Body Energy Conservation (BEC) model expands this concept by identifying the brain as the central broker of the organism’s finite energy budget (Shaulson et al., 2024). As molecular damage accumulates, somatic cells may enter a state of senescence, adopting a senescence-associated secretory phenotype (SASP)—a hypersecretory program marked by the sustained release of pro-inflammatory cytokines, chemokines, growth factors, and proteases (Coppé et al., 2008). While the SASP plays a role in signaling cellular stress and coordinating tissue repair and immune surveillance, its chronic activation imposes substantial metabolic demands and contributes to systemic inflammation. Over time, this persistent low-grade inflammatory state may evolve into inflammaging—a distinct, age-associated phenotype of chronic immune activation that contributes to tissue dysfunction and age-related disease. These hypermetabolic signals are sensed by the brain, which responds with energy-conservation strategies that suppress non-essential physiological processes. This includes downregulation of anabolic hormones, reduced voluntary activity, and simplification of immune and endocrine functions. Chronically elevated cortisol and sympathetic tone further reinforce this adaptive trade-off, promoting insulin resistance, visceral adiposity, and circadian disruption—hallmarks of allostatic load and declining systemic flexibility (Ryan & Ryznar, 2022; Sapolsky, 2004). Over time, these adaptations, though protective in the short term, contribute to the erosion of physiological resilience and the emergence of the ERM phenotype as a bioenergetically compromised state of accelerated aging. This neuroendocrine-driven substrate misallocation contributes to the ERM phenotype by creating an apparent paradox: energy is present but inaccessible to systems that need it most—undermining cellular repair and systemic resilience.
3.3. Immune Reprogramming and Inflammaging: Energy-Intensive Surveillance
In response to stress, the immune system rapidly adopts a pro-inflammatory phenotype, shifting macrophages, neutrophils, and T cells toward aerobic glycolysis—a metabolically costly state that supports cytokine production, phagocytosis, and rapid cell proliferation (Olenchock et al., 2017). While this reprogramming is essential for acute host defense, it becomes maladaptive under conditions of chronic stress and limited metabolic resources. Immune cells fail to revert to oxidative metabolism, locking into a glycolytic phenotype that sustains low-grade inflammation and impairs resolution (Franceschi et al., 2018; Willmann & Moita, 2024).
This loss of metabolic flexibility reflects a broader bioenergetic trade-off characteristic of ERM: when energy and substrate availability are constrained, immune surveillance is maintained at the expense of tissue regeneration, neuroprotection, and systemic homeostasis. Chronically activated or aging immune cells exhibit mitochondrial dysfunction and impaired mitochondrial quality control—including reduced mitophagy and elevated oxidative stress. These disturbances lead to the release of mitochondrial damage-associated molecular patterns (DAMPs), such as mitochondrial DNA (mtDNA), which may be oxidized depending on the cellular context. Once released into the cytosol, mtDNA activates innate immune pathways including the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome—particularly when oxidized—and the cyclic GMP–AMP synthase–stimulator of interferon genes (cGAS–STING) axis, thereby amplifying cytokine production and sustaining chronic inflammatory signaling (Wu et al., 2025).
As a result, immune cells shift from being metabolically flexible responders to chronically activated drivers of tissue injury, fibrosis, and energetic collapse—hallmarks of immunosenescence and inflammaging (Fulop et al., 2018). Persistent immune activation also depletes micronutrients critical to systemic resilience—such as zinc, selenium, and iron—compromising antioxidant defenses, mitochondrial function, and epithelial repair (Alack et al., 2019; Gulhar et al., 2024). This creates a self-reinforcing loop of micronutrient insufficiency, metabolic stress, and immune dysfunction.
Emerging technologies, such as Single-Cell Energetic metabolism by Translation Inhibition (SCENITH) and single-cell RNA sequencing (scRNA-seq), have revealed that aged immune cells display distinctive metabolic signatures—marked by diminished oxidative capacity and exaggerated inflammatory output (Wu et al., 2025). These findings align with ERM-associated metabolic adaptations, where immune vigilance is preserved while anabolic and reparative systems—such as skeletal muscle, neural tissue, and intestinal epithelium—are systematically deprioritized. Immune reprogramming under chronic metabolic strain thus exemplifies a maladaptive resolution phase of stress adaptation: energy is persistently funneled into defense and surveillance at the cost of long-term functional integrity and resilience.
3.4. Skeletal Muscle and Anabolic Resistance: The Energetic Reservoir Depleted
Skeletal muscle serves as the body’s primary amino acid reservoir, mobilized during physiological stress to support gluconeogenesis, immune activation, and the hepatic acute-phase response (Cahill, 2006). While this catabolic mobilization is adaptive in the short term, persistent stress drives a chronic depletion of muscle mass—transforming a protective mechanism into a source of dysfunction. In the Adapt phase of prolonged stress exposure, elevated catabolic hormones, such as cortisol, and pro-inflammatory cytokines promote proteolysis, while simultaneously impairing muscle protein synthesis (MPS) even in the presence of adequate protein intake (Ferreira & Duarte, 2023; Paulussen et al., 2021).
This impaired responsiveness to anabolic stimuli—termed anabolic resistance—reflects a systemic energy trade-off in which substrate allocation is redirected away from tissue maintenance and regeneration. Key anabolic pathways, including mechanistic target of rapamycin complex 1 (mTORC1) and insulin-like growth factor-1 (IGF-1) signaling, are suppressed under metabolic strain. Additionally, muscle stem cells (satellite cells) fail to initiate proper regeneration in the absence of sufficient energetic and micronutrient support (Bian et al., 2020; Langston & Mathis, 2024).
Over time, these deficits contribute to muscle atrophy and the development of sarcopenia—a progressive loss of muscle mass and function that is both a hallmark of biological aging and a potential clinical endpoint of undiagnosed ERM (Cederholm & Bosaeus, 2024; Walrand et al., 2021). Importantly, this decline in muscle integrity is not merely structural; it underpins a wide spectrum of clinical phenotypes, including fatigue, poor exercise tolerance, and delayed recovery following physical activity. These features reflect the energetic exhaustion of skeletal muscle as both an effector and a victim of chronic metabolic trade-offs—where maintaining defense and immune activation takes precedence over repair, performance, and resilience.
3.5. Cellular Integrated Stress Response: Translational Triage Under Strain
The Integrated Stress Response (ISR) is a highly conserved mechanism that regulates cellular adaptation to stress by modulating protein synthesis. In response to nutrient deprivation, redox imbalance, or proteotoxic stress, ISR is activated through phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α), which attenuates global protein translation while selectively enhancing the synthesis of adaptive transcription factors such as activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP) (Costa-Mattioli & Walter, 2020; Pakos-Zebrucka et al., 2016). This selective translational reprogramming enables cells to conserve resources and temporarily shift priorities toward stress mitigation and survival.
The ISR is functionally integrated with the endoplasmic reticulum (ER) stress response and the Unfolded Protein Response (UPRER), particularly through the PERK–eIF2α–ATF4 signaling axis. These systems converge on shared nodes to coordinate cellular responses to proteostatic disruption and nutrient imbalance. When misfolded proteins accumulate in the ER, the UPRER is activated to restore folding capacity or trigger apoptosis if damage is beyond repair (Hetz & Saxena, 2017).
This interplay exemplifies the principle of interorganelle collaboration, wherein stress signals from the ER, cytoplasm, and nutrient-sensing pathways are integrated to determine cellular fate. Importantly, the outcome of this collaboration is not fixed, but context dependent. According to the catabolic–anabolic cycling hormesis (CACH) model, adaptive responses to stress occur in two phases: an initial catabolic phase characterized by autophagy, translational suppression, and metabolic reallocation, followed by an anabolic recovery phase involving biosynthesis, growth, and repair (Calabrese & Mattson, 2024). The adaptive value of stress responses such as the ISR and UPRER depends on sufficient energy and substrates to sustain both the stress response and its recovery phase.
In C. elegans, targeted non-cell-autonomous activation of the UPRER through xbp-1s expression in specific neurons has been shown to enhance systemic proteostasis, increase ER stress resistance, and extend lifespan—largely through coordinated gut-directed signaling (Coakley et al., 2024; Higuchi-Sanabria et al., 2020; Taylor & Dillin, 2013). These benefits, however, appear contingent on spatiotemporal control and adequate metabolic buffering. By contrast, chronic or unresolvable stress, particularly under conditions of metabolic insufficiency, can lead to maladaptive persistence of catabolic signaling. Prolonged ISR or UPRER activation in this context disrupts proteostasis, impairs regenerative signaling, and may culminate in senescence or apoptosis, especially in high-turnover tissues such as intestinal epithelium and immune cells (Hetz & Papa, 2018). Similar mechanisms may also affect skeletal muscle, although this requires further empirical clarification.
