1. Introduction
In Western nations, gastroesophageal reflux disease (GERD) is currently the most common acid-related sickness. Although over 10% of persons in Japan, Taiwan, and India suffer from GERD, it is now recognised as an emerging issue in Asian countries, where it was once thought to be an unusual ailment [
1]. Proton pump inhibitors, or PPIs, remain the mainstay of therapy for GERD. Nevertheless, 15% of patients have Los Angeles C and D (LA-C/D) grade esophagitis do not improve after eight weeks of continuous double-dose PPI treatment [
2]. Additionally, it is well known that utilising PPIs to provide complete symptomatic relief is more difficult when compared to sealing mucosal fissures, which is why around one-third of GERD patients was not happy with their current treatment plan [
1].
In addition to twice-daily PPI, it is recommended that nocturnal acid breakthrough (NAB) be treated with a histamine receptor antagonist (H2RA) prior to bed in order to maximize therapeutic efficacy [
3]. The effectiveness of the additional H2RA declined as a result of drug tolerance, whereas NAB was successfully suppressed in the short term. It has been demonstrated that a sodium alginate–antacid combination that partially closes the "acid pocket" that develops after meals reduces post-prandial acid reflux, which helps control heartburn throughout the day. Therefore, it should go without saying that NAB is unable to employ this tactic. The gamma aminobutyric acid (GABA-B) receptor agonist baclofen has been demonstrated to have some [
4].
Impact on reflux reduction by constricting the lower oesophageal sphincter (LES); however, adverse effects such as tiredness make it difficult to use [
4]. Compared to conventional lansoprazole, modified-release dex-lansoprazole and long-acting PPIs such as tenatoprazole provide superior 24-hour pH regulation. But even with better dose and metabolic profile, MR formulation hasn't completely eliminated GERD symptoms [
5]. PPI therapy patients can need more intrusive procedures, which emphasises the necessity for more potent GERD medications. Vonoprazan, a new P-CAB, was licensed in 2014 to treat Helicobacter pylori eradication, gastric ulcers, duodenal ulcers, and erosive esophagitis. Recent studies indicate its safety and effectiveness in treating GERD [
6].
2. Objective and Scope of the Review
The primary purpose of conducting a systematic review is to ascertain the efficiency, safety, and clinical outcomes associated with vonoprazan fumarate as a potential cure of GERD, which is a disease that affects the esophagus. This review will be of the randomized controlled trials (RCTs), cohort studies, and meta-analyses which explore the vonoprazan’s role in the management of GERD. They involve case studies evaluating various doses, treatment durations, and patient populations while taking into account the patients with erosive reflux disease, as well as the patients with non-erosive reflux disease. The review also discusses possible safety precautions for vonoprazan, drug interactions, and side effects, thus offering an in-depth analysis of vonoprazan as an alternative to the traditional acid-suppressive therapies.
2. Methodology
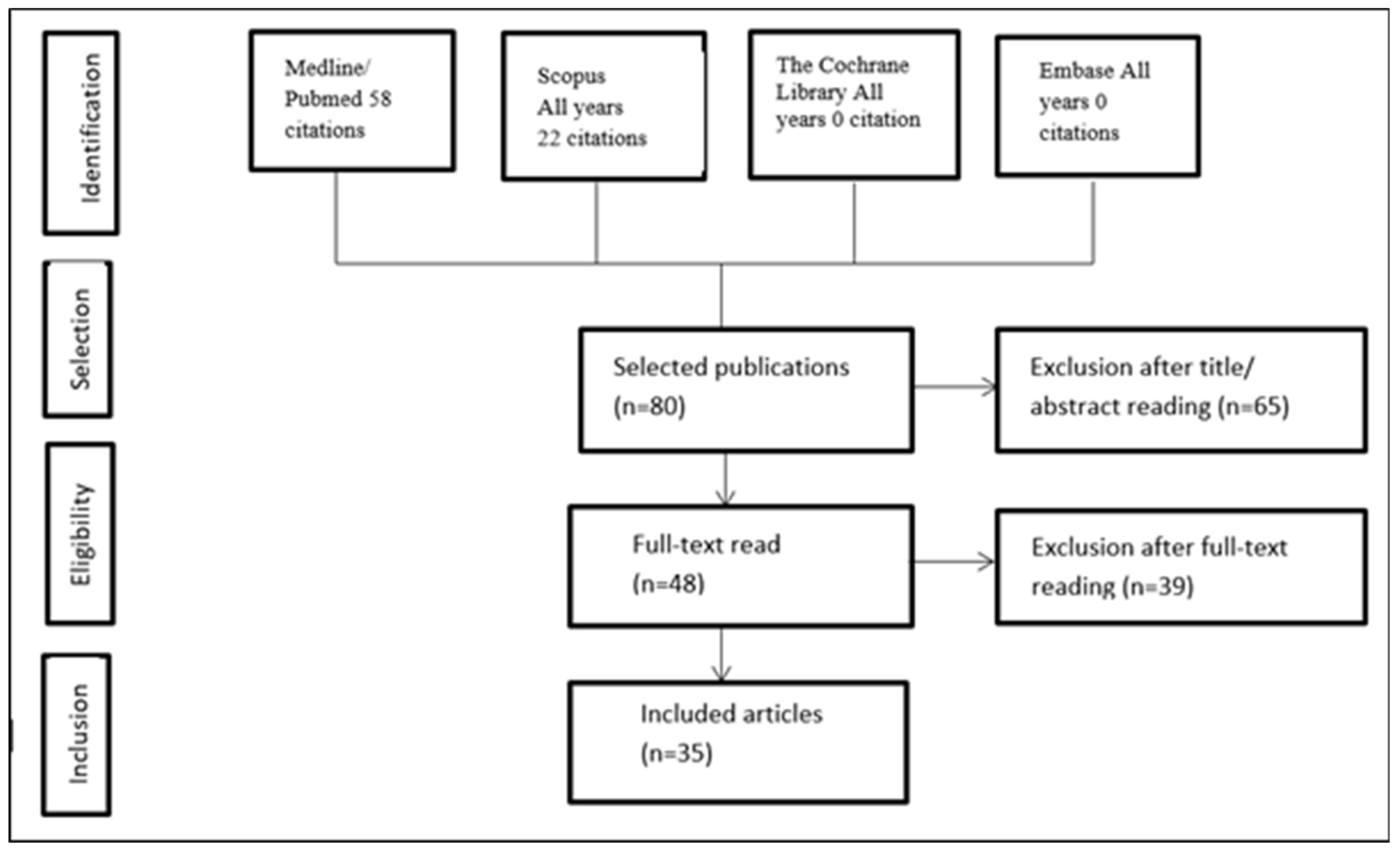
An exploration of literature was conducted on Medline-principally via electronic databases such as PubMed, Cochrane, Scopus, and Embase-through January 2024. Eighty reports were identified based on predefined criteria, which were analysed. Out of these, thirty-five were included in the current review. The automatic searching was then reinforced by a human evaluation of bibliography and a few abstracts of the selected articles.
