Submitted:
20 March 2025
Posted:
21 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
- a)
- intrinsic resistance of chitin is so high that in fact it was used as a dielectric material in capacitors before [11] and
- b)
- the low levels of “embedded” redox-active species (Fe, Ti, quinones, Cu) seen in (native, purified) chitin will not produce any electrochemical signal, probably for the same reasons. This does hold even when chitin samples were dissolved in dimethyl formamide containing Li salts like LiClO4. In terms of electrochemistry this solution is silent unless further ions are added [12] right from the DMF solution. The potential window which can be studied is very large, ranging from < - 2.5 vs SCE (e.g., TmII/III) up to the limits of anodic stability of the DMF solvent near some 1.7 V vs. SCE (chitin itself is much harder to oxidize both at an anode and by even very strong oxidants like Pb(IV) acetate, chromate, periodate [no reaction either unless for previous hydrolysis providing partly chitosan] or RuO4, MnO4-).
- c)
- There is an equivalent solvent (although viscous) which can be used down to almost the same potentials while providing a co-ordinative environment very similar to that in chitin which is why it was used in this work to simulate binding to chitin. This solvent is N-acetyl ethanolamine (N-AEA) [5]
- a)
- Replacing electrodes placed on one mobile ribbon or
- b)
- Replacing the electrolyte when separating the cations from freshwater, mine tailing waters or the like by passing through one cation exchanger, or
- c)
- adding selective ligands.
2. Materials and Methods
General Remarks on Materials, Setup
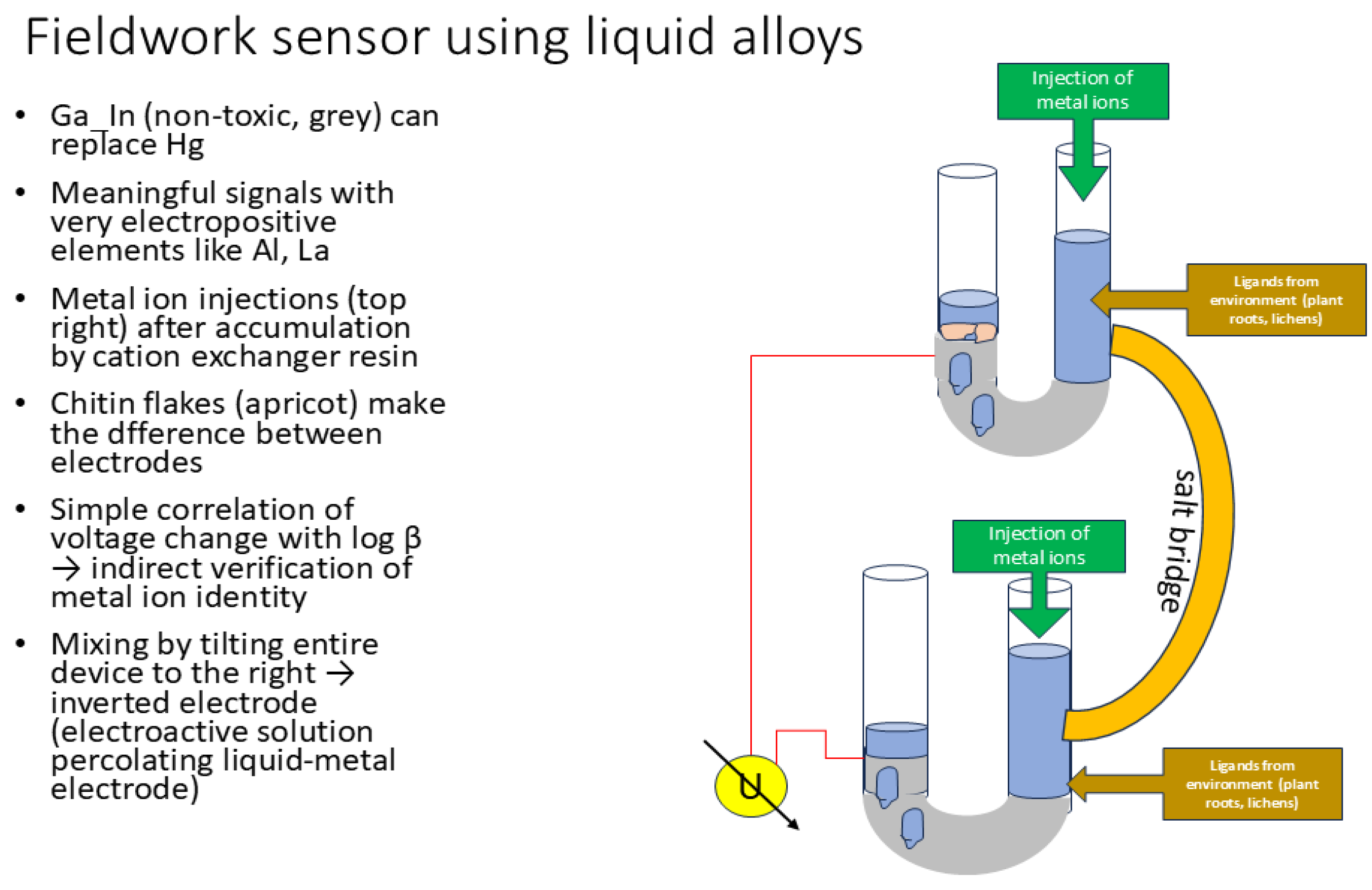
Setup
Preparation of Electrodes
| Required material | Formula | Source | Remarks, Purposes |
| Lanthanum nitrate | La(NO3)3 | Merck | |
| Cerium nitrate | Ce(NO3)3 | Sigma-Aldrich | Trivalent cerium, undergoes air oxidation on dry chitin; reversed when wet |
| Europium(III) triflate | Eu(CF3SO3)3 | Sigma-Aldrich | Used to identify response/possible perturbations of electrochemical signals by Eu-mediated photochemistry making radicals from ligands, other organics or changing oxidation state of dissolved ions bridging “bare” and chitin-covered electrodes |
| Liquid Ga_In alloy | Merck/Goodfellow | Eutectic mixture; by weight 75.5% Ga, 24.5% In | |
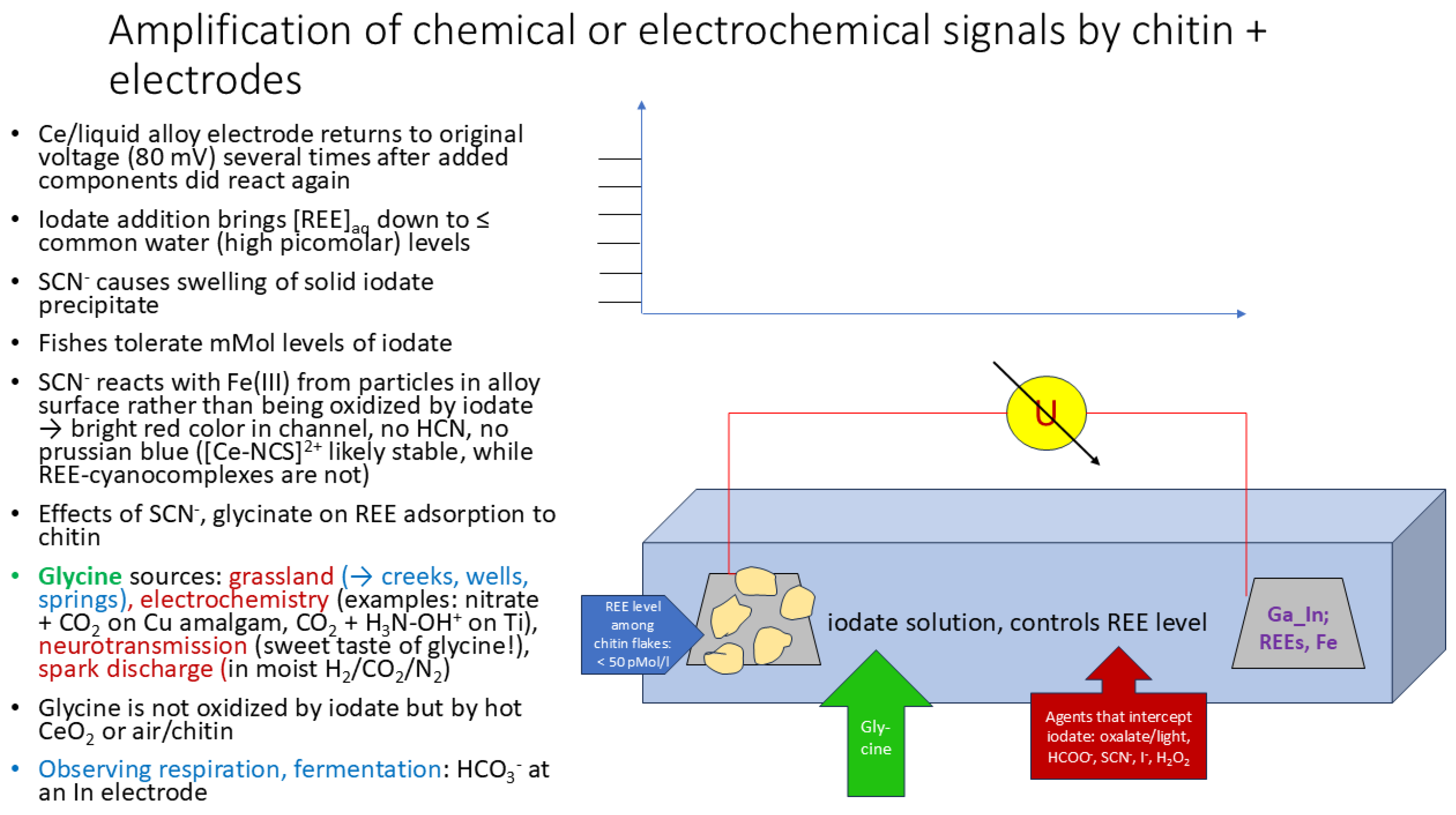
| Auer flintstone | approximate composition: main components are Ce, La, Fe, and Mg | Local retailer | Surroundings of Ga_In + Auer stone alloy gets bright red several hours after SCN- was added, oxidation of thiocyanate occurring only later |
