Submitted:
03 April 2025
Posted:
04 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
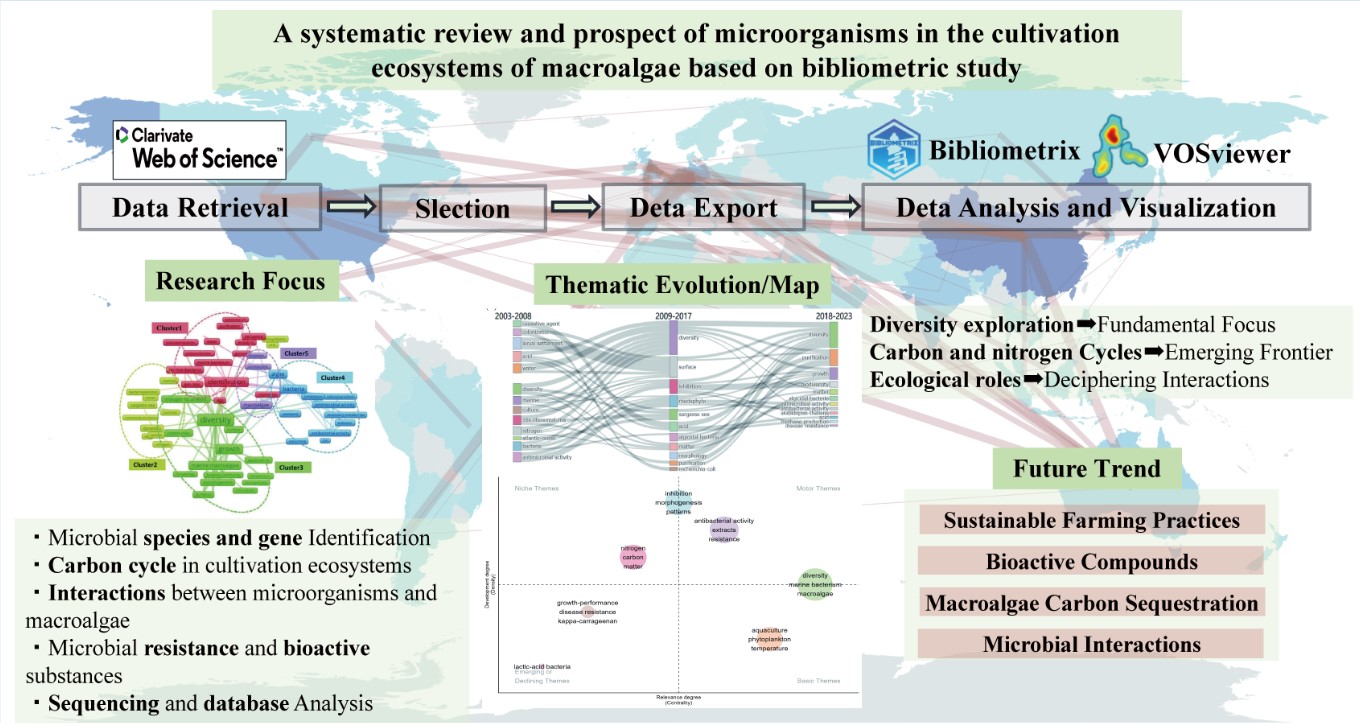
2. Methodology
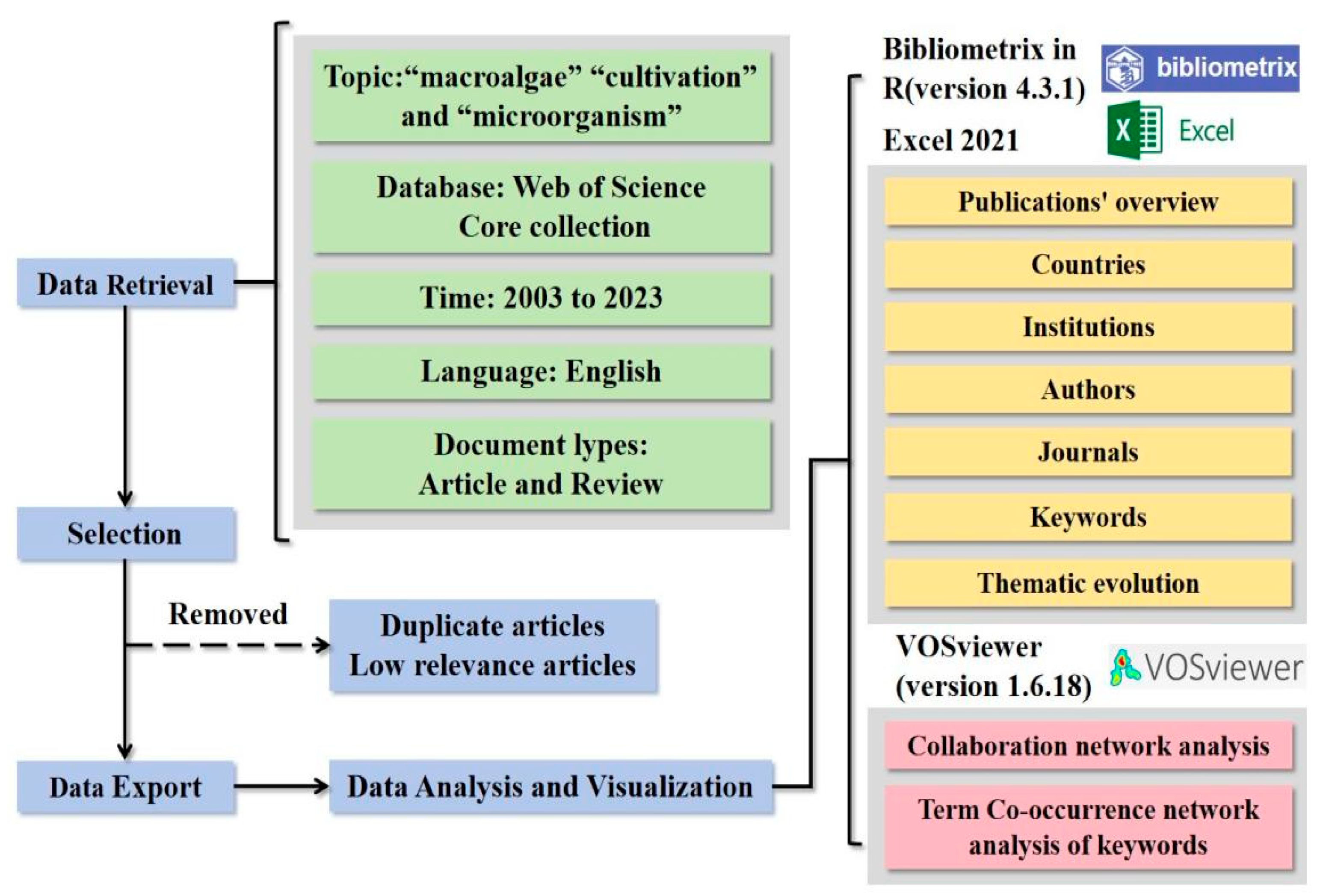
2.1. Study Framework and Data Sources
2.2. Search Strategy
2.3. Data Analysis and Visualization
3. Results and Discussions
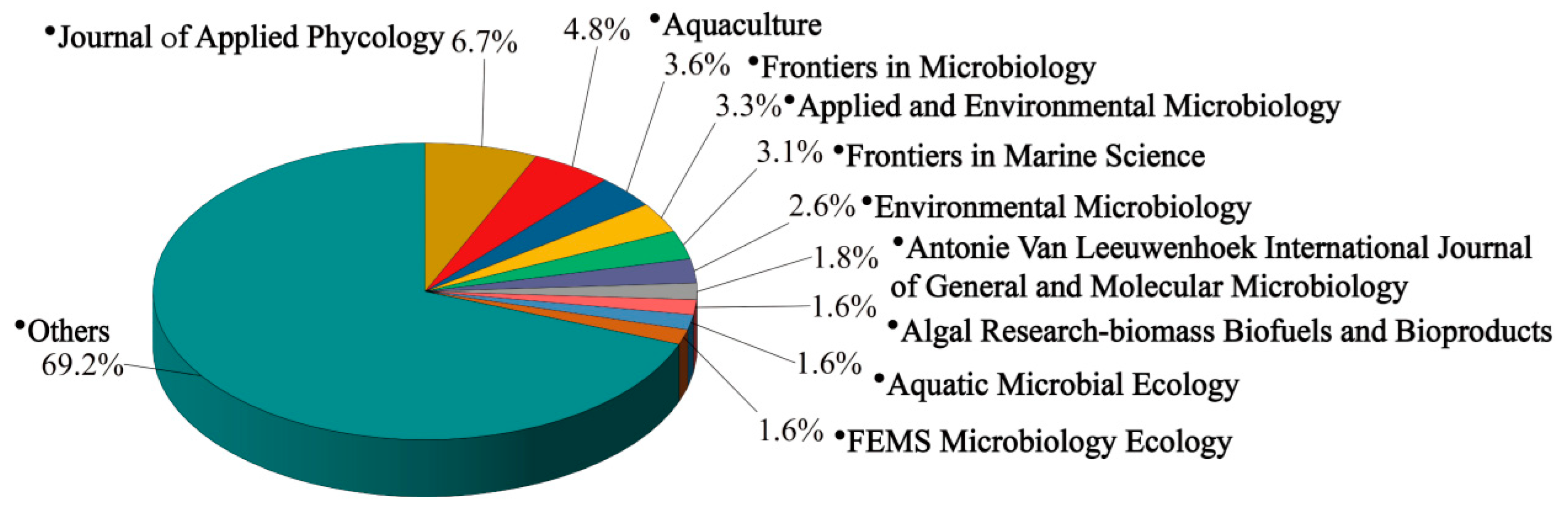
3.1. Quantitative Analysis of the Publications