2.1. Literature Search Strategy
This review includes studies from multiple reputable sources such as PubMed, Scopus, Web of Science, Cochrane Library, and Embase. The following keywords and phrases were used in various combinations to conduct the search:
"Vonoprazan fumarate in GERD treatment"
"Vonoprazan vs proton pump inhibitors"
"Potassium-competitive acid blockers in GERD"
"Acid suppression therapy with vonoprazan"
"Vonoprazan efficacy in reflux disease"
"Safety and tolerability of vonoprazan"
These search terms were selected to identify relevant studies evaluating the efficacy, safety, and comparative effectiveness of vonoprazan fumarate in the treatment of GERD. The search also aimed to include studies on its role in acid suppression, healing rates, symptom relief, and adverse event profiles.
Figure 1.
Search Concept Protocol.
Figure 1.
Search Concept Protocol.
2.2. Timeframe and Selection Criteria
Studies were selected based on predefined inclusion and exclusion criteria. Eligible studies included randomized controlled trials (RCTs), cohort studies, and meta-analyses evaluating the efficacy and safety of vonoprazan fumarate in GERD treatment. Only human studies published in English were considered. Exclusion criteria included case reports, editorials, conference abstracts, non-human studies, and articles unrelated to GERD. Studies comparing vonoprazan with proton pump inhibitors (PPIs) or other acid suppressants were prioritized.
2.3. Data Extraction
Studies that were selected were predetermined based on inclusion and exclusion criteria. RCTs, cohort studies, and meta-analyses of vonoprazan fumarate effectiveness and safety in hindering GERD were included in the eligible studies. Human studies which were only available in English were included. Case reports, editorials, conference abstracts, non-human studies, and articles not connected to GERD were the exclusion criteria. In addition, the trials that involved proton pump inhibitors or other acid suppressants were identified as priority studies.
2.4. Characteristics of Included Studies
The consisted of a mix of randomized controlled trials (RCTs) and cohort studies, which were mainly in Japan. A total of eight studies were examined, looking at the efficacy and safety of vonoprazan fumarate (20 mg) in the treatment of GERD, and comparing the different proton pump inhibitors (PPIs) such as lansoprazole, rabeprazole, and esomeprazole. The sample sizes had a big difference with the range of 19 to 1,435 patients. The durations of treatments also varied with a range of 1 to 8 weeks. The RCTs checked vonoprazan against the PPIs, while the cohort studies showed actual use and how effective it was. Miwa et al. (2011–2013) and Iwakiri et al. (2012–2013) did the long treatment trials (6–8 weeks), meanwhile, Nishizawa et al. (2002–2016), Sakurai et al. (2014–2015), and Mori et al. (2014–2016) concentrated on short-term therapy (1 week).
Table 1.
Included Studies on Vonoprazan Fumarate for GERD Treatment.
Table 1.
Included Studies on Vonoprazan Fumarate for GERD Treatment.
| AUTHORS |
COUNTRY |
STUDY DESIGN |
ENROLLMENT PERIOD |
DOSE OF VONOPRAZAN |
COMPARATOR (PPIs) |
NO.OF. PATIENTS INCLUDED (VONOPRAZAN/PPIs) |
DURATION OF TREATMENT |
| Nishizawa et al |
Japan |
Cohort |
2002.2-2016.6 |
20 mg |
Rabeprazole-10mg, Lansoprazole-20mg |
917 |
1 week |
| Iwakiri et al |
Japan |
RCT |
2012.8-2013.9 |
20 mg |
NA |
19 |
8 weeks |
| Ito et al |
Japan |
Cohort |
2014.6-2018.12 |
20 mg |
Lansoprazole, Rabeprazole |
1435 |
NA |
| Miwa et al |
Japan |
RCT |
2011.10-2013.2 |
20 mg |
Lansoprazole-30mg |
368 |
6 weeks |
| Miwa et al |
Japan |
RCT |
2011.11-2012.12 |
20 mg |
Lansoprazole-30mg |
482 |
8 weeks |
| Sakurai et al |
Japan |
RCT |
2016.5-2017.11 |
20 mg |
Esomeprazole-20mg |
60 |
4 weeks |
| Sakurai et al |
Japan |
Cohort |
2014.4-2015.12 |
20 mg |
Esomeprazole-20mg, Rabeprazole-10mg |
1353 |
1 week |
| Mori et al |
Japan |
Cohort |
2014.1-2016.12 |
20 mg |
Lansoprazole-30mg |
524 |
1 week |
On the whole, studies proved that 20 mg of vonoprazan were usually used and they had compared it with different PPI doses such as 10 mg rabeprazole, 20–30 mg lansoprazole, 20 mg esomeprazole. These studies give us helpful clinical information concerning the effectiveness of vonoprazan in the wrong-way movement of the stomach acid, namely, resolution rates, symptoms relief, and side effects in GERD treatment.
