Submitted:
10 April 2025
Posted:
11 April 2025
You are already at the latest version
Abstract
Keywords:
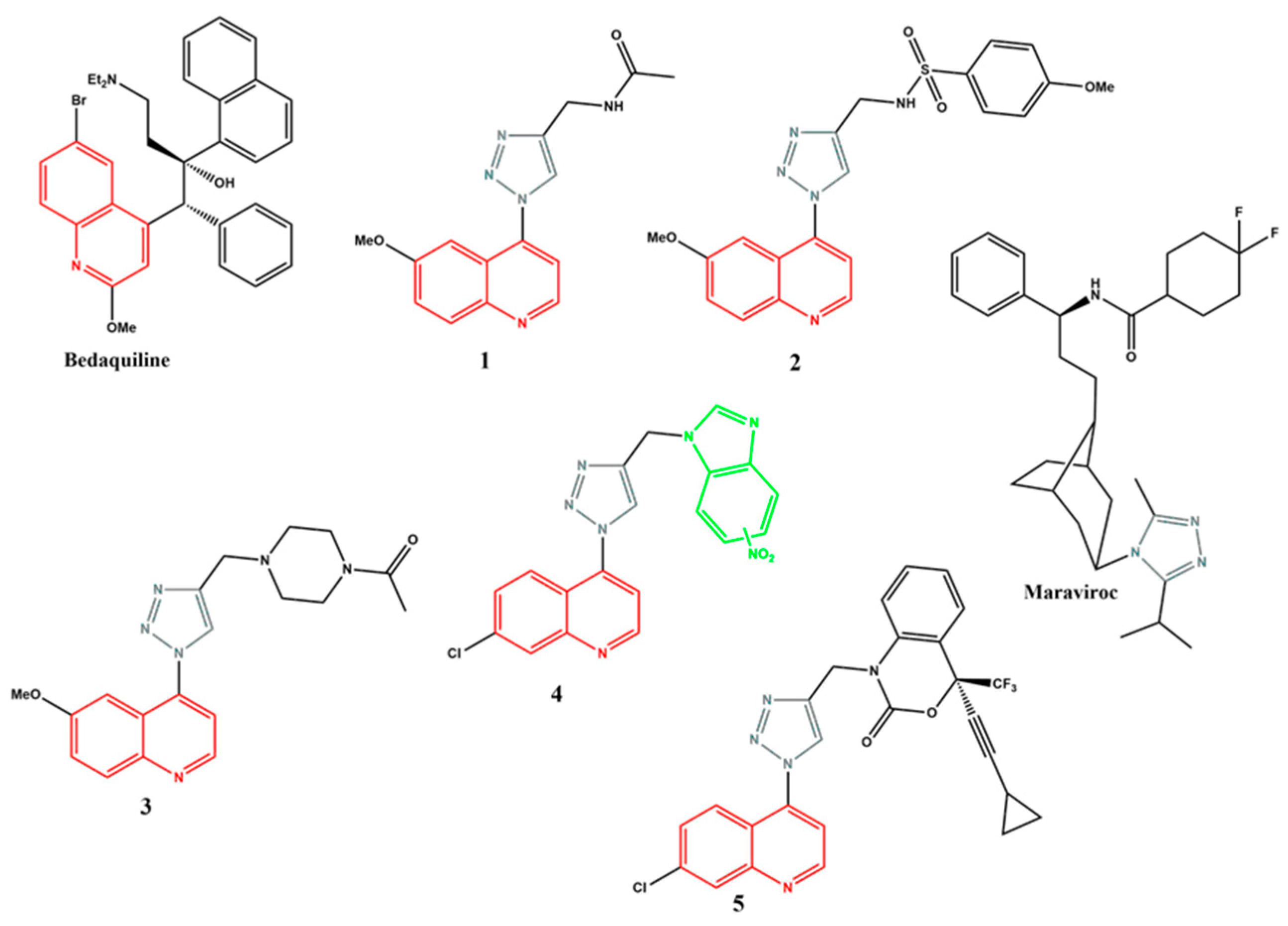
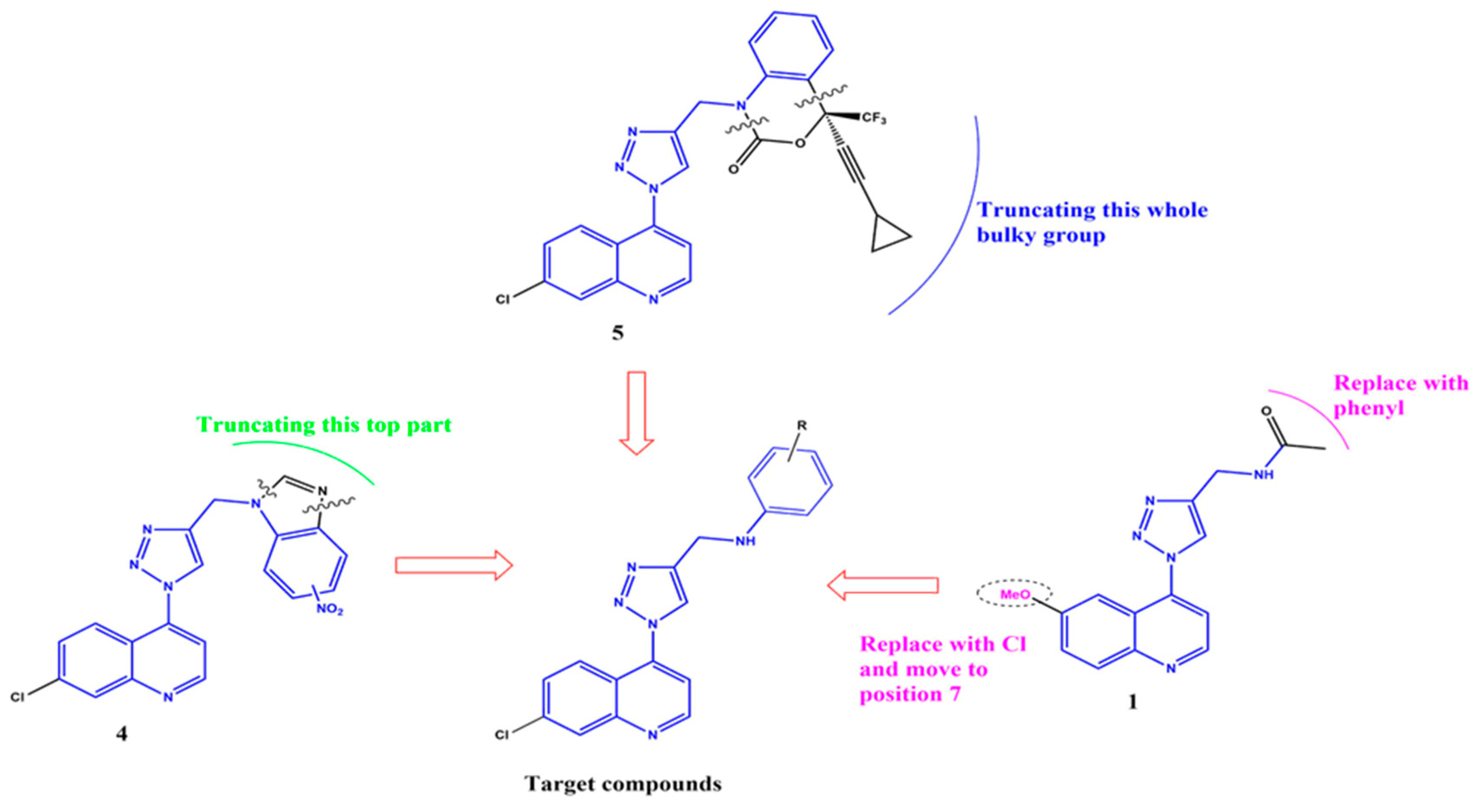
1. Introduction
2. Results and Discussion
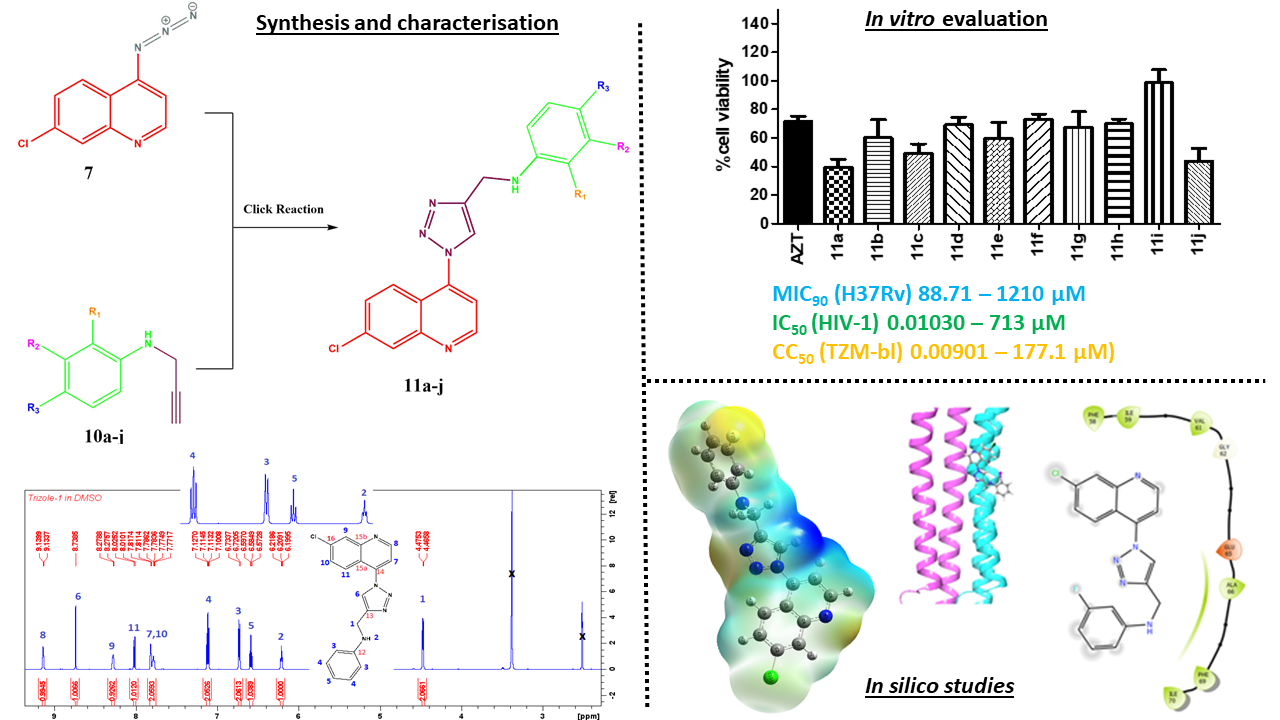
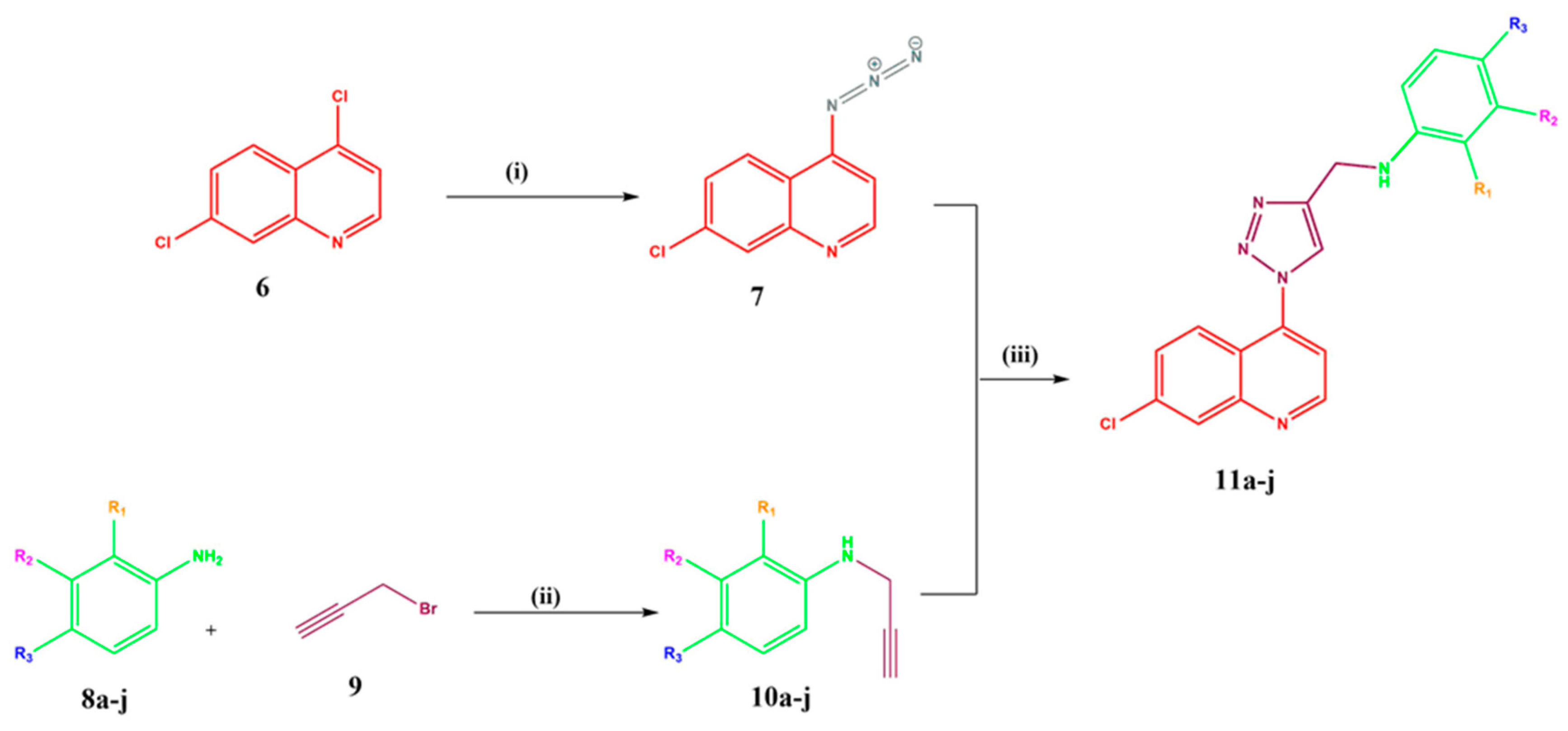
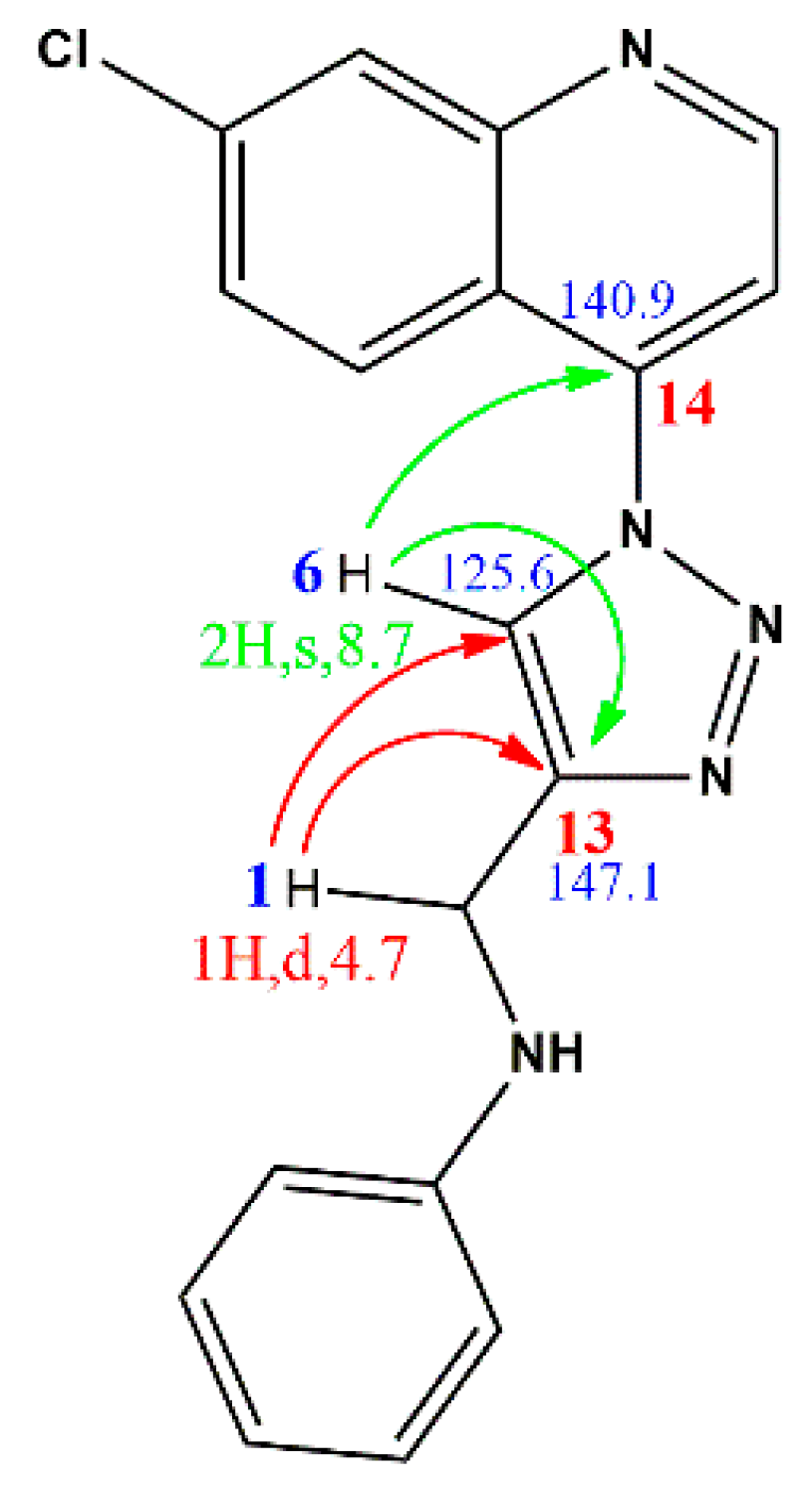
2.1. Chemistry
2.2. In Vitro Biological Activities
2.3. In Silico Studies
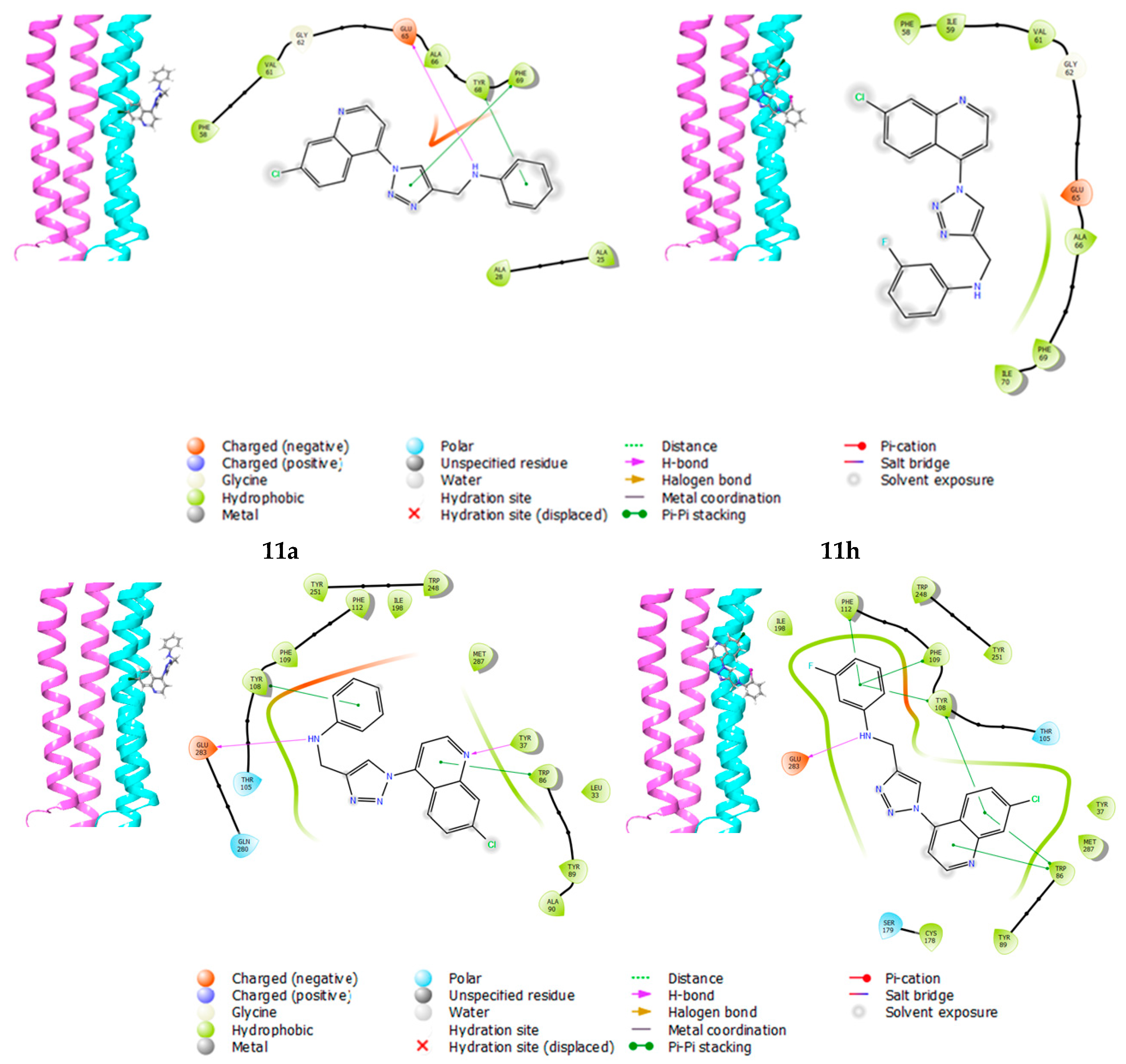
2.3.1. Molecular Docking
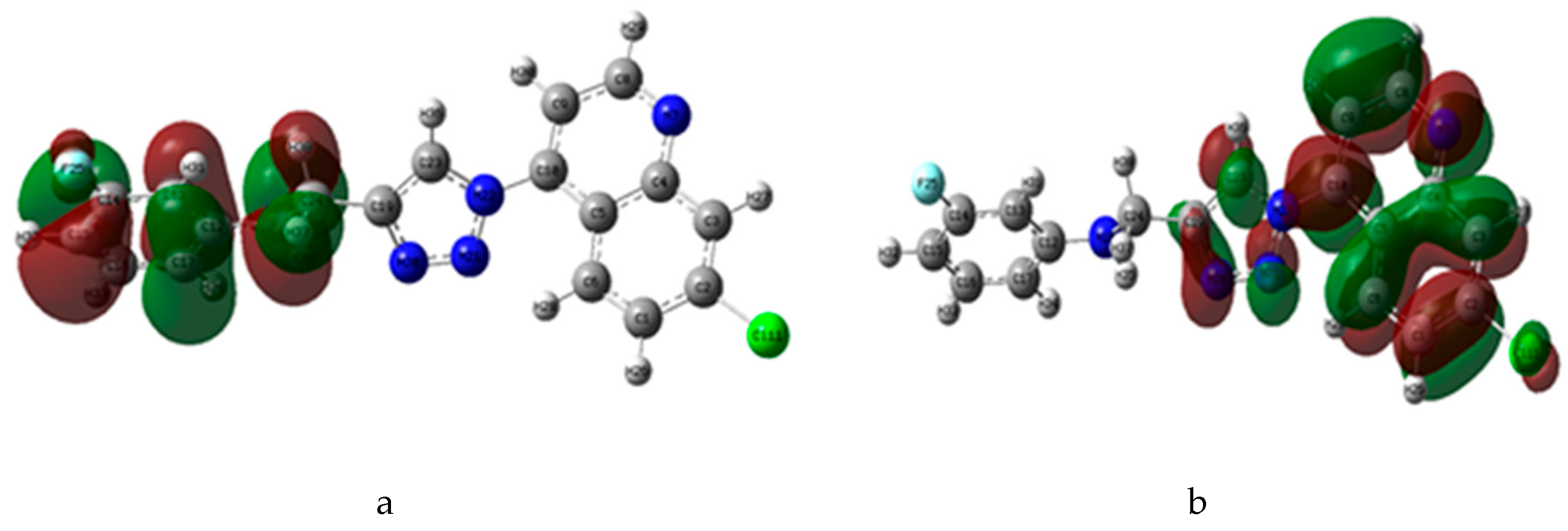
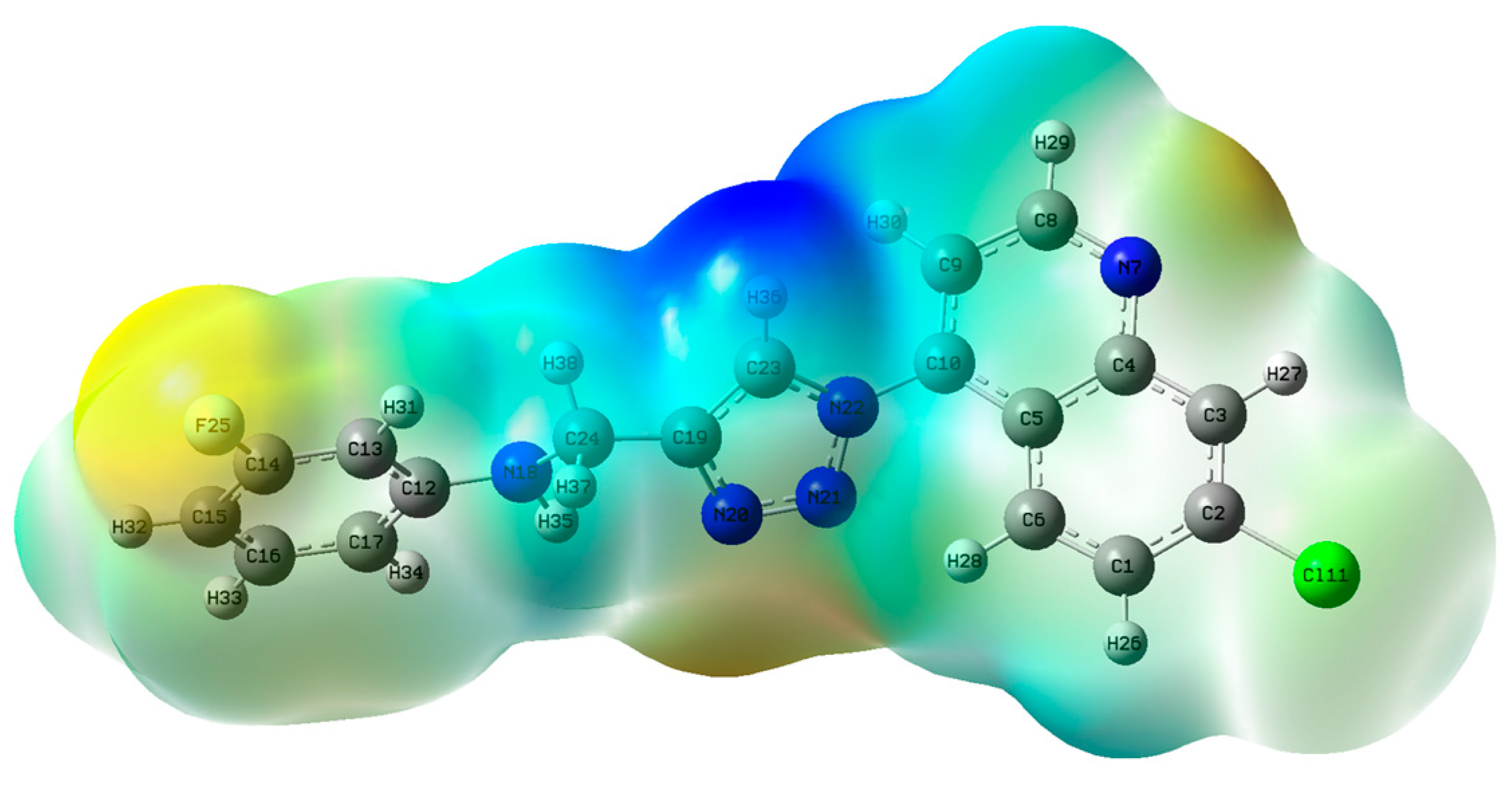
2.3.2. Density Functional Theory Studies
2.3.3. ADMET Predictions
3. Materials and Methods
3.1. Chemistry
3.2. Biology
3.2.1. Antimycobacterial Evaluation
3.2.2. MTT Cytotoxicity Evaluation
3.2.3. Luciferase-Based Antiviral Assay Evaluating Human Immunodeficiency Virus
Maintenance of Cell Lines
3.3. In Silico Studies
3.3.1. Molecular Docking
