Submitted:
18 May 2025
Posted:
20 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
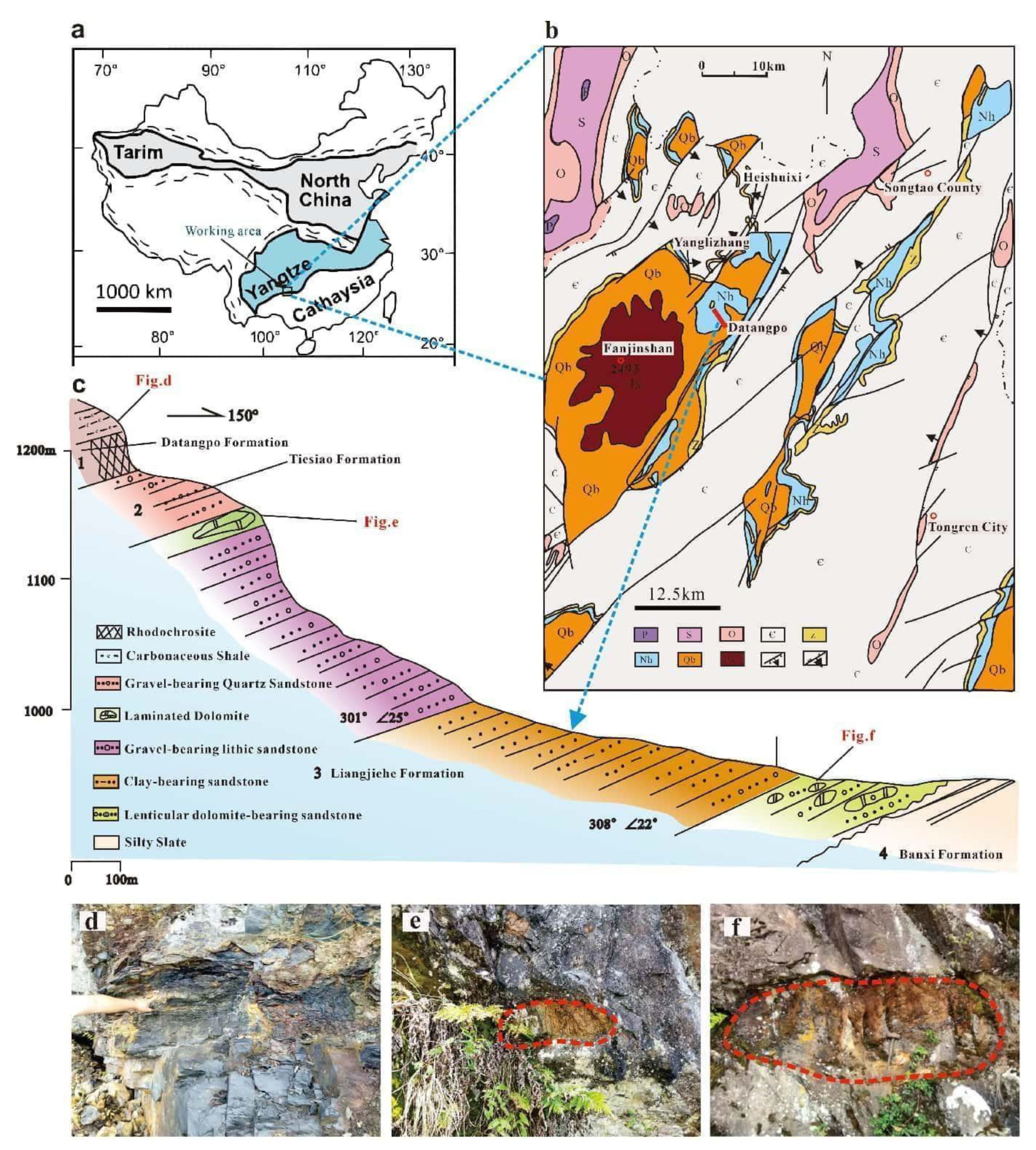
2. Geological setting
3. Material and Method
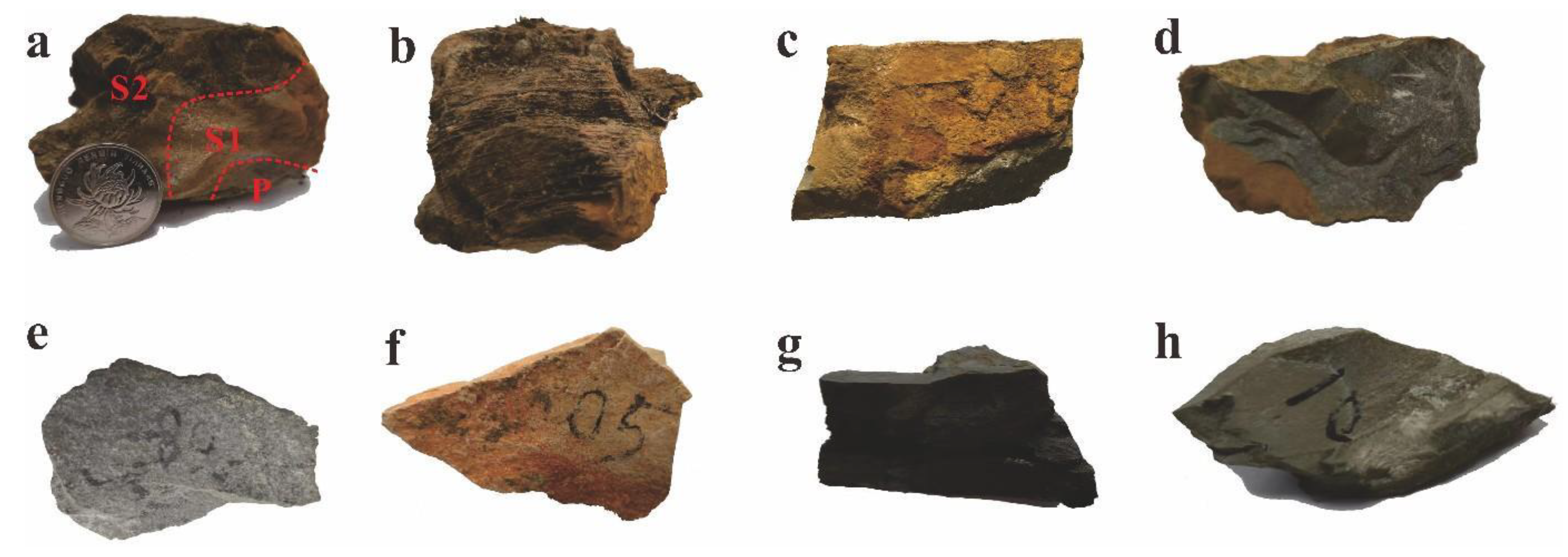
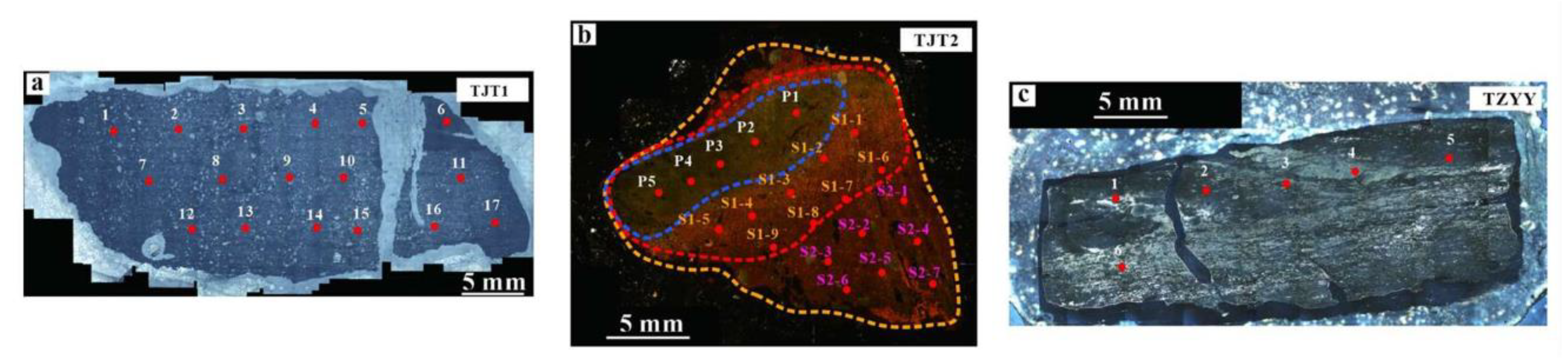
3.1. Petrographic Observation
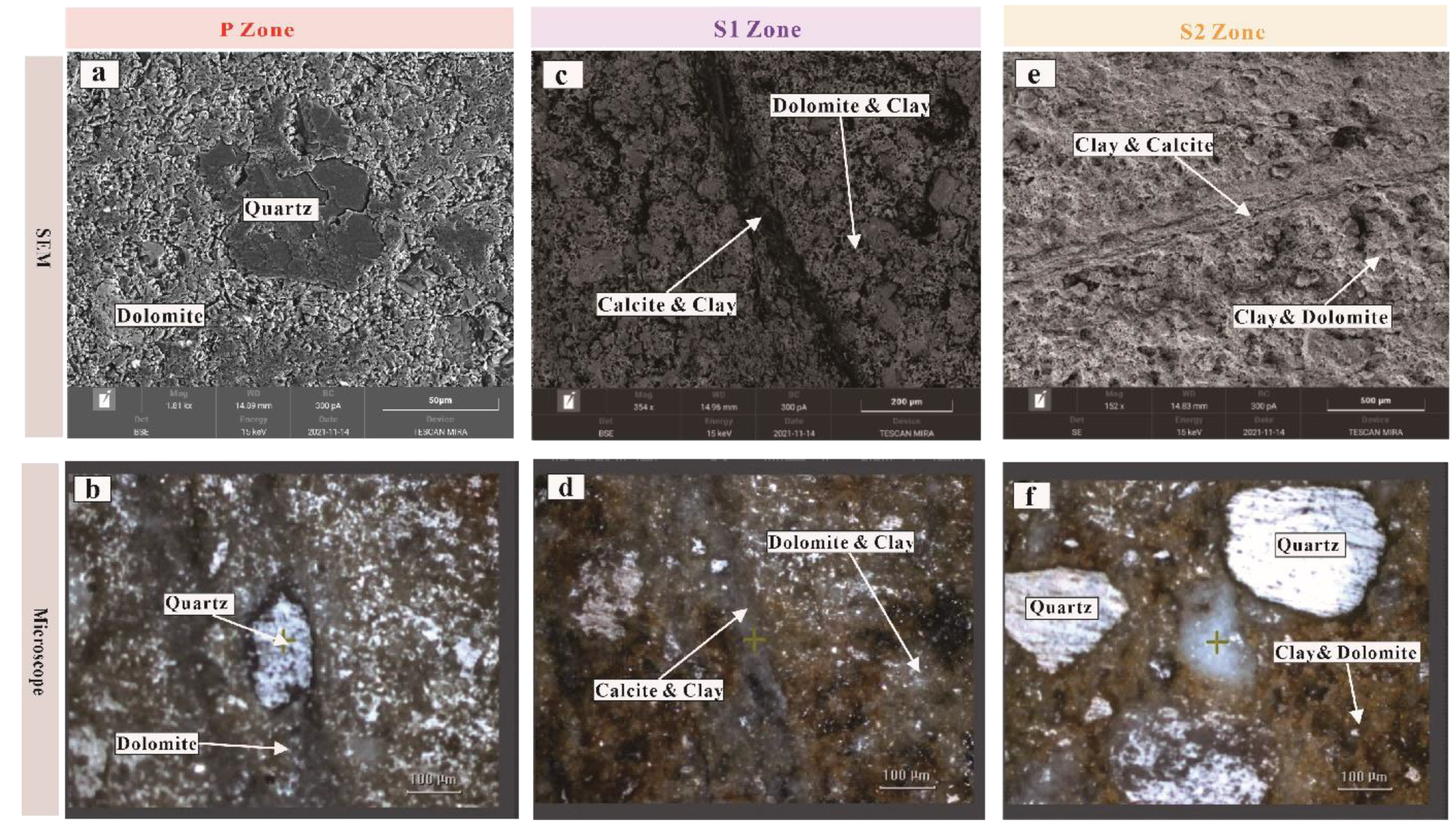
3.2. Scanning Electron Microscopy (SEM)
3.3. X-ray Diffraction (XRD) Mineral Identification
3.4. Trace and REEs Analysis
3.5. Carbon and Oxygen Isotope Analysis
3.6. Calculate Method
Migration Coefficient
Cerium (Ce) Anomaly
4. Result
4.1. SEM and Microscope
4.2. XRD Pattern
4.3. LA-ICP-MS Data
4.4. Carbon and Oxygen Isotopes
5. Discussion
5.1. Petrograph and Geochemistry Features Reveal Two Stages of Carbonate Incipient Weathering
5.2. Carbonate Incipient Weathering Indicates Lateritic Soil Formation Model
5.3. C-O Isotopes Variation During Carbonate Incipient Weathering
5.4. Carbonate Re-Precipitation During Carbonate Incipient Weathering
6. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, S.; Liu, Z.; Kaufmann, G. Sensitivity of the global carbonate weathering carbon-sink flux to climate and land-use changes. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lechuga-Crespo, J.L.; Sauvage, S.; Ruiz-Romera, E.; van Vliet, M.T.H.; Probst, J.L.; Fabre, C.; Sánchez-Pérez, J.M. Global carbon sequestration through continental chemical weathering in a climatic change context. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Z.; Davis, S.; Ciais, P. Monitoring global carbon emissions in 2022. Nat. Rev. Earth Environ. 2023, 4, 205–206. [Google Scholar] [CrossRef]
- Quinton, J.N.; Govers, G.; Van Oost, K.; Bardgett, R.D. The impact of agricultural soil erosion on biogeochemical cycling. Nat. Geosci. 2010, 3, 311–314. [Google Scholar] [CrossRef]
- Liu, L.; Sayer, E.J.; Deng, M.; Li, P.; Liu, W.; Wang, X.; Yang, S.; Huang, J.; Luo, J.; Su, Y.; et al. The grassland carbon cycle: Mechanisms, responses to global changes, and potential contribution to carbon neutrality. Fundam. Res. 2023, 3, 209–218. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, Z.; Groves, C. Large-scale CO2 removal by enhanced carbonate weathering from changes in land-use practices. Earth-Science Rev. 2022, 225, 103915. [Google Scholar] [CrossRef]
- Jiao, K.; Liu, Z.; Wang, W.; Yu, K.; Mcgrath, M.J.; Xu, W. Carbon cycle responses to climate change across China’s terrestrial ecosystem: Sensitivity and driving process. Sci. Total Environ. 2024, 915, 170053. [Google Scholar] [CrossRef]
- Knapp, W.J.; Tipper, E.T. The efficacy of enhancing carbonate weathering for carbon dioxide sequestration. Front. Clim. 2022, 4. [Google Scholar] [CrossRef]
- Bai X, Zhang S, Smith P, Li C, Xiong L, Du C, Xue Y, Li Z, Long M, Li M, Zhang X, Yang S, Luo Q, Shen X. Resolving controversies surrounding carbon sinks from carbonate weathering. Sci. Sin. Terrae 2024, 54, 2747–2761. [Google Scholar]
- Pan, Y.; Zhang, H.; Wang, C.; Zhou, Y. Impact of land use change on regional carbon sink capacity: Evidence from Sanmenxia, China. Ecol. Indic. 2023, 156, 111189. [Google Scholar] [CrossRef]
- Ott, R.; Gallen, S.F.; Helman, D. Erosion and weathering in carbonate regions reveal climatic and tectonic drivers of carbonate landscape evolution. Earth Surf. Dyn. 2023, 11, 247–257. [Google Scholar] [CrossRef]