| La metal | |||
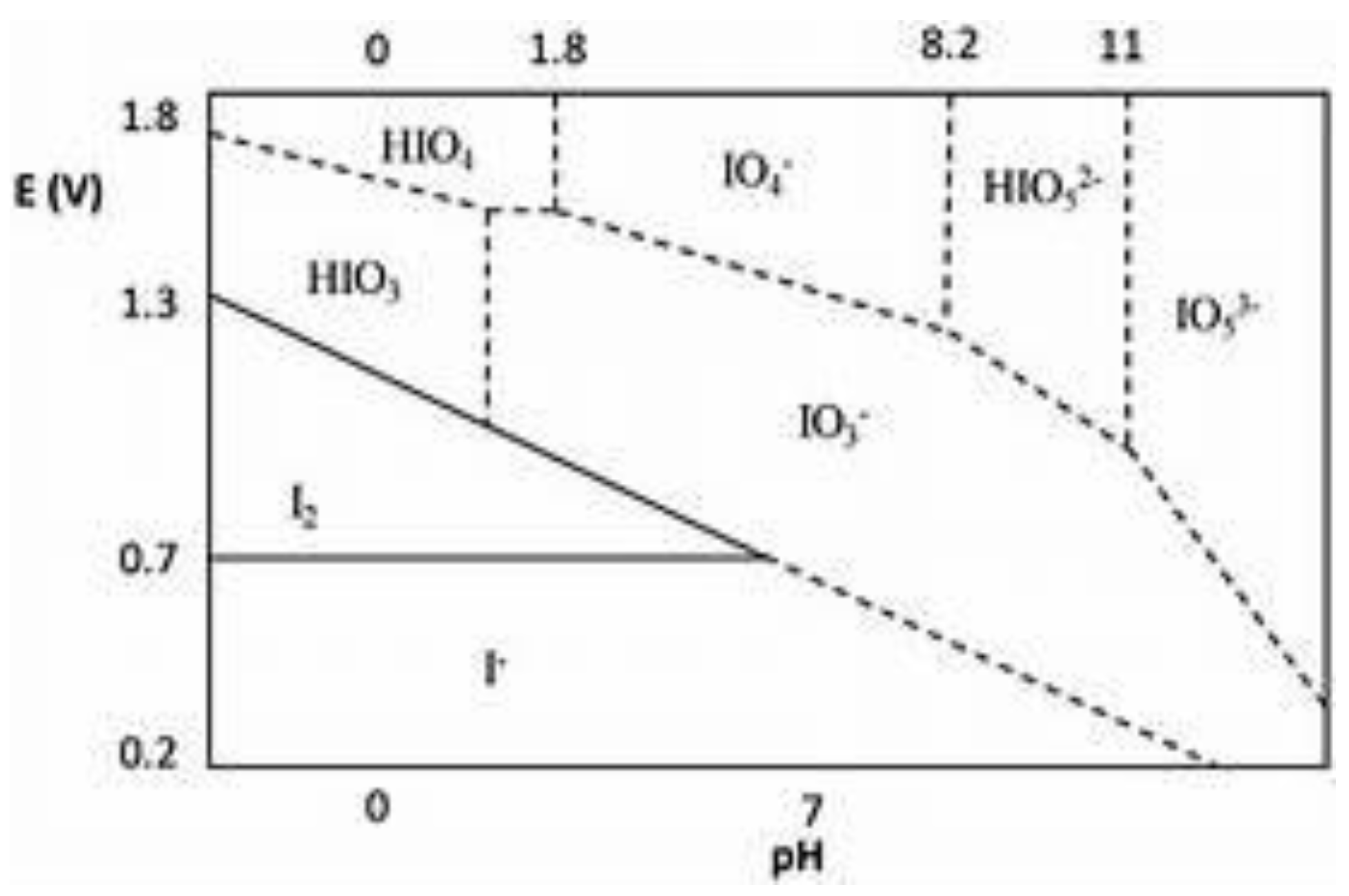
| Na iodate | NaIO3 | Sigma-Aldrich | does react with thiocyanate, formate and other reductants [24] |
| La iodate | La(IO3)3 | Prepared in lab by wet reaction | |
| Ce iodate | Ce(IO3)3 | same | |
| Na bicarbonate | NaHCO3 | Source of carbonate ions complexing all Ga, In [28], or REYs ≠ Y, Tm…Lu [29], added ion simulates respiration of arthropods or fungi introducing chitin into the system | |
| K thiocyanate | KSCN | ||
| glycine | H2N-CH2-COOH | Laborchemie Apolda | |
| Protease enzyme (papain etc.) | Raw, freshly-cut papaya slice; flesh and pressed juice taken as such | Local food retailer, papaya (ripe, yellow skin) from Brazil | |
| chitin | Purified, protein-free | For residual metal contents see [21] | |
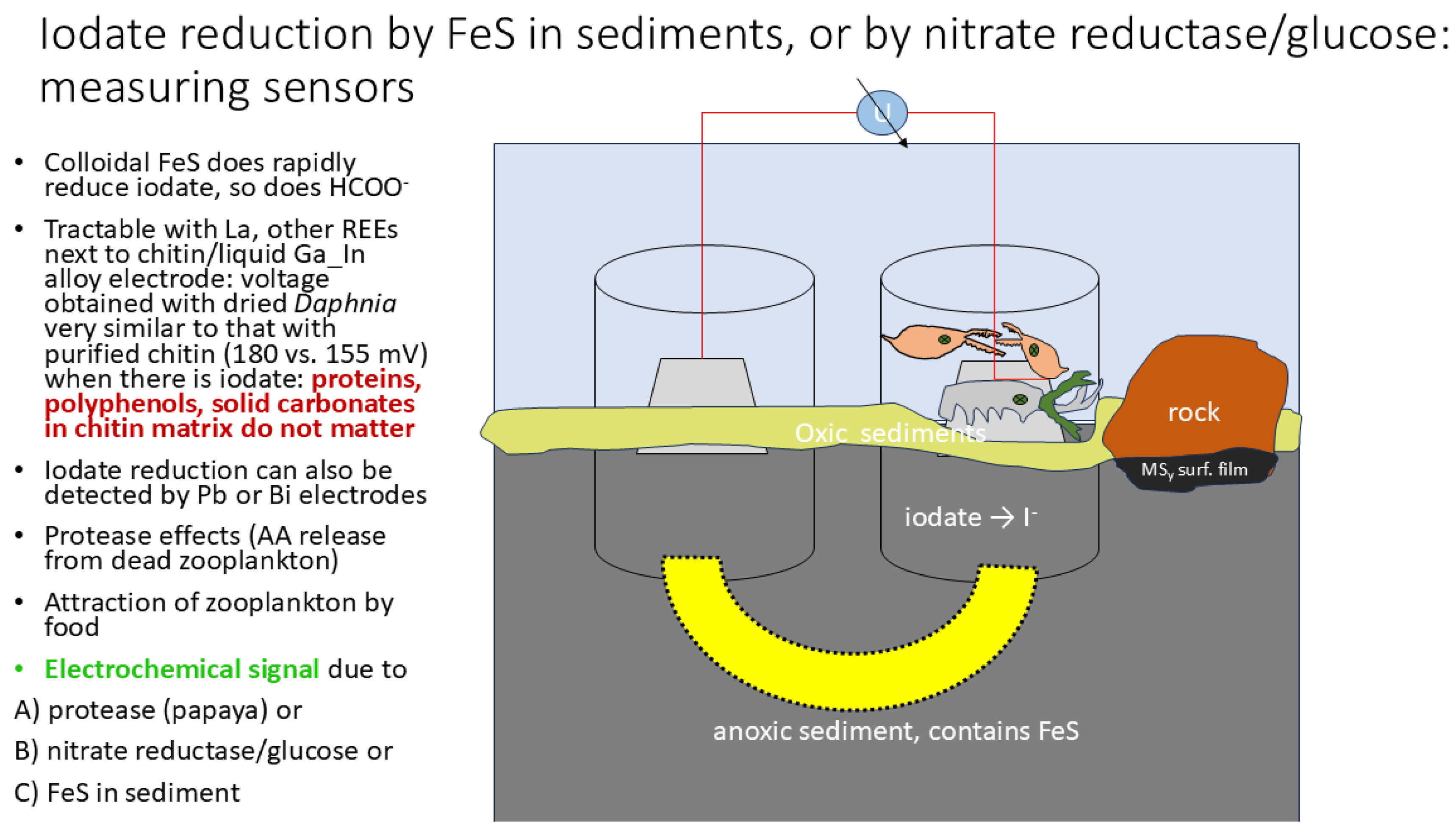
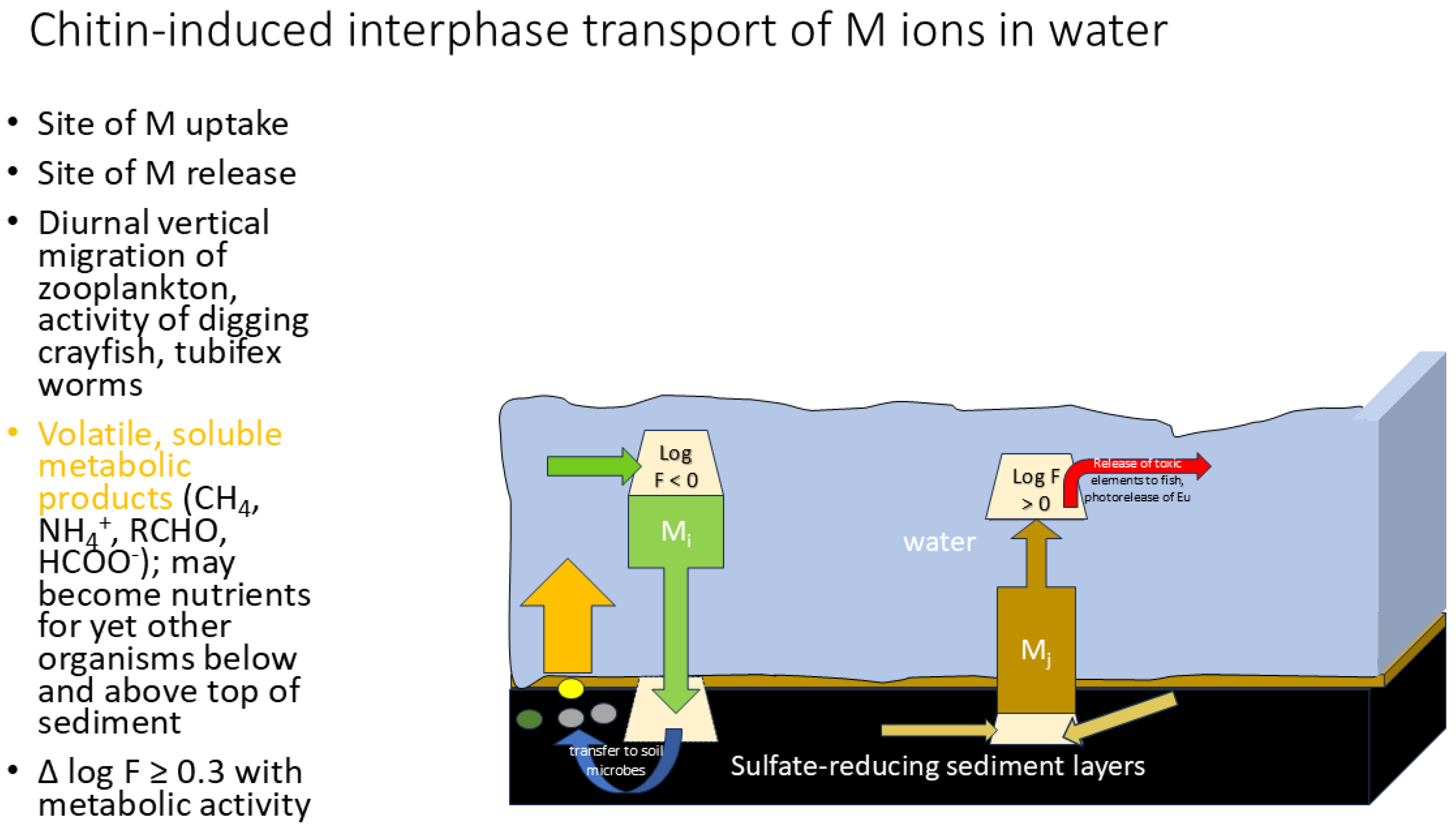
| chitin (arthropods) | dried Daphnia, sandhoppers and grasshoppers | Local pet shop | Preliminary comparisons using La in liquid alloy show that chitin behaves like pure chitin, movement away from the bare electrode does increase voltage and amino acids can be released by (plant-derived mixture of) proteases |
