1. Introduction
The term "psychedelic," derived from the Greek roots “psyche” (mind or soul) and “delos” (to reveal), was first coined in 1956 by psychiatrist Humphry Osmond (Tanne, 2004), who was researching lysergic acid diethylamide (LSD) (Johnson & Griffiths, 2017). By the late 1950s, Albert Hofmann from Sandoz Laboratories identified and synthesized the psychoactive compounds psilocybin and psilocin, found in Psilocybe mushrooms (Hoffman et al., 1958). Sandoz marketed psilocybin under the name Indocybin for basic psychopharmacological research and clinical studies. Psilocybin saw a rapid rise in popularity during the 1960s and was classified as a Schedule I drug in 1970 (Geiger et al., 2018).
Psychedelics are hallucinogenic substances that, once ingested, produce effects that alter the mind and distort reality, inducing hallucinations, delirium, emotional changes, and feelings of detachment. They are classified into dissociative drugs—such as dextromethorphan (DXM), ketamine, Salvia divinorum, and phencyclidine (PCP)—and serotoninergic and dopaminergic hallucinogens that interact with serotonin and dopamine receptors, respectively (Bogenschutz & Pommy 2012; Johnson et al., 2019).
In 1963, Aldous Huxley introduced the idea that psychedelics could be useful for treating "existential distress" at the end of life. On his deathbed, he requested two doses of LSD, passing away peacefully while his wife guided him "toward the light" (Huxley, 1968). Kast and Collins (1964) conducted a trial comparing the analgesic effects of LSD with opioids in terminal patients, demonstrating positive relief effects, although the research was discontinued in 1968 (Schimmers et al., 2022).
Psilocybin is a secondary metabolite found in mushrooms. It has played a crucial role in Aztec and Maya ceremonies, referred to as "the flesh of the gods" by the Aztecs (Ziff et al., 2022). Ethnographic documents such as the Florentine Codex (1529-1579) and records from botanist Schultes in the 16th century confirm the use of psilocybin mushrooms (Nichols, 2020; Ziff et al., 2022).
Psilocybin is now the subject of numerous investigations supporting its therapeutic potential in treating pain, headaches, mood disorders, and anxiety, including major depression, post-traumatic stress disorder, and obsessive-compulsive disorder (Johnson & Griffiths, 2017). Since psilocybin is a widely described natural compound, opportunities for patentability are limited. As a result, it does not easily fit into a conventional profit-driven pharmaceutical development model. Consequently, non-profit entities such as the Multidisciplinary Association for Psychedelic Studies (MAPS), the Usona Institute, and the Heffter Research Institute have generously funded initial development efforts and covered funding gaps. A shared goal of these organizations has been to advance the understanding of psilocybin's therapeutic effects and other consciousness-expanding substances while influencing policies to ultimately increase their accessibility (Geiger et al.,2018). Compass Pathways Ltd. and the Usona Institute have also received "breakthrough therapy" status from the FDA for psilocybin treatments, particularly for refractory depression and major depressive disorder (Albert et al., 2012; Lowe et al., 2021). The ability of psilocybin to promote the growth of cortical neurons has been hypothesized as responsible for the sustained therapeutic effects of psychedelics, highlighting their revolutionary potential in the neuropsychiatric field (Vargas et al., 2023).
1.1. Potential Ecological Role/Occurrence of Psilocybin
The ecological reasons for psilocybin production are a topic of ongoing debate. However, there must be a substantial ecological benefit to justify the energy cost of synthesizing and accumulating this secondary metabolite, which generally constitutes between 0.5% and 2% of the mushroom’s dry weight. Two primary hypotheses attempt to explain the occurrence of these metabolites (Lenz et al.,2021):
Monomer Hypothesis
The monomer hypothesis suggests that psilocybin acts as a defensive agent against animals that might consume psilocybin-producing mushrooms. In this context, crude methanolic extracts of Psilocybe (ranging from 2 to 23 μg/ml) have demonstrated lethal effects on brine shrimp (Arthropoda), though their effects on Caenorhabditis elegans (Nematoda) have been ambiguous. These data, along with the production of alkaloids triggered in the mushroom upon the formation of ephemeral fruiting bodies, suggest that nematodes may not be the primary target of psilocybin. Thus, the lack of solid evidence supporting this hypothesis indicates that its role as a defensive agent may be limited. Despite these considerations, some studies suggest that psilocybin-producing fungi might use the compound to protect themselves and influence the neurochemical behavior of small animals (Gasque et al., 2013)
Regarding the monomer hypothesis in mammals, such as humans, psilocybin exhibits negligible acute physiological toxicity, with an LD50/ED50 ratio > 1000 in mice. This limited interaction or lack of interaction with Psilocybe mushrooms in mammals raises questions about the evolutionary necessity of psilocybin as an ecological defense in these species. Moreover, it is argued that mammals evolved in an environment where fungi had already developed defense mechanisms against invertebrates over millions of years (Lenz et al.,2021).
Oligomer Hypothesis
Mushrooms containing psilocybin instantly turn blue when the mycelium is damaged or as they age. For decades, this distinctive phenomenon has intrigued both chemists and amateur mycologists. Oligomerization converts the indole core derived from L-tryptophan into a blue chromophore (Lenz et al.,2021).
Chemically, psilocybin can be viewed as the stabilized form of psilocin with a phosphate ester group as a protective moiety. This group is removed when the mycelium is damaged and psilocybin is exposed to intrinsic phosphatases or animals, followed by subsequent oligomerization of monomeric psilocin units. Based on this reaction, psilocybin may fulfill its true ecological role as a nascent or complete oligo-/polymer. The complex and dynamically developed coupling products share a polyphenolic character and an aryl coupling with tannins and melanins. These are families of substances with exceptional ecological importance, making tissues resistant to microbial attack and damage, such as from light. Like tannins, blue oligomers precipitate proteins. Additionally, it is assumed that several tannins serve defensive purposes by generating reactive oxygen species (Lenz et al.,2021).
In contrast to conditions that favor psilocin oxidation, tannin-based oxidations are also facilitated by a basic environment, such as the guts of arthropods feeding on living or decomposing plant matter. For example, studies with saprophagous larvae of Pentheria holosericea (which share an ecological niche with wood/dung-dwelling fungi like Psilocybe) maintain extraordinarily high pH values (10–12) and strongly oxidative conditions within their midguts, which aligns well with the "polymer hypothesis." In this scenario, psilocybin would represent the deactivated precursor of a "defense polymer upon consumption," with production potentially triggered instantaneously. Hypothetically, highly reactive psilocybin radicals might react directly with proteins and could have an immediate deleterious effect on insects feeding on the mushrooms (Lenz et al., 2021; Šustr et al 2014).
2. Structural, Chemical and Pharmacology Characteristics of Psilocybin
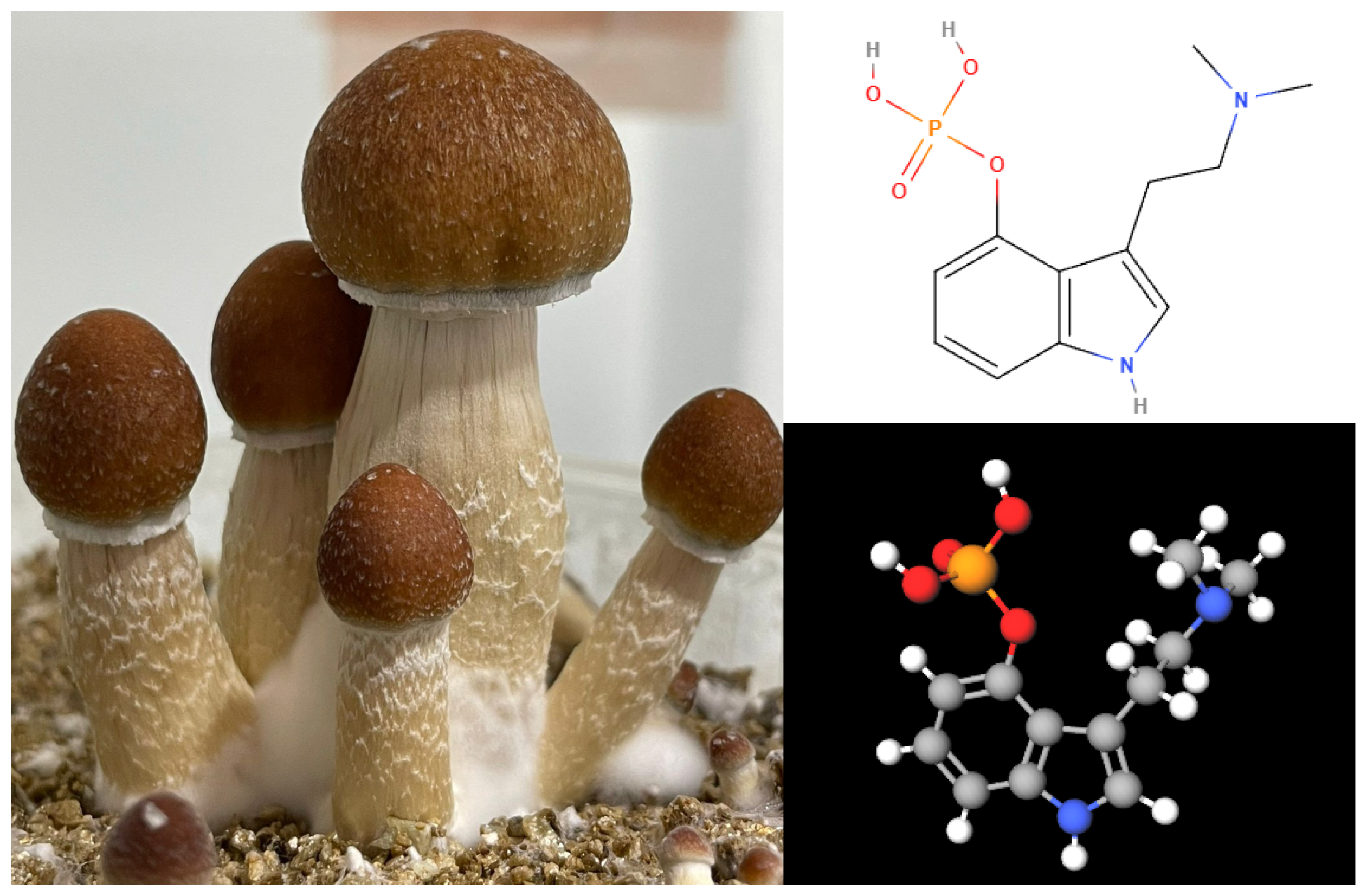
Psilocybe mushrooms belong to the Basidiomycota phylum and are part of the Strophariaceae family (Lee et al., 2020). The psychoactive effects of these mushrooms are due to two indole alkaloids: psilocybin and psilocin (see
Figure 1). Psilocybin is a phosphorylated ester of psilocin, which is found only in minimal quantities. These compounds were initially identified by Hoffmann and his team, who analyzed a sample of P. mexicana collected by Heim (Mastinu et al., 2023).
Psilocybin (CAS Number: [520-52-5], 3-[2-(dimethylamino)ethyl]-1H-indol-4-ol dihydrogen phosphate, C12H17N2O4P, MW = 248.2481 Da, mp = 224 ºC) is a natural psychedelic that degrades with heat and is water-soluble. It belongs to the class of indole-alkylamine hallucinogens and is considered to have minimal or no activity by itself, acting primarily as a prodrug for psilocin (CAS Number: [520-53-6], 3-[2-(dimethylamino)ethyl]-1H-indol-4-ol, C12H16N2O, MW = 204.268 Da, mp = 174.5 ºC). Psilocybin has six hydrogen bond acceptors, three hydrogen bond donors, and a logP of 0.03, which suggests it cannot easily cross the blood-brain barrier. In contrast, psilocin, the active metabolite of psilocybin, has three hydrogen bond acceptors, two hydrogen bond donors, and a logP of 1.32, making it significantly more lipophilic than its precursor. This increased lipophilicity allows psilocin to cross the blood-brain barrier more readily, thereby exerting its psychoactive effects. Visually, psilocybin is typically purified as white needle-like crystals, while psilocin appears as a dark brown to black oily liquid (Geiger et al., 2018).