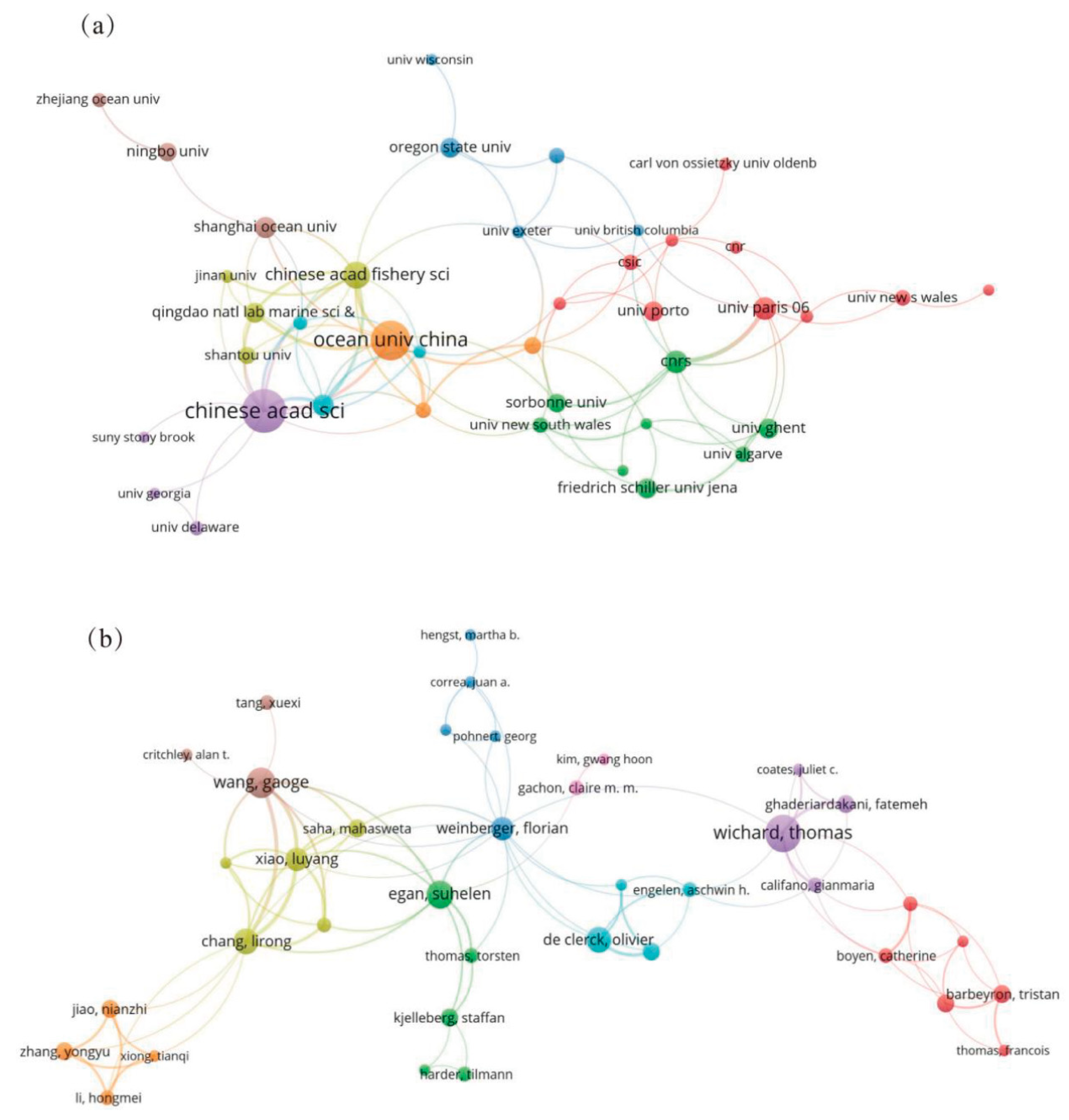
3.2. Collaboration Network Analysis
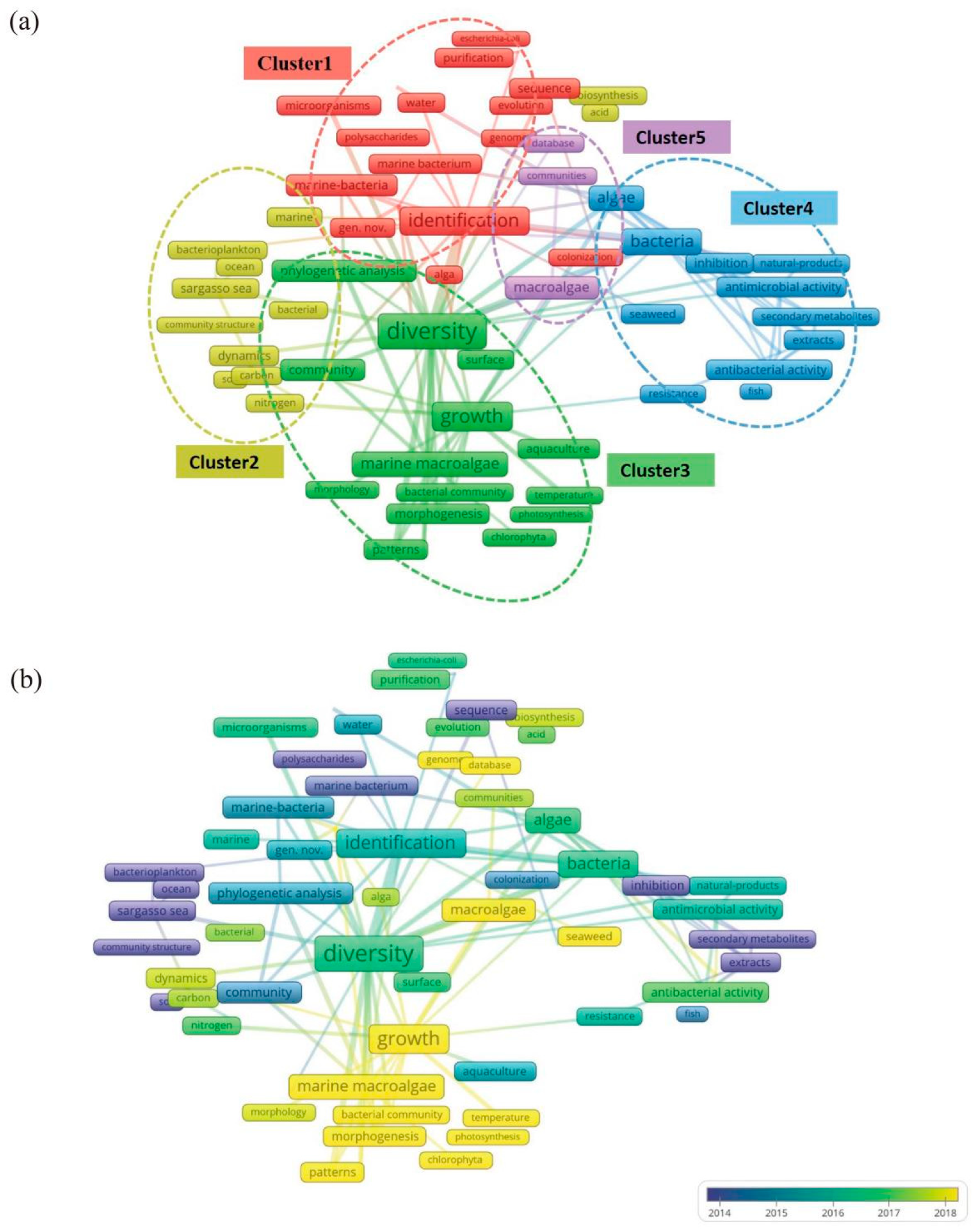
3.3. Term Co-Occurrence Network Analysis of Keywords
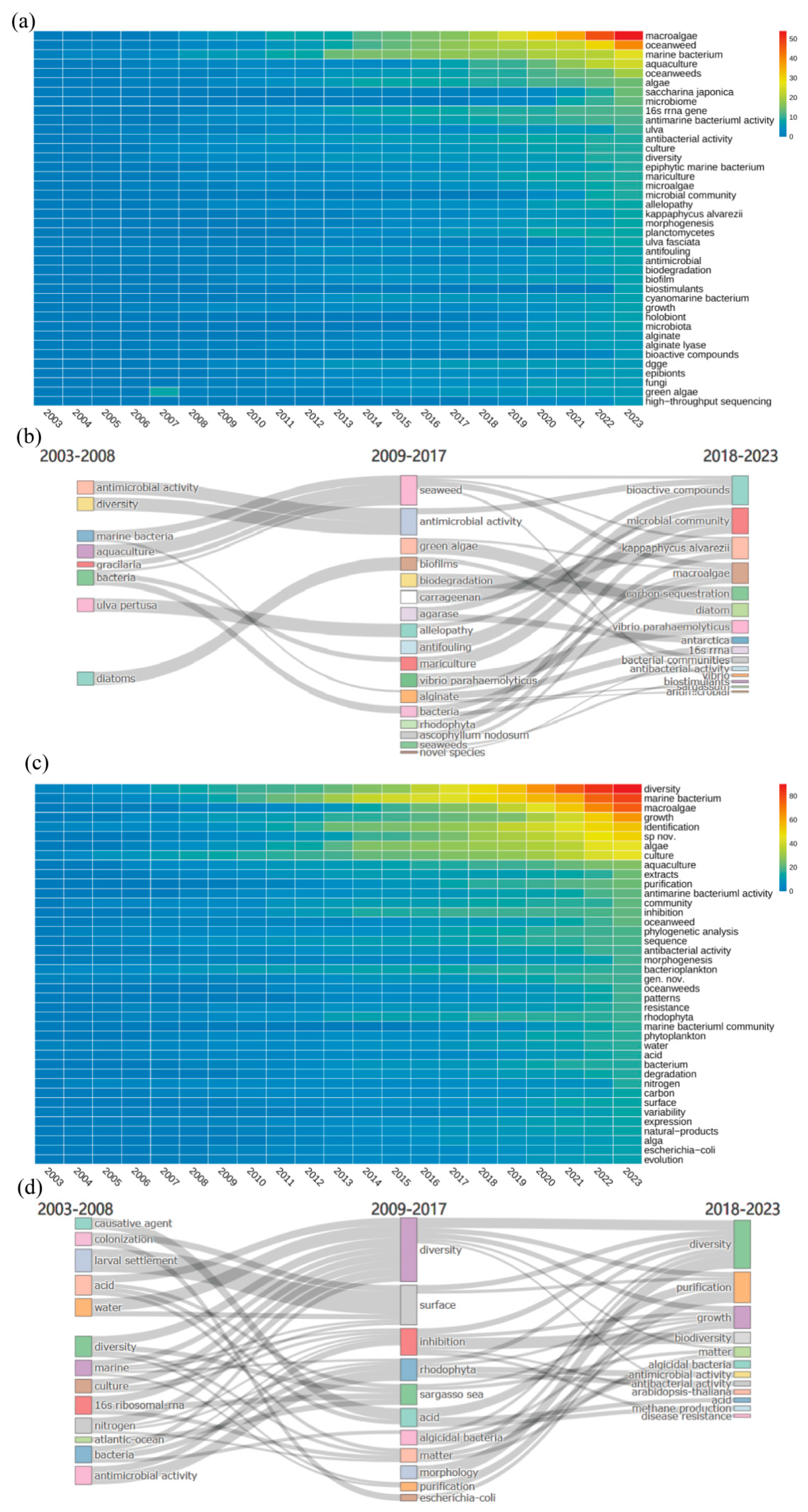
3.4. Thematic Evolution
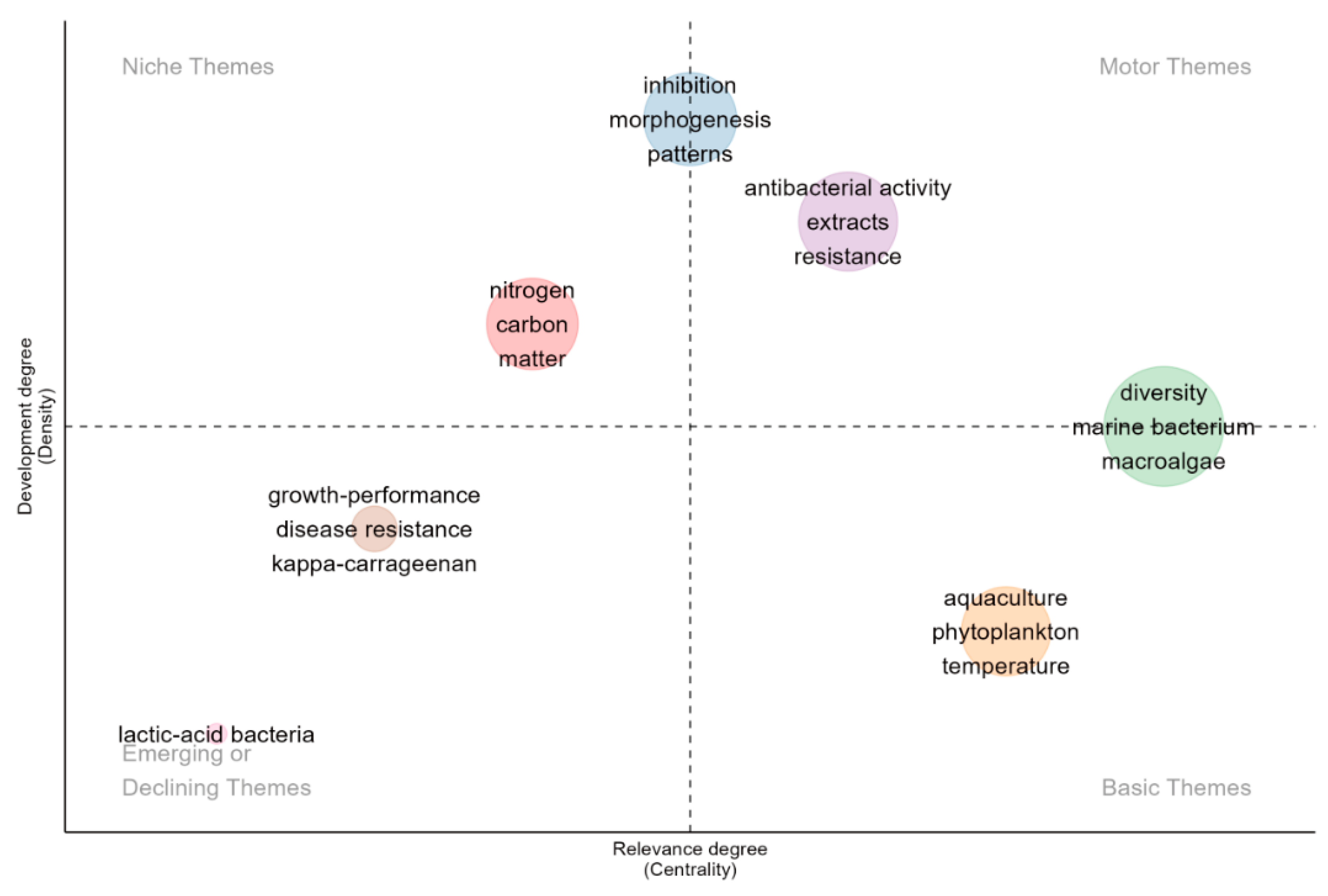
3.5. Thematic Map
3.6. Prospects of the Environmental Microbiology of Macroalgae Aquaculture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelrazek H M, Shams El-Din N G, Ghozlan H A, Sabry S A and Abouelkheir S S 2024 Distribution and functional perspective analysis of epiphytic and endophytic bacterial communities associated with marine seaweeds, Alexandria shores, Egypt. BMC Microbiology, 24:293. [CrossRef]
- Albakosh M A, Naidoo R K, Kirby B and Bauer R 2016 Identification of epiphytic bacterial communities associated with the brown alga Splachnidium rugosum. Journal of Applied Phycology, 28(3), 1891–1901. [CrossRef]
- Ashen J B and Goff L J 2000 Molecular and Ecological Evidence for Species Specificity and Coevolution in a Group of Marine Algal-Bacterial Symbioses. Applied and Environmental Microbiology, 66(7), 3024–3030. https://journals.asm.org/journal/aem.
