1. Introduction
Stereotactic body radiation therapy (SBRT) has become a standard treatment modali-ty for patients with inoperable early-stage non-small cell lung cancer, offering high rates of local control with a favorable toxicity profile [
1,
2,
3,
4]. However, the application of SBRT to centrally located lung tumors—those in close proximity to critical structures such as the central airways—presents significant challenges due to the heightened risk of severe toxicities [
5,
6].
Accurate target delineation is crucial in SBRT to maximize tumor control while minimizing exposure to surrounding healthy tissues [
7]. Respiratory-induced tumor mo-tion can introduce uncertainties in target localization, potentially compromising treat-ment efficacy and safety [
8]. Four-dimensional positron emission tomography/computed tomography (4D-PET/CT) has emerged as a valuable imaging modality that accounts for respiratory motion, thereby enhancing the precision of target volume delineation in tho-racic malignancies [
9,
10,
11,
12].
In cases of tumor recurrence within previously irradiated regions, particularly those exhibiting radiation-induced fibrosis, the complexities of re-irradiation are further ampli-fied [
13,
14,
15,
16,
17,
18,
19]. The fibrotic changes can obscure tumor boundaries, making accurate deline-ation challenging. The integration of 4D-PET/CT in such scenarios may provide critical insights, enabling more precise differentiation between recurrent tumor and fibrotic tissue [
20].
This short communication presents a clinical case illustrating the application of 4D-PET/CT for target delineation in the SBRT re-irradiation of a centrally located lung tu-mor recurrence within a radiation-induced fibrotic area. The case underscores the neces-sity of incorporating advanced imaging techniques to ensure safe and effective re-irradiation in complex clinical settings.
2. Detailed Case Description
2.1. Patient Description and Case History
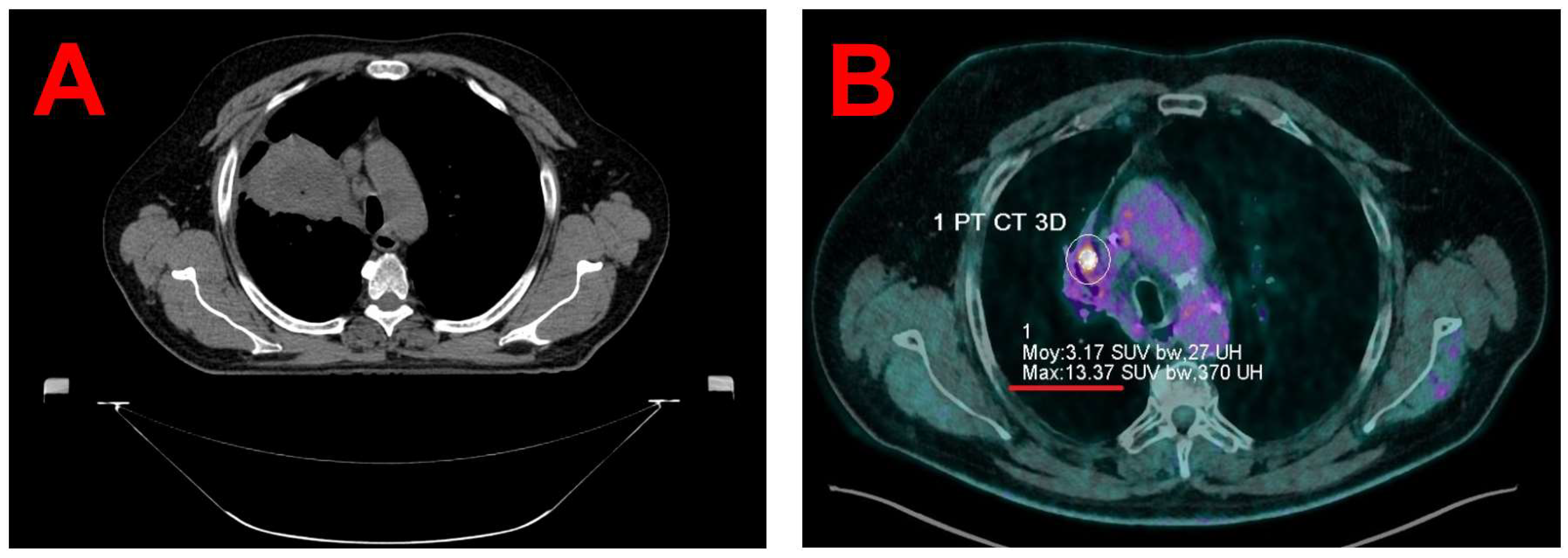
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. The patient is a 67-year-old male (1.70 m, 108 kg) who was diagnosed in 2020 with squamous cell carcinoma of the right upper lobe. Treatment included concurrent chemoradiotherapy (Carboplatin-Paclitaxel, 4 cycles, 66 Gy in 33 fractions; see