3.3.2. DFT Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report 2024. World Health Organisation: Geneva, 2024. https://iris.who.int/bitstream/handle/10665/379339/9789240101531-eng.pdf?sequence=1 (Accessed on 17 January 2025).
- Quimque, M.T.G.; Go, A.D.; Lim, J.A.K.; Vidar, W.S.; Macabeo, A.P.G. Mycobacterium tuberculosis Inhibitors Based on arylated quinoline carboxylic acid backbones with anti-Mtb gyrase activity. Int. J. Mol. Sci. 2023, 24 (14), 11632. https://doi.org/10.3390/ijms241411632. [CrossRef]
- Martinez, R.M. Bacillus subtilis, in Brenner's Encyclopedia of Genetics (2nd Ed), S. Maloy and K. Hughes, Editors. 2013, Academic Press: San Diego, 246-248.
- Smith, T., Wolff, K.A.; Nguyen, L. Molecular biology of drug resistance in Mycobacterium tuberculosis. Curr. Top. Microbiol. Immunol. 2013. 374: p. 53-80. https://doi.org/10.1007/82_2012_279. [CrossRef]
- Sawyer, E.B.; Grabowska, A.D.; Cortes, T. Translational regulation in mycobacteria and its implications for pathogenicity. Nucleic Acids Res. 2018, 46(14): 6950-6961. https://doi.org/10.1093/nar/gky574. [CrossRef]
- Bhat, Z.S.; Rather, M.A.; Maqbool, M.; Ahmad, Z. Drug targets exploited in Mycobacterium tuberculosis: Pitfalls and promises on the horizon. Biomed. Pharmacother. 2018, 103, 1733-1747. https://doi.org/10.1016/j.biopha.2018.04.176. [CrossRef]
- Amusengeri, A.; Khan, A.; Tastan Bishop, O. The structural basis of Mycobacterium tuberculosis RpoB drug-Resistant clinical mutations on rifampicin drug binding. Molecules, 2022, 27(3), 885. https://doi.org/10.3390/molecules27030885. [CrossRef]
- Goldberg, D.E.; Siliciano, R.F.; Jacobs, W.R. Outwitting evolution: Fighting drug-resistant TB, malaria, and HIV. Cell, 2012, 148(6), 1271-1283. https://doi.org/10.1016/j.cell.2012.02.021. [CrossRef]
- Aguilar Diaz, J.M.; Abulfathu, A.A.; te Brake, L.H.M.; van Ingen, J.; Kuipers, S.; Magis-Escurra, C.; Raiijmaker, J.; Svensson, E.M., Boeree, M.J. New