Introduction
Corynebacterium pseudotuberculosis, a Gram-positive bacterium, belongs to Actinomycetals order (Gomide et al., 2018; Eberle et al., 2018). It is also a β-hemolytic and non-motile pathogen causing caseous lymphadenitis (CLA). CLA or cheesy gland in sheep and goats caused by this animal pathogen often contributes to the significant economic losses and frequently observed across sheep rearing countries globally such as Australia, New Zealand, South Africa, United States, Canada, Brazil, Iran, Egypt and Malaysia (Soares et al., 2013). Common consequences of CLA are low milk production, reduction in body weight and death (Eberle et al., 2018).
Biofilms are structured communities of microorganisms encased in a self-produced extracellular matrix that adhere to surfaces, and they play a significant role in antibiotic resistance and persistent chronic infections (Safini et al. 2024). The matrix acts as a protective barrier, limiting the penetration of antibiotics and immune cells, while the close proximity of cells within the biofilm facilitates the exchange of resistance genes through horizontal gene transfer. In the last few years, the studies of protein expression in biofilm forming pathogens have dramatically increased (Yahya et al., 2017; Othman & Yahya 2019; Zawawi et al., 2020; Isa et al., 2022). This is because proteins are well understood to play major roles in the biofilm formation, a microbial survival strategy that allows microorganisms to form stable community in a dynamic environment. They initiate bacterial adhesion to surface, provide mechanical stability and form a cohesive network to support the biofilms cells. A large scale and systematic investigation of all proteins expressed in biofilm cells under specific conditions is called proteomics.
Previous works have characterized C. pseudotuberculosis biofilm in terms of structure, biochemical composition, and antimicrobial susceptibility (Yaacob et al., 2021a, Rashid et al., 2022), however, the study related to the proteomic changes during C. pseudotuberculosis biofilm development is still scarce. Thus, the objective of this work was to compare the whole-cell proteome profiles between planktonic and biofilm fractions of C. pseudotuberculosis and identify C. pseudotuberculosis proteins and biological pathways showing differential expressions. The present study used a combination of subtractive proteomic and protein-protein interaction network analyses (Yahya et al., 2017) to decipher the differential proteomic expression in C. pseudotuberculosis biofilm and to elucidate the unexplored functional links in understanding this veterinary pathogen.
Materials and Methods
Test Microorganism
Clinical isolate of C. pseudotuberculosis was provided by Veterinary Laboratory Service Unit at Universiti Putra Malaysia (UPM). The microorganism was maintained in nutrient broth at 37 ˚C overnight to achieve the optical density OD 600 = 0.5 for biofilm formation assay.
FESEM Analysis
The biofilm structure of C. pseudotuberculosis was studied using FESEM (Hitachi, Japan) (Amran et al. 2024). After fixation in 4% (v/v) formaldehyde for 3 h, the biofilm on the glass cover slip was rinsed with sterile distilled water thrice, dehydrated using absolute ethanol for 10 min and air dried overnight. Observation was performed at 5,000x magnification.
Extraction and Determination of Whole-Cell Proteins
After 24 h incubation, nutrient medium was transferred to a microcentrifuge tube, while the biofilm fraction was rinsed with 0.9% (w/v) NaCl twice and scraped from the surface before transferred to another microcentrifuge tube (Yahya et al., 2017). Both planktonic and biofilm fractions were analyzed for whole-cell proteome expression. They were pelleted at 10,000 rpm for 10 min. Then, they were incubated in a lysis buffer containing 1 mM PMSF and 0.5% (w/v) SDS at 95 ºC for 15 min and centrifuged at 10,000 rpm. Standard Bradford assay was performed to determine protein concentration.
1D-SDS-PAGE Analysis
The protein samples were separated by 1D-SDS PAGE (Yahya et al., 2017). 1X SDS sample buffer was used to solubilize the protein samples and heated at 95 ºC for 5 min. 12 % (v/v) polyacrylamide gel was used to separate protein samples and protein standards (10-245 kDa) for 50 min at 200 V. Gel staining was performed using G250 Coomassie-Blue and analyzed using ImageJ software. All protein bands were digested using trypsin and identified using LC-MS/MS.
Trypsin Digestion
The selected protein bands were incubated in acetonitrile: distilled water (50:50) containing 25 mM ammonium bicarbonate for 45 min, vacuum-dried and stored at -20 ºC (Yahya et al., 2017). Each of the gel slice was then treated with 10 μL of 12.5 µg/mL trypsin with 25 mM ammonium bicarbonate at 37 ºC overnight. The digested peptides were incubated in 10 mL of acetonitrile containing 1% (v/v) trifluoroacetic acid for 20 min, vacuum-dried and desalted. The concentration of eluted peptide was measured using the NanoDrop spectrophotometer.
LC-MS/MS Analysis
LC-MS/MS was used to determine the identity of C. pseudotuberculosis proteins. The tryptic peptides were analyzed using HPLC combined with TripleTOF mass spectrometer (AB SCIEX, USA) (Yahya et al., 2017). The parameter settings in mass spectrometer were as follows: Interface heater temperature (IHT) of 150, ion spray voltage floating (ISVF) of 2300 V and declustering potential (DP) of 70 V. A MS survey scan (350 – 1250) and MS/MS scan (100 and 1800) were used herein. Mass spectra were analysed using Mascot search engine (Matrix Science) and SwissProt database. The search parameters used were as follows: i) mass values: monoisotopic; ii) peptide mass tolerance: ±0.2 Da; iii) miss cleavage: 1; iv) variable modifications: phospho (ST), phos-pho (Y). Proteins with two or more peptides were chosen for high confidence protein identification.
Phosphoprotein Analysis
ProQ diamond assay was performed to validate biofilm phosphoproteins identified in the LC-MS/MS analysis (Yahya et al., 2017). The gels were treated using 10% (v/v) trichloroacetic acid, 50% (v/v) methanol for 30 min at 60 rpm, rinsed with ultrapure water for 10 min and incubated in the fluorescent dye in the dark for 60 min at 60 rpm. The gels were destained using 50 mM sodium acetate, 20% (v/v) acetonitrile, pH 4.0 in the dark for 30 min at 60 rpm. A gel documentation system (Alpha Innotech, CA) was used to visualize and photograph fluorescently labelled phosphoproteins.
Analysis of Functional Classification
Classification of protein function was performed by SwissProt/TrEMBL database search. All identified proteins were classified according to their functional categories and subcellular localizations.
Analysis of Functional Network of Interactive Proteins
The identified C. pseudotuberculosis proteins were used as queries in STRING database v.11.0 to construct the high-confidence network of protein linkages.
Analysis of Hypothetical Protein
The selected hypothetical protein was analyzed for transmembrane helices, functional domain, post-translational modification sites, physicochemical properties, 3D structure and functional linkages using TMHMM 2.0, SMART ScanProsite, ProtParam, SWISS-MODEL and STRING database, respectively.