- Marcé, R.; Obrador, B.; Morguí, J.A.; Lluís Riera, J.; López, P.; Armengol, J. Carbonate weathering as a driver of CO2 supersaturation in lakes. Nat. Geosci. 2015, 8, 107–111. [Google Scholar] [CrossRef]
- Nõges, P.; Cremona, F.; Laas, A.; Martma, T.; Rõõm, E.I.; Toming, K.; Viik, M.; Vilbaste, S.; Nõges, T. Role of a productive lake in carbon sequestration within a calcareous catchment. Sci. Total Environ. 2016, 550, 225–230. [Google Scholar] [CrossRef]
- Mo, C.; Xin, S.; Huang, F.; Cao, J.; Xiao, J. Characteristics of Dissolution Changes in Carbonate Rocks and Their Influencing Factors in the Maocun Basin, Guilin, China. Water (Switzerland) 2023, 15. [Google Scholar] [CrossRef]
- Morse, J.W.; Arvidson, R.S. The dissolution kinetics of major sedimentary carbonate minerals. Earth-Science Rev. 2002, 58, 51–84. [Google Scholar] [CrossRef]
- Boettger, J.D.; Kubicki, J.D. Equilibrium and kinetic isotopic fractionation in the CO2 hydration and hydroxylation reactions: Analysis of the role of hydrogen-bonding via quantum mechanical calculations. Geochim. Cosmochim. Acta 2021, 292, 37–63. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, X.; Yu, W.; Du, Y.; Du, Q. Redox conditions and manganese metallogenesis in the Cryogenian Nanhua Basin: Insight from the basal Datangpo Formation of South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 529, 39–52. [Google Scholar] [CrossRef]
- Wu, J.; Tan, Z.; Jia, W.; Chen, J.; Peng, P. Nitrogen isotopes and geochemistry of the basal Datangpo Formation: Contrasting redox conditions in the upper and lower water columns during the Cryogenian interglaciation period. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 637, 112005. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, C.; Hu, X.; Yang, B.; Zhang, X.; Du, Y.; Xu, K.; Yuan, L.; Ni, J.; Hu, D.; et al. A new metallogenic model for the giant manganese deposits in northeastern Guizhou, China. Ore Geol. Rev. 2022, 149, 105070. [Google Scholar] [CrossRef]
- Zhou, C.; Tucker, R.; Xiao, S.; Peng, Z.; Yuan, X.; Chen, Z. New constraints on the ages of Neoproterozoic glaciations in south China. Geology 2004, 32, 437–440. [Google Scholar] [CrossRef]
- Evans, D.A.D.; Li, Z.X.; Kirschvink, J.L.; Wingate, M.T.D. A high-quality mid-Neoproterozoic paleomagnetic pole from South China, with implications for ice ages and the breakup configuration of Rodinia. Precambrian Res. 2000, 100, 313–334. [Google Scholar] [CrossRef]
- Jiang, G.; Shi, X.; Zhang, S.; Wang, Y.; Xiao, S. Stratigraphy and paleogeography of the Ediacaran Doushantuo Formation (ca. 635-551Ma) in South China. Gondwana Res. 2011, 19, 831–849. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, X.; Xu, H.; Konishi, H.; Lin, Z.; Xu, L.; Chen, T.; Hao, X.; Lu, H.; Mann, J.P. Formation of dolomite catalyzed by sulfate-driven anaerobic oxidation of methane: Mineralogical and geochemical evidence from the northern South China Sea. Am. Mineral. 2018, 103, 720–734. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Li, D.; Lin, Z.; Chen, T.; Lin, H. Sediment Mineralogy and Geochemistry and Their Implications for the Accumulation of Organic Matter in Gashydrate Bearing Zone of Shenhu, South China Sea. Minerals 2023, 13, 1419. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Sun, X. Marine sediment nitrogen isotopes and their implications for the nitrogen cycle in the sulfate-methane transition zone. Front. Mar. Sci. 2023, 9, 1–16. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Li, D.; Lin, Z.; Lu, Y.; Liang, Y.; Zhang, Y. Elemental and isotopic response of different carbon components to anaerobic oxidation of methane: A case study of marine sediments in the Shenhu region, northern South China Sea. J. Asian Earth Sci. 2021, 206, 104577. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, X.; Lin, Z.; Sun, X.; Yang, Y.; Peckmann, J. Reducing microenvironments promote incorporation of magnesium ions into authigenic carbonate forming at methane seeps: Constraints for dolomite formation. Sedimentology 2021, 68, 2945–2964. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, X.; Peckmann, J.; Lu, Y.; Xu, L.; Strauss, H.; Zhou, H.; Gong, J.; Lu, H.; Teichert, B.M.A. How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chem. Geol. 2016, 440, 26–41. [Google Scholar] [CrossRef]
- Caetano-Filho, S.; Paula-Santos, G.M.; Dias-Brito, D. Carbonate REE + Y signatures from the restricted early marine phase of South Atlantic Ocean (late Aptian – Albian): The influence of early anoxic diagenesis on shale-normalized REE + Y patterns of ancient carbonate rocks. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 500, 69–83. [Google Scholar] [CrossRef]
- Liao, J.; Sun, X.; Li, D.; Sa, R.; Lu, Y.; Lin, Z.; Xu, L.; Zhan, R.; Pan, Y.; Xu, H. New insights into nanostructure and geochemistry of bioapatite in REE-rich deep-sea sediments: LA-ICP-MS, TEM, and Z-contrast imaging studies. Chem. Geol. 2019, 512, 58–68. [Google Scholar] [CrossRef]
- Bayon, G.; Birot, D.; Ruffine, L.; Caprais, J.C.; Ponzevera, E.; Bollinger, C.; Donval, J.P.; Charlou, J.L.; Voisset, M.; Grimaud, S. Evidence for intense REE scavenging at cold seeps from the Niger Delta margin. Earth Planet. Sci. Lett. 2011, 312, 443–452. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, D.; Peckmann, J.; Roberts, H.H.; Chen, D. New insights into cerium anomalies and mechanisms of trace metal enrichment in authigenic carbonate from hydrocarbon seeps. Chem. Geol. 2014, 381, 55–66. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M. Geochemical distribution of Co, Cu, Ni, and Zn in soil profiles of Fluvisols, Luvisols, Gleysols, and Calcisols originating from Germany and Egypt. Geoderma 2017, 307, 122–138. [Google Scholar] [CrossRef]
- Wang, D.; He, Y.; Chen, Y.; Yang, F.; He, Z.; Zeng, T.; Lu, X.; Wang, L.; Song, S.; Ma, J. Electron transfer enhancing the Mn(II)/Mn(III) cycle in MnO/CN towards catalytic ozonation of atrazine via a synergistic effect between MnO and CN. Water Res. 2023, 230, 119574. [Google Scholar] [CrossRef]
- Zhang Liankai, Ji Hongbing, Liu Xiuming, Wei Xiao, Luo Gang, Wang Shijie, Nguyen Dai Trung, Nguyen Quoc Dinh.Genetic mechanism and elemental evolution of weathering laterite crust overlying carbonate rocks in tropical areas[J]. Geology in China.2021, 48(2):651-660(in Chinese with English abstract).
- Zhang, li; Ji, H. ; Gao, J.; Li, J. Geochemistry of elements in typical carbonate weathered profiles of Guizhou Plateau.Geochemstry. 2015, 44, 323–336.