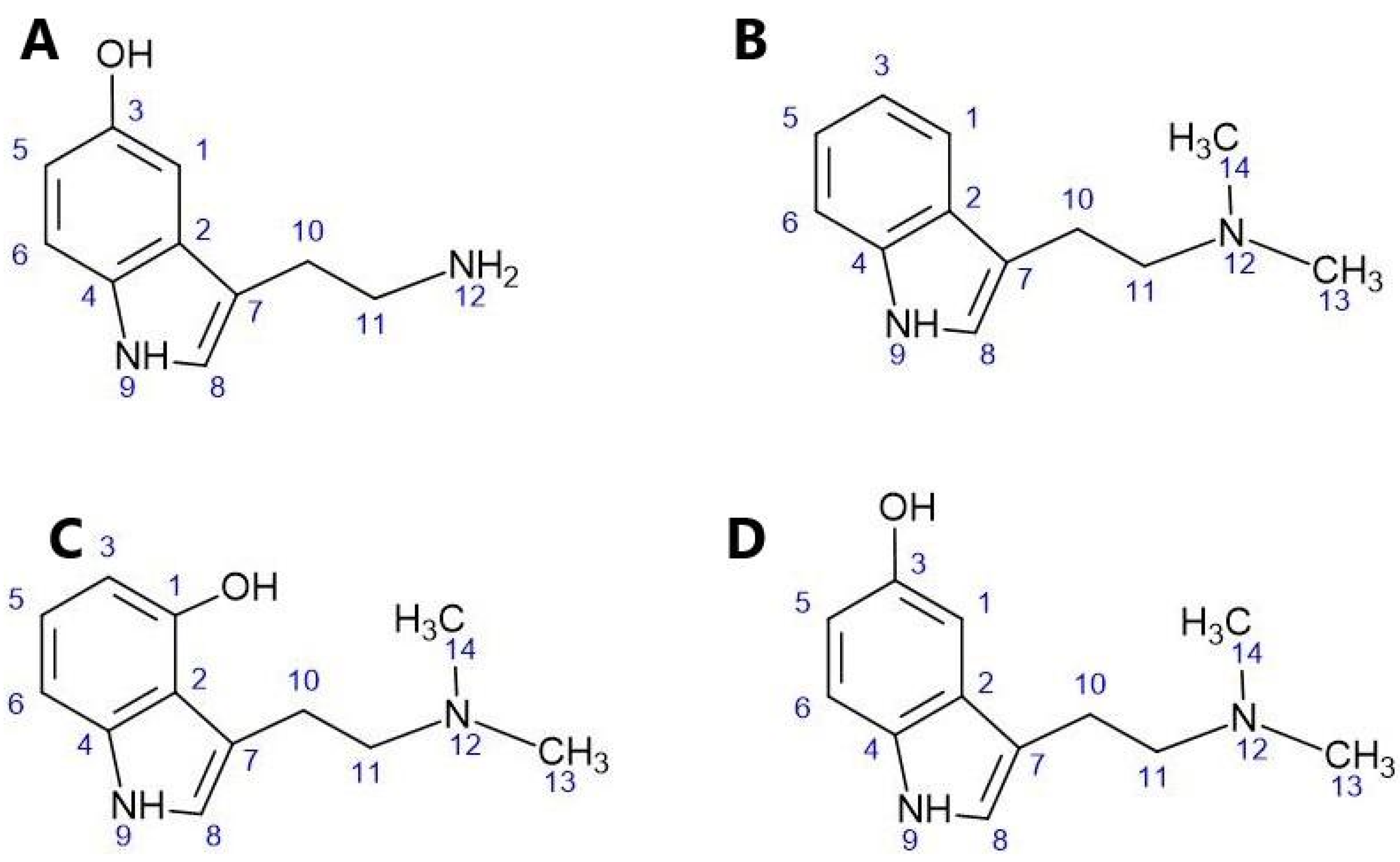
The structure of psilocin (C) and other indole-alkylamine psychedelics closely resembles that of the endogenous neurotransmitter serotonin (A), the hallucinogen N,N-dimethyltryptamine (B), and the hallucinogenic compound bufotenine (D) derived from toads. These structures are illustrated in
Figure 2.
Psychedelics are agonists of the 5-hydroxytryptamine 2A receptor (serotonin, 5HT2AR) and have the capacity to induce profound changes in perception, cognition, and mood. Recent studies suggest that these compounds may promote structural and functional neuroplasticity in the cerebral cortex through the activation of these receptors. However, the exact mechanism underlying this effect is not yet fully understood. It appears to be related to the activation of the tropomyosin receptor kinase B (TrkB), which regulates the mammalian target of rapamycin (mTOR) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor signaling (Vargas et al., 2023).
It remains unclear why some 5-HT2AR ligands promote neuroplasticity and produce lasting therapeutic responses while others do not. However, recent research suggests that the physicochemical properties of different ligands and the subcellular localization of receptors and proteins may provide a partial explanation for this phenomenon. For example, serotonin, due to its physicochemical properties, cannot passively diffuse through nonpolar membranes. In contrast, psychedelics from the tryptamine family, such as psilocybin, can traverse cellular membranes. This leads to the hypothesis that the activation of an intracellular population of 5-HT2AR is necessary for 5-HT2AR ligands to induce cortical structural plasticity and produce behavioral responses similar to antidepressants, which are more durable and stable over time (Vargas et al., 2023).
Although G protein-coupled receptors (GPCRs), such as 5-HT2A, are traditionally considered as initiators of signal transduction originating from the plasma membrane, there is growing evidence suggesting that GPCR signaling from intracellular compartments may play important roles in cellular responses to drugs. The concept of signaling bias has recently been proposed to explain signaling differences between endogenous peptide ligands that do not cross the membrane and membrane-permeable ligands in opioid receptors. Additionally, distinct signaling induced by ligands has been observed between intracellular populations and those located at the plasma membrane, as seen with δ-opioid receptors (Vargas et al., 2023).
In the case of 5-HT2AR ligands, a significant proportion of these receptors in cortical neurons are located in the Golgi apparatus. Intracellular compartments, such as the Golgi apparatus, are slightly more acidic compared to the cytosol and extracellular space. Therefore, it is plausible that the protonation of psychedelics within the Golgi apparatus leads to their retention and sustained signaling, resulting in neuronal growth even after transient stimulation. This persistent growth, even after the drugs have been cleared from the extracellular space, is a distinguishing feature of serotonergic psychoplastogens (Vargas et al., 2023).
Although the mechanistic details linking intracellular activation of 5-HT2AR with cortical neuronal growth are not yet fully understood, it is likely that they involve the signaling of AMPA receptors, TrkB, and mTOR, as previously established (Vargas et al., 2023). Future research should focus on examining in greater detail the signaling interactions among these proteins
2.1. Heteromers of 5-HT Receptors
A receptor heteromer is described as "a macromolecular complex consisting of at least two functional receptor units with biochemical properties that are demonstrably distinct from those of their individual components" (Ferré et al., 2009). This definition is accompanied by a set of criteria that a proposed receptor heteromer must meet to be considered a physiologically relevant heteromer. These standards can be summarized into three key criteria (
Table 1) that are necessary to demonstrate that a heteromer observed in a transfected in vitro environment has physiological relevance in an in vivo context. Firstly, proving that the receptor protomers have the capability to closely interact in vivo is a crucial initial step in validating the heteromeric complex. Appropriate evidence for this includes confirming that the receptor protomers are located within the same cell and sequestered within the same cellular compartments in native or primary cells (Dale et al., 2022; Ferré et al.,2009). This is most commonly achieved through immunoelectron microscopy, co-immunoprecipitation, and proximity-based biophysical techniques, such as Förster Resonance Energy Transfer (FRET) (Gomes et al., 2016) and proximity ligation assays (Dale et al., 2022; Trifilieffn et al., 2011). These techniques can also be used to provide direct evidence of heteromer formation by demonstrating the distance-dependent association of receptor protomers within physiologically relevant environments.
Secondly, to establish the biochemical distinctiveness of a heteromer compared to its individual monomers, it must exhibit unique biochemical properties. These differences can arise from allosteric modulation among protomers, which leads to unique signaling, trafficking, ligand binding, or other pharmacological characteristics. Verification can also involve identifying a specific ligand for the heteromer. Common techniques to assess these properties include ligand binding assays to observe changes in ligand affinity, assays to study how heteromers affect signaling cascades, and methods to investigate their trafficking, particularly regarding internalization mediated by surface and agonists (Dale et al., 2022; Gomes et al., 2016).
Finally, it is essential that its functional disruption be evident when a functional receptor heteromer is lost. This can be achieved using various methods, including membrane-permeable peptides targeting heteromer subunit interaction sites, transgenic animals expressing protomers that cannot form heteromers, and knockout animal models lacking one of the necessary protomers. Once a model of heteromer disruption is developed, several techniques can demonstrate changes in heteromer assembly and function. These techniques include comparing functional characteristics between systems with and without the relevant receptor heteromers, using heteromer-specific antibodies capable of detecting heteromers, and employing bivalent ligands selective for heteromers to show alterations in heteromer assembly and function (Dale et al., 2022; Gomes et al.,2016; Gupta et al., 2010).
To be recognized as a physiologically relevant receptor heteromer, it must meet at least two out of three criteria outlined by NC-IUPHAR guidelines. Historically, demonstrating these properties in receptor heteromers has been challenging due to the lack of suitable experimental tools. However, advancements in technology have made it more feasible to confirm the presence of receptor heteromers in native tissues. This progress has resulted in a greater number of identified and pharmacologically characterized receptor heteromers (Dale et al., 2022).
2.2. Examples of the Physiological Relevance of Heteromers in Pathophysiology
The diversity of changes in traffic profiles resulting from heteromerization has implications not only for heteromer formation, cellular localization, and surface expression but also for signaling. The formation of heteromers, such as D2R/5-HT2AR, can have significant implications for cellular signaling and the effects of psychoactive substances (Shah et al., 2020).
The activation of GPCRs can lead to G protein-dependent signaling events as well as G protein-independent signaling events, such as signaling pathways mediated by β-arrestin. Heterodimerization can induce changes in the conformation of the binding site and alterations in the pharmacological profiles of ligands. Biased ligands selectively activate one pathway over another. Biased ligands towards heteromers are molecules that exert a different downstream effect and a different pharmacological effect when binding to a heterodimeric complex compared to when binding to a receptor monomer (Shahet al., 2020).
The serotonin receptor 5-HT2A and the glutamate receptor mGluR2 send signals through Gαq and Gαi, respectively, and both are targets of atypical antipsychotics. Antipsychotics targeting 5-HT2A act as inverse agonists at the receptor to reduce Gαq signaling. Antipsychotics aimed at mGluR2 have an agonistic effect that increases signaling through Gαi. It has been shown that 5-HT2A and mGluR2 form a heteromer in native tissue, and in a non-pathological state, this heteromer acts to modulate signal transduction through each receptor protomer to enhance Gαi signaling and decrease Gαq signaling. Untreated schizophrenia patients exhibit upregulation of 5-HT2A alongside downregulation of mGluR2, which is thought to lead to a suboptimal ratio of receptors for heteromerization, resulting in reduced heteromer formation in schizophrenia (De Bartolomeis et al., 2013; Fribourg et al., 2011;Gonzalez- Maeso et al.,2008). Based on these findings, it has been suggested that combined therapy with a 5-HT2A inverse agonist like risperidone and an mGluR2 agonist like LY379268 could mimic the signaling balance provided by the heteromer, potentially leading to better outcomes for patients, supported by preclinical experiments. Despite this seemingly straightforward interaction between 5-HT2A and mGluR2 mediated by heteromerization, further research has revealed complexities in the function of this heteromer that may inform future targeting strategies (Moreno et al., 2016; Shah & González- Maeso, 2019).
