Submitted:
02 April 2025
Posted:
02 April 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Phosphate Homeostasis
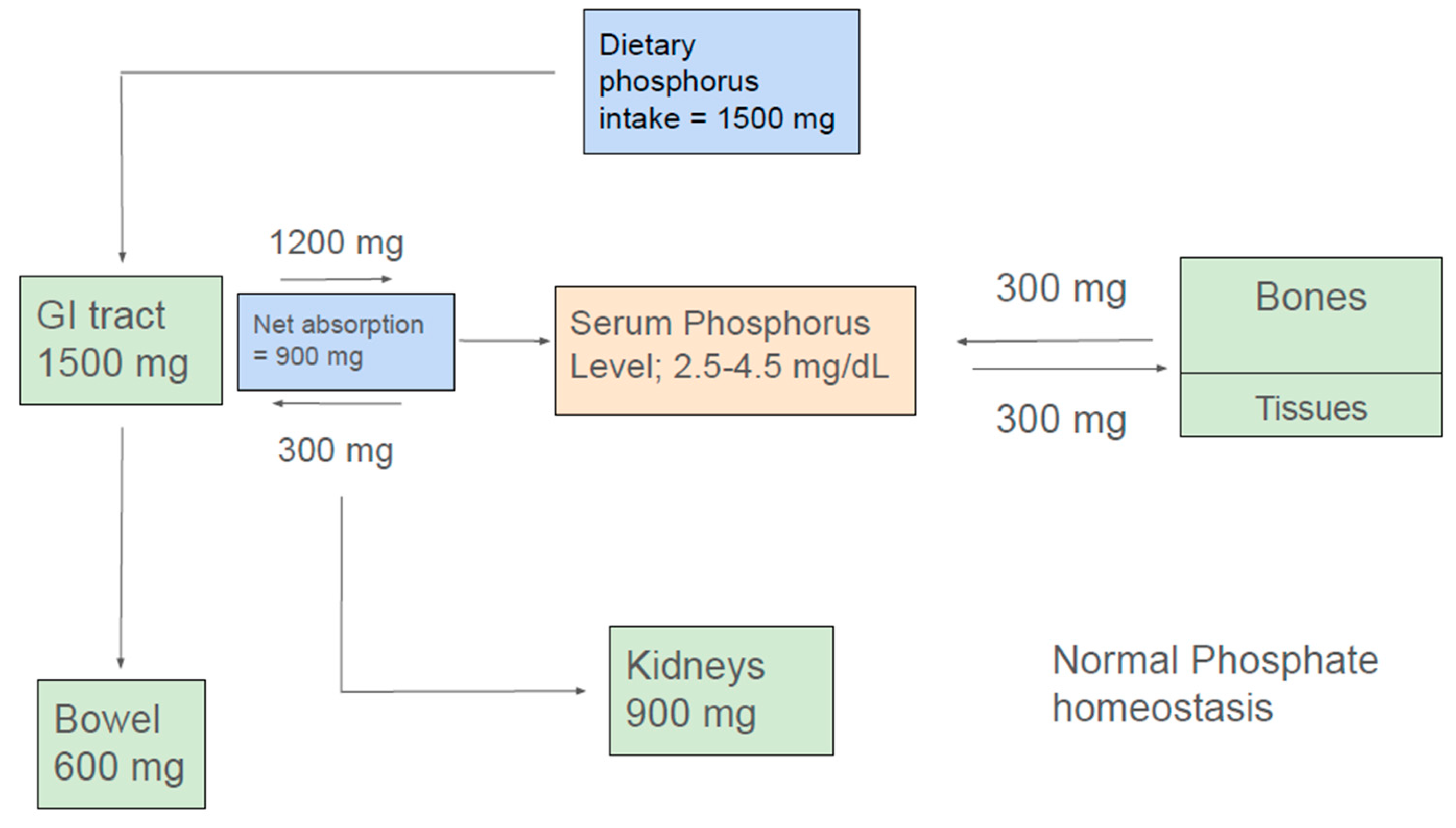
Phosphate Balance in Health:
Intestinal Phosphorus Handling:
Renal Phosphate Handling
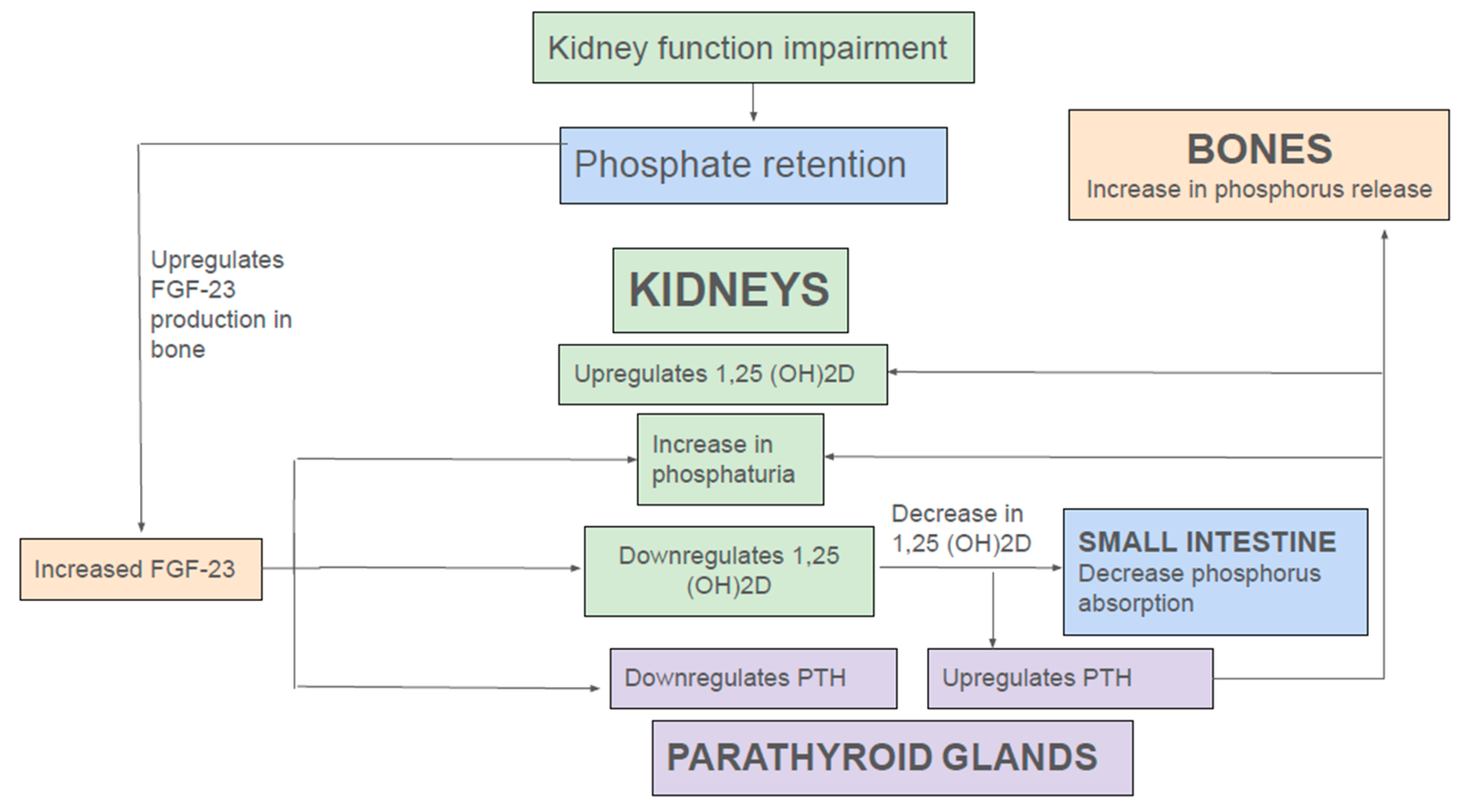
Endocrine Regulation of Phosphate Homeostasis in Health and Kidney Disease
Complications and Outcomes Associated with Hyperphosphatemia
Hyperphosphatemia: Cardiovascular Risks and Mortality
Hyperphosphatemia and Risk of Mortality and Progression of Renal Disease:
Hyperphosphatemia and Metabolic Bone Disease:
Management of Hyperphosphatemia
Removal of Phosphate by Dialysis:
Dietary Management of Hyperphosphatemia:
Forms of Phosphorus in the Diet: Organic and Inorganic
Intervention
Reducing Intestinal Phosphate Absorption:
- Metal-Based Phosphate Binders:
- Iron-based binders: Ferric Citrate and Sucroferric Oxyhydroxide
- Drugs inhibiting intestinal phosphate transport:
Controversies and Challenges in the Management of Hyperphosphatemia
Conflicts of Interest
References
- Heaney RP. Phosphorus. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:447-58).