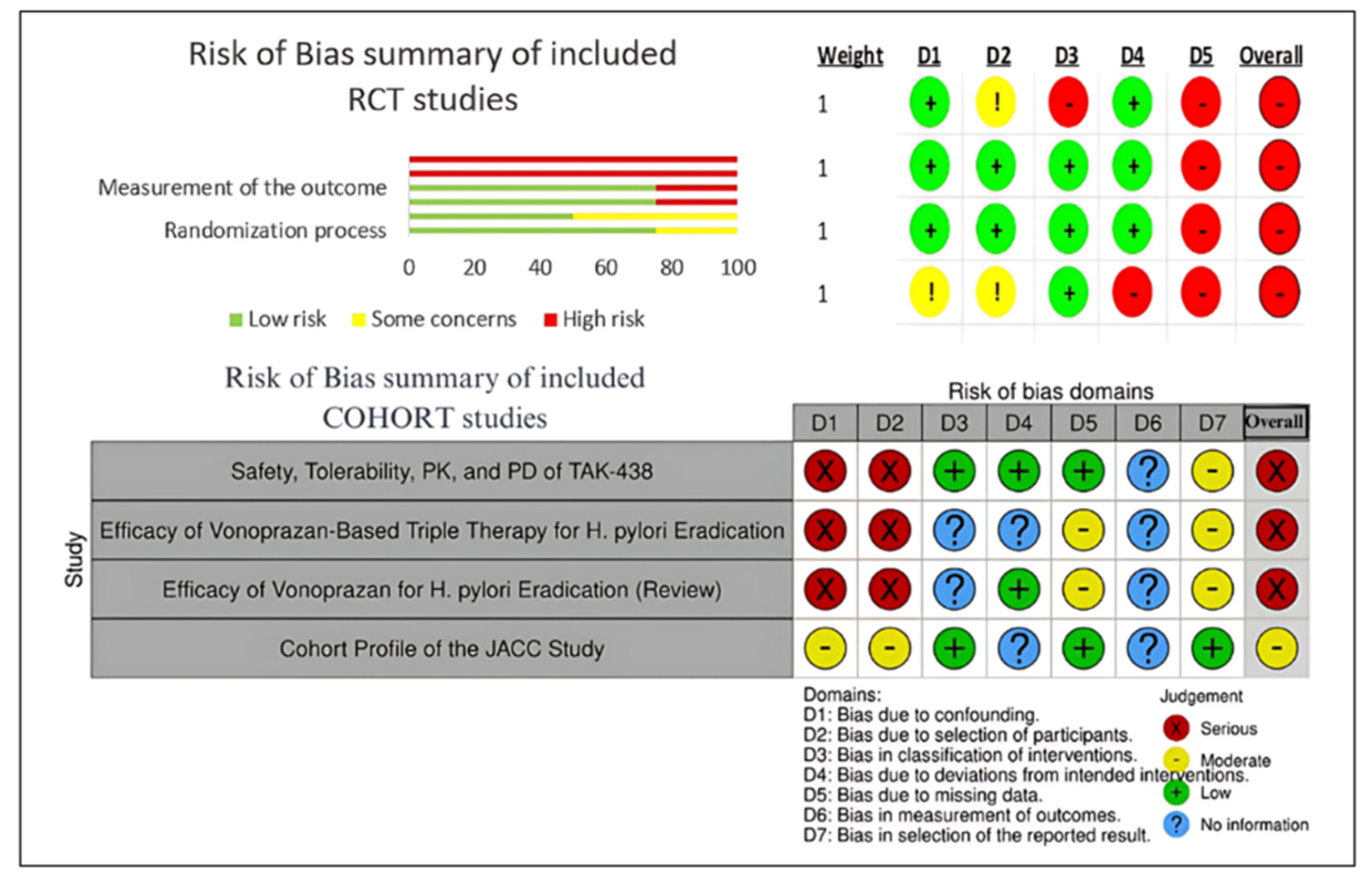
2.5. Risk Outcome of Included Trials
The studies that were picked were assessed for bias risk using the ROBINS-I tool 8 for cohort studies and the ROB 2 tool for randomized controlled trials (RCTs). The results reveal that all four RCTs are biasing with more of the errors spotted in various fields, in particular, in randomization and deviation from intended interventions. It is worth mentioning that non-concealed allocation was not done properly by all the RCTs, which led to a high risk of selection bias. On top of the aforementioned, unblinded outcome assessors were detected in three RCTs, hereby, adding to the detection bias. Due to multiple research studies lacking in the outcome data, attrition bias was counted as moderate to high. Three of the four cohort studies had a blue ribbon of bias because of confounding and the difficulties of classifying interventions. The JACC study was the only one that showed a moderate risk of bias since it tackled confounding factors more effectively and its cohort profile was clearer. In general, these findings imply that despite the useful information conveyed in these studies, their high and severe bias calls for the careful interpretation of the review's main points.
Figure 2.
Risk of Bias Assessment for RCT and COHORT Studies.
Figure 2.
Risk of Bias Assessment for RCT and COHORT Studies.
3. Results and Discussion
Vonoprazan fumarate has presented the ability to block gastric acid production by inhibiting the gastric proton pump in a reversible manner, with a higher pKa and better stability in acidic environments than previous potassium-competitive acid blockers (P-CABs) and proton pump inhibitors [
1].The efficacy of this antibiotic in the treatment of gastroesophageal reflux disease (GERD) has been investigated in a few case series on a limited scale, where it was used mainly as an adjuvant therapy in order to control symptoms, promote healing and maintenance therapy for GERD patients. In parallel, vonoprazan discovers many PPI restrictions and gives better results than the PPIs. For example, vonoprazan combines a better hydrogen-potassium ATPase (H, K-ATPase) inhibitory activity, acid stability, and favorable solubility and membrane permeability. The treatment of Helicobacter pylori infections with vonoprazan-based regimens revealed higher results and rates of eradication compared to the usually prescribed PPIs, which makes these targeted therapies a more efficient and novel choice in treating this pathogen. Studies on the safety of the oral vonoprazan fumarate dosages of 10 mg to 40 mg for seven days in healthy subjects show that these doses are well-tolerated. Nonetheless, GERD often results in severe complications like erosive esophagitis, strictures, and Barrett’s esophagus, especially in elderly males, thus making endoscopic investigation the top priority for severe esophagitis cases .Entirely, vonoprazan fumarate is a very good example of a significant goal in the field of treatment of acid-related disorders since it ensures the superiority of the acid suppression, increased H. pylori eradication rates, and efficient symptom control in GERD patients [
3]
-[
6].