Results
Distinct Whole-Cell Proteome Profiles
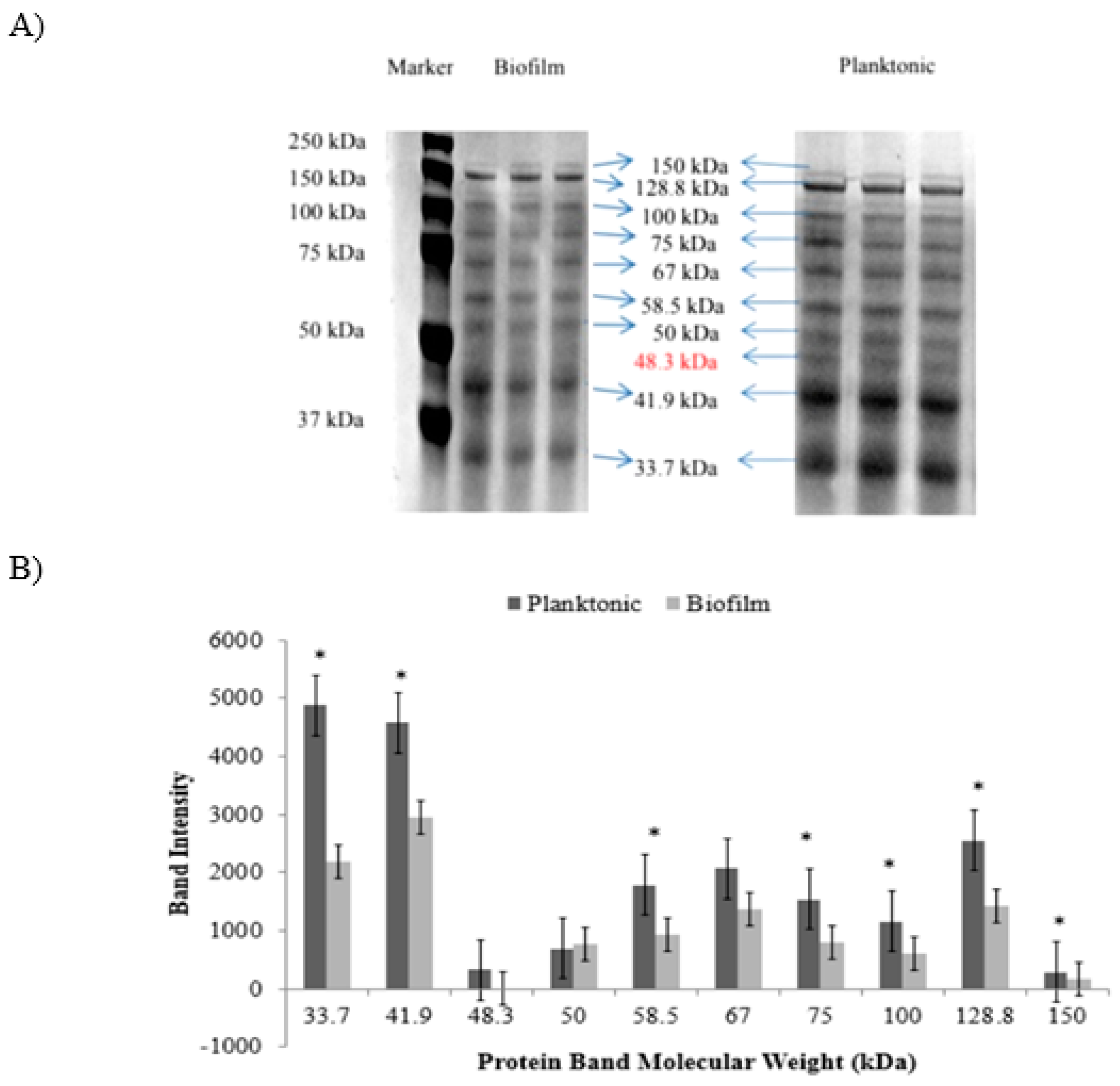
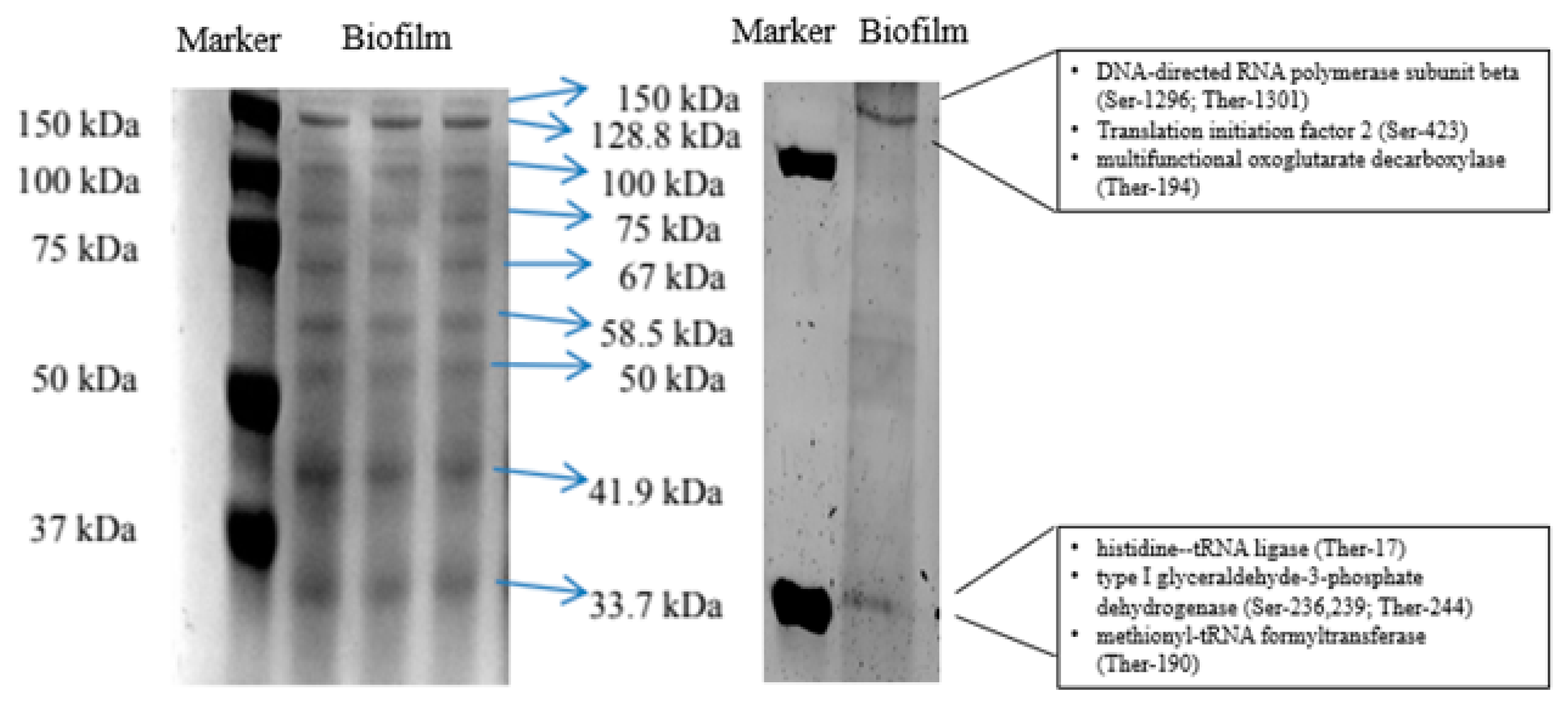
Figure 2 shows SDS-PAGE image of planktonic and biofilm fractions. Both fractions shared the same nine protein bands namely 33.7 kDa, 41.9 kDa, 50 kDa, 58.5 kDa, 67 kDa, 75 kDa, 100 kDa, 128.8 kDa and 150 kDa (
Figure 2A). On the other hand, 48.3 kDa protein band was found to be exclusively expressed in planktonic fraction.
Differential Protein Band Expression Level
Figure 2B shows densitometric analysis of all visible protein bands. Biofilm fraction demonstrated significantly (p<0.05) higher intensity of seven protein bands (33.7 kDa; 41.9 kDa, 58.5 kDa, 75 kDa, 100 kDa, 128.8 kDa, and 128.8 kDa) than planktonic fraction.