| differently shaped objects made from various metal foils, acting as electrodes | Merck, Goodfellow or local retailers (Cu, Sn) | Selected according to toxic relevance (Pb, Cd, Ni, V), possible cathodic reduction of CO2 on the material (In, Sn, Pb, Bi) [30] or the chance to deposit the very metal from water on a cathode (all) | |
| Conductive PLA | Polylactate plastics amended with soot | Non-metal electrode link to liquid alloy electrodes |
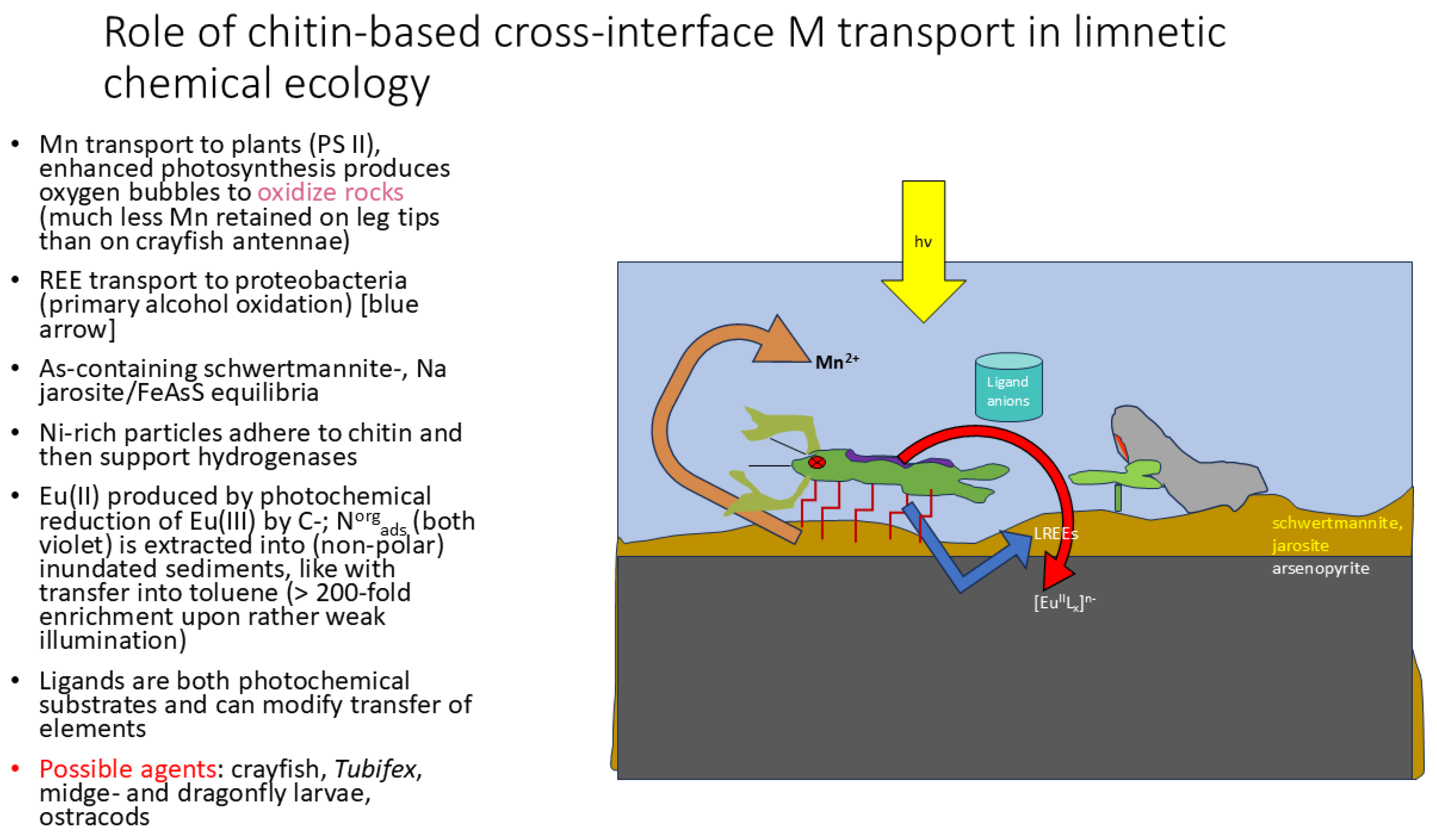
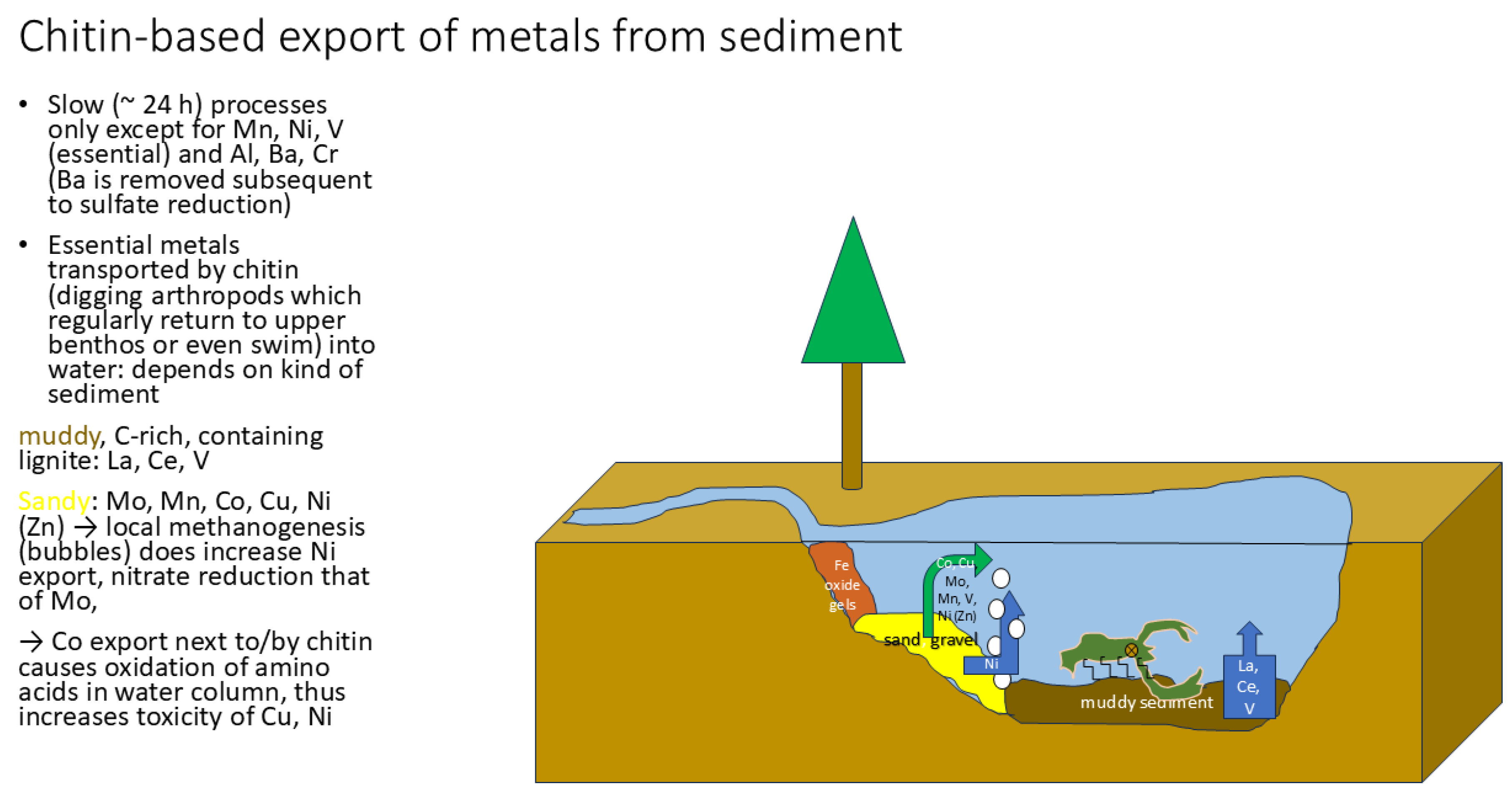
3. Results and Discussion
Replacing Purified Chitin with “Complete” Arthropods
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- B.A. Stankiewicz, M. Mastalerz, C.H.J. Hof, A. Bierstedt, M.B. Flannery, D.E.G. Briggs, R.P. Evershed, Biodegradation of the chitin-protein complex in crustacean cuticle, Org Geochem 1998, 28:67-76. [CrossRef]
- Digby, P.B.S. Calcification and its mechanism in the shore-crab, Carcinus maenas (L.). Proc. Linn. Soc. Lond. 1967, 178:129–146. [CrossRef]
- Retschke, D. Orientierende Untersuchungen zur Adsorption von Schwermetallen (Nickel) unter dem Einfluss Ausgewählter Komplexliganden Sowie in Arealen Potenzieller und Manifester Methanogenese. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2017.