- Beckford, H.O.; Chu, H.; Song, C.; Chang, C.; Ji, H. Geochemical characteristics and behaviour of elements during weathering and pedogenesis over karst area in Yunnan–Guizhou Plateau, southwestern China. Environ. Earth Sci. 2021, 80, 1–21. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Tripati, A.; Feng, S.; Elliott, B.; Whicker, C.; Arnold, A.; Kelley, A.M. Factors controlling the oxygen isotopic composition of lacustrine authigenic carbonates in Western China: implications for paleoclimate reconstructions. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Shi, X.; Zhang, H.; Cao, P.; Li, X.; Lukens, W.E. Applicability and Variability of Chemical Weathering Indicators and Their Monsoon-Controlled Mechanisms in the Bay of Bengal. Front. Earth Sci. 2021, 9. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Chen, S.; Chen, J.; Zhang, X.; Yan, J.; Chen, F. New insights on Chinese cave δ18O records and their paleoclimatic significance. Earth-Science Rev. 2020, 207, 103216. [Google Scholar] [CrossRef]
- Kuo, T.S.; Liu, Z.Q.; Li, H.C.; Wan, N.J.; Shen, C.C.; Ku, T.L. Climate and environmental changes during the past millennium in central western Guizhou, China as recorded by Stalagmite ZJD-21. J. Asian Earth Sci. 2011, 40, 1111–1120. [Google Scholar] [CrossRef]
- Consolaro, C.; Rasmussen, T.L.; Panieri, G.; Mienert, J.; Bünz, S.; Sztybor, K. Carbon isotope (d13C) excursions suggest times of major methane release during the last 14 kyr in Fram Strait, the deep-water gateway to the Arctic. Clim. Past 2015, 11, 669–685. [Google Scholar] [CrossRef]
- Kienast, M.; Calvert, S.E.; Pelejero, C.; Grimalt, J.O. A critical review of marine sedimentary δ13Corg-pCO2 estimates: New palaeorecords from the South China sea and a revisit of other low-latitude δ13Corg-pCO2 records. Global Biogeochem. Cycles 2001, 15, 113–127. [Google Scholar] [CrossRef]
- Meister, P.; Liu, B.; Khalili, A.; Böttcher, M.E.; Jørgensen, B.B. Factors controlling the carbon isotope composition of dissolved inorganic carbon and methane in marine porewater: An evaluation by reaction-transport modelling. J. Mar. Syst. 2019, 200, 103227. [Google Scholar] [CrossRef]