Distribution of Identified C. pseudotuberculosis Proteins
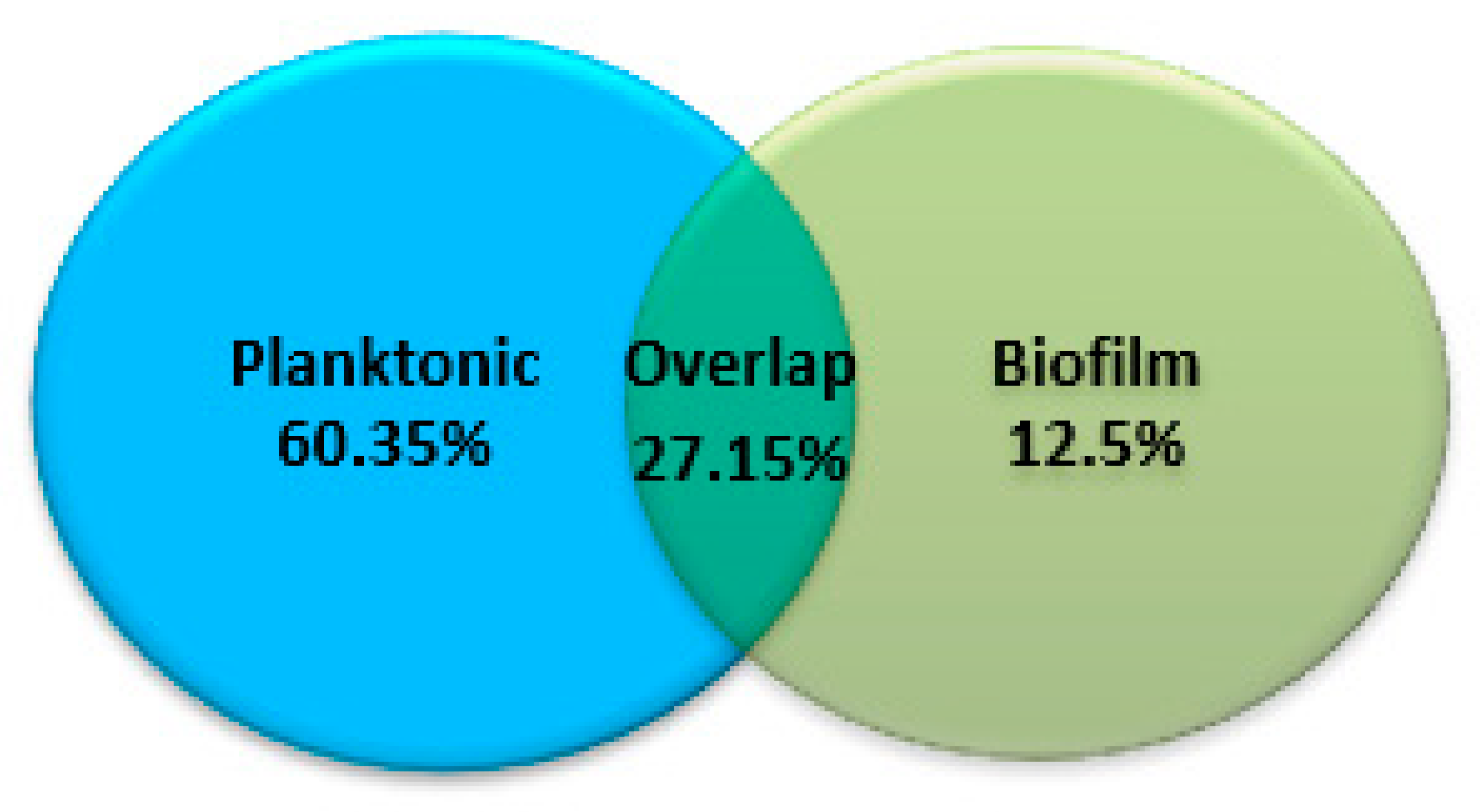
Figure 3 shows the number of unique and shared proteins between the planktonic and biofilm fractions of
C. pseudotuberculosis. A total of 854 and 387 proteins were successfully identified in planktonic and biofilm fractions, respectively. Of these, 589 proteins (60.35%) and 122 proteins (12.5%) were exclusively expressed in the planktonic and biofilm fractions, respectively. Meanwhile, 265 proteins (27.15%) were detected at both stages.
Differentially Expressed C. pseudotuberculosis Proteins
It should be noted that the differentially expressed proteins herein represent the proteins that were present only in planktonic or biofilm fraction. The basis of this consideration is that
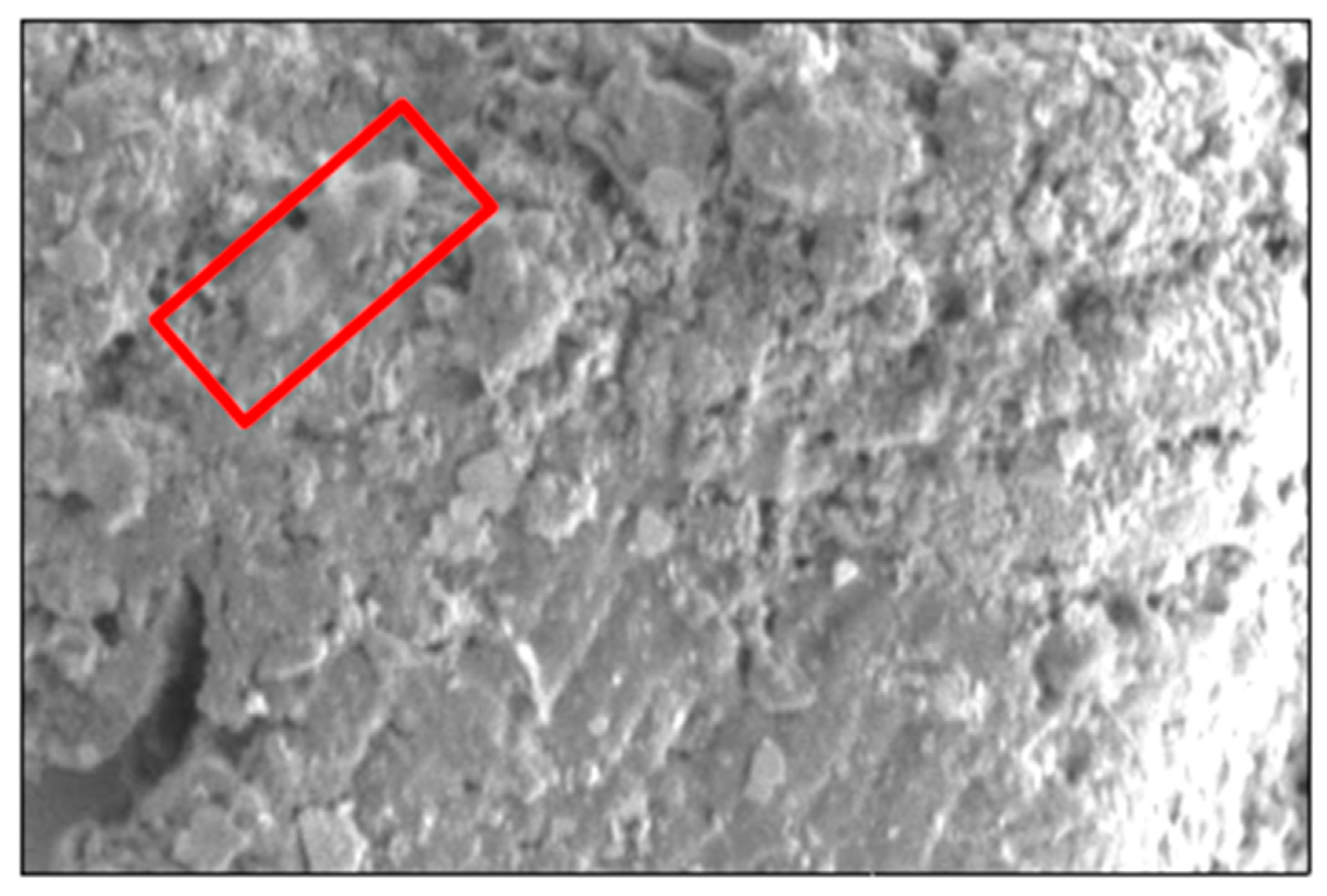
C. pseudotuberculosis clinical isolate tends to form heterogeneous biofilm on the surface (
Figure 1). Therefore, the subtractive approach is more suitable for the comparative proteomic analysis of biofilm (Yahya
et al., 2017). A total of 711 proteins were found to be differentially expressed in
C. pseudotuberculosis biofilm as compared to its planktonic counterpart. Ninety-four (13.22%) of them were found to be hypothetical proteins.