- Hu, M.C.; Moe, O.W. Phosphate and Cellular Senescence. Adv. Exp. Med. Biol. 2022, 1362, 55–72.
- Phosphorus. Facts Sheet For Health Professionals. https://ods.od.nih.gov/factsheets/Phosphorus-HealthProfessional/#en1, updated May 4, 2023. Accessed Mar. 24, 2025.
- The United States Renal Data System Annual Data Report 2024. www.usrds.org. Accessed March 24, 2025.
- Shroff, R.; Long, D.A.; Shanahan, C. Mechanistic insights into vascular calcification in CKD. J. Am. Soc. Nephrol. 2013, 24, 179–189. [CrossRef]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17.
- Paloian, N.J.; Giachelli, C.M. A current understanding of vascular calcification in CKD. Am. J. Physiol. Ren. Physiol. 2014, 307, F891–F900. [CrossRef]
- Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370-1378.
- Zhou W, Simic P, Zhou IY, Caravan P, Vela Parada X, Wen D, Washington OL, Shvedova M, Pierce KA, Clish CB, Mannstadt M, Kobayashi T, Wein MN, Jüppner H, Rhee EP. Kidney glycolysis serves as a mammalian phosphate sensor that maintains phosphate homeostasis. J Clin Invest.2023;133(8):e164610. [CrossRef]
- Sabbagh Y, Giral H, Caldas Y, Levi M, Schiavi SC. Intestinal phosphate transport. Adv Chronic Kidney Dis. 2011; 18(2): 85–90.
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004; 19(3): 429–35.
- Berndt TJ, Kumar R: Clinical Disturbances of Phosphate Homeostasis In Seldin and Giebisch’s The Kidney,Fifth Edition. [CrossRef]
- Walton J, Gray TK. Absorption of inorganic phosphate in the human small intestine. Clin Sci. 1979; 56(5): 407–12. [CrossRef]
- Danisi G, Straub RW. Unidirectional influx of phosphate across the mucosal membrane of rabbit small intestine. Pflugers Arch. 1980; 385(2): 117–22. [CrossRef]
- Davis GR, Zerwekh JE, Parker TF, Krejs GJ, Pak CY, Fordtran JS. Absorption of phosphate in the jejunum of patients with chronic renal failure before and after correction of vitamin D deficiency. Gastroenterology. 1983; 85(4): 908–16. [CrossRef]
- Marks J. Debnam ES, Unwin RJ. The role of the gastrointestinal tract in phosphate homeostasis in health and chronic kidney disease. Curr Opin Nephrol Hypertens 22: 481–487, 2013. [CrossRef]
- Sabbagh Y, O’Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, et al. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol. 2009; 20 (11): 2348–58.
- Marks J. Debnam ES, Unwin RJ Phosphate homeostasis and the renal-gastrointestinal axis. Am J Physiol Renal Physiol 299: F285–F296, 2010. [CrossRef]
- Larsson TE, Kameoka C, Nakajo I, Taniuchi Y, Yoshida S, Akizawa T, et al. NPT-IIb inhibition does not improve hyperphosphatemia in CKD. Kidney Int Rep. 2018; 3(1): 73–80. [CrossRef]
- Saurette M, Alexander RT. Intestinal phosphate absorption: the paracellular pathway predominates? Exp Biol Med. 2019; 244(8): 646–54.
- Knöpfel T, Himmerkus N, Günzel D, Bleich M, Hernando N, Wagner CA. Paracellular transport of phosphate along the intestine. Am J Physiol Gastrointest Liver Physiol. 2019; 317(2): G233–G41. [CrossRef]
- Lee DB, Walling MW, Corry DB. Phosphate transport across rat jejunum: influence of sodium, pH, and 1,25-dihydroxyvitamin D3. Am J Physiol. 1986; 251(1 Pt 1): G90–5. [CrossRef]
- King AJ, Siegel M, He Y, Nie B, Wang J, Koo-McCoy S, et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med. 2018; 10(456):eaam6474. [CrossRef]
- Dudeja PK, Rao DD, Syed I, Joshi V, Dahdal RY, Gardner C, et al. Intestinal distribution of human Na+/H+ exchanger isoforms NHE-1, NHE-2, and NHE-3 mRNA. Am J Physiol.1996; 271(3 Pt 1): G483–93. [CrossRef]
- Rosenbaum DP, Yan A, Jacobs JW. Pharmacodynamics, safety, and tolerability of the NHE3 inhibitor tenapanor: two trials in healthy volunteers. Clin Drug Investig. 2018; 38(4): 341–51. [CrossRef]
- Block GA, Rosenbaum DP, Yan A, Chertow GM. Efficacy and safety of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis: a randomized phase 3 trial. J Am Soc Nephrol. 2019; 30(4): 641–52. [CrossRef]
- Tatsumi, S.; Miyagawa, A.; Kaneko, I.; Shiozaki, Y.; Segawa, H.; Miyamoto, K. Regulation of renal phosphate handling: Inter-organ communication in health and disease. J. Bone Min. Metab. 2016, 34, 1–10. [CrossRef]
- Lederer, E. Renal phosphate transporters. Curr. Opin. Nephrol. Hypertens. 2014, 23, 502–506.