3.1. Pharmacological Action of Vonoprazan
The combination of vonoprazan and amoxicillin with clarithromycin is preferred for curing adult Helicobacter pylori (H. pylori) infections. Other options for adult H. pylori infection treatment are co-packaged medications containing monodrama and amoxicillin as well. It is also recommended for the treatment of adult H. pylori infection to take an additional co-packaged drug that contains just monodrama and amoxicillin [
7].
3.2. Pharmacodynamics Role of Vonoprazan
When vonoprazan is given, the intra-gastric pH soars more and more. If vonoprazan is regularly taken, its inhibitory effect on acid stashing is amplified (i.e., the inhibitory effect increases). After discontinuing vonoprazan, the anti-secretory effect becomes less, whereas the pH of the stomach remains high for around a day or two. In the clinic, vonoprazan and QT prolongation look the same and do not have any differences. While vonoprazan has a higher pK value (a pka of 9.06) than other potassium-competitive acid blockers, what makes it unique is that it has a higher point-positive charge. Therefore, the drugs can accumulate more in the stomach parietal cells' canalicular area (the binding of drugs H and K-ATPase in a competitive and reversible way) during this period. As a result, it inhibits H, K-ATPase, better than others. This occurs with drugs such as SCH28080 and proton-pump inhibitors such as lansoprazole too.Vonoprazan is a PCAB that blocks the potassium-competitive H, K-ATPase enzyme system. By inhibiting the secretory face of stomach parietal cells using this medium, vonoprazan prevents eager and assured gastric acid holding. Although H, K-ATPase, PPIs, and PCABs are all members of the family of drugs that are antagonized, but the drug families affect them in various ways. Whereas PPIs form a covalent disulphide bond with an enzyme's cysteine residue, thereby rendering the latter inactive, PCABs do not allow K to attach to the H, K-ATPase [
8].
3.3. Pharmacokinetics Role of Vonoprazan
Vonoprazan is absorbed quickly and undergoes maximum plasma concentration in the blood within 1.5 to 2.0 hours after oral administration. Food has a slight influence on its digestive tract absorption in animal studies, nevertheless, its bioavailability in humans by mouth still is unclear. Vonoprazan, in healthy individuals, is about 80% bound to plasma proteins.After a single 20 mg dose, vonoprazan’s apparent oral volume of distribution was 1001 liters, and its steady-state volume of distribution was about 782.7 liters. In people with no health problems, vonoprazan plasma protein links at 85-88% and this protein binding is constant and the different concentration levels do not affect the linking from 0.1 to 10 micrograms per milliliter.Vonoprazan is mainly metabolized by various cytochrome P450 (CYP) enzymes, particularly CYP3A4, while CYP3A5, CYP2C9, CYP2B6, CYP2C19, and CYP2D6 have lesser roles. Furthermore, the metabolism of vonoprazan takes place through sulfotransferases and glucuronyl transferases, however, they have no metabolites that possess the pharmacological activity. The CYP3A4 enzyme converts vonoprazan to metabolites M-I and M-II, and these are then still further bound with glucuronic acid to form M-I-G and M-II-G. Vonoprazan, on the other hand, is less dependent on CYP2C19 polymorphism compared to proton pump inhibitors in terms of drug pharmacokinetics [
9,
10].
3.4. Comparison Vonoprazan with Other Drugs
A variety of studies have set off vonoprazan fumarate versus other proton pump inhibitors (PPIs) in the treatment of acid-related disorders. In a research comparing the clinical effects of vonoprazan fumarate and omeprazole in the treatment of esophagitis, vonoprazan fumarate proved to be the most efficient drug in influencing mucosal healing and relief of symptoms. Likewise, studies assessing the drug's efficacy against lansoprazole GERD found that vonoprazan fumarate outperformed by providing better acid suppression and sped up the gastric mucosal healing.In the case of esophagitis treatment, vonoprazan fumarate caused a dramatic improvement with 24 hours of esophageal pH level and a decrease in the inflammatory products without an increase in the risk of drug allergic reactions. A study on the comparison between vonoprazan fumarate and esomeprazole in patients with chronic gastritis showed that overall they were almost the same in terms of efficacy. Although the drug-substance of vonoprazan fumarate did bring about slightly fewer after effects, both drugs were found to be equally well tolerated. These results infer that vonoprazan fumarate is competent traditional PPIs ant that it is able to provide a more advantageous acid suppression now and in the treatment of GERD and esophagitis [
11,
12].
3.5. Eradication Rate of H. pylori Using Vonoprazan Fumarate
H. pylori eradication treatment regimen should be started with either a proton pump inhibitor (PPI) or vonoprazan along with amoxicillin and clarithromycin (PPI-AC) as a first-line therapy. After that, it could be followed by a PPI or vonoprazan and amoxicillin and metronidazole (PPI-AM or VAM) as a second-line treatment. As vonoprazan is the mostly manufactured drug in Japan, hence it is mainly studied in that part of the world, the focus of most of the resources is on these three-drug therapy combinations [
13,
14].