Classification of Differentially Expressed C. pseudotuberculosis Proteins
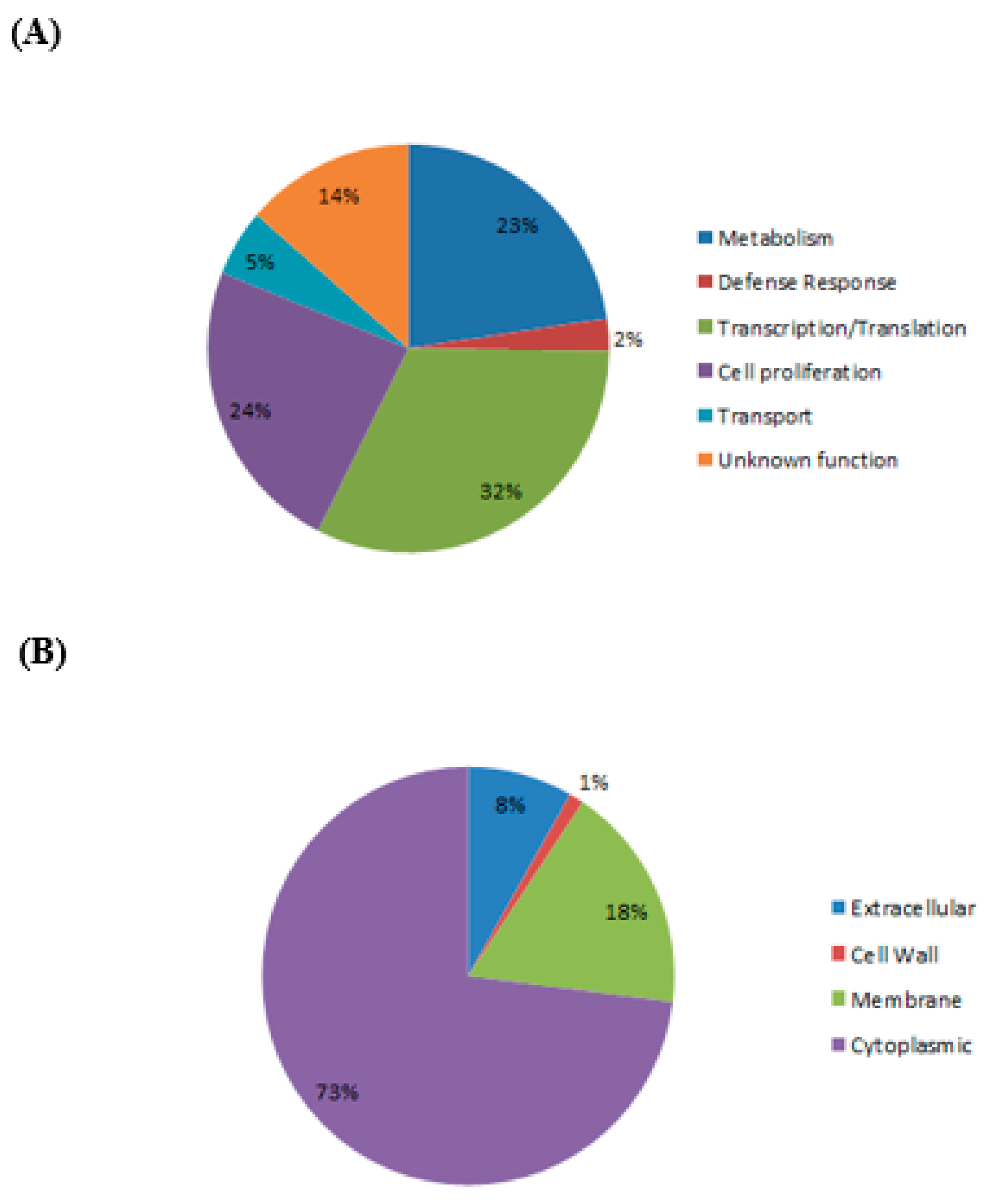
Figure 4 shows the different functional categories and subcellular localizations of differentially expressed
C. pseudotuberculosis proteins based on SwissProt/TrEMBL database. The majority of differentially expressed
C. pseudotuberculosis proteins were found to be linked to transcription and translation (32%), and cytoplasmic compartment (73%).
Validation of Proteomic Expression by Phosphoprotein Assay
In this study, the proteomic data was validated using phosphoprotein assay as previously reported (Yahya
et al., 2017).
Table 1 shows a list of phosphoprotein profile of
C. pseudotuberculosis biofilm. A total of 23 phosphoproteins in
C. pseudotuberculosis biofilm were also identified by LC-MS/MS. The presence of those phosphoproteins in
C. pseudotuberculosis biofilm was validated by ProQ diamond staining whereby two phosphoprotein bands, 33.7 kDa and 150 kDa, were detected (
Figure 5).
Functional Linkages Between Differentially Expressed C. pseudotuberculosis Proteins
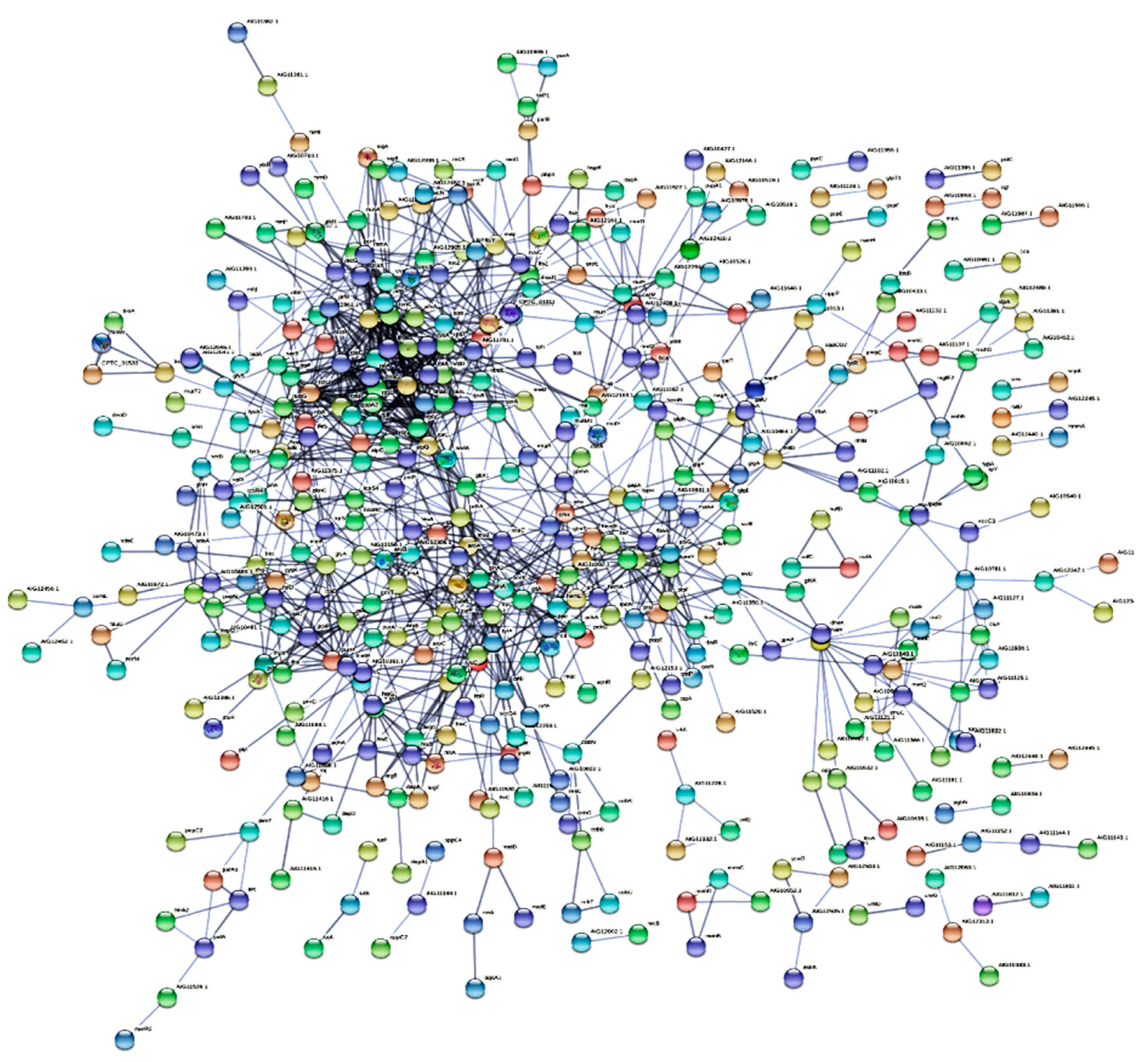
Figure 6 shows the protein-protein interaction network of differentially expressed
C. pseudotuberculosis proteins. A total of 1206 functional interactions were produced among the differentially expressed
C. pseudotuberculosis proteins. Fifty-seven
C. pseudotuberculosis proteins were considered as hub proteins as they showed more than 10 functional interactions in the network, including Phosphotransferase system II Component (24 interactions), Glycine hydroxy methyltransferase (25 interactions), Large subunit ribosomal protein L3 (26 interactions), Translation initiation factor IF-2 (29 interactions), multifunctional oxoglutarate decarboxylase (30 interactions) and DNA-dependent RNA polymerase (30 interactions).
