Submitted:
07 April 2025
Posted:
08 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ohmic Heating System
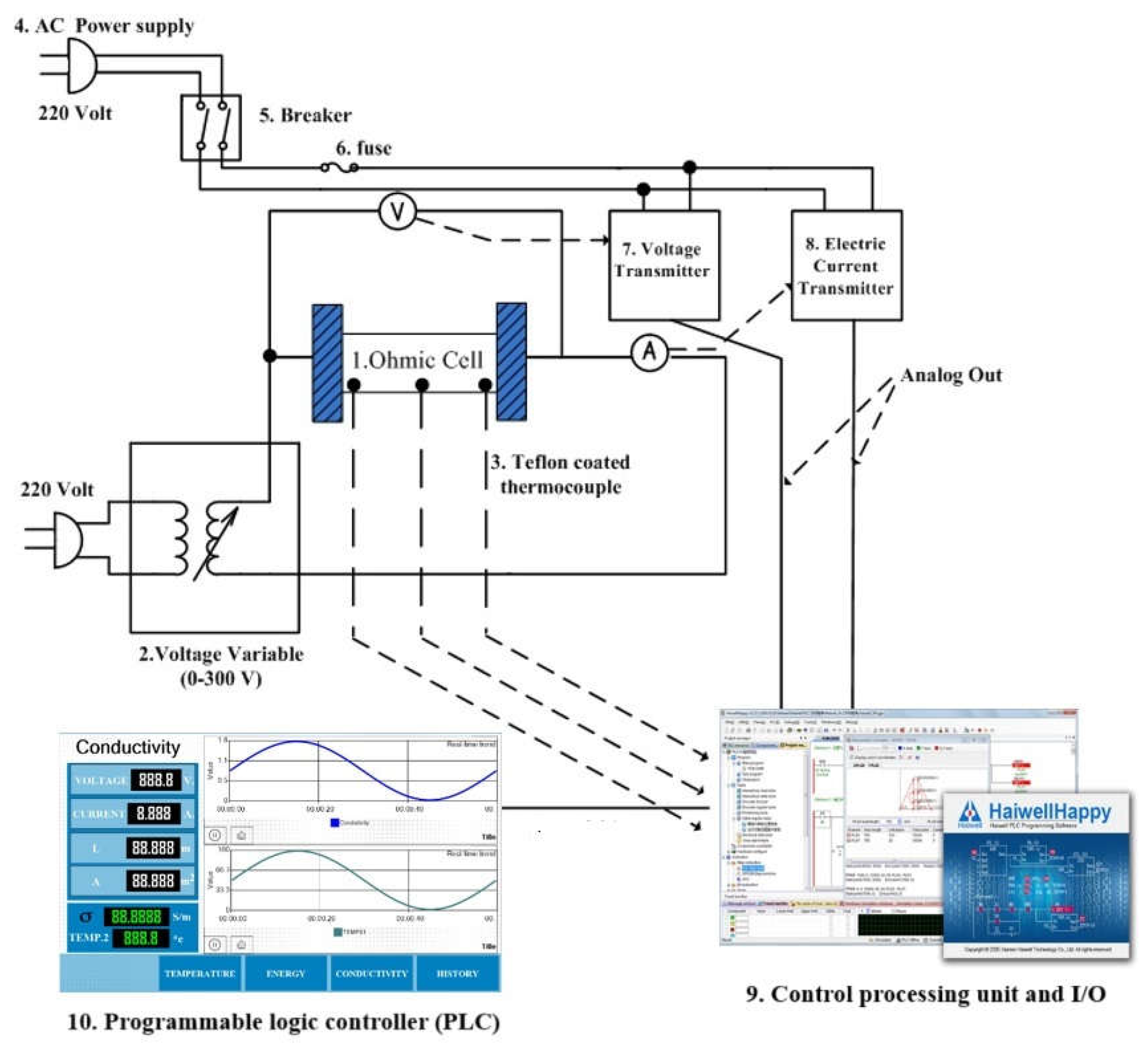
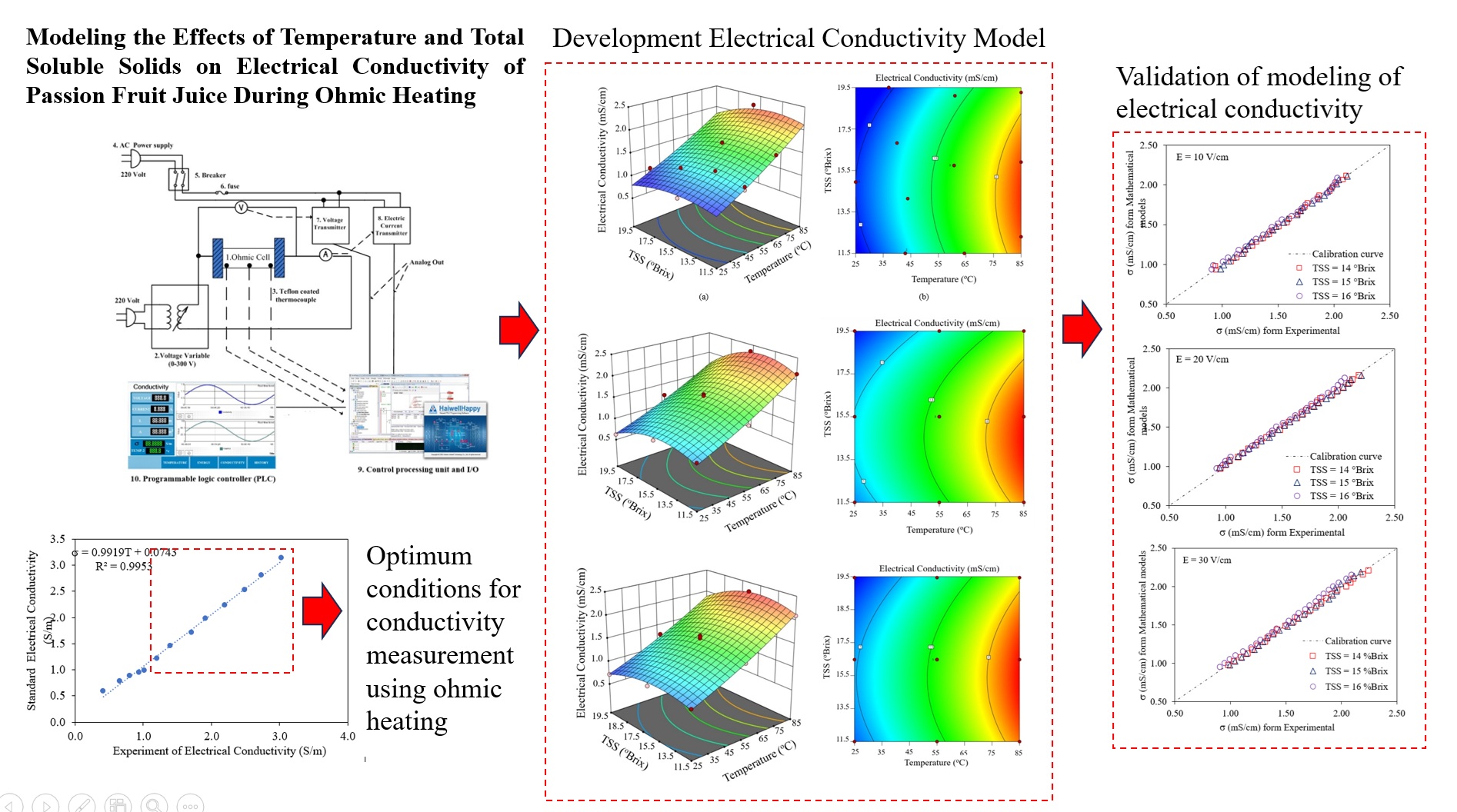
2.3. Electrical Conductivity of Fruit Juices Under Different Conditions
2.4. Electrical Conductivity Model
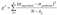

2.5. Statistical Evaluation
3. Results
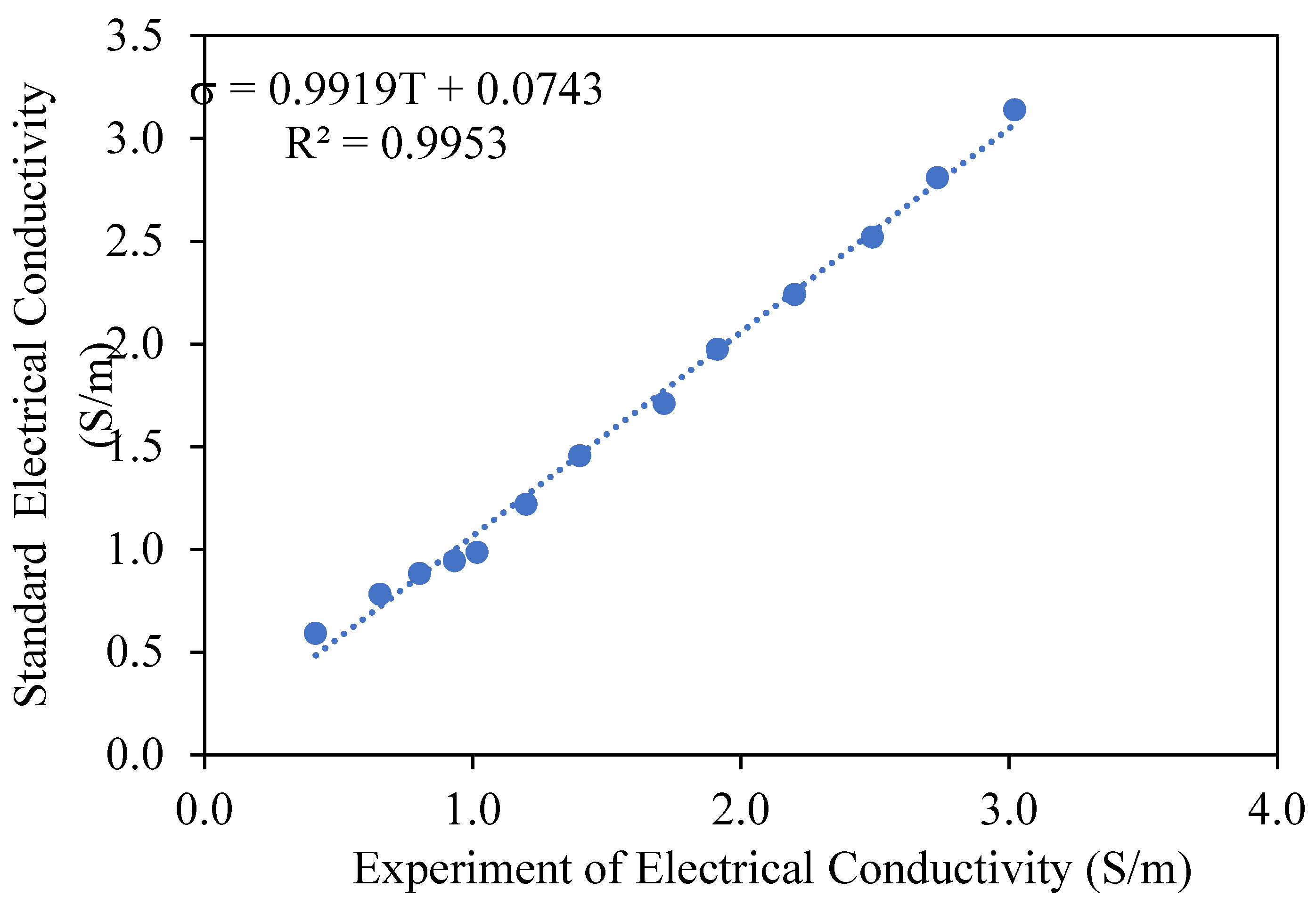
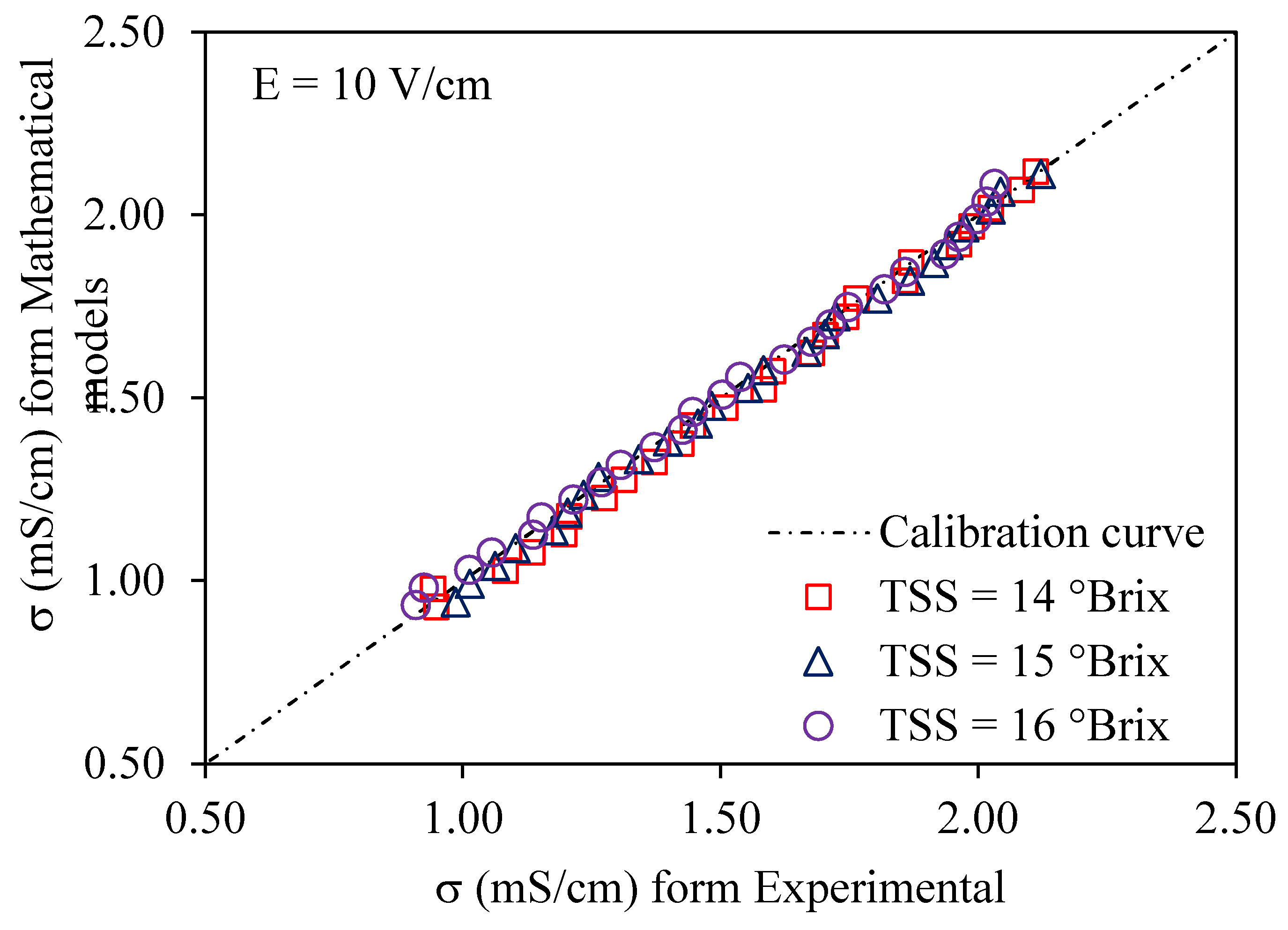
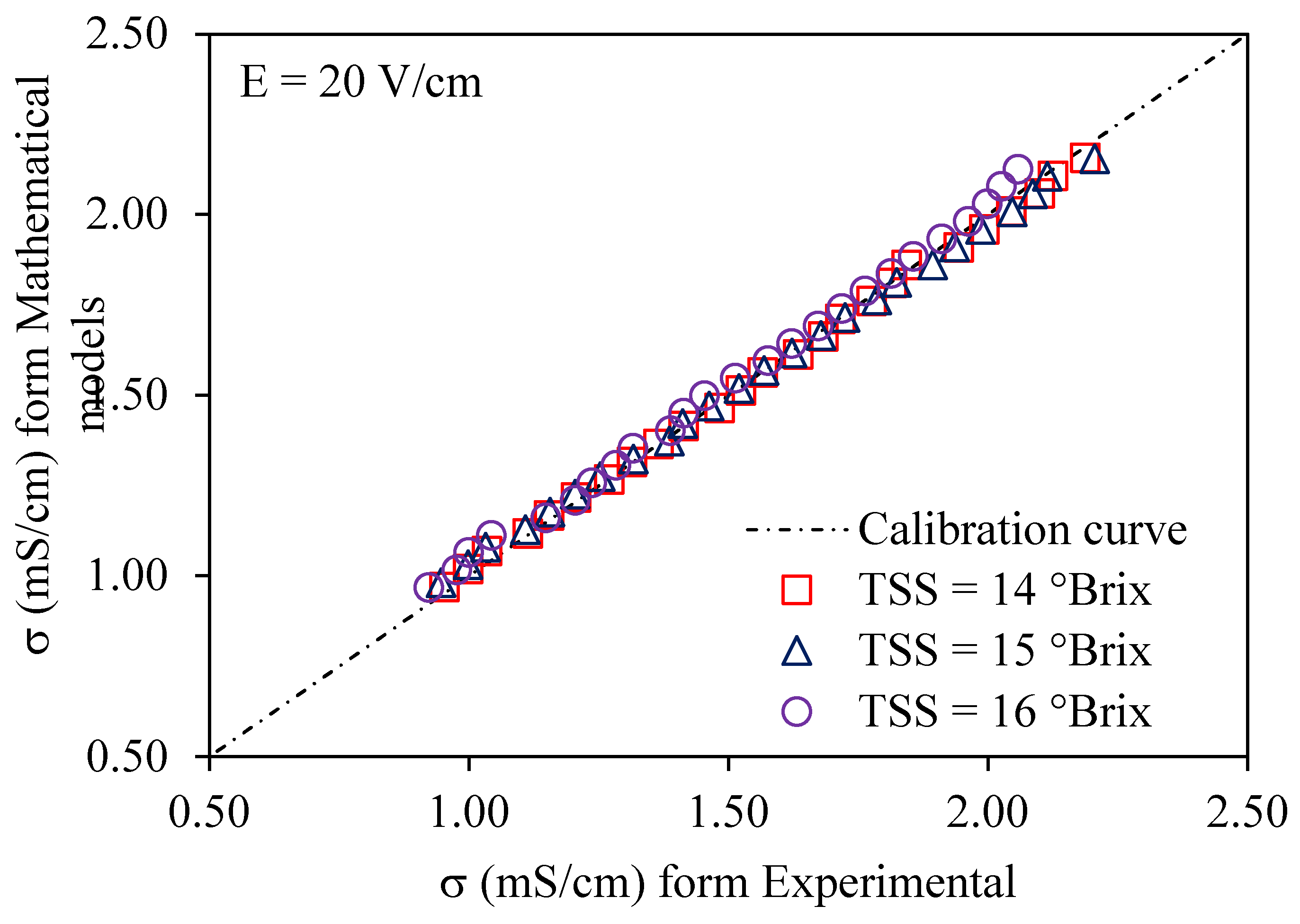
3.1. Ohmic Heating Electrical Conductivity Calibration and Accuracy
3.2. Determination Modeling of Electrical Conductivity During Ohmic Heating
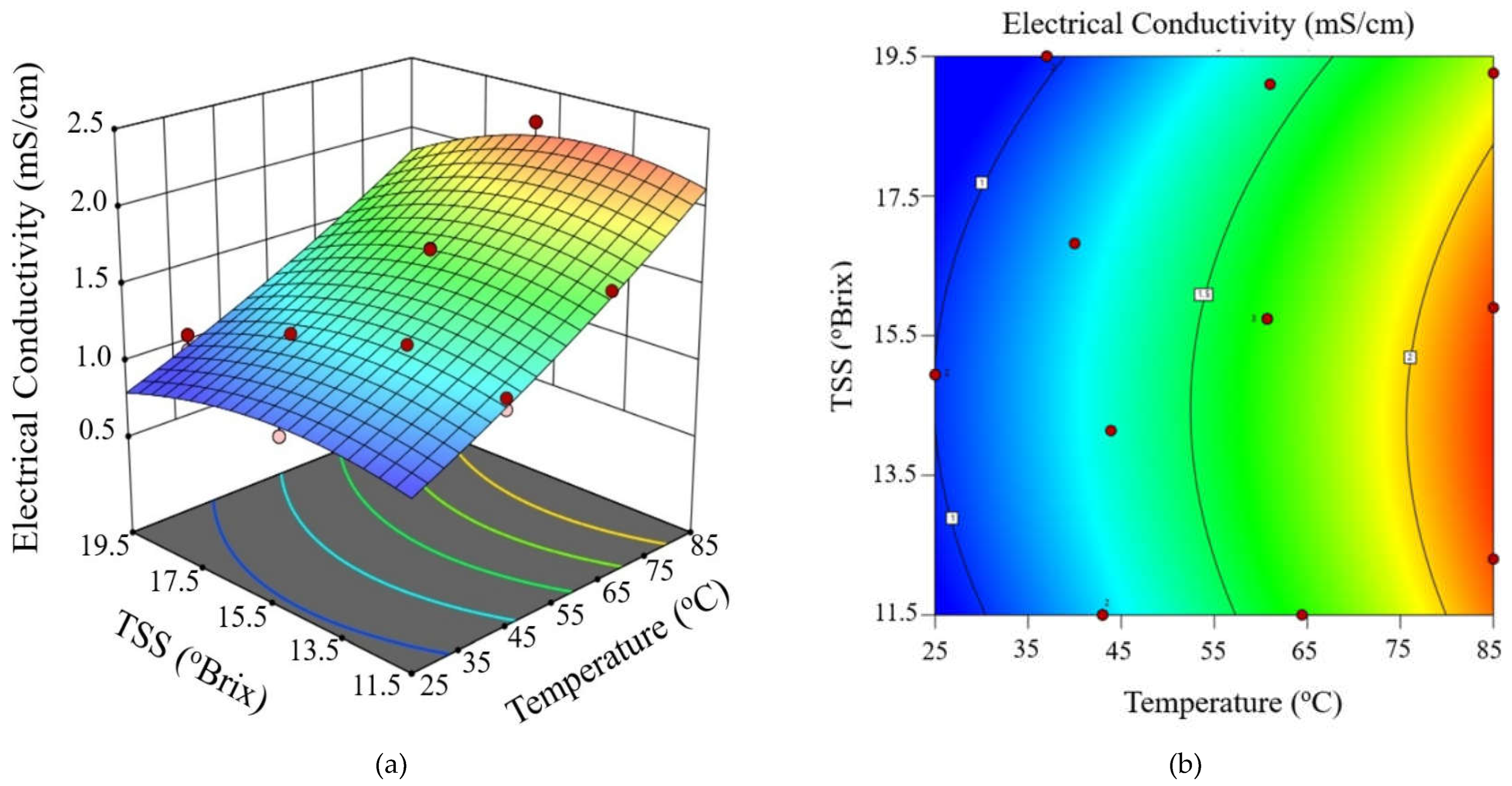
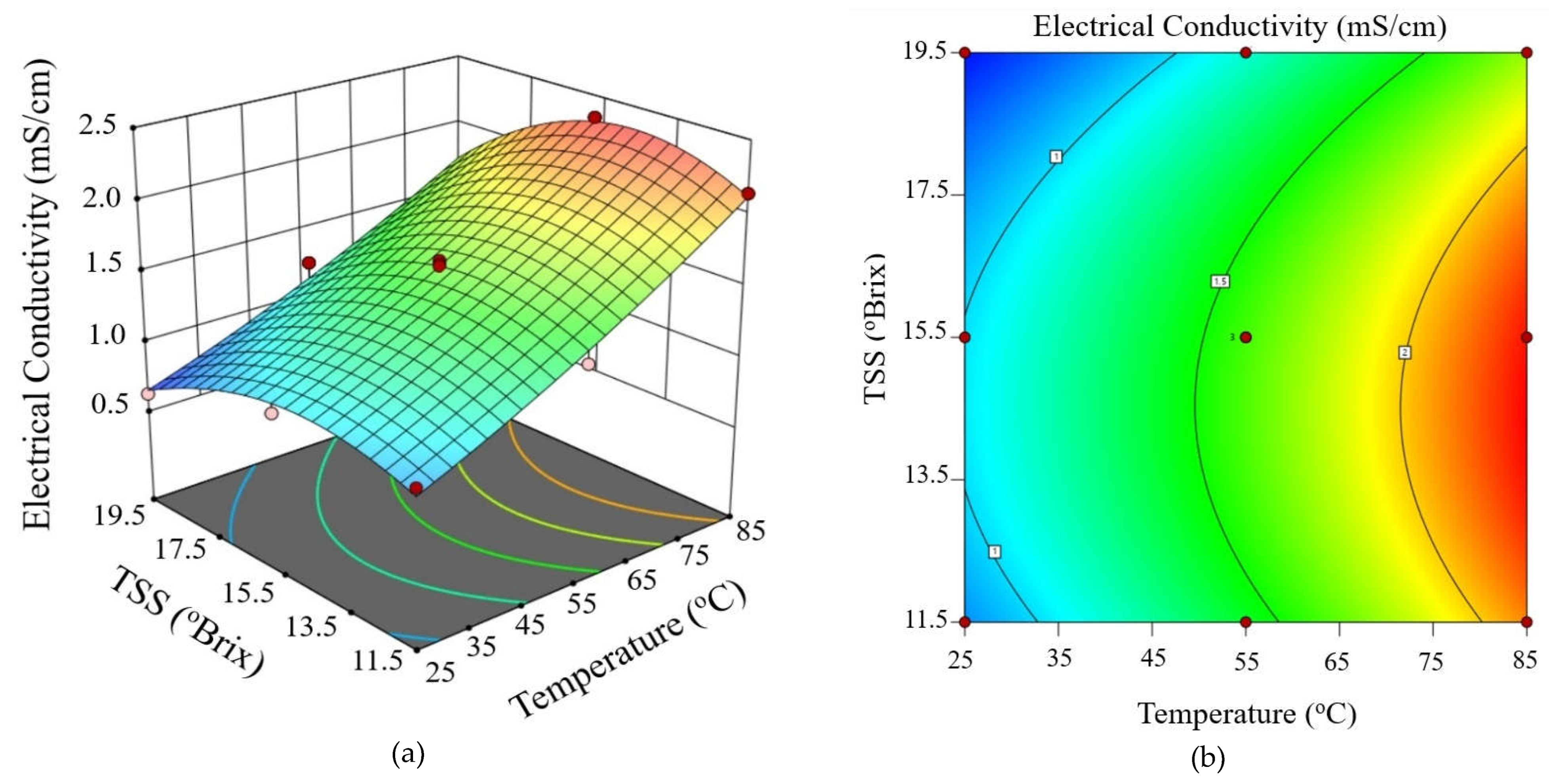

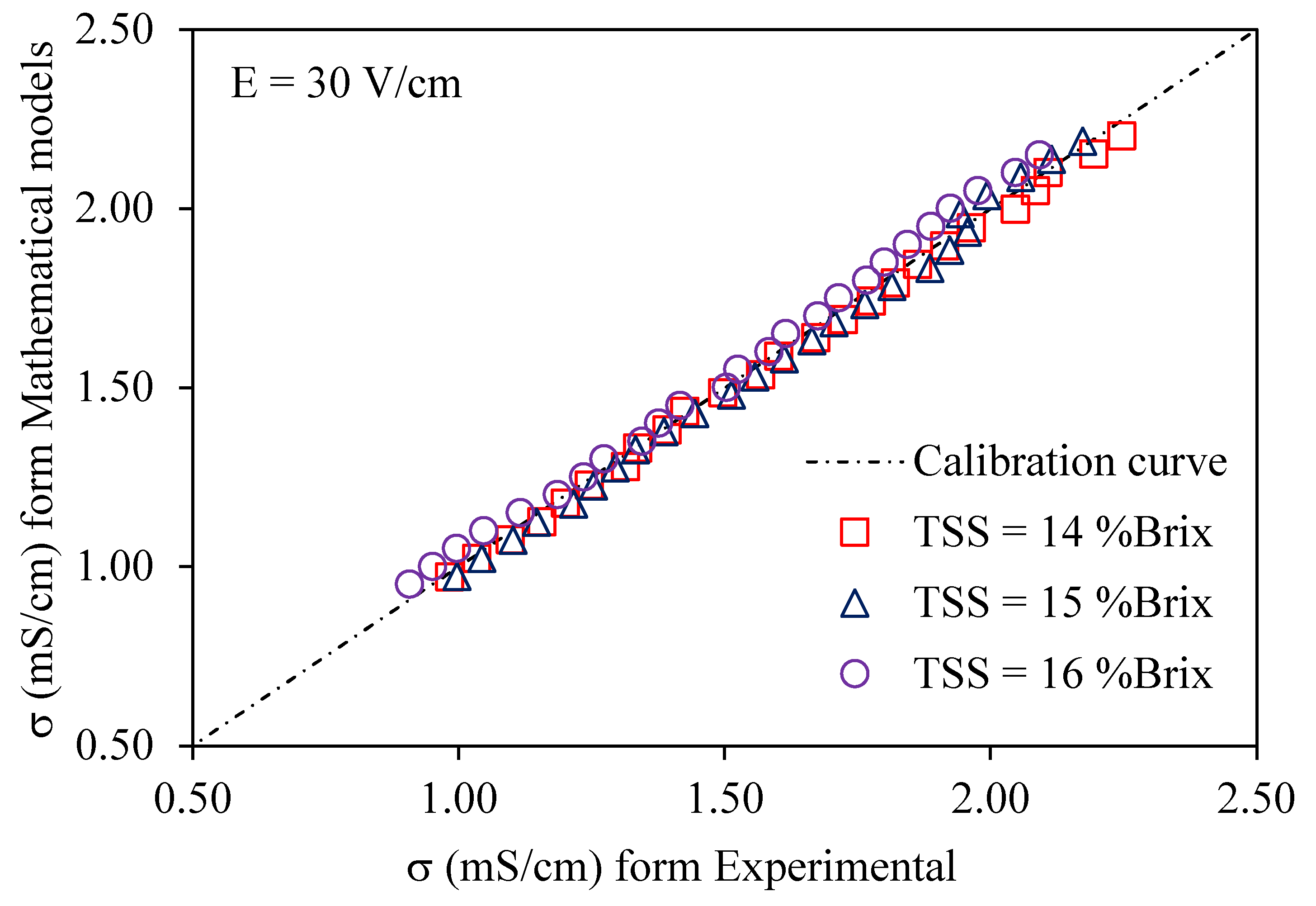
3.3. Validation of Modeling of Electrical Conductivity
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaur, M.; Kumar, S.; Samota, M.K.; Lalremmawii. Ohmic Heating Technology Systems, Factors Governing Efficiency and Its Application to Inactivation of Pathogenic Microbial, Enzyme Inactivation, and Extraction of Juice, Oil, and Bioactive Compounds in the Food Sector. Food Bioprocess Technol. 2024, 17, 299–324. [Google Scholar] [CrossRef]