3. Pharmacokinetics and Pharmacodynamics and Behavioral Effects of Psilocybin/Psilocin
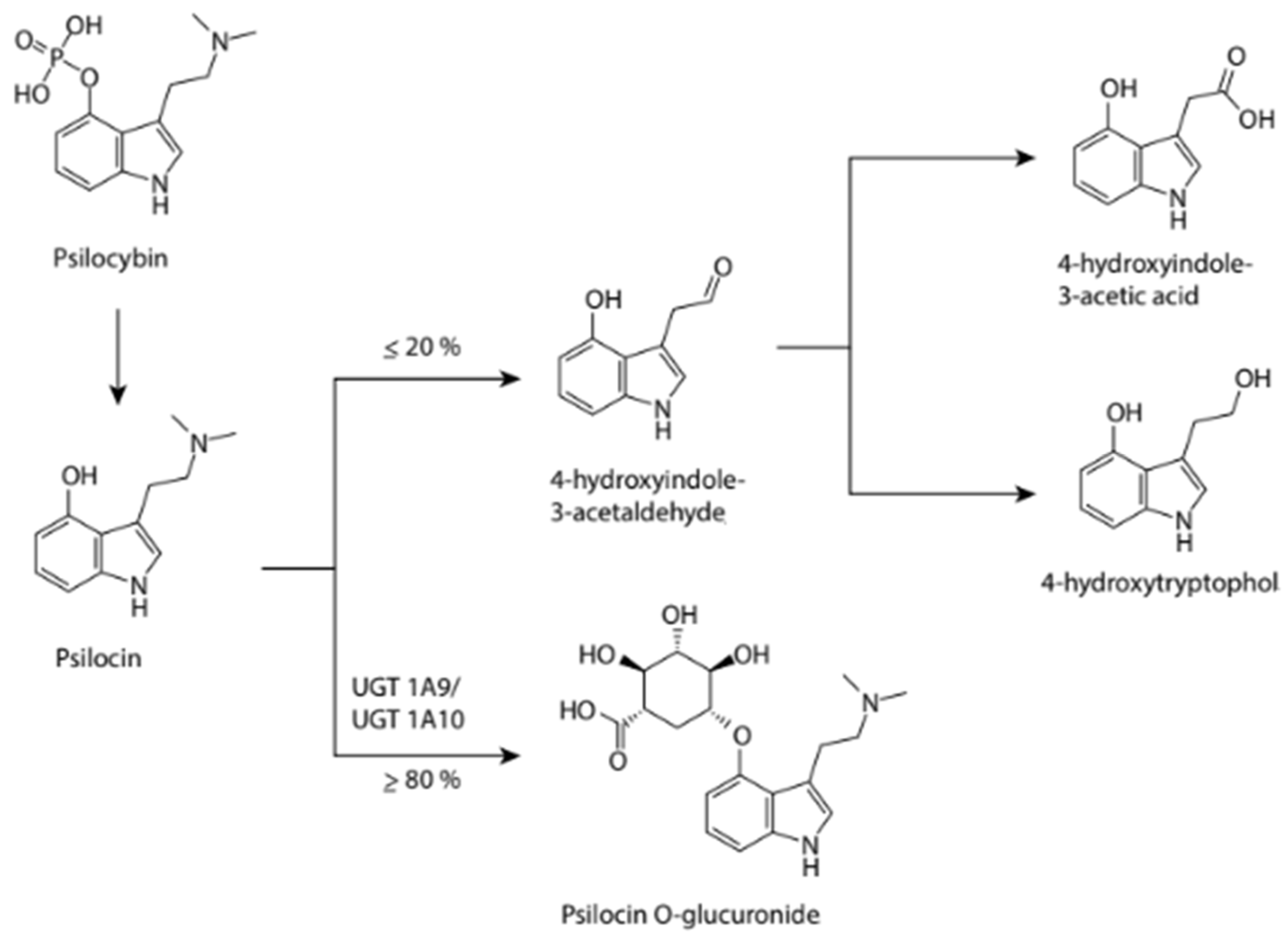
When ingested, psilocybin is absorbed and undergoes first-pass metabolism in the liver, where it is rapidly dephosphorylated into the psychoactive psilocin by an unidentified enzyme (
Figure 3). Psilocin then enters the systemic circulation and crosses into the brain, where it can exert its psychoactive effects. Psilocin undergoes both Phase I and Phase II metabolism. Although predominantly Phase II (≥80%), a significant portion still experiences Phase I reactions. Phase I metabolism first involves the oxidation of psilocin to 4-hydroxyindole-3-aldehyde and its subsequent oxidation to 4-hydroxyindole-3-acetic acid or reduction to 4-hydroxytryptophol; the enzymes involved in this process have not yet been identified. Phase II metabolism, via UGT1A10 in the small intestine and UGT1A9 in the liver, results in the formation of a psilocin glucuronide conjugate. These metabolites are then excreted. A recent study found that the elimination half-life of psilocin is approximately 3 hours in healthy adults, depending on individual characteristics and the route of administration. The complete metabolic pathway of psilocybin has been minimally studied, and much more information is needed to determine the exact mechanisms involved in its metabolism.
Figure 3 below outlines the series of reactions believed to be involved in the metabolism of psilocybin through urinary metabolite analysis (Geiger et al., 2018).
As previously mentioned, psilocybin is a prodrug that, when metabolized in the body, produces psilocin, which functions as an agonist of serotonin (5-hydroxytryptamine) 2A receptors (5-HT2AR) to produce a "mystical-like" hallucinogenic effect (Kargbo, 2020) due to induced hyperfrontality (Lowe et al., 2021), which in turn mediates its antidepressant and anxiolytic effects (Mithoefer et al., 2016).
A possible antidepressant mechanism of action of psilocybin is through the deactivation or normalization of hyperactivity in the medial prefrontal cortex (mPFC) (Hassan et al., 2017). During depression, the mPFC is typically hyperactive (Holtzheimer & Mayberg 2011). The antidepressant properties of psilocybin are mediated by the modulation of prefrontal and limbic brain regions, including the amygdala (Grimm et al., 2018). The amygdala plays an essential role in emotion perception and processing networks (Mahapatra & Gupta, 2017). In cases of depression, an individual typically loses responsiveness to emotional stimuli (Roseman et al., 2018). It is also suggested that the hyperfrontal metabolic pattern produced after psilocybin administration and 5-HT2A receptor activation is comparable to the metabolic patterns produced during acute psychotic episodes in chronic schizophrenics (Nichols, 2020).
Psilocybin is also reported to bind with high affinity to the 5-HT2A receptor subtype, but with low affinity to the 5-HT1A receptor subtype. The interaction of psilocybin and psilocin with 5-HT2A receptors to produce psychotomimetic effects has been confirmed in experiments with ketanserin, a 5-HT2A antagonist that attenuates psilocybin's effects (Lowe et al., 2021). In addition to the interaction with 5-HT2A receptors, it is also suggested that the psychopharmacological action of psilocybin may be mediated by receptors other than 5-HT2A (Halberstadt & Geyer, 2011; Mahapatra & Gupta, 2017). Psilocybin and psilocin also interact with 5-HT1D and 5-HT2C receptor subtypes (Lowe et al., 2021). Psilocybin is reported to produce significant changes in brain dynamics and functional connectivity (FC) between brain areas (Grimm et al., 2018). The psilocybin-induced alteration in brain connectivity involves the disintegration of associative networks and the integration of sensory function networks (Nichols, 2020). This dissociation may mediate the subjective effects of psilocybin use and an unrestricted cognition state (Nichols, 2020). Similarly, a possible mechanism behind the psychotomimetic effects of psilocybin is the interactions with feedback circuits between the cortex and thalamus([Lowe et al., 2021). Psilocybin administration produces general cortical activation (Nichols, 2020).
Upon oral administration, psilocybin converts to psilocin, which binds primarily to 5-HT2A receptors, inducing "mystical" hallucinatory effects through enhanced glutamatergic activity. Psilocin also promotes neuroplasticity and neuritogenesis via BDNF and mTOR pathways, contributing to its pharmacological actions, These actions are represented in
Figure 4 (Mastinu et al., 2023).
4. Effects of Psilocybin Ingestion
The physical effects of psilocybin ingestion depend on individual sensitivity to the components and the doses administered. Tolerance to psilocybin varies widely, so a safe practice is to start with low doses and assess specific reactions (Strumila et al., 2021). The effects of orally ingested psilocybin begin within 20-60 minutes, with a peak lasting 2 to 4 hours. A decrease in effects is typically perceived between 3-7 hours after consumption, and dose-dependent late effects can be experienced up to 24 hours post-ingestion (Geiger et al., 2018).
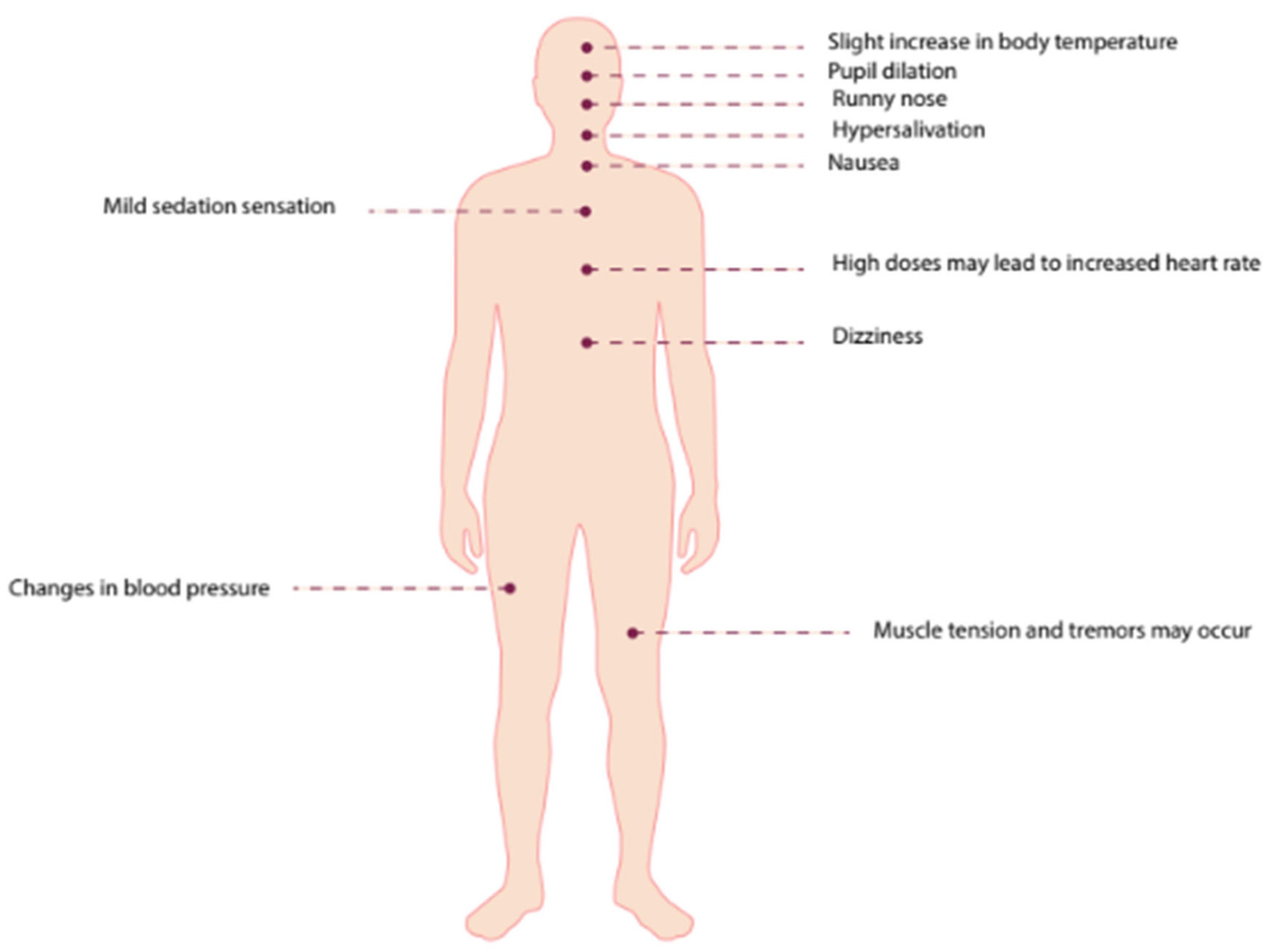
The usual dose in studies is 0.4 mg/kg. Psilocybin's effects are very similar to those of LSD. Symptoms reported include pupil dilation, changes in blood pressure, rhinorrhea, hypersalivation, slight increase in body temperature, mild sedation, dizziness, and nausea. Muscle tension and tremors may occur, and high doses can lead to increased heart rate (Greif & Šurkala, 2020).