- Erben RG. Physiological Actions of Fibroblast Growth Factor-23. Front Endocrinol 9: 267, 2018. [CrossRef]
- Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012 Jan;92(1):131–155. [CrossRef]
- Lee M, Partridge NC. Parathyroid hormone signaling in bone and kidney. Curr Opin Nephrol Hypertens. 2009 Jul;18(4):298–302. [CrossRef]
- Bargagli, M.; Arena, M.; Naticchia, A.; Gambaro, G.; Mazzaferro, S.; Fuster, D.; Ferraro, P.M. The Role of Diet in Bone and Mineral Metabolism and Secondary Hyperparathyroidism. Nutrients 2021, 13, 2328.
- Zhou W, Simic P, Zhou IY, Caravan P, Vela Parada X, Wen D, Washington OL, Shvedova M, Pierce KA, Clish CB, Mannstadt M, Kobayashi T, Wein MN, Jüppner H, Rhee EP. Kidney glycolysis serves as a mammalian phosphate sensor that maintains phosphate homeostasis. J Clin Invest.2023;133(8):e164610. [CrossRef]
- Ritz E, Gross M-L Hyperphosphatemia in renal failure. Blood Purif 2005, 23:6–9,.
- Chen W, Bushinsky D. Chronic kidney disease–mineral and bone disorder. In: Nissenson, A.R., Fine, R.N.B.T. Handbook of Dialysis Therapy, 5th edn. Elsevier, 2017, pp 685–697.e1.
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis, 31: 607-617, 1998 10.1053/ajkd.1998.v31.pm9531176.
- Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB, Sr., Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med, 167: 879-885, 2007. 10.1001/archinte.167.9.879.
- Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF (2011) Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 305(11):1119–1127.
- Schwartz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol. 2006;1: 825-831. [CrossRef]
- Tong J., Liu M., Li H., et al. Mortality and associated risk factors in dialysis patients with cardiovascular disease. Kidney Blood Press Res. 2016;41(4):479–487. [CrossRef]
- Cozzolino M., Mangano M., Stucchi A., Ciceri P., Conte F., Galassi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33(suppl 3):iii28–iii34. [CrossRef]
- Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK (2001) Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12(10):2131–2138.
- Cozzolino M, Dusso AS, Slatopolsky E (2001) Role of calcium-phosphate product and bone-associated proteins on vascular calcification in renal failure. J Am Soc Nephrol 12(11):2511–2516.
- Zhang D., Bi X., Liu Y., et al. High phosphate-induced calcification of vascular smooth muscle cells is associated with the TLR4/NF-κb signaling pathway. Kidney Blood Press Res. 2017;42(6):1205–1215. [CrossRef]
- Amann K (2008) Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol 3(6):1599–1605.
- El-Abbadi MM, Pai AS, Leaf EM et al. (2009) Phosphate feeding induces arterial medial calcification in uremic mice: role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int 75(12):1297–1307.
- Pai AS, Giachelli CM (2010) Matrix remodeling in vascular calcification associated with chronic kidney disease. J Am Soc Nephrol 21(10):1637–1640.
- Luong TTD, Schelski N, Boehme B, Makridakis M, Vlahou A, Lang F, Pieske B, Alesutan I, Voelkl J (2018) Fibulin-3 attenuates phosphate-induced vascular smooth muscle cell calcification by inhibition of oxidative stress. Cell Physiol Biochem 46:1305–1316. [CrossRef]
- Voelkl J, Lang F, Eckardt K et al. (2019) Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell Mol Life Sci 76(11):2077–2091. [CrossRef]
- Faul C., Amaral A.P., Oskouei B., et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. [CrossRef]
- Wannamethee S.G., Welsh P., Papacosta O., Lennon L., Whincup P.H., Sattar N. Elevated parathyroid hormone, but not vitamin D deficiency, is associated with increased risk of heart failure in older men with and without cardiovascular disease. Circulation Heart Fail. 2014;7(5):732–739. [CrossRef]
- Vogt I, Haffner D, Leifheit-Nestler M. FGF23 and Phosphate–Cardiovascular Toxins in CKD. Toxins Basel. 2019;11(11):647. [CrossRef]
- Ix J.H., Katz R., Kestenbaum B.R., et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2012;60(3):200–207. [CrossRef]
- Edmonston D, Grabner A, Wolf M: FGF23 and klotho at the intersection of kidney andcardiovascular disease. Nat Rev Cardiol, 21: 11-24, 2024 10.1038/s41569-023-00903-0.
- Zhong Z, Feng S, Fu D, et al. Serum fibroblast growth factor 23 concentration and the risk of mortality in patients undergoing peritoneal dialysis. Perit Dial Int 2024; 44:194.
- Cheng S.P., Liu C.L., Liu T.P., Hsu Y.C., Lee J.J. Association between parathyroid hormone levels and inflammatory markers among US adults. Mediators Inflamm. 2014;2014:709024. [CrossRef]
- Goldsmith D.J., Covic A.A., Venning M.C., Ackrill P. Blood pressure reduction after parathyroidectomy for secondary hyperparathyroidism: further evidence implicating calcium homeostasis in blood pressure regulation. Am J Kidney Dis. 1996;27(6):819–825. [CrossRef]
- Smogorzewski M., Perna A.F., Borum P.R., Massry S.G. Fatty acid oxidation in the myocardium: effects of parathyroid hormone and CRF. Kidney Int. 1988;34(6):797–803. [CrossRef]
- Rodríguez-Ayala E., Avila-Díaz M., Foyo-Niembro E., Amato D., Ramirez-San-Juan E., Paniagua R. Effect of parathyroidectomy on cardiac fibrosis and apoptosis: possible role of aldosterone. Nephron Physiol. 2006;103(3):112–118. [CrossRef]
- Saleh F.N., Schirmer H., Sundsfjord J., Jorde R. Parathyroid hormone and left ventricular hypertrophy. Eur Heart J. 2003;24(22):2054–2060. [CrossRef]
- Li Y., Chen C., Liu H.L., Qian G. Vitamin D, parathyroid hormone, and heart failure in a Chinese elderly population. Endocr Pract. 2015;21(1):30–40. [CrossRef]
- Kestenbaum, B.; Sampson, J.N.; Rudser, K.D.; Patterson, D.J.; Seliger, S.L.; Young, B.; Sherrard, D.J.; Andress, D.L. Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 520–528.
- Voormolen, N.; Noordzij, M.; Grootendorst, D.C.; Beetz, I.; Sijpkens, Y.W.; van Manen, J.G.; Boeschoten, E.W.; Huisman, R.M.;Krediet, R.T.; Dekker, F.W.; et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol. Dial. Transpl. 2007, 222, 909–916. [CrossRef]
- Tonelli M, Curhan G, Pfeffer M, Sacks F, Thadhani R, Melamed ML, Wiebe N, Muntner P (2009) Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation 120(18):1784–1792.