- Baltz R H 2017 Gifted microbes for genome mining and natural product discovery. Journal of Industrial Microbiology and Biotechnology, 44(4–5), 573–588. [CrossRef]
- Baltz R H 2021 Genome mining for drug discovery: Progress at the front end. In Journal of Industrial Microbiology and Biotechnology, Oxford University Press, 48, 9–10. [CrossRef]
- Bogolitsyn K, Dobrodeeva L, Samodova A, Parshina A 2024 In vitro Immunostimulant Activity of the Polyphenolic Extract from the Arctic Brown Algae Fucus vesiculosus. Plant Foods for Human Nutrition, 79(2), 511-517. [CrossRef]
- Brown A R, Lilley M, Shutler J, Lowe C, Artioli Y, Torres R, Berdalet E and Tyler C R 2020 Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Reviews in Aquaculture, 12(3), 1663-1688. [CrossRef]
- Cai J, Lovatelli A, Aguilar-Manjarrez J, Cornish L, Dabbadie L, Desrochers A, Diffey S, Garrido Gamarro E, Geehan J, Hurtado A, Lucente D, Mair G, Miao W, Potin P, Przybyla C, Reantaso M, Roubach R, Tauati M and Yuan X 2021 Seaweeds and microalgae: An overview for unlocking their potential in global aquaculture development. FAO Fisheries and Aquaculture Circular No. 1229. Rome: FAO. [CrossRef]
- Chai Z, Hu Z, Deng Y, Yang Y and Tang Y Z 2021 Interactions between the seaweed Gracilaria and dinoflagellate Akashiwo sanguinea in an indoor co-cultivation system and the interference of bacteria. Journal of Applied Phycology, 33(5), 3153–3163. [CrossRef]
- Chai Z Y, He Z L, Deng Y Y, Yang Y F and Tang Y Z 2018 Cultivation of seaweed Gracilaria lemaneiformis enhanced biodiversity in a eukaryotic plankton community as revealed via metagenomic analyses. Molecular Ecology, 27(4), 1081–1093. [CrossRef]
- Carrell A A, Veličković D, Lawrence T J, Bowen B P, Louie K B, Carper D L, Chu R K, Mitchell H D, Orr G, Markillie L M, Jawdy S S, Grimwood J, Shaw A J, Schmutz J, Northen T R, Anderton C R, Pelletier D A, Weston D J. Novel metabolic interactions and environmental conditions mediate the boreal peatmoss-cyanobacteria mutualism. ISME, 2022, 16(4): 1074–1085. [CrossRef]
- Chen J, Li H, Zhang Z, He C, Shi Q, Jiao N and Zhang Y 2020 DOC dynamics and bacterial community succession during long-term degradation of Ulva prolifera and their implications for the legacy effect of green tides on refractory DOC pool in seawater. Water Research, 185, 116268. [CrossRef]
- Chukwudulue U M, Barger N, Dubovis M and Luzzatto Knaan T 2023 Natural products and pharmacological properties of symbiotic Bacillota (Firmicutes) of marine macroalgae. Marine Drugs, 21(11), 569. [CrossRef]
- Croft M T, Lawrence A D, Raux-Deery E, Warren M J and Smith A G 2005 Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature, 438(7064), 90–93. [CrossRef]
- Ding Q, Song X, Yuan M, Sun R, Zhang J, Yin L and Pu Y 2022 Removal of microcystins from water and primary treatment technologies-A comprehensive understanding based on bibliometric and content analysis, 1991–2020. Journal of Environmental Management, 305, 114349. [CrossRef]
- Dittami S M, Duboscq-Bidot L, Perennou M, Gobet A, Corre E, Boyen C and Tonon T 2016 Host-microbe interactions as a driver of acclimation to salinity gradients in brown algal cultures. ISME Journal, 10(1), 51–63. [CrossRef]
- Dubois S, Marin-Léal J C, Ropert M and Lefebvre S 2007 Effects of oyster farming on macrofaunal assemblages associated with Lanice conchilega tubeworm populations: A trophic analysis using natural stable isotopes. Aquaculture, 271(1–4), 336–349. [CrossRef]
- FAO 2024 The State of World Fisheries and Aquaculture 2024 – Blue Transformation in action. Rome. [CrossRef]
- Gallagher J B, Shelamoff V and Layton C 2022 Seaweed ecosystems may not mitigate CO2 emissions. ICES Journal of Marine Science, 79(3), 585–592. [CrossRef]
- Gao G, Beardall J, Jin P, Gao L, Xie S and Gao K 2022 A review of existing and potential blue carbon contributions to climate change mitigation in the Anthropocene. Journal of Applied Ecology, 59(7), 1686–1699. [CrossRef]
- Gao G, Gao L, Jiang M, Jian A and He L 2022 The potential of seaweed cultivation to achieve carbon neutrality and mitigate deoxygenation and eutrophication. Environmental Research Letters, 17(1). [CrossRef]
- Garland G, Edlinger A, Banerjee S, Degrune F, García-Palacios P, Pescador D S, Herzog C, Romdhane S, Saghai A, Spor A, Wagg C, Hallin S, Maestre F T, Philippot L, Rillig M C and van der Heijden M G A 2021 Crop cover is more important than rotational diversity for soil multifunctionality and cereal yields in European cropping systems. Nature Food, 2(1), 28–37. [CrossRef]
- Goecke F, Labes A, Wiese J and Imhoff J F 2013 Phylogenetic analysis and antibiotic activity of bacteria isolated from the surface of two co-occurring macroalgae from the Baltic Sea. European Journal of Phycology, 48(1), 47–60. [CrossRef]
- Huang H, Zhan S, Shao K, Chen H and Fan J 2024 Spatial distribution characteristics and interaction effects of DOM and microbial communities in kelp cultivation areas. Science of the Total Environment, 10(920), 170511. [CrossRef]
- Jarone P, Laura G, Laura A, Maria M S 2006 Seasonal changes in bacterioplankton nutrient limitation and their effects on bacterial community composition in the NW Mediterranean Sea. Aquatic Microbal Ecology, 44, 241–252. [CrossRef]
- Jiao J Y, Liu L, Hua Z S, Fang B Z, Zhou E M, Salam N, Hedlund B P and Li W J 2021 Microbial dark matter coming to light: Challenges and opportunities. National Science Review, 8(3), 280. [CrossRef]
- Jiao N, Herndl G J, Hansell D A, Benner R, Kattner G, Wilhelm S W, Kirchman D L, Weinbauer M G, Luo T, Chen F and Azam F 2010 Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nature Reviews Microbiology, 8(8), 593–599. [CrossRef]
- Joint I, Tait K and Wheeler G 2007 Cross-kingdom signalling: Exploitation of bacterial quorum sensing molecules by the green seaweed Ulva. In Philosophical Transactions of the Royal Society B: Biological Sciences, 362(1483), 1223–1233. [CrossRef]
- Jonnadula R, Verma P, Shouche Y S and Ghadi S C 2009 Characterization of microbulbifer strain CMC-5, a new biochemical variant of microbulbifer elongatus type strain DSM6810T isolated from decomposing seaweeds. Current Microbiology, 59(6), 600–607. [CrossRef]
- Kaur M, Saini K C, Mallick A and Bast F 2023 Seaweed-associated epiphytic bacteria: Diversity, ecological and economic implications. Aquatic Botany, 189, 103698. [CrossRef]
- Kim G H, Klochkova T A, Lee D J and Im S H 2016 Chloroplast virus causes green-spot disease in cultivated Pyropia of Korea. Algal Research, 17, 293–299. [CrossRef]
- KleinJan H, Frioux C, Califano G, Aite M, Fremy E, Karimi E, Corre E, Wichard T, Siegel A, Boyen C and Dittami S M 2023 Insights into the potential for mutualistic and harmful host–microbe interactions affecting brown alga freshwater acclimation. Molecular Ecology, 32(3), 703–723. [CrossRef]
- Krause-Jensen D and Duarte C M 2016 Substantial role of macroalgae in marine carbon sequestration. Nature Geoscience, 9(10), 737–742. [CrossRef]
- Kwan V, Fong J, Ng C S L, and Huang D 2022 Temporal and spatial dynamics of tropical macroalgal contributions to blue carbon. Science of the Total Environment, 828, 154369. [CrossRef]
- Lamilla-Tamayo L, Escobar-Calderón F and Skalický M 2023 Reviewing the potential of algae species as a green alternative to produce nanoparticles: Findings from a database analysis. Water, 15(12), 2208. [CrossRef]
- Lead J C, Gamarro E G, Geehan J A, Lucente D, Mair G C, Miao W, Reantaso M B, Roubach R, Yuan X and Potin P 2021 Seaweeds and microalgae: an overview for unlocking their potential in global aquaculture development. https://openknowledge.fao.org/handle/20.500.14283/cb5670en.