Visual effects include color saturation, altered shape and color recognition, fusion of objects and colors, distorted perspective, and heightened auditory sensitivity. Hallucinations often involve vivid, colorful shapes and varying figures seen with both closed and open eyes(Geiger et al., 2018).
Psychological effects associated with psilocybin use include euphoria, a sense of weightlessness, increased empathy, simultaneous emotional experiences, heightened musical appreciation, a feeling of "ego dissolution," a need for "catharsis," and a sense of "rejuvenation." Psilocybin use is also associated with altered perceptions of time and space, derealization, and depersonalization. The effects can be perceived either positively or negatively depending on personal characteristics, knowledge of the objectives of psilocybin use, and the individual's particular life situation. Each psilocybinexperience is expected to yield a distinct result and perception from the user. A summary of these effects is illustrated in
Figure 5 (Geiger et al., 2018; Greif & Šurkala 2020).
The theory of "ego dissolution" refers to a state where the sense of self is perceived as "suspended." Psilocybin-induced experiences allow individuals to become more open to their social environment, fostering a heightened sense of connection with their surroundings and the people around them. Research has shown that psilocybin significantly improves emotional facial recognition and reduces feelings of social exclusion, making it particularly beneficial in treating affective disorders such as depression and even suicidal risk (Strumila et al., 2021). The personal and social changes reported with psilocybin ingestion are also advantageous in treating anxiety by diminishing persistent negative cognitions and emotional responses to chronic illnesses, such as cancer (Meade et al., 2022).
Adverse physiological effects of psychedelics are rare and generally transient, with cardiovascular responses linked to serotonergic and adrenergic actions being the most commonly reported. While precautions are advisable, severe adverse events are not typically observed. The primary risks arise from interactions with other medications, with psychological risks including adverse reactions in individuals predisposed to psychotic or manic episodes, trauma, or depression and anxiety. These negative emotional responses generally resolve with proper preparation and post-session psychological support, though more serious consequences such as existential crises, despair, or self-harm can occur (Agin-Liebes et al., 2020).
Interactions with other medications and other substances with psilocybin (Halman et al., 2024)reveals various effects. Pretreatment with anxiolytics such as buspirone significantly reduces psilocybin-induced visual distortion (Pokorny et al., 2016). Antipsychotics like chlorpromazine and haloperidol diminish psilocybin's effects on pupil dilation and visual distortions, though haloperidol can increase anxiety without affecting hallucinations (Keeler, 1967; Vollenweider et al., 1998). Risperidone also attenuates psilocybin's effects in a dose-dependent manner (Vollenweider et al., 1998). SSRIs like escitalopram decrease negative acute effects without altering the positive effects of psilocybin (Becker et al., 2022). Ketanserin, a 5-HT2A antagonist, blocks various psilocybin effects, including those on the Altered State of Consciousness Rating scale (Carter et al., 2005; Carter et al., 2007). Ergotamine does not significantly alter psilocybin experiences (Pokorny et al., 2016), and alcohol consumption does not notably affect psilocybin’s subjective effects, with most participants reporting unchanged effects (Barrett et al., 2000).
4. Psilocybin-Assisted Therapy in the Palliative Care Setting
Psychedelic-assisted therapies leverage altered states of consciousness induced by agents such as psilocybin, methylenedioxymethamphetamine (MDMA), or ketamine. Psilocybin has been shown to reduce depression, anxiety, and fear of death in patients with cancer-related depression and anxiety (Tai et al., 2021), with benefits persisting in 60% to 80% of patients at six months follow-up (Niles et al., 2021). Therefore, the goals of psilocybin treatment in palliative care aim to enhance comfort, improve quality of life, and alleviate pain and stress (Whinkin, 2023).
An important concept in palliative care is "existential distress," which is linked to the anticipation of responses to physical and psychosocial changes occurring during chronic illnesses, aggressive treatments, or conditions with high side effects, often seen in oncological diseases (Schimmel et al., 2022). "Existential distress" is a relatively new concept and lacks a clear definition; however, it is reported to affect 9% to 29% of terminal cancer patients (Schimmel et al., 2022). This distress is significantly related to the patient's personality before the oncological diagnosis, the presence of psychiatric conditions, prior life traumas such as loss of family members due to similar diagnoses or clinical illness, and the specific social and personal context at the time of disease onset. Anxiety, depression, and existential distress are some of the possible clinical manifestations at the end of life. Psychedelic therapies, with their range of physical and emotional reactions approaching integrative personal experiences, hold significant spiritual relevance (Schimmel et al., 2022).
In the context of palliative care, it has become essential to explore new treatment approaches with an integrative focus that includes individual psychological interventions, psychiatric evaluation (for symptoms that may impact treatment and patient choices), psychosocial interventions involving family (to ensure clear understanding of diagnosis, treatment options, and the best support methods tailored to each patient’s unique situation). Schimmel et al. review several important studies on the evidence of psilocybin in cancer patients. The prevalence of psychiatric symptoms in this population is estimated as follows: 14-21% anxiety, 10-25% mild depression, 15% major depression, and 6-19% adjustment disorders. A cross-sectional study found that 12% of cancer patients (n=377) had a serious desire for death, with 52% of these meeting criteria for anxiety and/or depression (Schimmel et al., 2022). An increasing number of oncology treatment units are expanding their therapeutic arsenal to address the mental health of patients with cancer diagnoses (Hernández et al., 2012).
Recent studies involving psilocybin, though limited in patient numbers, generally associate psilocybin with long-term improvements in "existential distress." Grob(2011) conducted an initial pilot study with advanced cancer patients (n=12) suffering from acute stress disorder, existential distress, generalized anxiety disorder, and cancer-related anxiety, all according to DSM-IV criteria. Patients received either psilocybin (0.2 mg/kg) or niacin (vitamin B3) as a placebo over two weeks. Improvements were observed in derealization and depersonalization dimensions associated with a positive mood (oceanic boundlessness) (p<0.001) and visionary restructuring (p<0.001). Ego dissolution results were marginally significant (p: 0.049). After one month, scores on the Beck Depression Inventory (BDI) decreased (p: 0.5), with more significant changes noted at six months (p: 0.03) (Grob et al., 2011). The study did not show a reduction in analgesic use or cancer-related pain intensity, and no adverse effects from the intervention were reported (Schimmel et al., 2022).
In 2016, Griffiths studied the effects of a high dose of psilocybin (22–30 mg/70 kg) versus a microdose considered as a placebo (1–3 mg/70 kg), building on previous studies (Griffiths et al.,2011;Griffiths et al., 2006) that established psychedelic and spiritual effects starting at 5 mg/70 kg. This study included patients with potentially terminal cancer and mood disorders (dysthymia, major depression, or mixed anxiety and depression disorder). The research demonstrated that high doses of psilocybin significantly improved depression in the long term, with notable effects observed both after the second administration and at six months follow-up. At six months, participants exhibited a clinical response rate of 78% to 83% in terms of reduced depression and anxiety symptoms. The psilocybin treatment also decreased demoralization and hopelessness, leading to improved spiritual well-being and quality of life, both in the short term (after 2 weeks) and the long term (after 6 months). Additionally, the treatment positively impacted measures related to spirituality, transcendence, life satisfaction, and acceptance of death, while reducing death-related distress.
Earlier studies by Griffiths and colleagues (2011; 2006) initially focused on the effects of psychedelics in healthy subjects without psychiatric comorbidities. These studies established the impact of psychedelics on mystical experiences, including feelings of unity and transcendence, fear of ego dissolution, and visionary attitudes. They also assessed scales of mysticism, spirituality, and transcendence related to death. The 2006 study compared high doses of psilocybin (30 mg/70 kg) with methylphenidate (Griffiths, 2006), while the 2011 study compared five doses of psilocybin (0, 5, 10, 20, and 30 mg/70 kg). It was found that subjective effects related to psychedelics began at 5 mg, with increased effects observed at higher doses of 20 to 30 mg. The higher doses were associated with mystical experiences, increased spirituality, and a greater sense of transcendence related to death (Griffiths et al., 2011).
In a randomized, crossover clinical trial with 29 patients suffering from cancer-related anxiety and depression, participants received either 0.3 mg/kg of psilocybin or 250 mg of niacin, combined with psychotherapy. The study found that psilocybin significantly improved anxiety and depressive symptoms, reduced demoralization and hopelessness, and enhanced spiritual well-being and quality of life. Serious adverse events were not reported, and no participants required psychiatric hospitalization. The most common adverse effects were increased blood pressure and heart rate (76%), headache (28%), and nausea (14%) (Ross et al., 2016).
Although it remains a debated topic, recent clinical trials involving oncology patients have shown significant reductions in death anxiety, as well as improved attitudes towards death and life (Ross et al., 2016; Griffiths et al., 2016). Even notions of transcendence in death have been observed in subjects without psychiatric comorbidities, as demonstrated by Griffiths (2011). Another dimension of psilocybin is its relationship to spiritual well-being. A Phase II trial administered a fixed dose of 25 mg of psilocybin to 30 patients with cancer and major depressive disorder, evaluating spiritual and psychosocial healing using the NIH-HEALS scale. The researchers found a significant improvement (p<0.001), although the study lacked a comparator and was not double-blinded, which limits its methodological rigor (Shnayder et al., 2023). Another pilot study, the HOPE trial, combined psilocybin with group psychotherapy for cancer patients with depression. This trial included three preparatory sessions, one high-dose psilocybin session (25 mg), and three integration sessions with a total of 12 participants. The sessions resulted in events such as nausea, headache, and hypertension, but no severe adverse events. The results showed significant improvement in depressive symptoms using the HAM-D questionnaire (p<0.001), based on the FACIT scale. The emotional, functional, spiritual meaning, spiritual peace, and spiritual faith domains showed statistically significant outcomes (p<0.05), and half of the group reported a mystical experience related to the transcendence of death scale (p: 0.014) (Lewis et al., 2023).
Demoralization, a form of existential suffering, was studied in older patients with a high-impact chronic illness like HIV/AIDS. This study involved 16 patients who received both individual and group psychotherapy, the latter using the Short-Term Supportive Group Therapy (SEGET) model, which is an existential psychotherapy centered on palliative care. The administration was divided into three groups: 0.3 mg/kg, 0.36 mg/kg, and a control group. This study demonstrated a decrease in patient demoralization with a standardized effect size of 0.47, and a confidence interval of 0.21 to 0.60 (Anderson et al., 2020).
In relation to palliative care there are few studies of psilocybin. It could be stated that the palliative conditions identified were oncology patients (Grobs, 2011, Ross, 2016, Griffths, 2016, Shnayder, 2023 and Lewis, 2024), and one study on patients with high impact chronic diseases such as HIV/AIDS (Anderson, 2020). In most studies these palliative conditions were related to psychiatric problems of anxiety and depression, but also to such important issues as demoralization, well-being, and acceptance of death. To conclude the studies that addressed the psychiatric problems of depression and anxiety in patients with oncological conditions, evaluating the use of psilocybin were those of Grobs (2011), Ross (2016), Griffths (2016) concluding that there was a significant decrease in the psilocybin intervention groups, versus placebo. In addition there was improvement in spiritual, emotional aspects as well as in well-being, and acceptance of death. In relation to high impact chronic pathologies, the use of psilocybin decreased demoralization (Anderson, 2020). Other studies explored the acute effects of psilocybin in healthy people without psychiatric comorbidities (Griffths, 2006 and Griffiths, 2011) that preceded the studies in palliative patients, and that allowed exploring the experiences and emotional dimensions of psilocybin use.