Thus, while interorganelle stress signaling pathways can be central to resilience, their effects depend on duration, localization, and metabolic context. When the catabolic phase is prolonged without anabolic resolution, adaptive responses may collapse into degenerative trade-offs, exhausting cellular reserves and accelerating vulnerability. This stress-induced shift—marked by sustained translational suppression and impaired renewal, characterizes early-stage ERM, in which the capacity for repair is sacrificed to preserve immediate survival.
3.6. Mitochondrial Stress Response and Mitokines: The Energetic Fulcrum and Feedback Signal
Mitochondria are increasingly recognized not only as producers of adenosine triphosphate (ATP) but also as central signal transduction hubs that integrate metabolic status, oxidative stress, immune activation, and intercellular communication (Picard & Shirihai, 2022). These organelles coordinate cellular responses to stress by modulating ATP output, redox balance, reactive oxygen species (ROS) generation, and the supply of metabolic intermediates. In response to mild or transient energetic stress, mitochondria engage in mitohormesis—a process in which low-level ROS production triggers adaptive signaling pathways that enhance mitochondrial biogenesis, repair, and overall resilience (Ristow & Schmeisser, 2014).
However, when energy demands exceed capacity over time, these adaptive processes fail. Mitochondrial dysfunction leads to excess ROS, loss of membrane potential, and the release of mitochondrial DNA (mtDNA) into the cytosol—activating inflammatory signaling via the NLRP3 inflammasome and cGAS–STING pathway (Ni et al., 2015; Qi et al., 2025). Beyond fueling inflammation, these mitochondrial disturbances also contribute to the induction of cellular senescence and SASP, creating a self-perpetuating cycle of immune activation, tissue damage, and bioenergetic decline (Martini & Passos, 2023; Victorelli et al., 2023). This cascade amplifies systemic inflammation and accelerates the progression of ERM phenotypes, senescence, and chronic disease.
In addition to local signaling, dysfunctional mitochondria produce mitokines—circulating stress signals such as fibroblast growth factor 21 (FGF21) and growth differentiation factor 15 (GDF15). These mitokines act on the central nervous system, particularly the hypothalamus and area postrema, to suppress appetite, reduce activity, and recalibrate energy expenditure (Lockhart et al., 2020; Zhang et al., 2024). GDF15, in particular, has been linked to anorexia, fatigue, and muscle catabolism, effectively informing the brain of peripheral energetic crisis and reinforcing systemic energy conservation (Shaulson et al., 2024).
Importantly, mitochondria also participate in intercellular rescue mechanisms through mitochondrial transfer—a process in which healthy mitochondria are transferred from donor cells (such as mesenchymal stem cells or astrocytes) to stressed or energy-depleted recipient cells via tunneling nanotubes, extracellular vesicles, or cell fusion (Diaz-Meco et al., 2025; Dong et al., 2023). This transfer restores oxidative capacity, reverses bioenergetic failure, and promotes tissue recovery, particularly in metabolically active or injured environments such as the brain, heart, and immune system (Islam et al., 2012; Spees et al., 2006). Mitochondrial transfer thus exemplifies a critical resilience mechanism—extending stress adaptation beyond the individual cell to a community-level response.
Together, these processes illustrate that mitochondria are not passive power plants, but energetic sentinels and signaling nodes that regulate both local and systemic adaptation. Through mitohormesis, mitokine signaling, and intercellular mitochondrial transfer, they act as the fulcrum of the stress response—balancing survival, repair, and functional decline across the trajectory of resilience and exhaustion.
3.7. Closing the Loop: From Peripheral Strain to Central Reprogramming
Collectively, these mechanistic pathways form a closed, self-reinforcing circuit of maladaptive stress adaptation. The loop begins with neuroendocrine activation, redirecting energy away from long-term maintenance toward immediate survival. This shift is reinforced by immune reprogramming that sustains inflammation, skeletal muscle catabolism that depletes physical capacity, translational triage that suppresses cellular renewal, and mitochondrial signaling that conveys energetic distress to the central nervous system. As resolution fails, this interconnected network transitions from adaptive to degenerative—locking the body into a chronically depleted, energy-conserving, and catabolic state.
The result is ERM— a subtle yet systemic bioenergetic phenotype that reflects the failure to resolve chronic physiological stress. This functional malnourishment is not characterized by overt starvation, but by persistent substrate rationing and misallocation, impaired anabolic signaling, and the chronic prioritization of immune and neural defense over cellular turnover and tissue repair. It represents the convergence of central command and peripheral exhaustion—a dynamic imbalance in which energy conservation becomes maladaptive, and the capacity for recovery is no longer energetically sustained.
By tracing this loop—from central regulation to peripheral strain and back—we recognize ERM as a bioenergetic signature of unresolved adaptation. It manifests as the metabolic cost of sustained defenses, the silent erosion of physiological plasticity, and the gradual loss of systemic resilience. Recognizing this loop presents a critical opportunity: to identify ERM early, disrupt its trajectory, and restore the bioenergetic balance essential for repair, recovery, and renewal—before chronic strain culminates in irreversible dysfunction.
4. Defining ERM as a Preclinical Aging Phenotype
Rather than repeating the definition of ERM, this section builds on the earlier conceptualization by outlining how ERM differs from classical malnutrition syndromes and how it serves as an early biomarker of resilience loss. ERM may occur despite normal or excessive caloric intake and arises in response to sustained stress exposure, bioenergetic strain, and substrate misallocation. The hallmark is not nutrient depletion per se, but maladaptive energy trade-offs that compromise long-term physiological functions.
4.1. ERM vs. Classical Malnutrition Syndromes
Unlike classical demand-driven syndromes—such as DRM, Chronic Energy Deficiency (CED), or Relative Energy Deficiency in Sport (REDs)—ERM may occur despite normal or even excessive caloric intake (Cederholm & Bosaeus, 2024; Mountjoy et al., 2018; Prisabela et al., 2023). DRM is typically characterized by inflammation-induced catabolism in the context of acute or chronic illness; CED often results from increased physiological demands such as pregnancy without sufficient intake; and REDs reflects chronic energy imbalance in athletes.
In contrast, ERM develops insidiously in response to sustained physiological stressors—including chronic inflammation, environmental toxicant burden, circadian misalignment, and psychosocial strain—that progressively elevate metabolic demand. These exposures challenge adaptive capacity over time, necessitating covert energy trade-offs that impair long-term resilience despite preserved or excessive energy intake.
ERM results from a chronic mismatch between metabolic demands and substrate availability, compounded by persistent misallocation of available resources. These demands are frequently amplified by prolonged inflammation, psychosocial stress, circadian disruption, and environmental exposures, all of which impose sustained strain on adaptive systems. In this context, neuroendocrine stress responses—particularly elevations in cortisol, insulin, and sympathetic activity—redirect metabolic substrates away from anabolic and regenerative pathways. A key feature of this maladaptive shift is the “insulin-induced substrate trap,” in which glucose and lipids are preferentially routed into storage compartments despite unresolved cellular energy needs (Friedman et al., 2024). While core survival systems remain supported, peripheral processes—such as skeletal muscle integrity, immune surveillance, and tissue repair—experience persistent energetic shortfall, undermining systemic resilience (Shaulson et al., 2024).
The hallmark of ERM is not overt nutrient depletion, but a characteristic pattern of maladaptive bioenergetic trade-offs—where physiological systems increasingly prioritize immediate survival at the expense of long-term functions such as repair, regeneration, and reproduction (Bobba-Alves et al., 2023; Ryan & Ryznar, 2022). ERM may precede, overlap with, or predispose individuals to later-stage malnutrition syndromes, including sarcopenia and frailty. Often clinically silent in its early stages, it represents a critical window for preventive detection and intervention.
4.2. ERM as the Early Metabolic Signature of Resilience Loss in Aging
ERM represents a transitional phase in which chronic activation of stress-adaptive systems gradually erodes physiological resilience, even in the absence of overt disease. In this context, chronic exposure to the exposome can be understood as a universal driver of adaptive demand. ERM may represent the early, maladaptive intersection between cumulative environmental and internal exposures, bioenergetic trade-offs, and declining physiological resilience. As an early metabolic hallmark of aging, it is characterized by substrate misallocation, diminished anabolic signaling, and impaired recovery—marking the shift from adaptive flexibility to cumulative vulnerability.
This perspective aligns with models that frame aging as the biological toll of chronic adaptation demands and allostatic load accumulation(Seeman et al., 2001), as well as with emerging views of mitochondria as central hubs that integrate and propagate cellular stress signals across systems (Picard & Shirihai, 2022; Seeman et al., 2001). Just as prediabetes signals metabolic dysfunction before the onset of diabetes, ERM reflects subclinical energy imbalance that, if left unresolved, increases vulnerability to chronic disease, immune decline, and accelerated aging.
Table 1 summarizes the key phenotypic distinctions between ERM, normative aging, and DRM, highlighting ERM as a distinct, stress-adapted phenotype shaped by cumulative adaptive trade-offs rather than overt nutrient deficiency or chronological age alone.