and repurposed drugs for the treatment of active tuberculosis: An Update for clinicians. Respiration, 2023, 102(2), 83-100. https://doi.org/10.1159/000528274. [CrossRef]
- Rendon, A.; Tiberi, S.; Scardigli, A.; D'Ambrosio, L.; Centis, R.; Caminero, J.A.; Migloori, G.B. Classification of drugs to treat multidrug-resistant tuberculosis (MDR-TB): evidence and perspectives. J. Thorac. Dis. 2016, 8(10), 2666-2671. https://doi.org/10.21037/jtd.2016.10.14. [CrossRef]
- Karim, Q.A.; Karim, S.S.A. The evolving HIV epidemic in South Africa. Int. J. Epidemol. 2002, 31(1), 37-40. https://doi.org/10.1093/ije/31.1.37. [CrossRef]
- The urgency of now: AIDS at a crossroads, Joint United Nations Programme on HIV/AIDS: Geneva, 2024. https://www.unaids.org/sites/default/files/media_asset/2024-unaids-global-aids-update_en.pdf (accessed 17 March 2025).
- Maeda, K.; Das, D.; Kobayakawa, T.; Tamamura, H.; Takeuchi, H. Discovery and development of anti-HIV therapeutic agents: Progress towards improved HIV medication. Curr. Top. Med. Chem. 2019, 19(18), 1621-1649. https://doi.org/10.2174/1568026619666190712204603. [CrossRef]
- Phanuphak, N.; Gulick, R.M. HIV treatment and prevention 2019: Current standards of care. Curr. Opin. HIV AIDS, 2020, 15(1), 4-12. https://doi.org/10.1097/COH.0000000000000588. [CrossRef]
- Arts, E.J.; Hazuda, D.J. HIV-1 antiretroviral drug therapy. Cold Spring Harb. Perspect. Med. 2012, 2(4): a007161. https://doi.org/10.1101/cshperpspect.a007161. [CrossRef]
- Holec, A.D.; Mandal, S.; Prathipati, P.K.; Destache, C.J. Nucleotide reverse transcriptase inhibitors: A thorough review, present status and tuture perspective as HIV therapeutics. Curr. HIV Res. 2017, 15(6), 411-421. https://doi.org/10.2174/1570162X15666171120110145. [CrossRef]
- Koren, D.E.. 225 Classes of antiretrovirals, in Fundamentals of HIV Medicine. 2020, Oxford University Press, New York. 225-238.