Figure 1A), followed by immunotherapy with Durvalumab for two years. A PET/CT scan on October 23, 2023, revealed progression of a hypermetabolic focus in the right upper lobe (maximum Standardized Uptake Values (SUVmax) 9.8 vs. 4.7 in January 2023), indicating a suspected local recurrence. A multidisciplinary tumor board on November 2, 2023, recommended SBRT and Nivolumab. A subsequent PET/CT scan on December 7, 2023, showed a further increase in metabolic activity, with a maximum SUVmax of 13.4 (see
Figure 1B).
2.2. Planning Images Acquisitions
For radiotherapy planning, a 4D-CT scan was acquired using a Canon Aquilion LB CT scanner (Canon Medical Systems Corporation, Ōtawara, Japan). Respiratory motion was recorded with the Respiratory Gating for SCanners (RGSC) system (Varian, a Siemens Healthineers company, Palo Alto, USA). Acquisitions were reconstructed into 10 respiratory-phase subsets with 2-mm slice thickness and exported to the Varian Eclipse 16.1 Treatment Planning System (TPS) (Varian, a Siemens Healthineers company, Palo Alto, USA), where an average intensity projection (AIP) was generated.
Given that the recurrence was located within a region of extensive fibrosis, delineation on the planning 4D-CT alone was challenging (see
Figure 1B). Consequently, a PET/CT scan was performed using a digital high-resolution PET/CT system (Biograph Vision 600, Siemens Healthineers, Knoxville, USA) following intravenous injection of 235 MBq of 18F-fluorodeoxyglucose (Gluscan, Advanced Accelerator Applications Molecular Imaging, Saint-Genis-Pouilly, France). PET data were acquired using FlowMotion technology at a table speed of 0.7 mm/second (total acquisition duration = 8 minutes). The patient's respiratory waveform during PET and CT acquisition was recorded with the RGSC system. PET/CT data were retrospectively reconstructed into two sets: a 4D-PET/CT with 10 gates corresponding to the breathing cycle and a static PET/CT. Reconstruction parameters for both the static and 4D PET images align with institutional protocols. A broader filter (4 mm versus 2 mm) was applied to the 4D PET reconstruction to mitigate noise due to lower counting statistics in each of the 10 respiratory gates.