- Salari, S.; Jafari, S.M. The Influence of Ohmic Heating on Degradation of Food Bioactive Ingredients. Food Eng. Rev. 2020, 12, 191–208. [Google Scholar] [CrossRef]
- Sastry, S.K.; Barach, J.T. Ohmic and Inductive Heating. J. Food Sci. 2000, 65, 42–46. [Google Scholar] [CrossRef]
- Javed, T.; Oluwole-Ojo, O.; Zhang, H.; Akmal, M.; Breikin, T.; O’Brien, A. System Design, Modelling, Energy Analysis, and Industrial Applications of Ohmic Heating Technology. Food and Bioprocess Technology 2025, 18, 2195–2217. [Google Scholar] [CrossRef]
- Doan, N.K.; Lai, D.Q.; Le, T.K.P. Ohmic Heating: Its Current and Future Application in Juice Processing. Food Rev. Int. 2023, 39, 6908–6933. [Google Scholar] [CrossRef]
- Cevik, M. Electrical Conductivity and Performance Evaluation of Verjuice Concentration Process Using Ohmic Heating Method. J. Food Process Eng. 2021, 44, e13672. [Google Scholar] [CrossRef]
- Kamonpatana, P.; Sastry, S.K. Electrical Conductivity of Foods and Food Components: The Influence of Formulation Processes. J. Food Process Eng. 2022, 45, e13992. [Google Scholar] [CrossRef]
- Afraz, M.T.; Xu, X.; Zeng, X.A.; Zhao, W.; Lin, S.; Woo, M.; Han, Z. The Science behind Physical Field Technologies for Improved Extraction of Juices with Enhanced Quality Attributes. Food Phys. 2024, 1, 100008. [Google Scholar] [CrossRef]