- Li JW, Xu C, Fan Y, Wang Y, Xiao YB (2014) Can serum levels of alkaline phosphatase and phosphate predict cardiovascular diseases and total mortality in individuals with preserved renal function? A systemic review and meta-analysis. PLoS One 9(7):e102276.
- Schwartz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol. 2006;1(4):825-831. [CrossRef]
- Maique J, Flores B, Shi M et al. (2020) High phosphate induces and Klotho attenuates kidney epithelial senescence and fibrosis. Front Pharmacol 11:1273. [CrossRef]
- Shen ZJ, Hu J, Shiizaki K et al. (2016) Phosphateinduced renal fibrosis requires the prolyl isomerase Pin1. PLoS One 11:e01500 . [CrossRef]
- Sim, J.J.; Bhandari, S.K.; Smith, N.; Chung, J.; Liu, I.L.; Jacobsen, S.J.; Kalantar-Zadeh, K. Phosphorus and risk of renal failure in subjects with normal renal function. Am. J. Med. 2013, 126, 311–318.
- Zoccali, C.; Ruggenenti, P.; Perna, A.; Leonardis, D.; Tripepi, R.; Tripepi, G.; Mallamaci, F.; Remuzzi, G.; REIN Study Group. Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J. Am. Soc. Nephrol. 2011, 22, 1923–1930.
- Da, J.; Xie, X.; Wolf, M.; Disthabanchong, S.; Wang, J.; Zha, Y.; Lv, J.; Zhang, L.; Wang, H. Serum phosphorus and progression of CKD and mortality: A meta-analysis of cohort studies. Am. J. Kidney Dis. 2015, 66, 258–265.
- Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370-1378. [CrossRef]
- Barrera-Baena P, Rodríguez-García M, Rodríguez-Rubio E, et al. Serum phosphate is associated with increased risk of bone fragility fractures in haemodialysis patients. Nephrol Dial Transplant. 2023;39(4):618-626. [CrossRef]
- Tentori, F.; McCullough, K.; Kilpatrick, R.D.; Bradbury, B.D.; Robinson, B.M.; Kerr, P.G.; Pisoni, R.L. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014, 85, 166–173. [CrossRef]
- Fusaro, M.; Holden, R.; Lok, C.; Iervasi, G.; Plebani, M.; Aghi, A.; Gallieni, M.; Cozzolino, M. Phosphate and bone fracture risk in chronic kidney disease patients. Nephrol. Dial. Transpl. 2021, 36, 405–412.
- Meleti, Z.; Shapiro, I.M.; Adams, C.S. Inorganic phosphate induces apoptosis of osteoblast-like cells in culture. Bone 2000, 27, 359–366. [CrossRef]
- Mozar, A.; Haren, N.; Chasseraud, M.; Louvet, L.; Mazière, C.; Wattel, A.; Mentaverri, R.; Morlière, P.; Kamel, S.; Brazier, M.; et al. High extracellular inorganic phosphate concentration inhibits RANK-RANKL signaling in osteoclast-like cells. J. Cell. Physiol.2008, 215, 47–54.
- Guedes M, Bieber B, Dasgupta I, et al. Serum Phosphorus Level Rises in US Hemodialysis Patients Over the Past Decade: A DOPPS Special Report. Kidney Med. 2022;5(2):100584. [CrossRef]
- Kidney Disease: Improving Global Outcomes CKDMBDUWG: KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2011), 7: 1-59, 2017 10.1016/j.kisu.2017.04.001.
- Kuhlmann MK. Phosphate elimination in modalities of hemodialysis and peritoneal dialysis. Blood Purif. 2010;29(2):137–44. [CrossRef] [PubMed]
- DeSoi CA, Umans JG. Phosphate kinetics during high-flux hemodialysis. J Am Soc Nephrol. Nov 1993;4(5):1214–8. [PubMed]
- Sugisaki H, Onohara M, Kunitomo T. Dynamic behavior of plasma phosphate in chronic dialysis patients. Trans Am Soc Artif Intern Organs. 1982;28:302–7. [PubMed]
- Zucchelli P, Santoro A. Inorganic phosphate removal during different dialytic procedures. Int J Artif Organs. 1987;10(3):173–8. [PubMed]
- Haas T, Hillion D, Dongradi G. Phosphate kinetics in dialysis patients. Nephrol Dial Transplant. 1991;6(Suppl 2):108–13. [PubMed]
- Man NK, Chauveau P, Kuno T, Poignet JL, Yanai M. Phosphate removal during hemodialysis, hemodiafiltration, and hemofiltration. A reappraisal. ASAIO Trans. Jul-Sep 1991;37(3):M463–5. [PubMed]
- Daugirdas JT. Removal of Phosphorus by Hemodialysis. Semin Dial. Nov-Dec 2015;28(6):620–3. [CrossRef]
- Kooienga L. Phosphorus balance with daily dialysis. Semin Dial. Jul-Aug 2007;20(4):342–5. [CrossRef] [PubMed]
- Minutolo R, Bellizzi V, Cioffi M, et al. Postdialytic rebound of serum phosphorus: pathogenetic and clinical insights. J Am Soc Nephrol. Apr 2002;13(4):1046–54. [PubMed]
- Stremke ER, Trevino L, Simit Doshi S, Moorthi RN, Gallant KMH. Moe SM, Post-Dialysis Serum Phosphate Equilibrium in Hemodialysis Patients on a controlled diet and no binders Hemodial Int . 2022 April ; 26(2): 255–263. [CrossRef]
- Perl, J.; Bargman, J.M. Peritoneal dialysis: From bench to bedside and bedside to bench. Am. J. Physiol. Renal Physiol. 2016, 311, F999–F1004. [CrossRef]
- Courivaud, C.; Davenport, A. Phosphate Removal by Peritoneal Dialysis: The Effect of Transporter Status and Peritoneal Dialysis Prescription. Perit. Dial. Int. 2016, 36, 85–93. [CrossRef]
- Bammens, B.; Evenepoel, P.; Verbeke, K.; Vanrenterghem, Y. Removal of middle molecules and protein-bound solutes by peritoneal dialysis and relation with uremic symptoms. Kidney Int. 2003, 64, 2238–2243. [CrossRef]
- Debowska, M.; Gomez, R.; Pinto, J.; Waniewski, J.; Lindholm, B. Phosphate clearance in peritoneal dialysis. Sci. Rep. 2020, 10, 17504.