- Lee S J, Hwang M S, Park M A, Baek J M, Ha D S, Lee J E and Lee S R 2015 Molecular identification of the algal pathogen Pythium chondricola (Oomycetes) from Pyropia yezoensis (Rhodophyta) using ITS and cox1 markers. Algae, 30(3), 217–222. [CrossRef]
- Li J, Weinberger F, de Nys R, Thomas T, Egan S 2023 A pathway to improve seaweed aquaculture through microbiota manipulation. Trends in Biotechnology, 41(4), 545–556. [CrossRef]
- Li J, Majzoub ME, Marzinelli EM, Dai Z, Thomas T, Egan S 2022 Bacterial controlled mitigation of dysbiosis in a seaweed disease. ISME., 16(2), 378-387. [CrossRef]
- Li T, Huang J, Du H, Liu X, Zhong C and Lin S 2022 Coral bleaching from a nutrient perspective is understudied: A bibliometric survey. Frontiers in Marine Science, 9. [CrossRef]
- Lu D C, Wang F Q, Amann R I, Teeling H and Du Z J 2023 Epiphytic common core bacteria in the microbiomes of co-located green (Ulva), brown (Saccharina) and red (Grateloupia, Gelidium) macroalgae. Microbiome, 11(1), 126. [CrossRef]
- Lu K, Lin W and Liu J 2008 The characteristics of nutrient removal and inhibitory effect of Ulva clathrata on Vibrio anguillarum 65. Journal of Applied Phycology, 20(6), 1061–1068. [CrossRef]
- Marrone M and Linnenluecke M K 2020 Interdisciplinary Research Maps: A new technique for visualizing research topics. PLoS ONE, 15(11), e0242283. [CrossRef]
- Marshall K, Joint I, Callow M E and Callow J A 2006 Effect of marine bacterial isolates on the growth and morphology of axenic plantlets of the green alga Ulva linza. Microbial Ecology, 52(2), 302–310. [CrossRef]
- Matsuo Y, Suzuki M, Kasai H, Shizuri Y and Harayama S 2003 Isolation and phylogenetic characterization of bacteria capable of inducing differentiation in the green alga Monostroma oxyspermum. Environmental Microbiology, 5(1), 25-35. [CrossRef]
- Middelburg J 2011 Chemoautotrophy in the ocean. Geophysical Research Letters, 2011, 38(24): L24604.
- Mongeon P and Paul-Hus A 2016 The journal coverage of Web of Science and Scopus: a comparative analysis. Scientometrics, 106(1), 213–228. [CrossRef]
- Nair S, Zhang Z, Li H, Zhao H, Shen H, Kao S J, Jiao N and Zhang Y 2022 Inherent tendency of Synechococcus and heterotrophic bacteria for mutualism on long-term coexistence despite environmental interference. Science Advance, 8(39), eabf4792.
- Nalini MS, Prakash HS 2017 Diversity and bioprospecting of actinomycete endophytes from the medicinal plants. Letters in Applied Microbiology, 64(4), 261-270. [CrossRef]
- Ortega A, Geraldi N R, Alam I, Kamau A A, Acinas S G, Logares R, Gasol J M, Massana R, Krause-Jensen D and Duarte C M 2019 Important contribution of macroalgae to oceanic carbon sequestration. Nature Geoscience, 12(9), 748–754. [CrossRef]
- Pagarete A, Ramos A S, Puntervoll P, Allen M J and Verdelho V 2021 Antiviral potential of algal metabolites-a comprehensive review. Marine Drugs, 19(2), 94. [CrossRef]
- Paul J and Criado A R 2020 The art of writing literature review: What do we know and what do we need to know? International Business Review, 29(4), 101717. [CrossRef]
- Pedersen M F, Filbee-Dexter K, Norderhaug K M, Fredriksen S, Frisk N L, Fagerli C W and Wernberg T 2020 Detrital carbon production and export in high latitude kelp forests. Oecologia, 192(1), 227–239. [CrossRef]
- Pei P, Aslam M, Du H, Liang H, Wang H, Liu X and Chen W 2021 Environmental factors shape the epiphytic bacterial communities of Gracilariopsis lemaneiformis. Scientific Reports, 11(1), 13253. [CrossRef]
- Pei P, Aslam M, Wang H, Ye P, Li T, Liang H, Lin Q, Chen W and Du H 2024 Diversity and ecological function of urease-producing bacteria in the cultivation environment of Gracilariopsis lemaneiformis. Microbial Ecology, 87(1), 35. [CrossRef]
- Penesyan A, Kjelleberg S and Egan S 2010 Development of novel drugs from marine surface associated microorganisms. Marine Drugs. 8(3), 438–459. [CrossRef]
- Penesyan A, Marshall-Jones Z, Holmstrom C, Kjelleberg S and Egan S 2009 Antimicrobial activity observed among cultured marine epiphytic bacteria reflects their potential as a source of new drugs: Research article. FEMS Microbiology Ecology, 69(1), 113–124. [CrossRef]
- Penesyan A, Tebben J, Lee M, Thomas T, Kjelleberg S, Harder T and Egan S 2011 Identification of the antibacterial compound produced by the marine epiphytic bacterium Pseudovibrio sp. D323 and related sponge-associated bacteria. Marine Drugs, 9(8), 1391-1402. [CrossRef]
- Peng Z, Wang P, Luo X, Deng Q, Yang Z, Wu J, Xian W, Yan W, Mou X, Yuan Y, Li W and Li J 2024 Community structure and carbon metabolism functions of bacterioplankton in the Guangdong coastal zone. Marine Life Science and Technology, 6, 547-561. [CrossRef]