- Athmaselvi, K.A.; Viswanathan, R.; Balasubramanian, M.; Roy, I. The Effects of Concentration and Type of Electrode on Electrical Conductivity of Guava Pulp during Ohmic Heating. J. Food Res. Technol. 2014, 2, 113–123. [Google Scholar]
- Srivastav, S.; Roy, S. Changes in Electrical Conductivity of Liquid Foods during Ohmic Heating. Int. J. Agric. Biol. Eng. 2014, 7, 133–138. [Google Scholar] [CrossRef]
- Lamsal, B.; Jindal, V. Variation in Electrical Conductivity of Selected Fruit Juices during Continuous Ohmic Heating. KMUTNB Int. J. Appl. Sci. Technol. 2014, 7, 47–56. [Google Scholar] [CrossRef]
- Assawarachan, R. Estimation Model for Electrical Conductivity of Red Grape Juice. Int. J. Agric. Biol. Eng. 2010, 3, 52–57. [Google Scholar] [CrossRef]
- Sabanci, S.; Kaya, K.; Göksu, A. Modeling the Electrical Conductivity Value of the Model Solution. An. Acad. Bras. Ciênc. 2023, 95, e20210062. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Militão, A.; da Silva, T.I.; do Nascimento, A.M.; da Costa, F.B.; de Castro, A.K.G.; Macêdo, L.F.; Santos, S.C.L. Storage Increases Soluble Sugars and Decreases Bioactive Compounds in Wild Passion Fruit (Passiflora cincinnata). Horticulturae Environ. Biotechnol. 2025, 1, 1–16. [Google Scholar] [CrossRef]