- Fränzle, S.; Erler, M.; Blind, F.; Ariuntsetseg, L.; Narangarvuu, D. Chitin adsorption in environmental monitoring: Not an alternative to moss monitoring but a method providing (lots of) bonus information. J. Sci. Arts. Univ. Valahia 2019, 19, 659–674.
- S. Fränzle, F. Blind, paper in revision: Chitin Employed in an Electrochemical Cell: Batteries, Sensors, Deposition of Metals, submitted to Compounds in 2025.
- Y. Luoga. Master thesis IHI Zittau/TU Dresden, 2021.
- Picone, Role of rare earth elements in methanol oxidation, Curr. Opin. Chem. Biol. 2018:39.
- Budelmann, P. Verbreitung der Flusskrebse (Decapoda) in der südlichen Oberlausitz und die Eignung des invasiven Kamberkrebses (Orconectes limosus) für Chitin-basiertes Monitoring von Schwermetallen in limnischen Ökosystemen. Master‘s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2021. [Google Scholar].
- Digby, P.B.S. Semi-conduction and electrode processes in biological material. I. Crustacea and certain soft-bodied forms. Transact. Linn. Soc. Lond. 1965, 176, 504–525. [CrossRef]
- Ishay JS, Benshalom-Shimony T, Ben-Shalom A, Kristianpoller N, Photovoltaic effects in the Oriental hornet, Vespa orientalis, J Insect Physiol 1992, 38:37-48. [CrossRef]
- D. Kasprzak, J. Liu, Chitin and cellulose as constituents of efficient, sustainable, and flexible zinc-ion hybrid supercapacitors, Sustain Mater Technol 2023, 38:e00726.
- Blind, F. Orientierende Untersuchungen zur Platinmetall freien Aktivierung von CH-Bindungen für Europium basierte Brennstoffzellenanwendungen. Master‘s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2018.
- S.A. Wood, The aqueous geochemistry of the rare-earth elements: Critical stability constants for complexes with simple carboxylic acids at 25°C and 1 bar and their application to nuclear waste management, Engineer Geol 1993, 34:229-59. [CrossRef]
- Ł. Klapiszewski et al., Preparation and characterization of multifunctional chitin/lignin materials, J Nanomat 2013, 1:425726.
- D. Lambertin, S. Chedhomme, G. Bourges, S. Sanchez, G. S. Picard, Activity coefficients of plutonium and cerium in liquid gallium at 1073 K: Application to a molten salt/solvent metal separation concept, J Nucl Mater 2005, 341:131–40. [CrossRef]
- H. Seelmann-Eggebert et al., Karlsruhe map of nuclides, 6th ed., Karlsruhe 1981.
- S. Dedyukhin, A. V. Shchetinskiy, V. A. Volkovich, L. F.Yamschchikov, Solubility of lanthanum in liquid alloys with gallium and indium, Alloys 2024, 2:242-55. [CrossRef]
- Kiss T, Sövago I, Gergely A, Critical survey of stability constants of complexes of glycine, Pure Appl Chem 1991, 63:597-638. [CrossRef]
- Heller, O. Rönitz, A. Barkleit, G. Bernhard, J.-U. Ackermann, Complexation of europium(III) with the zwitterionic form of amino acids studied with ultraviolet-visible and time-resolved laser-induced fluorescence spectroscopy, Appl Spectrosc 2010, 64:930-5. [CrossRef]
- Bahta, G.A. Parker, D.G. Tuck, Critical survey of stability constants of complexes of thiocyanate ion, Pure Appl Chem 1997, 69:1489-1548. [CrossRef]
- Erler, M. Untersuchung des Bindungsverhaltens Ausgewählter Elemente und Ihrer Bodenrelevanten Komplexe an Chitin. Master’s Thesis, IHI Zittau/Technische Universität Dresden, Dresden, Germany, 2020.
- F.H. Firsching, T.R. Paul, The solubilities of rare earth iodates, Inorg nucl Chem 1966, 28:2414-16. [CrossRef]
- Berka A, Vultenin J, Zýka J (1964): Massanalytische Oxydations- und Reduktionsmethoden. Geest & Portig, Leipzig.
- R. Gauguin, Remarques sur l´oxydation des thiocyanates par l´iodate, Anal Chim Acta 1949, 3:272-75. [CrossRef]
- M. I. Kakayannis, D. P. Nikolelis, Reaction-Rate of Thiocyanate: on the lodate-Hypophosphite Method for the Determination of SCN- Based on Its Inhibitory Effect on Reaction, Microchim Acta 1977, 22:356-61.
- Novoselova, V. Smolenski, The influence of the temperature and Ga-In alloy composition on the separation of uranium from neodymium in molten Ga-In/3LiCl-2KCl system during the recycling of high-level waste, Nucl Mater 2018, 519:313-17. [CrossRef]
- M. Wang, Y. Lin, Gallium-based liquid metals as reaction media for nanomaterials synthesis, Nanoscale 2024, 16:6915-33. [CrossRef]
- G. Jander, E. Blasius: Lehrbuch der analytischen und präparativen Chemie. Hirzel, Stuttgart 1979 (11th German edition).
- J.H. Lee, R.H. Byrne, Complexation of trivalent rare earth elements (Ce, Eu, Gd, Tb, Yb) by carbonate ions, Geochimica Cosmochimica Acta 1993, 57:295-302. [CrossRef]
- Y. Hori, K. Kikuchi, S. Suzuki, Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution, Chem Lett 1985:1695-98. [CrossRef]
- T. Ozaki, T. Kimura, T. Ohnuki, A. Kirishima, T. Yoshida, H. Isobe, A. J. Francis, Association of europium(III), americium(III), and curium(III) with cellulose, chitin, and chitosan, Environ Toxic Chem 2006, 25:51-58. [CrossRef]
- M. Pendzhiev, Proteolytic enzymes of papaya: medical applications, Pharmac Chem J 2002, 36:32–34 (translated from Khimiko-Farmatsevticheskii Zhurnal).