- Pfister C A, Altabet M A and Weigel B L 2019 Kelp beds and their local effects on seawater chemistry, productivity, and microbial communities. Ecology, 100(8), e02798. [CrossRef]
- Pfister C A and Altabet M A 2019 Enhanced microbial nitrogen transformations in association with macrobiota from the rocky intertidal. Biogeosciences, 16, 193-206. [CrossRef]
- Qian P Y, Cheng A, Wang R and Zhang R 2022 Marine biofilms: diversity, interactions and biofouling. Nature Reviews Microbiology. 20(11), 671–684. [CrossRef]
- Racine P, Marley A C, Froehlich H E, Gaines S D, Ladner I, MacAdam–Somer I, and Bradley D 2021 A case for seaweed aquaculture inclusion in U.S. nutrient pollution management. Marine Policy, 129, 104506. [CrossRef]
- Reisky L, Préchoux A, Zühlke M K, Bäumgen M, Robb C S, Gerlach N, Roret T, Stanetty C, Larocque R, Michel G, Song T, Markert S, Unfried F, Mihovilovic M D, Trautwein–Schult A, Becher D, Schweder T, Bornscheuer U T and Hehemann J H 2019 A marine bacterial enzymatic cascade degrades the algal polysaccharide ulvan. Nature Chemical Biology, 15(8), 803–812. [CrossRef]
- Saravanan P, Chatterjee A, Kiran K J, Bhowmick G D, Sappati P K and Nagarajan V 2024 Exploring Seaweed–Associated Marine Microbes: Growth Impacts and Enzymatic Potential for Sustainable Resource Utilization. Indian Journal of Microbiology 64, 593602. [CrossRef]
- Sarfatis A, Wang Y, Twumasi-Ankrah N, Moffitt J R 2025 Highly multiplexed spatial transcriptomics in bacteria. Science, 387(6732): eadr0932. [CrossRef]
- Singh R P and Reddy C R K 2014 Seaweed–microbial interactions: Key functions of seaweed–associated bacteria. FEMS Microbiology Ecology, 88(2), 213–230. [CrossRef]
- Spencer S J, Tamminen M V, Preheim S P, Guo M T, Briggs A W, Brito I L A, Weitz D, Pitkänen L K, Vigneault F, Virta M P and Alm E J 2016 Massively parallel sequencing of single cells by epicPCR links functional genes with phylogenetic markers. ISME Journal, 10(2), 427–436. [CrossRef]
- Sun Y, Li H, Wang X, Li H and Deng Y 2023 Kelp Culture Enhances Coastal Biogeochemical Cycles by Maintaining Bacterioplankton Richness and Regulating Its Interactions. mSystems, 8(2), 14. [CrossRef]
- Sylvers P H and Gobler C J 2021 Mitigation of harmful algal blooms caused by Alexandrium catenella and reduction in saxitoxin accumulation in bivalves using cultivable seaweeds. Harmful Algae, 105, 102056. [CrossRef]
- Tang Q 2014 Management strategies of marine food resources undermultiple stressors with particular reference of the Yellow Sea large marine ecosystem. Frontiers in Agricultural Science and Engineering, 1(1), 85-90. [CrossRef]
- Thomas F, Barbeyron T and Michel G 2011 Evaluation of reference genes for real–time quantitative PCR in the marine flavobacterium Zobellia galactanivorans. Journal of Microbiological Methods, 84(1), 61–66. [CrossRef]
- Ulrich J F, Gräfe M S, Dhiman S, Wienecke P, Arndt H D and Wichard T 2022 Thallusin Quantification in Marine Bacteria and Algae Cultures. Marine Drugs, 20(11), 609. [CrossRef]
- Ulfah M, Kasanah N, Wijayanti N. Antivibriosis and cytotoxicity of Actinobacteria associated with red seaweed Gelidiella acerosa. Aquaculture Research, 2021, 1, 1-9. [CrossRef]
- Van Eck N J and Waltman L 2007 VOS: A New Method for Visualizing Similarities Between Objects. In: Decker, R, Lenz, H.J eds) Advances in Data Analysis. Studies in Classification, Data Analysis, and Knowledge Organization. Springer, Berlin, Heidelberg. [CrossRef]
- Viju N, Punitha S M J and Satheesh S 2021 An Analysis of Biosynthesis Gene Clusters and Bioactivity of Marine Bacterial Symbionts. Current Microbiology, 78(7), 2522–2533. Springer. [CrossRef]
- Wang G, Shuai L, Li Y, Lin W, Zhao X and Duan D 2008 Phylogenetic analysis of epiphytic marine bacteria on Hole–Rotten diseased sporophytes of Laminaria japonica. Journal of Applied Phycology, 20(4), 403–409. [CrossRef]
- Wang W, Wu L, Xu K, Xu Y, Ji D, Chen C and Xie C 2020 The cultivation of Pyropia haitanensis has important impacts on the seawater microbial community. Journal of Applied Phycology, 32, 2561–2573. [CrossRef]
- Wang Q, Sun X, Lin S, Dong Y, Shen H, He Z, Luo H, Zou L, Chung I K, Yang Y 2025 Large-scale seaweed cultivation as a nature solution for carbon-negative economy and restorative environmental stewardship: Lessons from China. Renewable and Sustainable Energy Reviews, 207, 114954. [CrossRef]
- Wang Z, Xiao T, Pang S, Liu M and Yue H 2009 Isolation and identification of bacteria associated with the surfaces of several algal species. Chinese Journal of Oceanology and Limnology, 27(3), 487–492. [CrossRef]
- Wani H M, Chen C W, Huang C Y, Singhania R R, Sung Y J, Dong C and Patel A K 2023 Development of Bioactive Peptides Derived from Red Algae for Dermal Care Applications: Recent Advances. Sustainability, 15(11), 8506. [CrossRef]