Regarding the doses administered, all the studies handled different doses and administration schedules, although low doses could be differentiated from high doses. From this it was concluded that in the long term, high doses allow sustained effects in the improvement of depression, positive attitudes towards life, self-perception, spirituality and increased acceptance and positive attitudes towards death. While these positive effects are of great importance for palliative patients, this must be weighed against the adverse effects, even more so when dealing with patients with high morbid burden. Consequently, adverse events were associated with high doses, with increased blood pressure and heart rate, headache, nausea and emesis, as well as physical discomfort being the most prevalent (Griffiths, 2016, Grobs, 2011, Lewis, 2023, Shnayder, 2023). (See
Table 2)
4.1. End of Life
One of the central goals of palliative care is to support individuals at the end of life by addressing suffering and managing symptoms, with pain and anxiety management being crucial for both patients and their families. Additionally, addressing existential and spiritual concerns is essential (Yaden et al., 2022). Pharmacotherapy options include opioids, serotoninergic antidepressants, sedative-hypnotics, and neuroleptics. However, these do not always provide immediate relief; for example, selective serotonin reuptake inhibitors (SSRIs) for depression do not have an immediate effect. In fact, in the first wave of psychedelic studies, these substances were administered to terminal patients at the end of life.
For the administration of psilocybin, it is recommended to discontinue SSRIs for at least 4 to 5 half-lives to prevent serotonin syndrome, often referred to as "serotonin washout." Although the concurrent use with other psychotropic drugs has not been extensively studied, some research suggests that combining psilocybin with haloperidol may lead to experiences of derealization accompanied by agitation and anxiety, while lithium has been associated with seizures. Caution is advised when using steroids concurrently due to the risk of inducing mania, and when using opioids due to sedation. Nevertheless, psilocybin's potential analgesic effects have also been investigated, with studies indicating pain reduction at low doses (Yaden et al., 2022).
Letheby (2024) reflects on how these effects are achieved, proposing that reduced fear of death may result from inducing experiences that diminish such fears. She connects this to acute psychedelic experiences, drawing parallels with non-drug-induced experiences such as mystical-religious experiences, meditation, and near-death experiences, which share phenomenological and psychological benefits. Letheby concludes that psychedelic experiences involve relaxation and a re-evaluation of abstract, high-level beliefs about the self and the world. This process can dissolve and reconstruct mental models, potentially leading to decreased fear of death, improved depressive and anxiety symptoms, and acceptance of life's processes (Letheby, 2024).
5. Legal Status of Psilocybin
Psychedelic mushrooms have a long history intertwined with human rituals, religious practices, medical uses, and recreational activities. In Mexico, the genus Psilocybe includes 53 known psychedelic species, historically used by indigenous cultures such as the Nahuatl, who referred to them as "teonanácatl," meaning "flesh of the gods." This term was first documented by the Franciscan friar Bernardino de Sahagún between 1569 and 1582. However, during the Spanish colonization, the ceremonial use of these mushrooms was suppressed and omitted from literature (Guzmán, 2008).
It was not until the 20th century that these mushrooms were rediscovered academically. Austrian physician Blas Pablo Reko first described the religious practices of the Mazatec indigenous people in northern Oaxaca using mushrooms. In 1938, botanist Richard Evans Schultes visited Huautla with Reko to observe ceremonies, collect samples, and identify the mushrooms, which were Psilocybe caerulescens, Panacolus campanulatus, and Stropharia cubensis. The identity of the Mazatec sacred mushrooms was further elucidated by writer and ethnobotanist R. Gordon Wasson and his wife, mycologist Valentina Pavlovna, who, guided by María Sabina, became the first Westerners to experience and describe the religious and mystical experiences induced by these mushrooms. Mycologist Roger Heim identified the sacred mushroom as Psilocybe, and chemist Albert Hofmann isolated the compound, naming it psilocybin (Guzmán, 2008).
In the 1950s and 1960s, psilocybin and other psychedelics garnered significant scientific and psychiatric interest. However, political and cultural factors, particularly during the 1970s, led to increased regulation and a halt in research. Recently, there has been renewed interest in the potential of psychedelics for treating psychiatric conditions and psychological effects related to end-of-life processes. Psilocybin and MDMA are being studied in conjunction with psychotherapy for their therapeutic potential. Psilocybin shows promise in treating psychological alterations associated with oncological diseases (Apud et al., 2023), depression, anxiety (Schimmel et al., 2022), and substance addiction.
Psilocybin has been designated as a "breakthrough therapy" by the FDA and is currently in Phase II clinical trials (COMPASS, 2018). This research involves teams from the US, UK, and Switzerland, with initial studies conducted at Johns Hopkins University, New York University, and Harbor-UCLA. In the UK, the Medical Research Council supported psilocybin studies for treatment-resistant depression in 2015. COMPASS Pathways is conducting the first large-scale clinical trial of psilocybin therapy for treatment-resistant depression in Europe and North America (Lowe et al., 2021).
Although emerging evidence supports the use of psilocybin for treating PTSD (Dos Santos et al., 2018; Gill et al., 2020; Goldberg et al., 2020; Illingworth et al., 2021; Nutt et al., 2020; Vargas et al., 2020), its efficacy for anxiety disorders, substance abuse, and end-of-life disturbances is also being explored (Kisely et al., 2023).
In Australia, no psychedelic substances have been approved for clinical use. In February 2020, Australia's Therapeutic Goods Administration (TGA) rejected a proposal to reclassify MDMA and psilocybin from Schedule 9 (Prohibited Substances) to Schedule 8 (Controlled Drugs), which are substances with potential for abuse or dependence. Controlled drugs have legitimate therapeutic uses and can be prescribed under strict legislative controls, whereas prohibited substances lack established therapeutic use and are only available for medical or scientific research, analytical, educational, or training purposes (Kisely et al., 2023).
Countries where psilocybin is decriminalized or legally used include Jamaica, the Netherlands, the Bahamas, Brazil, Nepal, Mexico, Peru, and Portugal. In the United States and Canada, its use is restricted to approved clinical studies or religious groups that sanction its use (Whinkin et al., 2023).
Recent systematic reviews have highlighted that the number of studies remains limited, with few participants (Andersen et al., 2021; Dos Santos et al., 2018; [Kisely et al., 2023]. Additionally, reviews have generally focused on specific diagnoses, and it is not always clear if there were language restrictions in bibliographic searches. Only one review assessed the overall credibility of results using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework recommended by Cochrane Collaboration, but this was limited to PTSD, and how the guidelines were applied remains unclear [Varker et al., 2021]. For drugs with a rapid onset of action, it is important to consider studies with both inactive and active controls in separate comparisons. This is because if participants are told there will be a brief interval before the onset of any effects, it becomes very apparent that they are receiving an inactive placebo. Thus, there is a risk that any response in the intervention group may be amplified by expectancy effects, while in control groups, it may be diminished by disappointment from receiving a placebo (Muthukumaraswamy et al., 2021). Such contrasting reactions can artificially inflate the treatment effect [Kisely et al., 2023].
The future of psilocybin in palliative care is contingent on several key developments:
1. *Regulatory Changes*: Legislative shifts toward decriminalization and medicalization of psilocybin are crucial. Efforts to reclassify psilocybin from a Schedule I substance to a controlled but medically accessible drug are underway in various regions. Success in these areas will facilitate broader clinical application and acceptance.
2. *Clinical Research*: Expanding the body of clinical evidence through large-scale, randomized controlled trials will solidify psilocybin’s efficacy and safety profile. Research should focus on optimal dosing, administration protocols, and long-term outcomes to establish standardized guidelines for use in palliative care.
3. *Interdisciplinary Integration*: Incorporating psilocybin therapy into existing palliative care frameworks requires collaboration across medical, psychological, and spiritual disciplines. Training healthcare providers in psychedelic-assisted therapy and integrating these practices into holistic care models will be vital for seamless adoption.
4. *Public Perception and Education*: Addressing public misconceptions and educating both the general public and healthcare professionals about the benefits and risks of psilocybin is essential. Initiatives to destigmatize psychedelic therapy and highlight its scientific backing can foster a more supportive environment for its integration.
5. *Ethical and Cultural Considerations*: Ethical considerations, including informed consent, patient autonomy, and cultural sensitivity, must guide the use of psilocybin in palliative care. Respecting diverse cultural perspectives on psychedelics and ensuring equitable access to therapy will be important as the field progresses.
While significant challenges remain, the potential of psilocybin to transform palliative care is immense. Through continued research, advocacy, and interdisciplinary collaboration, psilocybin can become a valuable tool in the holistic management of terminal illness, providing relief and comfort to patients during their most vulnerable times.
- 2.
Conclusions
Psilocybin emerges as a promising therapeutic option in palliative care, demonstrating significant potential to enhance the quality of life for terminally ill patients. Its ability to alleviate psychological distress, improve emotional well-being, and facilitate profound existential experiences highlights its utility beyond traditional psychiatric applications. Current research supports the notion that psilocybin can effectively reduce symptoms of anxiety, depression, and existential distress in patients with advanced illness, offering a holistic approach that addresses both physical and psychological suffering.
The investigation of psilocybin as a therapeutic agent is increasingly complex when considering the potential "entourage effect" of whole mushroom extracts versus isolated psilocybin. While psilocybin, a naturally occurring tryptamine alkaloid, has shown promise in treating various psychiatric disorders, recent preclinical studies highlight that psilocybin-containing mushroom extracts (PME) may produce different biological effects compared to chemically synthesized psilocybin (PSIL). Comparative analyses reveal that PME exhibits more pronounced and sustained impacts on synaptic plasticity markers and metabolomic profiles in animal models. Specifically, PME has been observed to significantly increase neuroplasticity-related proteins such as GAP43 and synaptophysin in several brain regions, effects not as robustly seen with PSIL alone. Additionally, metabolomic data suggest a gradient of metabolic effects between vehicle, PSIL, and PME, indicating that the full spectrum of compounds in PME may contribute synergistically to its therapeutic efficacy. This suggests that the complex mixture of bioactive compounds in PME could be crucial for its enhanced effects (ref). Further research is essential to elucidate the specific molecules responsible for these differential effects and to clarify how the entourage effect may influence the therapeutic outcomes of psilocybin-based treatments.
The integration of psilocybin into palliative care could represent a significant shift in end-of-life care paradigms, underscoring the importance of addressing mental and emotional health alongside physical needs. Despite these promising findings, the advancement of psilocybin therapy faces challenges due to existing stigmatization and legal restrictions. Addressing these barriers through continued advocacy, rigorous clinical trials, and comprehensive policy reforms is crucial for unlocking the full therapeutic potential of psilocybin.
In the realm of Psychedelic-Assisted Therapies (PAT), the patient-doctor relationship plays a pivotal role. The success of such therapies is closely tied to the patient's trust in their physician, the thoroughness of the information provided about the medication, and the clarity of communication regarding expected physical and psychological effects. Patients must be well-informed about their diagnosis, treatment alternatives, and the specific goals of the prescribed therapy. Ensuring that patients are comfortable with the treatment plan and have the option to discontinue if desired aligns with ethical standards and bioethical principles.
To mitigate the risk of adverse reactions, it is essential to have a comprehensive understanding of the patient’s medical history, including any psychiatric conditions, recreational substance use, and diagnostic health issues. Rigorous clinical assessments should be conducted to monitor vital signs and other variables potentially impacted by psilocybin. An open dialogue regarding the patient’s expectations and concerns about psilocybin, along with a clear explanation of its potential benefits and risks, is fundamental.
Oncological diseases often disrupt patients' lives by affecting their physical capabilities, causing chronic exhaustion, and limiting daily activities, while also impacting work and economic situations, and eliciting reactions from their support network. In such contexts, a lack of personal relationships or adequate social support can exacerbate existential stress. Addressing these multifaceted challenges with an integrated approach that includes innovative therapies like psilocybin could significantly improve the overall well-being of patients in palliative care.