Functional Enrichment
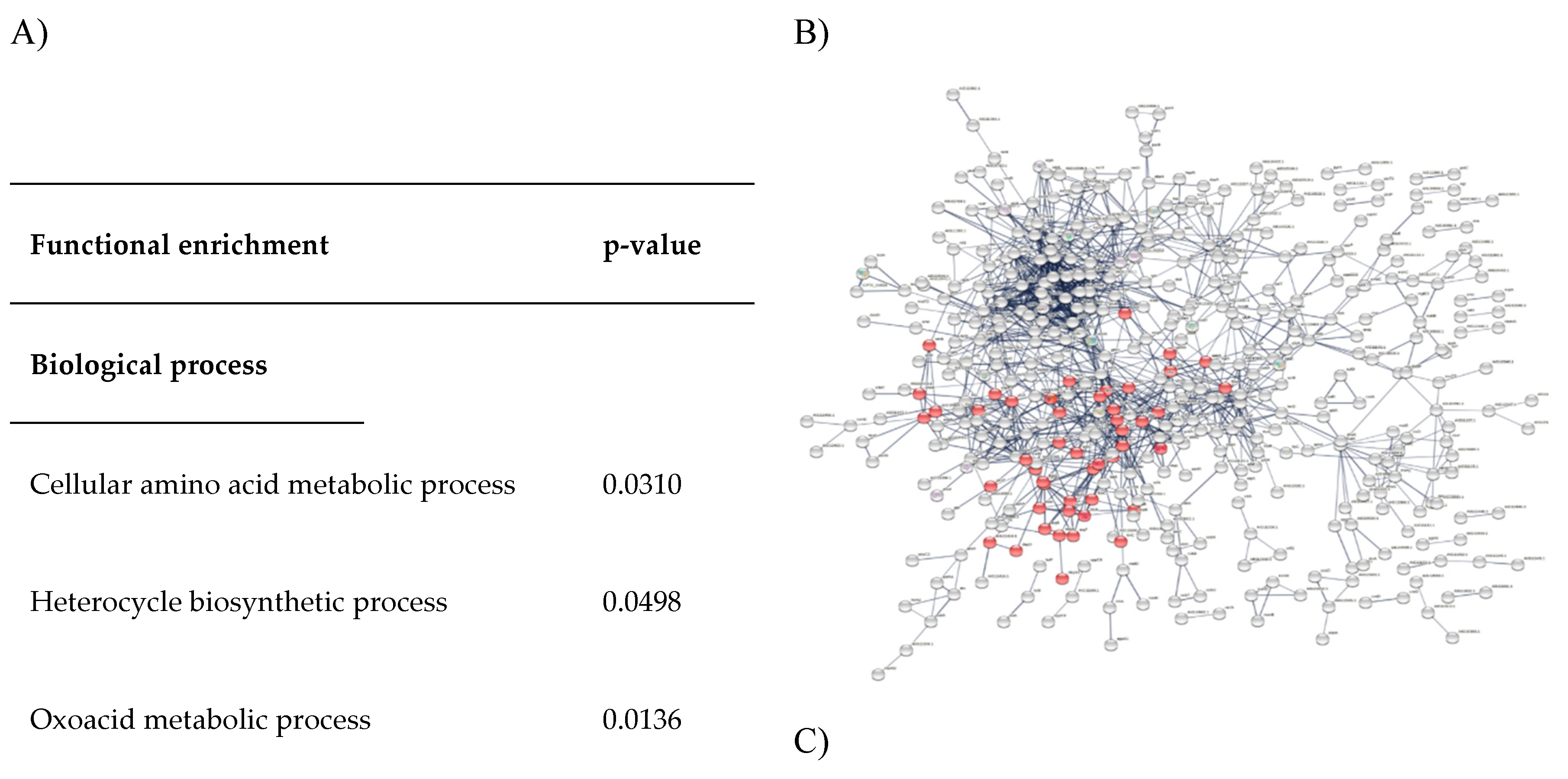
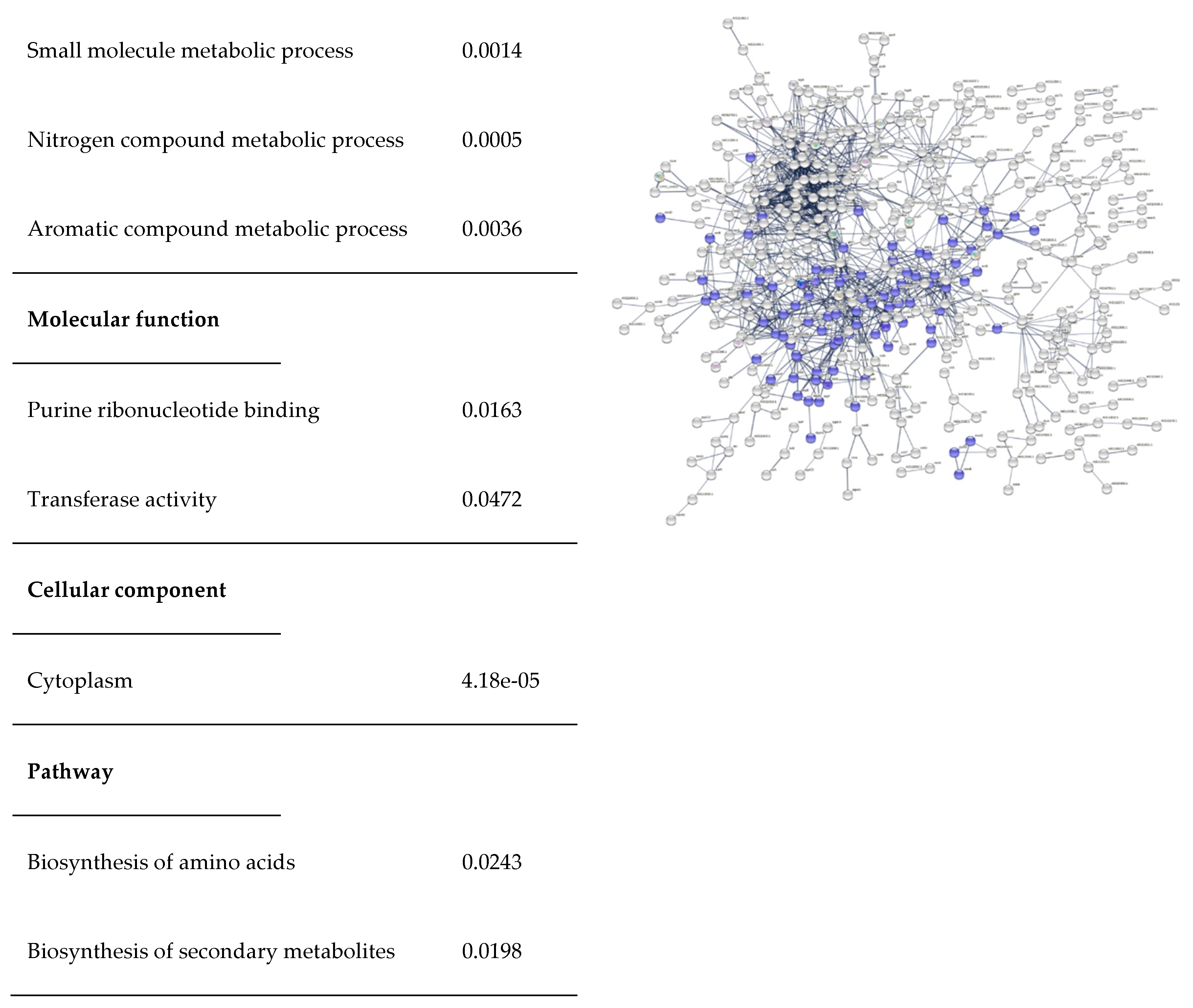
Figure 7 shows the functional enrichment based on the network of the differentially expressed
C. pseudotuberculosis proteins. All the data from the network enrichment analysis were significant (p-value<0.05) (
Figure 7A).