- Weigel B L, Miranda K K, Fogarty E C, Watson A R and Pfister C A 2022 Functional Insights into the Kelp Microbiome from Metagenome–Assembled Genomes. MSystems, 7(3), e01422–21. [CrossRef]
- West J A, Klochkova T A, Kim G H and Loiseaux–De Goër S 2006 Olpidiopsis sp, an oomycete from Madagascar that infects Bostrychia and other red algae: Host species susceptibility. Phycological Research, 54(1), 72–85. http://www.botany.unimelb.
- Wichard T 2015 Exploring bacteria–induced growth and morphogenesis in the green macroalga order Ulvales (Chlorophyta Frontiers in Plant Science, 6, 1–19. [CrossRef]
- Xie X, He Z, Hu X, Yin H, Liu X and Yang Y 2017 Large–scale seaweed cultivation diverges water and sediment microbial communities in the coast of Nan’ao Island, South China Sea. Science of the Total Environment, 598, 97–108. [CrossRef]
- Xie X, He Z, Wang Q and Yang Y 2024 Diversity, composition and ecological networks of bacterial communities in response to a full cultivation cycle of the seaweed, Gracilariopsis lemaneiformis. Environmental Research, 240, 117453. [CrossRef]
- Xiong T, Li H, Hu Y, Zhai W, Zhang Z, Liu Y, Zhang J, Lu L, Chang L, Xue L, Wei Q, Jiao N and Zhang Y 2024 Seaweed farming environments do not always function as CO2 sink under synergistic influence of macroalgae and microorganisms. Agriculture Ecosystems and Environment, 361, 108824. [CrossRef]
- Xiong Z, Wang R, Xia T, Zhang S, Ma S, Guo Z. Natural Products and Biological Activity from Actinomycetes Associated with Marine Algae. Molecules, 2023, 28(13), 5138. [CrossRef]
- Xu N, Wang W, Xu K, Xu Y, Ji D, Chen C and Xie C 2022 Cultivation of different seaweed species and seasonal changes cause divergence of the microbial community in coastal seawaters. Frontiers in Microbiology, 13. [CrossRef]
- Yang Y, Luo H, Wang Q, He Z and Long A 2021 Large–scale Cultivation of Seaweed is Effective Approach to Increase Marine Carbon Sequestration and Solve Coastal Environmental Problems. Bulletin of Chinese Academy of Sciences, 36(3), 259–269. [CrossRef]
- Zhang J, Wei L, Yang J, Ahmed W, Wang Y, Fu L, Ji G 2020 Probiotic Consortia: Reshaping the Rhizospheric Microbiome and Its Role in Suppressing Root-Rot Disease of Panax notoginseng. Frontiers in Microbiology, 11:701. [CrossRef]
- Zhang J, Zhao J, Jin H, Lv R, Shi H, De G, Yang B, Sun Z, Zhang H 2020 Probiotics maintain the intestinal microbiome homeostasis of the sailors during a long sea voyage. Gut Microbes, 11(4):930-943. [CrossRef]
- Zhang Y, Ji P, Wang J and Zhao F 2016 RiboFR–Seq: A novel approach to linking 16S rRNA amplicon profiles to metagenomes. Nucleic Acids Research, 44(10), 99. [CrossRef]
- Zheng R, Cai R, Liu R, Liu G and Sun C 2021 Maribellus comscasis sp. nov, a novel deep–sea Bacteroidetes bacterium, possessing a prominent capability of degrading cellulose. Environmental Microbiology, 23(8), 4561–4575. [CrossRef]
- Zheng W, Zhao S, Yin Y, Zhang H, Needham D M, Evans E D, Dai C L, Lu P J, Alm E J and Weitz D A 2022 High–throughput, single–microbe genomics with strain resolution, applied to a human gut microbiome. Science, 376(6597), 1483. [CrossRef]
- Zheng Y, Jin R, Zhang X, Wang Q and Wu J 2019 The considerable environmental benefits of seaweed aquaculture in China. Stochastic Environmental Research and Risk Assessment, 33(4–6), 1203–1221. [CrossRef]
- Zhong C: Li T, Bi R, Sanganyado E, Huang J, Jiang S, Zhang Z and Du H 2023 A systematic overview, trends and global perspectives on blue carbon: A bibliometric study (2003–2021). Ecological Indicators, 148, 110063. [CrossRef]

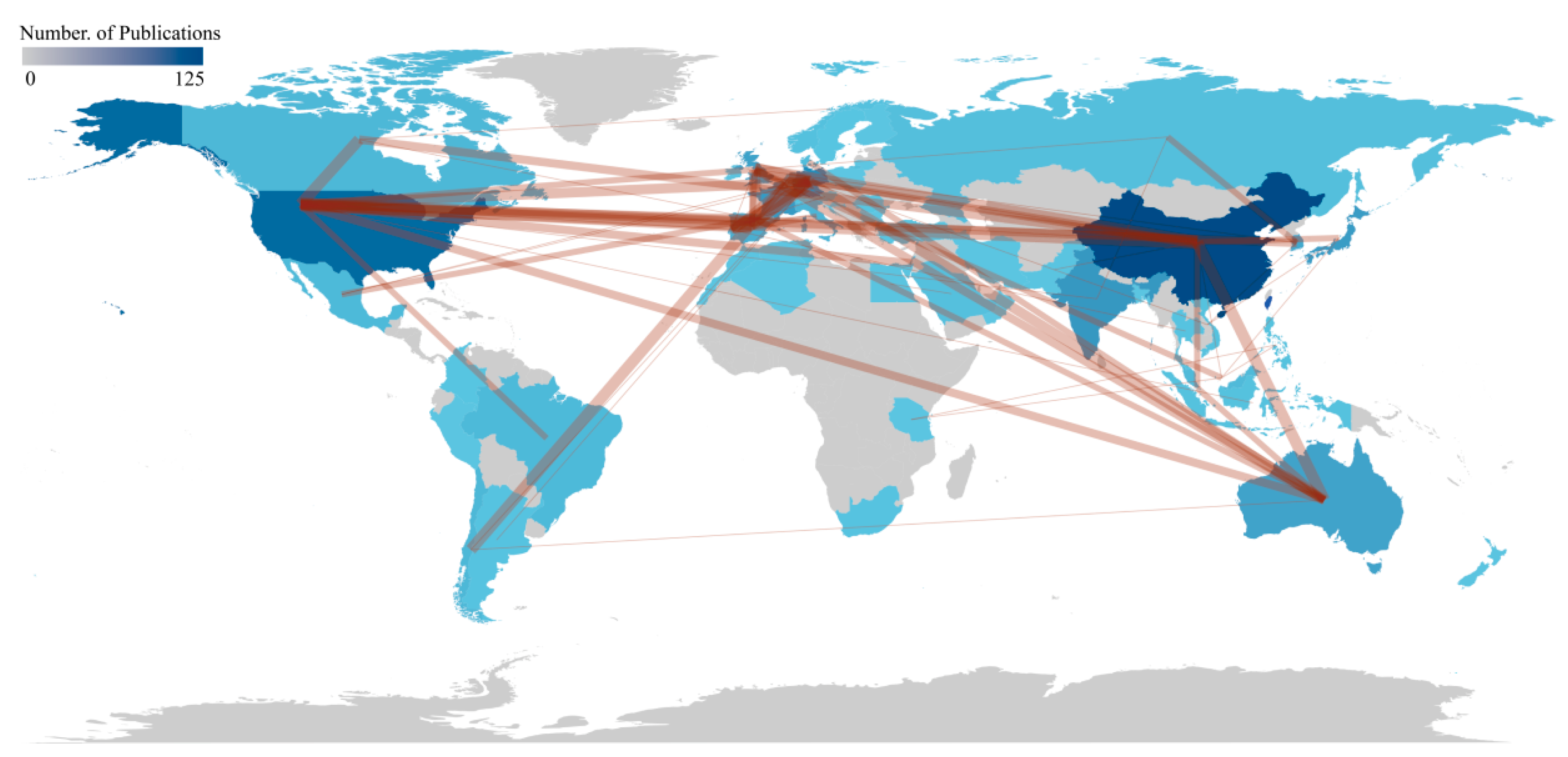
| Country | Articles | SCP | MCP | Freq. | MCP Ratio | TC | AAC |
|---|---|---|---|---|---|---|---|
| China | 125 | 101 | 24 | 0.205 | 0.192 | 2117 | 16.9 |
| USA | 73 | 61 | 12 | 0.12 | 0.164 | 7043 | 96.5 |
| India | 45 | 41 | 4 | 0.074 | 0.089 | 756 | 16.8 |
| Germany | 41 | 19 | 22 | 0.067 | 0.537 | 1660 | 40.5 |
| Japan | 37 | 34 | 3 | 0.061 | 0.081 | 815 | 22 |
| Korea | 25 | 19 | 6 | 0.041 | 0.24 | 581 | 23.2 |
| France | 24 | 16 | 8 | 0.039 | 0.333 | 505 | 21 |
| Australia | 21 | 12 | 9 | 0.034 | 0.429 | 1263 | 60.1 |
| United Kingdom | 20 | 10 | 10 | 0.033 | 0.5 | 774 | 38.7 |
| Italy | 14 | 10 | 4 | 0.023 | 0.286 | 311 | 22.2 |
| Portugal | 14 | 7 | 7 | 0.023 | 0.5 | 529 | 37.8 |
| Spain | 14 | 7 | 7 | 0.023 | 0.5 | 624 | 44.6 |
| Chile | 13 | 8 | 5 | 0.021 | 0.385 | 301 | 23.2 |
| Belgium | 12 | 3 | 9 | 0.02 | 0.75 | 300 | 25 |
| Brazil | 12 | 10 | 2 | 0.02 | 0.167 | 312 | 26 |
| Malaysia | 12 | 6 | 6 | 0.02 | 0.5 | 224 | 18.7 |
| Canada | 11 | 7 | 4 | 0.018 | 0.364 | 309 | 28.1 |
| Denmark | 10 | 5 | 5 | 0.016 | 0.5 | 549 | 54.9 |
| Mexico | 9 | 7 | 2 | 0.015 | 0.222 | 82 | 9.1 |
| Russia | 6 | 4 | 2 | 0.01 | 0.333 | 311 | 51.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