4.3. Recognizing ERM Phenotypes
ERM rarely presents with overt clinical signs in its early stages. Individuals often maintain a normal or elevated body mass index (BMI), masking underlying physiological compromise. Yet, they may exhibit subtle but functionally significant and progressive patterns of decline— including chronic fatigue, delayed physical recovery, increased susceptibility to infections, cognitive slowing, declining reproductive capacity, and persistent or unexplained chronic pain such as myofascial pain syndromes. These symptoms reflect sustained bioenergetic strain, impaired tissue repair, and neuromuscular inefficiency—hallmarks of maladaptive stress physiology.
Crucially, these features do not occur in isolation but rather as part of a recognizable constellation—a patterned signal of systemic compromise resulting from chronic substrate misallocation. Under conditions of persistent psychosocial stress, low-grade inflammation, and neuroendocrine dysregulation, energy allocation is progressively reprogrammed away from long-term maintenance toward short-term survival, often before classical signs of malnutrition appear (Fülöp et al., 2025). Yet, such early-stage dysfunction is frequently misattributed to aging, lifestyle, or mood disorders—leading to clinical underrecognition and missed opportunities for preventive intervention.
A recent case series of three Thai men with late-onset idiopathic Parkinson’s disease offers a compelling example of how ERM phenotypes may be present and clinically silent for decades (Tippairote et al., 2025). These individuals exhibited persistently elevated mercury levels due to lifelong fish and seafood consumption, compounded by chronic vitamin D insufficiency. Long before clinical diagnosis, they demonstrated functional ERM characteristics—fatigue, cognitive slowing, anabolic resistance, and elevated markers of oxidative stress, inflammation, and metabolic dysregulation. This trajectory exemplifies a “functional ERM” state: one in which apparent homeostasis is maintained through hidden energy trade-offs and micronutrient depletion, quietly eroding resilience.
Importantly, these bioenergetic compromises are not readily captured by conventional assessments. Instead, they manifest as emergent physiological patterns—integrating functional symptoms, signs, compositional shifts, and biochemical trade-offs—that signal maladaptive adaptation under chronic stress. Recognizing ERM as a distinct, stress-mediated malnutrition phenotype requires moving beyond isolated markers toward a systems-level interpretation of early resilience loss.
Functional Biomarkers: The First Line of ERM Detection
Emerging consensus emphasizes the value of functional tests—such as handgrip strength, gait speed, one-leg standing time, and calf circumference—as early, accessible indicators of systemic decline. These tests reflect neuromuscular performance, metabolic reserve, and organ system coordination. In fact, they outperform many molecular biomarkers in predicting mortality and frailty across populations (Furrer & Handschin, 2025a, 2025b).
Figure 2 summarizes the early phenotype of ERM, integrating functional symptoms, physical signs, and measurable biochemical trade-offs. This visualization supports clinical recognition of ERM beyond traditional malnutrition criteria.
Handgrip strength and muscle power (dynapenia/powerpenia) have stronger associations with morbidity and mortality than muscle mass alone, and reflect early disruption in anabolic signaling and mitochondrial energy metabolism.
Gait speed and balance tests capture neuromuscular coordination and are sensitive to cognitive and central nervous system compromise.
Calf circumference, particularly in the context of preserved BMI, serves as a practical surrogate for declining peripheral muscle mass and functional reserve.
These physical assessments should not be interpreted as isolated values, but as part of an integrated functional pattern that reflects ERM’s early-stage bioenergetic erosion.
ERM is an early-stage maladaptive state marked by subtle but systemic bioenergetic compromise. It manifests as a patterned triad of functional symptoms (e.g., fatigue, slowed recovery, anabolic resistance), physical performance signs (e.g., grip strength, gait speed, calf circumference), and distinctive biochemical trade-offs. Biomarker patterns reflect adaptive substrate prioritization: preservation of acute-phase reactants, decline in housekeeping proteins and cellular repair capacity, and suppression of anabolic and reproductive functions
From Structure to Cellular Function: Compositional and Biophysical Markers
In addition to performance-based tests, bioelectrical impedance analysis (BIA) provides insight into the body’s internal reallocation of resources:
Progressive decline in skeletal muscle mass and bone mineral content signals a shift away from long-term structural investment, consistent with catabolic resource diversion.
Accumulation of visceral fat, particularly in the presence of stable or rising BMI, reflects a maladaptive redistribution of energy stores—often sustained by hyperinsulinemia and glucose-driven metabolic programming under stress.
Reduction in phase angle (PhA), a marker of cell membrane integrity and intracellular water balance, reflects impaired cellular vitality and bioenergetic efficiency. Rather than relying on a single cutoff, declining trends in PhA may indicate cumulative stress effects and loss of physiological plasticity. Reduced PhA is also linked to anabolic resistance, sarcopenia, and increased frailty (Akamatsu et al., 2022; Norman et al., 2012).
Recent findings indicate that PhA is particularly sensitive in women, where it correlates not only with global cognitive function but also with specific domains such as memory, executive function, and attention—suggesting its potential as an early marker of physiological vulnerability prior to the development of overt malnutrition or dementia (Ikeue et al., 2025).
Patterns Over Points: The ERM Signature
ERM is best recognized not by single diagnostic thresholds, but by emergent physiological patterns over time. This concept aligns with recent findings showing that hematologic and metabolic biomarkers exhibit stable, individualized setpoints in healthy individuals. Deviations from personal baselines—even within population-normal ranges—can signal early adaptation failure (Foy et al., 2024).
These changes occur within the broader context of the integrated stress response (ISR), nutrient triage, and brain–body energy conservation, which prioritize short-term survival over long-term maintenance and repair (Ames, 2006; Shaulson et al., 2024; Wang & Zhang, 2025).
Pattern of Biochemical Trade-Offs: Systemic Signals of Strain
The underlying biochemical constellation reinforces the pattern-based signature of ERM:
Preservation or elevation of acute-phase reactants (e.g., CRP, ferritin), coagulation factors, and stress proteins indicate active immune prioritization (Cederholm & Bosaeus, 2024; Sganga et al., 1985).
Decline in housekeeping proteins such as transthyretin and transferrin marks hepatic reprioritization away from maintenance functions (Evans et al., 2021; Paulussen et al., 2021).
Suppression of long-term anabolic markers, including IGF-1, sex hormones, and proteins related to muscle, bone, and reproductive function, reflects deeper systemic sacrifice in favor of short-term homeostasis (Bian et al., 2020; Payea et al., 2024; Ryan & Ryznar, 2022).
These physiological and biochemical trends are often amplified by mitochondrial dysfunction and redox imbalance. Recognizing these patterns allows for a more precise clinical understanding of early resilience loss (Brzezniakiewicz-Janus et al., 2025).
4.4. ERM and the GLIM Criteria: A Missing Middle
The Global Leadership Initiative on Malnutrition (GLIM) provides an internationally accepted framework for diagnosing malnutrition in clinical and aging populations. Diagnosis requires the presence of at least one phenotypic criterion, e.g., non-volitional weight loss, low BMI, reduced muscle mass, and one etiologic criterion, e.g., reduced food intake, disease burden, or inflammation (Cederholm et al., 2025). While GLIM represents a major advance in standardizing clinical nutrition assessment, it may overlook a biologically significant but subclinical stage of nutrient misallocation and bioenergetic exhaustion.
This is where the concept of ERM offers critical complementary insight. Unlike GLIM-defined malnutrition, ERM can present in individuals who do not meet GLIM criteria, yet are experiencing metabolic compromise due to chronic stress adaptation, systemic inflammation, and substrate misallocation (Bobba-Alves et al., 2023; Ryan & Ryznar, 2022). The hallmark phenotypes of ERM—such as chronic fatigue, cognitive dysfunction, physical intolerance, immune dysregulation, shifts in body composition, and coordinated biomarker trade-off patterns—are not captured by the static thresholds defined in the GLIM criteria.
ERM may therefore be conceptualized as either:
a “pre-GLIM” phenotype, representing an earlier, subclinical stage of adaptation failure, or
a parallel subtype of functional malnutrition, primarily driven by maladaptive stress physiology rather than overt intake deficiency.
This distinction is especially relevant in older adults and individuals with chronic illness or psychosocial stress exposure, where conventional indicators—such as BMI or body weight—may remain within normal ranges even
as physiological reserve and resilience quietly erode. These differences are further clarified in
Table 2, which contrasts the GLIM and ERM frameworks across key domains—highlighting how ERM expands malnutrition recognition to include earlier, subclinical phases of bioenergetic compromise.
As shown, GLIM prioritizes observable structural loss, whereas ERM focuses on functional and metabolic vulnerability under stress. The two are not mutually exclusive: ERM may precede GLIM-defined malnutrition or persist in parallel. Recognizing this distinction allows for earlier, resilience-preserving interventions—particularly in aging populations where intake remains stable but physiological reserve is already compromised.