Biosynthesis of Amino acids
Figure 7B shows the network of amino acid biosynthesis. A total of 48 differentially expressed
C. pseudotuberculosis proteins were found to be associated with amino acid biosynthesis.
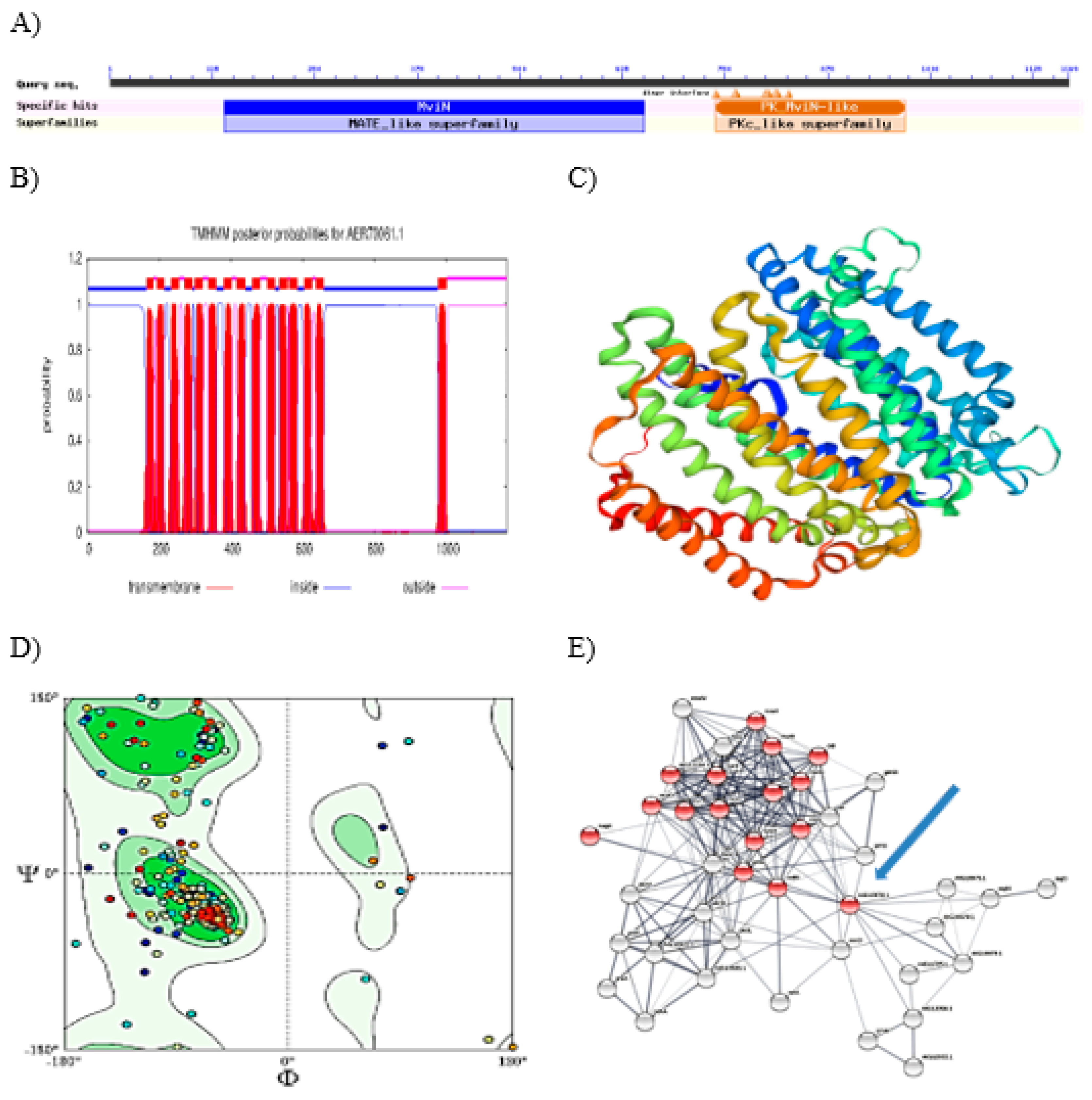
Hypothetical Protein Cp106_2054
Hypothetical protein Cp106_2054 was further analyzed for its functional characteristics as it has more than 1000 amino residues. Functional characteristics of this hypothetical protein are shown in
Figure 8. It was predicted to contain 15 transmembrane helix, MviN domain which plays role in peptidoglycan biosynthesis, and several post-translational modification sites such as casein kinase II phosphorylation and cGMP-dependent protein kinase phosphorylation site. It also showed an instability index of 35.01 classifying the protein as stable. Template 6cc4.1.A (a soluble cytochrome from
Escherichia coli) was used to construct the 3D model of this hypothetical protein. The sequence similarity between the target and template was found to be 22.83%. The 3D model showed Ramachandran-favored area of 93.18%. Hypothetical protein Cp106_2054 was also predicted to have functional linkages with other proteins involved in peptidoglycan metabolic pathway (p < 0.05).
Discussion
C. pseudotuberculosis is the etiologic agent of CLA. While many reports have shown that this disease primarily affects goats and sheep, it also infects humans. This disease has been shown to be prevalent in commercial regions of small ruminant-based products. In the last few years, the study of C. pseudotuberculosis biofilm has dramatically increased. By considering the fact that the biofilm is key to microbial pathogenesis, our current focus is to elucidate the proteomic expression during the development of C. pseudotuberculosis biofilm.
The morphology of C. pseudotuberculosis biofilm has previously been characterized using FESEM (Yaacob et al., 2021a, Rashid et al., 2022; Abd Rashid et al. 2024). They demonstrated extracellular matrix, heterogenous 3D structure and pores in C. pseudotuberculosis biofilm. Therefore, the present study used the same experimental approach to verify the biofilm formation before proteomic analysis.
The present study demonstrated that both planktonic and biofilm fractions shared the same nine protein bands (33.7 kDa - 150 kDa). Braithwaite et al. (1993) reported the expression of 38 kDa and 58 kDa protein bands in the whole-cell lysate of C. pseudotuberculosis isolates. However, they did not identify these protein bands in the biofilm fraction. Therefore, the present study provides a novel finding whereby both protein bands are differentially expressed in C. pseudotuberculosis during biofilm formation. On the other hand, 100 kDa protein band has also been shown to appear in the electrophoretic profile of C. pseudotuberculosis secreted proteins (Santos et al., 2022).
The present study successfully identified a total of 976 C. pseudotuberculosis proteins. Subtractive analysis revealed 711 proteins that were differentially expressed between biofilm and planktonic fractions. The majority of identified proteins were related to transcription/translation and cytoplasmic compartment. Several lines of proteomic works have used the subtractive approach to analyze complex and heterogeneous proteome samples to solve the potential problem of large range of protein expression level (Schirmer et al., 2003; Yahya et al., 2017). Meanwhile, Sá et al. (2021) has identified 40 proteins that were differentially expressed between biofilm producer and non-biofilm producer of C. pseudotuberculosis. Differential proteomic expression observed in the present study also corroborates the fact that biofilm state exhibits different proteome expression profile as compared to that of planktonic state (Yaacob et al., 2021b). Proteins associated with carbohydrate metabolism (phosphoglyceromutase and pyruvate dehydrogenase) were noticed to be differentially expressed in C. pseudotuberculosis biofilm. They probably play roles in the oxygen-limited environment and the synthesis of core biofilm matrix (Suryaletha et al., 2019). Furthermore, proteins associated with amino acid metabolism (Cysteine synthase A and Glutamine synthetase II) were also found to be differentially expressed in C. pseudotuberculosis biofilm. They may participate in the synthesis of extracellular matrix of robust biofilm (Suryaletha et al., 2019).