- Peruzzo, D.; Guedes, M.; Larkin, J.W.; Yokoyama, G.; Dos Santos, T.L.; Pecoits-Filho, R.; Ribeiro, S.C.; Ramos, A.; Barretti, P.; de Moraes, T.P.; et al. Peritoneal dialysis modality transition and impact on phosphate and potassium serum levels. PLoS ONE 2021, 16, e0257140. [CrossRef]
- Calvo MS, Park YK. Changing phosphorus content of the U.S. diet: potential for adverse effects on bone. J Nutr. 1996;126:1168S–1180S. [CrossRef]
- Kalantar-Zadeh, K., Gutekunst, L., Mehrotra, R., Kovesdy, C. P., Bross, R., Shinaberger, C. S., Noori, N., Hirschberg, R., Benner, D., Nissenson, A. R., & Kopple, J. D. (2010). Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clinical Journal of the American Society of Nephrology, 5(3), 519-530. [CrossRef]
- Calvo, M. S., & Uribarri, J. (2011). Dietary phosphorus intake and the risk for cardiovascular disease in the general population. Advances in Chronic Kidney Disease, 18(4), 266-272.
- Dang Z, He Y, Xie R, Chen P, Dong F, Plant-based Diet and Chronic Kidney Disease: A Systematic Review and Meta-analysis, Journal of Renal Nutrition (2025).
- Huml AM, Sullivan CM, Leon JB, et al. The adequacy of phosphorus binder prescriptions among American hemodialysis patients. Ren Fail. 2012;34:1258–1263. KDIGO-2017. [CrossRef]
- Brown-Tortorici, A. R., Narasaki, Y., You, A. S., Norris, K. C., Streja, E., Peralta, R. A., Guerrero, Y., Daza, A., Arora, R., Lo, R., Nakata, T., Nguyen, D. V., Kalantar-Zadeh, K., & Rhee, C. M. (2022). The interplay between dietary phosphorus, protein intake, and mortality in a prospective hemodialysis cohort. Nutrients, 14(15), 3070. [CrossRef]
- Fenton, T. R., et al. (2017). Phosphorus additives in food and their impact on human health. Current Opinion in Clinical Nutrition & Metabolic Care, 20(3), 192-196.
- Younes, M., Aquilina, G., Castle, L., Engel, K.-H., Fowler, P., Frutos Fernandez, M. J., Fürst, P., Gürtler, R., Husøy, T., Mennes, W., Moldeus, P., Oskarsson, A., Shah, R., Waalkens-Berendsen, I., Wölfle, D., Aggett, P., Cupisti, A., Fortes, C., Kuhnle, G., Lillegaard, I. T., Scotter, M., Giarola, A., Rincon, A., Tard, A., & Gundert-Remy, U. (2019). Re-evaluation of phosphoric acid–phosphates – di-, tri- and polyphosphates (E 338–341, E 343, E 450–452) as food additives and the safety of proposed extension of use. EFSA Panel on Food Additives and Flavourings (FAF). Adopted 4 June 2019; Published 12 June 2019. [CrossRef]
- Capra BT, Hudson S, Helder M, Laskaridou E, Johnson AL, Gilmore C, Marinik E, Hedrick VE, Savla J, David LA, Davy KP, Davy BM. Ultra-processed food intake, gut microbiome, and glucose homeostasis in mid-life adults: Background, design, and methods of a controlled feeding trial. Contemp Clin Trials. 2024 Feb;137:107427. Epub 2024 Jan 4. [CrossRef] [PubMed] [PubMed Central]
- FoodNavigator-USA. (2014, July 17). Until phosphorus gets on the USDA’s radar, labeling policy won’t change – NKF. FoodNavigator-USA. https://www.foodnavigator-usa.com/Article/2014/07/17/Until-phosphorus-gets-on-the-USDA-s-radar-labeling-policy-won-t-change-NKF/ (Accessed on March 23, 2025).
- Nelson SM, Sarabia SR, Christilaw E, et al. Phosphate-containing prescription medications contribute to the daily phosphate intake in a third of hemodialysis patients. J Ren Nutr. 2017;27:91–96.
- Sherman RA, Ravella S, Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int. 2015;87:1097–1099. [CrossRef]
- Moe SM, Chen NX, Seifert MF, et al. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009;75(2):176-184. [CrossRef]
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(2):257-264.
- Azadbakht L, Esmaillzadeh A. Soy-protein consumption and kidney-related biomarkers among type 2 diabetics: a crossover, randomized clinical trial. J Ren Nutr. 2009;19(6):479-486.
- Wu T, Chang C, Hsu W, et al. Nutritional status of vegetarians on maintenance haemodialysis. Nephrology (Carlton). 2011;16(6):582-587. [CrossRef]
- Garcia-Torres R, Young L, Murray DP, Kheda M, Nahman NS Jr. Dietary protein source and phosphate levels in patients on hemodialysis. J Ren Nutr. 2020:1-7 . [CrossRef]
- Kistler BM, Moore LW, Benner D, et al. The International Society of Renal Nutrition and Metabolism commentary on the National Kidney Foundation and Academy of Nutrition and Dietetics KDOQI clinical practice guideline for nutrition in chronic kidney disease. J Ren Nutr 2021;31(2):116-120.e1. [CrossRef]
- Stevens PE, Ahmed SB, Carrero JJ, et al. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105(4):S117- S314. [CrossRef]
- Kalantar-Zadeh, K., & Kopple, J. D. (2013). Preventing and correcting metabolic acidosis in chronic kidney disease: Implications for bone and mineral disease. American Journal of Kidney Diseases, 61(3), 508-516. [CrossRef]
- Goraya, N., & Wesson, D. E. (2020). Whole-Food Low-Protein Plant-Based Nutrition to Prevent or Slow Progression of Chronic Kidney Disease. Journal of Renal Nutrition, 30(6), 480–488.
- KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. (2020). American Journal of Kidney Diseases, 76(3), S1–S107.
- Zarantonello, D., & Brunori, G. (2023). Plant-Based Diets in Preventing and Mitigating Chronic Kidney Disease. Journal of Clinical Medicine, 12(19), 6137. [CrossRef]
- Joshi, S., Shah, S., & Kalantar-Zadeh, K. (2019). Adequacy of plant-based proteins in chronic kidney disease. Journal of Renal Nutrition, 29(2), 112-117. [CrossRef]
- Consultant360. (n.d.). Dietary modification in renal disease. Retrieved from https://www.consultant360.com/blog/consultant360/nephrology/dietary-modification-renal-disease (Accessed on March 23, 2025).
- Escoffier. (n.d.). The true costs of processed foods on health and the planet. Retrieved from https://www.escoffier.edu/blog/world-food-drink/true-costs-processed-foods-health-planet/#:~:text=So%20although%20processed%20food%20is,And%20agricultural%20byproducts%20are%20destroyed. (Accessed on March 23, 2025).
- Springmann, M., Clark, M. A., Rayner, M., Scarborough, P., & Webb, P. (2021). The global and regional costs of healthy and sustainable dietary patterns: A modelling study. The Lancet Planetary Health, 5(11), e797–e807. [CrossRef]
- Silva, A. R., Rodrigues, A. M., & Lima, M. L. (2021). Do plant-based consumers spend more on food? Frontiers in Nutrition, 8, 732129. [CrossRef]
- Kahleova, H., Levin, S., & Barnard, N. D. (2023). Vegan diet and food costs among adults with overweight: A secondary analysis of a randomized clinical trial. JAMA Network Open, 6(9), e2332106. [CrossRef]
- GFI. (2022). Reducing the price of alternative proteins. Retrieved from https://gfi.org/reducing-the-price-of-alternative-proteins/ (Accessed on March 23, 2025).