- Icier, F.; Yildiz, H.; Sabanci, S.; Cevik, M.; Cokgezme, O.F. Ohmic Heating Assisted Vacuum Evaporation of Pomegranate Juice: Electrical Conductivity Changes. Innov. Food Sci. Emerg. Technol. 2017, 39, 241–246. [Google Scholar] [CrossRef]
- Sastry, S.K.; Li, Q. Modeling the Ohmic Heating of Solid–Liquid Mixtures: Model Development and Validation. J. Food Eng. 1996, 27, 241–260. [Google Scholar] [CrossRef]
- Abdulstar, A.R.; Altemimi, A.; Al-HiIphy, A.R.; Watson, D.G.; Lakhssassi, N. Water Distillation Using an Ohmic Heating Apparatus. Int. J. Ambient Energy 2022, 43, 2748–2758. [Google Scholar] [CrossRef]
- Wiroonsri, P.; Wattanachant, S. Electrical Conductivity as a Precise Method for Salt Content Estimation in Raw and Cooked Tuna Meat. J. Food Compos. Anal. 2025, 137, 106953. [Google Scholar] [CrossRef]
- Hardinasinta, G.; Salengke, S.; Mursalim, M.; Muhidong, J. Effect of Ohmic Heating on the Rheological Characteristics and Electrical Conductivity of Mulberry (Morus nigra) Puree. Pol. J. Food Nutr. Sci. 2021, 71, 289–297. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Barbosa, V.P.; Gut, J.A.W.; Tadini, C.C. Predicting Dielectric Properties of Fruit Juices at 915 and 2450 MHz Using Machine Learning and Physicochemical Measurements. Meas. Food 2024, 14, 100158. [Google Scholar] [CrossRef]