5. ERM and the Hallmarks of Aging: Reframing Aging as a Failure to Resolve Adaptation
This section builds upon the earlier definition of ERM and maps it onto the established hallmarks of aging (Lopez-Otin et al., 2013; López-Otín et al., 2023). ERM exemplifies a reversible phase of stress maladaptation that converges with features such as mitochondrial dysfunction, impaired autophagy, and altered nutrient sensing—traditionally considered hallmarks of aging (Lopez-Otin et al., 2013). Rather than redefining ERM again, we focus on how its bioenergetic characteristics align with aging mechanisms and present opportunities for targeted intervention (Bobba-Alves et al., 2023; Schmauck-Medina et al., 2022).
This evolving perspective aligns closely with the concept of ERM, which characterizes a metabolically constrained but potentially reversible state of chronic adaptation failure. In ERM, the body reallocates limited energy and substrates toward short-term survival functions at the expense of maintenance, repair, and regeneration. This systemic triage can activate features classically associated with aging—such as mitochondrial inefficiency, anabolic suppression, immune dysregulation, and impaired tissue renewal—not because of biological age, but because of unresolved stress.
ERM thus reframes aging as a dynamic consequence of adaptation failure rather than a fixed outcome of time. Crucially, many of the functional changes observed in ERM—such as suppressed IGF-1 signaling, mitochondrial stress, reduced autophagy, and stem cell dormancy—are not necessarily irreversible. With timely intervention to restore nutrient availability, redox balance, and anabolic signaling, biological aging trajectories may be interrupted, and resilience potentially recovered.
5.1. Mitochondrial Dysfunction → Bioenergetic Reversibility
Mitochondrial dysfunction is a central hallmark of aging, characterized by impaired oxidative phosphorylation, increased reactive oxygen species (ROS), mitochondrial DNA instability, and defective mitophagy. These changes reduce cellular energy availability and contribute to inflammatory signaling and tissue degeneration (Picard & Shirihai, 2022; Qi et al., 2025).
However, such impairments are not always irreversible. In the context of chronic metabolic stress—such as that seen in ERM—mitochondrial fragmentation, redox imbalance, and ATP insufficiency may reflect adaptive energy reallocation rather than permanent failure. The mitochondrial integrated stress response (mt-ISR) downregulates energetically costly processes to preserve core functions under resource scarcity.
Importantly, when substrate availability and redox conditions are restored, mitochondrial dynamics and ATP production can often recover. This suggests that mitochondrial dysfunction in ERM represents a reversible metabolic bottleneck, offering a window to restore bioenergetic function and interrupt decline before structural damage becomes entrenched.
5.2. Altered Nutrient Sensing → Adaptive Metabolic Flexibility
Disruption of nutrient-sensing pathways—including insulin-like growth factor 1 (IGF-1), mechanistic target of rapamycin (mTOR), AMP-activated protein kinase (AMPK), and insulin signaling—is another hallmark of aging. These disruptions impair anabolic signaling, autophagy, and metabolic flexibility, undermining the capacity to balance growth and maintenance under fluctuating conditions (Chrousos, 2009).
A similar pattern is observed in ERM, where chronic energy insufficiency and stress induce systemic downregulation of these pathways. Suppressed IGF-1 signaling, insulin resistance, and impaired mTOR activity reflect an energy-conserving response to prolonged substrate scarcity. In skeletal muscle, this contributes to anabolic resistance—a reduced responsiveness to anabolic stimuli such as amino acids or mechanical loading—closely mirroring sarcopenia (Paulussen et al., 2021).
Unlike irreversible endocrine failure, these adaptations may be functionally reversible. When energetic capacity is restored and chronic stress resolved, nutrient-sensing pathways can recover their regulatory flexibility. ERM thus represents a state of adaptive metabolic plasticity, not permanent breakdown.
5.3. Cellular Senescence: Metabolically Driven Vulnerability
Cellular senescence is canonically defined as a state of irreversible cell cycle arrest, typically triggered by DNA damage, telomere attrition, oncogenic signaling, or oxidative stress (López-Otín & Kroemer, 2024; Wiley & Campisi, 2021). Senescent cells undergo distinctive metabolic remodeling and adopt a SASP that promotes chronic inflammation, tissue remodeling, and age-related pathology. Importantly, senescence is not merely a loss of proliferative capacity—it reflects a hyperfunctional, pro-inflammatory state with substantial bioenergetic demands.
In the context of ERM, chronic metabolic stress, including mitochondrial dysfunction, redox imbalance, and AMP/ATP depletion, may not directly induce senescence but may predispose cells to enter or maintain the senescent state. Metabolic factors such as NAD+/NADH ratios, AMPK activation, and ROS signaling are known to modulate the SASP and influence the inflammatory output of senescent cells (Wiley & Campisi, 2021). These shifts may determine whether a stressed cell proceeds toward full senescence or can recover from metabolic arrest. However, once the senescent state is established, it is generally considered irreversible, and metabolic modulation—though it may attenuate the SASP—is unlikely to restore proliferative capacity (Blagosklonny, 2011).
Rather than causing senescence directly, ERM may induce transient, non-proliferative states driven by nutrient deprivation, ISR activation, and translational repression (Costa-Mattioli & Walter, 2020; Wek, 2018). These metabolically adaptive responses—such as quiescence or ISR-induced dormancy—are distinct from senescence in that they retain the potential for recovery. However, persistent stress or failure to resolve the bioenergetic deficit may result in loss of proliferative potential and transition into true senescence. ERM may initially present as a reversible, stress-adaptive state such as cellular quiescence or ISR-induced dormancy. However, once cells undergo irreversible senescence, reversing ERM is unlikely to restore regenerative or proliferative function, and thus cannot reverse established aging-related decline.
5.4. Stem Cell Exhaustion → Resource-Dependent Dormancy
Stem cell exhaustion is a hallmark of aging, marked by reduced self-renewal, differentiation, and tissue maintenance across systems such as muscle, immune, and hematopoietic lineages (López-Otín & Kroemer, 2021). This decline arises from accumulated genomic damage, disrupted niche signaling, and chronic inflammatory environments, contributing to impaired regeneration and frailty.
However, not all stem cell dysfunction stems from irreversible depletion. Under prolonged stress or energy scarcity, stem cells can enter a quiescent state—a protective dormancy that suppresses proliferation to conserve energy and reduce damage risk (Cheung & Rando, 2013; Ryall et al., 2015). This response is particularly relevant in ERM, where inflammation, mitochondrial stress, and downregulated mTORC1/IGF-1 signaling create a hostile metabolic environment.
In this context, stem cells may remain metabolically silenced rather than lost—preserved in dormancy until conditions improve. Restoration of nutrient flow, redox balance, and growth signaling can reactivate regenerative function. This concept of resource-dependent dormancy positions ERM as a reversible stage within the trajectory of stem cell exhaustion.
5.5. Bioenergetic Inadequacies: A Hidden Cost of Chronic Adaptation
Daily adaptation to internal and external exposomes—ranging from chronic inflammation and environmental toxicants to psychological stress and circadian disruption—sustains a state of low-grade metabolic activation. This persistent activation places ongoing demands not only on macronutrient reserves, but also on micronutrients essential for enzymatic activity, redox homeostasis, and mitochondrial function (Alack et al., 2019; Raiten et al., 2015). Over time, this sustained pressure may result in subtle but cumulative bioenergetic inadequacy, where nutrient availability fails to keep pace with the prolonged demands of adaptation and repair.
Population-based studies reveal widespread nutrient insufficiencies, particularly among older adults, individuals with chronic illnesses, and those exposed to sustained psychosocial or environmental stressors (Kiani et al., 2022). While overt deficiency syndromes are relatively rare in developed countries, chronic functional depletion and physiological misallocation of essential micronutrients—such as magnesium, zinc, selenium, B vitamins, iron, and vitamin D—are common and frequently underrecognized (Bailey et al., 2015; Han et al., 2022; Wessells & Brown, 2012).
Macronutrients provide the foundational substrates for energy production, protein synthesis, and membrane integrity, while micronutrients serve as catalytic cofactors, coenzymes, and regulators across all domains of cellular metabolism. Under chronic stress, however, a mismatch emerges between rising metabolic demands and the body’s ability to supply and allocate resources. Stress-induced neuroendocrine signals also favor substrate storage and active acute-phase responses while suppressing longer-term regenerative and anabolic functions. This substrate trapping creates a hidden trade-off: nutrients may be present, but are rendered functionally unavailable to systems responsible for repair and resilience (Friedman et al., 2024).