- Di, L. and E.H. Kerns. Drug-like properties: Concepts, structure design and methods from ADME to toxicity optimization. 2012, Academic Press, Califonia.
- Sluis-Cremer, N.; Tachedjian, G. Mechanisms of inhibition of HIV replication by non-nucleoside reverse transcriptase inhibitors. Virus Res. 2008, 134(1-2), 147-156. https://doi.org/10.1016/j.virusres.2008.01.002. [CrossRef]
- Ramprasad, J.; Sthalam, V.K.; Thampunuri, R.L.M.; Bhukya, S.; Ummanni, R.; Balasubramanian, S.; Pabbaraja, S. Synthesis and evaluation of a novel quinoline-triazole analogs for antitubercular properties via molecular hybridization approach. Bioorg. Med. Chem. Lett. 2019, 29(20), 126671. https://doi.org/10.1016/j.bmcl.2019.126671. [CrossRef]
- Thomas, K.D.; Adhikari, A.V.; Chowdhury, I.H.; Sumesh, E.; Pal, N.K. New quinolin-4-yl-1,2,3-triazoles carrying amides, sulphonamides and amidopiperazines as potential antitubercular agents. Eur. J. Med. Chem. 2011, 46 (6), 2503-2512. https://doi.org/10.1016/j.ejmech.2011.03.039. [CrossRef]
- Nyoni, N.T.P.; Ncube, N.B.; Kubheka, M.X.; Mkhwanazi, N.P.; Senzani, S.; Tukulula, M. Synthesis, characterization, in vitro antimycobacterial and cytotoxicity evaluation, DFT calculations, molecular docking and ADME studies of new isomeric benzimidazole-1,2,3-triazole-quinoline hybrid mixtures. Bioorg. Chem. 2023, 141: 106904. https://doi.org/10.1016/j.bioorg.2023.106904. [CrossRef]
- Reddyrajula, R.; Dalimba, U. Quinoline-1,2,3-triazole hybrids: Design and synthesis through Click Reaction, evaluation of antitubercular activity, molecular docking and in silico ADME studies. ChemistrySelect, 2019, 4(9), 2685-2693. https://doi.org/10.1002/slct.201803946. [CrossRef]
- Ganesan, M.S.; Raja, K.K.; Murugesan, S.; Karankumar, B.; Faheem, F.; Thirunavukkarasu, S.; Shetye, G.; Ma, R.; Franzablau, S.G.; Wan, B.; Rajagopal, J. Quinoline-proline, triazole hybrids: Design, synthesis, antituberculosis, molecular docking, and ADMET studies. J. Heterocycl. Chem. 2021, 58(4), 952-968. https://doi.org/10.1002/jhet.4229. [CrossRef]
- Yadav, J.; Kaushik, C.P. Quinoline-1,2,3-triazole hybrids: Design, synthesis, antimalarial and antimicrobial evaluation. J. Mol. Struct. 2024, 1316: 138882. https://doi.org/10.1016/j.molstruc.2024.1388882. [CrossRef]
- Thakare, P.P.; Shinde, A.D.; Chavan, A.P.; Nyayanit, N.V.; Bobade, V.D.; Mhaske, P.C. Synthesis and biological evaluation of new 1,2,3-triazolyl-pyrazolyl-quinoline derivatives as potential antimicrobial agents. ChemistrySelect, 2020, 5(15), 4722-4727. https://doi.org/10.1002/slct.201904455. [CrossRef]
- Jamshidi, H.; Naimi-Jamal, M.R.; Safavi, M.; Sanati, K.R.; Azerang, P.; Tahhighi, A. Synthesis and biological activity profile of novel triazole/quinoline hybrids. Chem. Biol. Drug Des. 2022, 100(6), 935-946. https://doi.org/10.1111/cbdd.14031. [CrossRef]
- Bhoye, M.R.; Shinde, A.; Shaik, A.L.N.; Shisode, V.; Chavan, A.; Maliwal, D.; Pisssurlenkar, R.R.S. New thiazolyl-isoxazole derivatives as potential anti-infective agents: design, synthesis, in vitro and in silico antimicrobial efficacy. J. Biomol. Struct. Dynamic. 2024, 23, 1-15. https://doi.org/10.1080/07391102.2024.2306497. [CrossRef]
- Costa, C.C.P.; Boechat, N.; Bastos, M.M.; da Silva, F-de.C.; Marttorelli, A.; Souza, T.M.L; Baptista, M.S.; Hoelz, L.V.B.; Caffarena, E.R. New efavirenz derivatives and 1,2,3-triazolyl-phosphonates as inhibitors of reverse transcriptase of HIV-1. Curr. Top. Med. Chem. 2018, 18(17), 1494-1505. https://doi.org/10.2174/1568026618666181029150118. [CrossRef]
- Feng, L.S.; Zheng, M-J.; Zhao, F.; Liu, D. 1,2,3-Triazole hybrids with anti-HIV-1 activity. Arch. Pharm. 2021, 354(1), e2000163. https://doi.org/10.1002/ardp.202000163. [CrossRef]
- Tian, Y., Liu, Z.; Huang, B.; Kang, D.; Zhang, H.; De Clercq, E.; Daelemans, D.; Pannecouque, C.; Leé, K-H.; Chen, C-H.; Zhan, P.; Liu, Z. Targeting the entrance channel of NNIBP: Discovery of diarylnicotinamide 1,4-disubstituted 1,2,3-triazoles as novel HIV-1 NNRTIs with high potency against wild-type and E138K mutant virus. Eur. J. Med. Chem. 2018, 151, 339-350. https://doi.org/10.1016/j.ejmech.2018.03.059. [CrossRef]
- Zhou, Z.; Liu, T.; Wu, G.; Kang, D.; Fu, Z.; Wang, Z.; De Clercq, E.; Pannecouque, C.; Zhan, P. Liu, X. Targeting the hydrophobic channel of NNIBP: discovery of novel 1,2,3-triazole-derived diarylpyrimidines as novel HIV-1 NNRTIs with high potency against wild-type and K103N mutant virus. Org. Biomol. Chem. 2019, 17, 3202-3217. https://doi.org/10.1039/C9OB00032A. [CrossRef]
- Ahmad, A.; Akthar, J.; Ahmad, M.; Khan, M.I.; Wasim, R.; Islam, A.; Singh. A. Bedaquiline: An Insight Into its clinical use in multidrug-resistant pulmonary tuberculosis. Drug. Res. 2024, 74(6), 269-279. https://doi.org/10.1055/a-2331-7061. [CrossRef]
- Sutherland, H.S.; Tong, A.S.T.; Choi, P.J.; Blaser, A.; Conobe, D.; Franzblau, S.G.; Lotlikar, M.U.; Cooper, C.B.; Upton, A.M.; Denny, W.A.; Palmer, B.D. 3,5-Dialkoxypyridine analogues of bedaquiline are potent antituberculosis agents with minimal inhibition of the hERG channel. Bioorg. Med. Chem. 2019, 27(7), 1292-1307. https://doi.org/10.1016/j.bmc.2019.02.026. [CrossRef]