- Food & Wine. (2024, April 5). Why going vegetarian is a recipe for a cheaper grocery bill. Retrieved from https://www.foodandwine.com/vegetarian-cheapest-groceries-report-11685113 (Accessed on March 23, 2025).
- The Guardian. (2024, August 28). “Don’t be scared of beans”: how readers are handling US grocery inflation. Retrieved from https://www.theguardian.com/environment/article/2024/aug/28/inflation-groceries-tips (Accessed on March 23, 2025).
- Kimura M, Itokawa Y. Cooking losses of minerals in foods and its nutritional significance. J Nutr Sci Vitaminol (Tokyo). 1990;36 Suppl 1: S25–S32. discussion S33.
- Jones WL. Demineralization of a wide variety of foods for the renal patient. J Ren Nutr. 2001;11:90–96.
- Naber, T., & Purohit, S. (2021). Chronic Kidney Disease: Role of Diet for a Reduction in the Severity of the Disease. Nutrients, 13(9), 3277. [CrossRef]
- Ikizler TA, Burrowes JD, Byham-Gray LD ∙ Katrina L. Campbell KL, Carrero JJ, Chan W, Fouque D, Friedman AN, Ghaddar S, Goldstein-Fuchs DJ, Kaysen GA, Kopple JD. Teta D, Wang AY-M, Cuppari L. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. [CrossRef]
- Noce, A., Marrone, G., Wilson Jones, G., Di Lauro, M., Pietroboni Zaitseva, A., Ramadori, L., Celotto, R., Mitterhofer, A. P., & Di Daniele, N. (2021). Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients, 13(12), 4299.
- Kalantar-Zadeh, K., Bellizzi, V., Piccoli, G. B., Shi, Y., Lim, S. K., Riaz, S., Urbina Arronte, R., Lau, W. P., & Fouque, D. (2023). Caring for Patients With Advanced Chronic Kidney Disease: Dietary Considerations. Nutrition in Clinical Practice, 38(1), 22–34.
- US-DOPPS Practice Monitor, May 2021; http://www.dopps.org/DPM.
- Fresenius Medical Care North America. PhosLo® gelcaps (calcium acetate): 667 mg [prescribing information]. Waltham, MA: Fresenius Medical Care North America; 2011.
- Fresenius Medical Care North America. VELPHORO ® (sucroferric oxyhydroxide) [prescribing information]. Waltham, MA: Fresenius Medical Care North America; 2013.
- Shire US Inc. FOSRENAL® (lanthanum carbonate) [prescribing information]. Lexington, MA: Shire US Inc.; 2016.
- Keryx Biopharmaceuticals Inc. AURYXIA® (ferric citrate) tablets [prescribing information]. Cambridge, MA: Keryx Biopharmaceuticals Inc.; 2017.
- Genzyme Corp. RENVELA® (sevelamer carbonate) [prescribing information]. Cambridge, MA: Genzyme Corp.; 2020.
- Salusky IB, Foley J, Nelson P, Goodman WG. Aluminum accumulation during treatment with aluminum hydroxide and dialysis in children and young adults with chronic renal disease. N Engl J Med1991;324:527-31.
- Elliott HL, Dryburgh F, Fell GS, Sabet S, Macdougall AI. Aluminium toxicity during regular haemodialysis. BMJ 1978;1:1101-3. [CrossRef]
- Parkinson IS, Ward MK, Feest TG, Fawcett RW, Kerr DN. Fracturing dialysis osteodystrophy and dialysis encephalopathy: an epidemiological survey. Lancet 1979;1:406-9. [CrossRef]
- Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2005;67:1179-87. [CrossRef]
- K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42: Suppl 3:S1-S201.
- Janssen MJ, van der Kuy A, ter Wee PM, van Boven WP. Aluminum hydroxide, calcium carbonate and calcium acetate in chronic intermittent hemodialysis patients. Clin Nephrol 1996;45:111-9.
- Navaneethan SD, Palmer SC, Craig JC, Elder GJ, Strippoli GF. Benefits and harms of phosphate binders in CKD: a systematic review of randomized controlled trials. Am J Kidney Dis 2009;54:619-37. [CrossRef]
- Suki WN, Zabaneh R, Cangiano JL, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 2007;72: 1130-7. [CrossRef]
- Tonelli M, Wiebe N, Culleton B, et al. Systematic review of the clinical efficacy and safety of sevelamer in dialysis patients. Nephrol Dial Transplant 2007;22: 2856-66. [CrossRef]
- Block GA, Wheeler DC, Persky MS, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23:1407–1415.
- Hill KM, Martin BR, Wastney ME, et al. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int. 2013;83:959–966. [CrossRef]
- Spiegel DM, Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int. 2012;81:1116–1122. [CrossRef]
- Di Iorio B, Bellasi A, Russo D. Mortality in kidney disease patients treated with phosphate binders: a randomized study. Clin J Am Soc Nephrol. 2012;7: 87–493.
- Di Iorio B, Molony D, Bell C, et al. Sevelamer versus calcium carbonate in incident hemodialysis patients: results of an open-label 24-monthandomized clinical trial. Am J Kidney Dis. 2013;62:771–778.
- Zheng C, Liu J, Wang T, Hu H, Chen Y. A network meta-analysis of therapies for hyperphosphatemia in CKD based on randomized trials. Scientific Reports | (2025) 15:2012. [CrossRef]
- Perry, C. M. & Plosker, G. L. Sevelamer carbonate: A review in hyperphosphataemia in adults with chronic kidney disease. Drugs 74, 771–792. (2014). [CrossRef]
- Raggi, P., Vukicevic, S., Moysés, R. M., Wesseling, K. & Spiegel, D. M. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin. J. Am. Soc. Nephrol. 5(Suppl 1), S31-40. (2010). [CrossRef]
- Vervloet, M. G. et al. The role of phosphate in kidney disease. Nat. Rev. Nephrol. 13, 27–38. (2017). [CrossRef]
- Lenglet, A. et al. Does the administration of sevelamer or nicotinamide modify uremic toxins or endotoxemia in chronic hemodialysis patients?. Drugs 79, 855–862. (2019). [CrossRef]
- Wrong O, Harland C. Sevelamer. Nephrol Dial Transplant 2008;23:2101-2.
- Pai AB, Shepler BM. Comparison of sevelamer hydrochloride and sevelamer carbonate: risk of metabolic acidosis and clinical implications. Pharmacotherapy 2009;29:554-61. [CrossRef]
- Delmez J, Block G, Robertson J, et al. A randomized, double-blind, crossover design study of sevelamer hydrochloride and sevelamer carbonate in patients on hemodialysis. Clin Nephrol 2007;68:386-91. [CrossRef]
- Fissell, R.B.; Karaboyas, A.; Bieber, B.A.; Sen, A.; Li, Y.; Lopes, A.A.; Akiba, T.; Bommer, J.; Ethier, J.; Jadoul, M.; et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: Findings from the DOPPS. Hemodial. Int. 2016, 20, 38–49.