2.3. Target Volume Segmentation
A custom Python script was utilized to generate the maximal intensity projection (MIP) of the 4D-PET image. Both the static PET and 4D-MIP PET images were imported into the Varian Eclipse 16.1 TPS and fused with the planning 4D-CT. On each PET image, a 5-cm diameter spherical Region Of Interest (ROI) was delineated in a homogeneous uptake area of the liver. The noise level in the reconstructed image was defined as the standard deviation (SD) of SUV within this ROI. The Gross Tumor Volume (GTV) and the Internal Target Volume (ITV) were segmented on the static and 4D-MIP PET images, respectively, using an SUV threshold that accounts for both the maximal SUV in the lesion and the noise level, as defined in Equation 1:
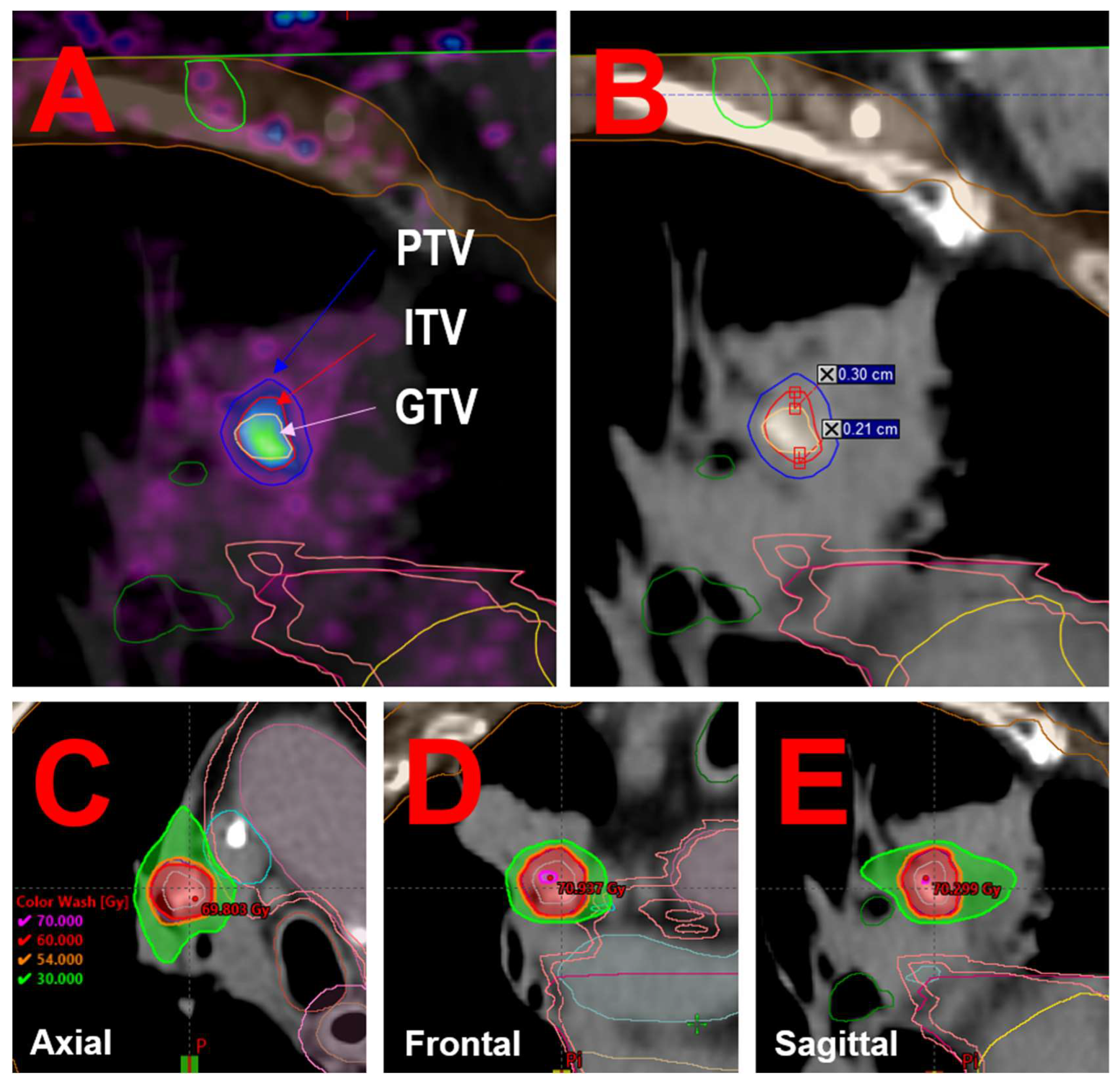
The volumes of the GTV and ITV were 0.5 cc and 0.9 cc, respectively. No lateral or anterior-posterior geometric differences were observed between the GTV and ITV. However, in the inferior-superior direction, the GTV was significantly smaller (maximum 8.0 mm) than the ITV (maximum 13.1 mm), with a maximum discrepancy of 5.1 mm (see
Figure 2A and 2sB). The Planning Target Volume (PTV) was defined as a 3 mm isotropic expansion of the ITV [
21].