- L. E. Koffi, G. S. E. Ekissi, H. K. Konan, J. O. Gbotognon, J. P. N’Guessan Kouadio, Composition of organic acids and anti-nutritional factors of papaya (Carica papaya L. var. SOLO 8) at different stages of maturity, World J Pharmac Medical Res 2020, 10:5-10.
- T. B. Councell, E. R. Landa, D.R. Lovley, Microbial reduction of iodate, Water, Air Soil Poll 1997, 100:99-106. [CrossRef]
- Y. Kosto, P. Matvija, G. Barcaro, I. Matolı ´nova, V. Kalinovych, K. C. Prince, S. Franchi, V. Matolı ´n, T. Ska ´la, N. Tsud, V. Carravetta, Role of the redox state of cerium oxide on glycine adsorption: an experimental and theoretical study, PhysChem ChemPhys 2025, 25:6693.
- M. J. Laverock, M. Stephenson, C. R. Macdonald, Toxicity of iodine, iodide, and iodate to Daphnia magna and rainbow trout (Oncorhynchus mykiss), Arch Environm Contam Toxicology 1995, 29:344-50. [CrossRef]
- G. B. Porter, S. N. Chen, H. L. Schläfer, H. Gausmann, Excited states in the photochemistry of chromium(III) complexes, Theor Chim Acta (Berl.) 1971, 20:81-91. [CrossRef]
- M. Bright, S. Gollner, S. Espada-Hinojosa, S. Hourdez, C. Karthäuser , A. Luiz de Oliveira, A. Fulford, I. V. Hughes, I. Kolar, N. Krause, V. Le Layec, T. Makovec, A. Messora, J. Mitchell, P. Pröts, S. M. Sievert, I. Rodríguez-Ramírez, J. Steger, F. Sieler, T. Tinta, T. Rosa Maria Winter, Z. Bright, R. Coffield, C. Hill, K. Ingram, A. Paris, Animal life in the shallow subseafloor crust at deep-sea hydrothermal vents, Nature Comm 2024, 15:8466. [CrossRef]
- E. Jeppesen, P. Nöges, T. A. Davidson, J. Haberman, T Nöges, K. Blank, T. L. Lauridsen, M. Søndergaard, C. Sayer, R. Laugaste, L. S. Johansson, R. Bjerring, S. L. Amsinck, Zooplankton as indicators in lakes: a scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD), Hydrobiologia 2011, 676:279-97. [CrossRef]