- Goldsmith, D. R., Scott, L. J., Cvetković, R. S. & Plosker, G. L. Sevelamer hydrochloride: A review of its use for hyperphosphataemia in patients with end-stage renal disease on haemodialysis. Drugs 68, 85–104. (2008). [CrossRef]
- Pai, A. B. & Shepler, B. M. Comparison of sevelamer hydrochloride and sevelamer carbonate: Risk of metabolic acidosis and clinical implications. Pharmacotherapy 29, 554–561. (2009). [CrossRef]
- Swanson BJ, Limketkai BN, Liu TC, Nazari K, Park JY, Santiago WC, Torbenson MS, Voltaggio LY, Martha M, Arnold CA. Sevelamer Crystals in the Gastrointestinal Tract (GIT) A New Entity Associated With Mucosal Injury.; Am J Surg Pathol. 37 :1686-1693, 2013. [CrossRef]
- Elkalashy A, Rainwater RR, Ali U, Elbahnasawy E, Singh M, Karakala N, Gastrointestinal mucosal cell injury caused by sevelamer crystals- Case series and literature review, J Renal Nutrition (2025). [CrossRef]
- Braunlin W, Zhorov E, Guo A, et al. Bile acid binding to sevelamer HCl Kidney Int, 62 (2002), pp. 611-619.
- Burke SK, Dillon MA, Hemken DE, et al. Meta-analysis of the effect of sevelamer on phosphorus, calcium, PTH, and serum lipids in dialysis patients Adv Ren Replace Ther, 10 (2003), pp. 133-145.
- Susantitaphong P, Jaber BL. Potential interaction between sevelamer and fat-soluble vitamins: a hypothesis. Am J Kidney Dis, 59 (2012), pp. 165-167.
- Vemuri N, Michelis MF, Matalon A. Conversion to lanthanum carbonate monotherapy effectively controls serum phosphorus with a reduced tablet burden: a multicenter open-label study. BMC Nephrol. 2011;12:49. [CrossRef]
- Dellanna F, Reichel H, Seibt F. Efficacy and safety of lanthanum carbonate in German patients on dialysis. Clin Nephrol. 2012;78:382–390. [CrossRef]
- Wilson RJ, Keith MS, Preston P, Copley JB. The real-world dose-relativity of sevelamer hydrochloride and lanthanum carbonate monotherapy in patients with end-stage renal disease. Adv Ther. 2013;30:1100–1110. [CrossRef]
- Takahara Y, Matsuda Y, Takahashi S, et al. Ef cacy and safety of lanthanum carbonate in pre-dialysis CKD patients with hyperphosphatemia: a randomized trial. Clin Nephrol. 2014;82:181–190. [CrossRef]
- Toida T, Fukudome K, Fujimoto S, et al. Effect of lanthanum carbonate vs. calcium carbonate on serum calcium in hemodialysis patients: a crossover study. Clin Nephrol. 2012;78:216–223.
- Zhang C, Wen J, Li Z, Fan J. Efficacy and safety of lanthanum carbonate on chronic kidney disease mineral and bone disorder in dialysis patients: a systematic review. BMC Nephrol. 2013;14:226. [CrossRef]
- Lacour B, Lucas A, Auchere D, et al. Chronic renal failure is associated with increased tissue deposition of lanthanum after 28-day oral administration. Kidney Int. 2005;67:1062–1069.
- Hutchison AJ, Barnett ME, Krause R, et al. Lanthanum carbon- ate treatment, for up to 6 years, is not associated with adverse effects on the liver in patients with chronic kidney disease stage 5 receiving hemodialysis. Clin Nephrol. 2009;71:286–295.
- Spasovski GB, Sikole A, Gelev S, et al. Evolution of bone and plasma concentration of lanthanum in dialysis patients before, during 1 year of treatment with lanthanum carbonate and after 2 years of follow-up. Nephrol Dial Transplant. 2006;21:2217–2224.
- Hutchison, A.J.; Wilson, R.J.; Garafola, S.; Copley, J.B. Lanthanum carbonate: Safety data after 10 years. Nephrology 2016, 21, 987–994.
- Lewis JB, Sika M, Koury MJ, et al. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26:493–503.
- Umanath K, Jalal DI, Greco BA, et al. Ferric citrate reduces intravenous iron and erythropoiesis-stimulating agent use in ESRD. J Am Soc Nephrol. 2015;26:2578–258. [CrossRef]
- Yokoyama, K.; Hirakata, H.; Akiba, T.; Fukagawa, M.; Nakayama, M.; Sawada, K.; Kumagai, Y.; Block, G.A. Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin. J. Am. Soc. Nephrol. 2014, 9, 543–552. [CrossRef]
- Van Buren PN, Lewis JB, Dwyer JP, Greene T, Middleton J, Sika M, Umanath K, Abraham JD, Arfeen SS, Bowline IG, Chernin G, Fadem SZ, Goral S, Koury M, Sinsakul MV, Weiner DE; Collaborative Study Group. The Phosphate Binder Ferric Citrate and Mineral Metabolism and Inflammatory Markers in Maintenance Dialysis Patients: Results From Prespecified Analyses of a Randomized Clinical Trial. Am J Kidney Dis. 2015 Sep;66(3):479-88. Epub 2015 May 7. [CrossRef] [PubMed] [PubMed Central]
- Floege J. Phosphate binders in chronic kidney disease: a systematic review of recent data. J Nephrol. 2016;29:329–340. [CrossRef]
- Cozzolino M, Funk F, Rakov V, et al. Preclinical pharmacokinetics, pharmacodynamics and safety of sucroferric oxyhydroxide. Curr Drug Metab. 2014;15:953–965.
- Floege J, Covic AC, Ketteler M, et al. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015;30:1037–1046. [CrossRef]
- Floege J, Covic AC, Ketteler M, et al. A phase III study of the efficacy and safety of a novel iron based phosphate binder in dialysis patients. Kidney Int. 2014;86:638–647.
- Ketteler, M.; Sprague, S.M.; Covic, A.C.; Rastogi, A.; Spinowitz, B.; Rakov, V.; Walpen, S.; Floege, J. Effects of sucroferric oxyhydroxide and sevelamer carbonate on chronic kidney disease-mineral bone disorder parameters in dialysis patients. Nephrol. Dial. Transpl. 2019, 34, 1163–1170. [CrossRef]
- Covic, A.C.; Floege, J.; Ketteler, M.; Sprague, S.M.; Lisk, L.; Rakov, V.; Rastogi, A. Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide. Nephrol. Dial. Transpl. 2017, 32, 1330–1338.