3.6. First-Line Eradication: Efficacy of Vonoprazan Fumarate
A multicenter, double-blind, randomized, parallel-group study was performed to evaluate the efficacy of vonoprazan-based and PPI-based regiments in patients' stomach ulcers infected with H. pylori. The study compared a lansoprazazole-based regimen (30 mg lansoprazole + 750 mg amoxicillin + 200 mg either 400 mg clarithromycin bid for seven days) with a vonoprazan-based regimen (20 mg vonoprazan + 750 mg amoxicillin). The eradication rates were 92.6% for lansoprazole and 75.9% for VAC therapy, respectively. It is shown that both the regiments were successful in the treatment of the H. pylori infection.Most studies indicate that vonoprazan achieves higher eradication rates than PPIs. More randomized controlled trials (RCTs) and meta-analyses should be performed to finally approve potassium-competitive acid blockers (PCABs) for being the primary drugs in the process of H. pylori elimination. Notably, VAC therapy showed the highest success rate when used to treat clarithromycin-resistant H. pylori strains. Different from PPIs which are mainly metabolized by CYP2C19, vonoprazan is mainly metabolized by CYP3A4/5, thus ensuring consistent acid suppression in all patients without inter-individual variability.A systematic review and meta-analysis found no significant difference in the incidence of adverse events in both vonoprazan- and PPI-based triple therapies. However, the non-randomized controlled studies have reported higher adverse event rates in vonoprazan-treated patients [
15].
3.7. Second-Line Eradication: Efficacy of Vonoprazan Fumarate
In Japan, the first alternative to standard second-line H. pylori eradication treatment is a combination of vonoprazan or PPI, amoxicillin, and metronidazole (VAM or PPI-AM). A study in which the two regimens were tested found no difference between them in their ability to eradicate bacteria. Because metronidazole is a DNA-specific antibiotic, it is not affected when vonoprazan, which inhibits gastric acid production, is also taken. There were adverse reactions in 0–30% of the VAM-containing regimen group of participants, with diarrhea being the most common of them. Several other studies have also reported no marked differences in patients with VAM vs. PPI-AM therapy [
16].
3.8. Third-Line Eradication: Efficacy of Vonoprazan Fumarate
At present a randomized trial is in progress to evaluate the effectiveness of vonoprazan- and PPI-based seven-day triple regimens that include amoxicillin and ciprofloxacin as a third-line treatment. With respect to the metronidazole- and clarithromycin-based therapies, the development of H. pylori infections resistant to them is the target that the new regimen is currently being investigated. According to the results of the trial, vonoprazan-based regimen led to a higher eradication rate than the PPI-AS regimen. Nevertheless, the ITT analyses displayed no statistically significant differences between the two groups. The study's findings are, purprisingly, not covered by Japan's national health insurance program as the fact is that third-line therapy is not listed in the program [
17].
3.9. Uses of Vonoprazan Fumarate