- Hare, V.J.; Loftus, E.; Jeffrey, A.; Ramsey, C.B. Atmospheric CO2 effect on stable carbon isotope composition of terrestrial fossil archives. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Lavallee, J.M. Soil organic matter formation, persistence, and functioning: A synthesis of current understanding to inform its conservation and regeneration; 1st ed.; Elsevier Inc., 2022; Vol. 172; ISBN 9780323989534.
- Wu, C.; Xia, G.; Wagreich, M.; Rodríguez-López, J.P.; Sun, X.; Liu, C.; Yi, H. Early Miocene expansion of C4 vegetation on the northern Tibetan Plateau. Glob. Planet. Change 2019, 177, 173–185. [Google Scholar] [CrossRef]
- Jolivet, M.; Boulvais, P. Global significance of oxygen and carbon isotope compositions of pedogenic carbonates since the Cretaceous. Geosci. Front. 2021, 12, 101132. [Google Scholar] [CrossRef]
- Shengqiang, Y.; Shiguo, W.; Thomas, L.; Genshun, Y.; Fuliang, L.; Feng, C.; Hairong, W.; Li, L. Fine-grained Pleistocene deepwater turbidite channel system on the slope of Qiongdongnan Basin, northern South China Sea. Mar. Pet. Geol. 2009, 26, 1441–1451. [Google Scholar] [CrossRef]
- Peng, Y.; Bao, H.; Jiang, G.; Crockford, P.; Feng, D.; Xiao, S.; Kaufman, A.J.; Wang, J. A transient peak in marine sulfate after the 635-Ma snowball Earth. Proc. Natl. Acad. Sci. U. S. A. 2022, 119, 1–7. [Google Scholar] [CrossRef]
- Guo, Y.; Deng, W.; Wei, G. Experimental Constraints on Clumped Isotope Fractionation During BaCO3 Precipitation. Geochemistry, Geophys. Geosystems 2022, 23, 1–15. [Google Scholar] [CrossRef]
- Chen, L.; Jin, M.; Wang, X.; Wang, H.; Li, N. The effects of diagenetic processes and fluid migration on rare earth element and organic matter distribution in seep-related sediments: A case study from the South China Sea. J. Asian Earth Sci. 2020, 191, 104233. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Lyons, W.B.; Bird, D.A. Rare earth element concentrations and speciation in alkaline lakes from the western U.S.A. Geophys. Res. Lett. 1994, 21, 773–776. [Google Scholar] [CrossRef]
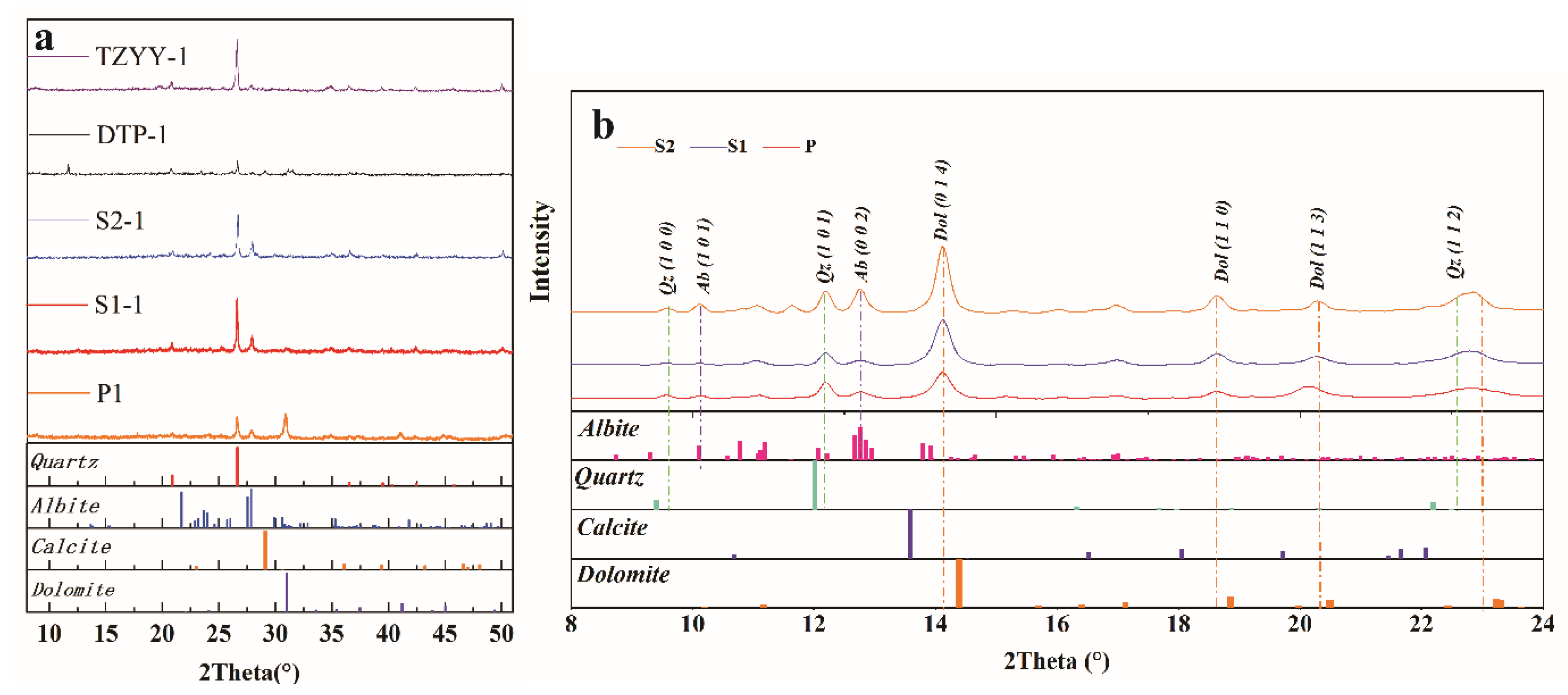
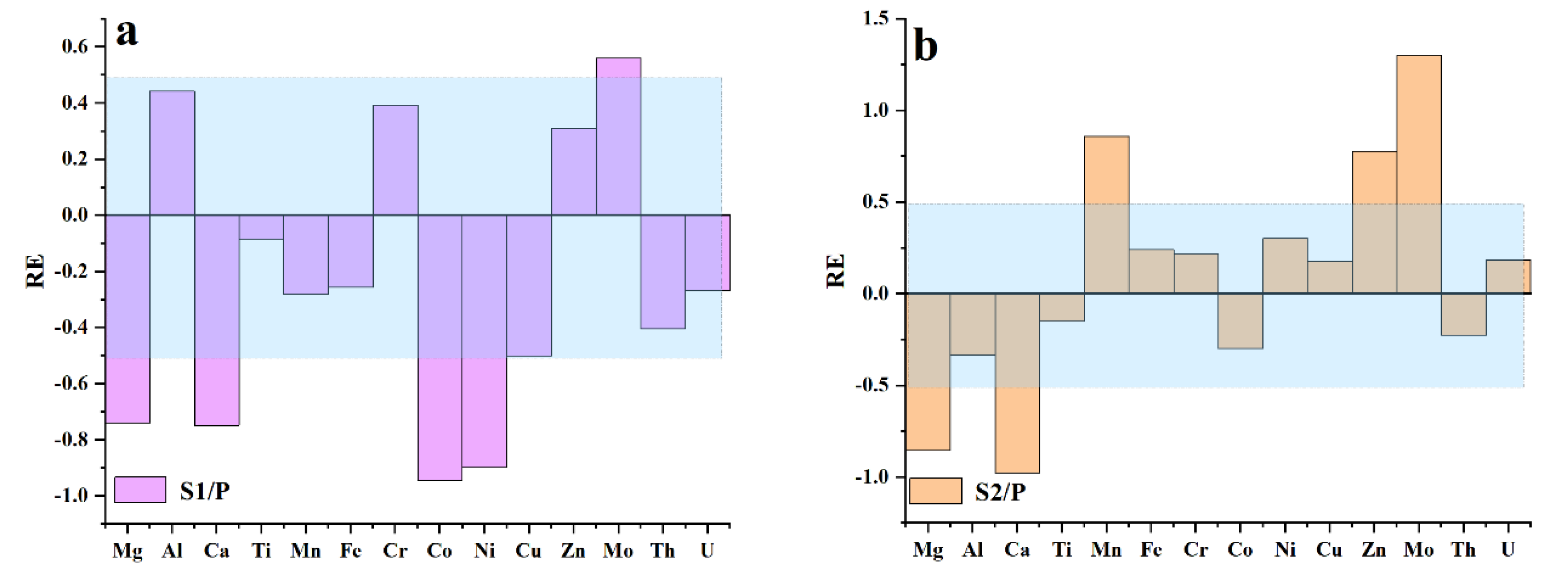
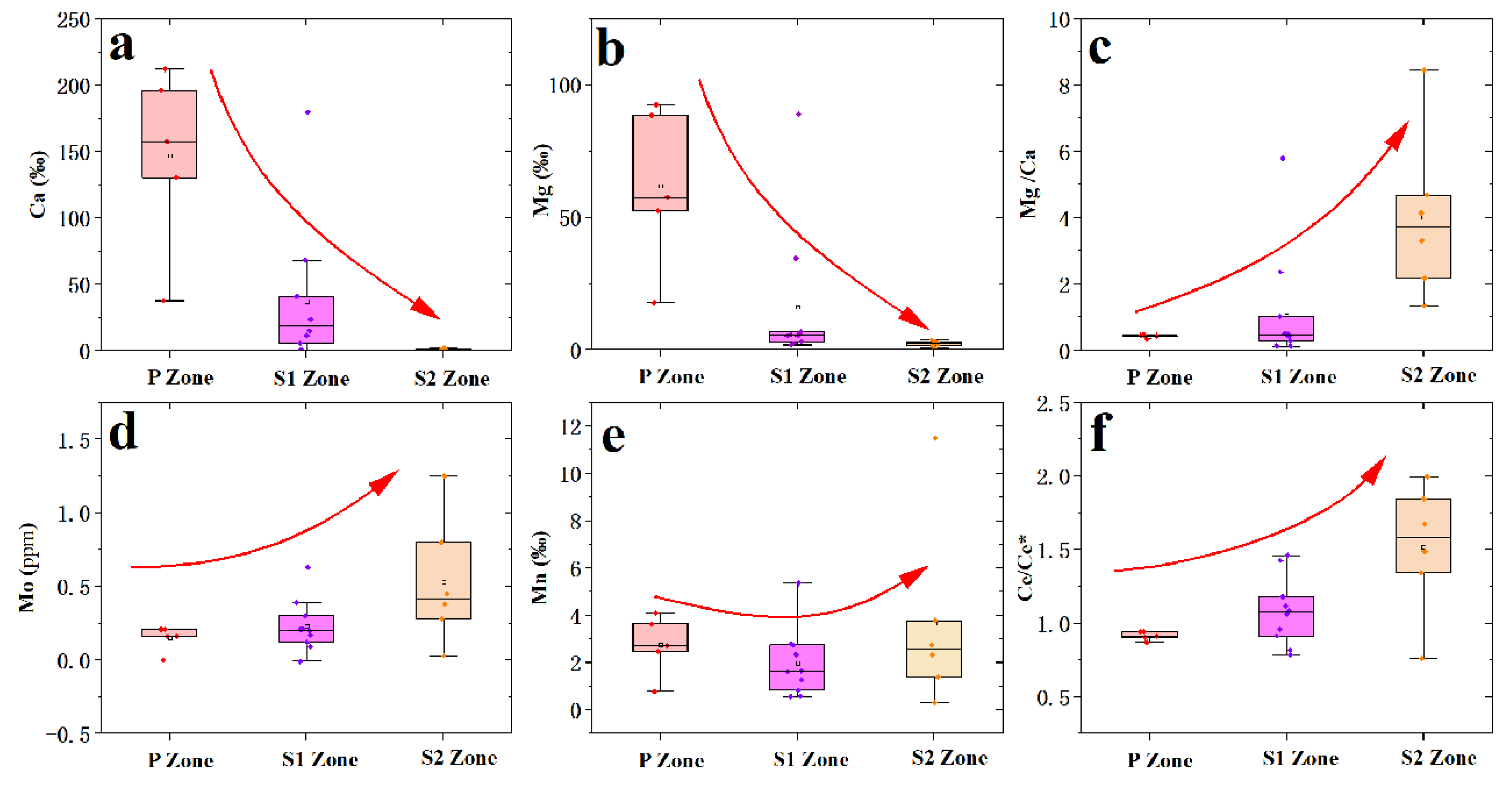
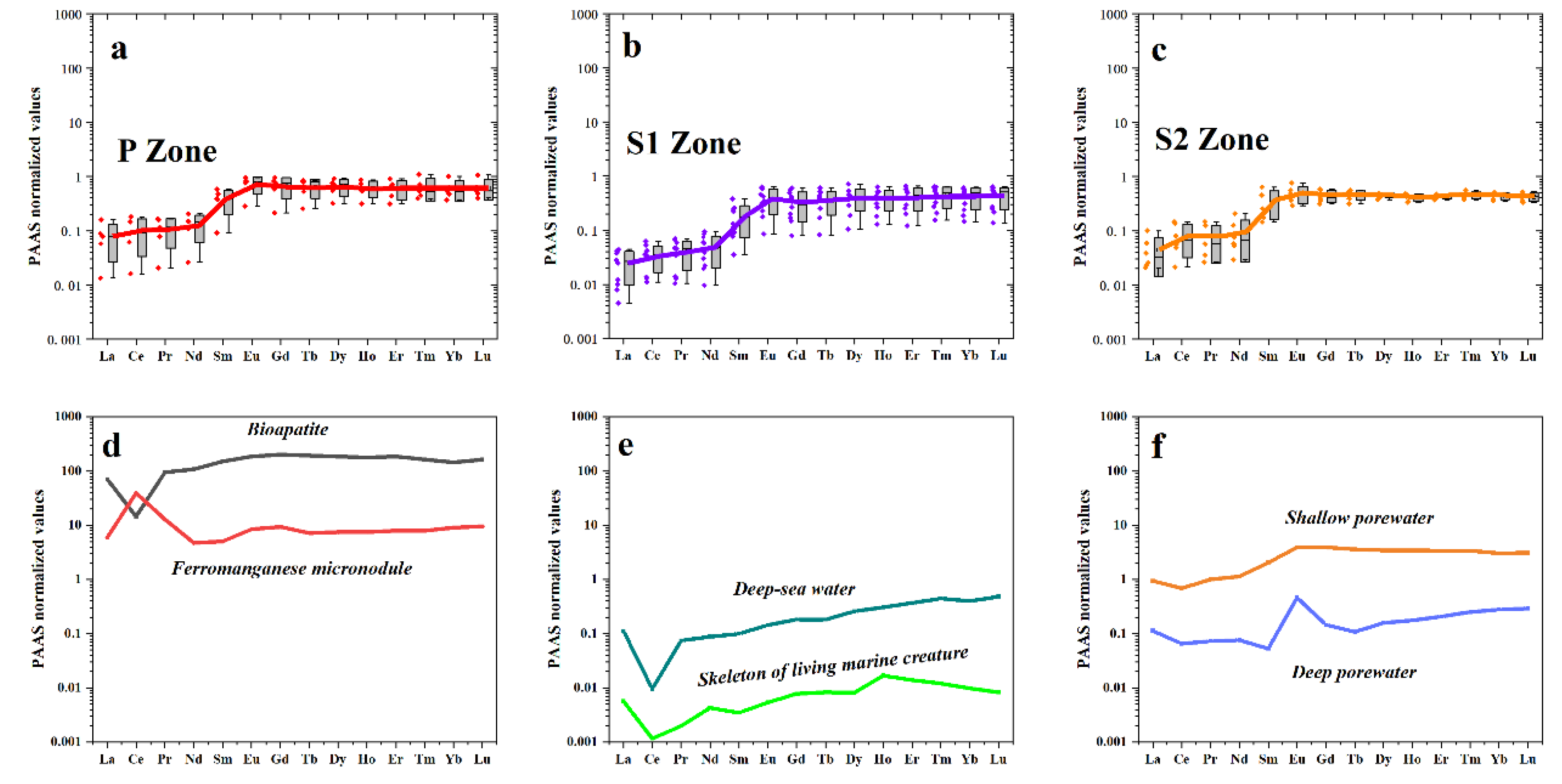
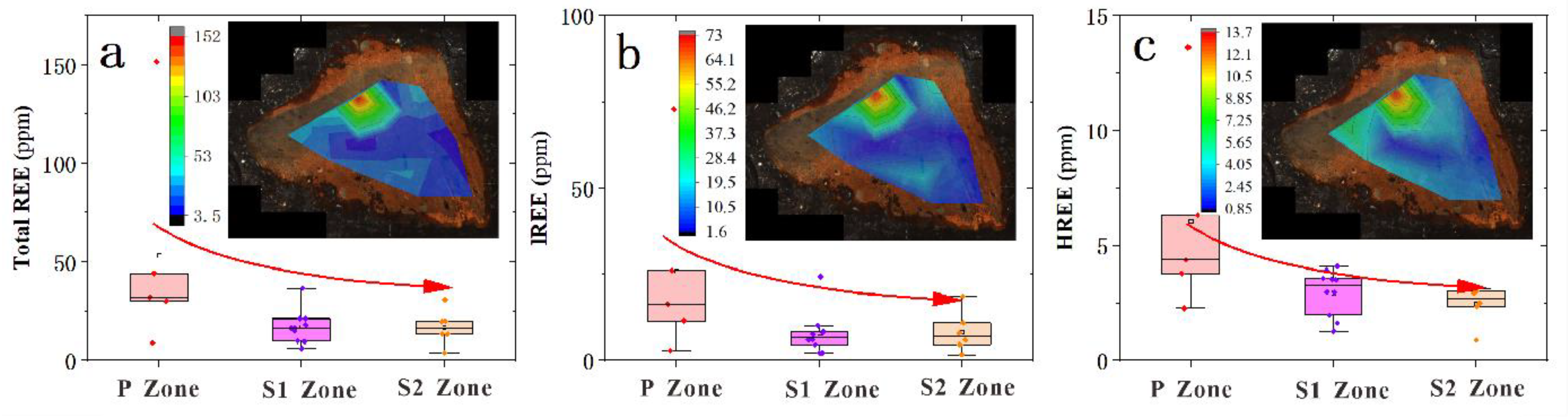
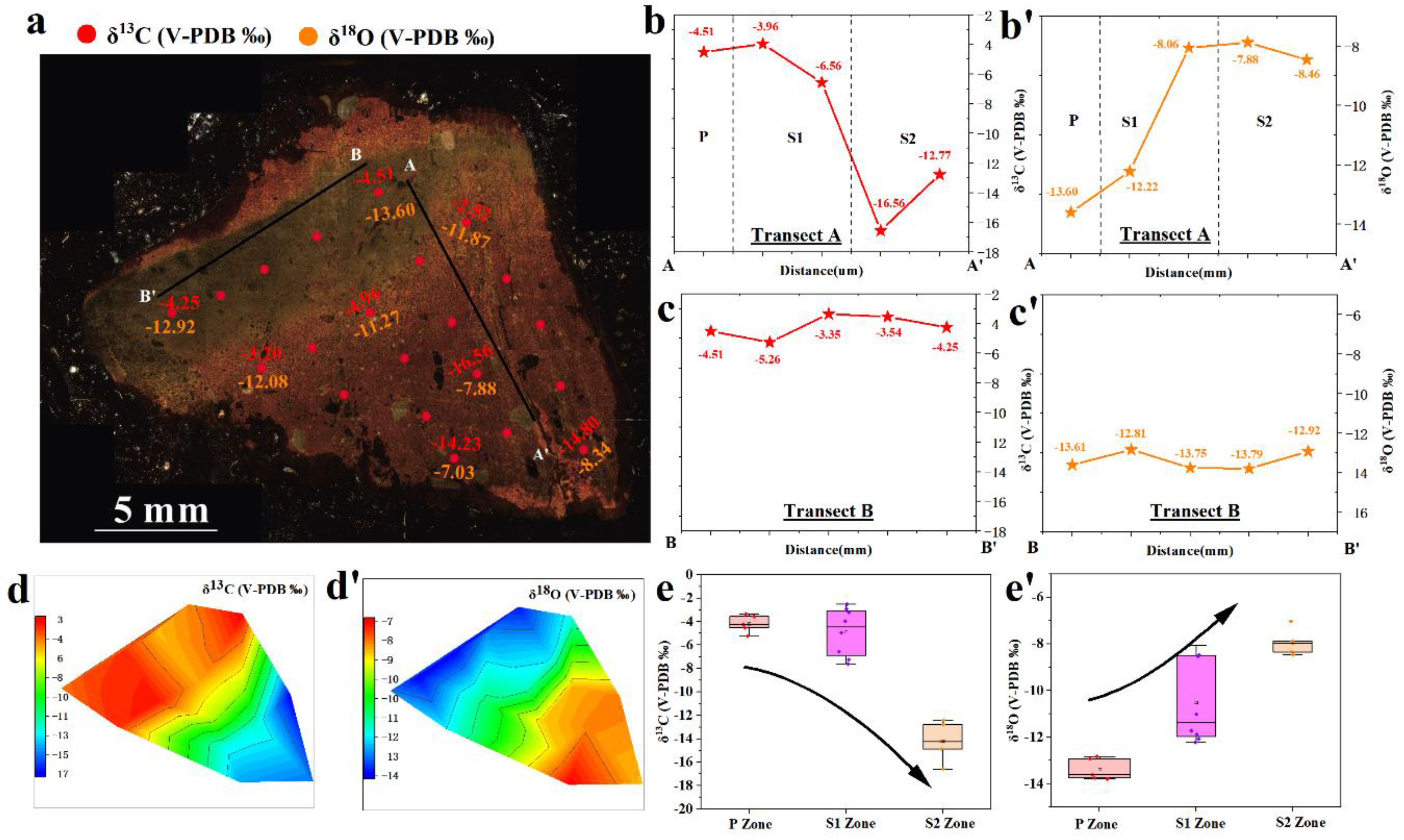
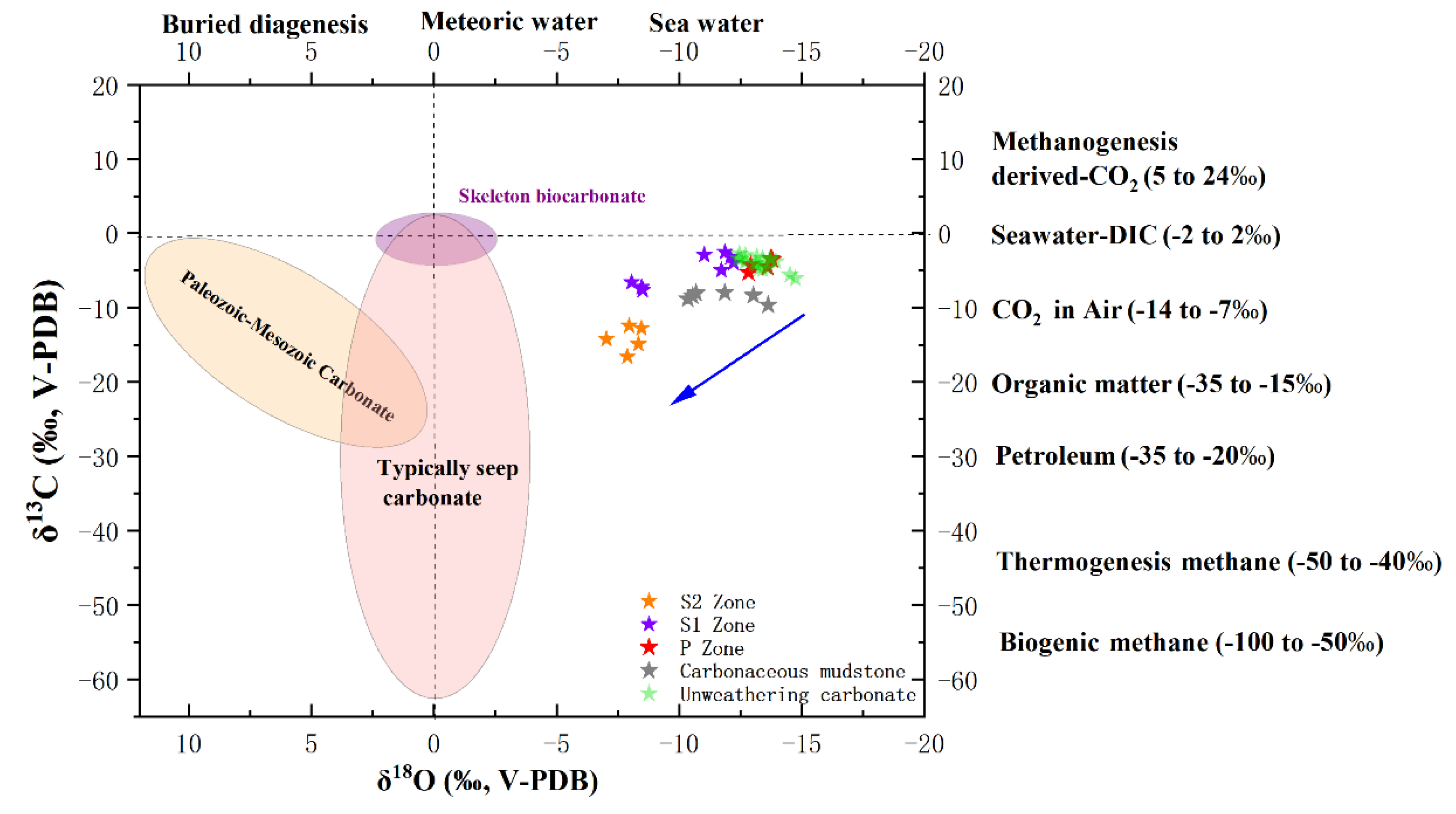
| Sample No.* | Mg | Al | Ca | Ti | Mn | Fe | Cr | Co | Ni | Cu | Zn | Mo | Th | U | Mn/Fe | Ni/Co | U/Th |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | ‰ | ‰ | ‰ | ‰ | ‰ | ‰ | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | |||