2.4. Treatment Planning and Delivery
The ITV was situated approximately 1 cm from the proximal bronchial tree, classifying the tumor as central per the LungTech trial criteria [
5]. Consequently, the patient was prescribed a total dose of 60 Gy, administered in eight fractions of 7.5 Gy each. According to the LungTech protocol, 95% of the PTV received at least the nominal fraction dose, and 99% of the PTV received a minimum of 54 Gy. The maximum dose within the PTV ranged from 66 to 78 Gy. The patient was treated every 2 days except during the week-end.
Treatment planning was performed using the Varian Eclipse 16.1 TPS. A volumetric modulated arc therapy plan was optimized and calculated on the AIP dataset using the Acuros XB algorithm (version 15.6.04), with a 1-mm dose calculation grid size and dose reported in terms of dose to water [
22]. The plan consisted of two partial arcs rotating clockwise and counterclockwise from 181° to 0°, with collimator angles set to 45° and 315°, respectively. Dose distribution is illustrated in
Figure 2C, D and E.
Treatment was delivered on a Varian TrueBeam STx linear accelerator (Varian Medical Systems, Palo Alto, CA, USA) using 6 MV flattening filter-free beams. Before each fraction, patient positioning was verified using onboard kilovoltage orthogonal imaging, followed by a 4D cone-beam computed tomography to optimize soft-tissue alignment. Final positioning approval was given by a radiation oncologist prior to beam delivery in free-breathing with an ITV strategy and an intrafraction patient motion tracking done using a combined surface optical/thermal imaging using the Brainlab ExacTrac Dynamic system (Brainlab AG, Munich, Germany).
2.5. Clinical Outcomes
The SBRT was administered between February 26 and March 14, 2024. The patient exhibited excellent immediate tolerance, with no acute adverse effects such as skin reactions, esophageal pain, dysphagia, increased dyspnea, or cough. A follow-up PET/CT on June 20, 2024, demonstrated a reduction in the hypermetabolic focus (SUVmax 7.6 compared to 13.4 in December 2023). During a clinical evaluation on July 1, 2024, the patient reported stable dyspnea, absence of bleeding, and no pain, indicating good acute tolerance to the radiotherapy. A subsequent PET/CT on October 10, 2024, showed further regression of the local hypermetabolism (SUVmax 5.8 compared to 7.6 in June 2024).
4. Discussion
The integration of 4D-PET/CT into radiotherapy planning has been recognized as a superior approach for accurate target volume delineation in thoracic malignancies, particularly when addressing challenges posed by respiratory-induced tumor motion. [
9,
11,
23]. The global interest in 4D-PET/CT stems from its ability to account for tumor motion due to respiration, leading to more accurate tumor localization and characterization. This modality captures the full range of tumor motion, allowing for the creation of motion-inclusive target volumes that better represent the tumor's behavior during the respiratory cycle [
20]. Consequently, 4D-PET/CT reduces the uncertainties associated with tumor motion, enabling more precise radiation delivery and potentially improving treatment outcomes [
12].
In this clinical case, a 67-year-old male presented with a recurrence of squamous cell carcinoma in the right upper lobe, situated within a post-irradiation fibrosis region and approximately 1 cm from the proximal bronchial tree. The proximity to critical structures and the presence of fibrosis underscored the necessity for precise target delineation to minimize radiation exposure to healthy tissues while ensuring effective treatment of the tumor.
Utilizing 4D-PET/CT, we observed that the metabolic target volume delineation based on the 4D-MIP PET (ITV) was 0.9 cc, nearly double the size of the GTV delineated on static PET, which measured 0.5 cc. Additionally, a maximum discrepancy of 5.1 mm was noted in the inferior-superior direction between the GTV and ITV, surpassing our institution's standard PTV margins of 3 mm [
21]. This significant variation highlights the potential for under-treatment if relying solely on static imaging for target delineation.
