Submitted:
08 April 2025
Posted:
08 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
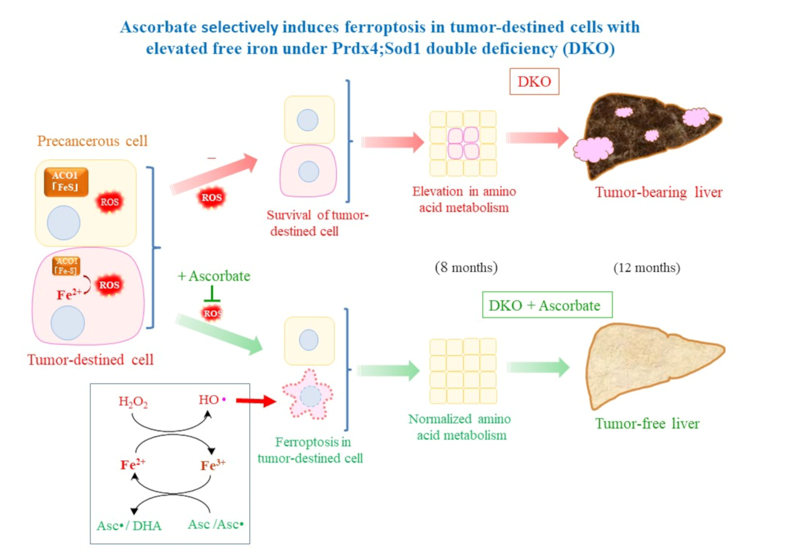
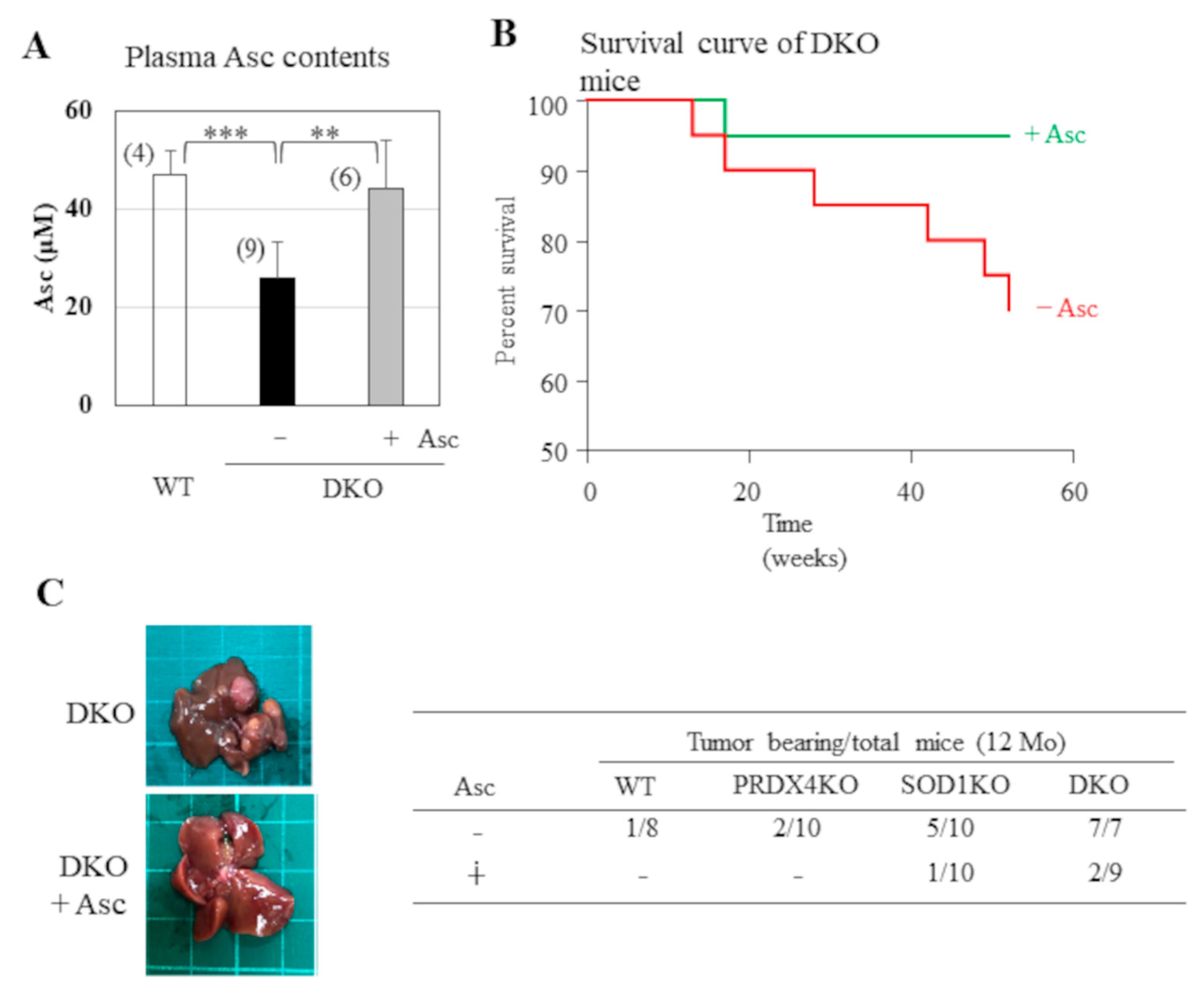
2.1. Hepatic Tumor Development in Sod1- and Prdx4 Double-Deficient Mice Was Suppressed by Supplementation with Asc
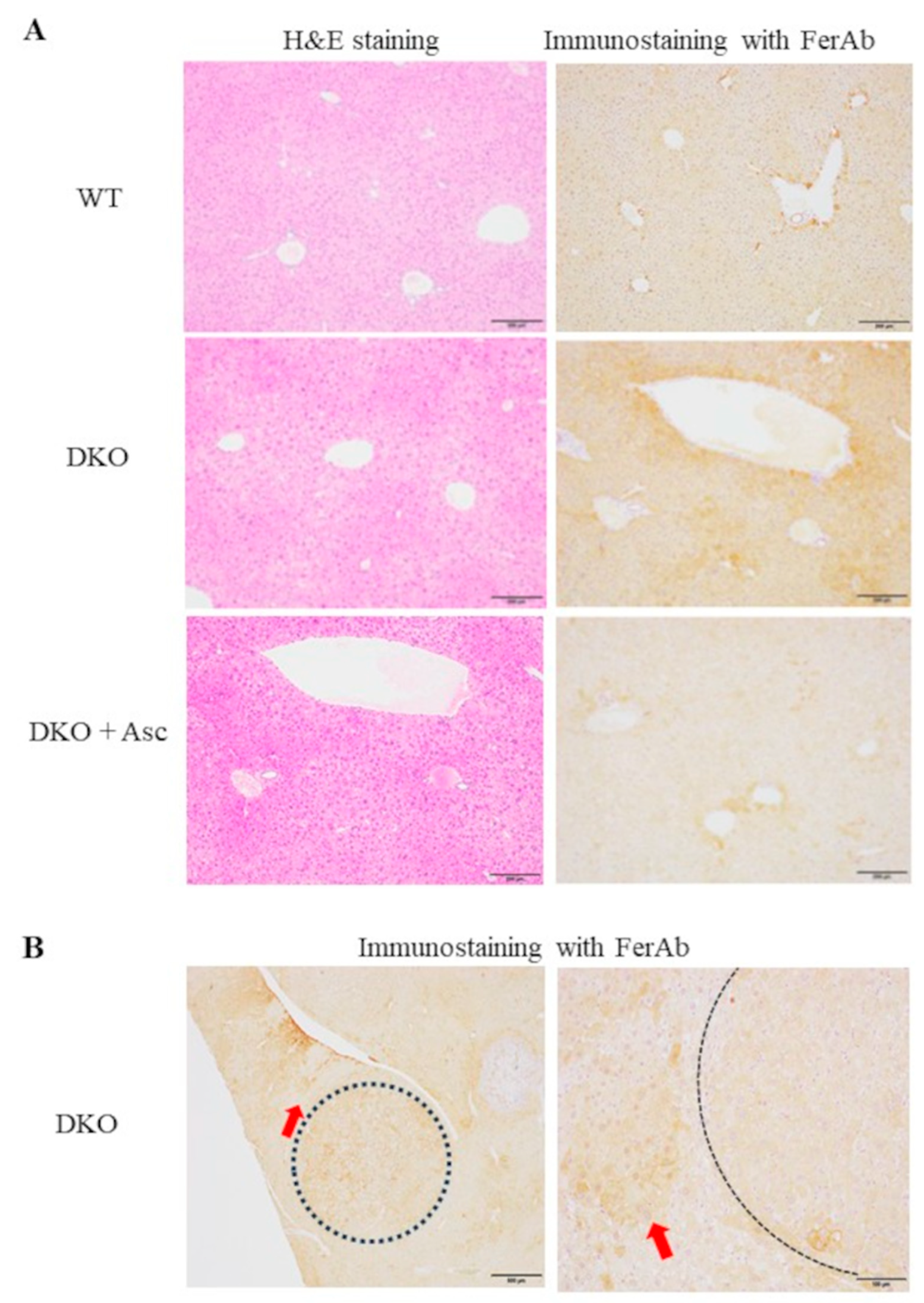
2.2. Histopathological Analyses of the Livers in Tumor-Bearing Mice
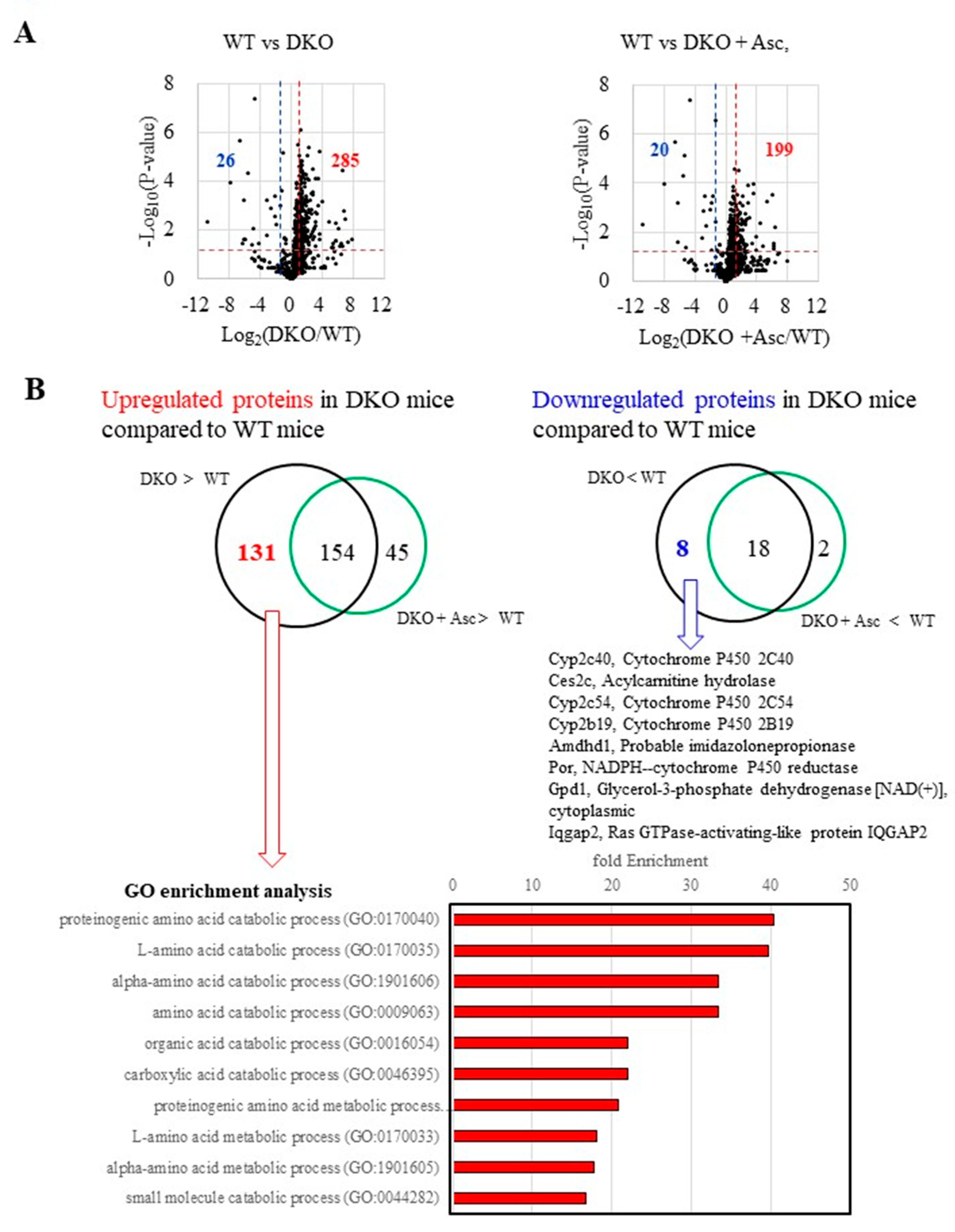
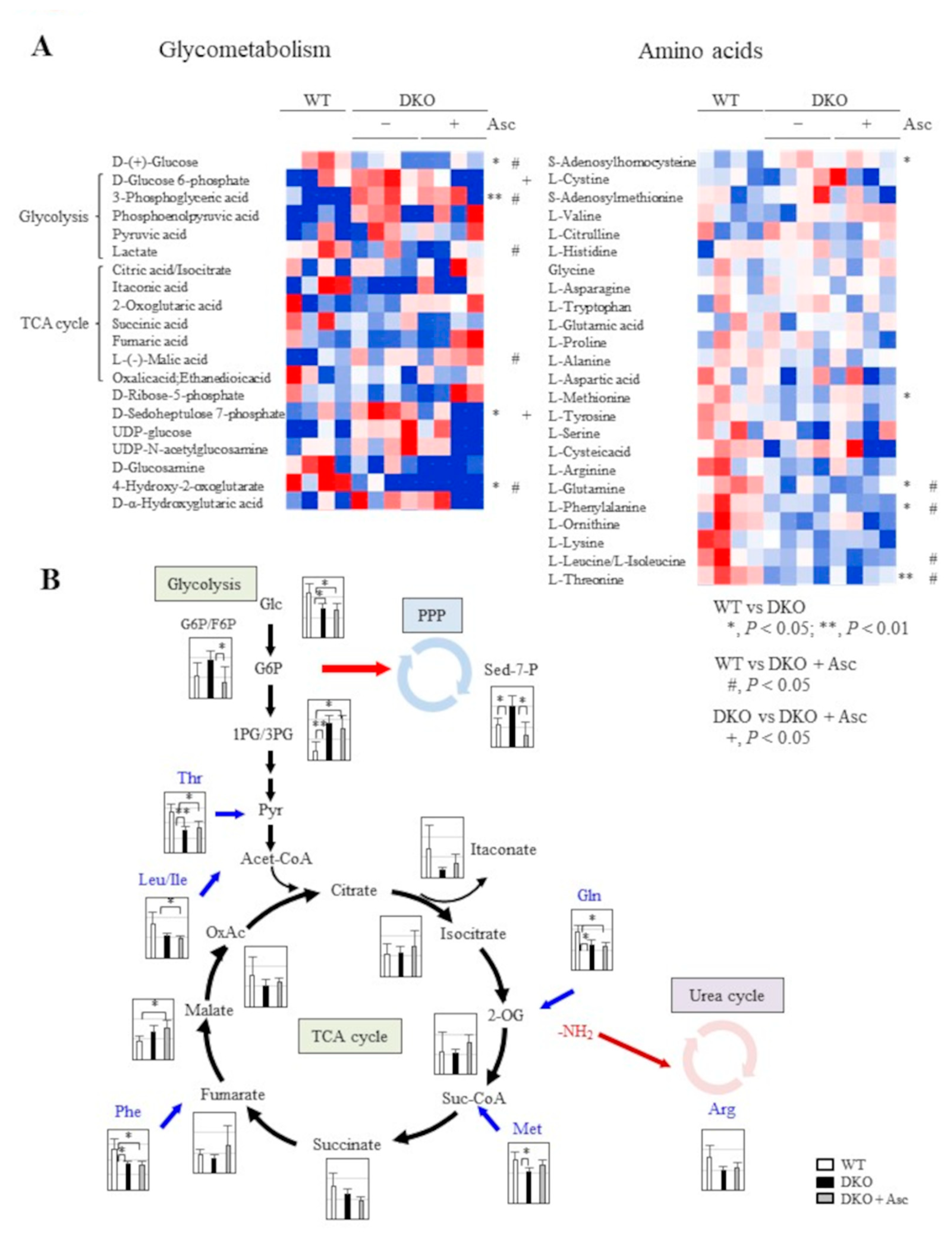
2.3. Proteomics Analysis Revealed an Elevation of Amino Acid Metabolism in Precancerous Livers
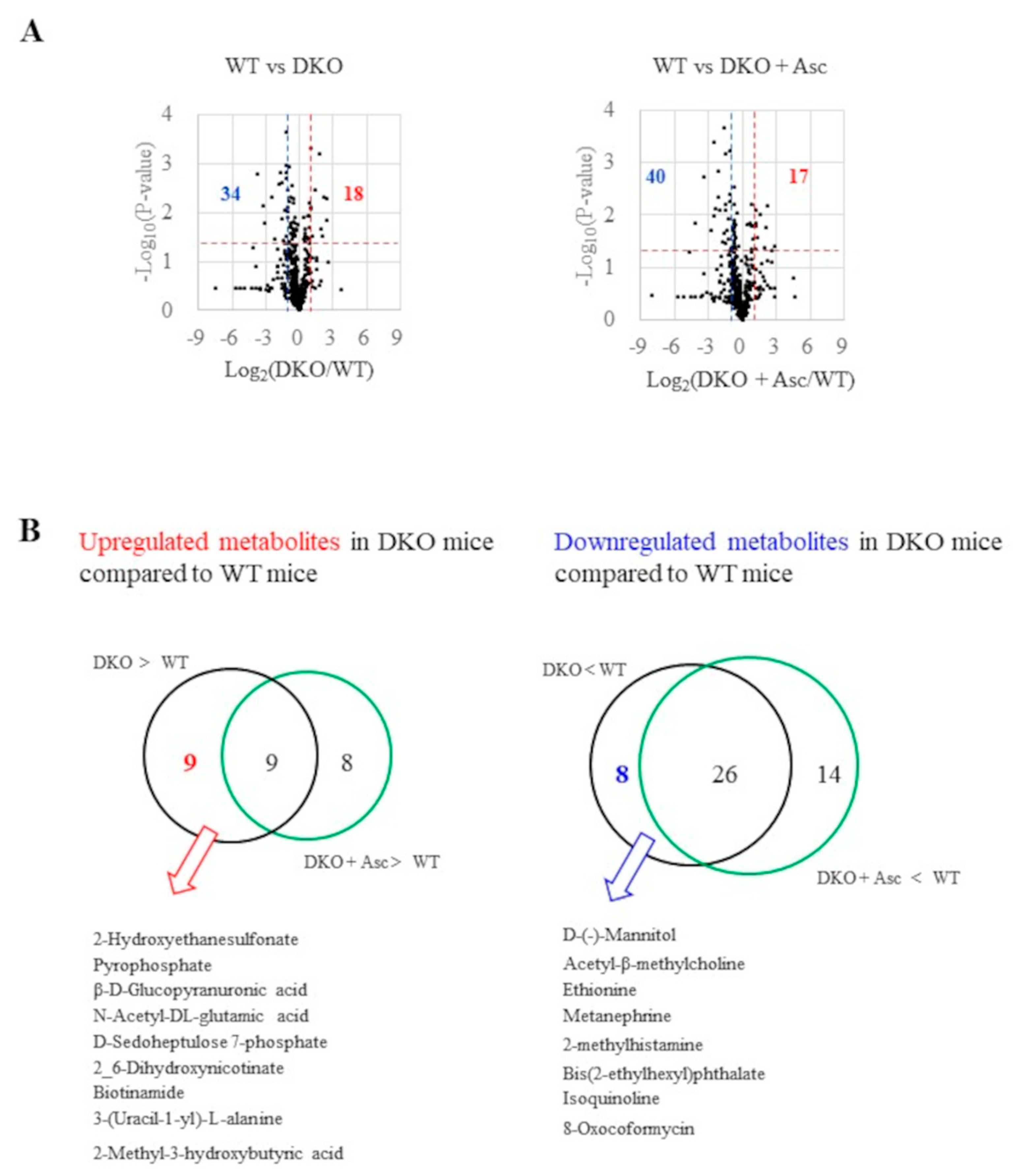
2.4. Analyses of Metabolites in Precancerous Livers
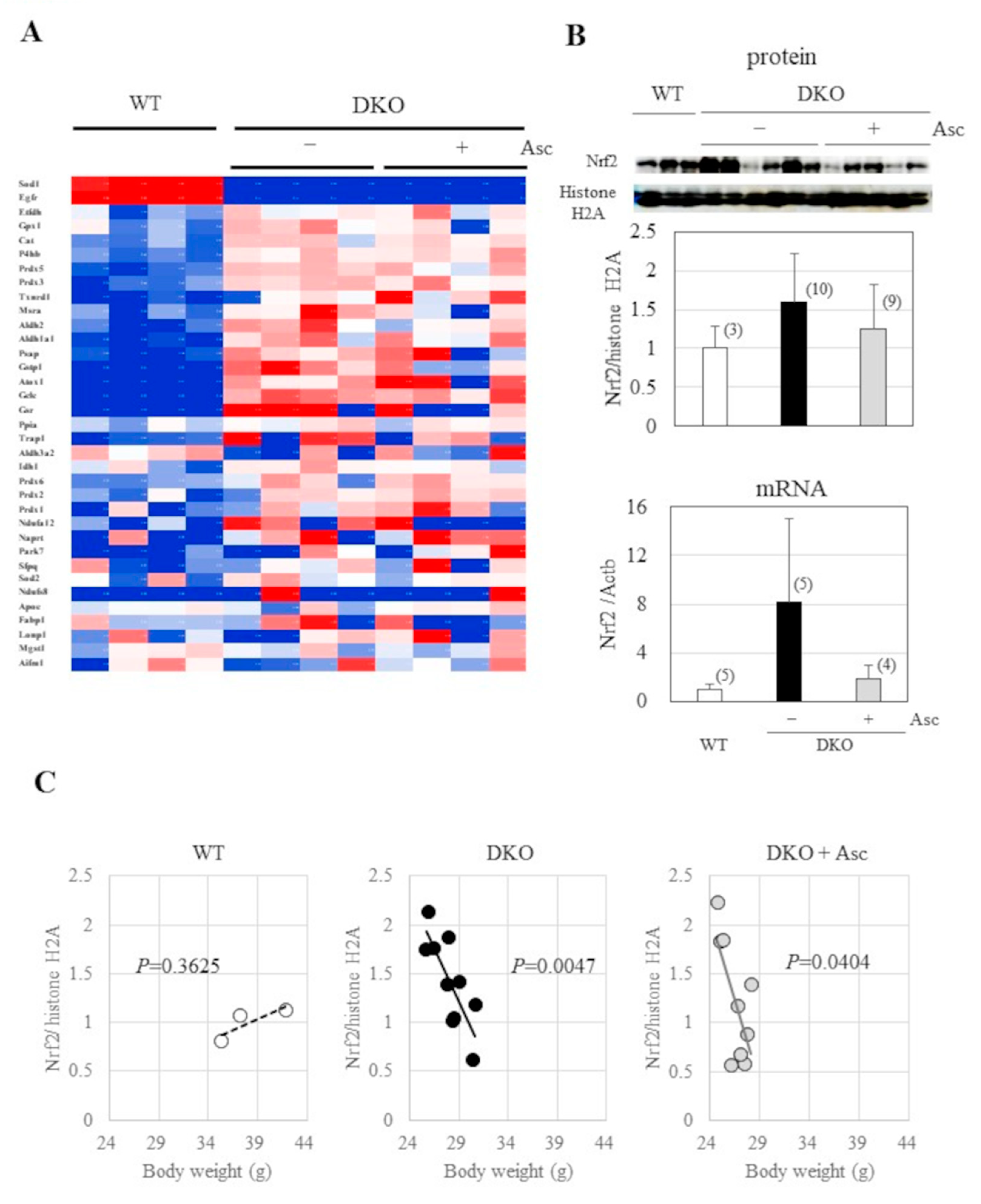
2.5. Upregulation in Antioxidative Proteins and Stimulated Proteolysis as Consequences of Oxidative Stress
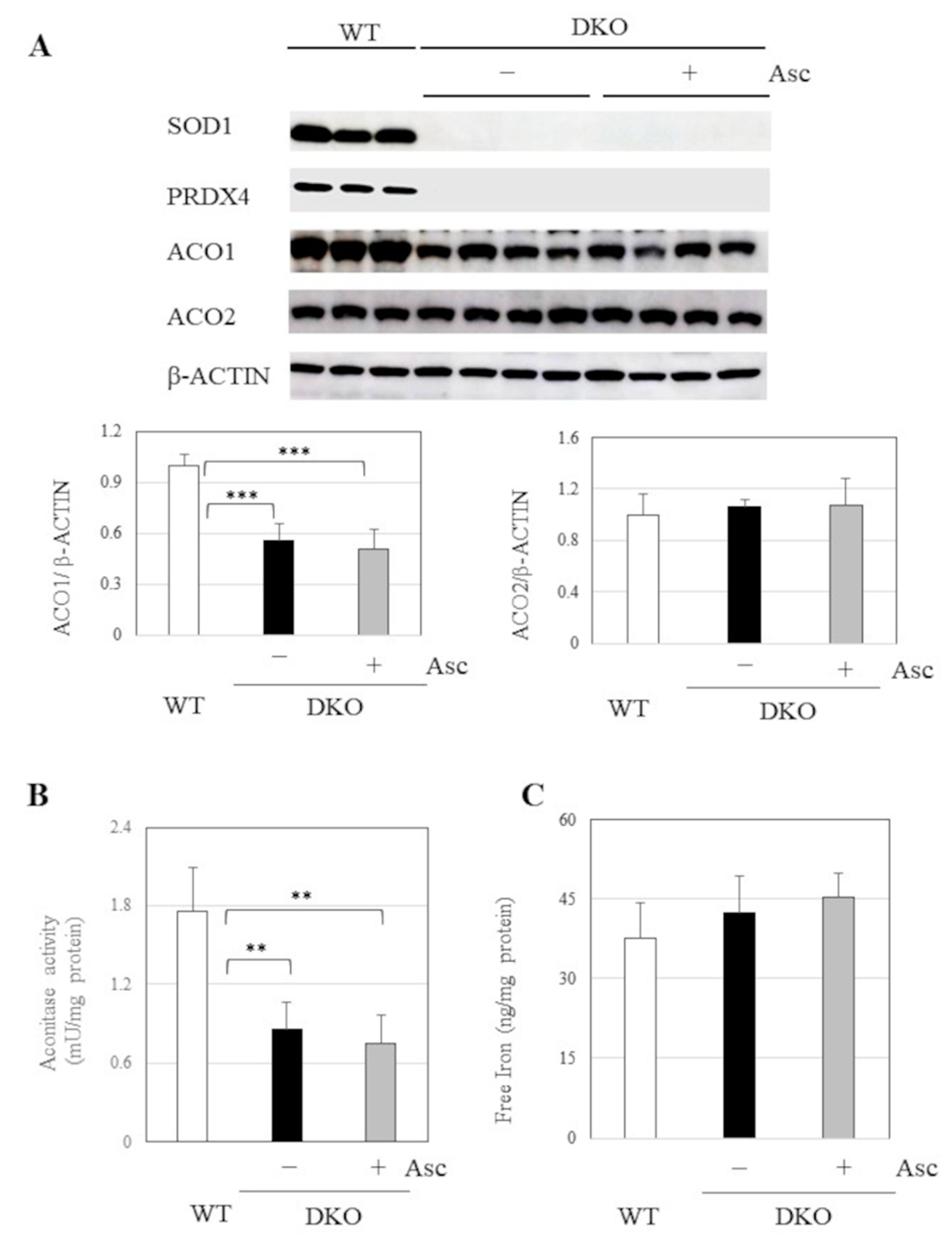
2.6. Implications of Aberrant Iron Metabolism in DKO Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Blood Cell Counting
4.3. Histological Analyses of Livers
4.4. Measurement of the Reduced Form of Asc
4.5. Preparation of Plasma and Liver Lysate
4.6. Proteomics Analysis
4.7. Metabolite Analysis
4.8. Protein Data Annotation
4.9. Isolation and Cultivation of Elicited Peritoneal Macrophages
4.10. Flow Cytometric Analyses of LPS-Treated Macrophages In Vitro
4.11. Preparation of Nuclear Fractions to Detect Nrf2
4.12. Immunoblotting
4.13. Detection of Carbonylated Proteins
4.14. Detection of Ubiquitinated Proteins
4.15. Measurement of Aconitase Activity
4.16. Reverse Transcription (RT)-PCR and Quantitative RT-PCR Analyses of the Produced DNA
4.17. Measurement of Free Iron in the Liver Homogenate
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ER | endoplasmic reticulum |
| NAFLD | non-alcoholic fatty liver disease |
| KO | knockout |