- X. Xu, F. Wang, W. Xu, H. Lu, L. Lv, H. Sha, X. Jiang, S. Wu, and S. Wang, Wide-bandgap rare-earth iodate single crystals for superior X-ray detection and imaging, Adv Sci 2023, 10:2206833.
- K. Abea, K. Sunadab, Y. Mochizukia, T. Isobea, S. Matsushitaa, T. Nagaib, H. Ishigurob, A. Nakajima, Preparation of rare earth iodates and their decomposition activity on organic dyes and antibacterial/antiviral activities Ceram Intern 2023, 49:14681-88.
- P. Pastorino, P. Brizio, M. C. Abete, M. Bertoli, A. G. Oss Noser, G Piazza, M. Prearo, A. C. Elia, E. Pizzul, S. Squadrone, Macrobenthic invertebrates as tracers of rare earth elements in freshwater watercourses, SciTot Environ 2020, 698:134282. [CrossRef]
- M.T.M. Koper, J.H. Sluyters, Electrochemical oscillators: an experimental study of the indium/thiocyanate oscillator, J Electroanal Chem 1991, 303:65-69. [CrossRef]
- D. H. Dang, W. Wang, G. Winkler, A. Chatzis, Rare earth element uptake mechanisms in plankton in the Estuary and Gulf of St. Lawrence, Sci Tot Environ 2023, 860:160394. [CrossRef]
- O. Kretschmer, BA thesis IHI Zittau/TU Dresden, 2018.
- Sendra, A. S. P. S. Reboleira, The world's deepest subterranean community – Krubera–Voronja Cave (Western Caucasus), Intern J Speleology 2012, 42:221–230. [CrossRef]
- V. Vahtera, P. Stoev, N. Akkari, Five million years in the darkness: A new troglomorphic species of Cryptops Leach, 1814 (Chilopoda, Scolopendromorpha) from Movile Cave, Romania", ZooKeys 2020, 1004: 1–26.
- S. Fränzle, F. Blind, Electronic Devices Made from Chitin: NAND Gates Made from Chitin Sorbates and Unsaturated Bridging Ligands—Possible Integration Levels and Kinetics of Operation, Nanomanufactoring 2023, 4:381-400. [CrossRef]
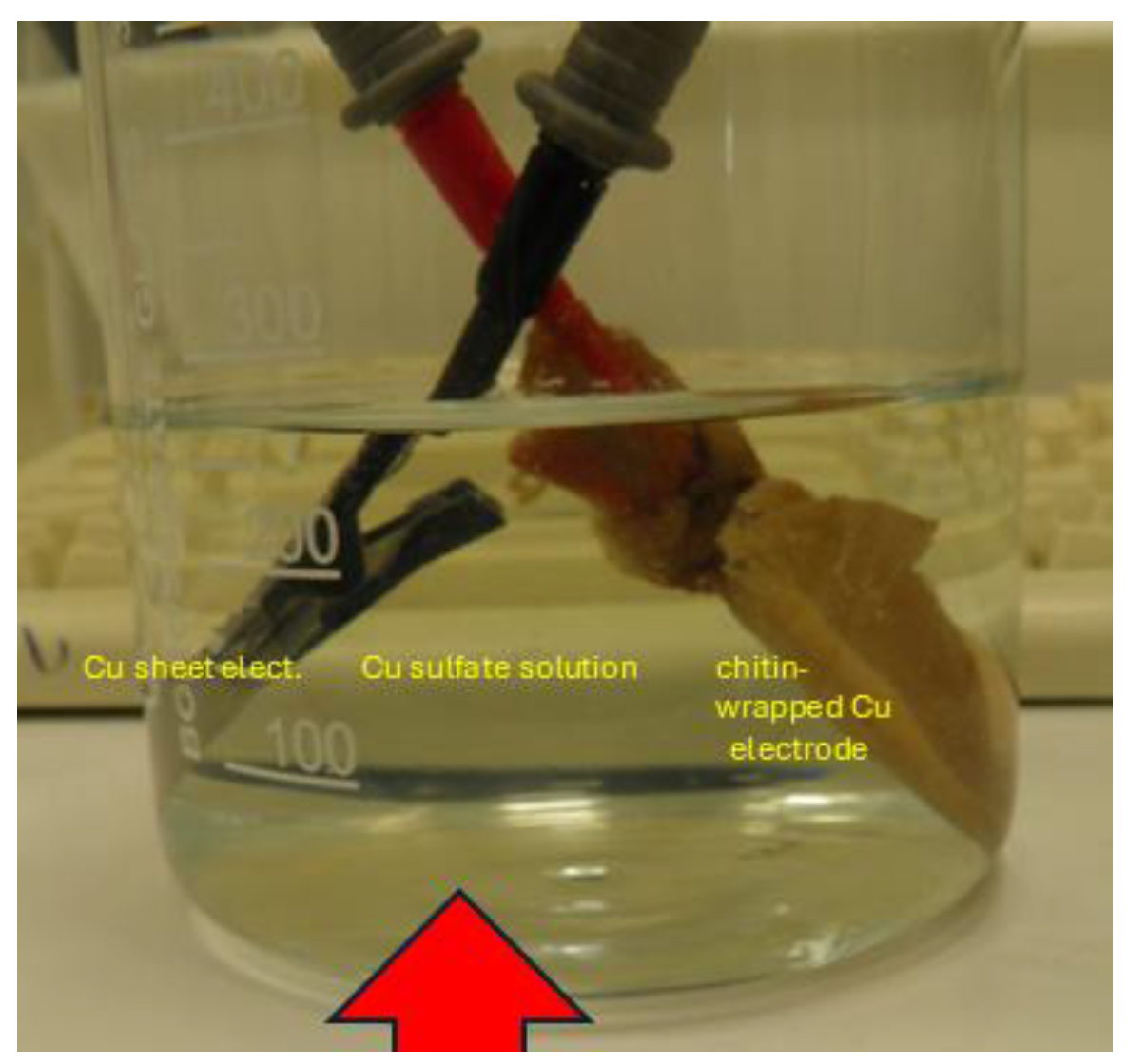
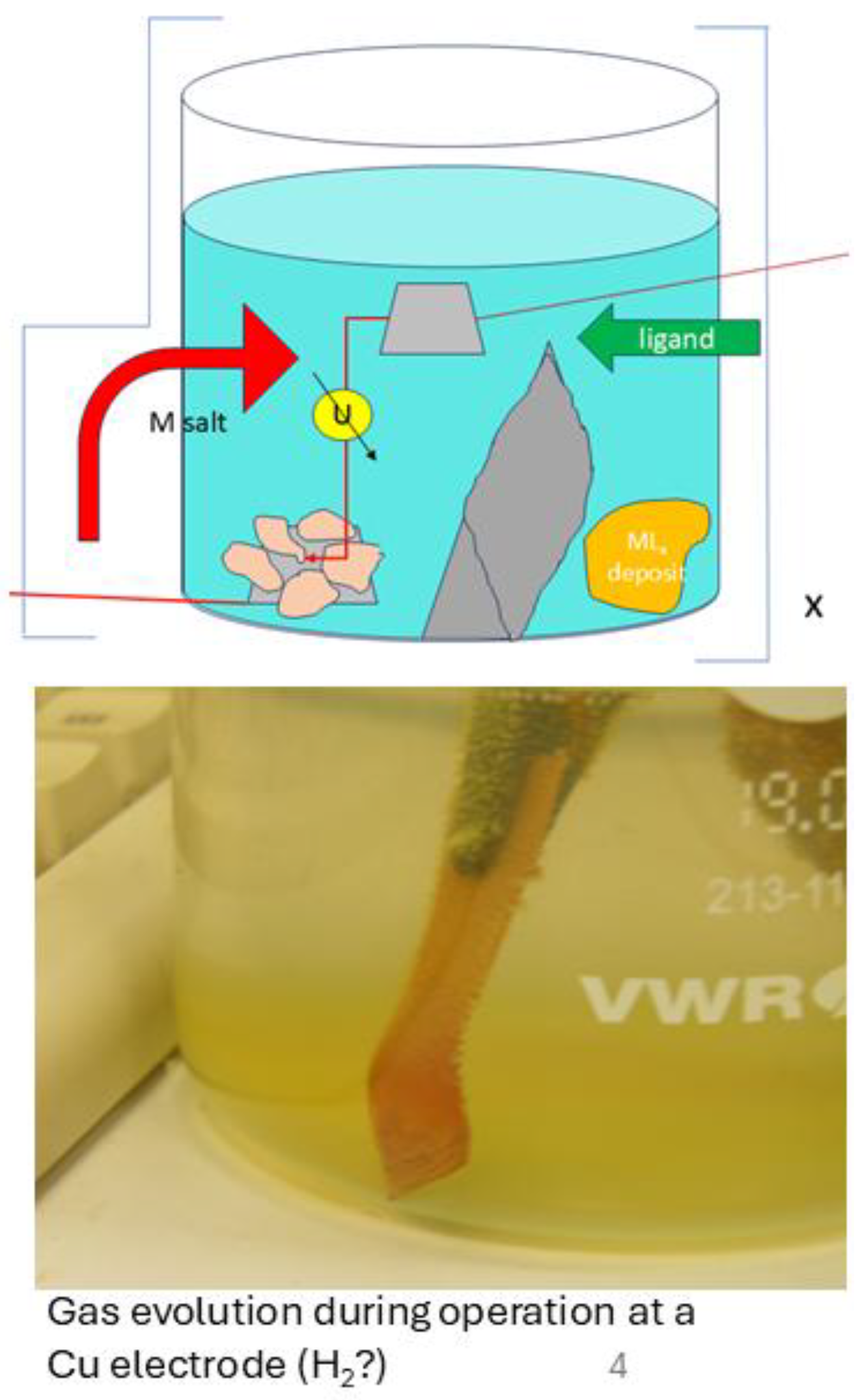

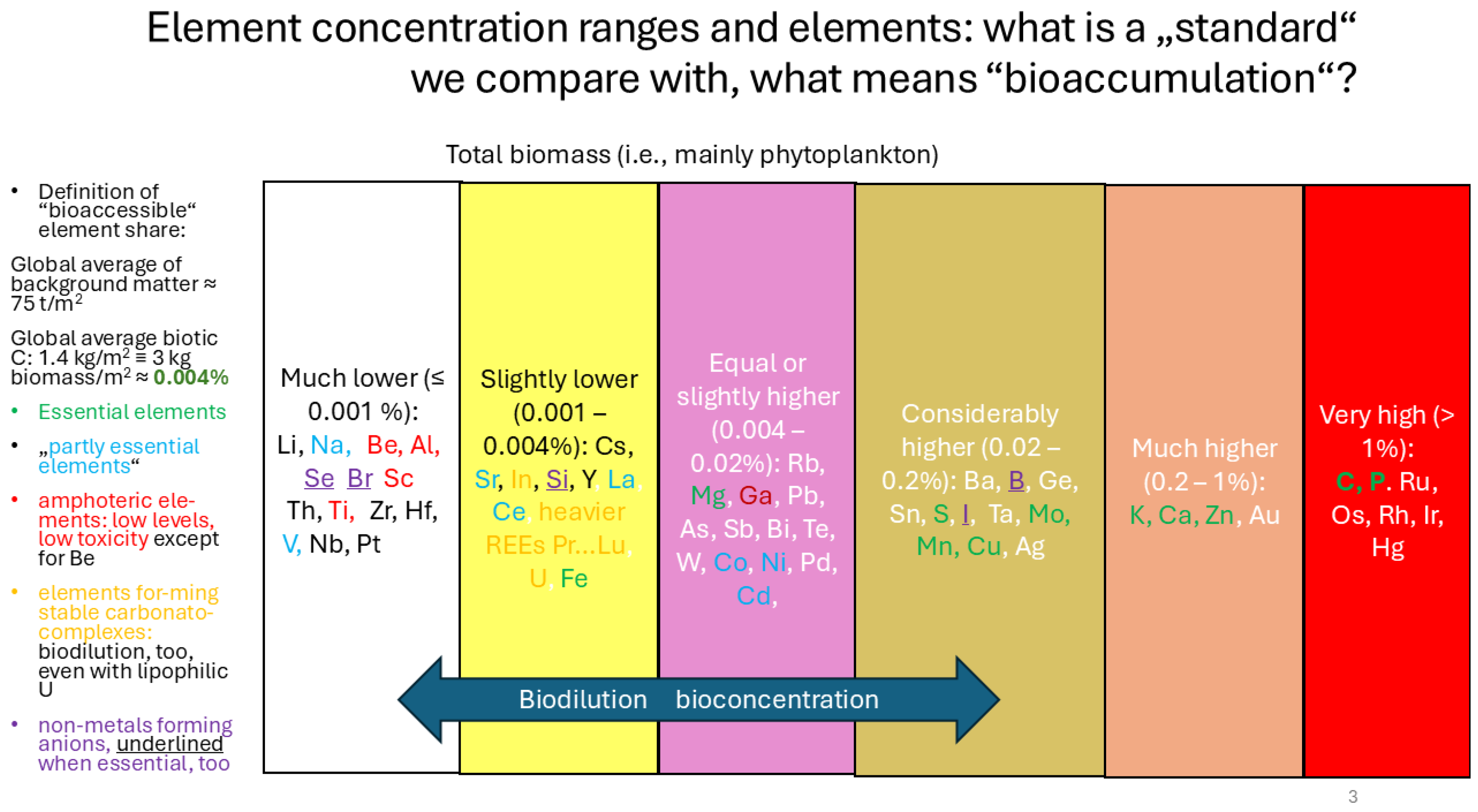
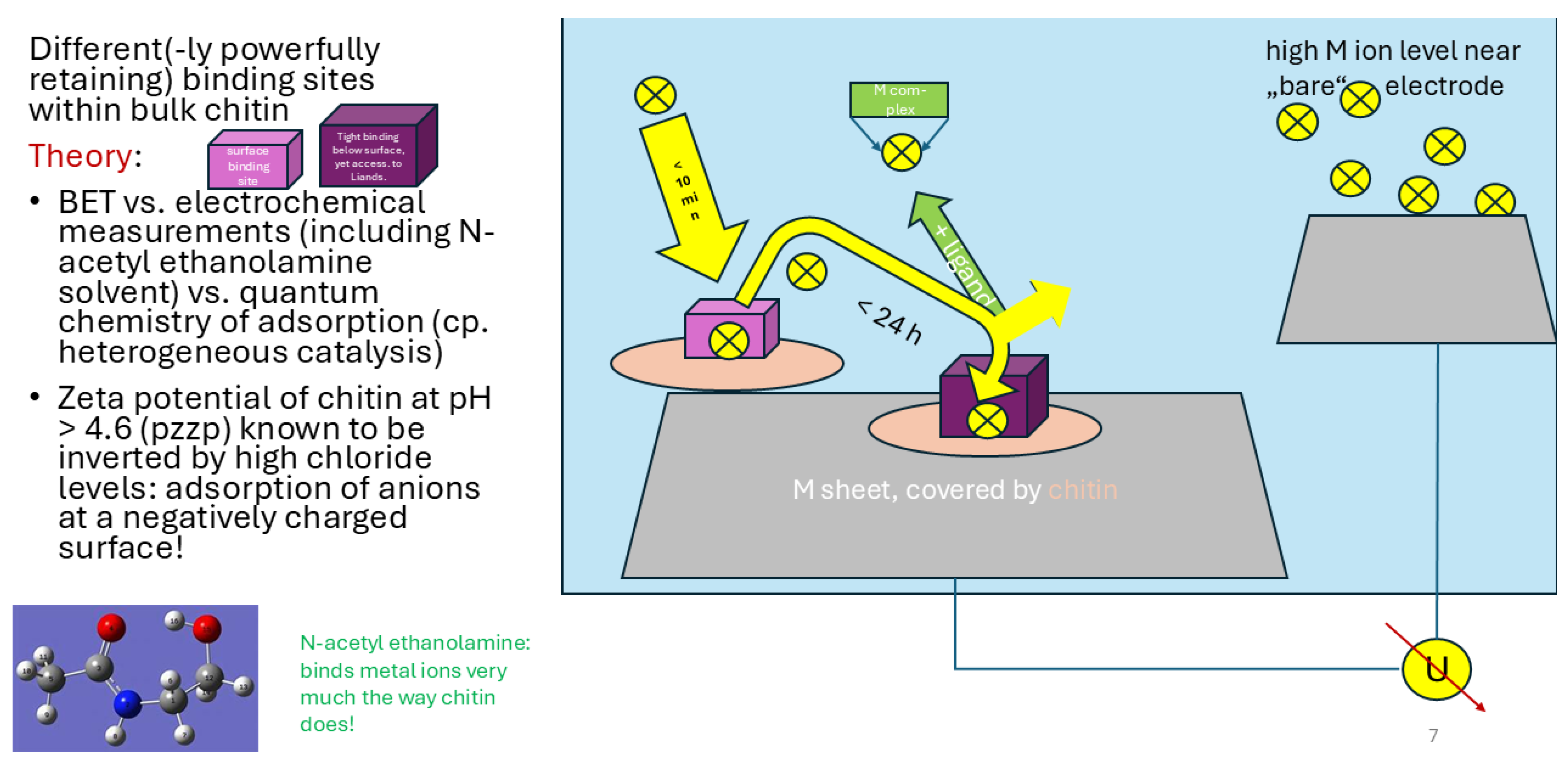