Author Contributions
Andres David Turizo-Smith: Methodology, Conceptualization, Validation, Investigation, Resources, Writing - review & editing. Natalia Botero Jaramillo: Conceptualization,Investigation, Writing - original draft. Diana Milena Berrio Cuartas: Writing - review & editing,Investigation, Conceptualization.
Funding
No funding was received for the publication of this work.
Acknowledgements
We would like to express our sincere gratitude to Edgar Gonzalez Rodriguez, a cultivator of mushrooms and fungi, for providing us with the photograph of Psilocybe. We also extend our heartfelt thanks to Dr. Viviana Peskin, psychiatrist, for her valuable feedback and thorough review of the draft.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article. The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics Statement
This study did not involve human participants or clinical interventions requiring registration. Therefore, a clinical trial number is not applicable.
References
- Agin-Liebes, G. I., Malone, T., Yalch, M. M., Mennenga, S. E., Ponté, K. L., Guss, J., Bossis, A. P., Grigsby, J., Fischer, S., & Ross, S. (2020). Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. Journal of psychopharmacology (Oxford, England), 34(2), 155–166. [CrossRef]
- Albert, P. R., Benkelfat, C., & Descarries, L. (2012). The neurobiology of depression--revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 367(1601), 2378–2381. [CrossRef]
- Anderson, B. T., Danforth, A., Daroff, P. R., Stauffer, C., Ekman, E., Agin-Liebes, G., Trope, A., Boden, M. T., Dilley, P. J., Mitchell, J., & Woolley, J. (2020). Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: An open-label safety and feasibility pilot study. EClinicalMedicine, 27, 100538. [CrossRef]
- Apud S, Montero F & Craig I. (2023).Revisión sistemática de la terapia con psilocibina en ansiedad y depresión de pacientes oncológicos. Cuadernos de Neuropsicología / Panamerican Journal of Neuropsychology ISSN: 0718-4123, Vol. 17 Nº 3, p. 30 - 49. [CrossRef]
- Barrett, S. P., Archambault, J., Engelberg, M. J., & Pihl, R. O. (2000). Hallucinogenic drugs attenuate the subjective response to alcohol in humans. Human psychopharmacology, 15(7), 559–565. [CrossRef]
- Becker, A. M., Holze, F., Grandinetti, T., Klaiber, A., Toedtli, V. E., Kolaczynska, K. E., Duthaler, U., Varghese, N., Eckert, A., Grünblatt, E., & Liechti, M. E. (2022). Acute Effects of Psilocybin After Escitalopram or Placebo Pretreatment in a Randomized, Double-Blind, Placebo-Controlled, Crossover Study in Healthy Subjects. Clinical pharmacology and therapeutics, 111(4), 886–895. [CrossRef]
- Bogenschutz, M. P., & Pommy, J. M. (2012). Therapeutic mechanisms of classic hallucinogens in the treatment of addictions: from indirect evidence to testable hypotheses. Drug testing and analysis, 4(7-8), 543–555. [CrossRef]
- Carter, O. L., Burr, D. C., Pettigrew, J. D., Wallis, G. M., Hasler, F., & Vollenweider, F. X. (2005). Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. Journal of cognitive neuroscience, 17(10), 1497–1508. [CrossRef]
- Carter, O. L., Hasler, F., Pettigrew, J. D., Wallis, G. M., Liu, G. B., & Vollenweider, F. X. (2007). Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology, 195(3), 415–424. [CrossRef]
- Dale, N. C., Johnstone, E. K. M., & Pfleger, K. D. G. (2022). GPCR heteromers: An overview of their classification, function and physiological relevance. Frontiers in endocrinology, 13, 931573. [CrossRef]
- De Bartolomeis, A., Buonaguro, E. F., & Iasevoli, F. (2013). Serotonin-glutamate and serotonin-dopamine reciprocal interactions as putative molecular targets for novel antipsychotic treatments: from receptor heterodimers to postsynaptic scaffolding and effector proteins. Psychopharmacology, 225(1), 1–19. [CrossRef]
- De Gregorio, D., Aguilar-Valles, A., Preller, K. H., Heifets, B. D., Hibicke, M., Mitchell, J., & Gobbi, G. (2021). Hallucinogens in Mental Health: Preclinical and Clinical Studies on LSD, Psilocybin, MDMA, and Ketamine. The Journal of neuroscience : the official journal of the Society for Neuroscience, 41(5), 891–900. [CrossRef]
- Dos Santos, R. G., Hallak, J. E., Baker, G., & Dursun, S. (2021). Hallucinogenic/psychedelic 5HT2A receptor agonists as rapid antidepressant therapeutics: Evidence and mechanisms of action. Journal of Psychopharmacology, 35(4), 453-458. DOI: 0269881120986422.
- Ferré, S., Baler, R., Bouvier, M., Caron, M. G., Devi, L. A., Durroux, T., Fuxe, K., George, S. R., Javitch, J. A., Lohse, M. J., Mackie, K., Milligan, G., Pfleger, K. D., Pin, J. P., Volkow, N. D., Waldhoer, M., Woods, A. S., & Franco, R. (2009). Building a new conceptual framework for receptor heteromers. Nature chemical biology, 5(3), 131–134. [CrossRef]
- Fribourg, M., Moreno, J. L., Holloway, T., Provasi, D., Baki, L., Mahajan, R., Park, G., Adney, S. K., Hatcher, C., Eltit, J. M., Ruta, J. D., Albizu, L., Li, Z., Umali, A., Shim, J., Fabiato, A., MacKerell, A. D., Jr, Brezina, V., Sealfon, S. C., Filizola, M., … Logothetis, D. E. (2011). Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell, 147(5), 1011–1023. [CrossRef]
- Gasque, G., Conway, S., Huang, J., Rao, Y., & Vosshall, L. B. (2013). Small molecule drug screening in Drosophila identifies the 5HT2A receptor as a feeding modulation target. Scientific reports, 3, srep02120. [CrossRef]
- Geiger, H. A., Wurst, M. G., & Daniels, R. N. (2018). DARK Classics in Chemical Neuroscience: Psilocybin. ACS chemical neuroscience, 9(10), 2438–2447. [CrossRef]
- Greif, A., & Šurkala, M. (2020). Compassionate use of psychedelics. Medicine, health care, and philosophy, 23(3), 485–496. [CrossRef]
- Goldberg, S. B., Pace, B. T., Nicholas, C. R., Raison, C. L., & Hutson, P. R. (2020). The experimental effects of psilocybin on symptoms of anxiety and depression: A meta-analysis. Psychiatry research, 284, 112749. [CrossRef]
- Gomes I, Ayoub MA, Fujita W, Jaeger WC, Pfleger KDG, Devi LA. G Protein–coupled receptor heteromers. Annu Rev Pharmacol Toxicol (2016) 56:403–25. [CrossRef]
- González-Maeso, J., Ang, R. L., Yuen, T., Chan, P., Weisstaub, N. V., López-Giménez, J. F., Zhou, M., Okawa, Y., Callado, L. F., Milligan, G., Gingrich, J. A., Filizola, M., Meana, J. J., & Sealfon, S. C. (2008). Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature, 452(7183), 93–97. [CrossRef]
- Greif, A., & Šurkala, M. (2020). Compassionate use of psychedelics. Medicine, health care, and philosophy, 23(3), 485–496. [CrossRef]
- Griffiths, R. R., Johnson, M. W., Richards, W. A., Richards, B. D., McCann, U., & Jesse, R. (2011). Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology, 218(4), 649–665. [CrossRef]
- Griffiths, R. R., Johnson, M. W., Carducci, M. A., Umbricht, A., Richards, W. A., Richards, B. D., Cosimano, M. P., & Klinedinst, M. A. (2016). Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. Journal of psychopharmacology (Oxford, England), 30(12), 1181–1197. [CrossRef]
- Grimm, O., Kraehenmann, R., Preller, K. H., Seifritz, E., & Vollenweider, F. X. (2018). Psilocybin modulates functional connectivity of the amygdala during emotional face discrimination. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology, 28(6), 691–700. [CrossRef]
- Grob, C. S., Danforth, A. L., Chopra, G. S., Hagerty, M., McKay, C. R., Halberstadt, A. L., & Greer, G. R. (2011). Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Archives of general psychiatry, 68(1), 71–78. [CrossRef]
- Gupta, A., Mulder, J., Gomes, I., Rozenfeld, R., Bushlin, I., Ong, E., Lim, M., Maillet, E., Junek, M., Cahill, C. M., Harkany, T., & Devi, L. A. (2010). Increased abundance of opioid receptor heteromers after chronic morphine administration. Science signaling, 3(131), ra54. [CrossRef]
- Guzmán, G. (2008). Hallucinogenic Mushrooms in Mexico: An Overview. Econ Bot 62, 404–412. [CrossRef]
- Halberstadt, A. L., & Geyer, M. A. (2011). Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology, 61(3), 364–381. [CrossRef]
- Halman, A., Kong, G., Sarris, J., & Perkins, D. (2024). Drug-drug interactions involving classic psychedelics: A systematic review. Journal of psychopharmacology (Oxford, England), 38(1), 3–18. [CrossRef]
- Hassan, Z., Bosch, O. G., Singh, D., Narayanan, S., Kasinather, B. V., Seifritz, E., Kornhuber, J., Quednow, B. B., & Müller, C. P. (2017). Novel Psychoactive Substances-Recent Progress on Neuropharmacological Mechanisms of Action for Selected Drugs. Frontiers in psychiatry, 8, 152. [CrossRef]
- Hernández M, Cruzado JA, Prado C,Rodríguez E, Hernández C, González MA, Martín JC.(2012) SALUD MENTAL Y MALESTAR EMOCIONAL EN PACIENTES CON CÁNCER. PSICOONCOLOGÍA.9 (2-3),233-257. ISSN:1696-7240. [CrossRef]
- Hoffman, A., Heim, R., Brack, A., & Kobel, H. (1958). Psilocybin, ein psychotroper Wirkstoff aus dem mexikanischen Rauschpilz Psilocybe mexicana Heim [Psilocybin, a psychotropic substance from the Mexican mushroom Psilicybe mexicana Heim]. Experientia, 14(3), 107–109. Experientia. [CrossRef]
- Holtzheimer, P. E., & Mayberg, H. S. (2011). Stuck in a rut: rethinking depression and its treatment. Trends in neurosciences, 34(1), 1–9. [CrossRef]
- Huxley, J. (1968). Transhumanism. Journal of Humanistic Psychology, 8(1), 73-76. [CrossRef]
- Johnson, M. W., & Griffiths, R. R. (2017). Potential Therapeutic Effects of Psilocybin. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics, 14(3), 734–740. [CrossRef]
- Kargbo R. B. (2020). Psilocybin Therapeutic Research: The Present and Future Paradigm. ACS medicinal chemistry letters, 11(4), 399–402. [CrossRef]
- Johnson, M. W., Hendricks, P. S., Barrett, F. S., & Griffiths, R. R. (2019). Classic psychedelics: An integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacology & therapeutics, 197, 83–102. [CrossRef]
- Kast, E. C., & Collins, V. J. (1964). Study of lysergic acid diethylamide as an analgesic agent. Anesthesia and analgesia, 43, 285–291.
- Kargbo R. B. (2020). Psilocybin Therapeutic Research: The Present and Future Paradigm. ACS medicinal chemistry letters, 11(4), 399–402. [CrossRef]
- Keeler MH. (1967) Chlorpromazine antagonism of psilocybin effect. International Journal of Neuropsychiatry 3: 66–71.