| P-1 | 17.77 | 45.76 | 37.79 | 1.66 | 0.78 | 8.51 | 24.78 | 1.61 | 2.56 | 1.25 | 34.94 | 0.00 | 2.05 | 0.51 | 0.09 | 1.59 | 0.25 |
| P-2 | 52.59 | 27.58 | 157.64 | 2.01 | 2.48 | 25.56 | 14.75 | 1.56 | 2.96 | 1.26 | 57.71 | 0.16 | 8.81 | 2.29 | 0.10 | 1.89 | 0.26 |
| P-3 | 92.45 | 9.44 | 212.06 | 0.91 | 4.1 | 33.40 | 9.27 | 2.38 | 3.22 | 1.18 | 77.48 | 0.21 | 2.44 | 0.44 | 0.12 | 1.35 | 0.18 |
| P-4 | 57.51 | 24.09 | 130.39 | 1.76 | 2.73 | 40.88 | 16.63 | 407.35 | 314.18 | 68.18 | 88.00 | 0.16 | 4.70 | 1.00 | 0.07 | 0.77 | 0.21 |
| P-5 | 88.57 | 13.25 | 196.03 | 0.40 | 3.65 | 33.10 | 10.21 | 1.70 | 3.21 | 1.63 | 102.24 | 0.21 | 1.80 | 0.25 | 0.11 | 1.89 | 0.14 |
| S1-1 | 89.06 | 9.9 | 179.80 | 0.51 | 5.36 | 54.65 | 23.52 | 21.48 | 10.75 | 7.76 | 249.17 | 0.63 | 2.12 | 0.96 | 0.10 | 0.50 | 0.45 |
| S1-2 | 34.48 | 16.14 | 68.05 | 0.85 | 2.35 | 27.91 | 14.98 | 2.37 | 5.01 | 13.01 | 101.97 | 0.30 | 2.47 | 0.75 | 0.08 | 2.12 | 0.31 |
| S1-3 | 5.78 | 41.96 | 5.67 | 1.94 | 0.56 | 12.60 | 16.61 | 1.02 | 3.77 | 1.61 | 31.13 | -0.01 | 4.07 | 0.90 | 0.04 | 3.68 | 0.22 |
| S1-4 | 5.44 | 108.74 | 11.59 | 2.73 | 0.84 | 9.10 | 61.11 | 1.18 | 3.70 | 1.71 | 57.14 | 0.12 | 1.14 | 0.29 | 0.09 | 3.13 | 0.25 |
| S1-5 | 6.74 | 41.5 | 23.80 | 1.12 | 1.66 | 18.69 | 15.92 | 2.39 | 6.45 | 2.65 | 75.03 | 0.09 | 2.69 | 0.53 | 0.09 | 2.70 | 0.20 |
| S1-6 | 1.89 | 12.54 | 0.80 | 0.56 | 2.79 | 22.80 | 10.57 | 2.97 | 7.36 | 5.19 | 62.84 | 0.21 | 2.08 | 0.66 | 0.12 | 2.48 | 0.32 |
| S1-7 | 5.32 | 20.14 | 41.11 | 1.47 | 1.61 | 23.09 | 13.13 | 2.72 | 8.64 | 15.31 | 150.29 | 0.39 | 2.85 | 0.70 | 0.07 | 3.17 | 0.25 |
| S1-8 | 6.65 | 46.18 | 15.06 | 1.94 | 0.58 | 11.73 | 33.33 | 2.72 | 6.40 | 2.46 | 79.07 | 0.20 | 3.29 | 0.95 | 0.05 | 2.35 | 0.29 |
| S1-9 | 3.04 | 17 | 23.39 | 0.31 | 1.26 | 12.52 | 4.88 | 1.12 | 3.65 | 2.81 | 44.89 | 0.17 | 1.04 | 0.19 | 0.10 | 3.26 | 0.18 |
| S2-1 | 2.32 | 32.36 | 0.40 | 0.93 | 2.75 | 17.04 | 16.74 | 7.41 | 10.11 | 20.91 | 93.10 | 0.21 | 1.87 | 0.64 | 0.16 | 1.36 | 0.34 |
| S2-2 | 0.76 | 11.53 | 0.09 | 0.59 | 0.3 | 3.33 | 5.42 | 0.44 | 0.88 | 0.85 | 21.90 | 0.03 | 0.95 | 0.33 | 0.09 | 2.01 | 0.35 |
| S2-3 | 2.96 | 33.06 | 1.36 | 1.61 | 3.78 | 26.55 | 22.90 | 5.17 | 11.79 | 11.98 | 199.42 | 0.38 | 1.87 | 0.86 | 0.14 | 2.28 | 0.46 |