Vonoprazan fumarate is utilized for waiver of various acid linked to the problems, including the erosive esophagitis which influences the esophagus. Moreover, it may be used in combination with other drugs for the treatment of the ailments such as gastrointestinal infection caused by the Helicobacter pylori (H. pylori), the bacterium which causes stomach and intestinal ulcers, and the irritation of the stomach lining. Being an acid blockage agent, Vonoprazan fumarate helps by reducing the excessive stomach juice, facilitating gastric ulcer healing, and relieving the main disadvantaging symptoms like heartburn and stomach pain [
18].
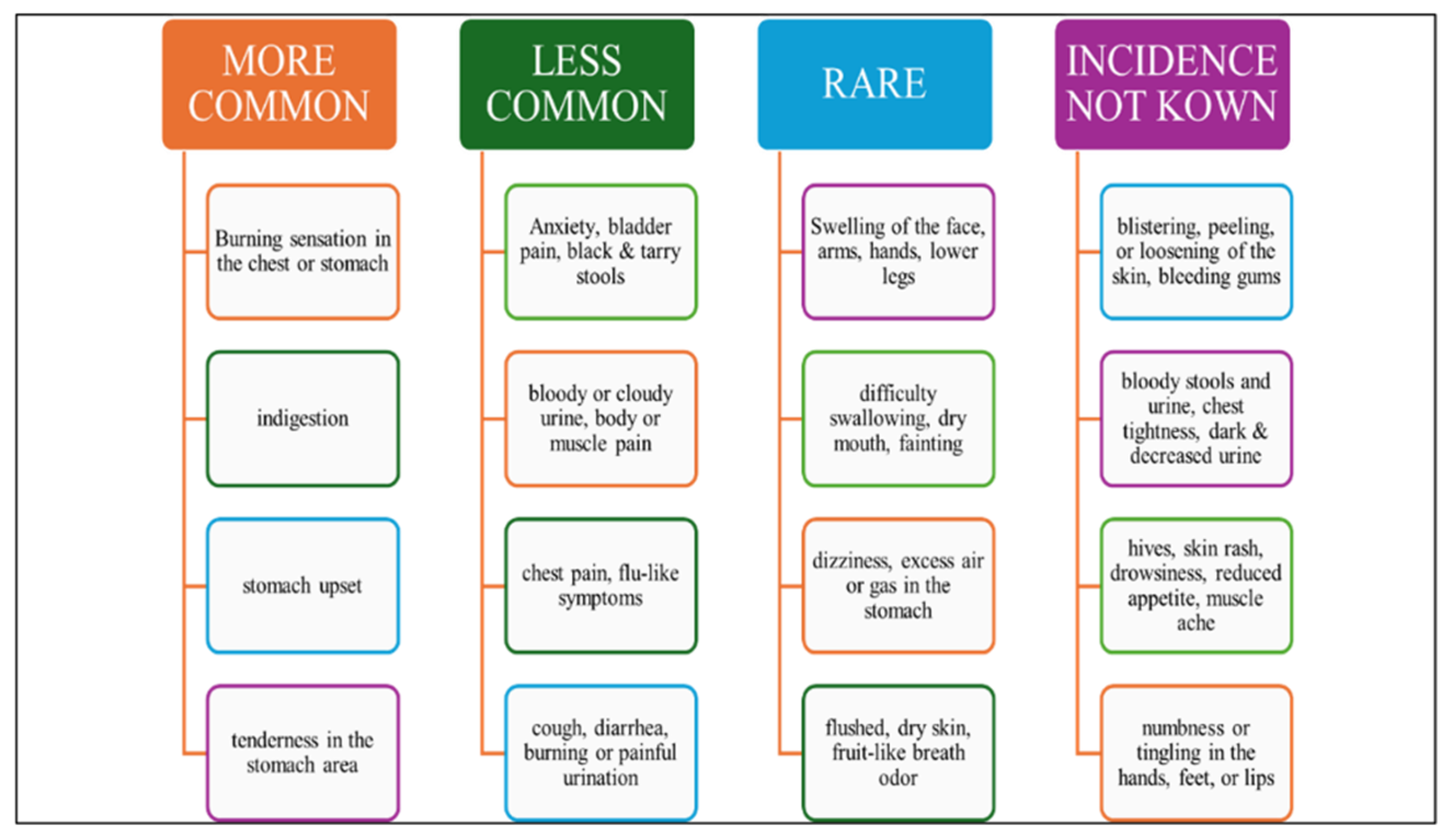
3.10. Adverse Reaction of Vonoprazan Fumarate:
Some of the commonly reported adverse events associated with vonoprazan and proton pump inhibitors (PPIs) include diarrhea, dizziness, cough, stomach pain, fatigue, hypersensitivity reactions, and white or brownish vaginal discharge. Chronic use of PPIs has been linked to several adverse effects due to underlying physiological changes. Hypochlorhydria can increase the risk of pneumonia, Clostridium difficile infection, spontaneous bacterial peritonitis, and excessive small intestinal bacterial proliferation. Hypergastrinemia, a consequence of prolonged acid suppression, may lead to gastric endocrine hyperplasia and carcinoid tumors. Malabsorption-related complications include iron deficiency anemia, osteoporosis, hypocalcemia, hypomagnesemia, fractures, and vitamin B12 deficiency. Other potential risks associated with long-term PPI use include tubulointerstitial nephritis, collagenous and lymphocytic colitis, myocardial infarction (MI), and dementia [
19,
20].
Figure 3.
Various Side Effects Caused by Vonoprazan Fumarate.
Figure 3.
Various Side Effects Caused by Vonoprazan Fumarate.
3.11. Toxicity Associated with Vonoprazan
There has been no reports about overdoses by Vonoprazan. The substance did not induce any unpleasant symptoms during the clinical trial in which patients took 120 mg single doses of vonoprazan. Haemodialysis is not an adequate method for the removal of vonoprazan from the body. Voquezna Dual Pak and Voquezna Triple Pak, as per the FDA label, both suggest symptomatic care in cases of an overdose. The vonoprazan mutagenicity test, which is animal-based, has no positive outcomes. The fertility and reproductive ability were not influenced by the oral doses of vonoprazan rat was treated with 300 mg/kg/day (133 times the highest human dose recommended). Oral dosages of vonoprazan of 6, 20, 60, and 200 mg/kg/day (0.04, 4.19, and 93 times the maximal recommended human dose) caused the occurrence of gastropathy, neuroendocrine cell hyperplasia, and benign or malignant neuroendocrine cell tumors (carcinoids) in the stomach of the mice [
20,
21,
23].
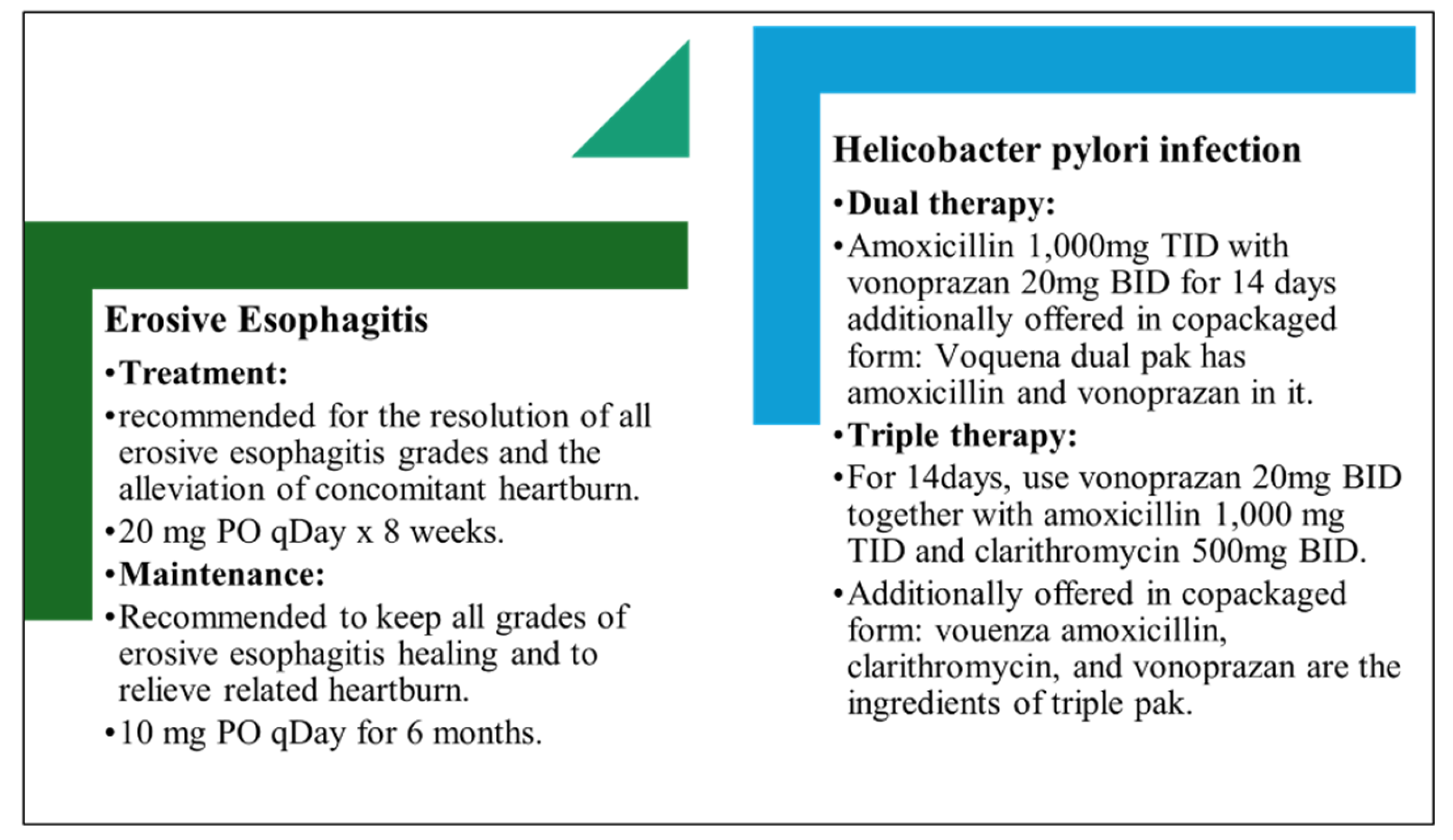
3.12. Dosage Regimen of Vonoprazan
Vonoprazan is typically given by mouth at a dose of 20 mg, once a day, to heal gastroduodenal ulcers. To eliminate and prevent reflux esophagitis, the drug is also used in 10 mg and 20 mg doses once a day. On the other hand, a daily dose of 10 mg is adopted for the treatment and prevention of peptic ulcers resulting from low-dose aspirin or non-steroidal anti-inflammatory drugs (NSAIDs). In a combination treatment with vonoprazan plus amoxicillin and clarithromycin, 20 mg of vonoprazan is given twice daily to get rid of H. pylori. You can obtain 10 mg or 20 mg tablets [
24,
26,
27].