- Zia, H.; Slatnar, A.; Košmerl, T.; Korošec, M. A Review Study on the Effects of Thermal and Non-Thermal Processing Techniques on the Sensory Properties of Fruit Juices and Beverages. Front. Food Sci. Technol. 2024, 4, 1405384. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Zhou, Y.; Li, G.H.; Feng, X.S. Progress in Pretreatment and Analysis of Organic Acids: An Update Since 2010. Food Chem. 2021, 360, 129977. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yan, J.K.; Jin, M.Y.; Li, L.Q.; Yu, Y.H.; Xu, L. Preparation, Physicochemical and Functional Characterization of Pectic Polysaccharides from Fresh Passion Fruit Peel by Magnetic-Induced Electric Field-Assisted Three-Phase Partitioning. Food Hydrocoll. 2024, 156, 110292. [Google Scholar] [CrossRef]
- Du, H.; Olawuyi, I.F.; Said, N.S.; Lee, W.Y. Comparative Analysis of Physicochemical and Functional Properties of Pectin from Extracted Dragon Fruit Waste by Different Techniques. Polymers 2024, 16, 1097. [Google Scholar] [CrossRef]
- Huo, D.; Dai, J.; Yuan, S.; Cheng, X.; Pan, Y.; Wang, L.; Wang, R. Eco-Friendly Simultaneous Extraction of Pectins and Phenolics from Passion Fruit (Passiflora edulis Sims) Peel: Process Optimization, Physicochemical Properties, and Antioxidant Activity. Int. J. Biol. Macromol. 2023, 243, 125229. [Google Scholar] [CrossRef]