| Sod1 | superoxide dismutase 1 |
| DKO | double knockout |
| Prdx4 | peroxiredoxin 4 |
| NASH | nonalcoholic steatohepatitis |
| ROS | reactive oxygen species |
| ATM | Ataxia-telangiectasia mutated |
| db | obese diabetic |
| SREBPs | sterol regulatory element binding transcription proteins |
| ERO1 | endoplasmic reticulum oxidoreductin 1 |
| Asc | ascorbate |
| WT | wild-type |
| EDTA | ethylenediaminetetraacetic acid |
| WBCs | white blood cells |
| H&E | hematoxylin-eosin |
| Naph-DiPy | 15-(Naphthalen-1-ylamino)-7-aza-3, 11-dioxadispiro [5.1.58.36] hexadecan-7-oxyl |
| nLC-MS/MS | nanoflow liquid chromatography tandem mass spectrometry |
| iBAQ | intensity-based absolute quantification |
| GO | Gene ontology |
| RBCs | red blood cells |
| SDS-PAGE | SDS-polyacrylamide gel electrophoresis |
| TBST | tris-buffered saline containing 0.1% Tween-20 |
| HRP | horseradish peroxidase |
| PVDF | polyvinylidene difluoride |
| Ub | ubiquitin |
| RT | Reverse transcription |
| SE | standard error |
| TCA | tricarboxylic acid cycle |
References
- Goh, G.B.; McCullough, A.J. Natural History of Nonalcoholic Fatty Liver Disease. Dig. Dis. S. 2016, 61, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N. Epidemiology and risk-stratification of NAFLD-associated HCC. J. Hepatol. 2021, 2021. 75, 1476–1484. [Google Scholar] [CrossRef]
- Marengo, A.; Rosso, C.; Bugianesi, E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu. Rev. Med. 2016, 67, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Kohlhepp, M.S.; Liu, H.; Tacke, F.; Guillot, A. The contradictory roles of macrophages in non-alcoholic fatty liver disease and primary liver cancer-Challenges and opportunities. Front. Mol. Biosci. 2023, 10, 1129831. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 15, 116–141. [Google Scholar] [CrossRef]