Figure 4.
Dosage Recommendations for Treatment.
Figure 4.
Dosage Recommendations for Treatment.
3.13. Drug Interaction of Vonoprazan
Drug interactions can have serious potential harm or change the working of the medications. It is very important to keep a written down list of all the medications you are taking like prescription drugs, over-the-counter medications and herbal supplements and give it to your doctor or pharmacist. Always take advice from your doctor before starting, stopping, or making any change in the dose. The way the body excretes vonoprazan may be changed by other drugs that affect the process such as rifamycin. Apart from that, vonoprazan decreases stomach acid production, which might hinder the absorption of some drugs that are dependent on stomach acid for their proper absorption.Vonoprazan has been reported to have a number of drug interactions. Honestin increases the concentration of vonoprazan in the blood stream, while lumetison goes the other way by slowing its metabolism. Vonoprazan affects the absorption of rilpivirine in the stomach and thus, the latter’s effectiveness is decreased. The difference in the metabolism of CYP3A4 enzymes in the stomach or the liver can be changed by bosentan, this in turn can decrease the whole concentration and efficacy of vonoprazan. On the other hand, ergotamine causes an increase its levels or effects by modifying the CYP3A4 enzyme metabolism.Using these examples, on one hand, vonoprazan’s absorption could be slowed down by consuming meals that are high in fat. However, these changes are considered to be not clinically relevant. As with drug-disease interactions, people who are hypersensitive to any product excipient, including vonoprazan fumarate, are prohibited from its use. It is important to understand the interactions so that the drugs are used correctly and safely [
28,
29,
30,
31].
3.13. Cautions of Vonaprazan
Once the diagnosis of acute tubulointerstitial nephritis is confirmed, vonoprazan must be immediately stopped, and the patient should be thoroughly assessed. Besides Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), which are painful skin conditions, also reported were the serious cutaneous adverse reaction of this drug. If there are any signs of hypersensitivity or any severe cutaneous adverse reactions, then stop the medication and get more tests done. A Bloating of stomach and a Decrease or absence of stomach acid caused by long-term acid-suppressing medications, such as vonoprazan intake, can lead to vitamin B12 malabsorption. These medications have been available since then, so besides it, there are few reported cases of vitamins deficiencies; and patients should have the B12 tests done if any of the clinical symptoms show a deficiency.In patients suspected to have a cancerous (malignant) or if cancer is already known to be present in the stomach, vonoprazan's curing of symptoms does not guarantee the absence of stomach cancer. In patients who do not show improvement to treatment or have an early symptomatic relapse, it is better to do a follow-up, along with other diagnostic tests, including endoscopy, especially in geriatrics.Along with other proton pump inhibitors (PPIs) vonoprazan may lead to the occurrence of Clostridium difficile-associated diarrhea (CDAD) in the hospital setting. If you have continuing diarrhea, be prepared to have CDAD investigated too. Use vonoprazan for the shortest duration you can get by and practitioners should be cautious of how the medication is used.Regular use of PPIs is connected with an augmented fracture possibility mainly in the hip, wrist, or spine area related to osteoporosis. Long-lasting therapy, at particularly high doses, could be the reason for fractures. Also, vonoprazan has been found to be a risk factor for fractures, and thus its usage should be restricted to the shortest treatment period only with special care of patients having osteoporosis-related breaks, thereby following the standard treatment protocols. Hypomagnesemia has been confirmed in patients receiving long-term PPI treatment, which includes vonoprazan. Patients under extended therapy or needing other medications such as digoxin, which is likely to become toxic due to hypomagnesemia, should check out their magnesium levels both before and during the treatment. The need for a vonoprazan discontinuation and magnesium supplementation to treatment due to hypomagnesemia is a possible solution if hypomagnesemia is detected [
32,
33,
34,
35].