- Emu, B.; Fessel, J.; Schrader, S.; Kumar, P.; Richmond, G.; Win, S.; Weinheimer, S. Marsolais, C.; Lewis, S. Phase 3 study of ibalizumab for multidrug-resistant HIV-1. N. Engl. J. Med. 2018, 379(7), 645-654. https://doi.org/10.1056/NEJMoa1711460. [CrossRef]
- Xu, G.G.; Guo, J.; Wu, Y. Chemokine Receptor CCR5 antagonist Maraviroc: Medicinal chemistry and clinical applications. Curr. Top. Med. Chem. 2014, 14(13), 1504-1514. https://doi.org/10.2174/1568026614666140827143745. [CrossRef]
- Price, D.A.; Armour, D.; de Groot, M.; Leishman, D.; Napier, C.; Perros, M.; Stammen, B.L.; Wood, A. Overcoming hERG affinity in the discovery of maraviroc; a CCR5 antagonist for the treatment of HIV. Bioorg. Med. Chem. Lett. 2006, 16(17), 4633-4637. https://doi.org/10.1016/j.bmcl.2006.06.012. [CrossRef]
- Manosuthi, W.; Wiboonchutikul, S; Sungkanuparph, S. Integrated therapy for HIV and tuberculosis. AIDS Res. Ther. 2016, 13:22. https://doi.org/10.1186/s12981-016-0106-y. [CrossRef]
- Khan, S.A.; Akthar, M.J.; Gogoi, U.; Meenakshi, D.U.; Das, A. An overview of 1,2,3-triazole-containing hybrids and their potential anticholinesterase activities. Pharmaceuticals, 2023, 16(2): 179. https://doi.org/10.3390/ph16020179. [CrossRef]
- Eswaran, S.; Adhikari, A.V.; Shetty, N.S. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur. J. Med. Chem. 2009, 44(11), 4637-4647. https://doi.org/10.1016/j.ejmech.2009.06.031. [CrossRef]
- Casano, G.; Dumétre, A.; Panneouque, C.; Hutter, S.; Azas, N.; Robin, M. Anti-HIV and antiplasmodial activity of original flavonoid derivatives. Bioorg. Med. Chem. 2010, 18 (16), 6012-6023. https://doi.org/10.1016/j.bmc.2010.06.067. [CrossRef]
- Indrayanto, G., Putra, G.S.; Suhud, F. Chapter Six - Validation of in-vitro bioassay methods: Application in herbal drug research, in Profiles of Drug Substances, Excipients and Related Methodology, A.A. Al-Majed, Editor. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273-307. https://doi.org/10.1016/bs.podrm.2020.07.005. [CrossRef]
- Kumar, S.; Mehra, R.; Sharma, S., Bokolia, N.P.; Raina, D.; Nargotra, A.; Singh, P.P.; Khan, I.A. Screening of antitubercular compound library identifies novel ATP synthase inhibitors of Mycobacterium tuberculosis. Tuberculosis, 2018, 108, 56-63. https://doi.org/10.1016/j.tube.2017.10.008. [CrossRef]
- El-Zohairy, M.A.; Zlotos, D.P.; Berger, M.R.; Adwan, H.H.; Mandour, Y.M. Discovery of Novel CCR5 Ligands as anticolorectal cancer agents by sequential virtual screening. ACS Omega, 2021, 6 (16), 10921-10935. https://doi.org/10.1021/acsomega.1c00681. [CrossRef]
- Vadivelu, A.; Gopal, V.; Reddy, C.U.M. Molecular docking studies of 1, 3, 4-thiadiazoles as novel peptide deformylase inhibitors as potential antibacterial agents. Int. J. Pharm. Sci. Rev. Res. 2015, 31(1) 58-62. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=327843d2a59588c3263726dc53a4b9d2f04c519f.
- Shah, P.; Naik. D.; Jariwala, N.; Bhadane, D.; Kumar, S.; Kulkarni, S.; Bhutani, K.K.; Singh, I.P. Synthesis of C-2 and C-3 substituted quinolines and their evaluation as anti-HIV-1 agents. Bioorg. Chem. 2018, 80, 591-601. https://doi.org/10.1016/j.bioorg.2018.07.016. [CrossRef]
- Ibrahim, T.S.; Bokhtia, R.; Al-Mahmoudy, A.M.M.; Taher, E.S.; AlAwadh, M.A.; Elagawany, M.; Abdel-Aal, E.H.; Panda, S.; Gouda, A.M.; Asfour, H.Z.; Alhakamy, N.A.; Youssif, B.G.M. Design, synthesis and biological evaluation of novel 5-((substituted quinolin-3-yl/1-naphthyl) methylene)-3-substituted imidazolidin-2,4-dione as HIV-1 fusion inhibitors. Bioorg. Chem. 2020, 99,103782. https://doi.org/10.1016/j.bioorg.2020.103782. [CrossRef]
- Oyeneyin, O.E.; Ojo, N.D.; Ipinlogu, N.; James, A.C.; Agbaffa, E.B. Investigation of corrosion inhibition potentials of some aminopyridine Schiff bases using density functional theory and Monte Carlo simulation. Chem. Afr. 2022, 5, 319-322. https://doi.org/10.1007/s42250-021-00304-1. [CrossRef]
- Zhan, C.-G.; Nichols, J.A.; Dixon, D.A. Ionization potential, electron affinity, electronegativity, dardness, and electron excitation energy: Molecular properties from density functional theory orbital energies. J. Phys. Chem. A, 2003, 107(20). 4184-4195. https://doi.org/10.1021/jp0225774. [CrossRef]
- Kadi, I.; Güldeniz, S.; Bouled, H.; Zebbiche, Z.; Suat, T.; Fatümetüzzehra, K.; Gönül, Z.; Küçükbay, H.; Boumoud, T. Synthesis, cytotoxicity, antioxidant activity, DFT calculations, and docking studies of new pyridine-malonate derivatives as potential anticancer agents. Polycycl. Aromat. Compd. 2022, 44(10), 6615-6629. https://doi.org/10.1080/10406638.2023.2281468. [CrossRef]
- Hussain, Z.; Ibrahim, M.A.; Hassanin, N.M.; Badran, A-S. Synthetic approaches for novel annulated pyrido[2,3-d]pyrimidines: Design, structural sharacterization, Fukui functions, DFT calculations, molecular docking and anticancer efficiency. J. Mol. Struct. 2024, 1318(1), 139335. https://doi.org/10.1016/j.molstruc.2024.139335. [CrossRef]