- Sutti, S.; Albano, E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 81–92. [Google Scholar] [CrossRef]
- Fridovich. I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Shimizu, T.; Shirasawa, T. CuZn-SOD deficiency causes ApoB degradation and induces hepatic lipid accumulation by impaired lipoprotein secretion in mice. J. Biol. Chem. 2006, 281, 31713–31719. [Google Scholar] [CrossRef]
- Kurahashi, T.; Konno, T.; Otsuki, N.; Kwon, M.; Tsunoda, S.; Ito, J.; Fujii, J. A malfunction in triglyceride transfer from the intracellular lipid pool to apoB in enterocytes of SOD1-deficient mice. FEBS Lett. 2012, 586, 4289–4295. [Google Scholar] [CrossRef]
- Elchuri, S.; Oberley, T.D.; Qi, W.; Eisenstein, R.S.; Jackson Roberts, L.; Van Remmen, H.; Epstein, C.J.; Huang, T.T. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 2005, 24, 367–380. [Google Scholar] [CrossRef]
- Erker, L.; Schubert, R.; Elchuri, S.; Huang, T.T.; Tarin, D.; Mueller, K.; Zielen, S.; Epstein, C.J.; Wynshaw-Boris, A. Effect of the reduction of superoxide dismutase 1 and 2 or treatment with alpha-tocopherol on tumorigenesis in Atm-deficient mice. Free Radic. Biol. Med. 2006, 41, 590–600. [Google Scholar] [CrossRef]
- Ito, J.; Ishii, N.; Akihara, R.; Lee, J.; Kurahashi, T.; Homma, T.; Kawasaki, R.; Fujii, J. A high-fat diet temporarily renders Sod1-deficient mice resistant to an oxidative insult. J. Nutr. Biochem. 2017, 40, 44–52. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer. 2014, 14, 581–597. [Google Scholar] [CrossRef]
- Luna-Marco, C.; Ubink, A.; Kopsida, M.; Heindryckx, F. Endoplasmic Reticulum Stress and Metabolism in Hepatocellular Carcinoma. Am. J. Pathol. 2023, 193, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Hiraishi, A.; Touyama, M.; Sakamoto, K. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem. Biophys. Res. Commun. 2008, 375, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Homma, T.; Kurahashi, T.; Kang, E.S.; Fujii, J. Oxidative stress triggers lipid droplet accumulation in primary cultured hepatocytes by activating fatty acid synthesis. Biochem. Biophys. Res. Commun. 2015, 464, 229–235. [Google Scholar] [CrossRef]
- Sato, Y.; Kojima, R.; Okumura, M.; Hagiwara, M.; Masui, S.; Maegawa, K.; Saiki, M.; Horibe, T.; Suzuki, M.; Inaba, K. Synergistic cooperation of PDI family members in peroxiredoxin 4-driven oxidative protein folding. Sci. Rep. 2013, 3, 2456. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Ochi, H.; Yamada, S. A comprehensive review of peroxiredoxin 4, a redox protein evolved in oxidative protein folding coupled with hydrogen peroxide detoxification. Free Radic. Biol. Med. 2024, 227, 336–354. [Google Scholar] [CrossRef]
- Iuchi, Y.; Okada, F.; Tsunoda, S.; Kibe, N.; Shirasawa, N.; Ikawa, M.; Okabe, M.; Ikeda, Y.; Fujii, J. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem. J. 2009, 419, 149–158. [Google Scholar] [CrossRef]
- Zito, E; Chin, K. T.; Blais, J.; Harding, H.P.; Ron, D. ERO1-beta, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J. Cell Biol. 2010, 188, 821–832. [Google Scholar] [CrossRef]
- Zito, E.; Hansen, H.G.; Yeo, G.S.; Fujii, J.; Ron, D. Endoplasmic reticulum thiol oxidase deficiency leads to ascorbic acid depletion and noncanonical scurvy in mice. Mol. Cell 2012, 48, 39–51. [Google Scholar] [CrossRef]
- Nabeshima, A.; Yamada, S.; Guo, X.; Tanimoto, A.; Wang, K.Y.; Shimajiri, S.; Kimura, S.; Tasaki, T.; Noguchi, H.; Kitada, S.; Watanabe, T.; Fujii, J.; Kohno, K.; Sasaguri, Y. Peroxiredoxin 4 protects against nonalcoholic steatohepatitis and type 2 diabetes in a nongenetic mouse model. Antioxid. Redox Signal. 