5. Conclusions
Vonoprazan is a new medicine that is currently under development for the treatment of reflux and eradication of Helicobacter pylori. The new anti-secretory effect along with a quick-acting and diminished anti-secretory variability are the reasons on its use instead of proton pump inhibitors (PPIs). The studies have come up with a finding that the efficacy of the vonoprazane fumarate in the mucosal healing process and symptomatic relief was superior to the omeprazole product. It is also important to say that it is more effective because it cures gastric mucosa by suppressing the action of gastric acid secretion of the drug. Twenty-four hours oesophageal PH is subsequently raised, resulting in the effective treatment of esophagitis by Vonoprazan tablets. Nevertheless, it was not found in the cases of overdose, and the FDA tag encourages only symptomatically supportive measures. The animal mutagenicity test revealed that no such positive findings have been detected yet. Vonoprazan is a type of long-term intake of NSAID medications that has already been demonstrated as a better treatment for the stomach ulcers caused by PUD and ESD in the H. pylori-positive individuals. It is also suggested for the treatment of artificial ulcers generated after the ESD. On the other hand, the research on the topic of vonoprazan is limited to Japan. Therefore, clinical trials are strongly recommended in prospective, randomized studies. Amlost all forms of artificial ulcers can be treated through surgical intervention; some of those ulcers do not replate only with medications. In addition, research on vonoprazan is limited to Japan, so further clinical research is needed to confirm its efficacy, especially in Japan.
Author Contributions
Kiran Khanna contributed to manuscript writing, results interpretation, and discussion. Lisiya S was responsible for data collection and compilation. Rahul Kannan M contributed to data collection, results analysis, and discussion. Karthik Thiyagarajan performed the risk of bias assessment and contributed to manuscript writing.
Ethics approval
Not applicable, as this study is a systematic review and does not involve human or animal subjects.
Conflicts of Interest
none to declare.
References
- Martinucci I, Blandizzi C, Bodini G, Marabotto E, Savarino V, Marchi S, et al. Vonoprazan fumarate for the management of acid-related diseases. Expert Opin Pharmacother. 2017, 18, 1145–52. [Google Scholar]
- Echizen, H. The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: pharmacokinetic and pharmacodynamic considerations. Clin Pharmacokinet. 2016, 55, 409–18. [Google Scholar] [PubMed]
- Sugano, K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: safety and clinical evidence to date. Ther Adv Gastroenterol. 2018, 11, 1756283X17745776. [Google Scholar]
- Otake K, Sakurai Y, Nishida H, Fukui H, Tagawa Y, Yamasaki H, et al. Characteristics of the novel potassium-competitive acid blocker vonoprazan fumarate (TAK-438). Adv Ther. 2016, 33, 1140–57. [Google Scholar]
- Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983, 1, 1273–5. [Google Scholar]
- Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch’s postulates for pyloric Campylobacter. Med J Aust. 1985, 142, 436–9. [Google Scholar]
- Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987, 82, 192–9. [Google Scholar]
- Asaka M, Kato M, Kudo M, et al. Atrophic changes of gastric mucosa are caused by Helicobacter pylori infection rather than aging: studies in asymptomatic Japanese adults. Helicobacter. 1996, 1, 52–6. [Google Scholar] [CrossRef]
- Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. The long-term impact of Helicobacter pylori eradication on gastric histology: a systematic review and meta-analysis. Helicobacter. 2007, 12, 32–38.
- Ford AC, Gurusamy KS, Delaney B, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive people. Cochrane Database Syst Rev. 2016, 4, CD003840. [Google Scholar] [CrossRef]
- Nakamura S, Sugiyama T, Matsumoto T, et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012, 61, 507–13. [Google Scholar]
- Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014, 348, g3174.
- Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008, 372, 392–7. [Google Scholar]
- Yoon SB, Park JM, Lim CH, Cho YK, Choi MG. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: a meta-analysis. Helicobacter. 2014, 19, 243–8. [Google Scholar]
- Satake M, Nishikawa J, Fukagawa Y, et al. The long-term efficacy of Helicobacter pylori eradication therapy in patients with idiopathic thrombocytopenic purpura. J Gastroenterol Hepatol. 2007, 22, 2233–7. [Google Scholar]
- Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010, 15, 1–20. [Google Scholar]
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut. 2017, 66, 6–30. [Google Scholar]
- Smith SM, O’Morain C, McNamara D. Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World J Gastroenterol. 2014, 20, 9912–21. [Google Scholar]
- Mori Y, Imanishi A, Matsukawa J, et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther. 2010, 335, 231–8. [Google Scholar]
- Otake K, Sakurai Y, Nishida H, Fukui H, Tagawa Y, Yamasaki H, et al. Characteristics of the novel potassium-competitive acid blocker vonoprazan fumarate (TAK-438). Adv Ther. 2016, 33, 1140–57. [Google Scholar] [CrossRef]
- Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018, 67, 430–40. [Google Scholar]
- Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017, 390, 2383–96. [Google Scholar]
- El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014, 63, 871–80. [Google Scholar]
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013, 108, 308–28. [Google Scholar]
- Fass, R. Proton pump inhibitor failure—what are the therapeutic options? Am J Gastroenterol. 2009, 104 (Suppl 2), S33–8. [Google Scholar] [PubMed]
- Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep. 2010, 12, 448–57. [Google Scholar]
- Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects—a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015, 42, 719–28. [Google Scholar]
- Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015, 41, 636–48. [Google Scholar]
- Miwa H, Uedo N, Watari J, et al. Randomised clinical trial: efficacy and safety of vonoprazan vs. lansoprazole in patients with gastric or duodenal ulcers—results from two phase 3, non-inferiority randomised controlled trials. Aliment Pharmacol Ther. 2017, 45, 240–52. [Google Scholar]
- Iwakiri K, Sakurai Y, Shiino M, et al. A randomized, double-blind study to evaluate the acid-inhibitory effect of vonoprazan (20 mg and 40 mg) in patients with proton-pump inhibitor-resistant erosive esophagitis. Ther Adv Gastroenterol. 2017, 10, 439–51. [Google Scholar]
- Shin JM, Sachs G. Differences in binding properties of two proton pump inhibitors on the gastric H+,K+-ATPase in vivo. Biochem Pharmacol. 2004, 68, 2117–27. [Google Scholar]
- Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013, 12, CD008337. [Google Scholar]
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017, 152, 706–15. [Google Scholar] [CrossRef]
- Vakil N, Van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006, 101, 1900–20. [Google Scholar]
- Shaw MJ, Talley NJ, Beebe TJ, et al. Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol. 2001, 96, 52–7. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).