| S2-4 | 2.91 | 8.96 | 2.17 | 0.48 | 11.51 | 51.73 | 52.54 | 5.70 | 15.82 | 16.49 | 256.20 | 1.25 | 1.28 | 0.41 | 0.22 | 2.78 | 0.32 |
| S2-5 | 2.25 | 31.26 | 0.48 | 1.25 | 1.39 | 19.47 | 31.65 | 3.08 | 8.71 | 7.50 | 172.93 | 0.45 | 1.70 | 0.73 | 0.07 | 2.83 | 0.43 |
| S2-6 | 3.69 | 32.6 | 0.89 | 1.35 | 2.76 | 37.79 | 31.98 | 3.65 | 10.70 | 10.55 | 254.11 | 0.80 | 2.84 | 1.72 | 0.07 | 2.93 | 0.61 |
| S2-7 | 1.49 | 20.79 | 0.45 | 1.04 | 2.32 | 17.69 | 9.52 | 1.09 | 3.53 | 4.49 | 103.79 | 0.28 | 2.33 | 0.62 | 0.13 | 3.24 | 0.26 |
| Sample No.* | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Ce/Ce* | Eu/Eu* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-1 | 0.51 | 1.29 | 0.18 | 0.89 | 0.51 | 0.30 | 0.99 | 0.20 | 1.49 | 0.31 | 0.89 | 0.16 | 1.05 | 0.17 | 0.95 | 1.85 |
| P-2 | 9.01 | 27.43 | 4.90 | 31.47 | 19.54 | 5.51 | 21.89 | 2.64 | 13.34 | 2.20 | 5.92 | 0.82 | 5.98 | 0.90 | 0.87 | 1.24 |
| P-3 | 6.08 | 11.64 | 1.46 | 6.97 | 3.22 | 1.03 | 4.44 | 0.65 | 3.59 | 0.72 | 2.03 | 0.29 | 1.78 | 0.28 | 0.90 | 1.24 |
| P-4 | 2.21 | 4.92 | 0.69 | 3.68 | 2.11 | 0.82 | 3.58 | 0.61 | 4.30 | 0.86 | 2.58 | 0.44 | 2.83 | 0.46 | 0.91 | 1.33 |
| P-5 | 3.04 | 7.31 | 1.01 | 4.99 | 2.63 | 0.87 | 3.81 | 0.62 | 3.39 | 0.63 | 1.86 | 0.22 | 1.47 | 0.24 | 0.94 | 1.25 |
| S1-1 | 3.40 | 14.41 | 1.40 | 5.07 | 2.17 | 0.89 | 2.73 | 0.41 | 2.56 | 0.53 | 1.24 | 0.18 | 1.34 | 0.21 | 1.46 | 1.69 |
| S1-2 | 1.59 | 4.98 | 0.62 | 2.88 | 1.37 | 0.65 | 2.28 | 0.40 | 2.52 | 0.50 | 1.53 | 0.25 | 1.51 | 0.24 | 1.12 | 1.64 |
| S1-3 | 1.54 | 2.84 | 0.30 | 1.32 | 0.73 | 0.37 | 1.25 | 0.27 | 2.09 | 0.51 | 1.64 | 0.26 | 1.75 | 0.28 | 0.96 | 1.73 |
| S1-4 | 0.30 | 0.88 | 0.11 | 0.76 | 0.51 | 0.34 | 0.69 | 0.13 | 0.88 | 0.18 | 0.57 | 0.08 | 0.53 | 0.09 | 1.06 | 2.62 |
| S1-5 | 1.69 | 3.34 | 0.51 | 2.54 | 1.45 | 0.62 | 2.58 | 0.47 | 3.35 | 0.63 | 1.89 | 0.26 | 1.73 | 0.22 | 0.82 | 1.42 |
| S1-6 | 1.08 | 4.23 | 0.41 | 1.96 | 1.27 | 0.46 | 1.57 | 0.30 | 2.07 | 0.42 | 1.17 | 0.19 | 1.40 | 0.22 | 1.43 | 1.49 |
| S1-7 | 0.95 | 2.60 | 0.41 | 2.06 | 1.23 | 0.50 | 1.91 | 0.39 | 2.50 | 0.51 | 1.67 | 0.23 | 1.43 | 0.24 | 0.91 | 1.46 |
| S1-8 | 0.38 | 1.04 | 0.12 | 0.67 | 0.43 | 0.19 | 0.93 | 0.20 | 1.75 | 0.41 | 1.41 | 0.21 | 1.63 | 0.26 | 1.09 | 1.28 |
| S1-9 | 1.44 | 3.30 | 0.60 | 3.17 | 2.11 | 0.68 | 2.81 | 0.33 | 1.76 | 0.31 | 0.73 | 0.11 | 0.68 | 0.11 | 0.79 | 1.28 |