This stress-induced reallocation reflects Bruce Ames’ nutrient triage hypothesis, which proposes that when resources are limited, the body prioritizes short-term survival at the expense of long-term maintenance (Ames, 2006). Even modest degree of nutrient insufficiencies—if chronically diverted—can undermine tissue repair, immune regulation, and mitochondrial integrity, contributing to the emergence of ERM as a systemic energy-constrained state of maladaptive adaptation.
These nutrient-based trade-offs do not operate in isolation; rather, they converge with the core hallmarks of aging—fueling mitochondrial dysfunction, impairing nutrient-sensing pathways, and disrupting regenerative signaling. By undermining the biochemical foundations of repair and resolution, chronic bioenergetic inadequacy accelerates the transition from adaptive stress response to maladaptive exhaustion. In this light, ERM may be understood as a nutrient-sensitive phenotype of early aging—subclinical yet mechanistically aligned with canonical hallmarks, and potentially reversible if identified and addressed in time.
5.6. From Irreversibility to Intervention: ERM as a Critical Inflection Point
By mapping the functional features of ERM onto canonical hallmarks of aging, this framework reconceptualizes biological aging not as an inevitable outcome of time, but as an accumulation of unresolved adaptation. ERM represents a critical inflection point—where physiological strain has begun to compromise resilience, yet functional systems remain responsive to intervention.
ERM thus reframes aging as a dynamic consequence of adaptation failure (Ryan & Ryznar, 2022). Many functional changes observed in ERM—such as suppressed IGF-1 signaling or mitochondrial stress—are not necessarily irreversible (Picard & Shirihai, 2022; Wang & Zhang, 2025). With timely intervention, biological aging trajectories may be interrupted (Calabrese & Mattson, 2024; Shaulson et al., 2024).
6. Translational and Clinical Relevance: Reframing Healthspan Through Adaptive Recovery
ERM challenges the traditional view of aging as a passive, time-driven decline. Instead, it reframes many hallmarks of aging—such as mitochondrial dysfunction, anabolic resistance, and immune dysregulation—as the downstream effects of unresolved physiological adaptation. ERM represents a chronic shift in bioenergetic priorities, where sustained stress redirects substrates away from maintenance and repair toward immediate survival, progressively compromising immune integrity, tissue regeneration, and neuroendocrine balance (Martel et al., 2024; Yiallouris et al., 2019).
6.1. A Window for Preventive Intervention
Despite its clinical relevance, ERM often remains invisible to conventional diagnostics. Individuals may fall within normal ranges for weight and laboratory values, yet present with signs of declining resilience—fatigue, brain fog, chronic pain, impaired recovery, immune susceptibility, and anabolic resistance. These early indicators often precede structural decline and mirror preclinical frailty (Arron et al., 2024; Walrand et al., 2021).
Japan’s national health model illustrates how early-stage metabolic compromise can be identified and mitigated. Since 2008, routine metabolic syndrome screening has included waist circumference—highlighting central adiposity as an early marker of energy misallocation driven by chronic glucose, insulin, and cortisol exposure. This simple anthropometric indicator reflects a core feature of ERM: substrate accumulation in visceral fat at the expense of lean tissue and repair capacity. Japan’s model, which integrates personalized lifestyle guidance into workplace and community health systems, has led to measurable improvements in metabolic outcomes (Shirai & Tsushita, 2024).
While the Japanese model provides a particularly integrated example, it is not the only cultural tradition supporting resilience and healthy aging. The Mediterranean lifestyle—characterized by plant-based dietary patterns, social meal rituals, and moderate physical activity—has also been associated with reduced allostatic load and improved metabolic health. These traditions, though geographically distinct, share key features: nutrient density, circadian-aligned behaviors, and community integration—each contributing to sustained adaptive capacity across the lifespan.
This proactive framework aligns with the clinical potential of ERM. By recognizing functional symptoms and biomarker trade-offs—before irreversible damage occurs—interventions can be deployed to restore bioenergetic availability, reactivate regenerative pathways, and preserve long-term physiological resilience.
6.2. Strategies to Restore Metabolic Governance
Because ERM stems from chronic resource misallocation and maladaptive stress responses, recovery requires a systems-level approach targeting both upstream causes and downstream consequences.
-
Lifestyle-Circadian Synchronization
Synchronizing daily behaviors with circadian rhythms—through structured sleep, timed meals, and light exposure—plays a vital role in restoring metabolic tempo, optimizing insulin sensitivity, and recalibrating neuroendocrine function (Ryan & Ryznar, 2022; Shaulson et al., 2024; Tippairote et al., 2021). Central to this process is the alignment of the cortisol rhythm, a core output of the circadian system that governs energy mobilization, immune modulation, and stress response. Disruptions to this rhythm—whether through irregular sleep patterns, nighttime eating, or insufficient light exposure—can desynchronize the HPA axis, promoting metabolic dysfunction.
Traditional Japanese lifestyle patterns, such as early dinners, seasonal meal timing, and exposure to natural light, inherently support circadian alignment. These culturally embedded practices may contribute to Japan’s exceptional longevity by sustaining hormonal rhythms and reducing bioenergetic strain (Shirai & Tsushita, 2024).
-
Mitochondrial and Nutritional Support for Resilience
Mitochondrial resilience and nutritional sufficiency are tightly intertwined. Essential cofactors—magnesium, zinc, selenium, B vitamins, alpha-lipoic acid—are critical for mitochondrial redox balance and energy production. Subclinical deficiencies often manifest as low alkaline phosphatase, elevated homocysteine, or reduced transport proteins (e.g., prealbumin, transferrin), signaling the need for targeted repletion and consistent protein intake (Beck & Rosenthal, 2002; Ray et al., 2017).
Complementary mitohormetic stimuli—such as moderate exercise, thermal stress, and intermittent fasting—enhance mitochondrial biogenesis and adaptive signaling. These effects are reinforced by polyphenols like quercetin and epigallocatechin gallate (EGCG), which reduce senescent cell burden and promote tissue repair (Martel et al., 2024; Ristow & Schmeisser, 2014).
Japan’s traditional diet offers a real-world validation: nutrient-dense, anti-inflammatory, and phytonutrient-rich, it supports immune competence, muscle integrity, and systemic recovery. This dietary pattern has been associated with reduced chronic disease burden and sustained physical and cognitive function into advanced age (Li et al., 2024; Shirai & Tsushita, 2024).
Improving dietary quality is a critical foundation in addressing ERM. Strategies such as reducing refined carbohydrate and sugar intake may help modulate insulin and stress hormone dynamics, while adequate protein intake supports tissue repair and regeneration. Repletion of specific micronutrients—particularly vitamin D, a key regulator of counter-regulatory immune responses—is essential to reduce the risk of sustained inflammation and impaired resolution in individuals with high metabolic demand.
However, nutritional repletion alone may be insufficient in the presence of unresolved stress, inflammation, or toxicant exposure, which can impair nutrient utilization and reinforce maladaptive metabolic states. Therefore, dietary strategies should be implemented as part of a broader integrative approach. Ultimately, successful reversal of ERM requires not just the availability of nutrients, but the physiological capacity to utilize them—highlighting the importance of synchronized, resilience-informed interventions.
-
Neuroendocrine and Stress Axis Modulation
The capacity to dynamically regulate stress through the HPA and SAM axes is essential for survival and healthy aging. In ERM, chronic overactivation of these axes leads to persistent catabolism, inflammation, and autonomic imbalance.
Mind-body interventions—such as mindfulness-based stress reduction (MBSR), yoga, and vagal breathing—help restore parasympathetic tone, lower cortisol output, and improve neuroendocrine flexibility (Chrousos, 2009; Srour & Keyes, 2025). These practices support systemic recovery and energy conservation.
Cultural constructs also matter. In Japan, ikigai—a sense of meaning and purpose—has been linked to lower allostatic load and longer lifespan, highlighting the role of psychosocial integration in sustaining adaptive capacity (Shirai & Tsushita, 2024).
Targeting these neuroendocrine hubs—where emotion, metabolism, and inflammation intersect—can help reverse the maladaptive cycle of ERM and reestablish the physiological adaptability required for resilience.
6.3. Toward Resilience-Informed Healthspan Strategies
ERM reframes healthcare goals—from treating disease to preserving adaptive potential. A resilience-informed approach emphasizes:
Monitoring of functional reserve
Recognition of dynamic biomarker constellations rather than reliance on static thresholds
Restoration of energy availability and systemic plasticity
This model is not aspirational—it is already being implemented. Japan’s Health Promotion Law, Smart Life Project, and community-based integrated care prioritize functional maintenance and social participation. These policies have contributed to Japan’s status as a global leader in healthy life expectancy (Shirai & Tsushita, 2024).
ERM provides a practical framework to apply similar principles in clinical settings. By identifying reversible patterns of depletion and intervening before overt disease, we can extend healthspan, delay frailty, and support aging as a process of sustained adaptation—not inevitable decline.