| Sample ID | Ga content of chitin after saturation [µg/g] | Ga [µmol/g] | In content of chitin after saturation [µg/g] | In (µmol/g] | Stoichiometric ratio on chitin [ - ] | remarks | Contents of other metals [µmol/g] |
|---|---|---|---|---|---|---|---|
| No (alloy) | 1082.86 | 213.38 | 5.075 | No chitin added | No | ||
| Pure chitin (background values) | Al 0.32 µmol/g, Cu 0.21, Fe 0.36; that is, Σ ≤ 2 % of saturation | ||||||
| Ga, In | 66.263 | 21.295 | 3.112 | Substantial relative Ga depletion on chitin when there are no additives other than HCO3-; Ga about “common” saturation level in chitin | |||
| Al, Ga, In | 35.503 | 2.4306 | 14.61 | Al mainly reduces In retention by chitin, less so of Ga | |||
| Ga, In, Fe, La, Ce | 165.18 | 31.2 | 5.294 | Fe, La, Ce from “auerstein“; Ga/In ratio close to that of alloy; very high potentials obtained upon adding SCN- | Ce (about 120 µmol/g)/La ≈ 23, probably due to air oxidation of Ce on drying chitin while free [REE(III)]aq. is limited by iodate precip.; Fe rather low (12.6 µg/g) but extractable from alloy |
| Metal ions, oxidation state | Set of elements | Regression equation (F2 – F1) | Critical value of a for log F < 0, lignite pits only | Regression equation (F3 – F1) | Critical value of a for log F < 0, clay pit vs. lignite pools | Remarks |
|---|---|---|---|---|---|---|
| +II | Mn, Co, Ni, Cu, Zn, Pb | 0.1261 a + 0.433 r2 = 0.66 |
-3.43 smaller than critical for Pb, Cu only | 0.0559 a + 0.478 Poor correlation (r = 0.163) only |
-8.55 (unrealistic) | Ba omitted, transition metals and Pb only; |
| +III | Al, La, Ce | 0.4583 – 0.0276 a, r2 = 0.8876 | 16.6 | 0.0490 a – 0.816 |
16.65 | No a value known for Bi, incomplete data for Cr. Critical values include all trivalent M save Al, incl. all REEs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