| S2-1 | 0.46 | 2.70 | 0.40 | 1.01 | 0.63 | 0.25 | 0.79 | 0.20 | 1.35 | 0.26 | 0.84 | 0.13 | 0.88 | 0.12 | 1.18 | 1.60 |
| S2-2 | 0.17 | 1.09 | 0.09 | 0.33 | 0.20 | 0.09 | 0.37 | 0.06 | 0.49 | 0.13 | 0.34 | 0.06 | 0.41 | 0.06 | 1.84 | 1.49 |
| S2-3 | 1.49 | 6.38 | 0.50 | 2.60 | 1.42 | 0.46 | 2.06 | 0.38 | 2.07 | 0.34 | 1.06 | 0.15 | 0.99 | 0.14 | 1.68 | 1.23 |
| S2-4 | 2.25 | 10.90 | 1.11 | 4.29 | 2.52 | 0.82 | 2.62 | 0.43 | 2.30 | 0.47 | 1.34 | 0.18 | 1.33 | 0.18 | 1.49 | 1.50 |
| S2-5 | 0.88 | 3.91 | 0.23 | 0.99 | 0.80 | 0.31 | 1.44 | 0.24 | 1.75 | 0.34 | 1.11 | 0.16 | 1.07 | 0.15 | 1.99 | 1.28 |
| S2-6 | 0.98 | 4.47 | 0.51 | 1.96 | 3.56 | 0.60 | 1.84 | 0.30 | 1.94 | 0.42 | 1.23 | 0.23 | 1.43 | 0.23 | 1.34 | 1.08 |
| S2-7 | 0.80 | 1.70 | 0.31 | 1.78 | 0.97 | 0.39 | 1.68 | 0.33 | 2.13 | 0.45 | 1.26 | 0.19 | 1.27 | 0.20 | 0.76 | 1.36 |
| Sample No. | δ13CV-PDB | δ18OV-PDB | Sample No* | δ13CV-PDB | δ18OV-PDB | |
|---|---|---|---|---|---|---|
| TJT1-1 | -6.07 | -14.73 | P-1 | -4.51 | -13.60 | |
| TJT1-2 | -5.58 | -14.52 | P-2 | -5.26 | -12.83 | |
| TJT1-3 | -4.83 | -13.40 | P-3 | -3.35 | -13.75 | |
| TJT1-4 | -4.68 | -13.61 | P-4 | -3.54 | -13.79 | |
| TJT1-5 | -3.45 | -13.68 | P-5 | -4.25 | -12.92 | |
| TJT1-6 | -3.78 | -13.94 | S1-1 | -2.52 | -11.87 | |
| TJT1-7 | -3.12 | -13.17 | S1-2 | -3.96 | -12.22 | |
| TJT1-8 | -3.47 | -12.51 | S1-3 | -4.96 | -11.72 | |
| TJT1-9 | -4.23 | -13.16 | S1-4 | -2.90 | -11.02 | |
| TJT1-10 | -4.73 | -13.27 | S1-5 | -3.20 | -12.08 | |
| TJT1-11 | -3.48 | -13.75 | S1-6 | - | - | |
| TJT1-12 | -3.36 | -13.40 | S1-7 | -6.56 | -8.06 | |
| TJT1-13 | -2.95 | -12.69 | S1-8 | -7.61 | -8.52 | |
| TJT1-14 | -2.76 | -12.45 | S1-9 | -7.23 | -8.47 | |
| TJT1-15 | -4.03 | -13.09 | S2-1 | - | - | |
| TJT1-16 | -3.83 | -12.69 | S2-2 | -16.56 | -7.88 | |
| TJT1-17 | -3.40 | -12.40 | S2-3 | -12.43 | -7.96 | |
| Average | -3.99 | -13.32 | S2-4 | -12.77 | -8.46 | |
| TZYY-1 | -8.03 | -10.68 | S2-5 | - | - | |
| TZYY-2 | -7.99 | -11.86 | S2-6 | -14.23 | -7.03 | |
| TZYY-3 | -8.44 | -10.55 | S2-7 | -14.8 | -8.34 | |
| TZYY-4 | -8.34 | -13.02 | Average | -7.26 | -10.58 | |
| TZYY-5 | -8.82 | -10.35 | ||||
| TZYY-6 | -9.65 | -13.63 | ||||
| Average | -8.55 | -11.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