7. Conclusions
Aging is not merely the passive accumulation of molecular damage over time—it is increasingly recognized as the cumulative cost of chronic adaptation under conditions of bioenergetic constraint. This review introduces ERM as a mechanistically grounded and clinically actionable phenotype of early maladaptation. ERM reflects a persistent state of stress-induced substrate misallocation, manifesting as a distinct constellation of metabolic trade-offs and progressive physiological inefficiency. Unlike classical forms of malnutrition, ERM does not result from overt nutrient deprivation but emerges from chronic bioenergetic compromise that prioritizes short-term survival at the expense of long-term maintenance and repair.
By aligning ERM with the established hallmarks of aging, this framework positions ERM as a potentially reversible driver of biological aging. It provides a new lens through which to detect early functional decline and metabolic fragility, often preceding the clinical onset of frailty, immune dysfunction, or sarcopenia.
To translate this concept into clinical and research practice, a phased and multidisciplinary strategy is essential:
A systematic review, registered with PROSPERO (ID: CRD420251033154), is currently underway to consolidate evidence across stress physiology, metabolic adaptation, and malnutrition domains. This review aims to identify converging biomarker constellations and physiological trade-offs that define ERM and support the development of pattern-based recognition criteria.
In parallel, a retrospective analysis of clinical data is in progress. This study will identify real-world ERM phenotypes, validate functional and biomarker signatures, and inform the development of a robust ERM staging model.
With phenotype recognition and staging in place, intervention trials can be designed to target the mechanisms underpinning ERM. These may include strategies to restore mitochondrial function, rebalance micronutrient and amino acid status, synchronize circadian and neuroendocrine rhythms, and promote recovery following sustained stress exposure.
Together, these research phases aim to establish ERM as a measurable, pattern-defined, and clinically meaningful constellation—a modifiable inflection point along the continuum of aging and chronic disease.
Reframing healthspan through the lens of adaptive recovery and metabolic flexibility offers a compelling new direction for preventive aging care. By detecting ERM in its earliest stages—and restoring the physiological conditions necessary for repair, regeneration, and energy balance—we may preserve systemic resilience, prolong functional independence, and intervene before chronic dysfunction becomes entrenched or irreversible.
Funding
The authors received no financial support from any organization for the submitted work.
List of Abbreviations
| Abbreviation |
FullTerm
|
| AMPK |
AMP-activated Protein Kinase |
| ATF4 |
Activating Transcription Factor 4 |
| ATP |
Adenosine Triphosphate |
| BEC |
Brain–Body Energy Conservation |
| BIA |
Bioelectrical Impedance Analysis |
| BMI |
Body Mass Index |
| cGAS–STING |
cyclic GMP–AMP synthase–stimulator of interferon genes |
| CED |
Chronic Energy Deficiency |
| CHOP |
C/EBP Homologous Protein |
| CRP |
C-Reactive Protein |
| DHEA |
Dehydroepiandrosterone |
| DRM |
Disease-Related Malnutrition |
| eIF2α |
eukaryotic Initiation Factor 2 Alpha |
| ERM |
Exposure-Related Malnutrition |
| FGF21 |
Fibroblast Growth Factor 21 |
| GDF15 |
Growth Differentiation Factor 15 |
| GLIM |
Global Leadership Initiative on Malnutrition |
| HPA |
Hypothalamic–Pituitary–Adrenal (axis) |
| IGF-1 |
Insulin-like Growth Factor 1 |
| ISR |
Integrated Stress Response |
| MBSR |
Mindfulness-Based Stress Reduction |
| mTORC1 |
mechanistic Target of Rapamycin Complex 1 |
| mt-ISR |
Mitochondrial Integrated Stress Response |
| mtDNA |
Mitochondrial DNA |
| MPS |
Muscle Protein Synthesis |
| NLRP3 |
NOD-like receptor family pyrin domain-containing 3 |
| PhA |
Phase Angle |
| RED-S |
Relative Energy Deficiency in Sport |
| ROS |
Reactive Oxygen Species |
| SAM |
Sympathetic–Adrenal–Medullary |
| SASP |
Senescence-Associated Secretory Phenotype |
| SCENITH |
Single-Cell Energetic metabolism by Translation Inhibition |
| scRNA-seq |
single-cell RNA sequencing |
| UPR |
Unfolded Protein Response |
Authors’ contributions
TT designed and supervised the study. PH, AS assisted in data collection. PT contributed to data analysis and drafted the manuscript. All authors reviewed and approved the final manuscript.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We appreciated the continuing support of the team of nurses and supporting staff in the Nutritional and Environment Medicine department, HP Medical Center, on this paper.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
Consent for Publication
Written consent for publication from all authors involved in this study is available
upon request.
Code availability
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used Elicit’s Notebooks to locate relevant papers, refine research questions, and Chat GPT for paraphrasing sentences. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
References
- Akamatsu, Y., Kusakabe, T., Arai, H., Yamamoto, Y., Nakao, K., Ikeue, K.,…Satoh-Asahara, N. (2022). Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J Cachexia Sarcopenia Muscle, 13(1), 180-189. [CrossRef]
- Alack, K., Pilat, C., & Krüger K*Shared, a. (2019). Current Knowledge and New Challenges in Exercise Immunology. Deutsche Zeitschrift für Sportmedizin, Volume 70(No. 10), 250-260. [CrossRef]
- Ames, B. N. (2006). Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A, 103(47), 17589-17594. [CrossRef]
- Arron, H. E., Marsh, B. D., Kell, D. B., Khan, M. A., Jaeger, B. R., & Pretorius, E. (2024). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: the biology of a neglected disease. Front Immunol, 15, 1386607. [CrossRef]
- Bailey, R. L., West, K. P., Jr., & Black, R. E. (2015). The epidemiology of global micronutrient deficiencies. Ann Nutr Metab, 66 Suppl 2, 22-33. [CrossRef]
- Beck, F. K., & Rosenthal, T. C. (2002). Prealbumin: a marker for nutritional evaluation. Am Fam Physician, 65(8), 1575-1578. https://www.aafp.org/afp/2002/0415/p1575.pdf.
- Bian, A., Ma, Y., Zhou, X., Guo, Y., Wang, W., Zhang, Y., & Wang, X. (2020). Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet Disord, 21(1), 214. [CrossRef]
- Blagosklonny, M. V. (2011). Cell cycle arrest is not senescence. Aging (Albany NY), 3(2), 94-101. [CrossRef]
- Bobba-Alves, N., Sturm, G., Lin, J., Ware, S. A., Karan, K. R., Monzel, A. S.,…Picard, M. (2023). Cellular allostatic load is linked to increased energy expenditure and accelerated biological aging. Psychoneuroendocrinology, 155, 106322. [CrossRef]
- Brzezniakiewicz-Janus, K., Jarczak, J., Konopko, A., Ratajczak, J., Kucia, M., & Ratajczak, M. Z. (2025). Mitochondria Express Functional Signaling Ligand-Binding Receptors that Regulate their Biological Responses – the Novel Role of Mitochondria as Stress-Response Sentinels. Stem Cell Reviews and Reports. [CrossRef]
- Cabre, H. E., Moore, S. R., Smith-Ryan, A. E., & Hackney, A. C. (2022). Relative Energy Deficiency in Sport (RED-S): Scientific, Clinical, and Practical Implications for the Female Athlete. Dtsch Z Sportmed, 73(7), 225-234. [CrossRef]
- Cahill, G. F., Jr. (2006). Fuel metabolism in starvation. Annu Rev Nutr, 26, 1-22. [CrossRef]
- Calabrese, E. J., & Mattson, M. P. (2024). The catabolic - anabolic cycling hormesis model of health and resilience. Ageing Res Rev, 102, 102588. [CrossRef]
- Calabrese, E. J., Nascarella, M., Pressman, P., Hayes, A. W., Dhawan, G., Kapoor, R.,…Agathokleous, E. (2024). Hormesis determines lifespan. Ageing Res Rev, 94, 102181. [CrossRef]
- Cederholm, T., & Bosaeus, I. (2024). Malnutrition in Adults. New England Journal of Medicine, 391(2), 155-165. [CrossRef]
- Cederholm, T., Jensen, G. L., Correia, M. I. T. D., Gonzalez, M. C., Fukushima, R., Pisprasert, V.,…Compher, C. (2025). The GLIM consensus approach to diagnosis of malnutrition: A 5-year update. Clin Nutr, 49, 11-20. [CrossRef]
- Cheung, T. H., & Rando, T. A. (2013). Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol, 14(6), 329-340. [CrossRef]
- Chrousos, G. P. (2009). Stress and disorders of the stress system. Nat Rev Endocrinol, 5(7), 374-381. [CrossRef]
- Coakley, A. J., Hruby, A. J., Wang, J., Bong, A., Nair, T., Ramos, C. M.,…Higuchi-Sanabria, R. (2024). Distinct mechanisms of non-autonomous UPR(ER) mediated by GABAergic, glutamatergic, and octopaminergic neurons. bioRxiv. [CrossRef]
- Coppé, J. P., Patil, C. K., Rodier, F., Sun, Y., Muñoz, D. P., Goldstein, J.,…Campisi, J. (2008). Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol, 6(12), 2853-2868. [CrossRef]
- Costa-Mattioli, M., & Walter, P. (2020). The integrated stress response: From mechanism to disease. Science, 368(6489), eaat5314. [CrossRef]
- Crane, P. A., Wilkinson, G., & Teare, H. (2022). Healthspan versus lifespan: new medicines to close the gap. Nature Aging, 2(11), 984-988. [CrossRef]
- Demirovic, D., & Rattan, S. I. S. (2013). Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Experimental gerontology, 48, 94-98.