In this case, the application of 4D-PET/CT-informed stereotactic body radiation therapy (SBRT) resulted in favorable outcomes. The patient exhibited excellent tolerance to the treatment, with no reported acute toxicities such as skin reactions, esophageal pain, dysphagia, increased dyspnea, or cough. Follow-up PET-CT scans demonstrated a progressive reduction in the hypermetabolic focus, with the SUVmax decreasing from 13.4 in December 2023 to 5.8 in October 2024, indicating a sustained metabolic response.
5. Conclusions
This case exemplifies the critical role of 4D-PET/CT in enhancing the precision of target volume delineation, particularly in complex scenarios involving tumor recurrence within post-irradiation fibrosis and proximity to critical structures. The observed discrepancies between static PET (GTV) and 4D-MIP PET (ITV) volumes underscore the potential risks of underestimating tumor motion and extent when relying solely on static imaging. These findings advocate for the broader adoption of 4D-PET/CT in radiotherapy planning for thoracic malignancies, especially in highly complex clinical cases.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to (specify the reason for the restriction).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SBRT |
Stereotactic Body Radiation Therapy |
| 4D-PET/CT |
Four-Dimensional Positron Emission Tomography/Computed Tomography |
| SUV |
Standardized Uptake Value |
| TPS |
Treatment Planning System |
| AIP |
Average Intensity Projection |
| ROI |
Region Of Interest |
| SD |
Standard Deviation |
| GTV |
Gross Tumor Volume |
| ITV |
Internal Garget Volume |
| PTV |
Planning Target Volume |
References
- Vlaskou Badra E, Baumgartl M, Fabiano S, Jongen A, Guckenberger M. Stereotactic radiotherapy for early stage non-small cell lung cancer: current standards and ongoing research. Transl Lung Cancer Res 2021;10:1930–49. [CrossRef]
- Yan M, Louie AV, Kotecha R, Ashfaq Ahmed M, Zhang Z, Guckenberger M, et al. Stereotactic body radiotherapy for Ultra-Central lung Tumors: A systematic review and Meta-Analysis and International Stereotactic Radiosurgery Society practice guidelines. Lung Cancer 2023;182:107281. [CrossRef]
- Buchberger DS, Videtic GMM. Stereotactic Body Radiotherapy for the Management of Early-Stage Non–Small-Cell Lung Cancer: A Clinical Overview. JCO Oncology Practice 2023;19:239–49. [CrossRef]
- Ladbury C, Sidiqi B, Cantrell N, Jones G, Skalina KA, Fekrmandi F, et al. Stereotactic Body Radiation Therapy for Primary Lung Cancer and Metastases: A Case-Based Discussion on Challenging Cases. Practical Radiation Oncology 2024:S1879850024002753. [CrossRef]
- Adebahr S, Collette S, Shash E, Lambrecht M, Le Pechoux C, Faivre-Finn C, et al. LungTech, an EORTC Phase II trial of stereotactic body radiotherapy for centrally located lung tumours: a clinical perspective. BJR 2015;88:20150036. [CrossRef]
- Lindberg S, Grozman V, Karlsson K, Onjukka E, Lindbäck E, Jirf KA, et al. Expanded HILUS Trial: A Pooled Analysis of Risk Factors for Toxicity From Stereotactic Body Radiation Therapy of Central and Ultracentral Lung Tumors. International Journal of Radiation Oncology*Biology*Physics 2023;117:1222–31. [CrossRef]
- Gandhidasan S, Woody NM, Stephans KL, Videtic GMM. Does Motion Management Technique for Lung SBRT Influence Local Control? A Single Institutional Experience Comparing Abdominal Compression to Breath-Hold Technique. Practical Radiation Oncology 2021;11:e180–5. [CrossRef]
- Yoganathan S, Maria Das K, Agarwal A, Kumar S. Magnitude, impact, and management of respiration-induced target motion in radiotherapy treatment: A comprehensive review. J Med Phys 2017;42:101. [CrossRef]
- Aristophanous M, Berbeco RI, Killoran JH, Yap JT, Sher DJ, Allen AM, et al. Clinical Utility of 4D FDG-PET/CT Scans in Radiation Treatment Planning. International Journal of Radiation Oncology*Biology*Physics 2012;82:e99–105. [CrossRef]
- Chi A, Nguyen NP. 4D PET/CT as a Strategy to Reduce Respiratory Motion Artifacts in FDG-PET/CT. Front Oncol 2014;4. [CrossRef]
- Sindoni A, Minutoli F, Pontoriero A, Iatì G, Baldari S, Pergolizzi S. Usefulness of four dimensional (4D) PET/CT imaging in the evaluation of thoracic lesions and in radiotherapy planning: Review of the literature. Lung Cancer 2016;96:78–86. [CrossRef]
- Dhingra J, Brandon D, Halkar R. 4D PET/CT integration: optimizing radiation and therapy. J Nucl Med 2021;62:1706.