2013, 19, 1983–1998. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Noguchi, H.; Ishii, N.; Homma, T.; Hamada, T.; Hiraki, T.; Zhang, J.; Matsuo, K.; Yokoyama, S.; Ishibashi, H.; Fukushige, T.; Kanekura, T.; Fujii, J.; Uramoto, H.; Tanimoto, A.; Yamada, S. The Association of Peroxiredoxin 4 with the Initiation and Progression of Hepatocellular Carcinoma. Antioxid. Redox Signal. 2019, 30, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Kurahashi, T.; Lee, J.; Nabeshima, A.; Yamada, S.; Fujii, J. Double Knockout of Peroxiredoxin 4 (Prdx4) and Superoxide Dismutase 1 (Sod1) in Mice Results in Severe Liver Failure. Oxid. Med. Cell. Longev. 2018, 2018, 2812904. [Google Scholar] [CrossRef] [PubMed]
- Sentman, M.L.; Granström, M.; Jakobson, H.; Reaume, A.; Basu, S.; Marklund, S.L. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J. Biol. Chem. 2006, 281, 6904–6909. [Google Scholar] [CrossRef]
- Ishii, N.; Homma, T.; Lee, J.; Mitsuhashi, H.; Yamada, K.I.; Kimura, N.; Yamamoto, Y.; Fujii, J. Ascorbic acid and CoQ10 ameliorate the reproductive ability of superoxide dismutase 1-deficient female mice. Biol. Reprod. 2020, 102, 102–115. [Google Scholar] [CrossRef]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA. 1976, 73, 3685–3689. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. CANCER. Revisiting vitamin C and cancer. Science 2015, 350, 1317–1318. [Google Scholar] [CrossRef]
- Iuchi, Y.; Okada, F.; Onuma, K.; Onoda, T.; Asao, H.; Kobayashi, M.; Fujii, J. Elevated oxidative stress in erythrocytes due to a SOD1 deficiency causes anaemia and triggers autoantibody production. Biochem. J. 2007, 402, 219–227. [Google Scholar] [CrossRef]
- Horn, P; Tacke, F. Metabolic reprogramming in liver fibrosis. Cell Metab. 2024, 36, 1439–1455. [Google Scholar] [CrossRef]