- Diaz-Meco, M. T., Linares, J. F., & Moscat, J. (2025). Hijacking the powerhouse: Mitochondrial transfer and mitophagy as emerging mechanisms of immune evasion. Molecular Cell, 85(7), 1258-1259. [CrossRef]
- Dong, L.-F., Rohlena, J., Zobalova, R., Nahacka, Z., Rodriguez, A.-M., Berridge, M. V., & Neuzil, J. (2023). Mitochondria on the move: Horizontal mitochondrial transfer in disease and health. Journal of Cell Biology, 222(3). [CrossRef]
- Evans, D. C., Corkins, M. R., Malone, A., Miller, S., Mogensen, K. M., Guenter, P.,…Committee, A. M. (2021). The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr Clin Pract, 36(1), 22-28. [CrossRef]
- Ferreira, R. P., & Duarte, J. A. (2023). Protein Turnover in Skeletal Muscle: Looking at Molecular Regulation towards an Active Lifestyle. Int J Sports Med, 44(11), 763-777. [CrossRef]
- Foy, B. H., Petherbridge, R., Roth, M. T., Zhang, C., De Souza, D. C., Mow, C.,…Higgins, J. M. (2024). Haematological setpoints are a stable and patient-specific deep phenotype. Nature. [CrossRef]
- Franceschi, C., Garagnani, P., Parini, P., Giuliani, C., & Santoro, A. (2018). Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology, 14(10), 576-590. [CrossRef]
- Friedman, M. I., Sørensen, T. I. A., Taubes, G., Lund, J., & Ludwig, D. S. (2024). Trapped fat: Obesity pathogenesis as an intrinsic disorder in metabolic fuel partitioning. Obesity Reviews, n/a(n/a). [CrossRef]
- Fülöp, B., Borbély, É., & Helyes, Z. (2025). How does chronic psychosocial distress induce pain? Focus on neuroinflammation and neuroplasticity changes. Brain, Behavior, & Immunity - Health, 44, 100964. [CrossRef]
- Fulop, T., Larbi, A., Dupuis, G., Le Page, A., Frost, E. H., Cohen, A. A.,…Franceschi, C. (2018). Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? [Review]. Front Immunol, 8(1960). [CrossRef]
- Furrer, R., & Handschin, C. (2025a). Biomarkers of aging: from molecules and surrogates to physiology and function. Physiol Rev, 105(3), 1609-1694. [CrossRef]
- Furrer, R., & Handschin, C. (2025b). Biomarkers of aging: functional aspects still trump molecular parameters. npj Aging, 11(1), 15. [CrossRef]
- Gulhar, R., Ashraf, M. A., & Jialal, I. (2024). Physiology, Acute Phase Reactants. In StatPearls. StatPearls Publishing.
- Han, X., Ding, S., Lu, J., & Li, Y. (2022). Global, regional, and national burdens of common micronutrient deficiencies from 1990 to 2019: A secondary trend analysis based on the Global Burden of Disease 2019 study. EClinicalMedicine, 44. [CrossRef]
- Harrell, C. S., Gillespie, C. F., & Neigh, G. N. (2016). Energetic stress: The reciprocal relationship between energy availability and the stress response. Physiol Behav, 166, 43-55. [CrossRef]
- Hetz, C., & Papa, F. R. (2018). The Unfolded Protein Response and Cell Fate Control. Molecular Cell, 69(2), 169-181. [CrossRef]
- Hetz, C., & Saxena, S. (2017). ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol, 13(8), 477-491. [CrossRef]
- Higuchi-Sanabria, R., Durieux, J., Kelet, N., Homentcovschi, S., de Los Rios Rogers, M., Monshietehadi, S.,…Dillin, A. (2020). Divergent Nodes of Non-autonomous UPR(ER) Signaling through Serotonergic and Dopaminergic Neurons. Cell Rep, 33(10), 108489. [CrossRef]
- Ikeue, K., Kato, H., Tanaka, M., Yamakage, H., Kato, S., Iwasa, M.,…Satoh-Asahara, N. (2025). Phase Angle Is a Potential Novel Early Marker for Sarcopenia and Cognitive Impairment in the General Population. J Cachexia Sarcopenia Muscle, 16(3), e13820. [CrossRef]
- Islam, M. N., Das, S. R., Emin, M. T., Wei, M., Sun, L., Westphalen, K.,…Bhattacharya, J. (2012). Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med, 18(5), 759-765. [CrossRef]
- Kiani, A. K., Dhuli, K., Donato, K., Aquilanti, B., Velluti, V., Matera, G.,…Bertelli, M. (2022). Main nutritional deficiencies. Journal of preventive medicine and hygiene, 63(2 Suppl 3), E93-e101. [CrossRef]
- Kivimäki, M., Bartolomucci, A., & Kawachi, I. (2023). The multiple roles of life stress in metabolic disorders. Nature Reviews Endocrinology, 19(1), 10-27. [CrossRef]
- Langston, P. K., & Mathis, D. (2024). Immunological regulation of skeletal muscle adaptation to exercise. Cell Metab. [CrossRef]
- Li, Y., Wang, K., Jigeer, G., Jensen, G., Tucker, K. L., Lv, Y.,…Gao, X. (2024). Healthy Lifestyle and the Likelihood of Becoming a Centenarian. JAMA Network Open, 7(6), e2417931-e2417931. [CrossRef]
- Lockhart, S. M., Saudek, V., & O’Rahilly, S. (2020). GDF15: A Hormone Conveying Somatic Distress to the Brain. Endocr Rev, 41(4). [CrossRef]
- Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194-1217. [CrossRef]
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2023). Hallmarks of aging: An expanding universe. Cell. [CrossRef]
- López-Otín, C., & Kroemer, G. (2021). Hallmarks of Health. Cell, 184(1), 33-63. [CrossRef]
- López-Otín, C., & Kroemer, G. (2024). The missing hallmark of health: psychosocial adaptation. Cell Stress, 8, 21-50. [CrossRef]
- Martel, J., Ojcius, D. M., & Young, J. D. (2024). Lifestyle interventions to delay senescence. Biomedical Journal, 47(2), 100676. [CrossRef]
- Martini, H., & Passos, J. F. (2023). Cellular senescence: all roads lead to mitochondria. The FEBS Journal, 290(5), 1186-1202. [CrossRef]
- McEwen, B. S. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev, 87(3), 873-904. [CrossRef]
- McEwen, B. S., & Wingfield, J. C. (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior, 43(1), 2-15. [CrossRef]
- McEwen, C. A. (2022). Connecting the biology of stress, allostatic load and epigenetics to social structures and processes. Neurobiol Stress, 17, 100426. [CrossRef]
- Monzel, A. S., Levin, M., & Picard, M. (2023). The energetics of cellular life transitions. Life Metabolism, 3(3). [CrossRef]
- Mountjoy, M., Sundgot-Borgen, J. K., Burke, L. M., Ackerman, K. E., Blauwet, C., Constantini, N.,…Budgett, R. (2018). IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br J Sports Med, 52(11), 687-697. [CrossRef]
- Muscaritoli, M., Imbimbo, G., Jager-Wittenaar, H., Cederholm, T., Rothenberg, E., di Girolamo, F. G.,…Molfino, A. (2023). Disease-related malnutrition with inflammation and cachexia. Clin Nutr, 42(8), 1475-1479. [CrossRef]
- National Academies of Sciences, E., & Medicine. (2024). Toward a Common Research Agenda in Infection-Associated Chronic Illnesses: Proceedings of a Workshop. The National Academies Press. [CrossRef]
- Ni, H.-M., Williams, J. A., & Ding, W.-X. (2015). Mitochondrial dynamics and mitochondrial quality control. Redox Biology, 4, 6-13. [CrossRef]
- Norman, K., Stobäus, N., Pirlich, M., & Bosy-Westphal, A. (2012). Bioelectrical phase angle and impedance vector analysis – Clinical relevance and applicability of impedance parameters. Clin Nutr, 31(6), 854-861. [CrossRef]
- Olenchock, B. A., Rathmell, J. C., & Vander Heiden, M. G. (2017). Biochemical Underpinnings of Immune Cell Metabolic Phenotypes. Immunity, 46(5), 703-713. [CrossRef]
- Pakos-Zebrucka, K., Koryga, I., Mnich, K., Ljujic, M., Samali, A., & Gorman, A. M. (2016). The integrated stress response. EMBO reports, 17(10), 1374-1395. [CrossRef]
- Paulussen, K. J. M., McKenna, C. F., Beals, J. W., Wilund, K. R., Salvador, A. F., & Burd, N. A. (2021). Anabolic Resistance of Muscle Protein Turnover Comes in Various Shapes and Sizes. Front Nutr, 8, 615849. [CrossRef]