- Brito, I.P.C.; Silva, E.K. Pulsed Electric Field Technology in Vegetable and Fruit Juice Processing: A Review. Food Res. Int. 2024, 173, 114207. [Google Scholar] [CrossRef] [PubMed]
- Khuenpet, K.; Jittanit, W. The Effects of Pasteurization by Conventional and Ohmic Heating Methods and Concentration Processes on the Madan (Garcinia schomburgkiana Pierre) Juice Properties. Appl. Eng. Agric. 2020, 36, 205–219. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Cheng, Y.; Gao, Z.; Qu, K.; Chen, Z.; Guan, W. Effects of Pulsed Electric Field and High-Pressure Processing Treatments on the Juice Yield and Quality of Sea Buckthorn. Foods 2024, 13, 1829. [Google Scholar] [CrossRef] [PubMed]
- Sarbatly, R.; Sariau, J.; Krishnaiah, D. Recent Developments of Membrane Technology in the Clarification and Concentration of Fruit Juices. Food Eng. Rev. 2023, 15, 420–437. [Google Scholar] [CrossRef]
- Chutia, H.; Begum, F.; Rohilla, S.; Mahanta, C.L. Impact of Thermosonication Treatment on Passion Fruit Juice: ANN/GA Optimization, Predictive Modelling for Shelf Life and Quality Changes during Storage. Int. J. Food Eng. 2024, 20, 463–474. [Google Scholar] [CrossRef]