- Shen, X.; Yu, Z.; Wei, C.; Hu, C.; Chen, J. Iron metabolism and ferroptosis in nonalcoholic fatty liver disease: what is our next step? Am. J. Physiol. Endocrinol. Metab. 2024, 326, E767–E775. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J. Protein damage and degradation by oxygen radicals. I. general aspects. J. Biol. Chem. 1987, 262, 9895–9901. [Google Scholar] [CrossRef]
- Demasi, M.; Netto, L.E.; Silva, G.M.; Hand, A.; de Oliveira, C.L.; Bicev, R.N.; Gozzo, F.; Barros, M.H.; Leme, J.M.; Ohara, E. Redox regulation of the proteasome via S-glutathionylation. Redox Biol. 2013, 2, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Motooka, Y.; Toyokuni, S. Ferroptosis As Ultimate Target of Cancer Therapy. Antioxid. Redox Signal. 2023, 39, 206–223. [Google Scholar] [CrossRef]
- Rouault. T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Fujii, J.; Osaki, T.; Bo, T. Ascorbate Is a Primary Antioxidant in Mammals. Molecules 2022, 27, 6187. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; Levine, M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA. 2007, 104, 8749–8754. [Google Scholar] [CrossRef] [PubMed]
- Villagran, M.; Ferreira, J.; Martorell, M.; Mardones, L. The Role of Vitamin C in Cancer Prevention and Therapy: A Literature Review. Antioxidants 2021, 10, 1894. [Google Scholar] [CrossRef]
- Ghanem, A.; Melzer, A.M.; Zaal, E.; Neises, L.; Baltissen, D.; Matar, O.; Glennemeier-Marke, H.; Almouhanna, F.; Theobald, J.; Abu El Maaty, M.A.; Berkers, C.; Wölfl, S. Free Radic. Biol. Med. 2021, 163, 196–209. [CrossRef]
- Pozzer, D.; Invernizzi, R.W.; Blaauw, B.; Cantoni, O.; Zito, E. Ascorbic Acid Route to the Endoplasmic Reticulum: Function and Role in Disease. Antioxid. Redox Signal. 2021, 34, 845–855. [Google Scholar] [CrossRef]