- Payea, M. J., Dar, S. A., Anerillas, C., Martindale, J. L., Belair, C., Munk, R.,…Maragkakis, M. (2024). Senescence suppresses the integrated stress response and activates a stress-remodeled secretory phenotype. Molecular Cell, 84(22), 4454-4469.e4457. [CrossRef]
- Peters, A. (2004). The selfish brain: competition for energy resources. American Journal of Human Biology, 23(1), 29-34. [CrossRef]
- Peters, A., Sprengell, M., & Kubera, B. (2022). The principle of ‘brain energy on demand’ and its predictive power for stress, sleep, stroke, obesity and diabetes. Neuroscience & Biobehavioral Reviews, 141, 104847. [CrossRef]
- Picard, M., McEwen, B., Epel, E., & Sandi, C. (2018). An Energetic View of Stress: Focus on Mitochondria. Front Neuroendocrinol, 49, 72-85. [CrossRef]
- Picard, M., & Shirihai, O. S. (2022). Mitochondrial signal transduction. Cell Metab, 34(11), 1620-1653. [CrossRef]
- Prisabela, M., Nadhiroh, S. R., & Isaura, E. R. (2023). Characteristics of Pregnant Woman with Chronic Energy Deficiency in Puskesmas Gesang, Lumajang on 2020: Descriptive Analysis. Media Gizi Kesmas, 12(2), 643-648. [CrossRef]
- Qi, Y., Rajbanshi, B., Hao, R., Dang, Y., Xu, C., Lu, W.,…Zhang, X. (2025). The dual role of PGAM5 in inflammation. Exp Mol Med, 57(2), 298-311. [CrossRef]
- Raiten, D. J., Sakr Ashour, F. A., Ross, A. C., Meydani, S. N., Dawson, H. D., Stephensen, C. B.,…van Ommen, B. (2015). Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J Nutr, 145(5), 1039s-1108s. [CrossRef]
- Rappaport, S. M., Barupal, D. K., Wishart, D., Vineis, P., & Scalbert, A. (2014). The blood exposome and its role in discovering causes of disease. Environ Health Perspect, 122(8), 769-774. [CrossRef]
- Rattan, S. I. S. (2020). Homeostasis, Homeodynamics and Aging. In S. I. S. Rattan (Ed.), Encyclopedia of Biomedical Gerontology (pp. 238-241). Academic Press. [CrossRef]
- Ray, C. S., Singh, B., Jena, I., Behera, S., & Ray, S. (2017). Low Alkaline Phosphatase (ALP) In Adult Population an Indicator of Zinc (Zn) and Magnesium (Mg) Deficiency. Current Research in Nutrition and Food Science Journal, 5, 347-352.
- Ristow, M., & Schmeisser, K. (2014). Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS). Dose-Response, 12(2), dose-response.13-035.Ristow. [CrossRef]
- Ryall, J. G., Cliff, T., Dalton, S., & Sartorelli, V. (2015). Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell, 17(6), 651-662. [CrossRef]
- Ryan, M., & Ryznar, R. (2022). The Molecular Basis of Resilience: A Narrative Review [Review]. Frontiers in Psychiatry, 13. [CrossRef]
- Sapolsky, R. M. (2004). Why zebras don’t get ulcers: The acclaimed guide to stress, stress-related diseases, and coping (3rd ed.). Henry Holt.
- Schmauck-Medina, T., Molière, A., Lautrup, S., Zhang, J., Chlopicki, S., Madsen, H. B.,…Fang, E. F. (2022). New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging (Albany NY). [CrossRef]
- Seeman, T. E., McEwen, B. S., Rowe, J. W., & Singer, B. H. (2001). Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A, 98(8), 4770-4775. [CrossRef]
- Selye, H. (1950). Stress and the General Adaptation Syndrome. British Medical Journal, 1(4667), 1383-1392. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2038162/.
- Sganga, G., Siegel, J. H., Brown, G., Coleman, B., Wiles, C. E., III, Belzberg, H.,…Placko, R. (1985). Reprioritization of Hepatic Plasma Protein Release in Trauma and Sepsis. Archives of Surgery, 120(2), 187-199. [CrossRef]
- Shaulson, E. D., Cohen, A. A., & Picard, M. (2024). The brain–body energy conservation model of aging. Nature Aging, 4(10), 1354-1371. [CrossRef]
- Shirai, T., & Tsushita, K. (2024). Lifestyle Medicine and Japan’s Longevity Miracle. American Journal of Lifestyle Medicine, 18(4), 598-607. [CrossRef]
- Spees, J. L., Olson, S. D., Whitney, M. J., & Prockop, D. J. (2006). Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A, 103(5), 1283-1288. [CrossRef]
- Srour, R. A., & Keyes, D. (2025). Lifestyle Mindfulness In Clinical Practice. In StatPearls. StatPearls Publishing.
- Copyright © 2025, StatPearls Publishing LLC.
- Taylor, R. C., & Dillin, A. (2013). XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell, 153(7), 1435-1447. [CrossRef]
- Tippairote, T., Bjørklund, G., & Yaovapak, A. (2021). The continuum of disrupted metabolic tempo, mitochondrial substrate congestion, and metabolic gridlock toward the development of non-communicable diseases. Critical Reviews in Food Science and Nutrition. [CrossRef]
- Tippairote, T., Hoonkaew, P., Suksawang, A., & Tippairote, P. (2025). Exploring the Association Between Low-Level Mercury Exposure, Gut Barrier Dysfunction, and Parkinson’s Disease: A Case Series of Three Thai Patients. SN Comprehensive Clinical Medicine, 7(1), 70. [CrossRef]
- Tsigos, C., & Chrousos, G. P. (2002). Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53(4), 865-871. [CrossRef]
- Vermeulen, R., Schymanski, E. L., Barabási, A. L., & Miller, G. W. (2020). The exposome and health: Where chemistry meets biology. Science, 367(6476), 392-396. [CrossRef]
- Victorelli, S., Salmonowicz, H., Chapman, J., Martini, H., Vizioli, M. G., Riley, J. S.,…Passos, J. F. (2023). Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature, 622(7983), 627-636. [CrossRef]
- Walrand, S., Guillet, C., & Boirie, Y. (2021). Nutrition, Protein Turnover and Muscle Mass. In Sarcopenia (pp. 45-62). [CrossRef]
- Wang, X., & Zhang, G. (2025). The mitochondrial integrated stress response: A novel approach to anti-aging and pro-longevity. Ageing Res Rev, 103, 102603. [CrossRef]
- Wek, R. C. (2018). Role of eIF2α Kinases in Translational Control and Adaptation to Cellular Stress. Cold Spring Harb Perspect Biol, 10(7). [CrossRef]
- Wessells, K. R., & Brown, K. H. (2012). Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One, 7(11), e50568. [CrossRef]
- WHO. (2025). Noncommunicable diseases. WHO. Retrieved March 25 from https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1.
- Wiley, C. D., & Campisi, J. (2021). The metabolic roots of senescence: mechanisms and opportunities for intervention. Nature Metabolism, 3(10), 1290-1301. [CrossRef]
- Willmann, K., & Moita, L. F. (2024). Physiologic disruption and metabolic reprogramming in infection and sepsis. Cell Metab. [CrossRef]
- Wu, F., Mu, W.-C., Markov, N. T., Fuentealba, M., Halaweh, H., Senchyna, F.,…Furman, D. (2025). Immunological biomarkers of aging. The Journal of Immunology, 214(5), 889-902. [CrossRef]
- Wu, Z., Qu, J., Zhang, W., & Liu, G.-H. (2024). Stress, epigenetics, and aging: Unraveling the intricate crosstalk. Molecular Cell, 84(1), 34-54. [CrossRef]
- Yiallouris, A., Tsioutis, C., Agapidaki, E., Zafeiri, M., Agouridis, A. P., Ntourakis, D., & Johnson, E. O. (2019). Adrenal Aging and Its Implications on Stress Responsiveness in Humans [Review]. Frontiers in Endocrinology, 10(54). [CrossRef]
- Zhang, B., Chang, J. Y., Lee, M. H., Ju, S.-H., Yi, H.-S., & Shong, M. (2024). Mitochondrial Stress and Mitokines: Therapeutic Perspectives for the Treatment of Metabolic Diseases. Diabetes Metab J, 48(1), 1-18. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).