- Kisely, S., Connor, M., Somogyi, A. A., & Siskind, D. (2023). A systematic literature review and meta-analysis of the effect of psilocybin and methylenedioxymethamphetamine on mental, behavioural or developmental disorders. The Australian and New Zealand journal of psychiatry, 57(3), 362–378. [CrossRef]
- Lee, J. W., Park, M. S., Park, J. H., Cho, Y., Kim, C., Kim, C. S., Jo, J. W., & Lim, Y. W. (2020). Taxonomic Study of the Genus Pholiota (Strophariaceae, Basidiomycota) in Korea. Mycobiology, 48(6), 476–483. [CrossRef]
- Lenz, C., Sherwood, A., Kargbo, R., & Hoffmeister, D. (2021). Taking Different Roads: l-Tryptophan as the Origin of Psilocybe Natural Products. ChemPlusChem, 86(1), 28–35. [CrossRef]
- Letheby, C. ¿Cómo reducen los psicodélicos el miedo a la muerte? 2024. Neuroethics 17, 27.
https://doi-org.ez.unisabana.edu.co/10.1007/s12152-024-09564-3.
- Lewis, B. R., Garland, E. L., Byrne, K., Durns, T., Hendrick, J., Beck, A., & Thielking, P. (2023). HOPE: A Pilot Study of Psilocybin Enhanced Group Psychotherapy in Patients With Cancer. Journal of pain and symptom management, 66(3), 258–269. [CrossRef]
- Lowe, H., Toyang, N., Steele, B., Valentine, H., Grant, J., Ali, A., Ngwa, W., & Gordon, L. (2021). The Therapeutic Potential of Psilocybin. Molecules (Basel, Switzerland), 26(10), 2948. [CrossRef]
- Mahapatra, A., & Gupta, R. (2017). Role of psilocybin in the treatment of depression. Therapeutic advances in psychopharmacology, 7(1), 54–56. [CrossRef]
- Mastinu, A., Anyanwu, M., Carone, M., Abate, G., Bonini, S. A., Peron, G., Tirelli, E., Pucci, M., Ribaudo, G., Oselladore, E., Premoli, M., Gianoncelli, A., Uberti, D. L., & Memo, M. (2023). The Bright Side of Psychedelics: Latest Advances and Challenges in Neuropharmacology. International journal of molecular sciences, 24(2), 1329. [CrossRef]
- Meade, E., Hehir, S., Rowan, N., & Garvey, M. (2022). Mycotherapy: Potential of Fungal Bioactives for the Treatment of Mental Health Disorders and Morbidities of Chronic Pain. Journal of fungi (Basel, Switzerland), 8(3), 290. [CrossRef]
- Mithoefer, M. C., Grob, C. S., & Brewerton, T. D. (2016). Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. The lancet. Psychiatry, 3(5), 481–488. [CrossRef]
- Moreno, J. L., Miranda-Azpiazu, P., García-Bea, A., Younkin, J., Cui, M., Kozlenkov, A., Ben-Ezra, A., Voloudakis, G., Fakira, A. K., Baki, L., Ge, Y., Georgakopoulos, A., Morón, J. A., Milligan, G., López-Giménez, J. F., Robakis, N. K., Logothetis, D. E., Meana, J. J., & González-Maeso, J. (2016). Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Science signaling, 9(410), ra5. [CrossRef]
- Nichols D. E. (2020). Psilocybin: from ancient magic to modern medicine. The Journal of antibiotics, 73(10), 679–686. [CrossRef]
- Niles, H., Fogg, C., Kelmendi, B., & Lazenby, M. (2021). Palliative care provider attitudes toward existential distress and treatment with psychedelic-assisted therapies. BMC palliative care, 20(1), 191. [CrossRef]
- Pokorny, T., Preller, K. H., Kraehenmann, R., & Vollenweider, F. X. (2016). Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur Neuropsychopharmacol: J Eur Coll Neuropsychopharmacol 26: 756–766.
- Roseman, L., Nutt, D. J., & Carhart-Harris, R. L. (2018). Quality of Acute Psychedelic Experience Predicts Therapeutic Efficacy of Psilocybin for Treatment-Resistant Depression. Frontiers in pharmacology, 8, 974. [CrossRef]
- Ross, S., Bossis, A., Guss, J., Agin-Liebes, G., Malone, T., Cohen, B., Mennenga, S. E., Belser, A., Kalliontzi, K., Babb, J., Su, Z., Corby, P., & Schmidt, B. L. (2016). Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. Journal of psychopharmacology (Oxford, England), 30(12), 1165–1180. [CrossRef]
- Shah, U. H., & González-Maeso, J. (2019). Serotonin and Glutamate Interactions in Preclinical Schizophrenia Models. ACS chemical neuroscience, 10(7), 3068–3077. [CrossRef]
- Shah, U., Pincas, H., Sealfon, S. C., González-Maeso, J. (2020). Structure and function of serotonin GPCR heteromers. In C. P. Müller & K. A. Cunningham (Eds.), Handbook of Behavioral Neuroscience (Vol. 31, pp. 217-238). Elsevier. [CrossRef]
- Shahar, O., Botvinnik, A., Shwartz, A., Lerer, E., Golding, P., Buko, A., Hamid, E., Kahn, D., Guralnick, M., Blakolmer, K., Wolf, G., Lotan, A., Lerer, L., Lerer, B., & Lifschytz, T. (2024). Effect of chemically synthesized psilocybin and psychedelic mushroom extract on molecular and metabolic profiles in mouse brain. Molecular psychiatry, 10.1038/s41380-024-02477-w. Advance online publication. [CrossRef]
- Shnayder, S., Ameli, R., Sinaii, N., Berger, A., & Agrawal, M. (2023). Psilocybin-assisted therapy improves psycho-social-spiritual well-being in cancer patients. Journal of affective disorders, 323, 592–597. [CrossRef]
- Schimmers, N., Breeksema, J. J., Smith-Apeldoorn, S. Y., Veraart, J., van den Brink, W., & Schoevers, R. A. (2022). Psychedelics for the treatment of depression, anxiety, and existential distress in patients with a terminal illness: a systematic review. Psychopharmacology, 239(1), 15–33. [CrossRef]
- Strumila, R., Nobile, B., Korsakova, L., Lengvenyte, A., Olie, E., Lopez-Castroman, J., Guillaume, S., & Courtet, P. (2021). Psilocybin, a Naturally Occurring Indoleamine Compound, Could Be Useful to Prevent Suicidal Behaviors. Pharmaceuticals (Basel, Switzerland), 14(12), 1213. [CrossRef]
- Šustr, V., Stingl, U., & Brune, A. (2014). Microprofiles of oxygen, redox potential, and pH, and microbial fermentation products in the highly alkaline gut of the saprophagous larva of Penthetria holosericea (Diptera: Bibionidae). Journal of insect physiology, 67, 64–69. [CrossRef]
- Tai, S. J., Nielson, E. M., Lennard-Jones, M., Johanna Ajantaival, R. L., Winzer, R., Richards, W. A., Reinholdt, F., Richards, B. D., Gasser, P., & Malievskaia, E. (2021). Development and Evaluation of a Therapist Training Program for Psilocybin Therapy for Treatment-Resistant Depression in Clinical Research. Frontiers in psychiatry, 12, 586682. [CrossRef]
- Tanne J. H. (2004). Humphry Osmond. BMJ : British Medical Journal, 328(7441),713. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC381240/.
- Trifilieff, P., Rives, M. L., Urizar, E., Piskorowski, R. A., Vishwasrao, H. D., Castrillón, J., Schmauss, C., Slättman, M., Gullberg, M., & Javitch, J. A. (2011). Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. BioTechniques, 51(2), 111–118. [CrossRef]
- Vargas, M. V., Dunlap, L. E., Dong, C., Carter, S. J., Tombari, R. J., Jami, S. A., Cameron, L. P., Patel, S. D., Hennessey, J. J., Saeger, H. N., McCorvy, J. D., Gray, J. A., Tian, L., & Olson, D. E. (2023). Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science (New York, N.Y.), 379(6633), 700–706. [CrossRef]
- Varker, T., Jones, K. A., Arjmand, H.-A., Hinton, M., Hiles, S. A., Freijah, I., Forbes, D., Kartal, D., Phelps, A., Bryant, R. A., McFarlane, A., Hopwood, M., & O'Donnell, M. (2021). Dropout from guideline-recommended psychological treatments for posttraumatic stress disorder: A systematic review and meta-analysis. Journal of Affective Disorders Reports, 4, 100093. [CrossRef]
- Vollenweider, F. X., Vollenweider-Scherpenhuyzen, M. F., Bäbler, A., Vogel, H., & Hell, D. (1998). Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport, 9(17), 3897–3902. [CrossRef]
- Williams, M. L., Korevaar, D., Harvey, R., Fitzgerald, P. B., Liknaitzky, P., O'Carroll, S., Puspanathan, P., Ross, M., Strauss, N., & Bennett-Levy, J. (2021). Translating Psychedelic Therapies From Clinical Trials to Community Clinics: Building Bridges and Addressing Potential Challenges Ahead. Frontiers in psychiatry, 12, 737738. [CrossRef]
- Whinkin, E., Opalka, M., Watters, C., Jaffe, A., & Aggarwal, S. (2023). Psilocybin in Palliative Care: An Update. Current geriatrics reports, 12(2), 50–59. [CrossRef]
- Yaden, D. B., Nayak, S. M., Gukasyan, N., Anderson, B. T., & Griffiths, R. R. (2022). The Potential of Psychedelics for End of Life and Palliative Care. Current topics in behavioral neurosciences, 56, 169–184. [CrossRef]
- Zamaria, J.A. (2016) A Phenomenological Examination of Psilocybin and its Positive and Persisting After effects. NeuroQuantologym 13 (2), 285- 296. [CrossRef]
- Ziff, S., Stern, B., Lewis, G., Majeed, M., & Gorantla, V. R. (2022). Analysis of Psilocybin-Assisted Therapy in Medicine: A Narrative Review. Cureus, 14(2), e21944. [CrossRef]
Figure 1.
Psilocybe mushrooms and Psilocybin. (A) Psilocybe cubensis (Photo donated by Edgar Gonzalez Rodriguez). (B) Chemical structure of psilocybin in 2D. (C) Chemical structure of psilocybin in 3D.All molecular visualization were Created by the author with MolView (n.d.). Retrieved August 25, 2024, from
https://molview.org/?cid=10624.
Figure 1.
Psilocybe mushrooms and Psilocybin. (A) Psilocybe cubensis (Photo donated by Edgar Gonzalez Rodriguez). (B) Chemical structure of psilocybin in 2D. (C) Chemical structure of psilocybin in 3D.All molecular visualization were Created by the author with MolView (n.d.). Retrieved August 25, 2024, from
https://molview.org/?cid=10624.
Figure 2.
Naturally Occurring Tryptamine Analogues of Psilocybin: (A)Serotonin, (B) Dimethyltryptamine, (C)Psilocin, (D)bufotenin Source: Created by the author.
Figure 2.
Naturally Occurring Tryptamine Analogues of Psilocybin: (A)Serotonin, (B) Dimethyltryptamine, (C)Psilocin, (D)bufotenin Source: Created by the author.
Figure 3.
Phase I and phase II metabolites of psilocybin, Source: Created by the author.
Figure 3.
Phase I and phase II metabolites of psilocybin, Source: Created by the author.
Figure 4.
After oral administration, psilocybin loses its phosphate group and is fully converted into psilocin, which consequently represents the primary derivative responsible for its pharmacological activity. (A) Psilocin has a high affinity for the 5-HT2A receptor, and this binding is responsible for the "mystical" hallucinatory effects induced by psilocin. In order of increasing affinity, psilocin can also bind to 5-HT2B, 5-HT1D, dopamine D1, 5-HT1E, 5-HT1A, 5-HT5A, 5-HT7, 5-HT6, D3, 5-HT2C, and 5-HT1B receptors. (B) Activation of the 5-HT2A receptor in the prefrontal cortex by psilocin results in increased glutamatergic activity with the release of glutamate at AMPA and NMDA receptors on cortical pyramidal neurons. (C) Psilocin has been observed to exert its pharmacological action by enhancing neuroplasticity and neuritogenesis through the BDNF and mTOR pathways. Taken from Mastinu et al., 2023, The Bright Side of Psychedelics: Latest Advances and Challenges in Neuropharmacology. International journal of molecular sciences, 24(2), 1329. licensed under CC BY 4.0.