Protein-protein interaction network is characterized by nodes corresponding to proteins, and edges connecting different proteins. The network is useful to annotate a wide range of protein functions, and thus enhancing the existing knowledge on diverse metabolic reactions and cellular responses. Our analysis using STRING database demonstrated 57 highly connected nodes or hub proteins in the functional network of C. pseudotuberculosis. This finding shows that the formation of C. pseudotuberculosis biofilm is carefully coordinated by multiple biological pathways (Huang et al., 2019). According to Folador et al. (2016), hub proteins in C. pseudotuberculosis represent essential proteins which cause death upon removal from the organism. Therefore, hub proteins identified in C. pseudotuberculosis biofilm formation may have potential as therapeutic targets for CLA disease control. Previous works have also reported the presence of hub proteins in other biofilm forming pathogens (Yahya et al., 2017; Isa et al., 2022; Nematiasgarabad et al. 2024).
Functional enrichment analysis has been widely used to link the properties of protein sequences with relevant functional categories. Most functional enrichment tools are web-based and focused on specific contexts. Functional enrichment analysis using STRING database demonstrated in the present study successfully characterized
C. pseudotuberculosis proteome. It allows identification of various biological processes and pathways involved in the formation of
C. pseudotuberculosis biofilm by quantitative measurement using a common and well-known statistical method namely Fisher's exact test (Szklarczyk
et al., 2017). We found that biosynthesis of amino acids and biosynthesis of secondary metabolites (
Figure 8) were significantly enriched (p<0.005) in the network of differentially expressed proteins. This result agrees with Santos
et al. (2018) reporting the similar significant pathways in the proteome dataset of
C. pseudotuberculosis collected from the NCBI GenPept database. The use of bioinformatics tools is vital to advance global functional analysis of
C. pseudotuberculosis genome (da Silva
et al., 2021)
GalT is a protein responsible for breaking down galactose via Leloir pathway which plays role in exopolysaccharides biosynthesis. The catalytic activity of this protein is dependent on metallic trace element such as zinc and iron. In the present study, galactose-1-phosphate uridyltransferase was found to be expressed in planktonic fraction but was not expressed in biofilm fraction. This result corroborates de Sá et al. (2021) demonstrating differential expression of GalT in C. pseudotuberculosis biofilm (CAPJ4 strain). The GalT family member is required for the regulation of biofilm formation through purine biosynthesis (Monds et al., 2010). It is possible that the expression of this protein family is required during early biofilm stage but is not required when C. pseudotuberculosis has reached the mature and heterogeneous biofilm stage.
clpB plays role in ATP binding, protein folding and stress response which promotes the survival of microbial cells. The present study showed that ATP-dependent chaperone clpB was expressed in biofilm fraction but was not expressed in planktonic fraction. According to Petrova and Sauer (2012), clpB is required for the function of chemotaxis protein BdlA in dispersion of Pseudomonas aeruginosa biofilm. This has led us to assume that the clpB expression may also be required for dispersion of C. pseudotuberculosis biofilm.
GrpE is a cochaperone that promotes dissociation of ADP from nucleotide-binding cleft of DnaK. GrpE plays prominent roles in protein folding. The present study showed that nucleotide exchange factor GrpE was expressed in planktonic fraction but was not expressed in biofilm fraction. Changes in the expression of this protein during biofilm formation is not well investigated, however, Gomide et al. (2018) has demonstrated differential expression of grpE gene in C. pseudotuberculosis following heat shock stress. It is likely that GrpE is required during the transition from planktonic stage to biofilm stage of C. pseudotuberculosis.
Our in silico data has predicted that this hypothetical protein may function as a transmembrane protein that participates in peptidoglycan metabolism. This suggestion is in line with the fact that transmembrane protein complexes play important roles in peptidoglycan metabolism (Formstone et al., 2008). Differential expression of this hypothetical protein during the biofilm formation by C. pseudotuberculosis is consistent with Li et al. (2014) demonstrating differential expression of proteins involved in peptidoglycan biosynthesis during the formation of Actinobacillus pleuropneumoniae biofilm. In the present study, we used re-centering function available in STRING database to analyze the network of differentially expressed C. pseudotuberculosis proteins in detail, thereby allowing identification of peptidoglycan biosynthetic pathway. That database function enables user to identify what types of proteins exist around the protein of interest in protein-protein interaction network which is useful to unravel new protein function that is hidden in large and complex biological networks (Praneenararat et al., 2012).
Conclusion
The present study demonstrates the whole-cell proteome expression of C. pseudotuberculosis biofilm. The biofilm formation by C. pseudotuberculosis was found to result in differential expression of proteins and pathways associated with multiple metabolic pathways including amino acid biosynthesis and secondary metabolite biosynthesis. The findings from this research may be useful to enhance understanding on how to contain CLA disease.
Acknowledgements
This research was funded by the Pembiayaan Yuran Penerbitan Artikel (PYPA), Universiti Teknologi MARA.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Statement
This research does not require ethical approval.
References
- Abd Rashid, S. A.; Ahmad, A.; Idris, H.; Yahya, M. F. Z. R. Evaluation of antibiofilm efficacy of actinomycetes isolated from BRIS soil of Terengganu against Corynebacterium pseudotuberculosis. In International Conference on Science Technology and Social Sciences–Biology Track (ICONSTAS-BIO 2023); Atlantis Press, 2024; pp. 17–28. [Google Scholar]
- Akanuma, G.; Nanamiya, H.; Natori, Y.; Yano, K.; Suzuki, S.; Omata, S.; Ishizuka, M.M.; Sekine, Y.; Kawamura, F. Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation. Journal of Bacteriology 2012, 194, 6282–6291. [Google Scholar] [CrossRef] [PubMed]
- Amran, S. S. D.; Syaida, A. A. R.; Jalil, M. T. M.; Nor, N. H. M.; Yahya, M. F. Z. R. Preparation of Biofilm Assay Using 96-Well and 6-Well Microplates for Quantitative Measurement and Structural Characterization: A Review. Science Letters 2024, 18(2), 121–134. [Google Scholar]
- Araújo, C.L.; Alves, J.; Nogueira, W.; Pereira, L.C.; Gomide, A.C.; Ramos, R.; Azevedo, V.; Silva, A.; Folador, A. Prediction of new vaccine targets in the core genome of Corynebacterium pseudotuberculosis through omics approaches and reverse vaccinology. Gene 2019, 702, 36–45. [Google Scholar] [CrossRef]
- Braithwaite, C.E.; Smith, E.E.; Songer, J.G.; Reine, A.H. Characterization of detergent-soluble proteins of Corynebacterium pseudotuberculosis. Veterinary Microbiology 1993, 38(1-2), 59–70. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.M.; Seyffert, N.; Silva, A.; Azevedo, V. A journey through the Corynebacterium pseudotuberculosis proteome promotes insights into its functional genome. PeerJ 2021, 9, e12456. [Google Scholar] [CrossRef]