- Saruan, N.; Abdullah, N.; Malik, N.H.; Muhammad, N. Effect of Ultrasound Treatment on the Physicochemical Properties and Bioactive Compounds of Yellow Passion Fruit Juice. In Proceedings of the 5th International Conference on Agricultural and Food Engineering (CAFEi2022), No. 1, 030012. Melaka, Malaysia, 21–23 February 2022; AIP Publishing: Melville, NY, USA, 2023; Volume 2682. [Google Scholar] [CrossRef]
- Angami, T.; Assumi, S.R.; Kalita, H.; Saloi, B.; Singh, K.S.; Touthang, L.; Tasung, A. Preparation and Evaluation of Fresh Pineapple, Passion Fruit and Ginger Blended Ready-to-Serve Drink. Environ. Conserv. J. 2023, 24, 63–66. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Zendeboodi, F.; Mirsaeedghazi, H. Effect of Membrane Clarification on the Physicochemical Properties of Fruit Juices: A Review. Iran. J. Chem. Chem. Eng. 2022, 41, 51–66. [Google Scholar]
| Model Type | Regression Equation | Voltage gradient |
R2 | χ² | RMSE |
|---|---|---|---|---|---|
| Linear: | σ (mS/cm) = 0.6163+ 0.0193 (T) -0.0174 (TSS) |
10 V/cm | 0.9111 | 0.2129 | 0.0879 |
| σ (mS/cm) = 0.6624+ 0.02007 (T) -0.02065 (TSS) |
20 V/cm | 0.8791 | 0.2763 | 0.1028 | |
| σ (mS/cm) = 0.7933+ 0.0202 (T) -0.0282 (TSS) |