Figure 4.
After oral administration, psilocybin loses its phosphate group and is fully converted into psilocin, which consequently represents the primary derivative responsible for its pharmacological activity. (A) Psilocin has a high affinity for the 5-HT2A receptor, and this binding is responsible for the "mystical" hallucinatory effects induced by psilocin. In order of increasing affinity, psilocin can also bind to 5-HT2B, 5-HT1D, dopamine D1, 5-HT1E, 5-HT1A, 5-HT5A, 5-HT7, 5-HT6, D3, 5-HT2C, and 5-HT1B receptors. (B) Activation of the 5-HT2A receptor in the prefrontal cortex by psilocin results in increased glutamatergic activity with the release of glutamate at AMPA and NMDA receptors on cortical pyramidal neurons. (C) Psilocin has been observed to exert its pharmacological action by enhancing neuroplasticity and neuritogenesis through the BDNF and mTOR pathways. Taken from Mastinu et al., 2023, The Bright Side of Psychedelics: Latest Advances and Challenges in Neuropharmacology. International journal of molecular sciences, 24(2), 1329. licensed under CC BY 4.0.

Figure 5.
Effects of Psilocybin in Humans. This figure summarizes the effects of psilocybin in humans, which include pupil dilation, changes in blood pressure, rhinorrhea, hypersalivation, a slight increase in body temperature, mild sedation, dizziness, and nausea. Muscle tension and tremors may occur, with high doses potentially increasing heart rate (Greif & Šurkala 2020). Visually, psilocybin causes color saturation, altered shape and color recognition, fusion of objects and colors, distorted perspective, and heightened auditory sensitivity. Hallucinations often feature vivid, colorful shapes and varying figures seen with both closed and open eyes. Source: Created by the author.
Figure 5.
Effects of Psilocybin in Humans. This figure summarizes the effects of psilocybin in humans, which include pupil dilation, changes in blood pressure, rhinorrhea, hypersalivation, a slight increase in body temperature, mild sedation, dizziness, and nausea. Muscle tension and tremors may occur, with high doses potentially increasing heart rate (Greif & Šurkala 2020). Visually, psilocybin causes color saturation, altered shape and color recognition, fusion of objects and colors, distorted perspective, and heightened auditory sensitivity. Hallucinations often feature vivid, colorful shapes and varying figures seen with both closed and open eyes. Source: Created by the author.
Table 1.
Summary of Characteristics of Heteromers with Physiological Relevance.
Table 1.
Summary of Characteristics of Heteromers with Physiological Relevance.
| 1 |
Receptor protomers must have the ability to closely interact in vivo.
|
| 2 |
Exhibit biochemical properties distinct from their individual monomers. |
| 3 |
Alteration of the heteromer’s function should be observable after the loss of functionality of the heteromeric receptor. |
Table 2.
Summary of clinical studies with psilocybin in healthy and palliative patients.
Table 2.
Summary of clinical studies with psilocybin in healthy and palliative patients.
| Research |
Methodology |
Research instruments and measurements |
Conclusions |
| Palliative patients |
| Lewis et.al (Lewis, 2023) HOPE trial (A Pilot Study of Psilocybin Enhanced Group Psychotherapy in Patients with Cancer) |
Open-label, single-arm, pilot study with psilocybin-assisted group therapy in cancer patients with DSM-5 depressive disorder. High dose of 25 mg psilocybin. Included 12 participants. |
It included 3 preparatory sessions, a high-dose psilocybin intake session of 25 mg and 3 integration sessions with 12 participants, follow-up at 2 weeks and 26 weeks.
The primary outcome was the measurement of depressive symptoms, measured by the 17-Item-HAM-D scale at day 0 and at 2 weeks and 26 weeks after the intervention.Secondary outcomes included the Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being Scale version 4 (FACIT-sp) and The Death Transcendence Scale. MED-30 (Mystical experience questionnaire) was administered at the end of the day of dose administration.
|
Nausea and headache were reported as adverse effects. The primary outcome was measured with the HAM-D scale, 3/12 patients showed a clinically significant change (4-6 points) and 7-12 patients a clinically substantial change (7-12 points). 6 of 12 patients showed remission criteria (<7 points), these outcomes had a statistically significant p value of <0.001. In relation to the FACIT-sp. Outcomes with statistical significance were in the emotional, functional, spiritual meaning, spiritual peace and spiritual faith dimensions with p <0.05. |
| Schneider et. al (Shnayder, 2023) |
This is Phase II, single-center, open label trial, 30 cancer patients with major depressive disorder received a fixed dose of 25 mg of psilocybin. |
The NIH-HEALS, a self-administered, 35-item, measure of psycho-social spiritual healing was completed at baseline and post-treatment at day 1, week 1, week 3, and week 8 following psilocybin therapy. |
Reported adverse eventsincluded headache (80%), nausea (40%), tearfulness (27%), anxiety (23%), euphoria (23%), fatigue (23%), and mild impairment of psychomotor functioning (10%). All three factors of NIH-HEALS: connection, reflection & Introspection, and Trust & Acceptance demonstrated positive change with a p value less than 0,05. . |
| Anderson et. al(Anderson, 2020) |
Single-arm, open-label, single-arm pilot study of psilocybin-assisted group therapy in a group of 18 cisgender men with HIV/AIDS aged ≥ 50 years and with moderate to severe demoralization assessed with the Demoralization Scale-II (DS-II) of ≥8/32. A dose of 0.3 mg/kg was administered for cohort 1, 0.36 mg/kg for cohort 2 and 3. Overall the average dose was 27.1 mg/dose. |
Seven psychotherapy sessions were conducted.
The scale used to evaluate benefits and harms was a 7-point Likert scale. The Schedule of Attitudes towards Hastened Death (SAHD) was administered at baseline, end-of-treatment and 3-month follow-up. The Montreal Cognitive Assessment (MoCA) was performed at enrollment and end-of-treatment. The primary clinical outcome was administered at least at baseline, end-of-treatment, and 3-month follow-up on the 16-item self-report DS-II. |
Moderate to severe adverse effects reported were severe anxiety reaction, paranoia, hypotension, and hypertension, but none were serious.A clinically significant change in demoralization was detected from baseline to 3-month follow-up, with a standardized effect size of 0.47, and a confidence interval of 0.21 to 0.60. |
| Ross et. al(Ross et al., 2016), |
Randomized, double-blind, crossover clinical trial in which a single dose of psilocybin (0.3 mg/kg) versus a control of niacin (250 mg) is administered to 29 oncology patients suffering from anxiety or depression. |
Hospital Anxiety and Depression Scales (HADS) in its measures of Anxiety, Depression and Total; STAI in its measures of Trait and State Anxiety; BDI with a baseline measurement, at one week and then at 26 weeks after the second dose. and at 6.5 months follow-up. |
The percentages of symptom remission and anxiolytic/antidepressant response were significantly higher in the psilocybin group compared to the control. No significant differences were observed between groups after the administration of psilocybin in the second session to the group that previously received placebo. At 6.5 months follow-up, clinical response rates for measures of anxiety and depression ranged from 60 to 80% for all participants as a whole and when compared to baseline. |
| Griffiths et. al s (Griffiths, 2016) |
Randomized, double-blind, crossover, clinical trial comparing low to high doses of psilocybin 1 to 3 mg/70 kg vs 22 to 30 mg/70 kg, in 56 patients with a diagnosis of life-threatening cancer and a DSM IV diagnosis of anxiety or mood disorder (dysthymia, depression, major, or mixed).. |
Meetings with monitors: before consumption (mean of 3 sessions), the day after psilocybin consumption (mean of 1.2 hours), meetings between the first and second psilocybin session (mean of 2.7 sessions), and after the second session and follow-up at 6 months (mean of 2.5 sessions). Monitoring of cardiovascular measures. Questionnaire carried out by the monitors. HRS (Hallucinogen Rating Scale), 5D-ASC (Dimension Altered State of Consciousness), MEQ30 (Mystical Experience Questionnaire, Four factor scores (Mystical, Positive mood, Transcendence of time and space, and Ineffability). For depression GRID-HAM-D-17, and anxiety HAM-A with SIGH-A. LAP-R Death Acceptance, Death Transcendence Scale |
Adverse effects reported: increased blood pressure more in high than in low doses (34 vs 17%), nausea and emesis in high doses (15%), physical discomfort of any type more in high than in low doses (21 vs 8%).
High doses cause cardiovascular changes, as well as result in visual effects and increased happiness. At the level of depression questionnaires, in the first session there was no difference between high and low doses, but in the second session and at 6 months, high doses were more effective. Measures of spirituality showed similar increases. High doses produced higher ratings on persistent positive effects on attitudes about life, self, mood change, behavior change, and spirituality. There were increases in positive attitudes toward death and acceptance of death, greater with higher doses.
|
| Grobs et. al (Grobs, 2011) |
Randomized, single-blind, crossover, placebo-controlled clinical trial in patients with advanced cancer and anxiety. use of moderate doses of psilocybin (0.2mg/kg). 12 adults with advanced cancer and anxiety (according to DSM-IV). |
The following scales were used: BID (Beck Depression Inventory), Profile of Mood States, State-Trait Anxiety Inventory, 5D-ASC (5-Dimension Altered States of Consciousness) y Brief Psychiatric Rating Scale ,with follow-up at 6 months after treatment. |
Psilocybin administration increased blood pressure and heart rate. In relation to 5D-ASC psilocybin had effects on anxious ego-dissolution and auditory disturbances. It also led to positive depersonalization, positive mood, manic experiences, elementary hallucinations, change of meaning of perceptions, facilitated recall and facilitated imagination. Psilocybin helped to decrease anxious and depressive symptoms. Mood improved for 2 weeks, and the sustained improvement in BID was sustained for 6 months. |
| Healthy adults without psychiatric disorders |
| Griffiths et. al(Griffiths, 2011) |
Double-blind, cross-over, semi-randomized clinical trial. Use of psilocybin doses of 0, 5, 10, 10, 20 and 30 mg/70 kg. Each participant went through the different doses including the 0 dose which would be the equivalent to placebo. Eighteen healthy adults with no history of psychiatric disorders or dependence on alcohol or psychoactive substances participated. |
Five sessions were carried out, with follow-up at 1 month and 14 months. Cardiovascular parameters were measured and questionnaires were applied by monitors. Use of Hallucinogen Rating Scale, APZ, which includes three scales: OSE (oceanic immensity: mystical experience that includes feelings of unity and transcendence), AIA (fear of ego dissolution), and VUS (visionary). ARCI (Addiction Research Center Inventory), States of Consciousness Questionnaire, Mysticism Scale, and Death Transcendence Scale. |
At the level of cardiovascular parameters, elevated blood pressure was related to higher doses. The subjective effects related to hallucinogens were experienced with doses starting at 5 mg, but increased with higher doses, and the mystical experience increased with the dose. However, with the highest dose 30 mg/70 kg, fear or anxiety was experienced (39% of participants). Persistent effects were dose-related. 61% considered the experience as the most spiritually significant in their lives, and 83% among their top five experiences. |
| Griffiths et. al(Griffiths, 2006) |
Randomized, double-blind, crossover clinical trial to evaluate the acute (7h) and long-term (2 months) effects of psilocybin (30mg/70kg) compared to methylphenidate (40mg/70kg), in 36 healthy volunteers without psychiatric comorbidities, without previous experience with hallucinogens. 30 patients had 2 sessions and 6 patients 3 (2 with methylphenidate, 1 with unblinded psilocybin), the latter 6 were excluded from the analyses. |
The following scales were used: HRA (Hallucinogen Rating Scale), APZ, ARCI (Addiction research center inventor), States of consciousness questionnaire, Mysticism Scale.
|
Methylphenidate and psilocybin presented alterations in heart rate and blood pressure. Psilocybin obtained higher scores in HRS, APZ, and ARCI. The respective scales of the mythical experience obtained higher scores for psilocybin. At two months, psilocybin produced higher scores on positive attitudes, mood, social effects, and behavior, and at two months the mysticism scales were higher for psilocybin. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).