- de Sá, M.C.A.; Da Silva, W.M.; Rodrigues, C.C.S.; Rezende, C.P.; Marchioro, S.B.; Rocha; Filho, J.T. R.; de Sousa, T.J.; Oliveira, H.P.; Costa, M.M.; Figueiredo, H.C.P.; Portela, R.D.; Castro, T.L.P.; Azevedo, V.; Seyffert, N.; Meyer, R. Comparative proteomic analyses between biofilm-forming and non-biofilm-forming strains of Corynebacterium pseudotuberculosis isolated from goats. Frontiers in Veterinary Science 2021, 8, 614011. [Google Scholar]
- dos Santos, R.M.; Cerqueira, S.M.A.; Andrade, C.L.B.; Müller, G.S.; de Menezes Santos, V.C.; de Araújo, H.R.; Queiroz, S.; Conceicao, R.R.; Oliveira, L.G.F.; Ribeiro, M.B.; Marchioro, S.; de Moura-costa, L.F.; de Sousa, F.S.C.; de Sá, M.D.C.A.; Filho, J.T.R.R.; Trindade, S.C.; Netto, E. M.; Meyer, R.; Freire, S.M. Human seroreactivity to secreted molecules of Corynebacterium pseudotuberculosis. Advances in Microbiology 2022, 12(3), 150–158. [Google Scholar] [CrossRef]
- Eberle, R.J.; Kawai, L.A.; de Moraes, F.R.; Tasic, L.; Arni, R.K.; Coronaso, M.A. Biochemical and biophysical characterization of a mycoredoxin protein glutaredoxin A1 from Corynebacterium pseudotuberculosis. International Journal of Biological Macromolecules 2017, 107(Part B), 1999–2007. [Google Scholar] [CrossRef]
- Folador, E.L.; de Carvalho, P.V.S.D.; Silva, W.M.; Ferreira, R.S.; Silva, A.; Gromiha, M.; Ghosh, P.; Barth, D.; Azevedo, V.; Röttger, R. In silico identification of essential proteins in Corynebacterium pseudotuberculosis based on protein-protein interaction networks. BMC Systems Biology 2016, 10, 103. [Google Scholar] [CrossRef]
- Formstone, A.; Carballido-López, R.; Noirot, P.; Errington, J.; Scheffers, D.J. Localization and interactions of teichoic acid synthetic enzymes in Bacillus subtilis. Journal of Bacteriology 2008, 190(5), 1812–1821. [Google Scholar] [CrossRef]
- Gomide, A.C.P.; de Sá, P.G.; Cavalcante, A.L.Q.; de Jesus Sousa, T.; Gomes, L.G.R.; Ramos, R.T.J.; Azevedo, V.; Silva, A.; Folador, A.R.C. Heat shock stress: Profile of differential expression in Corynebacterium pseudotuberculosis biovar equi. Gene 2017, 645, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Gomide, A.C.P.; Ibraim, I.C.; Alves, J.T.C.; de Sá, P.G.; de Oliveira Silva, Y.R.; Santana, M.P.; Silva, W.M.; Folador, E.L.; Mariano, D.C.B.; de Paula Castro, T.L.; Barbosa, S.; Dorella, F.A.; Carvalho, A.F.; Pereira, F.L.; Leal, C.A.G.; Figueiredo, H.C.P.; Azevedo, V.; Silva, A.; Folador, A.R.C. Transcriptome analysis of Corynebacterium pseudotuberculosis biovar equi in two conditions of the environmental stress. Gene 2018, 677, 349–360. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.H.; Zhu, H.Z.; Andrianova, E.P.; Jiang, C.Y.; Li, D.; Ma, L.; Feng, J.; Liu, Z.P.; Xiang, H.; Zhulin, I.B.; Liu, S.J. Cross talk between chemosensory pathways that modulate chemotaxis and biofilm formation. American Society for Microbiology 2019, 10(1), e02876–18. [Google Scholar] [CrossRef]
- Isa, S.F.M.; Hamida, U.M.A.; Yahya, M.F.Z.R. Treatment with the combined antimicrobials triggers proteomic changes in Pseudomonas aeruginosa–Candida albicans polyspecies biofilms. ScienceAsia 2022, 48(2), 215–222. [Google Scholar]
- Li, L.; Zhu, J.; Yang, K.; Xu, Z.; Liu, Z.; Zhou, R. Changes in gene expression of Actinobacillus pleuropneumoniae in response to anaerobic stress reveal induction of central metabolism and biofilm formation. Journal of Microbiology 2014, 52(6), 473–481. [Google Scholar] [CrossRef]
- Monds, R.D.; Newell, P.D.; Wagner, J.C.; Schwartzman, J.A.; Lu, W.; Rabinowitz, J.D.; O'Toole, G. A. Di-adenosine tetraphosphate (Ap4A) metabolism impacts biofilm formation by Pseudomonas fluorescens via modulation of c-di-GMP-dependent pathways. Journal of Bacteriology 2010, 192(12), 3011–3023. [Google Scholar] [CrossRef]
- Nematiasgarabad, P.; Hashim, N. A. N.; Yahya, M. F. Z. R. In silico elucidation of protein-protein interaction network in fish pathogen Flavobacterium Columnare. Malaysian Applied Biology 2024, 53(3), 137–146. [Google Scholar] [CrossRef]
- Othman, N.A.; Yahya, M.F.Z.R. In silico analysis of essential and non-homologous proteins in Salmonella typhimurium biofilm. Journal of Physics: Conference Series 2019, 1349(1), 012133. [Google Scholar] [CrossRef]
- Rashid, S.A.A.; Yaacob, M.F.; Aazmi, M.S.; Jesse, F.F.A.; Yahya, M.F.Z.R. Inhibition of Corynebacterium pseudotuberculosis biofilm by DNA and protein synthesis inhibitors. Journal of Sustainability Science and Management 2022, 17(4), 49–56. [Google Scholar] [CrossRef]
- Santos, E.M.S.; Almeida, A.C.; Santos, H.O.; Cangussu, A.S.R.; Almeida, D.A.; Costa, K.S. Leader gene of Corynebacterium pseudotuberculosis may be useful in vaccines against caseous lymphadenitis of goats: a bioinformatics approach. Journal of Veterinary Medical Science 2018, 80(8), 1317–1324. [Google Scholar] [CrossRef]
- Safini, I. N. M.; Zakaria, N. F. S.; Saad, M. I. H.; Yahya, M. F. Z.; Jamil, N. M. Understanding Bacterial Persistence under Antibiotic Pressure: A Review. Science Letters 2024, 18(2), 56–69. [Google Scholar]
- Schirmer, E.C.; Florens, L.; Guan, T.; Yates, J.R.; Gerace, L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science 2003, 301(5638), 1380–1382. [Google Scholar] [PubMed]
- Soares, S.C.; Trost, E.; Ramos, R.T.J.; Carneiro, A.R.; Santos, A.R.; Pinto, A.C.; Barbosa, E.; Aburjaile, F.; Ali, A.; Diniz, C.A.A.; Hassan, S.S.; Fiaux, K.; Guimarães, L.C.; Bakhtiar, S.M.; Pereira, U.; Almeida, S.S.; Abreu, V.A.C.; Rocha, F.S.; Dorella, F.A.; Miyoshi, A.; Silva, A.; Azevedo, V.; Tauch, A. Genome sequence of Corynebacterium pseudotuberculosis biovar equi strain 258 and prediction of antigenic targets to improve biotechnological vaccine production. Journal of Biotechnology 2013, 167(2), 135–141. [Google Scholar] [CrossRef]
- Suryaletha, K.; Narendrakumar, L.; John, J.; Radhakrishnan, M.P.; George, S.; Thomas, S. Decoding the proteomic changes involved in the biofilm formation of Enterococcus faecalis SK460 to elucidate potential biofilm determinants. BMC Microbiology 2019, 19, 146. [Google Scholar]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N. T.; Roth, A.; Bork, P.; Jensen, L. J.; von Mering, C. The STRING database in 2017: quality-controlled protein-protein interaction networks, made broadly accessible. Nucleic Acid Research 2017, 45(D1), D362–D368. [Google Scholar]
- Yaacob, M.F.; Murata, A.; Nor, N.H.M.; Jesse, F.F.A.; Yahya, M.F.Z.R. Biochemical composition, morphology and antimicrobial susceptibility pattern of Corynebacterium pseudotuberculosis biofilm. Journal of King Saud University–Science 2021a, 33(1), 101225. [Google Scholar]
- Yaacob, M.F.; Johari, N.A.; Kamaruzzaman, A.N.A.; Yahya, M.F.Z.R. Mass spectrometry-based proteomic investigation of heterogeneous biofilms: A review. Scientific Research Journal 2021b, 18(2), 67–87. [Google Scholar]
- Yahya, M.F.Z.R.; Alias, Z.; Karsani, S.A. Subtractive protein profiling of Salmonella typhimurium biofilm treated with DMSO. The Protein Journal 2017, 36, 286–298. [Google Scholar]
- Zawawi, W.M.A.W.M.; Ibrahim, M.S.A.; Rahmad, N.; Hamid, U.M.A.; Yahya, M.F.Z.R. Proteomic analysis of Pseudomonas aeruginosa biofilm treated with Chromolaena odorata extracts. Malaysian Journal of Microbiology 2020, 16(2), 124–133. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).