- Castro, L.; Tórtora, V.; Mansilla, S.; Radi, R. Aconitases: Non-redox Iron-Sulfur Proteins Sensitive to Reactive Species. Acc Chem. Res. 2019, 52, 2609–2619. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Hrycay, E.G.; Bandiera, S.M. Involvement of Cytochrome P450 in Reactive Oxygen Species Formation and Cancer. Adv. Pharmacol. 2015, 74, 35–84. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Homma, T.; Guo, X.; Yamada, K.I.; Yamada, S.; Fujii, J. Ascorbic acid prevents N-nitrosodiethylamine-induced hepatic injury and hepatocarcinogenesis in Akr1a-knockout mice. Toxicol. Lett. 2020, 333, 192–201. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O'Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Kaelin, W.G. Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Mikkelsen SU, Gillberg L, Lykkesfeldt J, Grønbæk K. The role of vitamin C in epigenetic cancer therapy. Free Radic. Biol. Med. 2021, 170, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Quagliariello, V.; Maurea, N.; Di Francia, R.; Sharifi, S.; Facchini, G.; Rinaldi, L.; Piezzo, M.; Manuela, C.; Nunnari, G.; Montopoli, M. Multiple Effects of Ascorbic Acid against Chronic Diseases: Updated Evidence from Preclinical and Clinical Studies. Antioxidants 2020, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Takeda, Y.; Nakano, T.; Akatsuka, S.; Kinoshita, D.; Kurahashi, T.; Saitoh, S.; Yamada, K.I.; Miyata, S.; Asao, H.; Goto, K.; Watanabe, T.; Watanabe, M.; Toyokuni, S.; Fujii, J. Defective biosynthesis of ascorbic acid in Sod1-deficient mice results in lethal damage to lung tissue. Free Radic. Biol. Med. 2021, 162, 255–265. [Google Scholar] [CrossRef]