- Lioulios, G.; Stangou, M.; Sarafidis, P.A.; Tsouchnikas, I.; Minasidis, I.; Vainas, A.; Faitatzidou, D.; Sampani, E.; Papagianni, A. Chronic therapy with sucroferric oxyhydroxide does not affect iron and anemia markers in dialysis patients. Blood Purif. 2020, 49. [CrossRef]
- Schiavi SC, Tang W, Bracken C, et al. Npt2b deletion attenuates hyperphosphatemia associated with CKD. J Am Soc Nephrol. 2012;23:1691–1700. [CrossRef]
- Müller D, Mehling H, Otto B, et al. Niacin lowers serum phosphate and increases HDL cholesterol in dialysis patients. Clin J Am Soc Nephrol 2007; 2:1249.
- Cheng SC, Young DO, Huang Y, et al. A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol 2008; 3:1131.
- Ix JH, Isakova T, Larive B, et al. Effects of Nicotinamide and Lanthanum Carbonate on Serum Phosphate and Fibroblast Growth Factor-23 in CKD: The COMBINE Trial. J Am Soc Nephrol 2019; 30:1096. [CrossRef]
- Ketteler M, Wiecek A, Rosenkranz AR, et al. Modified-release nicotinamide for the treatment of hyperphosphataemia in haemodialysis patients: 52-week efficacy and safety results of the phase 3 randomized controlled NOPHOS trial. Nephrol Dial Transplant 2023; 38:982.
- Gallant KMH, Sprague SM, Rosenbaum DP, Spiegel DM, Kozuka K, Edelstein S, Chertow GM. Tenapanor: A phosphate absorption inhibitor for the management of hyperphosphatemia in patients with kidney failure. J Renal Nutrition 35: 2025: pp 25-34. [CrossRef]
- Pergola PE, Rosenbaum DP, Yang Y, Chertow GM. A randomized trial of tenapanor and phosphate binders as a dual-mechanism treatment for hyperphosphatemia in patients on maintenance dialysis (AMPLIFY). J Am Soc Nephrol 32: 1465–1473, 2021. [CrossRef]
- https://ir.ardelyx.com/news-releases/news-release-details/fda-approves-xphozahr-tenapanor-first-class-phosphate-absorption#:~:text=FDA%20approval%20of%20XPHOZAH%20is,met%20their%20primary%20and%20key. Accessed March 24, 2025.
- Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R: Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol Jun 2009;4(6):1089-1096. [CrossRef]
- Nagano N, Ito K, Ono T, Ariyoshi Y, Masima S, Kobayashi H, Ando T, Tsutsui T, Ogawa T: Prescription characteristics of phosphate binders in a high pill burden for hemodialysis patients. Renal Replacement Therapy 2021/02/04 2021;7(1):5. [CrossRef]
- Forfang D, Edwards DP, Kalantar-Zadeh K: The Impact of Phosphorus Management Today on Quality of Life: Patient Perspectives. Kidney Med, 4: 100437, 2022. [CrossRef]
- Edmonston DL, Isakova T, Dember LM, Brunelli S, Young A, Brosch R, Beddhu Chakraborty H, Wolf M: Design and Rationale of HiLo: A Pragmatic, Randomized Trial of Phosphate Management for Patients on Maintenance Hemodialysis. Am J Kidney Dis, 2020 10.1053/j.ajkd.2020.10.008.
- Wald R, Walsh MW: In Search of the Optimal Target for Phosphate Control: Episode 1. J Am Soc Nephrol Mar 2021;32(3):526-528. [CrossRef]
- Greg LP, Hedayati SS. Management of Traditional Cardiovascular Risk Factors in CKD: What Are the Data? Am J Kidney Dis. 72(5): 728-744. 2018. [CrossRef]
| Dietary interventions | Comments |
|---|---|
| Limit processed meats, processed cheese and dairy products | Contain highly bioavailable (80-100%) inorganic phosphates |
| Choose fresh meat or fish without added phosphates | Contain organic phosphates of intermediate bioavailability (40-60%) |
| Choose plant based food (Like legumes, soy products, nuts, whole grains) | Contain organic phosphate complexed with phytates. Has low bioavailability (20-40%) |
| Be aware of hidden phosphates in prescription/ over-the counter medicines and supplements | May contain considerable amount of highly bioavailable (80-100%) inorganic phosphates |
| Reduction of intestinal phosphate absorption | |
| Phosphate Binders | Comments |
| Calcium acetate: Daily dose 1334-2001 mg) | High pill burden. Risk of calcium load and vascular calcification. Cheap |
| Calcium carbonate: Daily dose 1250-3750 mg) | High pill burden. Risk of calcium load and vascular calcification. Cheap |
| Sevelamer hydrochloride and sevelamer carbonate: Daily dose 2400-9600 mg | High pill burden. Gastrointestinal side effects. More expensive than Ca-based binders |
| Lanthanum carbonate: Daily dose 1500 mg Sucroferric oxyhydroxide: Daily dose 7.5-15 g Ferric citrate: Daily dose 630-1260 mg |
Low pill burden. Chewable tablets or powder preparations. Gastrointestinal side effects. No evidence of hepatotoxicity Low pill burden. Chewable. Low iron absorption Primary Gastrointestinal side effects. Low pill burden. High iron absorption. May help with anemia management. Primary Gastrointestinal side effects. Risk of aluminum toxicity as citrate increases absorption of aluminum |
| Intestinal phosphate transport inhibitors | Comments |
| Nicotinamide Tenapanor: Dose 10-30 mg twice a day |
Blocks small intestinal active transport of phosphate via NaPi-IIb. Limited efficacy and poor tolerance due to side effects (Diarrhea, pruritus, and thrombocytopenia). Not recommended for hyperphosphatemia management Inhibits small intestinal paracellular transport of phosphate by blocking sodium/hydrogen exchanger 3. Used as an add-on therapy with phosphate binders. The major adverse effect is an increase in stool frequency and Diarrhea |
| Removal of phosphate by Dialysis | |
| Dialysis modality | Comments |
| In-center hemodialysis 3-times a week | Inadequate in removing daily phosphate load. Patients need additional measures (Diet and phosphate lowering agents) to manage hyperphosphatemia |
| Short-daily or nocturnal hemodialysis | Much better in controlling hyperphosphatemia |
| Peritoneal dialysis | Inadequate in removing daily phosphate load. Patients need additional measures (Diet and phosphate lowering agents) to manage hyperphosphatemia |
| Sources of Phosphate | Nature of Phosphate | Bioavailability | Examples |
|---|---|---|---|
| Plant-based Foods | Organic phosphates Complexed with phytates |
20-40% | Nuts and seeds Legumes Whole grain Leafy greens Soy products |
| Animal-based Foods | Organic Phosphates | 40-60% | Chicken/Poultry Red meat Fish/ sea food Milk/ dairy products |
| Preservatives/Additives | Inorganic Phosphates | 80-100% | Soft drinks Processed foods Canned foods |
| Medicines/ supplements | Inorganic Phosphates | 80-100% | OTC* multivitamins/supplements Prescription Medicines |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