30 V/cm | 0.9471 | 0.2037 | 0.0918 | |
| Two-Factor Interaction | σ (mS/cm) = 0.3422+ 0.0243 (T) - 0.0002 (TSS) - 0.00032 (T)(TSS) |
10 V/cm | 0.9481 | 0.2041 | 0.0857 |
| σ (mS/cm) = 0.4535+ 0.0239 (T) -0.0072 (TSS) - 0.00025 (T)(TSS) |
20 V/cm | 0.9562 | 0.2542 | 0.0988 | |
| σ (mS/cm) = 0.5635+ 0.0243 (T) -0.0134 (TSS) + 0.00027 (T)(TSS) |
30 V/cm | 0.9488 | 0.1999 | 0.0903 | |
| Polynomial model | σ (mS/cm) = -2.2007+ 0.0243 (T) +0.3436 (TSS) - 0.00032 (T)(TSS) - 0.0111 (TSS)2 |
10 V/cm | 0.9974 | 0.0112 | 0.0191 |
| σ (mS/cm) = -2.486+ 0.0233 (T) + 0.3917 (TSS) - 0.00025 (T)(TSS) - 0.01287 (TSS)2 |
20 V/cm | 0.9948 | 0.0211 | 0.0298 | |
| σ (mS/cm) = -1.9071+ 0.0243 (T) +0.3203 (TSS) - 0.00027 (T)(TSS) - 0.0108 (TSS)2 |
30 V/cm | 0.9901 | 0.0265 | 0.0323 |
| Voltage gradients |
TSS ( oBrix) | R2 |
χ² |
RMSE |
|---|---|---|---|---|
| 14 | 0.9871 | 0.0380 | 0.0256 | |
| 10 V/cm | 15 | 0.9950 | 0.0240 | 0.0088 |
| 16 | 0.9953 | 0.0234 | 0.0095 | |
| 14 | 0.9967 | 0.0204 | 0.0060 | |
| 20 V/cm | 15 | 0.9956 | 0.0238 | 0.0091 |
| 16 | 0.9880 | 0.0367 | 0.0234 | |
| 14 | 0.9948 | 0.0260 | 0.0095 | |
| 30 V/cm | 15 | 0.9924 | 0.0295 | 0.0127 |
| 16 | 0.9825 | 0.0446 | 0.0307 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