- Roskoski Jr, R. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2024, 200: 107059. https://doi.org/10.1016/j.phrs.2024.107059. [CrossRef]
- Wei, X.; Decker, M.J.; Liu, H.; Zhang, Z.; Arani, R.B.; Kilny, J.M.; Saag, M.S.; Wu, X.; Shaw, G.M.; Kappes, J.C. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 2002, 46(6), 1896-1905. https://doi.org/10.1128/AAC.46.6.1896-1905.2002. [CrossRef]
- Naidu, D.; Oduro-Kwateng, E.; Solima, M.E.S.; Ndlovu, S.I.; Mkhwanazi, N.P. Alternaria alternata (Fr) Keissl crude extract inhibits HIV subtypes and integrase drug-resistant strains at different stages of HIV replication. Pharmaceuticals, 2025, 18(2), 189. https://doi.org/10.3390/ph18020189. [CrossRef]
- Sastry, M.G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27(3), 221-234. https://doi.org/10.1007/s10822-013-9644-8. [CrossRef]
- Mohammadpourasl, S.; de Biani, F.F.; Coppola, C.C.; Parisi, M.L.; Zani, L.; Dessi, A.; Calamante, M.; Reginato, G.; Basosi, R.; Sinicropi, A. Ground-State redox potentials calculations of D-π-A and D-A-π-A organic dyes for DSSC and visible-light-driven hydrogen production. Energies, 2020, 13(8) 2032. https://doi.org/10.3390/en13082032. [CrossRef]
- Aslam, R., Serdaoglu, G.; Zehra, S.; Verma, K.D.; Aslam, J.; Guo, L.; Verma, C.; Ebenzo, E.E.; Quraishi, M.A. Corrosion inhibition of steel using different families of organic compounds: Past and present progress. J. Mol. Liq. 2022, 348, 118373. https://doi.org/10.1016/j.molliq.2021.118373. [CrossRef]
- Ibrahim, M.A., Roushdy, N.; Atta, A.A.; Badran. A-S.; Farag, A.A.M. Comprehensive study on pyrano[3,2-c]quinoline-based indole: Synthesis, characterization, and potential for optoelectronic and photovoltaic applications. J. Mol. Struct. 2024, 1312, 138660. https://doi.org/10.1016/j.molstruc.2024.138660. [CrossRef]
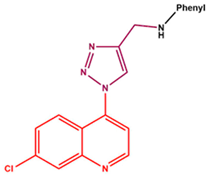 | |||||
| Compound | Phenyl | Appearance | % Yield | m.p. (°C) | MS data |
| 11a | 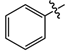 |
cream white solid | 88 | 150-152 | 306.0968 [(M-H)-N2]- |
| 11b | 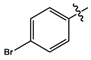 |
light brown solid | 87 | 188-190 | 449.9707 [(M+HCl]- |
| 11c | 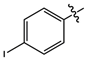 |
brown solid | 92 | 198-199 | 495.9821 [(M-H)+Cl]- |
| 11d | 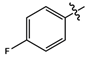 |
light grey solid | 79 | 145-147 | 324.0866 [(M-H)-N2]- |
| 11e | 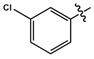 |
Yellow solid | 69 | 169-171 | 340.0580 [(M-H)-N2)]- |
| 11f | 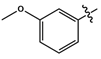 |
dark brown liquid | 43 | - | 388.1096 (M+Na)+ |
| 11g |  |
Yellow liquid | 48 | - | - |
| 11h | 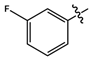 |
cream white solid | 85 | 145-147 | 324.0868 [(M-H)-N2]- |
| 11i | 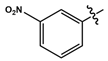 |
Orange solid | 79 | 192-194 | 381.2533 (M+1)- |
| 11j | 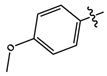 |
Brown solid | 86 | 169-172 | 336.1097 [(M-H)-N2]- |
| Compound | Anti-Mtb (µM) | Anti-HIV (µM) | Cytotoxicity CC50 (µM) | SIa |
|---|---|---|---|---|
| H37Rv MIC90 | HIV-1 Subtype B IC50 | TZM-bl cell line | (CC50/IC50) | |
| 11a | 186.52 | 3.013 | 177.1 | 58.75 |
| 11b | 1210.65 | 124.4 | 0.248 | 1.994 x 10-3 |
| 11c | 1084.6 | 23.20 | 156.9 | 6.76 |
| 11d | 176.52 | DNC* | 1320.0 | - |
| 11e | 168.81 | 713.7 | 834.6 | 1.17 |
| 11f | 171.19 | 22.75 | 3.599 | 0.158 |
| 11g | 155.05 | 0.3883 | 4414 | 11367.49 |
| 11h | 88.72 | 0.01032 | 25.52 | 2472.87 |
| 11i | nd* | 0.167 | 0.00901 | 0.05 |
| 11j | 1369.86 | 180.4 | 4.000 | 0.02 |
| 7 | 19.09 | nd* | nd* | - |
| 10a | 3814 | nd* | nd* | - |
| Ethambutol | 9.68 | - | - | - |
| AZT | - | 0.0909 | 1122.58 | 12349.59 |
| Compound | Docking Scores 4V1F | Docking Scores 4MBS |
|---|---|---|
| 11a | -2.540 | -6.990 |
| 11b | -2.291 | -6.729 |
| 11c | -2.339 | -6.899 |
| 11d | -2.528 | -7.561 |
| 11e | -2.879 | -7.371 |
| 11f | -2.035 | -4.815 |
| 11g | -2.714 | -6.427 |
| 11h | -2.606 | -7.362 |
| 11i | -2.479 | -5.825 |
| 11j | -2.570 | -5.301 |
| Compound | EHOMO (eV) | ELUMO (eV) | I (eV) | A (eV) | Eg (eV) | η (eV) | S (eV-1) | χ (eV) | ω (eV) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 11h | -5.89 | -2.64 | 5.89 | 2.64 | 3.25 | 1.63 | 0.615 | 4.27 | 5.59 | |
| Compound | QPlogKhsa | QPlogS | % Human oral absorption | QPlogBB | CNS | #metab | Ro5 |
|---|---|---|---|---|---|---|---|
| 11a | 0.555 | -5.804 | 100.000 | -0.293 | 0 | 5 | 0 |
| 11b | 0.697 | -6.667 | 100.000 | -0.127 | 0 | 4 | 0 |
| 11c | 0.723 | -6.793 | 100.000 | -0.116 | 0 | 4 | 1 |
| 11d | 0.597 | -6.169 | 100.000 | -0.186 | 0 | 4 | 0 |
| 11e | 0.673 | -6.548 | 100.000 | -0.137 | 0 | 5 | 0 |
| 11f | 0.549 | -5.946 | 100.000 | -0.329 | 0 | 6 | 0 |
| 11g | 0.779 | -6.922 | 100.000 | -0.022 | 0 | 6 | 1 |
| 11h | 0.598 | -6.169 | 100.000 | -0.187 | 0 | 6 | 0 |
| 11i | 0.500 | -5.932 | 88.989 | -1.381 | -2 | 6 | 0 |
| 11j | 0.545 | -5.938 | 100.000 | -0.369 | 0 | 5 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