- Käsmann L, Dietrich A, Staab-Weijnitz CA, Manapov F, Behr J, Rimner A, et al. Radiation-induced lung toxicity – cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat Oncol 2020;15:214. [CrossRef]
- John C, Dal Bello R, Andratschke N, Guckenberger M, Boda-Heggemann J, Gkika E, et al. In-field stereotactic body radiotherapy (SBRT) reirradiation for pulmonary malignancies as a multicentre analysis of the German Society of Radiation Oncology (DEGRO). Sci Rep 2021;11:4590. [CrossRef]
- Lee TH, Kim D-Y, Wu H-G, Lee JH, Kim HJ. Treatment outcomes of re-irradiation using stereotactic ablative radiotherapy to lung: a propensity score matching analysis. Radiat Oncol 2021;16:222. [CrossRef]
- Rulach R, Ball D, Chua KLM, Dahele M, De Ruysscher D, Franks K, et al. An International Expert Survey on the Indications and Practice of Radical Thoracic Reirradiation for Non-Small Cell Lung Cancer. Advances in Radiation Oncology 2021;6:100653. [CrossRef]
- Strange TA, Erasmus LT, Ahuja J, Agrawal R, Shroff GS, Truong MT, et al. Spectrum of Imaging Patterns of Lung Cancer following Radiation Therapy. Diagnostics 2023;13:3283. [CrossRef]
- Ross D, Chan D, Kuo E, Harkenrider M. Thoracic Reirradiation with Stereotactic Body Radiation Therapy (SBRT) for Recurrent Advanced Non-Small Cell Lung Cancer (NSCLC). Practical Radiation Oncology 2024;14:234–40. [CrossRef]
- Sahin A, Romano E, Casutt A, Moeckli R, Vallet V, El Chammah S, et al. Stereotactic Lung Re-Irradiation After a First Course of Stereotactic Radiotherapy with In-Field Relapse: A Valuable Option to Be Considered. Cancers 2025;17:366. [CrossRef]
- Yanlan Z, Xianfeng L, Lingjun H. Consideration of the target area of radiotherapy for lung cancer with 4DPET. Precision Radiation Oncology 2021;5:191–6. [CrossRef]
- Trémolières P, Gonzalez-Moya A, Paumier A, Mege M, Blanchecotte J, Theotime C, et al. Lung stereotactic body radiation therapy: personalized PTV margins according to tumor location and number of four-dimensional CT scans. Radiat Oncol 2022;17:5. [CrossRef]
- Retif P, Djibo Sidikou A, Letellier R, Verrecchia-Ramos E, Quetin P. Technical Note: A 3D-printed phantom for radiochromic film evaluation of moving lung tumor SBRT without dose convolution. Medical Physics 2021;48:3453–8. [CrossRef]
- Siva S, Chesson B, Callahan JW, Hardcastle N, Crawford L, Antippa P, et al. Dosimetric Consequences of 3D Versus 4D PET/CT for Target Delineation of Lung Stereotactic Radiotherapy. Journal of Thoracic Oncology 2015;10:1112–5. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).