- Kobayashi, S.; Harada, Y.; Homma, T.; Yokoyama, C.; Fujii, J. Characterization of a rat monoclonal antibody raised against ferroptotic cells. J. Immunol. Methods. 2021, 489, 112912. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Yamato, M.; Yamasaki, T.; Mito, F.; Yamada, K. Rapid and convenient detection of ascorbic acid using a fluorescent nitroxide switch. Free Radic. Biol. Med. 2012, 53, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Sugiyama, D.; Magari, Y.; Souri, M.; Ichinose, A. Rapid immunochromatographic test for detection of anti-factor XIII A subunit antibodies can diagnose 90 % of cases with autoimmune haemorrhaphilia XIII/13. Thromb. Haemost. 2015, 113, 1347–1356. [Google Scholar] [CrossRef]
- Homma, T.; Fujiwara, H.; Osaki, T.; Fujii, S.; Fujii, J. Consequences of a peroxiredoxin 4 (Prdx4) deficiency on learning and memory in mice. Biochem. Biophys. Res. Commun. 2022, 621, 32–38. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef]
- Kobayashi, S.; Homma, T.; Okumura, N.; Han, J.; Nagaoka, K.; Sato, H.; Konno, H.; Yamada, S.; Takao, T.; Fujii, J. Carnosine dipeptidase II (CNDP2) protects cells under cysteine insufficiency by hydrolyzing glutathione-related peptides. Free Radic. Biol. Med. 2021, 174, 12–27. [Google Scholar] [CrossRef]
- Kobayashi, S; Hamashima, S. ; Homma, T.; Sato, M.; Kusumi, R.; Bannai, S.; Fujii, J.; Sato, H. Cystine/glutamate transporter, system xc-, is involved in nitric oxide production in mouse peritoneal macrophages. Nitric Oxide 2018, 78, 32–40. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. "NIH Image to ImageJ: 25 years of image analysis". Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Watanabe, Y.; Moriya, Y.; Kawano, S.; Yamamoto, T.; Matsumoto, M.; Takami, T.; Kobayashi, D.; Araki, N.; Yoshizawa, A.C.; Tabata, T.; Sugiyama, N.; Goto, S.; Ishihama, Y. jPOSTrepo: an international standard data repository for proteomes. Nucleic Acids Res. 2017, 45, D1107–D1111. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




